Photoacoustic Properties of Polypyrrole Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanoparticle Synthesis
2.2. Physical Characterization of the Nanoparticles
2.3. In Vitro Photoacoustic Imaging
2.4. Nanoparticle Coating
- (1)
- An aqueous solution of PLL or Pluronic F-127 (0.2 mL, 30 mg/mL) filtered through a sterile syringe filter (polyvinylidene difluoride membrane (PVDF), 220 nm) was dissolved in 0.985 mL of water (Aqua pro injectione) and consequently 1.015 mL of PPy sterilized colloid (19.7 mg/mL) was added dropwise under sonication (1 mm tip diameter; 20 W) and finally sonicated for 2 min;
- (2)
- PPy sterilized colloid (1.015 mL, 19.7 mg/mL) was diluted with 1.985 mL of water (Aqua pro injectione) and 0.2 mL of filtered (PVDF, 220 nm) poly(N,N-dimethylacrylamide)-b-poly(2,2,2-trifluoroethyl acrylate), Mw = 10-b-10 kDa (abrev. pDMAAm-b-pTFEA; DB3) solution in acetone was added dropwise under sonication, sonicated for 2 min and finally reduced in septum sealed ampule on vacuo to volume 2 mL. pDMAAm was chosen for its previously reported suitability for particle biocompatible coating [38] and pTFEA was chosen due to its lipophilic character and light transparency. The main suggested coating mechanism is built on absorption of pTFEA block onto PPy surface, while water soluble pDMMAm block floats in dispersion medium and acts as steric stabilizer.
2.5. Cell Proliferation in the Presence of the Nanoparticles
2.6. In Vivo High-Frequency Ultrasound and Photoacoustic Imaging
3. Results
3.1. Chemical and Physical Characteristics
3.2. In Vitro Photoacoustics
3.3. Cell Proliferation in the Presence of the Nanoparticles
3.4. In Vivo Imaging
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.V.; Hu, S. Photoacoustic Tomography: In Vivo Imaging from Organelles to Organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attia, A.B.E.; Balasundaram, G.; Moothanchery, M.; Dinish, U.; Bi, R.; Ntziachristos, V.; Olivo, M. A review of clinical photoacoustic imaging: Current and future trends. Photoacoustics 2019, 16, 100144. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, I.; Huland, D.M.; Vermesh, O.; Frostig, H.E.; Tummers, W.S.; Gambhir, S.S. Photoacoustic clinical imaging. Photoacoustics 2019, 14, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.G. On the production and reproduction of sound by light. Am. J. Sci. 1880, 29, 305–324. [Google Scholar] [CrossRef] [Green Version]
- Razansky, D. Optoacoustic Imaging. In Comprehensive Biomedical Physics; Brahme, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 281–300. [Google Scholar] [CrossRef]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Maturi, M.; Locatelli, E.; Monaco, I.; Franchini, M.C. Current concepts in nanostructured contrast media development for in vivo photoacoustic imaging. Biomater. Sci. 2019, 7, 1746–1775. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Huang, L.; Jiang, M.S.; Jiang, H. Contrast Agents for Photoacoustic and Thermoacoustic Imaging: A Review. Int. J. Mol. Sci. 2014, 15, 23616–23639. [Google Scholar] [CrossRef]
- Borg, R.E.; Rochford, J. Molecular Photoacoustic Contrast Agents: Design Principles & Applications. Photochem. Photobiol. 2018, 94, 1175–1209. [Google Scholar] [CrossRef]
- Laramie, M.D.; Smith, M.K.; Marmarchi, F.; McNally, L.R.; Henary, M. Small Molecule Optoacoustic Contrast Agents: An Unexplored Avenue for Enhancing In Vivo Imaging. Molecules 2018, 23, 2766. [Google Scholar] [CrossRef] [Green Version]
- Benson, R.C.; Kues, H.A. Absorption and fluorescence properties of cyanine dyes. J. Chem. Eng. Data 1977, 22, 379–383. [Google Scholar] [CrossRef]
- Luke, G.; Yeager, D.; Emelianov, S.Y. Biomedical Applications of Photoacoustic Imaging with Exogenous Contrast Agents. Ann. Biomed. Eng. 2012, 40, 422–437. [Google Scholar] [CrossRef]
- Pan, D.; Kim, B.; Wang, L.; Lanza, G.M. A brief account of nanoparticle contrast agents for photoacoustic imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 517–543. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.; Ji, X.; Luo, J. Rational nanocarrier design towards clinical translation of cancer nanotherapy. Biomed. Mater. 2021, 16, 032005. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, S.M.; Fleischmann, D.; Goepferich, A. Biomedical nanoparticle design: What we can learn from viruses. J. Control. Release 2021, 329, 552–569. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Sreeharsha, N.; Chitrapriya, N.; Jang, Y.J.; Kenchappa, V. Evaluation of nanoparticle drug-delivery systems used in preclinical studies. Ther. Deliv. 2021, 12, 325–336. [Google Scholar] [CrossRef]
- Jain, P.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated Absorption and Scattering Properties of Gold Nanoparticles of Different Size, Shape, and Composition: Applications in Biological Imaging and Biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [Green Version]
- Gao, D.; Yuan, Z. Photoacoustic-Based Multimodal Nanoprobes: From Constructing to Biological Applications. Int. J. Biol. Sci. 2017, 13, 401–412. [Google Scholar] [CrossRef] [Green Version]
- Lemaster, J.E.; Jokerst, J.V. What is new in nanoparticle-based photoacoustic imaging? Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Reguera, J.; de Aberasturi, D.J.; Henriksen-Lacey, M.; Langer, J.; Espinosa, A.; Szczupak, B.; Wilhelm, C.; Liz-Marzán, L.M. Janus plasmonic–magnetic gold–iron oxide nanoparticles as contrast agents for multimodal imaging. Nanoscale 2017, 9, 9467–9480. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, X.; Zhao, C.; Shao, L.; Lu, J.; Tong, Y.; Chen, L.; Cui, X.; Sun, H.; Liu, J.; et al. From one to all: Self-assembled theranostic nanoparticles for tumor-targeted imaging and programmed photoactive therapy. J. Nanobiotechnol. 2019, 17, 1–12. [Google Scholar] [CrossRef]
- Ma, W.; Wang, J. Theranostics of Gold Nanoparticles with an Emphasis on Photoacoustic Imaging and Photothermal Therapy. Curr. Pharm. Des. 2018, 24, 2719–2728. [Google Scholar] [CrossRef]
- Men, X.; Yuan, Z. Multifunctional conjugated polymer nanoparticles for photoacoustic-based multimodal imaging and cancer photothermal therapy. J. Innov. Opt. Health Sci. 2019, 12, 1930001. [Google Scholar] [CrossRef] [Green Version]
- Phan, T.T.V.; Bui, N.Q.; Cho, S.-W.; Bharathiraja, S.; Manivasagan, P.; Moorthy, M.S.; Mondal, S.; Kim, C.-S.; Oh, J. Photoacoustic Imaging-Guided Photothermal Therapy with Tumor-Targeting HA-FeOOH@PPy Nanorods. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Luciano, M.; Erfanzadeh, M.; Zhou, F.; Zhu, H.; Bornhütter, T.; Röder, B.; Zhu, Q.; Brückner, C. In vivo photoacoustic tumor tomography using a quinoline-annulated porphyrin as NIR molecular contrast agent. Org. Biomol. Chem. 2017, 15, 972–983. [Google Scholar] [CrossRef] [Green Version]
- de Sousa, L.E.; de Paiva, L.S.R.; Filho, D.A.D.S.; Sini, G.; Neto, P.H.D.O. Assessing the effects of increasing conjugation length on exciton diffusion: From small molecules to the polymeric limit. Phys. Chem. Chem. Phys. 2021, 23, 15635–15644. [Google Scholar] [CrossRef]
- Gürbüz, O.; Şenkal, B.F.; Içelli, O. Structural, optical and electrical properties of polypyrrole in an ionic liquid. Polym. Bull. 2017, 74, 2625–2639. [Google Scholar] [CrossRef]
- Liang, X.; Li, Y.; Li, X.; Jing, L.; Deng, Z.; Yue, X.; Li, C.; Dai, Z. PEGylated Polypyrrole Nanoparticles Conjugating Gadolinium Chelates for Dual-Modal MRI/Photoacoustic Imaging Guided Photothermal Therapy of Cancer. Adv. Funct. Mater. 2015, 25, 1451–1462. [Google Scholar] [CrossRef]
- Manivasagan, P.; Bui, N.Q.; Bharathiraja, S.; Moorthy, M.S.; Oh, Y.-O.; Song, K.; Seo, H.; Yoon, M.; Oh, J. Multifunctional biocompatible chitosan-polypyrrole nanocomposites as novel agents for photoacoustic imaging-guided photothermal ablation of cancer. Sci. Rep. 2017, 7, 43593. [Google Scholar] [CrossRef] [Green Version]
- Zha, Z.; Deng, Z.; Li, Y.; Li, C.; Wang, J.; Wang, S.; Qu, E.; Dai, Z. Biocompatible polypyrrole nanoparticles as a novel organic photoacoustic contrast agent for deep tissue imaging. Nanoscale 2013, 5, 4462–4467. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Zhang, L.; Zhu, H.; Chen, J.; Wu, D.; Bouakaz, A.; Wan, M.; Feng, Y. A Highly Efficient One-for-All Nanodroplet for Ultrasound Imaging-Guided and Cavitation-Enhanced Photothermal Therapy. Int. J. Nanomed. 2021, 16, 3105–3119. [Google Scholar] [CrossRef]
- Theune, L.; Buchmann, J.; Wedepohl, S.; Molina, M.; Laufer, J.; Calderón, M. NIR- and thermo-responsive semi-interpenetrated polypyrrole nanogels for imaging guided combinational photothermal and chemotherapy. J. Control. Release 2019, 311–312, 147–161. [Google Scholar] [CrossRef]
- Zeng, W.; Wu, X.; Chen, T.; Sun, S.; Shi, Z.; Liu, J.; Ji, X.; Zeng, X.; Guan, J.; Mei, L.; et al. Renal-Clearable Ultrasmall Polypyrrole Nanoparticles with Size-Regulated Property for Second Near-Infrared Light-Mediated Photothermal Therapy. Adv. Funct. Mater. 2021, 31, 2008362. [Google Scholar] [CrossRef]
- Paúrová, M.; Taboubi, O.; Šeděnková, I.; Hromádková, J.; Matouš, P.; Herynek, V.; Šefc, L.; Babič, M. Role of dextran in stabilization of polypyrrole nanoparticles for photoacoustic imaging. Eur. Polym. J. 2021, 157, 110634. [Google Scholar] [CrossRef]
- Paúrová, M.; Šeděnková, I.; Hromádková, J.; Babič, M. Polypyrrole nanoparticles: Control of the size and morphology. J. Polym. Res. 2020, 27, 1–10. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Babič, M.; Horák, D.; Jendelová, P.; Glogarová, K.; Herynek, V.; Trchová, M.; Likavčanová, K.; Lesný, P.; Pollert, E.; Hájek, M.; et al. Poly(N,N-dimethylacrylamide)-Coated Maghemite Nanoparticles for Stem Cell Labeling. Bioconjug. Chem. 2009, 20, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Xu, M.; Wang, L.V. Photoacoustic imaging in biomedicine. Rev. Sci. Instrum. 2006, 77, 041101. [Google Scholar] [CrossRef] [Green Version]
- Shahbazi, K.; Frey, W.; Chen, Y.-S.; Aglyamov, S.; Emelianov, S. Photoacoustics of core–shell nanospheres using comprehensive modeling and analytical solution approach. Commun. Phys. 2019, 2, 1–11. [Google Scholar] [CrossRef]
- Babič, M.; Horák, D.; Trchová, M.; Jendelova, P.; Glogarová, K.; Lesný, P.; Herynek, V.; Hájek, M.; Syková, E. Poly(l-lysine)-Modified Iron Oxide Nanoparticles for Stem Cell Labeling. Bioconjugate Chem. 2008, 19, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Garello, F.; Pasquino, C.; Arena, F.; Giustetto, P.; Antico, F.; Terreno, E. Indocyanine green labeling for optical and photoacoustic imaging of mesenchymal stem cells after in vivo transplantation. J. Biophotonics 2019, 12, e201800035. [Google Scholar] [CrossRef]
- Okumura, K.; Yoshida, K.; Yoshioka, K.; Aki, S.; Yoneda, N.; Inoue, D.; Kitao, A.; Ogi, T.; Kozaka, K.; Minami, T.; et al. Photoacoustic imaging of tumour vascular permeability with indocyanine green in a mouse model. Eur. Radiol. Exp. 2018, 2, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Lan, M.; Shen, D.; Fang, K.; Zhu, L.; Liu, Y.; Hao, L.; Li, P. Targeted Nanobubbles Carrying Indocyanine Green for Ultrasound, Photoacoustic and Fluorescence Imaging of Prostate Cancer. Int. J. Nanomed. 2020, 15, 4289–4309. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.E.; Jang, H.J.; Park, S.W.; Wei, J.; Cho, S.; Park, W.I.; Lee, B.R.; Yang, C.D.; Jung, Y.K. Folic Acid Functionalized Carbon Dot/Polypyrrole Nanoparticles for Specific Bioimaging and Photothermal Therapy. ACS Appl. Bio Mater. 2021, 4, 3453–3461. [Google Scholar] [CrossRef]
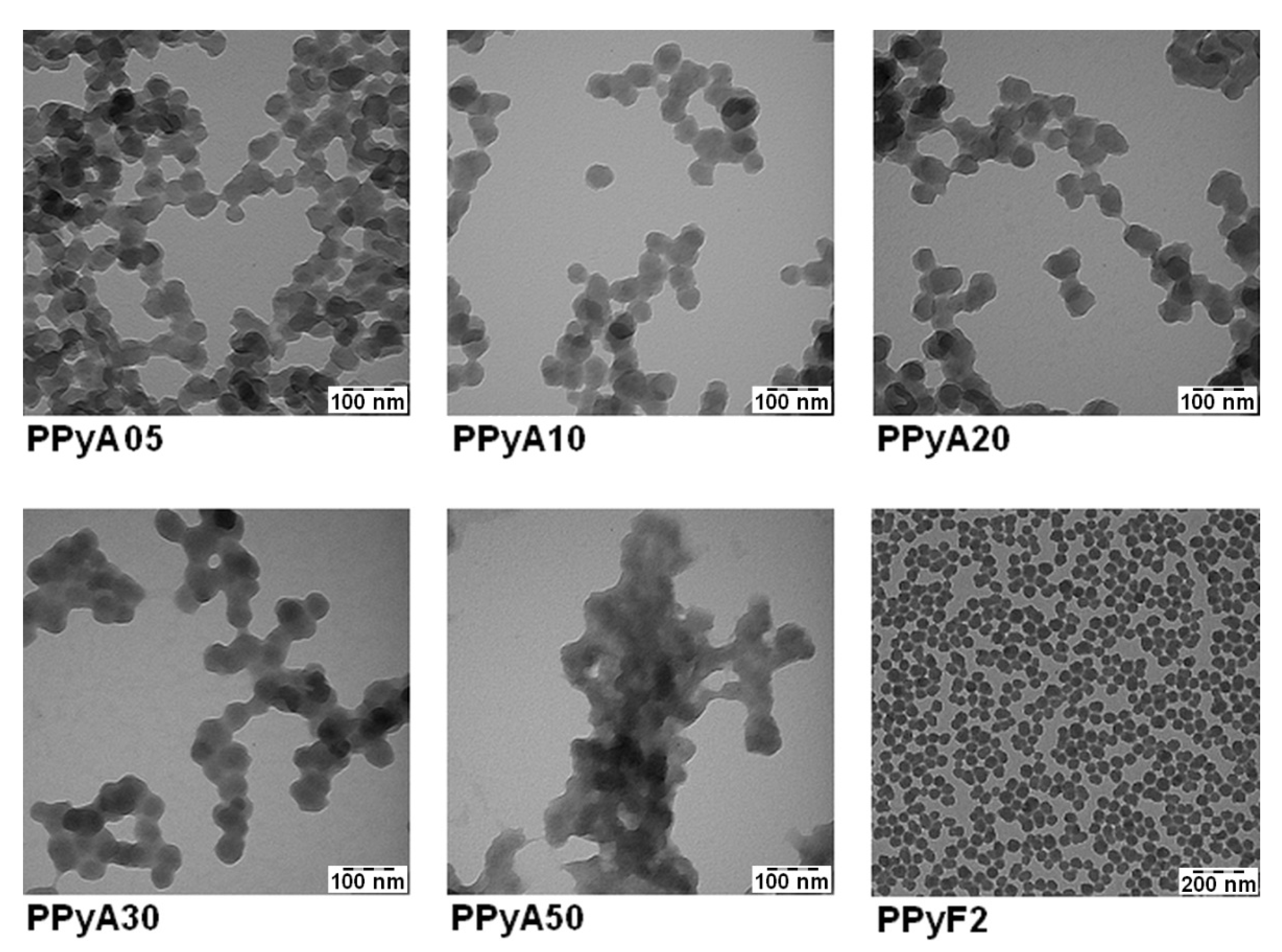
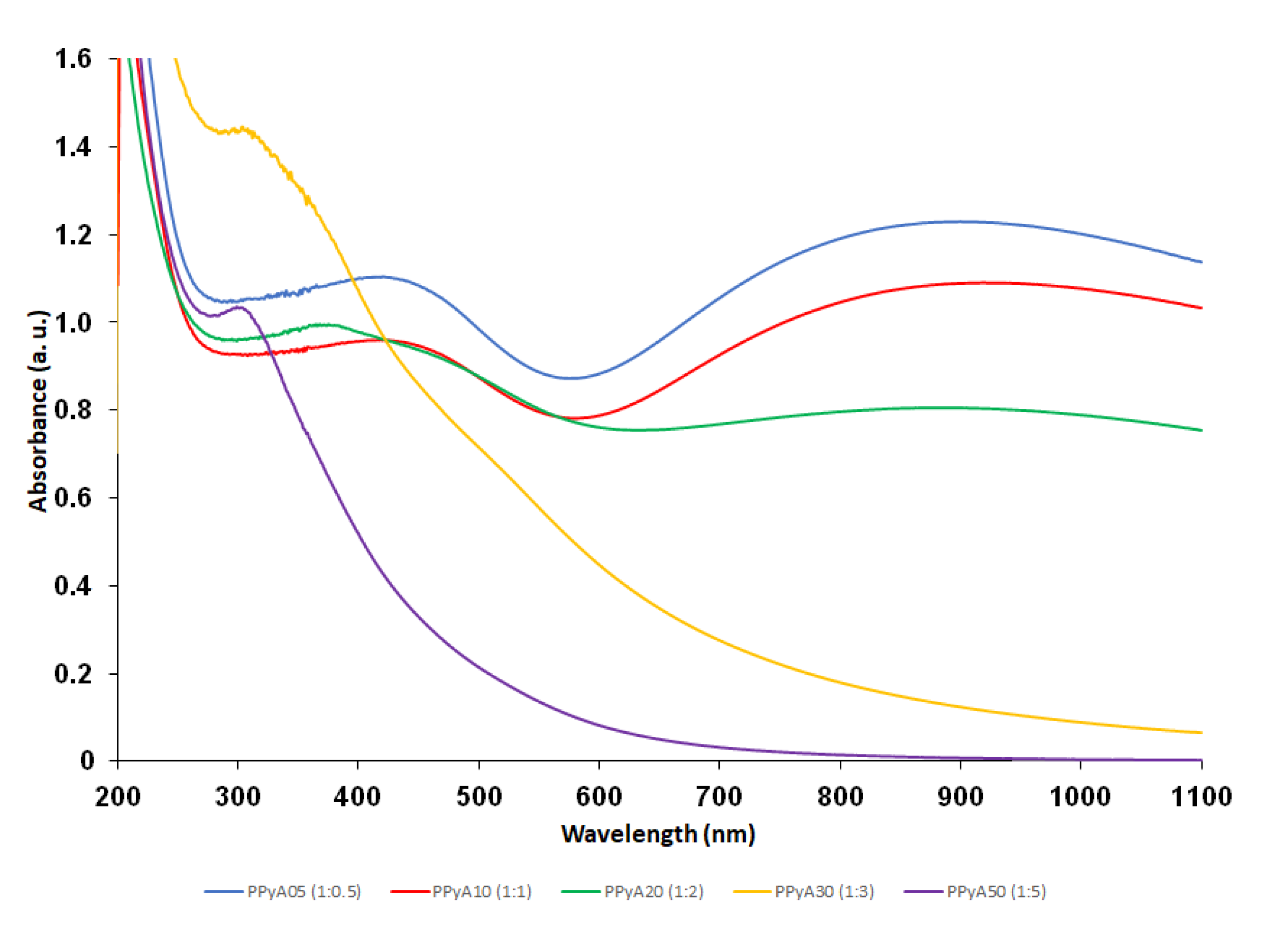
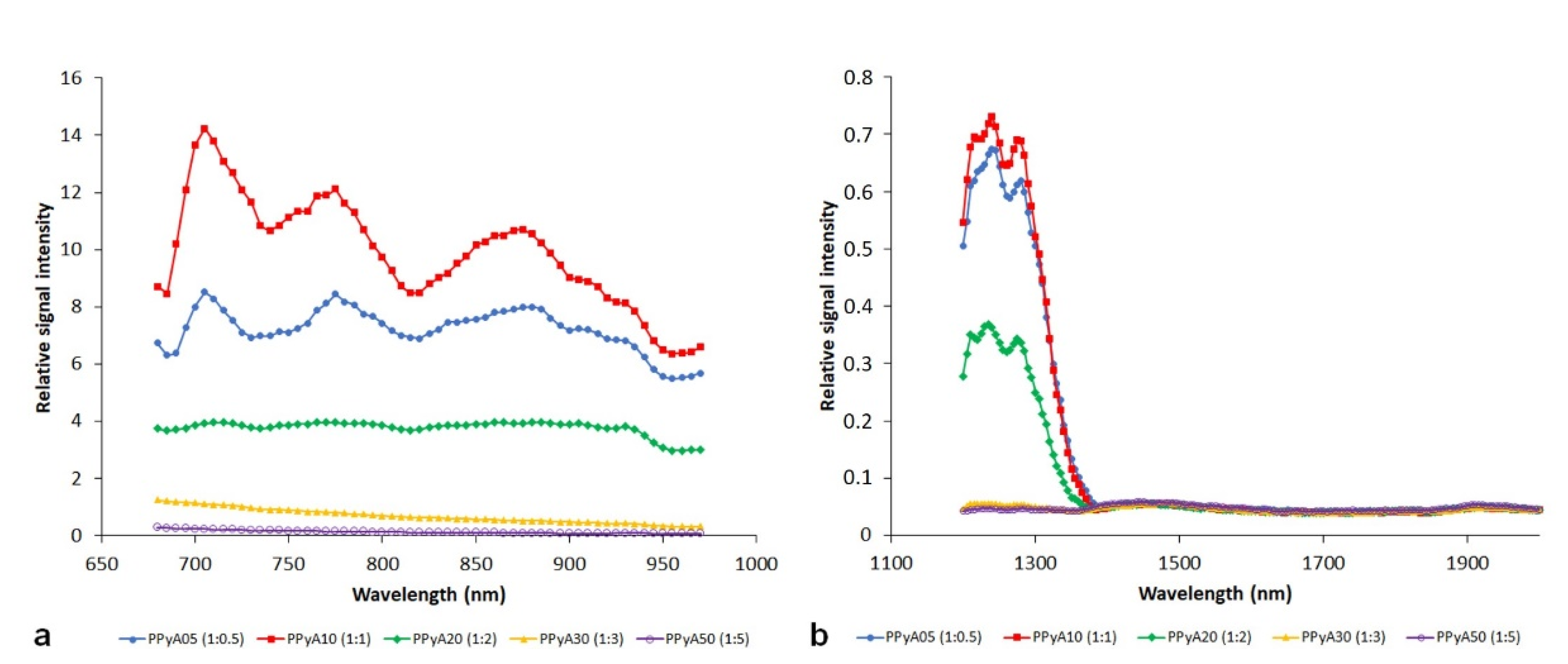
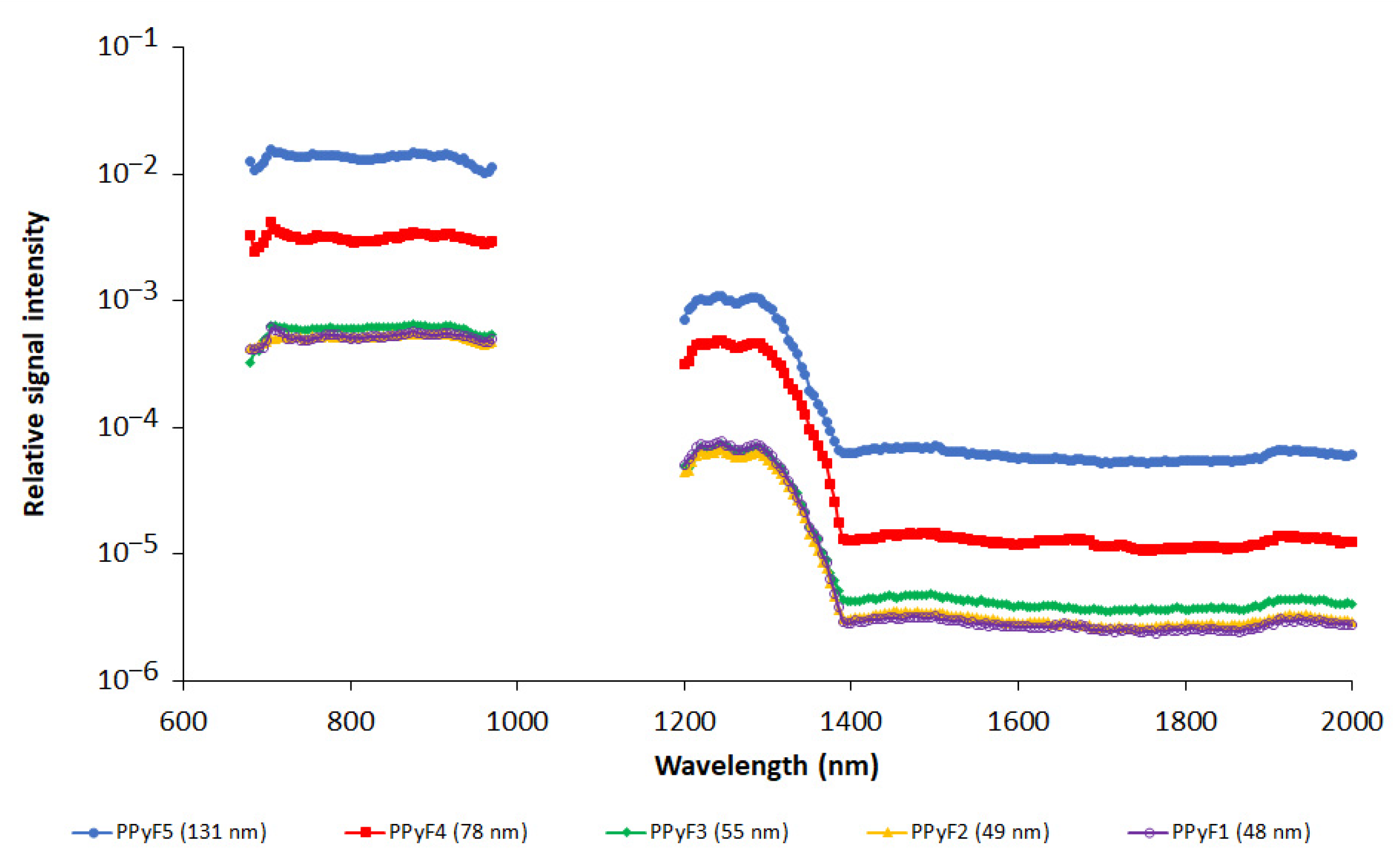
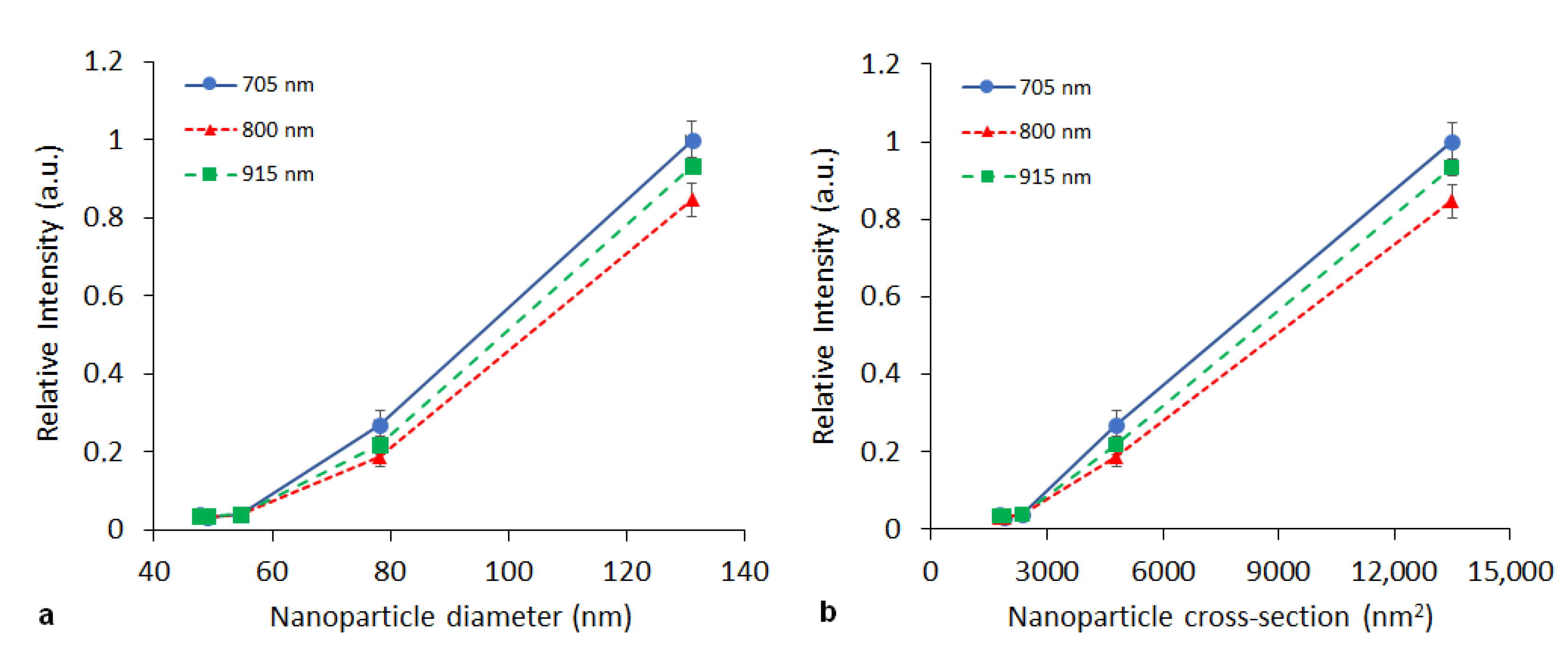
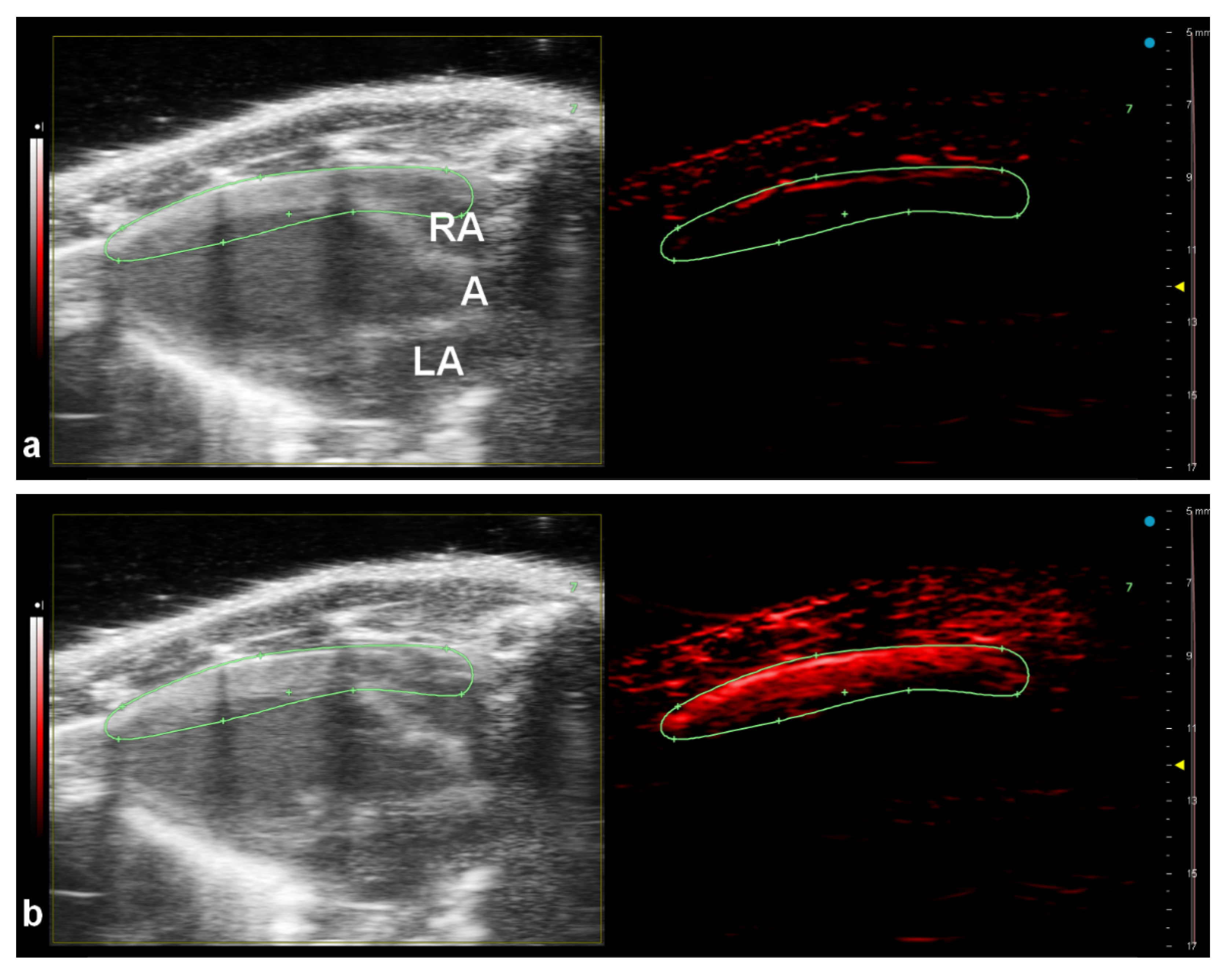
| Particle | nPy:nOxidant | Dn (nm) | Đ | Dh (nm) | PI | ζ (mV) * |
|---|---|---|---|---|---|---|
| PPyA05 | 1:0.5 APS | 37.8 | 1.06 | 218 ± 4 | 0.165 ± 0.006 | 2.2 ± 0.2 |
| PPyA10 | 1:1 APS | 42.3 | 1.04 | 302 ± 2 | 0.214 ± 0.013 | 1.57 ± 0.09 |
| PPyA20 | 1:2 APS | 43.2 | 1.05 | 452 ± 4 | 0.278 ± 0.012 | −0.1 ± 0.7 |
| PPyA30 | 1:3 APS | 50.1 | 1.11 | 428 ± 9 | 0.289 ± 0.160 | −4.7 ± 0.8 |
| PPyA50 | 1:5 APS | 44.5 | 1.09 | 490 ± 20 | 0.335 ± 0.027 | −8 ± 1 |
| PPyF1 | 1:2.3 FeCl3 | 47.6 | 1.15 | 110.3 ± 0.5 | 0.149 ± 0.008 | 28.2 ± 0.7 |
| PPyF2 | 1:2.3 FeCl3 | 49.1 | 1.04 | 112 ± 2 | 0.160 ± 0.010 | 21.3 ± 0.6 |
| PPyF3 | 1:2.3 FeCl3 | 54.6 | 1.04 | 126.0 ± 0.8 | 0.100 ± 0.012 | 29.2 ± 0.6 |
| PPyF4 | 1:2.3 FeCl3 | 78.2 | 1.04 | 134 ± 1 | 0.068 ± 0.014 | 36.5 ± 0.2 |
| PPyF5 | 1:2.3 FeCl3 | 131.1 | 1.12 | 226 ± 2 | 0.085 ± 0.015 | 42.5 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keša, P.; Paúrová, M.; Babič, M.; Heizer, T.; Matouš, P.; Turnovcová, K.; Mareková, D.; Šefc, L.; Herynek, V. Photoacoustic Properties of Polypyrrole Nanoparticles. Nanomaterials 2021, 11, 2457. https://doi.org/10.3390/nano11092457
Keša P, Paúrová M, Babič M, Heizer T, Matouš P, Turnovcová K, Mareková D, Šefc L, Herynek V. Photoacoustic Properties of Polypyrrole Nanoparticles. Nanomaterials. 2021; 11(9):2457. https://doi.org/10.3390/nano11092457
Chicago/Turabian StyleKeša, Peter, Monika Paúrová, Michal Babič, Tomáš Heizer, Petr Matouš, Karolína Turnovcová, Dana Mareková, Luděk Šefc, and Vít Herynek. 2021. "Photoacoustic Properties of Polypyrrole Nanoparticles" Nanomaterials 11, no. 9: 2457. https://doi.org/10.3390/nano11092457
APA StyleKeša, P., Paúrová, M., Babič, M., Heizer, T., Matouš, P., Turnovcová, K., Mareková, D., Šefc, L., & Herynek, V. (2021). Photoacoustic Properties of Polypyrrole Nanoparticles. Nanomaterials, 11(9), 2457. https://doi.org/10.3390/nano11092457






