One-Pot Synthesis of Chlorophyll-Assisted Exfoliated MoS2/WS2 Heterostructures via Liquid-Phase Exfoliation Method for Photocatalytic Hydrogen Production
Abstract
:1. Introduction
2. Materials and Methods
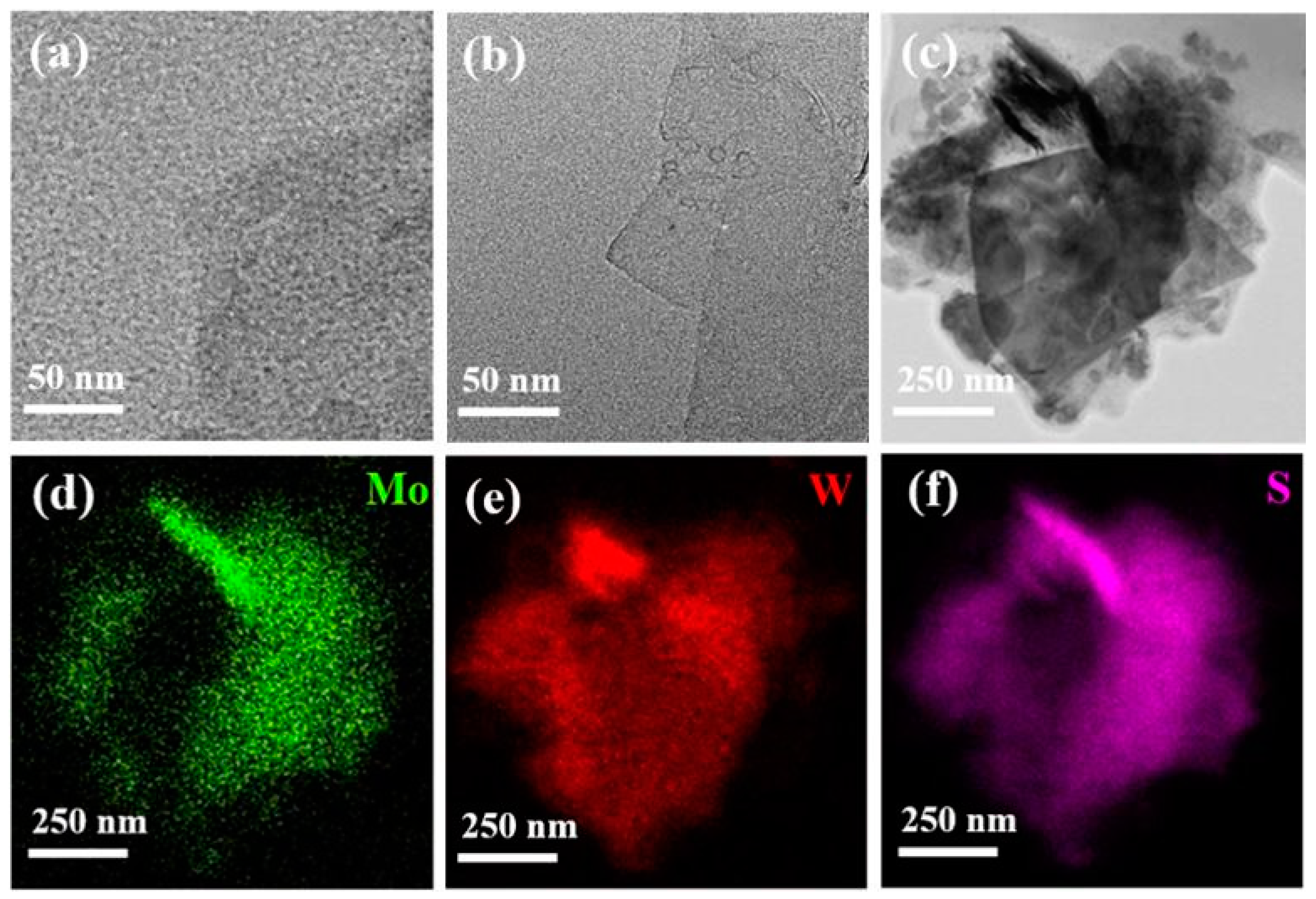
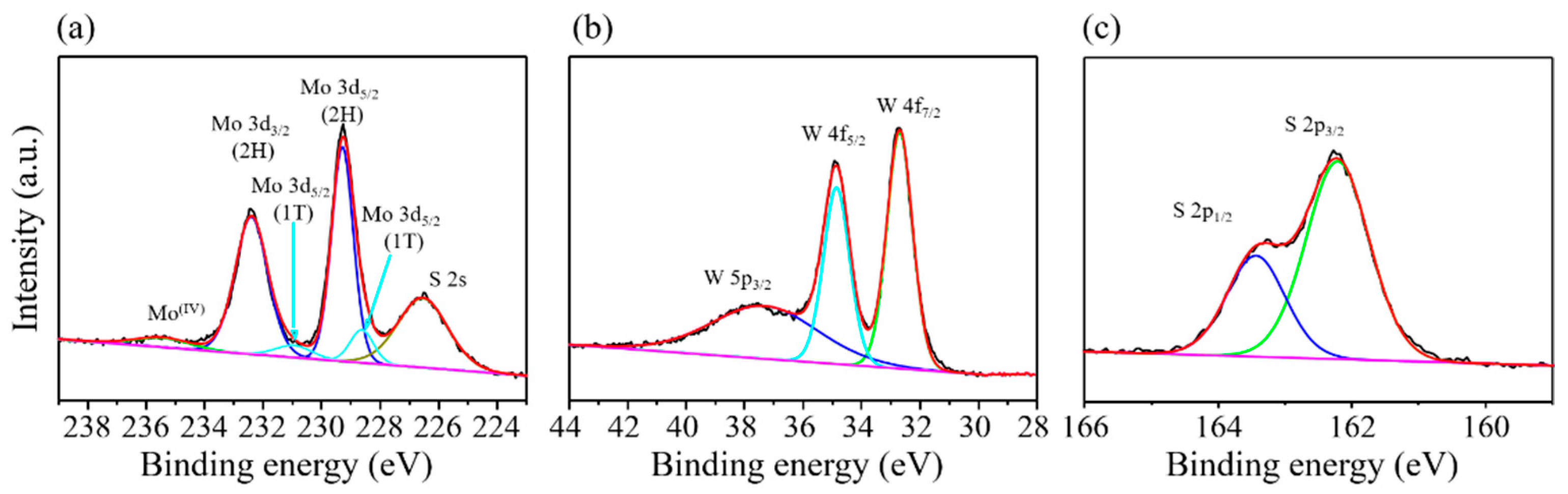
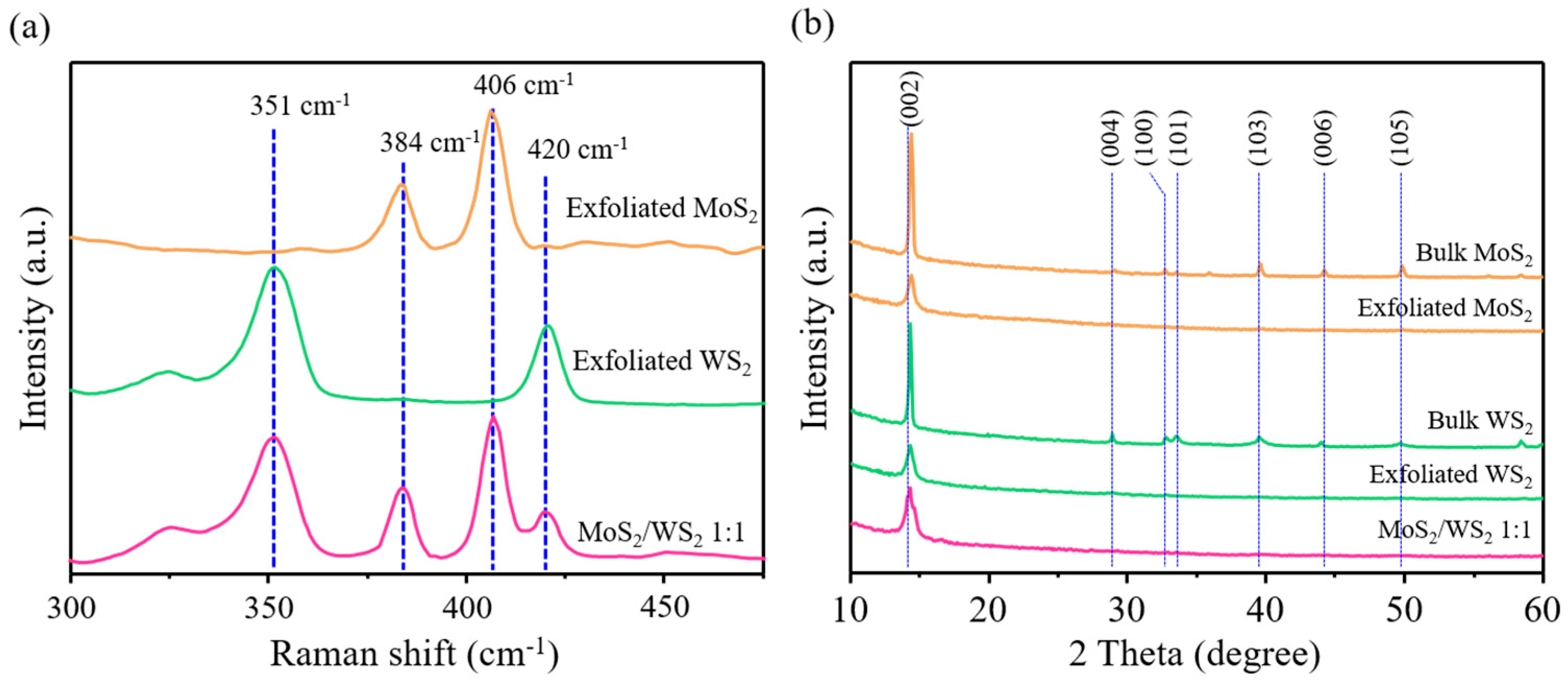
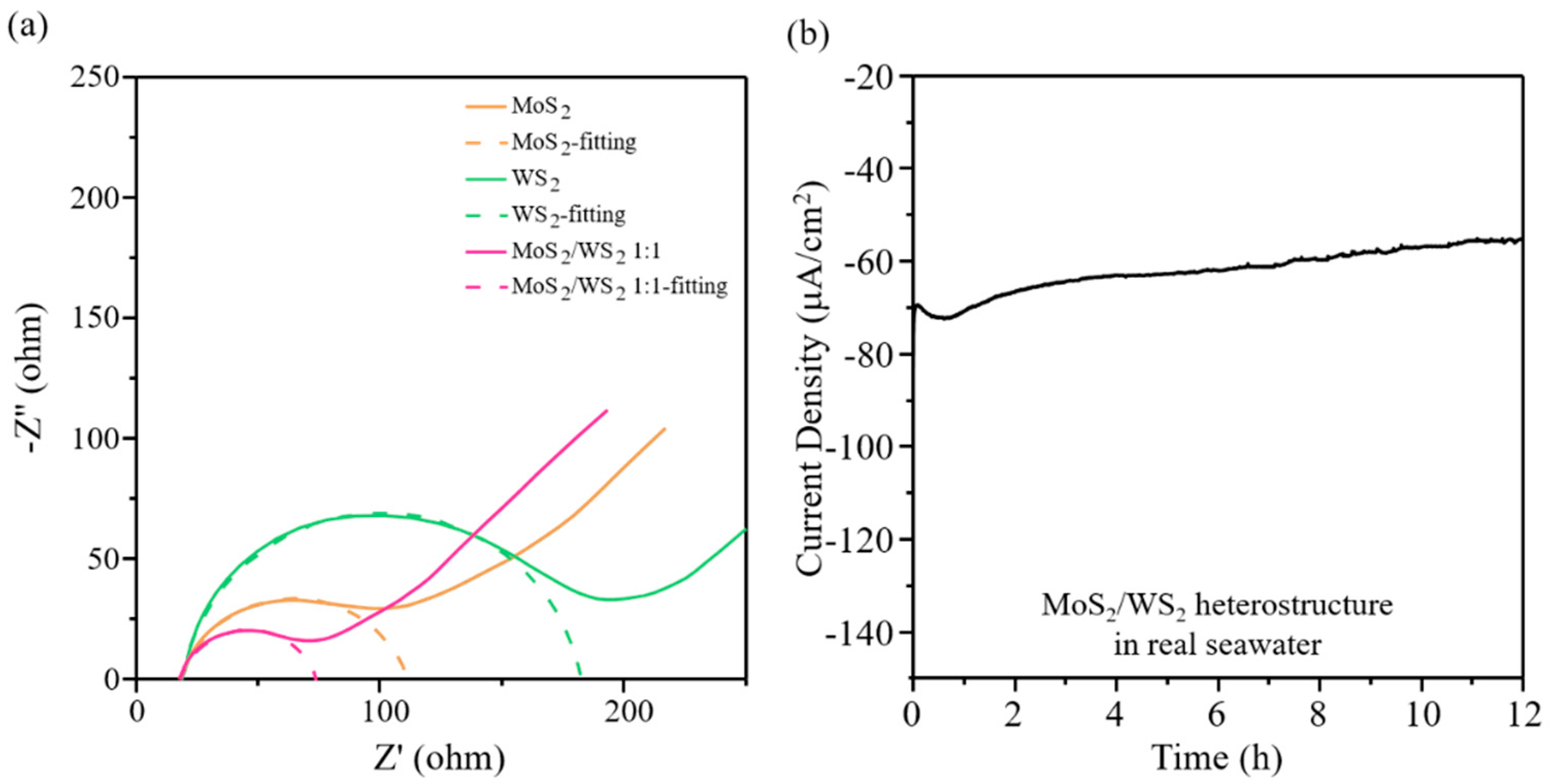
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dincer, I. Renewable Energy and Sustainable Development: A Crucial Review. Renew. Sust. Energy Rev. 2000, 4, 157–175. [Google Scholar] [CrossRef]
- Wang, J.; Feng, L.; Tang, X.; Bentley, Y.; Höök, M. The Implications of Fossil Fuel Supply Constraints on Climate Change Projections: A Supply-Side Analysis. Futures 2017, 86, 58–72. [Google Scholar] [CrossRef]
- Joshi, R.K.; Shukla, S.; Saxena, S.; Lee, G.H.; Sahajwalla, V.; Alwarappan, S. Hydrogen Generation via Photoelectrochemical Water Splitting Using Chemically Exfoliated MoS2 Layers. AIP Adv. 2016, 6, 015315. [Google Scholar] [CrossRef] [Green Version]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhao, Z.; Li, S.; Su, Y.; Huang, L.; Shao, N.; Liu, F.; Bu, Y.; Zhang, H.; Zhang, Z. Role of SnS2 in 2D-2D SnS2/TiO2 Nanosheet Heterojunctions for Photocatalytic Hydrogen Evolution. ACS Appl. Nano Mater. 2019, 2, 2144–2151. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of Cocatalysts in Photocatalysis and Photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, K.; Nan, F.; Wang, J.-H.; Yang, D.-J.; Zhou, L.; Wang, Q.-Q. Improved Hydrogen Production of Au-Pt-CdS Hetero-Nanostructures by Efficient Plasmon-Induced Multipathway Electron Transfer. Adv. Funct. Mater. 2016, 26, 6076–6083. [Google Scholar] [CrossRef]
- Luo, M.; Yao, W.; Huang, C.; Wu, Q.; Xu, Q. Shape Effects of Pt Nanoparticles on Hydrogen Production via Pt/CdS Photocatalysts Under Visible Light. J. Mater. Chem. A 2015, 3, 13884–13891. [Google Scholar] [CrossRef]
- Liu, W.; Shang, Y.; Zhu, A.; Tan, P.; Liu, Y.; Qiao, L.; Chu, D.; Xiong, X.; Pan, J. Enhanced Performance of Doped BiOCl Nanoplates for Photocatalysis: Understanding from Doping Insight Into Improved Spatial Carrier Separation. J. Mater. Chem. A 2017, 5, 12542–12549. [Google Scholar] [CrossRef]
- Wang, B.C.; Nisar, J.; Pathak, B.; Kang, T.W.; Ahuja, R. Band Gap Engineering in BiNbO4 for Visible-Light Photocatalysis. Appl. Phys. Lett. 2012, 100, 182102. [Google Scholar] [CrossRef]
- Zeng, W.; Bian, Y.; Cao, S.; Ma, Y.; Liu, Y.; Zhu, A.; Tan, P.; Pan, J. Phase Transformation Synthesis of Strontium Tantalum Oxynitride-Based Heterojunction for Improved Visible Light-Driven Hydrogen Evolution. ACS Appl. Mater. Interfaces 2018, 10, 21328–21334. [Google Scholar] [CrossRef] [PubMed]
- Lembke, D.; Bertolazzi, S.; Kis, A. Single-Layer MoS2 Electronics. Acc. Chem. Res. 2015, 48, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; He, S.; Wang, X.; Ma, Q.; Zhao, C. A BiOCl/β-FeOOH Heterojunction for HER Photocatalytic Performance Under Visible-Light Illumination. Int. J. Energy Res. 2019, 43, 2162–2171. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, Y.; Chen, H.; Xu, N.; Deng, S. Enhanced Performance of a Monolayer MoS2/WSe2 Heterojunction as a Photoelectrochemical Cathode. Nano-Micro Lett. 2018, 10, 60. [Google Scholar] [CrossRef] [Green Version]
- Kozawa, D.; Kumar, R.; Carvalho, A.; Kumar Amara, K.; Zhao, W.; Wang, S.; Toh, M.; Ribeiro, R.M.; Castro Neto, A.H.; Matsuda, K.; et al. Photocarrier Relaxation Pathway in Two-Dimensional Semiconducting Transition Metal Dichalcogenides. Nat. Commun. 2014, 5, 4543. [Google Scholar] [CrossRef]
- Singh, A.K.; Mathew, K.; Zhuang, H.L.; Hennig, R.G. Computational Screening of 2D Materials for Photocatalysis. J. Phys. Chem. Lett. 2015, 6, 1087–1098. [Google Scholar] [CrossRef]
- Su, T.; Shao, Q.; Qin, Z.; Guo, Z.; Wu, Z. Role of Interfaces in Two-Dimensional Photocatalyst for Water Splitting. ACS Catal. 2018, 8, 2253–2276. [Google Scholar] [CrossRef]
- Sherrell, P.C.; Palczynski, P.; Sokolikova, M.S.; Reale, F.; Pesci, F.M.; Och, M.; Mattevi, C. Large-Area CVD MoS2/WS2 Heterojunctions as a Photoelectrocatalyst for Salt-Water Oxidation. ACS Appl. Energy Mater. 2019, 2, 5877–5882. [Google Scholar] [CrossRef]
- Pesci, F.M.; Sokolikova, M.S.; Grotta, C.; Sherrell, P.C.; Reale, F.; Sharda, K.; Ni, N.; Palczynski, P.; Mattevi, C. MoS2/WS2 Heterojunction for Photoelectrochemical Water Oxidation. ACS Catal. 2017, 7, 4990–4998. [Google Scholar] [CrossRef] [Green Version]
- Chen, I.-W.P.; Shie, M.-Y.; Liu, M.-H.; Huang, W.-M.; Chen, W.-T.; Chao, Y.-T. Scalable Synthesis of Two-Dimensional Nano-Sheet Materials with Chlorophyll Extracts: Enhancing the Hydrogen Evolution Reaction. Green Chem. 2018, 20, 525–533. [Google Scholar] [CrossRef]
- Hong, X.; Kim, J.; Shi, S.F.; Zhang, Y.; Jin, C.; Sun, Y.; Tongay, S.; Wu, J.; Zhang, Y.; Wang, F. Ultrafast charge transfer in atomically thin MoS2/WS2 heterostructures. Nat. Nanotechnol. 2014, 9, 682–686. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Long, R.; Prezhdo, O.V. Charge Separation and Recombination in Two-Dimensional MoS2/WS2: Time-Domain ab Initio Modeling. Chem. Mater. 2017, 29, 2466–2473. [Google Scholar] [CrossRef]
- Bissett, M.A.; Kinloch, I.A.; Dryfe, R.A.W. Characterization of MoS2-Graphene Composites for High-Performance Coin Cell Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 17388–17398. [Google Scholar] [CrossRef]
- Huang, W.-M.; Liao, W.-S.; Lai, Y.-M.; Chen, I.-W.P. Tuning the Surface Charge Density of Exfoliated Thin Molybdenum Disulfide Sheets via Non-covalent Functionalization for Promoting Hydrogen Evolution Reaction. J. Mater. Chem. C 2020, 8, 510–517. [Google Scholar] [CrossRef]
- Xu, D.; Xu, P.; Zhu, Y.; Peng, W.; Li, Y.; Zhang, G.; Zhang, F.; Mallouk, T.E.; Fan, X. High Yield Exfoliation of WS2 Crystals into 1-2 Layer Semiconducting Nanosheets and Efficient Photocatalytic Hydrogen Evolution from WS2/CdS Nanorod Composites. ACS Appl. Mater. Interfaces 2018, 10, 2810–2818. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-W.; Shie, M.-Y.; Hsiao, C.-H.; Liang, Y.-C.; Wang, B.; Chen, I.-W.P. Synthesis of High-Quality Monolayer Tungsten Disulfide with Chlorophylls and Its Application for Enhancing Bone Regeneration. NPJ 2D Mater. Appl. 2020, 4, 34. [Google Scholar] [CrossRef]
- Dong, N.; Li, Y.; Feng, Y.; Zhang, S.; Zhang, X.; Chang, C.; Fan, J.; Zhang, L.; Wang, J. Optical Limiting and Theoretical Modelling of Layered Transition Metal Dichalcogenide Nanosheets. Sci. Rep. 2015, 5, 14646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Ji, J.; Jiang, Q.; Zhang, L.; Ao, Z.; Fan, X.; Wang, S.; Li, Y.; Zhang, F.; Zhang, G.; et al. Few-Layered Trigonal WS2 Nanosheet-Coated Graphite Foam as an Efficient Free-Standing Electrode for a Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2017, 9, 30591–30598. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, B.; Song, Y.; Dou, M.; Ji, J.; Wang, F. One-Pot Synthesis of MoS2/WS2 Ultrathin Nanoflakes with Vertically Aligned Structure on Indium Tin Oxide as a Photocathode for Enhanced Photo-Assistant Electrochemical Hydrogen Evolution Reaction. RSC Adv. 2017, 7, 49309–49319. [Google Scholar] [CrossRef] [Green Version]
- Balasingam, S.K.; Thirumurugan, A.; Lee, J.S.; Jun, Y. Amorphous MoSx Thin-Film-Coated Carbon Fiber Paper as A 3D Electrode for Long Cycle Life Symmetric Supercapacitors. Nanoscale 2016, 8, 11787–11791. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Li, N.; Su, C.; Zhao, H.; Xu, L.; Yin, Z.; Li, J.; Du, Y. Colloidal Synthesis of 1T’ Phase Dominated WS2 Towards Endurable Electrocatalysis. Nano Energy 2018, 50, 176–181. [Google Scholar] [CrossRef]
- Voiry, D.; Goswami, A.; Kappera, R.; Castro e Silva, C.D.C.; Kaplan, D.; Fujita, T.; Chen, M.; Asefa, T.; Chhowalla, M. Covalent Functionalization of Monolayered Transition Metal Dichalcogenides by Phase Engineering. Nat. Chem. 2015, 7, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Veeramani, V.; Yu, H.-C.; Hu, S.-F.; Liu, R.-S. Highly Efficient Photoelectrochemical Hydrogen Generation Reaction Using Tungsten Phosphosulfide Nanosheets. ACS Appl. Mater. Interfaces 2018, 10, 17280–17286. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Guha, P.K. An Effective Liquid-Phase Exfoliation Approach to Fabricate Tungsten Disulfide into Ultrathin Two-Dimensional Semiconducting Nanosheets. J. Mater. Sci. 2017, 52, 7256–7268. [Google Scholar] [CrossRef]
- Štengl, V.; Henych, J.; Slušná, M.; Ecorchard, P. Ultrasound Exfoliation of Inorganic Analogues of Graphene. Nanoscale Res. Lett. 2014, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Zeng, D.; Zhang, J.; Wu, C.; Wen, Y.; Shan, B.; Xie, C. Effect of Layer Number on Recovery Rate of WS2 Nanosheets for Ammonia Detection at Room Temperature. Appl. Surf. Sci. 2017, 414, 244–250. [Google Scholar] [CrossRef]
- Ye, L.; Wang, D.; Chen, S. Fabrication and Enhanced Photoelectrochemical Performance of MoS2/S-Doped g-C3N4 Heterojunction Film. ACS Appl. Mater. Interfaces 2016, 8, 5280–5289. [Google Scholar] [CrossRef]
- Kong, L.; Yan, J.; Liu, S.F. Carbonyl Linked Carbon Nitride Loading Few Layered MoS2 for Boosting Photocatalytic Hydrogen Generation. ACS Sustain. Chem. Eng. 2019, 7, 1389–1398. [Google Scholar] [CrossRef]
- Ashraf, W.; Bansal, S.; Singh, V.; Barman, S.; Khanuja, M. BiOCl/WS2 Hybrid Nanosheet (2D/2D) Heterojunctions for Visible-Light-Driven Photocatalytic Degradation of Organic/Inorganic Water Pollutants. RSC Adv. 2020, 10, 25073–25088. [Google Scholar] [CrossRef]
- Zheng, J.; Lei, Z. Incorporation of CoO Nanoparticles in 3D Marigold Flower-Like Hierarchical Architecture MnCo2O4 for Highly Boosting Solar Light Photo-Oxidation and Reduction Ability. Appl. Catal. B 2018, 237, 1–8. [Google Scholar] [CrossRef]
- Yin, W.; Bai, L.; Zhu, Y.; Zhong, S.; Zhao, L.; Li, Z.; Bai, S. Embedding Metal in the Interface of a p-n Heterojunction with a Stack Design for Superior Z-Scheme Photocatalytic Hydrogen Evolution. ACS Appl. Mater. Interfaces 2016, 8, 23133–23142. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Ma, J.; Si, K.; Xu, X.; Quan, C.; He, C.; Xu, X. Band Alignment of WS2/MoS2 Photoanodes with Efficient Photoelectric Responses based on Mixed Van der Waals Heterostructures. Phys. Status Solidi 2019, 216, 1900544. [Google Scholar] [CrossRef]


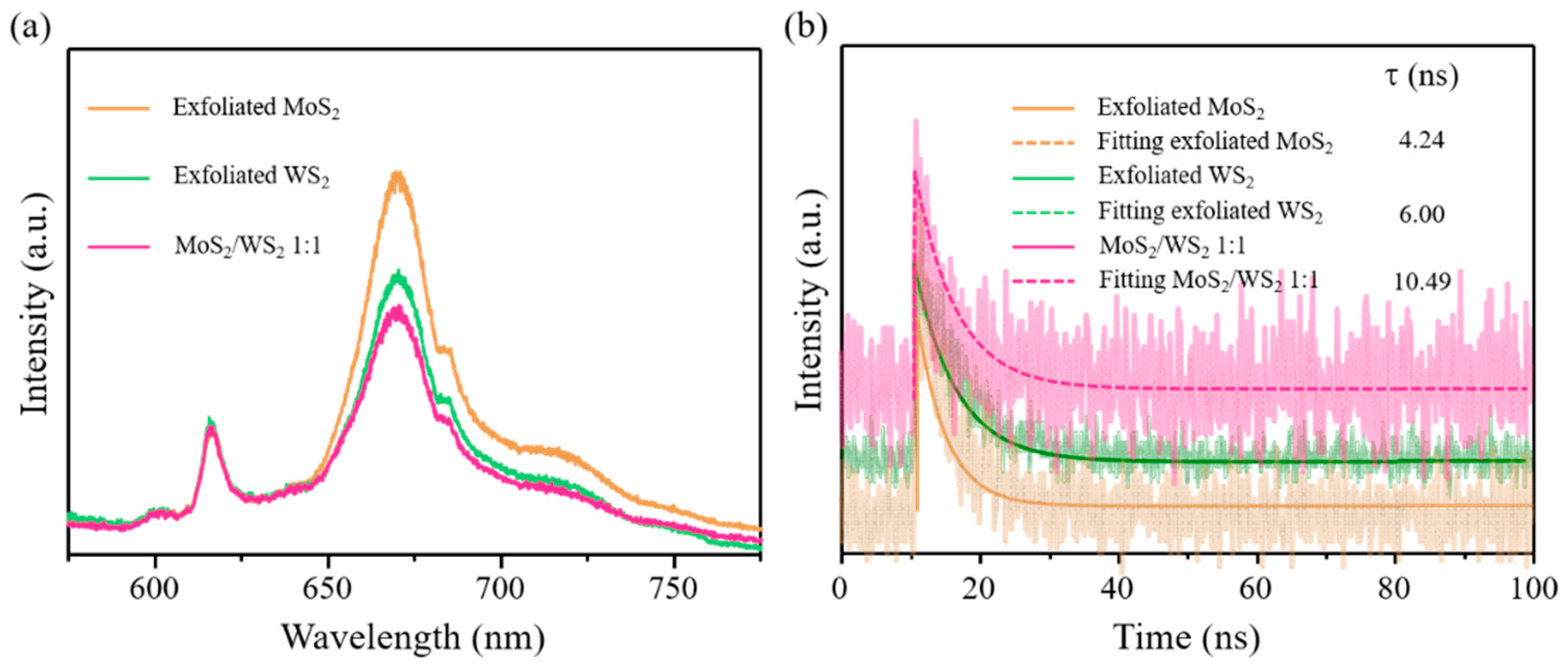
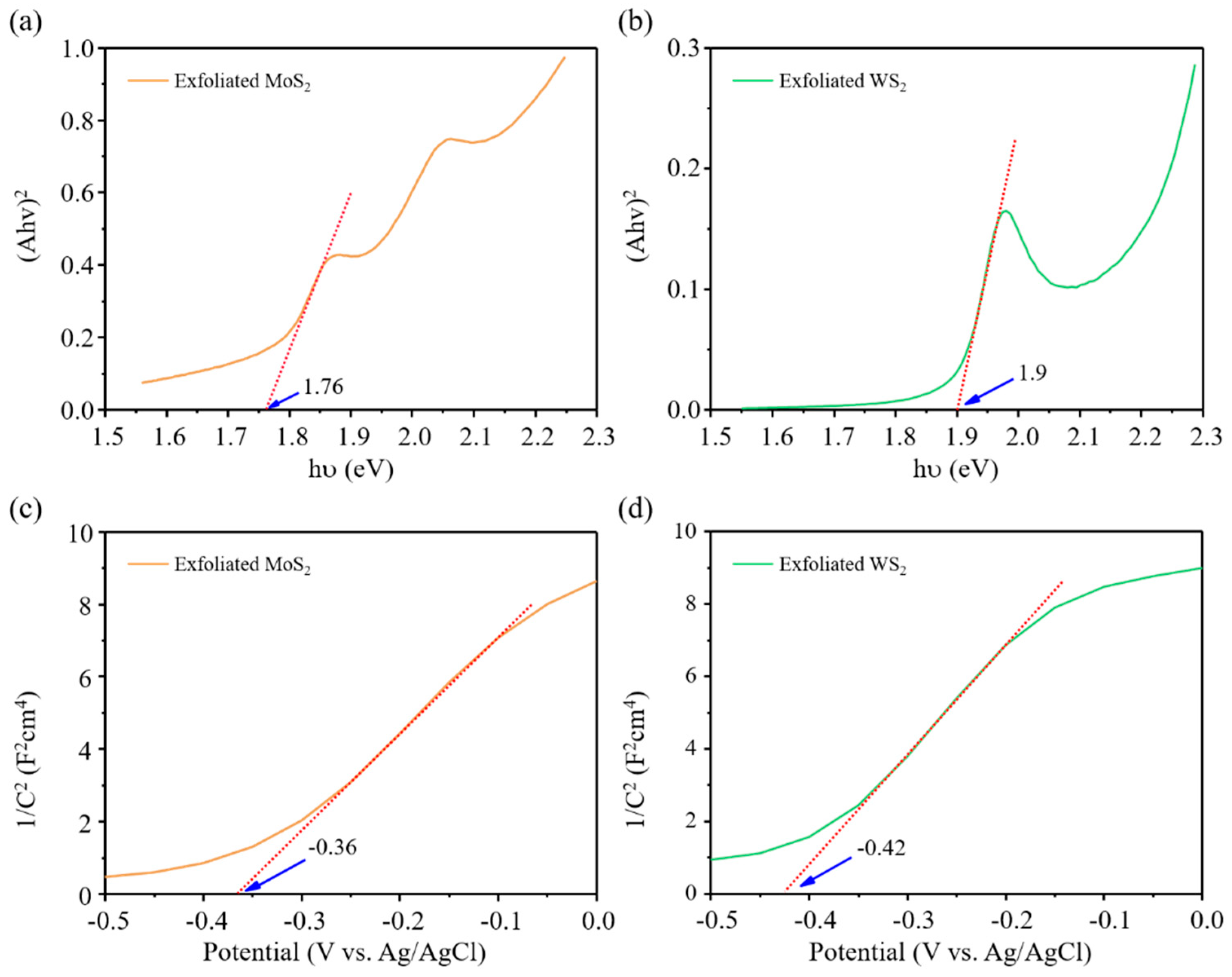
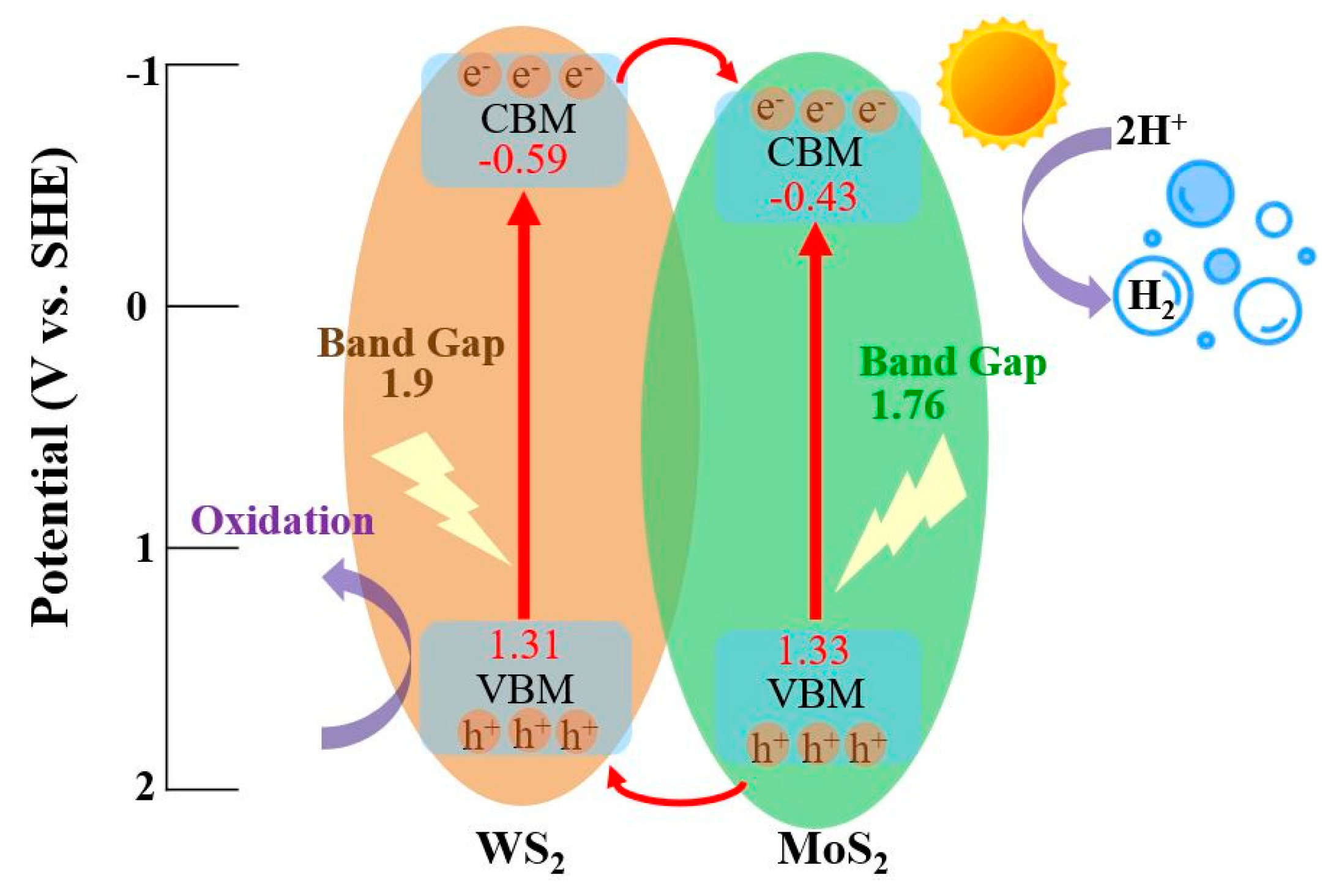
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, I.-W.P.; Lai, Y.-M.; Liao, W.-S. One-Pot Synthesis of Chlorophyll-Assisted Exfoliated MoS2/WS2 Heterostructures via Liquid-Phase Exfoliation Method for Photocatalytic Hydrogen Production. Nanomaterials 2021, 11, 2436. https://doi.org/10.3390/nano11092436
Chen I-WP, Lai Y-M, Liao W-S. One-Pot Synthesis of Chlorophyll-Assisted Exfoliated MoS2/WS2 Heterostructures via Liquid-Phase Exfoliation Method for Photocatalytic Hydrogen Production. Nanomaterials. 2021; 11(9):2436. https://doi.org/10.3390/nano11092436
Chicago/Turabian StyleChen, I-Wen P., Yan-Ming Lai, and Wei-Sheng Liao. 2021. "One-Pot Synthesis of Chlorophyll-Assisted Exfoliated MoS2/WS2 Heterostructures via Liquid-Phase Exfoliation Method for Photocatalytic Hydrogen Production" Nanomaterials 11, no. 9: 2436. https://doi.org/10.3390/nano11092436
APA StyleChen, I.-W. P., Lai, Y.-M., & Liao, W.-S. (2021). One-Pot Synthesis of Chlorophyll-Assisted Exfoliated MoS2/WS2 Heterostructures via Liquid-Phase Exfoliation Method for Photocatalytic Hydrogen Production. Nanomaterials, 11(9), 2436. https://doi.org/10.3390/nano11092436






