Photocatalytic Degradation of Tobacco Tar Using CsPbBr3 Quantum Dots Modified Bi2WO6 Composite Photocatalyst
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Materials
2.2. Synthesis of CsPbBr3/Bi2WO6
2.3. Photocatalytic Experiments
2.4. Characterization
3. Results and Discussion
3.1. Properties of the Composite Photocatalysts
3.2. Activity for Photodegradation of PAHs from Tobacco Tar
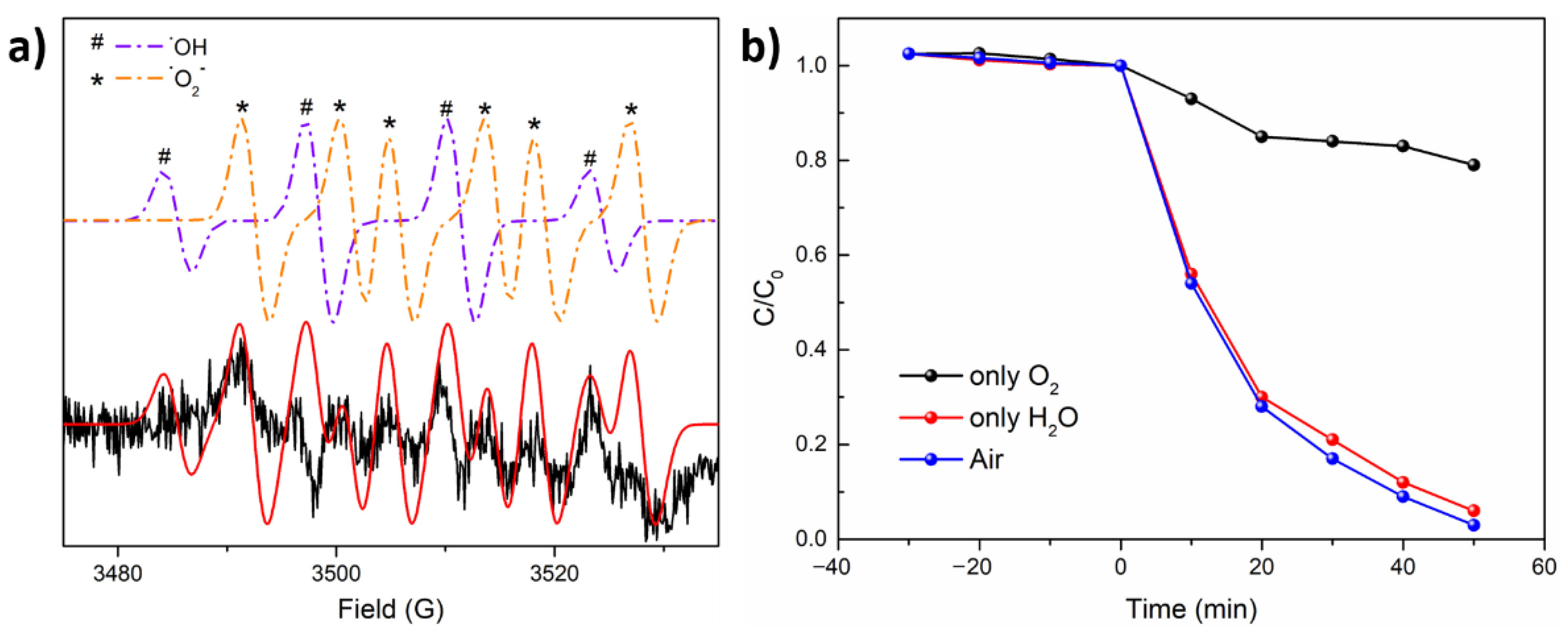
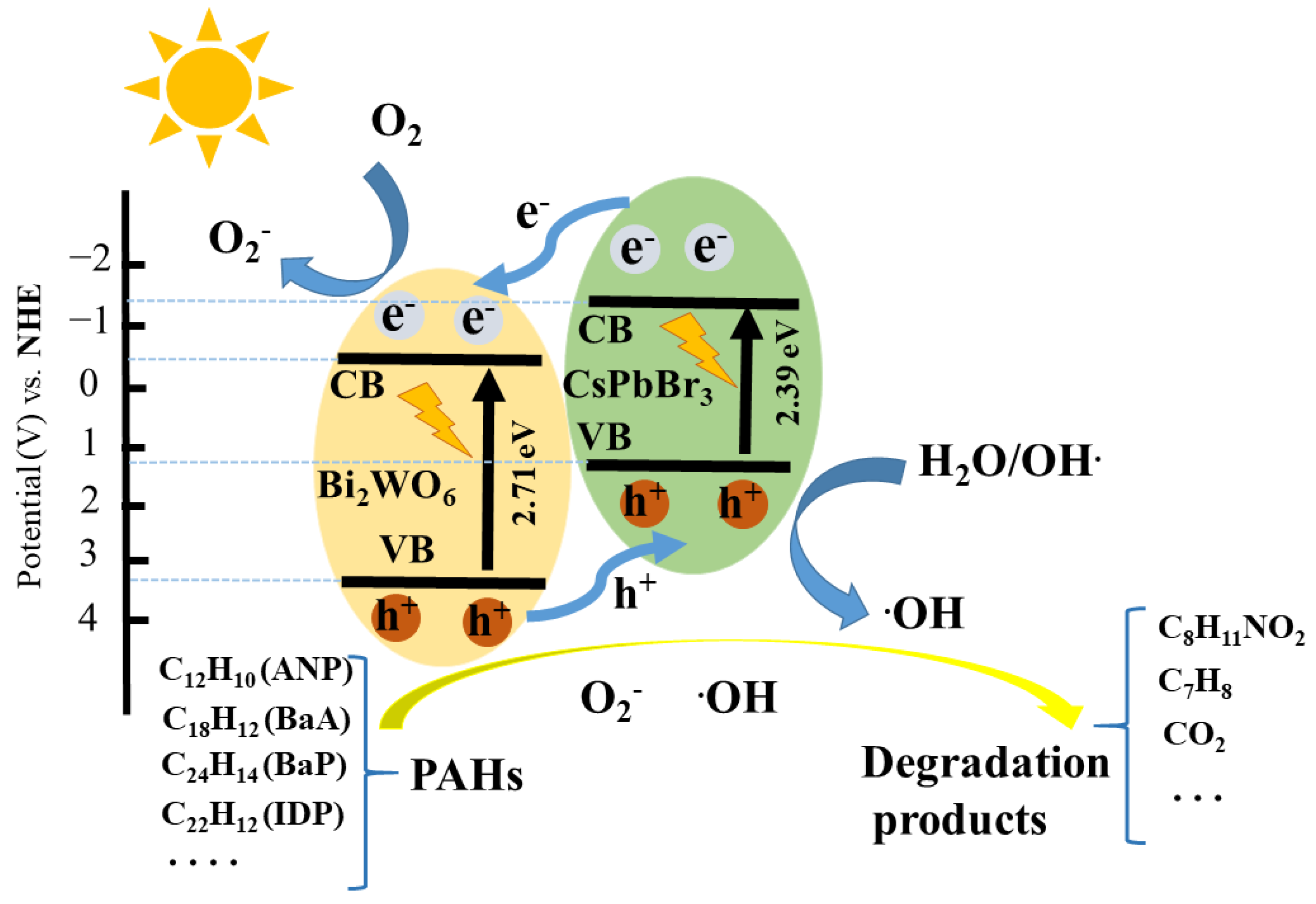
4. Photocatalytic Mechanism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, J.; Kumar, V.; Jolly, S.S.; Kim, K.-H.; Rawat, M.; Kukkar, D.; Tsang, Y.F. Biogenic synthesis of silver nanoparticles and its photocatalytic applications for removal of organic pollutants in water. J. Ind. Eng. Chem. 2019, 80, 247–257. [Google Scholar] [CrossRef]
- Soltan, W.B.; Ammar, S.; Olivier, C.; Toupance, T. Influence of zinc doping on the photocatalytic activity of nanocrystalline SnO2 particles synthesized by the polyol method for enhanced degradation of organic dyes. J. Alloys Compd. 2017, 729, 638–647. [Google Scholar] [CrossRef]
- Andreoli, R.; Spatari, G.; Pigini, D.; Poli, D.; Banda, I.; Goldoni, M.; Riccelli, M.G.; Petyx, M.; Protano, C.; Vitali, M.; et al. Urinary biomarkers of exposure and of oxidative damage in children exposed to low airborne concentrations of benzene. Environ. Res. 2015, 142, 264–272. [Google Scholar] [CrossRef]
- Yan, Z.; Xu, Z.; Yu, J.; Jaroniec, M. Enhanced formaldehyde oxidation on CeO2/AlOOH-supported Pt catalyst at room temperature. Appl. Catal. B Environ. 2016, 199, 458–465. [Google Scholar] [CrossRef]
- Husman, T. Health effects of indoor-air microorganisms. Scand. J. Work Environ. Health 1996, 22, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Alexandrov, L.B.; Ju, Y.S.; Haase, K.; Van Loo, P.; Martincorena, I.; Nik-Zainal, S.; Totoki, Y.; Fujimoto, A.; Nakagawa, H.; Shibata, T.; et al. Mutational signatures associated with tobacco smoking in human cancer. Science 2016, 354, 618–622. [Google Scholar] [CrossRef] [Green Version]
- Enguita, F.J.; Leitao, A.L. Hydroquinone: Environmental pollution, toxicity, and microbial answers. BioMed Res. Int. 2013, 2013, 542168. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Sun, H.; Zelekew, O.A.; Zhang, J.; Guo, Y.; Zeng, A.; Kuo, D.-H.; Lin, J. Biological renewable hemicellulose-template for synthesis of visible light responsive sulfur-doped TiO2 for photocatalytic oxidation of toxic organic and As(III) pollutants. Appl. Surf. Sci. 2020, 525, 146531. [Google Scholar] [CrossRef]
- Nagajyothi, P.; Vattikuti, S.; Devarayapalli, K.; Yoo, K.; Shim, J.; Sreekanth, T. Green synthesis: Photocatalytic degradation of textile dyes using metal and metal oxide nanoparticles-latest trends and advancements. Crit. Rev. Environ. Sci. Technol. 2019, 50, 2617–2723. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Zhang, Q.; Qin, P.; Wang, X.; He, G.; Li, L. One-step synthesis of a WO3-CuS nanosheet heterojunction with enhanced photocatalytic performance for methylene blue degradation and Cr(VI) reduction. J. Chem. Technol. Biotechnol. 2019, 95, 665–674. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Atchudan, R.; Parveen, A.S.; Kalaiarasan, K.; Lee, Y.R.; Kim, S.-C. Fabrication of ZnO nanoparticles adorned nitrogen-doped carbon balls and their application in photodegradation of organic dyes. Sci. Rep. 2019, 9, 19509. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Muliane, U.; Weon, S.; Choi, W. Substrate-specific mineralization and deactivation behaviors of TiO2 as an air-cleaning photocatalyst. Appl. Catal. B Environ. 2020, 275, 119145. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, X.; Liu, W.; Yang, D. Facile preparation of well-combined lignin-based carbon/ZnO hybrid composite with excellent photocatalytic activity. Appl. Surf. Sci. 2017, 426, 206–216. [Google Scholar] [CrossRef]
- Ullah, I.; Munir, A.; Muhammad, S.; Ali, S.; Khalid, N.; Zubair, M.; Sirajuddin, M.; Hussain, S.Z.; Ahmed, S.; Khan, Y.; et al. Influence of W-doping on the optical and electrical properties of SnO2 towards photocatalytic detoxification and electrocatalytic water splitting. J. Alloys Compd. 2020, 827, 154247. [Google Scholar] [CrossRef]
- Parida, K.M.; Reddy, K.H.; Martha, S.; Das, D.P.; Biswal, N. Fabrication of nanocrystalline LaFeO3: An efficient sol–gel auto-combustion assisted visible light responsive photocatalyst for water decomposition. Int. J. Hydrogen Energy 2010, 35, 12161–12168. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Mathe, V.L.; Kulkarni, S.B. Photocatalytic degradation of salicylic acid using BaTiO3 photocatalyst under ultraviolet light illumination. J. Mater. Sci. Mater. Electron. 2018, 29, 15069–15073. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, G.; Wang, L.; Irvine, J.T.S. Inorganic perovskite photocatalysts for solar energy utilization. Chem. Soc. Rev. 2016, 45, 5951–5984. [Google Scholar] [CrossRef] [Green Version]
- Xie, T.; Liu, Y.; Wang, H.; Wu, Z. Synthesis of α-Fe2O3/Bi2WO6 layered heterojunctions by in situ growth strategy with enhanced visible-light photocatalytic activity. Sci. Rep. 2019, 9, 7551. [Google Scholar] [CrossRef] [Green Version]
- Dumrongrojthanath, P.; Phuruangrat, A.; Thongtem, S.; Thongtem, T. Hydrothermal preparation of visible-light-driven Br-doped Bi2WO6 photocatalyst. Mater. Lett. 2017, 209, 501–504. [Google Scholar] [CrossRef]
- Zhang, P.; Teng, X.; Feng, X.; Ding, S.; Zhang, G. Preparation of Bi2WO6 photocatalyst by high-energy ball milled Bi2O3-WO3 mixture. Ceram. Int. 2016, 42, 16749–16757. [Google Scholar] [CrossRef]
- Kaur, A.; Kansal, S.K. Bi2WO6 nanocuboids: An efficient visible light active photocatalyst for the degradation of levofloxacin drug in aqueous phase. Chem. Eng. J. 2016, 302, 194–203. [Google Scholar] [CrossRef]
- Zhuo, Y.; Huang, J.; Cao, L.; Ouyang, H.; Wu, J. Photocatalytic activity of snow-like Bi2WO6 microcrystalline for decomposition of Rhodamine B under natural sunlight irradiation. Mater. Lett. 2013, 90, 107–110. [Google Scholar] [CrossRef]
- Liu, M.; Xue, X.; Yu, S.; Wang, X.; Hu, X.; Tian, H.; Chen, H.; Zheng, W. Improving Photocatalytic Performance from Bi2WO6@MoS2/graphene Hybrids via Gradual Charge Transferred Pathway. Sci. Rep. 2017, 7, 3637. [Google Scholar] [CrossRef]
- Zhao, S.; Jin, H.; Yang, Y.; Cui, J. Magnetically retrievable Bi2WO6/Fe3O4/Na-MMT composite: Fabrication and photocatalytic activity. Res. Chem. Intermed. 2020, 46, 4579–4593. [Google Scholar] [CrossRef]
- Shen, Z.; Li, H.; Hao, H.; Chen, Z.; Hou, H.; Zhang, G.; Bi, J.; Yan, S.; Liu, G.; Gao, W. Novel Tm3+ and Yb3+ co-doped bismuth tungstate up-conversion photocatalyst with greatly improved photocatalytic properties. J. Photochem. Photobiol. A Chem. 2019, 380, 111864. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Wei, L.; Yu, C.; Yi, J.; Ji, H. Regulate the crystal and optoelectronic properties of Bi2WO6 nanosheet crystals by Sm3+ doping for superior visible-light-driven photocatalytic performance. Appl. Surf. Sci. 2020, 508, 145309. [Google Scholar] [CrossRef]
- Hou, X.; Liu, J.; Guo, W.; Li, S.; Guo, Y.; Shi, Y.; Zhang, C. A novel 3D-structured flower-like bismuth tungstate/mag-graphene nanoplates composite with excellent visible-light photocatalytic activity for ciprofloxacin degradation. Catal. Commun. 2019, 121, 27–31. [Google Scholar] [CrossRef]
- Kadeer, K.; Tursun, Y.; Dilinuer, T.; Okitsu, K.; Abulizi, A. Sonochemical preparation and photocatalytic properties of CdS QDs/Bi2WO6 3D heterojunction. Ceram. Int. 2018, 44, 13797–13805. [Google Scholar] [CrossRef]
- Parnicka, P.; Mikolajczyk, A.; Pinto, H.P.; Lisowski, W.; Klimczuk, T.; Trykowski, G.; Bajorowicz, B.; Zaleska-Medynska, A. Experimental and DFT insights into an eco-friendly photocatalytic system toward environmental remediation and hydrogen generation based on AgInS2 quantum dots embedded on Bi2WO6. Appl. Surf. Sci. 2020, 525, 146596. [Google Scholar] [CrossRef]
- Ge, L.; Liu, J. Efficient visible light-induced photocatalytic degradation of methyl orange by QDs sensitized CdS-Bi2WO6. Appl. Catal. B Environ. 2011, 105, 289–297. [Google Scholar] [CrossRef]
- Schlaus, A.P.; Spencer, M.S.; Miyata, K.; Liu, F.; Wang, X.; Datta, I.; Lipson, M.; Pan, A.; Zhu, X.Y. How lasing happens in CsPbBr3 perovskite nanowires. Nat. Commun. 2019, 10, 265. [Google Scholar] [CrossRef]
- Liu, B.; Feng, S.; Yang, L.; Li, C.; Luo, Z.; Wang, T.; Gong, J. Bifacial passivation of n-silicon metal–insulator–semiconductor photoelectrodes for efficient oxygen and hydrogen evolution reactions. Energy Environ. Sci. 2020, 13, 221–228. [Google Scholar] [CrossRef]
- Corti, M.; Bonomi, S.; Chiara, R.; Romani, L.; Quadrelli, P.; Malavasi, L. Application of Metal Halide Perovskites as Photocatalysts in Organic Reactions. Inorganics 2021, 9, 56. [Google Scholar] [CrossRef]
- Zhu, X.; Lin, Y.; San Martin, J.; Sun, Y.; Zhu, D.; Yan, Y. Lead halide perovskites for photocatalytic organic synthesis. Nat. Commun. 2019, 10, 2843. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Yun, S.H. Structure and optical properties of perovskite-embedded dual-phase microcrystals synthesized by sonochemistry. Commun. Chem. 2020, 3, 15. [Google Scholar] [CrossRef]
- Mano, G.; Harinee, S.; Sridhar, S.; Ashok, M.; Viswanathan, A. Microwave assisted synthesis of ZnO-PbS heterojuction for degradation of organic pollutants under visible light. Sci. Rep. 2020, 10, 2224. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Yang, M.; Ke, X.; Yang, S.; Wang, K.; Huang, H.; Wang, W.; Luo, D.; Zheng, Z.; Huang, L.; et al. Cubic-cubic perovskite quantum dots/PbS mixed dimensional materials for highly efficient CO2 reduction. J. Power Sources 2021, 481, 228838. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, J.; Du, Y.; Zhou, C.; Zhang, M.; Wang, Z.; Weng, Y.; Long, J.; Hofkens, J.; Steele, J.A.; et al. Direct Z-Scheme Heterojunction of Semicoherent FAPbBr3/Bi2WO6 Interface for Photoredox Reaction with Large Driving Force. ACS Nano 2020, 14, 16689–16697. [Google Scholar] [CrossRef]
- Choi, H.; Stathatos, E.; Dionysiou, D.D. Photocatalytic TiO2 films and membranes for the development of efficient wastewater treatment and reuse systems. Desalination 2007, 202, 199–206. [Google Scholar] [CrossRef]
- Lin, R.; Wan, J.; Xiong, Y.; Wu, K.; Cheong, W.C.; Zhou, G.; Wang, D.; Peng, Q.; Chen, C.; Li, Y. Quantitative Study of Charge Carrier Dynamics in Well-Defined WO3 Nanowires and Nanosheets: Insight into the Crystal Facet Effect in Photocatalysis. J. Am. Chem. Soc. 2018, 140, 9078–9082. [Google Scholar] [CrossRef]
- Tekin, D.; Tekin, T.; Kiziltas, H. Photocatalytic degradation kinetics of Orange G dye over ZnO and Ag/ZnO thin film catalysts. Sci. Rep. 2019, 9, 17544. [Google Scholar] [CrossRef]
- Kasinathan, M.; Thiripuranthagan, S.; Sivakumar, A. A facile fabrication of Br-modified g-C3N4/rGO composite catalyst for enhanced visible photocatalytic activity towards the degradation of harmful dyes. Mater. Res. Bull. 2020, 130, 110870. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, X.; Hao, H.; Gan, W. Facile synthesis 3D flower-like Ag@WO3 nanostructures and applications in solar-light photocatalysis. J. Alloys Compd. 2018, 757, 134–141. [Google Scholar] [CrossRef]
- Pang, H.-F.; Xiang, X.; Li, Z.-J.; Fu, Y.-Q.; Zu, X.-T. Hydrothermal synthesis and optical properties of hexagonal tungsten oxide nanocrystals assisted by ammonium tartrate. Phys. Status Solidi 2012, 209, 537–544. [Google Scholar] [CrossRef]
- Bhagyalakshmi, S.B.; Ghimire, S.; Takahashi, K.; Yuyama, K.I.; Takano, Y.; Nakamura, T.; Biju, V. Nonradiative Energy Transfer through Distributed Bands in Piezochemically Synthesized Cesium and Formamidinium Lead Halide Perovskites. Chemistry 2020, 26, 2133–2137. [Google Scholar] [CrossRef]
- Thorat, A.A.; Dalvi, S.V. Liquid antisolvent precipitation and stabilization of nanoparticles of poorly water soluble drugs in aqueous suspensions: Recent developments and future perspective. Chem. Eng. J. 2012, 181–182, 1–34. [Google Scholar] [CrossRef]
- Chen, S.; Lu, W.; Shen, H.; Xu, S.; Chen, X.; Xu, T.; Wang, Y.; Chen, Y.; Gu, Y.; Wang, C.; et al. The development of new pigments: Colorful g-C3N4-based catalysts for nicotine removal. Appl. Catal. B Environ. 2019, 254, 500–509. [Google Scholar] [CrossRef]
- Liu, B.; Chen, B.; Zhang, B.; Song, X.; Zeng, G.; Lee, K. Photocatalytic ozonation of offshore produced water by TiO2 nanotube arrays coupled with UV-LED irradiation. J. Hazard. Mater. 2021, 402, 123456. [Google Scholar] [CrossRef]
- Woo, O.T.; Chung, W.K.; Wong, K.H.; Chow, A.T.; Wong, P.K. Photocatalytic oxidation of polycyclic aromatic hydrocarbons: Intermediates identification and toxicity testing. J. Hazard. Mater. 2009, 168, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Vela, N.; Martínez-Menchón, M.; Navarro, G.; Pérez-Lucas, G.; Navarro, S. Removal of polycyclic aromatic hydrocarbons (PAHs) from groundwater by heterogeneous photocatalysis under natural sunlight. J. Photochem. Photobiol. A Chem. 2012, 232, 32–40. [Google Scholar] [CrossRef]
- Lair, A.; Ferronato, C.; Chovelon, J.-M.; Herrmann, J.-M. Naphthalene degradation in water by heterogeneous photocatalysis: An investigation of the influence of inorganic anions. J. Photochem. Photobiol. A Chem. 2008, 193, 193–203. [Google Scholar] [CrossRef]
- Benkhennouche-Bouchene, H.; Mahy, J.G.; Wolfs, C.; Vertruyen, B.; Poelman, D.; Eloy, P.; Hermans, S.; Bouhali, M.; Souici, A.; Bourouina-Bacha, S.; et al. Green Synthesis of N/Zr Co-Doped TiO2 for Photocatalytic Degradation of p-Nitrophenol in Wastewater. Catalysts 2021, 11, 235. [Google Scholar] [CrossRef]
- Wen, S.; Zhao, J.; Sheng, G.; Fu, J.; Peng, P. Photocatalytic reactions of phenanthrene at TiO2/water interfaces. Chemosphere 2002, 46, 871–877. [Google Scholar] [CrossRef]
- Luo, H.; Zeng, Y.; He, D.; Pan, X. Application of iron-based materials in heterogeneous advanced oxidation processes for wastewater treatment: A review. Chem. Eng. J. 2021, 407, 127191. [Google Scholar] [CrossRef]
- Su, F.; Mathew, S.C.; Lipner, G.; Fu, X.; Antonietti, M.; Blechert, S.; Wang, X. mpg-C3N4-Catalyzed Selective Oxidation of Alcohols Using O-2 and Visible Light. J. Am. Chem. Soc. 2010, 132, 16299–16301. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Jin, C.; Li, Z.; Yang, D.; Xie, Y.; Wang, M. MoS2 and g-C3N4 nanosheet co-modified Bi2WO6 ternary heterostructure catalysts coupling with H2O2 for improved visible photocatalytic activity. Mater. Chem. Phys. 2019, 272, 124982. [Google Scholar] [CrossRef]
- Gong, Y.; Shen, J.; Zhu, Y.; Yan, W.; Zhu, J.; Hou, L.; Xie, D.; Li, C. Tailoring charge transfer in perovskite quantum dots/black phosphorus nanosheets photocatalyst via aromatic molecules. Appl. Surf. Sci. 2021, 545, 149012. [Google Scholar] [CrossRef]
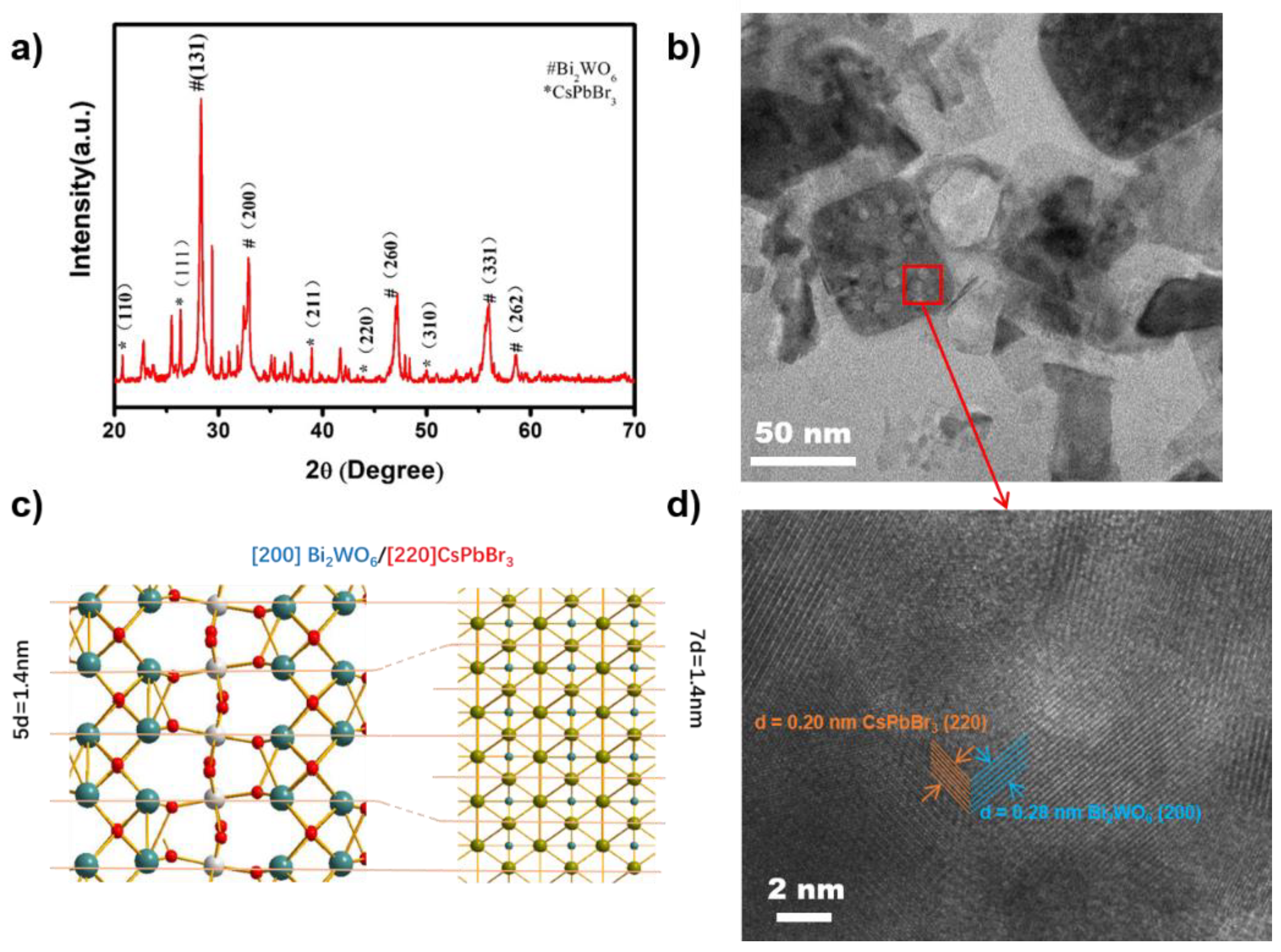
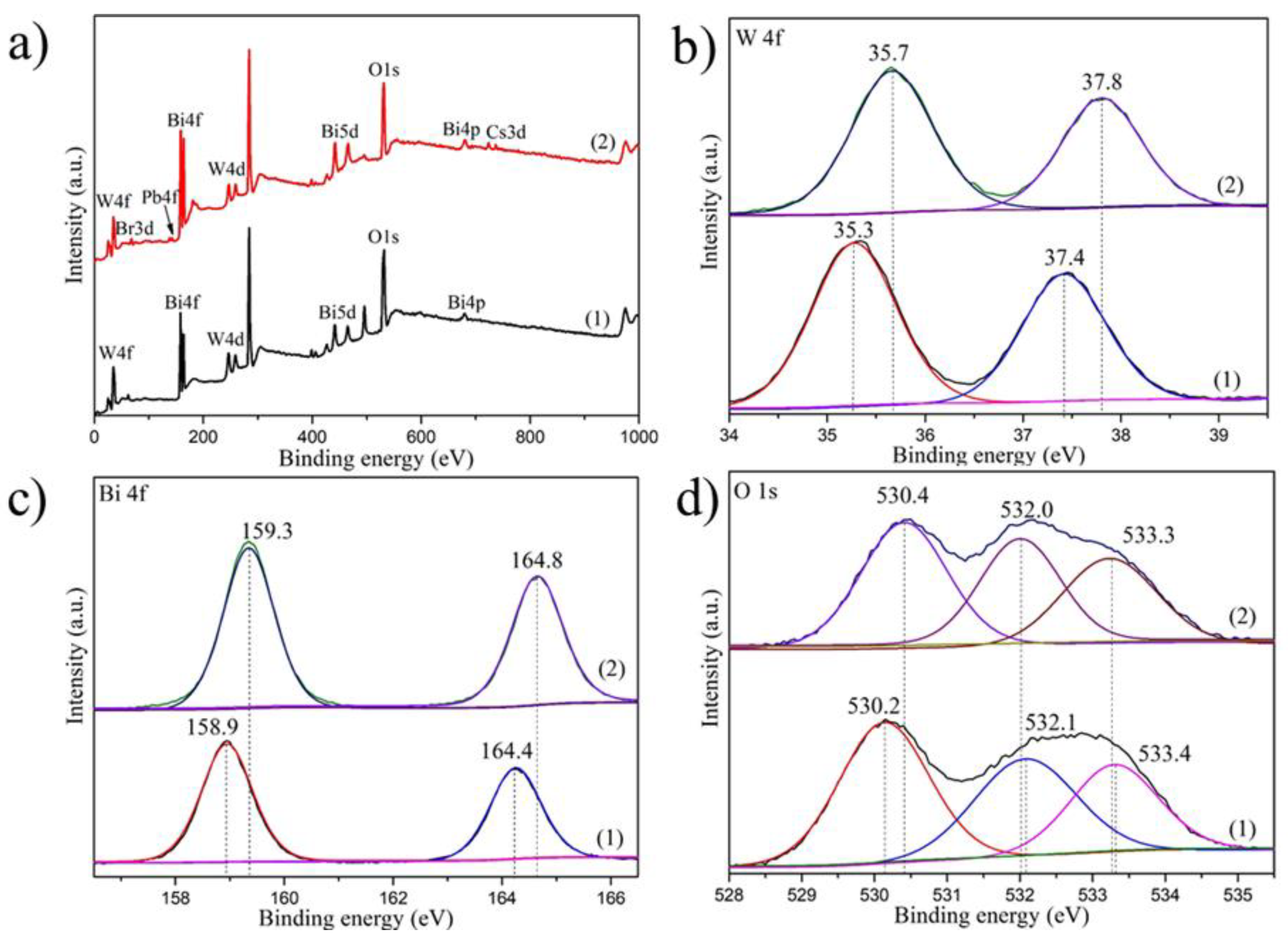

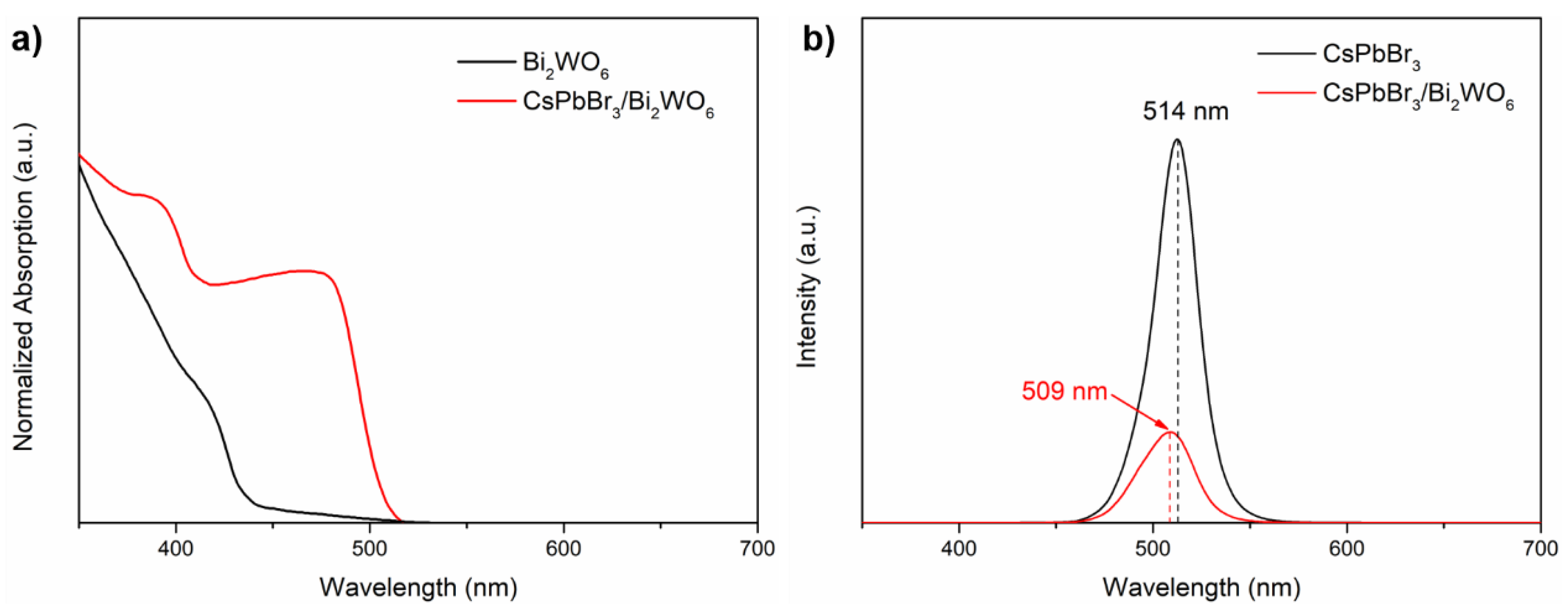
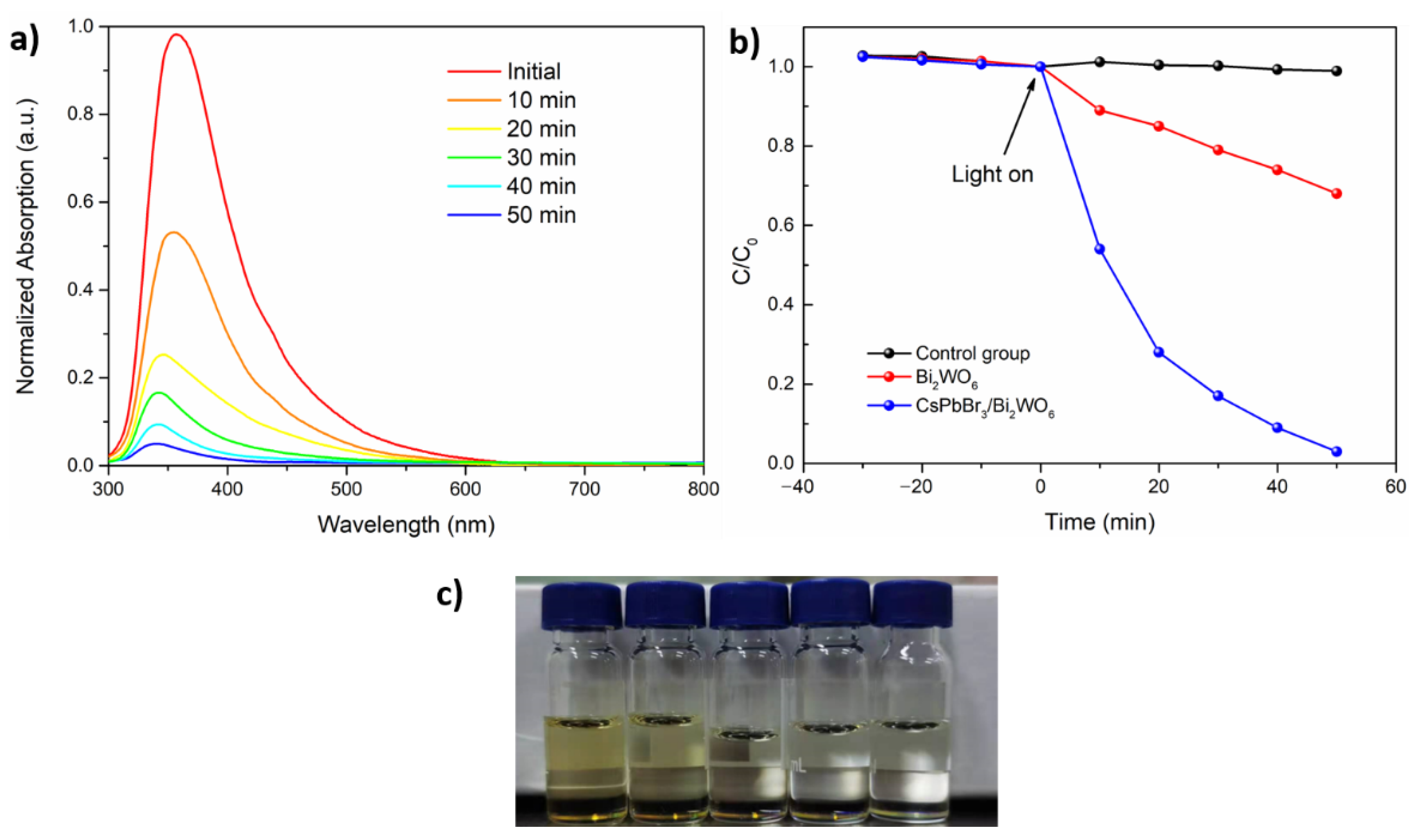
| Sample | Bi4f/% | W4f/% | O1s/% | C1s/% | Br3d/% | Pb4f/% | Cs4d/% |
|---|---|---|---|---|---|---|---|
| Bi2WO6 | 1.62 | 2.53 | 24.04 | 71.8 | 0 | 0 | 0 |
| CsPbBr3/Bi2WO6 | 2.21 | 2.15 | 21.7 | 72.74 | 0.96 | 0.09 | 0.16 |
| Sample | Carrier Density (cm−3) | Hall Mobility (cm2V−1s−1) | p/n Type |
|---|---|---|---|
| Bi2WO6 | 2.738 × 1011 | 25.316 | n |
| CsPbBr3/Bi2WO6 | 5.154 × 1014 | 4.672 | p |
| Catalyst | BET Surface Area (m2/g) | t-Plot External Surface Area (m2/g) |
|---|---|---|
| CsPbBr3/Bi2WO6 | 9.88 | 9.70 |
| Bi2WO6 | 5.04 | 5.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, R.; Zhang, M.; Zheng, Z.; Wang, K.; Liu, X.; Chen, Q.; Luo, D. Photocatalytic Degradation of Tobacco Tar Using CsPbBr3 Quantum Dots Modified Bi2WO6 Composite Photocatalyst. Nanomaterials 2021, 11, 2422. https://doi.org/10.3390/nano11092422
Huang R, Zhang M, Zheng Z, Wang K, Liu X, Chen Q, Luo D. Photocatalytic Degradation of Tobacco Tar Using CsPbBr3 Quantum Dots Modified Bi2WO6 Composite Photocatalyst. Nanomaterials. 2021; 11(9):2422. https://doi.org/10.3390/nano11092422
Chicago/Turabian StyleHuang, Runda, Menglong Zhang, Zhaoqiang Zheng, Kunqiang Wang, Xiao Liu, Qizan Chen, and Dongxiang Luo. 2021. "Photocatalytic Degradation of Tobacco Tar Using CsPbBr3 Quantum Dots Modified Bi2WO6 Composite Photocatalyst" Nanomaterials 11, no. 9: 2422. https://doi.org/10.3390/nano11092422
APA StyleHuang, R., Zhang, M., Zheng, Z., Wang, K., Liu, X., Chen, Q., & Luo, D. (2021). Photocatalytic Degradation of Tobacco Tar Using CsPbBr3 Quantum Dots Modified Bi2WO6 Composite Photocatalyst. Nanomaterials, 11(9), 2422. https://doi.org/10.3390/nano11092422






