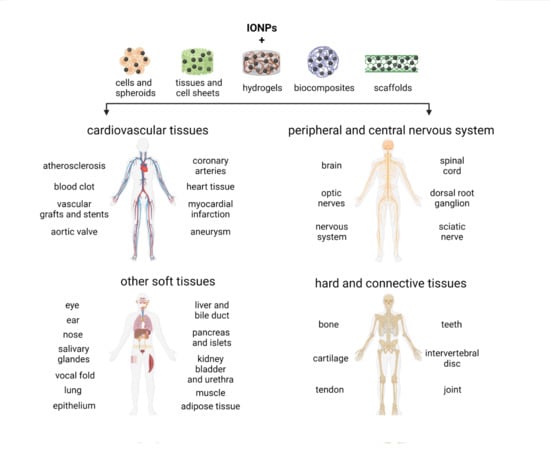
Iron Oxide Nanoparticles in Regenerative Medicine and Tissue Engineering
Abstract
1. Introduction
2. IONP Synthesis, Functionalization and Targeting
3. Cardiovascular Tissue Regeneration and Engineering
3.1. Thrombolysis
3.2. Vascular Grafts and Stents
3.2.1. IONP-Based MRT Monitoring of Grafts and Stents
3.2.2. IONP-Based Improvements of Vascular Scaffolds
3.2.3. IONP-Based Stent Improvements
3.3. Atherosclerosis
3.3.1. IONP-Based Atherosclerosis Imaging
3.3.2. IONP-Based Therapy of Atherosclerosis
3.3.3. Magnetic Drug Targeting to Atherosclerotic Plaques
3.3.4. Cell-Based Plaque Regeneration
3.4. IONPs as Modulator and Enhancer of Cardiovascular Regeneration
3.5. Stem Cell Therapy
3.5.1. IONP-Based In Vivo Monitoring
3.5.2. IONP-Based Cell Targeting
3.5.3. MNP-Based Cell Modulation
3.6. Cardiac Tissue Engineering and Regeneration
4. Hard and Connective Tissue Regeneration and Engineering
4.1. Cartilage
4.1.1. MRI-Assisted Stem Cell Therapy for Cartilage Regeneration
4.1.2. Magnetically-Based Targeted Cell Therapy for Cartilage Regeneration
4.1.3. Tissue-Engineered Cartilage
4.1.4. Drug Supported Cartilage Tissue Engineering
4.1.5. Scaffold-Free Cartilage Tissue Engineering
4.2. Bone Regeneration
4.2.1. Stem Cell Therapy for Bone Regeneration
4.2.2. Bone Tissue-Engineering
4.2.3. Magnetic Force-Based Bone Tissue-Engineering
4.2.4. Magnetic Force-Enhanced Stimulation of Engineered Tissues
4.2.5. Magnetic Force-Based Attraction of Agents to Engineered Tissues
4.2.6. Tissue-Engineered Osteochondral Scaffolds
4.3. Intervertebral Disc and Joint Repair
4.4. Tendon
4.5. Teeth
5. PNS and CNS Regeneration
5.1. PNS
5.1.1. Dorsal Root Ganglia
5.1.2. Sciatic Nerves
5.2. CNS
5.2.1. Optic Nerves
5.2.2. Spinal Cord
Spinal Cord Regeneration by IONP-Enhanced Cell Therapy
Regeneration by IONPs
Spinal Cord Regeneration by IONP-Containing Biocomposites
5.2.3. Brain
Monitoring by IONP-Labelled Cells
Enhanced Brain Regeneration by IONP-Labelled Cells
IONP-Based Magnetic Cell Targeting
Brain Regeneration by Application of Functionalized IONP
Brain Regeneration by IONP-Containing Biocomposites
6. Other Soft Tissue Regeneration and Engineering
6.1. Ear, Eye, Nose, Vocal Fold and Salivary Glands
6.1.1. Ear
6.1.2. Eye
6.1.3. Nose
6.1.4. Vocal Fold
6.1.5. Salivary Glands
6.2. Kidney
6.3. Liver and Bile Duct
6.4. Islets and Pancreas
6.5. Bladder and Urethra
6.6. Tissue Glue, Wound Regeneration and Skin Engineering
6.7. Muscle and Adipose Tissue
6.8. Lung Tissue
7. Conclusions and Discussion
References
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| APA | alginate-poly-L-lysine-alginate | MMPs | matrix metalloproteases |
| APTS | acetylated 3-aminopropyltrimethoxysilane | MPI | magnetic particle imaging |
| ARCs | adipose-derived regenerative cells | MPO | myeloperoxidase |
| ASCs | adipose-derived stem cells | MRI | magnetic resonance imaging |
| BC | bacterial cellulose | MRI | magnetic resonance imaging |
| Bcl-2 | B-cell lymphoma 2 | MSCs | mesenchymal stem cells |
| BDNF | brain-derived neurotrophic factor | NE | nanoemulsion |
| BM-EPCs | bone marrow-derived endothelial progenitor cells | NGF | nerve growth factor |
| BMMs | biomimetic magnetic microrobots | n-HA | nano-hydroxyapatite |
| BMP-2 | bone morphogenetic protein 2 | NIRF | near-infrared fluorescence |
| BM-SCs | bone marrow-derived stem cells | NK | nattokinase |
| BNC | bacterial nanocellulose | NPs | nanoparticles |
| CCR2 | chemokine (C-C motif) receptor 2 | OECs | olfactory ensheathing cells |
| CDK | chronic kidney disease | OMCs | olfactory mucosal cells |
| CECs | corneal endothelial cells | OxLDL | oxidized low-density lipoproteins |
| CF | cystic fibrosis | PAA | polyacrylic acid |
| ChABC | chondroitinase ABC | PCL | poly-ε-caprolactone |
| CMs | cardiomyocytes | PEG | polyethylene glycol |
| CNTs | carbon nanotubes | PEI | polyethylenimine |
| CPC | calcium phosphate cement | PEO | polyethylene oxide |
| cRGD | cyclic arginine-glycine-aspartate | PET-CT | positron emission tomography–computed tomography |
| CSCs | cardiosphere-derived stem cells | PGA | polyglycolic acid |
| CTA | computed tomographic angiography | PLA | poly(l-lactide) |
| dCCA | decellularized porcine common carotid artery | PLGA | poly(lactic-co-glycolic acid) |
| Dex | dexamethasone | PMAO | poly(maleicanhydride-alt-1-octadecene) |
| Dexa | dexamethasone phosphate | POC | poly(1,8-octamethylene citrate) |
| DGR | Ptx | paclitaxel | |
| DMSA | dimercaptosuccinic acid | PU | polyurethane |
| DPSCs | dental pulp stem cells | PVA | polyvinyl alcohol |
| DS | dextran sulfate | PVDF | polyvinylidene fluoride |
| ECCs | embryonic cardiac cells | PVDF | polyvinylidene difluoride |
| ECM | extracellular matrix | RGCs | retinal ganglion cells |
| ECs | endothelial cells | RGD | arginine-glycine-aspartate |
| EGFP-EGF1 | enhanced green fluorescent protein with the first epidermal growth factor domain | ROS | reactive oxygen species |
| EMMPRIN | ECM metalloproteinase inducer | scFv | single-chain variable fragment antibody |
| eNOS | endothelial nitric oxide synthase | SCs | Schwann cells |
| EPCs | endothelial progenitor cells | sGAG | sulfated glycosaminoglycan |
| ePTFE | expanded polytetrafluoroethylene | SK | streptokinase |
| ESCs | embryonic stem cells | SkMB | skeletal myoblasts |
| FGF2 | fibroblast growth factor 2 | SMCs | smooth muscle cells |
| FLI | fluorescence imaging | SPECT | single photon emission computed tomography |
| GDNF | glial derived neurotrophic factor | SR-A | macrophage scavenger receptor type A |
| GFP | green fluorescent protein | SS | stainless steel |
| HA | hydroxyapatite | SUI | stress urinary incontinence |
| hAECs | human aortic endothelial cells | TEVGs | tissue-engineered vascular grafts |
| HDL | high-density lipoproteins | TGF | transforming growth factor |
| hESCs | human embryonic stem cells | tPA | tissue plasminogen activator |
| hVEGF | human vascular endothelial growth factor | UK | urokinase |
| IBMIR | instant blood-mediated inflammatory responses | VCAM-1 | endothelial vascular adhesion molecule-1 |
| IONPs | iron oxide nanoparticles | VEGF | vascular endothelial growth factor |
| LIFU | low intensity focused ultrasound irradiation | VEGFR-2 | vascular endothelial growth factor receptor-2 |
| MCP-1 | monocyte chemoattractant protein-1 | WAT | white adipose tissue |
References
- Padmanabhan, P.; Kumar, A.; Kumar, S.; Chaudhary, R.K.; Gulyas, B. Nanoparticles in practice for molecular-imaging applications: An overview. Acta Biomater. 2016, 41, 1–16. [Google Scholar] [CrossRef]
- Heidt, T.; Nahrendorf, M. Multimodal iron oxide nanoparticles for hybrid biomedical imaging. NMR Biomed. 2013, 26, 756–765. [Google Scholar] [CrossRef]
- Kubinova, S.; Sykova, E. Nanotechnologies in regenerative medicine. Minim. Invasive Ther. Allied Technol. 2010, 19, 144–156. [Google Scholar] [CrossRef]
- Yi, D.K.; Nanda, S.S.; Kim, K.; Tamil Selvan, S. Recent progress in nanotechnology for stem cell differentiation, labeling, tracking and therapy. J. Mater. Chem. B 2017, 5, 9429–9451. [Google Scholar] [CrossRef]
- Pottler, M.; Cicha, I.; Unterweger, H.; Janko, C.; Friedrich, R.P.; Alexiou, C. Nanoparticles for regenerative medicine. Nanomedicine 2019, 14, 1929–1933. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, R.P.; Janko, C.; Unterweger, H.; Lyer, S.; Alexiou, C. SPIONs and magnetic hybrid materials: Synthesis, toxicology and biomedical applications. Phys. Sci. Rev. 2021. [Google Scholar] [CrossRef]
- Kumar, A.; Jena, P.K.; Behera, S.; Lockey, R.F.; Mohapatra, S.; Mohapatra, S. Multifunctional magnetic nanoparticles for targeted delivery. Nanomedicine 2010, 6, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Cicha, I.; Alexiou, C. Cardiovascular applications of magnetic particles. J. Magn. Magn. Mater. 2021, 518, 167428. [Google Scholar] [CrossRef]
- Mathiasen, A.B.; Hansen, L.; Friis, T.; Thomsen, C.; Bhakoo, K.; Kastrup, J. Optimal labeling dose, labeling time, and magnetic resonance imaging detection limits of ultrasmall superparamagnetic iron-oxide nanoparticle labeled mesenchymal stromal cells. Stem Cells Int. 2013, 2013, 353105. [Google Scholar] [CrossRef] [PubMed]
- Parashurama, N.; Ahn, B.C.; Ziv, K.; Ito, K.; Paulmurugan, R.; Willmann, J.K.; Chung, J.; Ikeno, F.; Swanson, J.C.; Merk, D.R.; et al. Multimodality Molecular Imaging of Cardiac Cell Transplantation: Part II. In Vivo Imaging of Bone Marrow Stromal Cells in Swine with PET/CT and MR Imaging. Radiology 2016, 280, 826–836. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knuchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef]
- Yamoah, M.A.; Moshref, M.; Sharma, J.; Chen, W.C.; Ledford, H.A.; Lee, J.H.; Chavez, K.S.; Wang, W.; Lopez, J.E.; Lieu, D.K.; et al. Highly efficient transfection of human induced pluripotent stem cells using magnetic nanoparticles. Int. J. Nanomed. 2018, 13, 6073–6078. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.E.; Osman, G.; Morris, G.E.; Markides, H.; Rotherham, M.; Bayoussef, Z.; El Haj, A.J.; Denning, C.; Shakesheff, K.M. Highly efficient delivery of functional cargoes by the synergistic effect of GAG binding motifs and cell-penetrating peptides. Proc. Natl. Acad. Sci. USA 2016, 113, E291–E299. [Google Scholar] [CrossRef] [PubMed]
- Budde, M.D.; Frank, J.A. Magnetic tagging of therapeutic cells for MRI. J. Nucl. Med. 2009, 50, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Ashammakhi, N.; Kaarela, O.; Ferretti, P. Pulling and Pushing Stem Cells to Control Their Differentiation. J. Craniofacial Surg. 2018, 29, 804–806. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef]
- Hasany, S.; Ahmed, I.; Rajan, J.; Rehman, A. Systematic review of the preparation techniques of iron oxide magnetic nanoparticles. Nanosci. Nanotechnol. 2012, 2, 148–158. [Google Scholar] [CrossRef]
- Wahajuddin, S.A. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar] [CrossRef]
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.-P. Magnetic nanoparticles in nanomedicine: A review of recent advances. Nanotechnology 2019, 30, 502003. [Google Scholar] [CrossRef]
- Majidi, S.; Sehrig, F.Z.; Farkhani, S.M.; Goloujeh, M.S.; Akbarzadeh, A. Current methods for synthesis of magnetic nanoparticles. Artif Cells Nanomed. Biotechnol. 2016, 44, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Ficiara, E.; Ruffinatti, F.A.; Stura, I.; Argenziano, M.; Abollino, O.; Cavalli, R.; Guiot, C.; D’Agata, F. Magnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Functionalization for Biomedical Applications in the Central Nervous System. Materials 2019, 12, 465. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Guo, Z.; Gao, F.; Gao, Q.; Wang, D.; Liaw, B.S.; Cai, Q.; Sun, X.; Wang, X.; Zhao, L. Shape-, size- and structure-controlled synthesis and biocompatibility of iron oxide nanoparticles for magnetic theranostics. Theranostics 2018, 8, 3284–3307. [Google Scholar] [CrossRef] [PubMed]
- Krasia-Christoforou, T.; Socoliuc, V.; Knudsen, K.D.; Tombacz, E.; Turcu, R.; Vekas, L. From Single-Core Nanoparticles in Ferrofluids to Multi-Core Magnetic Nanocomposites: Assembly Strategies, Structure, and Magnetic Behavior. Nanomaterials 2020, 10, 2178. [Google Scholar] [CrossRef]
- Amstad, E.; Textor, M.; Reimhult, E. Stabilization and functionalization of iron oxide nanoparticles for biomedical applications. Nanoscale 2011, 3, 2819–2843. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Monji, D.; Taromi, F.A. Bio-inspired surface modification of iron oxide nanoparticles for active stabilization in hydrogels. Soft Matter 2021, 17, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Margel, S. Review: Remotely controlled magneto-regulation of therapeutics from magnetoelastic gel matrices. Biotechnol. Adv. 2020, 44, 107611. [Google Scholar] [CrossRef]
- Ganguly, S.; Neelam; Grinberg, I.; Margel, S. Layer by layer controlled synthesis at room temperature of tri-modal (MRI, fluorescence and CT) core/shell superparamagnetic IO/human serum albumin nanoparticles for diagnostic applications. Polym. Adv. Technol. 2021. [Google Scholar] [CrossRef]
- Vallabani, N.V.S.; Singh, S. Recent advances and future prospects of iron oxide nanoparticles in biomedicine and diagnostics. 3 Biotech 2018, 8, 279. [Google Scholar] [CrossRef]
- Hola, K.; Markova, Z.; Zoppellaro, G.; Tucek, J.; Zboril, R. Tailored functionalization of iron oxide nanoparticles for MRI, drug delivery, magnetic separation and immobilization of biosubstances. Biotechnol. Adv. 2015, 33, 1162–1176. [Google Scholar] [CrossRef]
- Avasthi, A.; Caro, C.; Pozo-Torres, E.; Leal, M.P.; Garcia-Martin, M.L. Magnetic Nanoparticles as MRI Contrast Agents. Top. Curr. Chem. 2020, 378, 40. [Google Scholar] [CrossRef]
- Billings, C.; Langley, M.; Warrington, G.; Mashali, F.; Johnson, J.A. Magnetic Particle Imaging: Current and Future Applications, Magnetic Nanoparticle Synthesis Methods and Safety Measures. Int. J. Mol. Sci. 2021, 22, 7651. [Google Scholar] [CrossRef]
- Alphandery, E. Iron oxide nanoparticles for therapeutic applications. Drug Discov. Today 2020, 25, 141–149. [Google Scholar] [CrossRef]
- Ajinkya, N.; Yu, X.; Kaithal, P.; Luo, H.; Somani, P.; Ramakrishna, S. Magnetic Iron Oxide Nanoparticle (IONP) Synthesis to Applications: Present and Future. Materials 2020, 13, 4644. [Google Scholar] [CrossRef] [PubMed]
- Vangijzegem, T.; Stanicki, D.; Laurent, S. Magnetic iron oxide nanoparticles for drug delivery: Applications and characteristics. Expert Opin. Drug Deliv. 2019, 16, 69–78. [Google Scholar] [CrossRef]
- Vallabani, N.V.S.; Singh, S.; Karakoti, A.S. Magnetic Nanoparticles: Current Trends and Future Aspects in Diagnostics and Nanomedicine. Curr. Drug Metab. 2019, 20, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Andujar, C.; Walter, A.; Cotin, G.; Bordeianu, C.; Mertz, D.; Felder-Flesch, D.; Begin-Colin, S. Design of iron oxide-based nanoparticles for MRI and magnetic hyperthermia. Nanomedicine 2016, 11, 1889–1910. [Google Scholar] [CrossRef] [PubMed]
- El-Boubbou, K. Magnetic iron oxide nanoparticles as drug carriers: Clinical relevance. Nanomedicine 2018, 13, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Smit, F.E.; Dohmen, P.M. Cardiovascular tissue engineering: Where we come from and where are we now? Med. Sci. Monit. Basic Res. 2015, 21, 1–3. [Google Scholar] [CrossRef]
- Cicha, I.; Unterweger, H.; Lyer, S.; Janko, C.; Friedrich, R.P.; Pottler, M.; Alexiou, C. Nanomedicine for cardiovascular disorders. Nanomedicine 2019, 14, 3007–3012. [Google Scholar] [CrossRef]
- Saraste, A.; Nekolla, S.G.; Schwaiger, M. Cardiovascular molecular imaging: An overview. Cardiovasc. Res. 2009, 83, 643–652. [Google Scholar] [CrossRef]
- Hu, B.; Zeng, M.; Chen, J.; Zhang, Z.; Zhang, X.; Fan, Z.; Zhang, X. External Magnetic Field-Induced Targeted Delivery of Highly Sensitive Iron Oxide Nanocubes for MRI of Myocardial Infarction. Small 2016, 12, 4707–4712. [Google Scholar] [CrossRef]
- Wen, A.M.; Wang, Y.; Jiang, K.; Hsu, G.C.; Gao, H.; Lee, K.L.; Yang, A.C.; Yu, X.; Simon, D.I.; Steinmetz, N.F. Shaping bio-inspired nanotechnologies to target thrombosis for dual optical-magnetic resonance imaging. J. Mater. Chem. B 2015, 3, 6037–6045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Wu, J.C. Comparison of imaging techniques for tracking cardiac stem cell therapy. J. Nucl. Med. 2007, 48, 1916–1919. [Google Scholar] [CrossRef]
- Uppal, R.; Caravan, P. Targeted probes for cardiovascular MRI. Future Med. Chem. 2010, 2, 451–470. [Google Scholar] [CrossRef]
- Waters, E.A.; Wickline, S.A. Contrast agents for MRI. Basic Res. Cardiol. 2008, 103, 114–121. [Google Scholar] [CrossRef]
- Noukeu, L.C.; Wolf, J.; Yuan, B.; Banerjee, S.; Nguyen, K.T. Nanoparticles for Detection and Treatment of Peripheral Arterial Disease. Small 2018, 14, 1800644. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.R.; Weissleder, R. Multifunctional magnetic nanoparticles for targeted imaging and therapy. Adv. Drug Deliv. Rev. 2008, 60, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mascheri, N.; Dharmakumar, R.; Li, D. Cellular magnetic resonance imaging: Potential for use in assessing aspects of cardiovascular disease. Cytotherapy 2008, 10, 575–586. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liaw, N.; Liebeskind, D. Emerging therapies in acute ischemic stroke. F1000Research 2020, 9, F1000. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Ye, X.; Chen, Z.; Chen, Z.S. Mechanisms of thrombosis and research progress on targeted antithrombotic drugs. Drug Discov. Today 2021. [Google Scholar] [CrossRef]
- Zamanlu, M.; Farhoudi, M.; Eskandani, M.; Mahmoudi, J.; Barar, J.; Rafi, M.; Omidi, Y. Recent advances in targeted delivery of tissue plasminogen activator for enhanced thrombolysis in ischaemic stroke. J. Drug Target. 2018, 26, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Varna, M.; Juenet, M.; Bayles, R.; Mazighi, M.; Chauvierre, C.; Letourneur, D. Nanomedicine as a strategy to fight thrombotic diseases. Future Sci. OA 2015, 1, FSO46. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, R.P.; Zaloga, J.; Schreiber, E.; Toth, I.Y.; Tombacz, E.; Lyer, S.; Alexiou, C. Tissue Plasminogen Activator Binding to Superparamagnetic Iron Oxide Nanoparticle-Covalent Versus Adsorptive Approach. Nanoscale Res. Lett. 2016, 11, 297. [Google Scholar] [CrossRef]
- Heid, S.; Unterweger, H.; Tietze, R.; Friedrich, R.P.; Weigel, B.; Cicha, I.; Eberbeck, D.; Boccaccini, A.R.; Alexiou, C.; Lyer, S. Synthesis and Characterization of Tissue Plasminogen Activator-Functionalized Superparamagnetic Iron Oxide Nanoparticles for Targeted Fibrin Clot Dissolution. Int. J. Mol. Sci. 2017, 18, 1837. [Google Scholar] [CrossRef]
- Chen, J.P.; Yang, P.C.; Ma, Y.H.; Wu, T. Characterization of chitosan magnetic nanoparticles for in situ delivery of tissue plasminogen activator. Carbohydr. Polym. 2011, 84, 364–372. [Google Scholar] [CrossRef]
- Chen, J.P.; Yang, P.C.; Ma, Y.H.; Tu, S.J.; Lu, Y.J. Targeted delivery of tissue plasminogen activator by binding to silica-coated magnetic nanoparticle. Int. J. Nanomed. 2012, 7, 5137–5149. [Google Scholar] [CrossRef]
- Chen, J.P.; Yang, P.C.; Ma, Y.H.; Lu, Y.J. Superparamagnetic iron oxide nanoparticles for delivery of tissue plasminogen activator. J. Nanosci. Nanotechnol. 2011, 11, 11089–11094. [Google Scholar] [CrossRef]
- Chen, J.P.; Liu, C.H.; Hsu, H.L.; Wu, T.; Lu, Y.J.; Ma, Y.H. Magnetically controlled release of recombinant tissue plasminogen activator from chitosan nanocomposites for targeted thrombolysis. J. Mater. Chem. B 2016, 4, 2578–2590. [Google Scholar] [CrossRef]
- Chen, H.A.; Ma, Y.H.; Hsu, T.Y.; Chen, J.P. Preparation of Peptide and Recombinant Tissue Plasminogen Activator Conjugated Poly(Lactic-Co-Glycolic Acid) (PLGA) Magnetic Nanoparticles for Dual Targeted Thrombolytic Therapy. Int. J. Mol. Sci. 2020, 21, 2690. [Google Scholar] [CrossRef]
- Tu, S.J.; Wu, S.Y.; Wang, F.S.; Ma, Y.H. Retention assessment of magnetic nanoparticles in rat arteries with micro-computed tomography. Phys. Med. Biol. 2014, 59, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.W.; Hua, M.Y.; Lin, K.J.; Wey, S.P.; Tsai, R.Y.; Wu, S.Y.; Lu, Y.C.; Liu, H.L.; Wu, T.; Ma, Y.H. Bioconjugation of recombinant tissue plasminogen activator to magnetic nanocarriers for targeted thrombolysis. Int. J. Nanomed. 2012, 7, 5159–5173. [Google Scholar] [CrossRef]
- Huang, L.; Wang, J.; Huang, S.; Siaw-Debrah, F.; Nyanzu, M.; Zhuge, Q. Polyacrylic acid-coated nanoparticles loaded with recombinant tissue plasminogen activator for the treatment of mice with ischemic stroke. Biochem. Biophys. Res. Commun. 2019, 516, 565–570. [Google Scholar] [CrossRef]
- Xie, M.; Zhang, W.; Fan, C.; Wu, C.; Feng, Q.; Wu, J.; Li, Y.; Gao, R.; Li, Z.; Wang, Q.; et al. Bioinspired Soft Microrobots with Precise Magneto-Collective Control for Microvascular Thrombolysis. Adv. Mater. 2020, 32, e2000366. [Google Scholar] [CrossRef] [PubMed]
- Kempe, M.; Kempe, H.; Snowball, I.; Wallen, R.; Arza, C.R.; Gotberg, M.; Olsson, T. The use of magnetite nanoparticles for implant-assisted magnetic drug targeting in thrombolytic therapy. Biomaterials 2010, 31, 9499–9510. [Google Scholar] [CrossRef] [PubMed]
- Erdem, S.S.; Sazonova, I.Y.; Hara, T.; Jaffer, F.A.; McCarthy, J.R. Detection and treatment of intravascular thrombi with magnetofluorescent nanoparticles. Methods Enzymol. 2012, 508, 191–209. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, D.; Zhang, Y.; Wu, W.; Ran, H.; Wang, Z. Construction and evaluation of Fe(3)O(4)-based PLGA nanoparticles carrying rtPA used in the detection of thrombosis and in targeted thrombolysis. ACS Appl. Mater. Interfaces 2014, 6, 5566–5576. [Google Scholar] [CrossRef]
- Tadayon, A.; Jamshidi, R.; Esmaeili, A. Delivery of tissue plasminogen activator and streptokinase magnetic nanoparticles to target vascular diseases. Int. J. Pharm. 2015, 495, 428–438. [Google Scholar] [CrossRef]
- Ouyang, H.; Zheng, Z.; Chen, Y.; Liu, Y.; Hong, C.; Zhu, Y.; Deng, J.; Ding, X.; Zhou, W.; Wang, X. A magnetically modified black phosphorus nanosheet-based heparin delivery platform for preventing DVT accurately. J. Mater. Chem. B 2019, 7, 6099–6108. [Google Scholar] [CrossRef]
- Chang, M.; Lin, Y.H.; Gabayno, J.L.; Li, Q.; Liu, X. Thrombolysis based on magnetically-controlled surface-functionalized Fe3O4 nanoparticle. Bioengineered 2017, 8, 29–35. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Yuan, Z.; Yang, W.; Wu, Q.; Gu, H. Targeted thrombolysis by using of magnetic mesoporous silica nanoparticles. J. Biomed. Nanotechnol. 2012, 8, 624–632. [Google Scholar] [CrossRef]
- Li, Q.; Liu, X.; Chang, M.; Lu, Z. Thrombolysis Enhancing by Magnetic Manipulation of Fe(3)O(4) Nanoparticles. Materials 2018, 11, 2313. [Google Scholar] [CrossRef] [PubMed]
- Prilepskii, A.Y.; Fakhardo, A.F.; Drozdov, A.S.; Vinogradov, V.V.; Dudanov, I.P.; Shtil, A.A.; Bel’tyukov, P.P.; Shibeko, A.M.; Koltsova, E.M.; Nechipurenko, D.Y.; et al. Urokinase-Conjugated Magnetite Nanoparticles as a Promising Drug Delivery System for Targeted Thrombolysis: Synthesis and Preclinical Evaluation. ACS Appl. Mater. Interfaces 2018, 10, 36764–36775. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, J.; Liu, C.; Li, J.; Li, Z.; Zhao, J.; Liu, H. Synthesis of sustained release/controlled release nanoparticles carrying nattokinase and their application in thrombolysis. Pharmazie 2021, 76, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Zhang, S.F.; Lu, S.; Qi, T.; Yan, J.; Gao, C.; Liu, M.; Li, T.; Ji, Y. Synthesis of mesoporous silica/polyglutamic acid peptide dendrimer with dual targeting and its application in dissolving thrombus. J. Biomed. Mater. Res. Part A 2019, 107, 1824–1831. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, H.; Goel, L.; Kim, H.; Peng, C.; Kim, J.; Dayton, P.A.; Gao, Y.; Jiang, X. Magneto-sonothrombolysis with combination of magnetic microbubbles and nanodroplets. Ultrasonics 2021, 116, 106487. [Google Scholar] [CrossRef]
- Zhang, B.; Kim, H.; Wu, H.; Gao, Y.; Jiang, X. Sonothrombolysis with magnetic microbubbles under a rotational magnetic field. Ultrasonics 2019, 98, 62–71. [Google Scholar] [CrossRef]
- Wang, S.; Guo, X.; Xiu, W.; Liu, Y.; Ren, L.; Xiao, H.; Yang, F.; Gao, Y.; Xu, C.; Wang, L. Accelerating thrombolysis using a precision and clot-penetrating drug delivery strategy by nanoparticle-shelled microbubbles. Sci. Adv. 2020, 6, eaaz8204. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhou, J.; Zhong, Y.; Zhang, Y.; Liu, J.; Chen, Y.; Deng, L.; Sheng, D.; Wang, Z.; Ran, H.; et al. Phase Transition Nanoparticles as Multimodality Contrast Agents for the Detection of Thrombi and for Targeting Thrombolysis: In Vitro and in Vivo Experiments. ACS Appl. Mater. Interfaces 2017, 9, 42525–42535. [Google Scholar] [CrossRef]
- Liu, C.H.; Hsu, H.L.; Chen, J.P.; Wu, T.; Ma, Y.H. Thrombolysis induced by intravenous administration of plasminogen activator in magnetoliposomes: Dual targeting by magnetic and thermal manipulation. Nanomedicine 2019, 20, 101992. [Google Scholar] [CrossRef]
- Jeon, J.K.; Han, S.M.; Min, S.K.; Seo, S.J.; Ihm, K.; Chang, W.S.; Kim, J.K. Coulomb nanoradiator-mediated, site-specific thrombolytic proton treatment with a traversing pristine Bragg peak. Sci. Rep. 2016, 6, 37848. [Google Scholar] [CrossRef]
- Pashuck, E.T.; Stevens, M.M. Designing regenerative biomaterial therapies for the clinic. Sci. Transl. Med. 2012, 4, 160sr4. [Google Scholar] [CrossRef] [PubMed]
- Vellayappan, M.V.; Balaji, A.; Subramanian, A.P.; John, A.A.; Jaganathan, S.K.; Murugesan, S.; Supriyanto, E.; Yusof, M. Multifaceted prospects of nanocomposites for cardiovascular grafts and stents. Int. J. Nanomed. 2015, 10, 2785–2803. [Google Scholar] [CrossRef]
- Mironov, V.; Kasyanov, V.; Markwald, R.R. Nanotechnology in vascular tissue engineering: From nanoscaffolding towards rapid vessel biofabrication. Trends Biotechnol. 2008, 26, 338–344. [Google Scholar] [CrossRef]
- Antonyshyn, J.A.; D’Costa, K.A.; Santerre, J.P. Advancing tissue-engineered vascular grafts via their endothelialization and mechanical conditioning. J. Cardiovasc. Surg. 2020, 61, 555–576. [Google Scholar] [CrossRef]
- Gu, Z.; Rolfe, B.E.; Thomas, A.C.; Xu, Z.P. Restenosis treatments using nanoparticle-based drug delivery systems. Curr. Pharm. Des. 2013, 19, 6330–6339. [Google Scholar] [CrossRef]
- Jiang, B.; Perrin, L.; Kats, D.; Meade, T.; Ameer, G. Enabling non-invasive assessment of an engineered endothelium on ePTFE vascular grafts without increasing oxidative stress. Biomaterials 2015, 69, 110–120. [Google Scholar] [CrossRef]
- Luderer, F.; Begerow, I.; Schmidt, W.; Martin, H.; Grabow, N.; Bunger, C.M.; Schareck, W.; Schmitz, K.P.; Sternberg, K. Enhanced visualization of biodegradable polymeric vascular scaffolds by incorporation of gold, silver and magnetite nanoparticles. J. Biomater. Appl. 2013, 28, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Mertens, M.E.; Koch, S.; Schuster, P.; Wehner, J.; Wu, Z.; Gremse, F.; Schulz, V.; Rongen, L.; Wolf, F.; Frese, J.; et al. USPIO-labeled textile materials for non-invasive MR imaging of tissue-engineered vascular grafts. Biomaterials 2015, 39, 155–163. [Google Scholar] [CrossRef]
- Wolf, F.; Paefgen, V.; Winz, O.; Mertens, M.; Koch, S.; Gross-Weege, N.; Morgenroth, A.; Rix, A.; Schnoering, H.; Chalabi, K.; et al. MR and PET-CT monitoring of tissue-engineered vascular grafts in the ovine carotid artery. Biomaterials 2019, 216, 119228. [Google Scholar] [CrossRef]
- Harrington, J.K.; Chahboune, H.; Criscione, J.M.; Li, A.Y.; Hibino, N.; Yi, T.; Villalona, G.A.; Kobsa, S.; Meijas, D.; Duncan, D.R.; et al. Determining the fate of seeded cells in venous tissue-engineered vascular grafts using serial MRI. FASEB J. 2011, 25, 4150–4161. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.N.; Roh, J.D.; Mirensky, T.L.; Wang, Y.; Yi, T.; Tellides, G.; Pober, J.S.; Shkarin, P.; Shapiro, E.M.; Saltzman, W.M.; et al. Initial evaluation of the use of USPIO cell labeling and noninvasive MR monitoring of human tissue-engineered vascular grafts in vivo. FASEB J. 2008, 22, 3888–3895. [Google Scholar] [CrossRef]
- Karbasian, M.; Eftekhari, S.A.; Karimzadeh Kolamroudi, M.; Kamyab Moghadas, B.; Nasri, P.; Jasemi, A.; Telloo, M.; Saber-Samandari, S.; Khandan, A. Therapy with new generation of biodegradable and bioconjugate 3D printed artificial gastrointestinal lumen. Iran. J. Basic Med. Sci. 2021, 24, 391–399. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, F.; Bai, Q.; Song, D.; Zheng, Z.; Wang, Y.; Liu, X.; Abdulrahman, A.A.; Bian, Y.; Xu, X.; et al. Oscillating Magnetic Field Regulates Cell Adherence and Endothelialization Based on Magnetic Nanoparticle-Modified Bacterial Cellulose. ACS Appl. Mater. Interfaces 2020, 12, 52467–52478. [Google Scholar] [CrossRef]
- Arias, S.L.; Shetty, A.; Devorkin, J.; Allain, J.P. Magnetic targeting of smooth muscle cells in vitro using a magnetic bacterial cellulose to improve cell retention in tissue-engineering vascular grafts. Acta Biomater. 2018, 77, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Perea, H.; Aigner, J.; Heverhagen, J.T.; Hopfner, U.; Wintermantel, E. Vascular tissue engineering with magnetic nanoparticles: Seeing deeper. J. Tissue Eng. Regen. Med. 2007, 1, 318–321. [Google Scholar] [CrossRef]
- Perea, H.; Aigner, J.; Hopfner, U.; Wintermantel, E. Direct magnetic tubular cell seeding: A novel approach for vascular tissue engineering. Cells Tissues Organs 2006, 183, 156–165. [Google Scholar] [CrossRef]
- Singh, R.; Eitler, D.; Morelle, R.; Friedrich, R.P.; Dietel, B.; Alexiou, C.; Boccaccini, A.R.; Liverani, L.; Cicha, I. Optimization of cell seeding on electrospun PCL-silk fibroin scaffolds. Eur. Polym. J. 2020, 134, 109838. [Google Scholar] [CrossRef]
- Shimizu, K.; Ito, A.; Arinobe, M.; Murase, Y.; Iwata, Y.; Narita, Y.; Kagami, H.; Ueda, M.; Honda, H. Effective cell-seeding technique using magnetite nanoparticles and magnetic force onto decellularized blood vessels for vascular tissue engineering. J. Biosci. Bioeng. 2007, 103, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Fayol, D.; Le Visage, C.; Ino, J.; Gazeau, F.; Letourneur, D.; Wilhelm, C. Design of biomimetic vascular grafts with magnetic endothelial patterning. Cell Transplant. 2013, 22, 2105–2118. [Google Scholar] [CrossRef]
- Gonzalez-Molina, J.; Riegler, J.; Southern, P.; Ortega, D.; Frangos, C.C.; Angelopoulos, Y.; Husain, S.; Lythgoe, M.F.; Pankhurst, Q.A.; Day, R.M. Rapid magnetic cell delivery for large tubular bioengineered constructs. J. R. Soc. Interface 2012, 9, 3008–3016. [Google Scholar] [CrossRef]
- Neamtu, I.; Chiriac, A.P.; Diaconu, A.; Nita, L.E.; Balan, V.; Nistor, M.T. Current concepts on cardiovascular stent devices. Mini Rev. Med. Chem. 2014, 14, 505–536. [Google Scholar] [CrossRef]
- Qi, P.; Chen, S.; Liu, T.; Chen, J.; Yang, Z.; Weng, Y.; Chen, J.; Wang, J.; Maitz, M.F.; Huang, N. New strategies for developing cardiovascular stent surfaces with novel functions (Review). Biointerphases 2014, 9, 029017. [Google Scholar] [CrossRef] [PubMed]
- Jana, S. Endothelialization of cardiovascular devices. Acta Biomater. 2019, 99, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Uthamaraj, S.; Tefft, B.J.; Hlinomaz, O.; Sandhu, G.S.; Dragomir-Daescu, D. Ferromagnetic Bare Metal Stent for Endothelial Cell Capture and Retention. J. Vis. Exp. 2015, 103, 53100. [Google Scholar] [CrossRef]
- Tefft, B.J.; Uthamaraj, S.; Harburn, J.J.; Hlinomaz, O.; Lerman, A.; Dragomir-Daescu, D.; Sandhu, G.S. Magnetizable stent-grafts enable endothelial cell capture. J. Magn. Magn. Mater. 2017, 427, 100–104. [Google Scholar] [CrossRef]
- Uthamaraj, S.; Tefft, B.J.; Klabusay, M.; Hlinomaz, O.; Sandhu, G.S.; Dragomir-Daescu, D. Design and validation of a novel ferromagnetic bare metal stent capable of capturing and retaining endothelial cells. Ann. Biomed. Eng. 2014, 42, 2416–2424. [Google Scholar] [CrossRef]
- Polyak, B.; Fishbein, I.; Chorny, M.; Alferiev, I.; Williams, D.; Yellen, B.; Friedman, G.; Levy, R.J. High field gradient targeting of magnetic nanoparticle-loaded endothelial cells to the surfaces of steel stents. Proc. Natl. Acad. Sci. USA 2008, 105, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, W.; Zhang, J.; Sun, W.; Zhang, R.; Gu, H. Fabrication of a novel polymer-free nanostructured drug-eluting coating for cardiovascular stents. ACS Appl. Mater. Interfaces 2013, 5, 10337–10345. [Google Scholar] [CrossRef]
- Lee, J.S.; Han, P.; Song, E.; Kim, D.; Lee, H.; Labowsky, M.; Taavitsainen, J.; Yla-Herttuala, S.; Hytonen, J.; Gulcher, M.; et al. Magnetically Coated Bioabsorbable Stents for Renormalization of Arterial Vessel Walls after Stent Implantation. Nano Lett. 2018, 18, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Tefft, B.J.; Uthamaraj, S.; Harbuzariu, A.; Harburn, J.J.; Witt, T.A.; Newman, B.; Psaltis, P.J.; Hlinomaz, O.; Holmes, D.R., Jr.; Gulati, R.; et al. Nanoparticle-Mediated Cell Capture Enables Rapid Endothelialization of a Novel Bare Metal Stent. Tissue Eng. Part A 2018, 24, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Adamo, R.F.; Fishbein, I.; Zhang, K.; Wen, J.; Levy, R.J.; Alferiev, I.S.; Chorny, M. Magnetically enhanced cell delivery for accelerating recovery of the endothelium in injured arteries. J. Control Release 2016, 222, 169–175. [Google Scholar] [CrossRef][Green Version]
- Polyak, B.; Medved, M.; Lazareva, N.; Steele, L.; Patel, T.; Rai, A.; Rotenberg, M.Y.; Wasko, K.; Kohut, A.R.; Sensenig, R.; et al. Magnetic Nanoparticle-Mediated Targeting of Cell Therapy Reduces In-Stent Stenosis in Injured Arteries. ACS Nano 2016, 10, 9559–9569. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, S.; Wu, Z.; Wei, Z.; Zhang, W.; Li, W. Anti-CD34-Grafted Magnetic Nanoparticles Promote Endothelial Progenitor Cell Adhesion on an Iron Stent for Rapid Endothelialization. ACS Omega 2019, 4, 19469–19477. [Google Scholar] [CrossRef]
- Kodama, T.; Yoshihara, A.; Goel, I.; Sekino, M.; Kuwahata, A.; Yoshimori, A.; Murayama, Y.; Ishihara, K.; Ekdahl, K.N.; Nilsson, B.; et al. Identification of Metal-Binding Peptides and Their Conjugation onto Nanoparticles of Superparamagnetic Iron Oxides and Liposomes. ACS Appl. Mater. Interfaces 2020, 12, 24623–24634. [Google Scholar] [CrossRef] [PubMed]
- Chorny, M.; Fishbein, I.; Forbes, S.; Alferiev, I. Magnetic nanoparticles for targeted vascular delivery. IUBMB Life 2011, 63, 613–620. [Google Scholar] [CrossRef]
- Chorny, M.; Fishbein, I.; Adamo, R.F.; Forbes, S.P.; Folchman-Wagner, Z.; Alferiev, I.S. Magnetically targeted delivery of therapeutic agents to injured blood vessels for prevention of in-stent restenosis. Methodist DeBakey Cardiovasc. J. 2012, 8, 23–27. [Google Scholar] [CrossRef]
- Rathel, T.; Mannell, H.; Pircher, J.; Gleich, B.; Pohl, U.; Krotz, F. Magnetic stents retain nanoparticle-bound antirestenotic drugs transported by lipid microbubbles. Pharm. Res. 2012, 29, 1295–1307. [Google Scholar] [CrossRef]
- Johnson, B.; Toland, B.; Chokshi, R.; Mochalin, V.; Koutzaki, S.; Polyak, B. Magnetically responsive paclitaxel-loaded biodegradable nanoparticles for treatment of vascular disease: Preparation, characterization and in vitro evaluation of anti-proliferative potential. Curr. Drug Deliv. 2010, 7, 263–273. [Google Scholar] [CrossRef]
- Chorny, M.; Fishbein, I.; Yellen, B.B.; Alferiev, I.S.; Bakay, M.; Ganta, S.; Adamo, R.; Amiji, M.; Friedman, G.; Levy, R.J. Targeting stents with local delivery of paclitaxel-loaded magnetic nanoparticles using uniform fields. Proc. Natl. Acad. Sci. USA 2010, 107, 8346–8351. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guo, X.; Ren, L.; Wang, B.; Hou, L.; Zhou, H.; Gao, Q.; Gao, Y.; Wang, L. Targeting and deep-penetrating delivery strategy for stented coronary artery by magnetic guidance and ultrasound stimulation. Ultrason. Sonochem. 2020, 67, 105188. [Google Scholar] [CrossRef] [PubMed]
- Schoenhagen, P.; Conyers, J.L. Nanotechnology and atherosclerosis imaging: Emerging diagnostic and therapeutic applications. Recent Pat. Cardiovasc. Drug Discov. 2008, 3, 98–104. [Google Scholar] [CrossRef]
- Vaidyanathan, K.; Gopalakrishnan, S. Nanomedicine in the Diagnosis and Treatment of Atherosclerosis-A Systematic Review. Cardiovasc. Hematol. Disord. Drug Targets 2017, 17, 119–131. [Google Scholar] [CrossRef]
- Syed, M.B.; Fletcher, A.J.; Forsythe, R.O.; Kaczynski, J.; Newby, D.E.; Dweck, M.R.; van Beek, E.J. Emerging techniques in atherosclerosis imaging. Br. J. Radiol. 2019, 92, 20180309. [Google Scholar] [CrossRef]
- Skajaa, T.; Cormode, D.P.; Jarzyna, P.A.; Delshad, A.; Blachford, C.; Barazza, A.; Fisher, E.A.; Gordon, R.E.; Fayad, Z.A.; Mulder, W.J. The biological properties of iron oxide core high-density lipoprotein in experimental atherosclerosis. Biomaterials 2011, 32, 206–213. [Google Scholar] [CrossRef]
- Kanwar, R.K.; Chaudhary, R.; Tsuzuki, T.; Kanwar, J.R. Emerging engineered magnetic nanoparticulate probes for molecular MRI of atherosclerosis: How far have we come? Nanomedicine 2012, 7, 899–916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zu, Y.; Dhanasekara, C.S.; Li, J.; Wu, D.; Fan, Z.; Wang, S. Detection and treatment of atherosclerosis using nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1412. [Google Scholar] [CrossRef] [PubMed]
- Talev, J.; Kanwar, J.R. Iron Oxide Nanoparticles as Imaging and Therapeutic Agents for Atherosclerosis. Semin. Thromb. Hemost. 2020, 46, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Montiel Schneider, M.G.; Lassalle, V.L. Magnetic iron oxide nanoparticles as novel and efficient tools for atherosclerosis diagnosis. Biomed. Pharmacother. 2017, 93, 1098–1115. [Google Scholar] [CrossRef]
- Vazquez-Prada, K.X.; Lam, J.; Kamato, D.; Xu, Z.P.; Little, P.J.; Ta, H.T. Targeted Molecular Imaging of Cardiovascular Diseases by Iron Oxide Nanoparticles. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Cicha, I.; Lyer, S.; Alexiou, C.; Garlichs, C.D. Nanomedicine in diagnostics and therapy of cardiovascular diseases: Beyond atherosclerotic plaque imaging. Nanotechnol. Rev. 2013, 2, 449–472. [Google Scholar] [CrossRef]
- Palekar, R.U.; Jallouk, A.P.; Lanza, G.M.; Pan, H.; Wickline, S.A. Molecular imaging of atherosclerosis with nanoparticle-based fluorinated MRI contrast agents. Nanomedicine 2015, 10, 1817–1832. [Google Scholar] [CrossRef]
- Juenet, M.; Varna, M.; Aid-Launais, R.; Chauvierre, C.; Letourneur, D. Nanomedicine for the molecular diagnosis of cardiovascular pathologies. Biochem. Biophys. Res. Commun. 2015, 468, 476–484. [Google Scholar] [CrossRef]
- Uca, Y.O.; Hallmann, D.; Hesse, B.; Seim, C.; Stolzenburg, N.; Pietsch, H.; Schnorr, J.; Taupitz, M. Microdistribution of Magnetic Resonance Imaging Contrast Agents in Atherosclerotic Plaques Determined by LA-ICP-MS and SR-muXRF Imaging. Mol. Imaging Biol. 2021, 23, 382–393. [Google Scholar] [CrossRef]
- Jarrett, B.R.; Correa, C.; Ma, K.L.; Louie, A.Y. In vivo mapping of vascular inflammation using multimodal imaging. PLoS ONE 2010, 5, e13254. [Google Scholar] [CrossRef]
- Millon, A.; Dickson, S.D.; Klink, A.; Izquierdo-Garcia, D.; Bini, J.; Lancelot, E.; Ballet, S.; Robert, P.; Mateo de Castro, J.; Corot, C.; et al. Monitoring plaque inflammation in atherosclerotic rabbits with an iron oxide (P904) and (18)F-FDG using a combined PET/MR scanner. Atherosclerosis 2013, 228, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Schnorr, J.; Ludwig, A.; Stangl, V.; Ebert, M.; Hamm, B.; Taupitz, M. Contrast-enhanced MR imaging of atherosclerosis using citrate-coated superparamagnetic iron oxide nanoparticles: Calcifying microvesicles as imaging target for plaque characterization. Int. J. Nanomed. 2013, 8, 767–779. [Google Scholar] [CrossRef][Green Version]
- Kaneko, C.; Nitta, N.; Tsuchiya, K.; Watanabe, S.; Nitta-Seko, A.; Ohta, S.; Otani, H.; Sonoda, A.; Murata, K.; Shiomi, M. MRI study of atherosclerotic plaque progression using ultrasmall superparamagnetic iron oxide in Watanabe heritable hyperlipidemic rabbits. Br. J. Radiol. 2015, 88, 20150167. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.M.; Du, L.; Wu, W.H.; Li, D.Y.; Hao, J.; Gong, L.; Deng, L.; Zhang, T.; Zhang, C.; Zhang, Y. Detection of Vulnerable Atherosclerotic Plaques in Experimental Atherosclerosis with the USPIO-Enhanced MRI. Cell Biochem. Biophys. 2015, 73, 331–337. [Google Scholar] [CrossRef]
- Briley-Saebo, K.C.; Mani, V.; Hyafil, F.; Cornily, J.C.; Fayad, Z.A. Fractionated Feridex and positive contrast: In vivo MR imaging of atherosclerosis. Magn. Reson. Med. 2008, 59, 721–730. [Google Scholar] [CrossRef]
- Tang, T.Y.; Howarth, S.P.; Miller, S.R.; Graves, M.J.; JM, U.K.-I.; Li, Z.Y.; Walsh, S.R.; Hayes, P.D.; Varty, K.; Gillard, J.H. Comparison of the inflammatory burden of truly asymptomatic carotid atheroma with atherosclerotic plaques in patients with asymptomatic carotid stenosis undergoing coronary artery bypass grafting: An ultrasmall superparamagnetic iron oxide enhanced magnetic resonance study. Eur. J. Vasc. Endovasc. Surg. 2008, 35, 392–398. [Google Scholar] [CrossRef]
- Moonen, R.P.M.; Coolen, B.F.; Sluimer, J.C.; Daemen, M.; Strijkers, G.J. Iron Oxide Nanoparticle Uptake in Mouse Brachiocephalic Artery Atherosclerotic Plaque Quantified by T2-Mapping MRI. Pharmaceutics 2021, 13, 279. [Google Scholar] [CrossRef]
- Sadat, U.; Usman, A.; Gillard, J.H. Imaging pathobiology of carotid atherosclerosis with ultrasmall superparamagnetic particles of iron oxide: An update. Curr. Opin. Cardiol. 2017, 32, 437–440. [Google Scholar] [CrossRef]
- Hedgire, S.; Krebill, C.; Wojtkiewicz, G.R.; Oliveira, I.; Ghoshhajra, B.B.; Hoffmann, U.; Harisinghani, M.G. Ultrasmall superparamagnetic iron oxide nanoparticle uptake as noninvasive marker of aortic wall inflammation on MRI: Proof of concept study. Br. J. Radiol. 2018, 91, 20180461. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.R.; Stirrat, C.; Richards, J.; Mirsadraee, S.; Semple, S.I.; Tse, G.; Henriksen, P.; Newby, D.E. Vascular and plaque imaging with ultrasmall superparamagnetic particles of iron oxide. J. Cardiovasc. Magn. Reson. 2015, 17, 83. [Google Scholar] [CrossRef]
- Tang, T.Y.; Muller, K.H.; Graves, M.J.; Li, Z.Y.; Walsh, S.R.; Young, V.; Sadat, U.; Howarth, S.P.; Gillard, J.H. Iron oxide particles for atheroma imaging. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1001–1008. [Google Scholar] [CrossRef]
- Usman, A.; Patterson, A.J.; Sadat, U.; Tang, T.Y.; Graves, M.J.; Gillard, J.H. Assessment of Carotid Plaque Inflammation in Diabetic and Nondiabetic Patients-An Exploratory Ultrasmall Superparamagnetic Iron Oxide-Enhanced Magnetic Resonance Imaging Study. J. Stroke Cerebrovasc. Dis. 2017, 26, 858–862. [Google Scholar] [CrossRef]
- Usman, A.; Sadat, U.; Patterson, A.J.; Tang, T.Y.; Varty, K.; Boyle, J.R.; Armon, M.P.; Hayes, P.D.; Graves, M.J.; Gillard, J.H. Use of ultrasmall superparamagnetic iron oxide particles for imaging carotid atherosclerosis. Nanomedicine 2015, 10, 3077–3087. [Google Scholar] [CrossRef] [PubMed]
- Degnan, A.J.; Patterson, A.J.; Tang, T.Y.; Howarth, S.P.; Gillard, J.H. Evaluation of ultrasmall superparamagnetic iron oxide-enhanced MRI of carotid atherosclerosis to assess risk of cerebrovascular and cardiovascular events: Follow-up of the ATHEROMA trial. Cerebrovasc. Dis. 2012, 34, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Herborn, C.U.; Vogt, F.M.; Lauenstein, T.C.; Dirsch, O.; Corot, C.; Robert, P.; Ruehm, S.G. Magnetic resonance imaging of experimental atherosclerotic plaque: Comparison of two ultrasmall superparamagnetic particles of iron oxide. J. Magn. Reson. Imaging 2006, 24, 388–393. [Google Scholar] [CrossRef]
- Klug, G.; Kampf, T.; Ziener, C.; Parczyk, M.; Bauer, E.; Herold, V.; Rommel, E.; Jakob, P.M.; Bauer, W.R. Murine atherosclerotic plaque imaging with the USPIO Ferumoxtran-10. Front Biosci. 2009, 14, 2546–2552. [Google Scholar] [CrossRef]
- Smits, L.P.; Tiessens, F.; Zheng, K.H.; Stroes, E.S.; Nederveen, A.J.; Coolen, B.F. Evaluation of ultrasmall superparamagnetic iron-oxide (USPIO) enhanced MRI with ferumoxytol to quantify arterial wall inflammation. Atherosclerosis 2017, 263, 211–218. [Google Scholar] [CrossRef]
- Stein-Merlob, A.F.; Hara, T.; McCarthy, J.R.; Mauskapf, A.; Hamilton, J.A.; Ntziachristos, V.; Libby, P.; Jaffer, F.A. Atheroma Susceptible to Thrombosis Exhibit Impaired Endothelial Permeability In Vivo as Assessed by Nanoparticle-Based Fluorescence Molecular Imaging. Circ. Cardiovasc. Imaging 2017, 10, e005813. [Google Scholar] [CrossRef]
- Morishige, K.; Kacher, D.F.; Libby, P.; Josephson, L.; Ganz, P.; Weissleder, R.; Aikawa, M. High-resolution magnetic resonance imaging enhanced with superparamagnetic nanoparticles measures macrophage burden in atherosclerosis. Circulation 2010, 122, 1707–1715. [Google Scholar] [CrossRef]
- Kawahara, I.; Nakamoto, M.; Kitagawa, N.; Tsutsumi, K.; Nagata, I.; Morikawa, M.; Hayashi, T. Potential of magnetic resonance plaque imaging using superparamagnetic particles of iron oxide for the detection of carotid plaque. Neurol. Med. Chir. 2008, 48, 157–161. [Google Scholar] [CrossRef]
- Nakamura, M.; Kosuge, H.; Oyane, A.; Kuroiwa, K.; Shimizu, Y.; Aonuma, K. In vivostudy of iron oxide-calcium phosphate composite nanoparticles for delivery to atherosclerosis. Nanotechnology 2021, 32, 345101. [Google Scholar] [CrossRef]
- Tu, C.; Ng, T.S.; Sohi, H.K.; Palko, H.A.; House, A.; Jacobs, R.E.; Louie, A.Y. Receptor-targeted iron oxide nanoparticles for molecular MR imaging of inflamed atherosclerotic plaques. Biomaterials 2011, 32, 7209–7216. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kim, B.; Lim, E.K.; Choi, Y.; Choi, J.; Kim, E.; Jang, E.; Park, H.S.; Suh, J.S.; Huh, Y.M.; et al. Magnetic nanoclusters engineered by polymer-controlled self-assembly for the accurate diagnosis of atherosclerotic plaques via magnetic resonance imaging. Macromol. Biosci. 2014, 14, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tian, X.Y.; Chan, C.K.W.; Bai, Q.; Cheng, C.K.; Chen, F.M.; Cheung, M.S.H.; Yin, B.; Yang, H.; Yung, W.Y.; et al. Promoting the Delivery of Nanoparticles to Atherosclerotic Plaques by DNA Coating. ACS Appl. Mater. Interfaces 2019, 11, 13888–13904. [Google Scholar] [CrossRef] [PubMed]
- Terashima, M.; Uchida, M.; Kosuge, H.; Tsao, P.S.; Young, M.J.; Conolly, S.M.; Douglas, T.; McConnell, M.V. Human ferritin cages for imaging vascular macrophages. Biomaterials 2011, 32, 1430–1437. [Google Scholar] [CrossRef]
- Segers, F.M.; den Adel, B.; Bot, I.; van der Graaf, L.M.; van der Veer, E.P.; Gonzalez, W.; Raynal, I.; de Winther, M.; Wodzig, W.K.; Poelmann, R.E.; et al. Scavenger receptor-AI-targeted iron oxide nanoparticles for in vivo MRI detection of atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1812–1819. [Google Scholar] [CrossRef]
- Kitagawa, T.; Kosuge, H.; Uchida, M.; Iida, Y.; Dalman, R.L.; Douglas, T.; McConnell, M.V. RGD targeting of human ferritin iron oxide nanoparticles enhances in vivo MRI of vascular inflammation and angiogenesis in experimental carotid disease and abdominal aortic aneurysm. J. Magn. Reson. Imaging 2017, 45, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Tarin, C.; Carril, M.; Martin-Ventura, J.L.; Markuerkiaga, I.; Padro, D.; Llamas-Granda, P.; Moreno, J.A.; Garcia, I.; Genicio, N.; Plaza-Garcia, S.; et al. Targeted gold-coated iron oxide nanoparticles for CD163 detection in atherosclerosis by MRI. Sci. Rep. 2015, 5, 17135. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Li, X.; Zhou, C.; Tian, Q.; Li, C.; Xia, S.; Wang, R.; Feng, Y.; Zhan, W. Identifying macrophage enrichment in atherosclerotic plaques by targeting dual-modal US imaging/MRI based on biodegradable Fe-doped hollow silica nanospheres conjugated with anti-CD68 antibody. Nanoscale 2018, 10, 20246–20255. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-H.; Fu, Y.-C.; Zhang, D.-W.; Yin, K.; Tang, C.-K. Foam cells in atherosclerosis. Clin. Chim. Acta 2013, 424, 245–252. [Google Scholar] [CrossRef]
- Wu, M.; Li, X.; Guo, Q.; Li, J.; Xu, G.; Li, G.; Wang, J.; Zhang, X. Magnetic mesoporous silica nanoparticles-aided dual MR/NIRF imaging to identify macrophage enrichment in atherosclerotic plaques. Nanomedicine 2021, 32, 102330. [Google Scholar] [CrossRef]
- Smith, B.R.; Heverhagen, J.; Knopp, M.; Schmalbrock, P.; Shapiro, J.; Shiomi, M.; Moldovan, N.I.; Ferrari, M.; Lee, S.C. Localization to atherosclerotic plaque and biodistribution of biochemically derivatized superparamagnetic iron oxide nanoparticles (SPIONs) contrast particles for magnetic resonance imaging (MRI). Biomed. Microdevices 2007, 9, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Li, X.; Zhang, C.; Tan, H.; Wang, C.; Pang, L.; Shi, H. Detection of vulnerable atherosclerosis plaques with a dual-modal single-photon-emission computed tomography/magnetic resonance imaging probe targeting apoptotic macrophages. ACS Appl. Mater. Interfaces 2015, 7, 2847–2855. [Google Scholar] [CrossRef]
- Kao, C.W.; Wu, P.T.; Liao, M.Y.; Chung, I.J.; Yang, K.C.; Tseng, W.I.; Yu, J. Magnetic Nanoparticles Conjugated with Peptides Derived from Monocyte Chemoattractant Protein-1 as a Tool for Targeting Atherosclerosis. Pharmaceutics 2018, 10, 62. [Google Scholar] [CrossRef]
- Kelly, K.A.; Allport, J.R.; Tsourkas, A.; Shinde-Patil, V.R.; Josephson, L.; Weissleder, R. Detection of vascular adhesion molecule-1 expression using a novel multimodal nanoparticle. Circ. Res. 2005, 96, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Jaffer, F.A.; Kelly, K.A.; Sosnovik, D.E.; Aikawa, E.; Libby, P.; Weissleder, R. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation 2006, 114, 1504–1511. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, S.; Zhou, Q.; Chen, W. VHPKQHR peptide modified magnetic mesoporous nanoparticles for MRI detection of atherosclerosis lesions. Artif Cells Nanomed. Biotechnol. 2019, 47, 2440–2448. [Google Scholar] [CrossRef] [PubMed]
- Michalska, M.; Machtoub, L.; Manthey, H.D.; Bauer, E.; Herold, V.; Krohne, G.; Lykowsky, G.; Hildenbrand, M.; Kampf, T.; Jakob, P.; et al. Visualization of vascular inflammation in the atherosclerotic mouse by ultrasmall superparamagnetic iron oxide vascular cell adhesion molecule-1-specific nanoparticles. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2350–2357. [Google Scholar] [CrossRef] [PubMed]
- Prevot, G.; Kauss, T.; Lorenzato, C.; Gaubert, A.; Lariviere, M.; Baillet, J.; Laroche-Traineau, J.; Jacobin-Valat, M.J.; Adumeau, L.; Mornet, S.; et al. Iron oxide core oil-in-water nanoemulsion as tracer for atherosclerosis MPI and MRI imaging. Int. J. Pharm. 2017, 532, 669–676. [Google Scholar] [CrossRef]
- Ta, H.T.; Prabhu, S.; Leitner, E.; Jia, F.; von Elverfeldt, D.; Jackson, K.E.; Heidt, T.; Nair, A.K.; Pearce, H.; von Zur Muhlen, C.; et al. Enzymatic single-chain antibody tagging: A universal approach to targeted molecular imaging and cell homing in cardiovascular disease. Circ. Res. 2011, 109, 365–373. [Google Scholar] [CrossRef]
- Lariviere, M.; Lorenzato, C.S.; Adumeau, L.; Bonnet, S.; Hemadou, A.; Jacobin-Valat, M.J.; Noubhani, A.; Santarelli, X.; Minder, L.; Di Primo, C.; et al. Multimodal molecular imaging of atherosclerosis: Nanoparticles functionalized with scFv fragments of an anti-alphaIIbbeta3 antibody. Nanomedicine 2019, 22, 102082. [Google Scholar] [CrossRef]
- Jacobin-Valat, M.J.; Deramchia, K.; Mornet, S.; Hagemeyer, C.E.; Bonetto, S.; Robert, R.; Biran, M.; Massot, P.; Miraux, S.; Sanchez, S.; et al. MRI of inducible P-selectin expression in human activated platelets involved in the early stages of atherosclerosis. NMR Biomed. 2011, 24, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Jacobin-Valat, M.J.; Laroche-Traineau, J.; Lariviere, M.; Mornet, S.; Sanchez, S.; Biran, M.; Lebaron, C.; Boudon, J.; Lacomme, S.; Cerutti, M.; et al. Nanoparticles functionalised with an anti-platelet human antibody for in vivo detection of atherosclerotic plaque by magnetic resonance imaging. Nanomedicine 2015, 11, 927–937. [Google Scholar] [CrossRef]
- Poon, C.; Gallo, J.; Joo, J.; Chang, T.; Banobre-Lopez, M.; Chung, E.J. Hybrid, metal oxide-peptide amphiphile micelles for molecular magnetic resonance imaging of atherosclerosis. J. Nanobiotechnol. 2018, 16, 92. [Google Scholar] [CrossRef]
- Evans, R.J.; Lavin, B.; Phinikaridou, A.; Chooi, K.Y.; Mohri, Z.; Wong, E.; Boyle, J.J.; Krams, R.; Botnar, R.; Long, N.J. Targeted Molecular Iron Oxide Contrast Agents for Imaging Atherosclerotic Plaque. Nanotheranostics 2020, 4, 184–194. [Google Scholar] [CrossRef]
- Kim, M.; Sahu, A.; Kim, G.B.; Nam, G.H.; Um, W.; Shin, S.J.; Jeong, Y.Y.; Kim, I.S.; Kim, K.; Kwon, I.C.; et al. Comparison of in vivo targeting ability between cRGD and collagen-targeting peptide conjugated nano-carriers for atherosclerosis. J. Control Release 2018, 269, 337–346. [Google Scholar] [CrossRef]
- Chaudhary, R.; Roy, K.; Kanwar, R.K.; Walder, K.; Kanwar, J.R. Engineered atherosclerosis-specific zinc ferrite nanocomplex-based MRI contrast agents. J. Nanobiotechnol. 2016, 14, 6. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Yang, B.; Qiao, H.; Gao, L.; Su, T.; Ma, S.; Zhang, X.; Li, X.; Liu, G.; et al. In vivo MR and Fluorescence Dual-modality Imaging of Atherosclerosis Characteristics in Mice Using Profilin-1 Targeted Magnetic Nanoparticles. Theranostics 2016, 6, 272–286. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Wang, J.; Shi, W.; Zhang, B.; Jiang, H.; Du, M.; Mei, H.; Hu, Y. Improved in vivo detection of atherosclerotic plaques with a tissue factor-targeting magnetic nanoprobe. Acta Biomater. 2019, 90, 324–336. [Google Scholar] [CrossRef]
- Jung, C.; Kaul, M.G.; Bruns, O.T.; Ducic, T.; Freund, B.; Heine, M.; Reimer, R.; Meents, A.; Salmen, S.C.; Weller, H.; et al. Intraperitoneal injection improves the uptake of nanoparticle-labeled high-density lipoprotein to atherosclerotic plaques compared with intravenous injection: A multimodal imaging study in ApoE knockout mice. Circ. Cardiovasc. Imaging 2014, 7, 303–311. [Google Scholar] [CrossRef]
- Wen, S.; Liu, D.F.; Liu, Z.; Harris, S.; Yao, Y.Y.; Ding, Q.; Nie, F.; Lu, T.; Chen, H.J.; An, Y.L.; et al. OxLDL-targeted iron oxide nanoparticles for in vivo MRI detection of perivascular carotid collar induced atherosclerotic lesions in ApoE-deficient mice. J. Lipid Res. 2012, 53, 829–838. [Google Scholar] [CrossRef]
- Li, H.; El-Dakdouki, M.H.; Zhu, D.C.; Abela, G.S.; Huang, X. Synthesis of beta-cyclodextrin conjugated superparamagnetic iron oxide nanoparticles for selective binding and detection of cholesterol crystals. Chem. Commun. 2012, 48, 3385–3387. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Huang, J.W.; Song, J.C.; Ma, Z.L.; Shi, H.B. In vivo MRI detection of atherosclerosis in ApoE-deficient mice by using tenascin-C-targeted USPIO. Acta Radiol. 2018, 59, 1431–1437. [Google Scholar] [CrossRef]
- Chen, H.; Chen, L.; Liang, R.; Wei, J. Ultrasound and magnetic resonance molecular imaging of atherosclerotic neovasculature with perfluorocarbon magnetic nanocapsules targeted against vascular endothelial growth factor receptor 2 in rats. Mol. Med. Rep. 2017, 16, 5986–5996. [Google Scholar] [CrossRef] [PubMed]
- Hossaini Nasr, S.; Tonson, A.; El-Dakdouki, M.H.; Zhu, D.C.; Agnew, D.; Wiseman, R.; Qian, C.; Huang, X. Effects of Nanoprobe Morphology on Cellular Binding and Inflammatory Responses: Hyaluronan-Conjugated Magnetic Nanoworms for Magnetic Resonance Imaging of Atherosclerotic Plaques. ACS Appl. Mater. Interfaces 2018, 10, 11495–11507. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Wang, Y.B.; Han, D.; Wang, J.; Qi, S.; Gao, L.; Shao, Y.H.; Qiao, H.Y.; Chen, J.W.; Liang, S.H.; et al. Multimodality Imaging of Angiogenesis in a Rabbit Atherosclerotic Model by GEBP11 Peptide Targeted Nanoparticles. Theranostics 2017, 7, 4791–4804. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Hui, H.; Shang, W.; Zhang, Y.; Tian, F.; Ma, Q.; Yang, X.; Tian, J.; Chen, Y. Highly sensitive magnetic particle imaging of vulnerable atherosclerotic plaque with active myeloperoxidase-targeted nanoparticles. Theranostics 2021, 11, 506–521. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Deng, L.; Li, D.; Wu, W.; Gong, L.; Li, Y.; Zhang, Q.; Zhang, T.; Zhang, C.; Zhang, Y. Identifying Vulnerable Atherosclerotic Plaque in Rabbits Using DMSA-USPIO Enhanced Magnetic Resonance Imaging to Investigate the Effect of Atorvastatin. PLoS ONE 2015, 10, e0125677. [Google Scholar] [CrossRef]
- Nurhidayah, D.; Maruf, A.; Zhang, X.; Liao, X.; Wu, W.; Wang, G. Advanced drug-delivery systems: Mechanoresponsive nanoplatforms applicable in atherosclerosis management. Nanomedicine 2019, 14, 3105–3122. [Google Scholar] [CrossRef]
- Banik, B.; Surnar, B.; Askins, B.W.; Banerjee, M.; Dhar, S. Dual-Targeted Synthetic Nanoparticles for Cardiovascular Diseases. ACS Appl. Mater. Interfaces 2020, 12, 6852–6862. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, W.; Gao, P.; Chen, W.; Zhou, Q. Construction of dual nanomedicines for the imaging and alleviation of atherosclerosis. Artif. Cells Nanomed. Biotechnol. 2020, 48, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Winter, P.M.; Neubauer, A.M.; Caruthers, S.D.; Harris, T.D.; Robertson, J.D.; Williams, T.A.; Schmieder, A.H.; Hu, G.; Allen, J.S.; Lacy, E.K.; et al. Endothelial alpha(v)beta3 integrin-targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2103–2109. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, Y.; Zhao, W.; Xu, Z.P.; Little, P.J.; Whittaker, A.K.; Zhang, R.; Ta, H.T. Novel iron oxide-cerium oxide core-shell nanoparticles as a potential theranostic material for ROS related inflammatory diseases. J. Mater. Chem. B 2018, 6, 4937–4951. [Google Scholar] [CrossRef]
- Bonnet, S.; Prevot, G.; Mornet, S.; Jacobin-Valat, M.J.; Mousli, Y.; Hemadou, A.; Duttine, M.; Trotier, A.; Sanchez, S.; Duonor-Cerutti, M.; et al. A Nano-Emulsion Platform Functionalized with a Fully Human scFv-Fc Antibody for Atheroma Targeting: Towards a Theranostic Approach to Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 5188. [Google Scholar] [CrossRef]
- Yao, Y.; Li, B.; Fu, C.; Teng, G.; Ma, G.; Liu, N. Anti-connective tissue growth factor detects and reduces plaque inflammation in early-stage carotid atherosclerotic lesions. Nanomedicine 2017, 13, 2385–2394. [Google Scholar] [CrossRef]
- Ye, M.; Zhou, J.; Zhong, Y.; Xu, J.; Hou, J.; Wang, X.; Wang, Z.; Guo, D. SR-A-Targeted Phase-Transition Nanoparticles for the Detection and Treatment of Atherosclerotic Vulnerable Plaques. ACS Appl. Mater. Interfaces 2019, 11, 9702–9715. [Google Scholar] [CrossRef]
- Oumzil, K.; Ramin, M.A.; Lorenzato, C.; Hemadou, A.; Laroche, J.; Jacobin-Valat, M.J.; Mornet, S.; Roy, C.E.; Kauss, T.; Gaudin, K.; et al. Solid Lipid Nanoparticles for Image-Guided Therapy of Atherosclerosis. Bioconjug. Chem. 2016, 27, 569–575. [Google Scholar] [CrossRef]
- Gao, B.; Xu, J.; Zhou, J.; Zhang, H.; Yang, R.; Wang, H.; Huang, J.; Yan, F.; Luo, Y. Multifunctional pathology-mapping theranostic nanoplatforms for US/MR imaging and ultrasound therapy of atherosclerosis. Nanoscale 2021, 13, 8623–8638. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, Y.; Zou, X.; Chen, L.; Li, Y. Imaging of carotid artery inflammatory plaques with superparamagnetic nanoparticles and an external magnet collar. J. Mater. Chem. B 2017, 5, 797–806. [Google Scholar] [CrossRef]
- Bietenbeck, M.; Florian, A.; Faber, C.; Sechtem, U.; Yilmaz, A. Remote magnetic targeting of iron oxide nanoparticles for cardiovascular diagnosis and therapeutic drug delivery: Where are we now? Int. J. Nanomed. 2016, 11, 3191–3203. [Google Scholar] [CrossRef]
- Matuszak, J.; Dorfler, P.; Zaloga, J.; Unterweger, H.; Lyer, S.; Dietel, B.; Alexiou, C.; Cicha, I. Shell matters: Magnetic targeting of SPIONs and in vitro effects on endothelial and monocytic cell function. Clin. Hemorheol. Microcirc. 2015, 61, 259–277. [Google Scholar] [CrossRef]
- Matuszak, J.; Zaloga, J.; Friedrich, R.P.; Lyer, S.; Nowak, J.; Odenbach, S.; Alexiou, C.; Cicha, I. Endothelial biocompatibility and accumulation of SPION under flow conditions. J. Magn. Magn. Mater. 2015, 380, 20–26. [Google Scholar] [CrossRef]
- Matuszak, J.; Lutz, B.; Sekita, A.; Zaloga, J.; Alexiou, C.; Lyer, S.; Cicha, I. Drug delivery to atherosclerotic plaques using superparamagnetic iron oxide nanoparticles. Int. J. Nanomed. 2018, 13, 8443–8460. [Google Scholar] [CrossRef]
- Wei, H.; Tan, T.; Cheng, L.; Liu, J.; Song, H.; Li, L.; Zhang, K. MRI tracing of ultrasmall superparamagnetic iron oxide nanoparticlelabeled endothelial progenitor cells for repairing atherosclerotic vessels in rabbits. Mol. Med. Rep. 2020, 22, 3327–3337. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Wang, H.; Feng, Y.; Li, Y.; Hua, X.; Pang, X.; Zhang, S.; Song, L.; Zhang, Y.; Gu, N. Cardioprotective activity of iron oxide nanoparticles. Sci. Rep. 2015, 5, 8579. [Google Scholar] [CrossRef] [PubMed]
- Leuschner, F.; Courties, G.; Dutta, P.; Mortensen, L.J.; Gorbatov, R.; Sena, B.; Novobrantseva, T.I.; Borodovsky, A.; Fitzgerald, K.; Koteliansky, V.; et al. Silencing of CCR2 in myocarditis. Eur. Heart J. 2015, 36, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Moura, J.M.; Wu, Y.L.; Ho, C. Immune cells detection of the in vivo rejecting heart in USPIO-enhanced magnetic resonance imaging. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2006, 2006, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, W.; Ou, L.; Wang, W.; Delyagina, E.; Lux, C.; Sorg, H.; Riehemann, K.; Steinhoff, G.; Ma, N. Targeted delivery of human VEGF gene via complexes of magnetic nanoparticle-adenoviral vectors enhanced cardiac regeneration. PLoS ONE 2012, 7, e39490. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Dormer, K.; Po, S.S. Autonomic denervation using magnetic nanoparticles. Trends Cardiovasc. Med. 2010, 20, 268–272. [Google Scholar] [CrossRef]
- Kiaie, N.; Emami, S.H.; Rabbani, S.; Aghdam, R.M.; Tafti, H.A. Targeted and Controlled Drug Delivery to a Rat Model of Heart Failure Through a Magnetic Nanocomposite. Ann. Biomed. Eng. 2020, 48, 709–721. [Google Scholar] [CrossRef]
- Sivaraman, B.; Ramamurthi, A. Multifunctional nanoparticles for doxycycline delivery towards localized elastic matrix stabilization and regenerative repair. Acta Biomater. 2013, 9, 6511–6525. [Google Scholar] [CrossRef]
- Sivaraman, B.; Swaminathan, G.; Moore, L.; Fox, J.; Seshadri, D.; Dahal, S.; Stoilov, I.; Zborowski, M.; Mecham, R.; Ramamurthi, A. Magnetically-responsive, multifunctional drug delivery nanoparticles for elastic matrix regenerative repair. Acta Biomater. 2017, 52, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, X.; Bao, L.; Liu, T.; Yuan, P.; Yang, X.; Qiu, X.; Gooding, J.J.; Bai, Y.; Xiao, J.; et al. Treatment of infarcted heart tissue via the capture and local delivery of circulating exosomes through antibody-conjugated magnetic nanoparticles. Nat. Biomed. Eng. 2020, 4, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.R.; Park, B.W.; Kim, J.; Choo, Y.W.; Kim, H.Y.; Yoon, J.K.; Kim, H.; Hwang, J.W.; Kang, M.; Kwon, S.P.; et al. Nanovesicles derived from iron oxide nanoparticles-incorporated mesenchymal stem cells for cardiac repair. Sci. Adv. 2020, 6, eaaz0952. [Google Scholar] [CrossRef] [PubMed]
- Santoso, M.R.; Ikeda, G.; Tada, Y.; Jung, J.H.; Vaskova, E.; Sierra, R.G.; Gati, C.; Goldstone, A.B.; von Bornstaedt, D.; Shukla, P.; et al. Exosomes From Induced Pluripotent Stem Cell-Derived Cardiomyocytes Promote Autophagy for Myocardial Repair. J. Am. Heart Assoc. 2020, 9, e014345. [Google Scholar] [CrossRef]
- Cao, Y. Therapeutic angiogenesis for ischemic disorders: What is missing for clinical benefits? Discov. Med. 2010, 9, 179–184. [Google Scholar]
- Gazeau, F.; Wilhelm, C. Magnetic labeling, imaging and manipulation of endothelial progenitor cells using iron oxide nanoparticles. Future Med. Chem. 2010, 2, 397–408. [Google Scholar] [CrossRef]
- Li, Y.; Yao, Y.; Sheng, Z.; Yang, Y.; Ma, G. Dual-modal tracking of transplanted mesenchymal stem cells after myocardial infarction. Int. J. Nanomed. 2011, 6, 815–823. [Google Scholar] [CrossRef]
- Santoso, M.R.; Yang, P.C. Magnetic Nanoparticles for Targeting and Imaging of Stem Cells in Myocardial Infarction. Stem. Cells Int. 2016, 2016, 4198790. [Google Scholar] [CrossRef]
- Rogers, W.J.; Meyer, C.H.; Kramer, C.M. Technology insight: In vivo cell tracking by use of MRI. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Li, C.; Yang, S.; Xu, J.; Shen, Y.; Xie, X.; Dai, Y.; Lu, H.; Gong, H.; Sun, A.; et al. Magnetic resonance hypointensive signal primarily originates from extracellular iron particles in the long-term tracking of mesenchymal stem cells transplanted in the infarcted myocardium. Int. J. Nanomed. 2015, 10, 1679–1690. [Google Scholar] [CrossRef][Green Version]
- Naumova, A.V.; Balu, N.; Yarnykh, V.L.; Reinecke, H.; Murry, C.E.; Yuan, C. Magnetic Resonance Imaging Tracking of Graft Survival in the Infarcted Heart: Iron Oxide Particles Versus Ferritin Overexpression Approach. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Terrovitis, J.; Stuber, M.; Youssef, A.; Preece, S.; Leppo, M.; Kizana, E.; Schar, M.; Gerstenblith, G.; Weiss, R.G.; Marban, E.; et al. Magnetic resonance imaging overestimates ferumoxide-labeled stem cell survival after transplantation in the heart. Circulation 2008, 117, 1555–1562. [Google Scholar] [CrossRef]
- Ma, N.; Cheng, H.; Lu, M.; Liu, Q.; Chen, X.; Yin, G.; Zhu, H.; Zhang, L.; Meng, X.; Tang, Y.; et al. Magnetic resonance imaging with superparamagnetic iron oxide fails to track the long-term fate of mesenchymal stem cells transplanted into heart. Sci. Rep. 2015, 5, 9058. [Google Scholar] [CrossRef] [PubMed]
- Amsalem, Y.; Mardor, Y.; Feinberg, M.S.; Landa, N.; Miller, L.; Daniels, D.; Ocherashvilli, A.; Holbova, R.; Yosef, O.; Barbash, I.M.; et al. Iron-oxide labeling and outcome of transplanted mesenchymal stem cells in the infarcted myocardium. Circulation 2007, 116, I38–I45. [Google Scholar] [CrossRef]
- Yao, Y.; Li, Y.; Ma, G.; Liu, N.; Ju, S.; Jin, J.; Chen, Z.; Shen, C.; Teng, G. In vivo magnetic resonance imaging of injected endothelial progenitor cells after myocardial infarction in rats. Mol. Imaging Biol. 2011, 13, 303–313. [Google Scholar] [CrossRef]
- Pacak, C.A.; Hammer, P.E.; MacKay, A.A.; Dowd, R.P.; Wang, K.R.; Masuzawa, A.; Sill, B.; McCully, J.D.; Cowan, D.B. Superparamagnetic iron oxide nanoparticles function as a long-term, multi-modal imaging label for non-invasive tracking of implanted progenitor cells. PLoS ONE 2014, 9, e108695. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Kee, K.; Barral, J.K.; Dash, R.; Kosuge, H.; Wang, X.; Weissman, I.; Robbins, R.C.; Nishimura, D.; Quertermous, T.; et al. In vivo molecular MRI of cell survival and teratoma formation following embryonic stem cell transplantation into the injured murine myocardium. Magn. Reson. Med. 2011, 66, 1374–1381. [Google Scholar] [CrossRef]
- Hung, T.C.; Suzuki, Y.; Urashima, T.; Caffarelli, A.; Hoyt, G.; Sheikh, A.Y.; Yeung, A.C.; Weissman, I.; Robbins, R.C.; Bulte, J.W.; et al. Multimodality evaluation of the viability of stem cells delivered into different zones of myocardial infarction. Circ. Cardiovasc. Imaging 2008, 1, 6–13. [Google Scholar] [CrossRef]
- Tallheden, T.; Nannmark, U.; Lorentzon, M.; Rakotonirainy, O.; Soussi, B.; Waagstein, F.; Jeppsson, A.; Sjogren-Jansson, E.; Lindahl, A.; Omerovic, E. In vivo MR imaging of magnetically labeled human embryonic stem cells. Life Sci. 2006, 79, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Skelton, R.J.; Khoja, S.; Almeida, S.; Rapacchi, S.; Han, F.; Engel, J.; Zhao, P.; Hu, P.; Stanley, E.G.; Elefanty, A.G.; et al. Magnetic Resonance Imaging of Iron Oxide-Labeled Human Embryonic Stem Cell-Derived Cardiac Progenitors. Stem Cells Transl. Med. 2016, 5, 67–74. [Google Scholar] [CrossRef]
- Sadek, H.; Latif, S.; Collins, R.; Garry, M.G.; Garry, D.J. Use of ferumoxides for stem cell labeling. Regen. Med. 2008, 3, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Wierzbinski, K.R.; Szymanski, T.; Rozwadowska, N.; Rybka, J.D.; Zimna, A.; Zalewski, T.; Nowicka-Bauer, K.; Malcher, A.; Nowaczyk, M.; Krupinski, M.; et al. Potential use of superparamagnetic iron oxide nanoparticles for in vitro and in vivo bioimaging of human myoblasts. Sci. Rep. 2018, 8, 3682. [Google Scholar] [CrossRef] [PubMed]
- Salamon, J.; Wicklein, D.; Didie, M.; Lange, C.; Schumacher, U.; Adam, G.; Peldschus, K. Magnetic resonance imaging of single co-labeled mesenchymal stromal cells after intracardial injection in mice. Rofo 2014, 186, 367–376. [Google Scholar] [CrossRef]
- Mohanty, S.; Jain, K.G.; Nandy, S.B.; Kakkar, A.; Kumar, M.; Dinda, A.K.; Singh, H.; Ray, A. Iron oxide labeling does not affect differentiation potential of human bone marrow mesenchymal stem cells exhibited by their differentiation into cardiac and neuronal cells. Mol. Cell. Biochem. 2018, 448, 17–26. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Y.N.; Shi, X.L.; Ma, G.T.; Kong, L.Y.; Xue, H.D.; Lei, J.; He, Y.L.; Jin, Z.Y. In vivo and in vitro imaging tracing of dual-labeled bone mesenchymal stem cells transplanted into myocardium of F344 rats. Acta Acad. Sin. 2012, 34, 474–479. [Google Scholar] [CrossRef]
- Cao, J.; Li, X.; Chang, N.; Wang, Y.; Lei, J.; Zhao, D.; Gao, K.; Jin, Z. Dual-modular molecular imaging to trace transplanted bone mesenchymal stromal cells in an acute myocardial infarction model. Cytotherapy 2015, 17, 1365–1373. [Google Scholar] [CrossRef]
- Hua, P.; Wang, Y.Y.; Liu, L.B.; Liu, J.L.; Liu, J.Y.; Yang, Y.Q.; Yang, S.R. In vivo magnetic resonance imaging tracking of transplanted superparamagnetic iron oxide-labeled bone marrow mesenchymal stem cells in rats with myocardial infarction. Mol. Med. Rep. 2015, 11, 113–120. [Google Scholar] [CrossRef]
- Drey, F.; Choi, Y.H.; Neef, K.; Ewert, B.; Tenbrock, A.; Treskes, P.; Bovenschulte, H.; Liakopoulos, O.J.; Brenkmann, M.; Stamm, C.; et al. Noninvasive in vivo tracking of mesenchymal stem cells and evaluation of cell therapeutic effects in a murine model using a clinical 3.0 T MRI. Cell Transplant. 2013, 22, 1971–1980. [Google Scholar] [CrossRef] [PubMed]
- Ruhparwar, A.; Ghodsizad, A.; Niehaus, M.; Bara, C.; Lotz, J.; Voelkel, T.; Makoui, M.; Martin, U.; Wolf, F.; Gams, E.; et al. Clinically applicable 7-Tesla magnetic resonance visualization of transplanted human adult stem cells labeled with CliniMACS nanoparticles. Thorac. Cardiovasc. Surg. 2006, 54, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Hnatiuk, A.P.; Ong, S.G.; Olea, F.D.; Locatelli, P.; Riegler, J.; Lee, W.H.; Jen, C.H.; De Lorenzi, A.; Gimenez, C.S.; Laguens, R.; et al. Allogeneic Mesenchymal Stromal Cells Overexpressing Mutant Human Hypoxia-Inducible Factor 1-alpha (HIF1-alpha) in an Ovine Model of Acute Myocardial Infarction. J. Am. Heart Assoc. 2016, 5, e003714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; An, Q.; Li, Q.; Huang, J.; Chen, X.; Chen, X.; Zhang, J.; Wang, Y.; Yang, G.Y.; Zhu, W. Therapeutic benefit of bone marrow-derived endothelial progenitor cell transplantation after experimental aneurysm embolization with coil in rats. PLoS ONE 2014, 9, e90069. [Google Scholar] [CrossRef]
- Qin, J.B.; Li, K.A.; Li, X.X.; Xie, Q.S.; Lin, J.Y.; Ye, K.C.; Jiang, M.E.; Zhang, G.X.; Lu, X.W. Long-term MRI tracking of dual-labeled adipose-derived stem cells homing into mouse carotid artery injury. Int. J. Nanomed. 2012, 7, 5191–5203. [Google Scholar] [CrossRef]
- Zheng, Y.; Qin, J.; Wang, X.; Peng, Z.; Hou, P.; Lu, X. Dynamic imaging of allogeneic adipose-derived regenerative cells transplanted in ischemic hind limb of apolipoprotein E mouse model. Int. J. Nanomed. 2017, 12, 61–71. [Google Scholar] [CrossRef]
- Wang, J.; Xiang, B.; Deng, J.X.; Lin, H.Y.; Freed, D.H.; Arora, R.C.; Tian, G.H. Hypoxia enhances the therapeutic potential of superparamagnetic iron oxide-labeled adipose-derived stem cells for myocardial infarction. J. Huazhong Univ. Sci. Technol. Med. Sci. 2017, 37, 516–522. [Google Scholar] [CrossRef]
- Elkhenany, H.; Abd Elkodous, M.; Ghoneim, N.I.; Ahmed, T.A.; Ahmed, S.M.; Mohamed, I.K.; El-Badri, N. Comparison of different uncoated and starch-coated superparamagnetic iron oxide nanoparticles: Implications for stem cell tracking. Int. J. Biol. Macromol. 2020, 143, 763–774. [Google Scholar] [CrossRef]
- Hill, J.M.; Dick, A.J.; Raman, V.K.; Thompson, R.B.; Yu, Z.X.; Hinds, K.A.; Pessanha, B.S.; Guttman, M.A.; Varney, T.R.; Martin, B.J.; et al. Serial cardiac magnetic resonance imaging of injected mesenchymal stem cells. Circulation 2003, 108, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Mathiasen, A.B.; Qayyum, A.A.; Jorgensen, E.; Helqvist, S.; Ekblond, A.; Ng, M.; Bhakoo, K.; Kastrup, J. In Vivo MRI Tracking of Mesenchymal Stromal Cells Labeled with Ultrasmall Paramagnetic Iron Oxide Particles after Intramyocardial Transplantation in Patients with Chronic Ischemic Heart Disease. Stem Cells Int. 2019, 2019, 2754927. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, X.; Huang, Z.; Pei, N.; Xu, J.; Li, Z.; Wang, Y.; Qian, J.; Ge, J. Comparison of Magnetic Intensities for Mesenchymal Stem Cell Targeting Therapy on Ischemic Myocardial Repair: High Magnetic Intensity Improves Cell Retention but Has no Additional Functional Benefit. Cell Transplant. 2015, 24, 1981–1997. [Google Scholar] [CrossRef]
- Naseroleslami, M.; Aboutaleb, N.; Parivar, K. The effects of superparamagnetic iron oxide nanoparticles-labeled mesenchymal stem cells in the presence of a magnetic field on attenuation of injury after heart failure. Drug Deliv. Transl. Res. 2018, 8, 1214–1225. [Google Scholar] [CrossRef]
- Huang, Z.; Shen, Y.; Sun, A.; Huang, G.; Zhu, H.; Huang, B.; Xu, J.; Song, Y.; Pei, N.; Ma, J.; et al. Magnetic targeting enhances retrograde cell retention in a rat model of myocardial infarction. Stem Cell Res. Ther. 2013, 4, 149. [Google Scholar] [CrossRef] [PubMed]
- Ottersbach, A.; Mykhaylyk, O.; Heidsieck, A.; Eberbeck, D.; Rieck, S.; Zimmermann, K.; Breitbach, M.; Engelbrecht, B.; Brugmann, T.; Hesse, M.; et al. Improved heart repair upon myocardial infarction: Combination of magnetic nanoparticles and tailored magnets strongly increases engraftment of myocytes. Biomaterials 2018, 155, 176–190. [Google Scholar] [CrossRef]
- Cheng, K.; Shen, D.; Hensley, M.T.; Middleton, R.; Sun, B.; Liu, W.; De Couto, G.; Marban, E. Magnetic antibody-linked nanomatchmakers for therapeutic cell targeting. Nat. Commun. 2014, 5, 4880. [Google Scholar] [CrossRef] [PubMed]
- Vandergriff, A.C.; Hensley, T.M.; Henry, E.T.; Shen, D.; Anthony, S.; Zhang, J.; Cheng, K. Magnetic targeting of cardiosphere-derived stem cells with ferumoxytol nanoparticles for treating rats with myocardial infarction. Biomaterials 2014, 35, 8528–8539. [Google Scholar] [CrossRef]
- Zhang, B.F.; Jiang, H.; Chen, J.; Hu, Q.; Yang, S.; Liu, X.P. Silica-coated magnetic nanoparticles labeled endothelial progenitor cells alleviate ischemic myocardial injury and improve long-term cardiac function with magnetic field guidance in rats with myocardial infarction. J. Cell. Physiol. 2019, 234, 18544–18559. [Google Scholar] [CrossRef]
- Wang, J.; Xiang, B.; Deng, J.; Lin, H.Y.; Zheng, D.; Freed, D.H.; Arora, R.C.; Tian, G. Externally Applied Static Magnetic Field Enhances Cardiac Retention and Functional Benefit of Magnetically Iron-Labeled Adipose-Derived Stem Cells in Infarcted Hearts. Stem Cells Transl. Med. 2016, 5, 1380–1393. [Google Scholar] [CrossRef]
- Riegler, J.; Liew, A.; Hynes, S.O.; Ortega, D.; O’Brien, T.; Day, R.M.; Richards, T.; Sharif, F.; Pankhurst, Q.A.; Lythgoe, M.F. Superparamagnetic iron oxide nanoparticle targeting of MSCs in vascular injury. Biomaterials 2013, 34, 1987–1994. [Google Scholar] [CrossRef]
- Vosen, S.; Rieck, S.; Heidsieck, A.; Mykhaylyk, O.; Zimmermann, K.; Bloch, W.; Eberbeck, D.; Plank, C.; Gleich, B.; Pfeifer, A.; et al. Vascular Repair by Circumferential Cell Therapy Using Magnetic Nanoparticles and Tailored Magnets. ACS Nano 2016, 10, 369–376. [Google Scholar] [CrossRef]
- Blumler, P.; Friedrich, R.P.; Pereira, J.; Baun, O.; Alexiou, C.; Mailander, V. Contactless Nanoparticle-Based Guiding of Cells by Controllable Magnetic Fields. Nanotechnol Sci. Appl. 2021, 14, 91–100. [Google Scholar] [CrossRef]
- Kyrtatos, P.G.; Lehtolainen, P.; Junemann-Ramirez, M.; Garcia-Prieto, A.; Price, A.N.; Martin, J.F.; Gadian, D.G.; Pankhurst, Q.A.; Lythgoe, M.F. Magnetic tagging increases delivery of circulating progenitors in vascular injury. JACC Cardiovasc. Interv. 2009, 2, 794–802. [Google Scholar] [CrossRef]
- Han, J.; Kim, B.; Shin, J.Y.; Ryu, S.; Noh, M.; Woo, J.; Park, J.S.; Lee, Y.; Lee, N.; Hyeon, T.; et al. Iron oxide nanoparticle-mediated development of cellular gap junction crosstalk to improve mesenchymal stem cells’ therapeutic efficacy for myocardial infarction. ACS Nano 2015, 9, 2805–2819. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhao, E.R.; Hableel, G.; Hu, T.; Kim, T.; Li, J.; Gonzalez-Pech, N.I.; Cheng, D.J.; Lemaster, J.E.; Xie, Y.; et al. Increasing the Efficacy of Stem Cell Therapy via Triple-Function Inorganic Nanoparticles. ACS Nano 2019, 13, 6605–6617. [Google Scholar] [CrossRef]
- Takanari, H.; Miwa, K.; Fu, X.; Nakai, J.; Ito, A.; Ino, K.; Honda, H.; Tonomura, W.; Konishi, S.; Opthof, T.; et al. A New In Vitro Co-Culture Model Using Magnetic Force-Based Nanotechnology. J. Cell. Physiol. 2016, 231, 2249–2256. [Google Scholar] [CrossRef] [PubMed]
- Blocki, A.; Beyer, S.; Dewavrin, J.Y.; Goralczyk, A.; Wang, Y.; Peh, P.; Ng, M.; Moonshi, S.S.; Vuddagiri, S.; Raghunath, M.; et al. Microcapsules engineered to support mesenchymal stem cell (MSC) survival and proliferation enable long-term retention of MSCs in infarcted myocardium. Biomaterials 2015, 53, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Mauricio, R.G.; Acarregui, A.; Sanchez-Margallo, F.M.; Crisostomo, V.; Gallo, I.; Hernandez, R.M.; Pedraz, J.L.; Orive, G.; Martin-Cancho, M.F. A preliminary approach to the repair of myocardial infarction using adipose tissue-derived stem cells encapsulated in magnetic resonance-labelled alginate microspheres in a porcine model. Eur. J. Pharm. Biopharm. 2013, 84, 29–39. [Google Scholar] [CrossRef]
- Nazari, H.; Heirani-Tabasi, A.; Hajiabbas, M.; Salimi Bani, M.; Nazari, M.; Pirhajati Mahabadi, V.; Rad, I.; Kehtari, M.; Ahmadi Tafti, S.H.; Soleimani, M. Incorporation of SPION-casein core-shells into silk-fibroin nanofibers for cardiac tissue engineering. J. Cell. Biochem. 2020, 121, 2981–2993. [Google Scholar] [CrossRef] [PubMed]
- Zwi-Dantsis, L.; Wang, B.; Marijon, C.; Zonetti, S.; Ferrini, A.; Massi, L.; Stuckey, D.J.; Terracciano, C.M.; Stevens, M.M. Remote Magnetic Nanoparticle Manipulation Enables the Dynamic Patterning of Cardiac Tissues. Adv. Mater. 2020, 32, e1904598. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Zhao, M.; Matsuura, Y.; Laurent, S.; Yang, P.C.; Bernstein, D.; Ruiz-Lozano, P.; Serpooshan, V. Infection-resistant MRI-visible scaffolds for tissue engineering applications. Bioimpacts 2016, 6, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.; Lv, S.; Xiong, F.; Han, Y.; Zhao, Y.; Li, J.; Gu, N.; Zhou, J. Effects of different doses of 2,3-dimercaptosuccinic acid-modified Fe2 O3 nanoparticles on intercalated discs in engineered cardiac tissues. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 121–130. [Google Scholar] [CrossRef]
- Dvir, T.; Kedem, A.; Ruvinov, E.; Levy, O.; Freeman, I.; Landa, N.; Holbova, R.; Feinberg, M.S.; Dror, S.; Etzion, Y.; et al. Prevascularization of cardiac patch on the omentum improves its therapeutic outcome. Proc. Natl. Acad. Sci. USA 2009, 106, 14990–14995. [Google Scholar] [CrossRef] [PubMed]
- Sapir, Y.; Ruvinov, E.; Polyak, B.; Cohen, S. Magnetically actuated alginate scaffold: A novel platform for promoting tissue organization and vascularization. Methods Mol. Biol. 2014, 1181, 83–95. [Google Scholar] [CrossRef]
- Sapir, Y.; Polyak, B.; Cohen, S. Cardiac tissue engineering in magnetically actuated scaffolds. Nanotechnology 2014, 25, 014009. [Google Scholar] [CrossRef] [PubMed]
- Blondiaux, E.; Pidial, L.; Autret, G.; Rahmi, G.; Balvay, D.; Audureau, E.; Wilhelm, C.; Guerin, C.L.; Bruneval, P.; Silvestre, J.S.; et al. Bone marrow-derived mesenchymal stem cell-loaded fibrin patches act as a reservoir of paracrine factors in chronic myocardial infarction. J. Tissue Eng. Regen. Med. 2017, 11, 3417–3427. [Google Scholar] [CrossRef] [PubMed]
- Vallee, J.P.; Hauwel, M.; Lepetit-Coiffe, M.; Bei, W.; Montet-Abou, K.; Meda, P.; Gardier, S.; Zammaretti, P.; Kraehenbuehl, T.P.; Herrmann, F.; et al. Embryonic stem cell-based cardiopatches improve cardiac function in infarcted rats. Stem Cells Transl. Med. 2012, 1, 248–260. [Google Scholar] [CrossRef]
- Ishii, M.; Shibata, R.; Shimizu, Y.; Yamamoto, T.; Kondo, K.; Inoue, Y.; Ouchi, N.; Tanigawa, T.; Kanemura, N.; Ito, A.; et al. Multilayered adipose-derived regenerative cell sheets created by a novel magnetite tissue engineering method for myocardial infarction. Int. J. Cardiol. 2014, 175, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Shibata, R.; Numaguchi, Y.; Kito, T.; Suzuki, H.; Shimizu, K.; Ito, A.; Honda, H.; Murohara, T. Enhanced angiogenesis by transplantation of mesenchymal stem cell sheet created by a novel magnetic tissue engineering method. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2210–2215. [Google Scholar] [CrossRef]
- Shimizu, K.; Ito, A.; Lee, J.K.; Yoshida, T.; Miwa, K.; Ishiguro, H.; Numaguchi, Y.; Murohara, T.; Kodama, I.; Honda, H. Construction of multi-layered cardiomyocyte sheets using magnetite nanoparticles and magnetic force. Biotechnol. Bioeng. 2007, 96, 803–809. [Google Scholar] [CrossRef]
- Akiyama, H.; Ito, A.; Sato, M.; Kawabe, Y.; Kamihira, M. Construction of cardiac tissue rings using a magnetic tissue fabrication technique. Int. J. Mol. Sci. 2010, 11, 2910–2920. [Google Scholar] [CrossRef] [PubMed]
- Kito, T.; Shibata, R.; Ishii, M.; Suzuki, H.; Himeno, T.; Kataoka, Y.; Yamamura, Y.; Yamamoto, T.; Nishio, N.; Ito, S.; et al. iPS cell sheets created by a novel magnetite tissue engineering method for reparative angiogenesis. Sci. Rep. 2013, 3, 1418. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, D.; Mehrotra, S.; Majumder, O.; Mandal, B.B. Magnetic Actuator Device Assisted Modulation of Cellular Behavior and Tuning of Drug Release on Silk Platform. ACS Biomater. Sci. Eng. 2019, 5, 92–105. [Google Scholar] [CrossRef]
- Martinez, C.; Henao, A.; Rodriguez, J.E.; Padgett, K.R.; Ramaswamy, S. Monitoring steady flow effects on cell distribution in engineered valve tissues by magnetic resonance imaging. Mol. Imaging 2013, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.R.; Xu, S.; Stains, J.P.; Bennett, C.H.; Lovering, R.M. Superparamagnetic Iron Oxide Nanoparticles in Musculoskeletal Biology. Tissue Eng. Part B Rev. 2017, 23, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xia, C.; Wang, Z.; Lv, F.; Gao, F.; Gong, Q.; Song, B.; Ai, H.; Gu, Z. Magnetic resonance imaging probes for labeling of chondrocyte cells. J. Mater. Sci. Mater. Med. 2011, 22, 601–606. [Google Scholar] [CrossRef]
- Saha, S.; Yang, X.B.; Tanner, S.; Curran, S.; Wood, D.; Kirkham, J. The effects of iron oxide incorporation on the chondrogenic potential of three human cell types. J. Tissue Eng. Regen. Med. 2013, 7, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Fayol, D.; Luciani, N.; Lartigue, L.; Gazeau, F.; Wilhelm, C. Managing magnetic nanoparticle aggregation and cellular uptake: A precondition for efficient stem-cell differentiation and MRI tracking. Adv. Healthc. Mater. 2013, 2, 313–325. [Google Scholar] [CrossRef]
- Jing, X.H.; Yang, L.; Duan, X.J.; Xie, B.; Chen, W.; Li, Z.; Tan, H.B. In vivo MR imaging tracking of magnetic iron oxide nanoparticle labeled, engineered, autologous bone marrow mesenchymal stem cells following intra-articular injection. Jt. Bone Spine 2008, 75, 432–438. [Google Scholar] [CrossRef]
- Wimpenny, I.; Markides, H.; El Haj, A.J. Orthopaedic applications of nanoparticle-based stem cell therapies. Stem Cell Res. Ther. 2012, 3, 13. [Google Scholar] [CrossRef]
- Chiang, C.S.; Chen, J.Y.; Chiang, M.Y.; Hou, K.T.; Li, W.M.; Chang, S.J.; Chen, S.Y. Using the interplay of magnetic guidance and controlled TGF-beta release from protein-based nanocapsules to stimulate chondrogenesis. Int. J. Nanomed. 2018, 13, 3177–3188. [Google Scholar] [CrossRef]
- Feng, Y.; Jin, X.; Dai, G.; Liu, J.; Chen, J.; Yang, L. In vitro targeted magnetic delivery and tracking of superparamagnetic iron oxide particles labeled stem cells for articular cartilage defect repair. J. Huazhong Univ. Sci. Technol. Med. Sci. 2011, 31, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Ochi, M.; Yanada, S.; Ishikawa, M.; Adachi, N.; Deie, M.; Arihiro, K. A novel cell delivery system using magnetically labeled mesenchymal stem cells and an external magnetic device for clinical cartilage repair. Arthroscopy 2008, 24, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Kamei, N.; Ochi, M.; Adachi, N.; Ishikawa, M.; Yanada, S.; Levin, L.S.; Kamei, G.; Kobayashi, T. The safety and efficacy of magnetic targeting using autologous mesenchymal stem cells for cartilage repair. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 3626–3635. [Google Scholar] [CrossRef]
- Mertens, M.E.; Hermann, A.; Buhren, A.; Olde-Damink, L.; Mockel, D.; Gremse, F.; Ehling, J.; Kiessling, F.; Lammers, T. Iron Oxide-labeled Collagen Scaffolds for Non-invasive MR Imaging in Tissue Engineering. Adv. Funct. Mater. 2014, 24, 754–762. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Z.; Lu, J.; Xia, C.; Gao, F.; Gong, Q.; Song, B.; Zhao, X.; Shuai, X.; Chen, X.; et al. Low molecular weight alkyl-polycation wrapped magnetite nanoparticle clusters as MRI probes for stem cell labeling and in vivo imaging. Biomaterials 2011, 32, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liang, Y.; Huang, Z.; Xiong, J.; Wang, D. Preparation, Characterization, and Biological Testing of Novel Magnetic Nanocomposite Hydrogels. ACS Omega 2020, 5, 9733–9743. [Google Scholar] [CrossRef]
- Yang, W.; Zheng, Y.; Chen, J.; Zhu, Q.; Feng, L.; Lan, Y.; Zhu, P.; Tang, S.; Guo, R. Preparation and characterization of the collagen/cellulose nanocrystals/USPIO scaffolds loaded kartogenin for cartilage regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 1362–1373. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, C.; Yan, S.; Liu, Q.; Hou, M.; Xu, Y.; Guo, R. Non-invasive monitoring of in vivo hydrogel degradation and cartilage regeneration by multiparametric MR imaging. Theranostics 2018, 8, 1146–1158. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, P.; Huang, H.; Zheng, Y.; Liu, J.; Feng, L.; Guo, H.; Tang, S.; Guo, R. Functionalization of Novel Theranostic Hydrogels with Kartogenin-Grafted USPIO Nanoparticles To Enhance Cartilage Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 34744–34754. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, S.; Greco, J.B.; Uluer, M.C.; Zhang, Z.; Zhang, Z.; Fishbein, K.W.; Spencer, R.G. Magnetic resonance imaging of chondrocytes labeled with superparamagnetic iron oxide nanoparticles in tissue-engineered cartilage. Tissue Eng. Part A 2009, 15, 3899–3910. [Google Scholar] [CrossRef] [PubMed]
- Nedopil, A.J.; Mandrussow, L.G.; Daldrup-Link, H.E. Implantation of ferumoxides labeled human mesenchymal stem cells in cartilage defects. J. Vis. Exp. 2010, 38, 1793. [Google Scholar] [CrossRef] [PubMed]
- Brady, M.A.; Talvard, L.; Vella, A.; Ethier, C.R. Bio-inspired design of a magnetically active trilayered scaffold for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 1298–1302. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.T.; Liu, T.Y.; Chiang, M.Y.; Chen, C.Y.; Chang, S.J.; Chen, S.Y. Cartilage Tissue-Mimetic Pellets with Multifunctional Magnetic Hyaluronic Acid-Graft-Amphiphilic Gelatin Microcapsules for Chondrogenic Stimulation. Polymers 2020, 12, 785. [Google Scholar] [CrossRef]
- Dankova, J.; Buzgo, M.; Vejpravova, J.; Kubickova, S.; Sovkova, V.; Vyslouzilova, L.; Mantlikova, A.; Necas, A.; Amler, E. Highly efficient mesenchymal stem cell proliferation on poly-epsilon-caprolactone nanofibers with embedded magnetic nanoparticles. Int. J. Nanomed. 2015, 10, 7307–7317. [Google Scholar] [CrossRef]
- Veloso, S.R.S.; Ferreira, P.M.T.; Martins, J.A.; Coutinho, P.J.G.; Castanheira, E.M.S. Magnetogels: Prospects and Main Challenges in Biomedical Applications. Pharmaceutics 2018, 10, 145. [Google Scholar] [CrossRef]
- Fan, M.; Yan, J.; Tan, H.; Miao, Y.; Hu, X. Magnetic biopolymer nanogels via biological assembly for vectoring delivery of biopharmaceuticals. J. Mater. Chem. B 2014, 2, 8399–8405. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, H.; Lee, J.W.; Lee, K.Y. Magnetic field-responsive release of transforming growth factor beta 1 from heparin-modified alginate ferrogels. Carbohydr. Polym. 2016, 151, 467–473. [Google Scholar] [CrossRef]
- Karahaliloğlu, Z.; Yalçın, E.; Demirbilek, M.; Denkbaş, E.B. Magnetic silk fibroin e-gel scaffolds for bone tissue engineering applications. J. Bioact. Compat. Polym. 2017, 32, 596–614. [Google Scholar] [CrossRef]
- Son, B.; Kim, H.D.; Kim, M.; Kim, J.A.; Lee, J.; Shin, H.; Hwang, N.S.; Park, T.H. Physical Stimuli-Induced Chondrogenic Differentiation of Mesenchymal Stem Cells Using Magnetic Nanoparticles. Adv. Healthc. Mater. 2015, 4, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Jo, J.I.; Yukawa, H.; Tsumaki, N.; Baba, Y.; Tabata, Y. Visualization of Human Induced Pluripotent Stem Cells-Derived Three-Dimensional Cartilage Tissue by Gelatin Nanospheres. Tissue Eng. Part C Methods 2020, 26, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ye, D.; Li, M.; Ma, M.; Gu, N. Adaptive Materials Based on Iron Oxide Nanoparticles for Bone Regeneration. Chemphyschem 2018, 19, 1965–1979. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Zheng, C.; Huang, N.; Chen, X.; Zhu, X.; Zhao, Y.; Yu, Q.; Liu, J. Ru nanoparticles coated with gamma-Fe2O3 promoting and monitoring the differentiation of human mesenchymal stem cells via MRI tracking. Colloids Surf. B Biointerfaces 2018, 170, 701–711. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, J.; Zhao, L.; Zhang, F.; Liang, X.J.; Guo, Y.; Weir, M.D.; Reynolds, M.A.; Gu, N.; Xu, H.H.K. Magnetic field and nano-scaffolds with stem cells to enhance bone regeneration. Biomaterials 2018, 183, 151–170. [Google Scholar] [CrossRef]
- Kerouredan, O.; Ribot, E.J.; Fricain, J.C.; Devillard, R.; Miraux, S. Magnetic Resonance Imaging for tracking cellular patterns obtained by Laser-Assisted Bioprinting. Sci. Rep. 2018, 8, 15777. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, B.; Cao, M.; Sun, J.; Wu, H.; Zhao, P.; Xing, J.; Yang, Y.; Zhang, X.; Ji, M.; et al. Response of MAPK pathway to iron oxide nanoparticles in vitro treatment promotes osteogenic differentiation of hBMSCs. Biomaterials 2016, 86, 11–20. [Google Scholar] [CrossRef]
- Schulze, F.; Gramoun, A.; Crowe, L.A.; Dienelt, A.; Akcan, T.; Hofmann, H.; Vallee, J.P.; Duda, G.N.; Ode, A. Accumulation of amino-polyvinyl alcohol-coated superparamagnetic iron oxide nanoparticles in bone marrow: Implications for local stromal cells. Nanomedicine 2015, 10, 2139–2151. [Google Scholar] [CrossRef]
- Henstock, J.; El Haj, A. Controlled mechanotransduction in therapeutic MSCs: Can remotely controlled magnetic nanoparticles regenerate bones? Regen. Med. 2015, 10, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Labusca, L.; Herea, D.D.; Danceanu, C.M.; Minuti, A.E.; Stavila, C.; Grigoras, M.; Gherca, D.; Stoian, G.; Ababei, G.; Chiriac, H.; et al. The effect of magnetic field exposure on differentiation of magnetite nanoparticle-loaded adipose-derived stem cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110652. [Google Scholar] [CrossRef]
- Sniadecki, N.J. A tiny touch: Activation of cell signaling pathways with magnetic nanoparticles. Endocrinology 2010, 151, 451–457. [Google Scholar] [CrossRef]
- Hu, B.; El Haj, A.J.; Dobson, J. Receptor-targeted, magneto-mechanical stimulation of osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. Int. J. Mol. Sci. 2013, 14, 19276–19293. [Google Scholar] [CrossRef]
- Rotherham, M.; Henstock, J.R.; Qutachi, O.; El Haj, A.J. Remote regulation of magnetic particle targeted Wnt signaling for bone tissue engineering. Nanomedicine 2018, 14, 173–184. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Q.; Yang, M.; Zhan, X.; Lan, F.; He, J.; Gu, Z.; Wu, Y. Protein Corona of Magnetic Hydroxyapatite Scaffold Improves Cell Proliferation via Activation of Mitogen-Activated Protein Kinase Signaling Pathway. ACS Nano 2017, 11, 3690–3704. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, P.; Luo, B.; Lan, F.; He, J.; Wu, Y. Dynamic protein corona influences immune-modulating osteogenesis in magnetic nanoparticle (MNP)-infiltrated bone regeneration scaffolds in vivo. Nanoscale 2019, 11, 6817–6827. [Google Scholar] [CrossRef]
- Heidari, F.; Razavi, M.; Bahrololoom, M.E.; Bazargan-Lari, R.; Vashaee, D.; Kotturi, H.; Tayebi, L. Mechanical properties of natural chitosan/hydroxyapatite/magnetite nanocomposites for tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 65, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Bianchi, M.; Sartori, M.; Boi, M.; Giavaresi, G.; Salter, D.M.; Jelic, M.; Maltarello, M.C.; Ortolani, A.; Sprio, S.; et al. Bone regeneration in a rabbit critical femoral defect by means of magnetic hydroxyapatite macroporous scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Sahmani, S.; Khandan, A.; Saber-Samandari, S.; Mohammadi Aghdam, M. Effect of magnetite nanoparticles on the biological and mechanical properties of hydroxyapatite porous scaffolds coated with ibuprofen drug. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110835. [Google Scholar] [CrossRef]
- Przekora, A.; Audemar, M.; Pawlat, J.; Canal, C.; Thomann, J.S.; Labay, C.; Wojcik, M.; Kwiatkowski, M.; Terebun, P.; Ginalska, G.; et al. Positive Effect of Cold Atmospheric Nitrogen Plasma on the Behavior of Mesenchymal Stem Cells Cultured on a Bone Scaffold Containing Iron Oxide-Loaded Silica Nanoparticles Catalyst. Int. J. Mol. Sci. 2020, 21, 4738. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Feng, L.; Chen, Z.; Lan, Y.; Liu, Y.; Li, D.; Yan, C.; Xu, Y. Ultrasmall Superparamagnetic Iron Oxide Labeled Silk Fibroin/Hydroxyapatite Multifunctional Scaffold Loaded With Bone Marrow-Derived Mesenchymal Stem Cells for Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 697. [Google Scholar] [CrossRef] [PubMed]
- Pistone, A.; Iannazzo, D.; Panseri, S.; Montesi, M.; Tampieri, A.; Galvagno, S. Hydroxyapatite-magnetite-MWCNT nanocomposite as a biocompatible multifunctional drug delivery system for bone tissue engineering. Nanotechnology 2014, 25, 425701. [Google Scholar] [CrossRef]
- Brett, E.; Zielins, E.R.; Luan, A.; Ooi, C.C.; Shailendra, S.; Atashroo, D.; Menon, S.; Blackshear, C.; Flacco, J.; Quarto, N.; et al. Magnetic Nanoparticle-Based Upregulation of B-Cell Lymphoma 2 Enhances Bone Regeneration. Stem Cells Transl. Med. 2017, 6, 151–160. [Google Scholar] [CrossRef]
- Cojocaru, F.D.; Balan, V.; Popa, M.I.; Lobiuc, A.; Antoniac, A.; Antoniac, I.V.; Verestiuc, L. Biopolymers—Calcium phosphates composites with inclusions of magnetic nanoparticles for bone tissue engineering. Int. J. Biol. Macromol. 2019, 125, 612–620. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, S.C.; Jee, S.C.; Sung, J.S.; Kadam, A.A. Surface functionalization of halloysite nanotubes with supermagnetic iron oxide, chitosan and 2-D calcium-phosphate nanoflakes for synergistic osteoconduction enhancement of human adipose tissue-derived mesenchymal stem cells. Colloids Surf. B Biointerfaces 2019, 173, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Lalande, C.; Miraux, S.; Derkaoui, S.M.; Mornet, S.; Bareille, R.; Fricain, J.C.; Franconi, J.M.; Le Visage, C.; Letourneur, D.; Amedee, J.; et al. Magnetic resonance imaging tracking of human adipose derived stromal cells within three-dimensional scaffolds for bone tissue engineering. Eur. Cell Mater. 2011, 21, 341–354. [Google Scholar] [CrossRef]
- Wang, Y.J.; Jeng, U.S.; Hsu, S.H. Biodegradable Water-Based Polyurethane Shape Memory Elastomers for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2018, 4, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhou, Y.; Zhao, Y.; Xu, Y.; Zhang, F.; Gu, N.; Ma, J.; Reynolds, M.A.; Xia, Y.; Xu, H.H.K. Enhanced bone regeneration and visual monitoring via superparamagnetic iron oxide nanoparticle scaffold in rats. J. Tissue Eng. Regen. Med. 2018, 12, e2085–e2098. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, X.; Song, Y.; Han, B.; Hu, X.; Wang, X.; Lin, Y.; Deng, X. Magnetic biodegradable Fe3O4/CS/PVA nanofibrous membranes for bone regeneration. Biomed. Mater. 2011, 6, 055008. [Google Scholar] [CrossRef]
- Grabska-Zielinska, S.; Sionkowska, A. How to Improve Physico-Chemical Properties of Silk Fibroin Materials for Biomedical Applications?-Blending and Cross-Linking of Silk Fibroin-A Review. Materials 2021, 14, 1510. [Google Scholar] [CrossRef] [PubMed]
- Lalegül-Ülker, Ö.; Vurat, M.T.; Elçin, A.E.; Elçin, Y.M. Magnetic silk fibroin composite nanofibers for biomedical applications: Fabrication and evaluation of the chemical, thermal, mechanical, and in vitro biological properties. J. Appl. Polym. Sci. 2019, 136, 48040. [Google Scholar] [CrossRef]
- Singh, R.K.; Patel, K.D.; Lee, J.H.; Lee, E.J.; Kim, J.H.; Kim, T.H.; Kim, H.W. Potential of magnetic nanofiber scaffolds with mechanical and biological properties applicable for bone regeneration. PLoS ONE 2014, 9, e91584. [Google Scholar] [CrossRef]
- Zhuang, J.; Lin, S.; Dong, L.; Cheng, K.; Weng, W. Magnetically Assisted Electrodeposition of Aligned Collagen Coatings. ACS Biomater. Sci. Eng. 2018, 4, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.S.; Santos, L.F.; Mendes, M.C.; Mano, J.F. Multi-layer pre-vascularized magnetic cell sheets for bone regeneration. Biomaterials 2020, 231, 119664. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.; Peluso, V.; Gloria, A.; Oliviero, O.; Rinaldi, L.; Improta, G.; De Santis, R.; D’Anto, V. Combination Design of Time-Dependent Magnetic Field and Magnetic Nanocomposites to Guide Cell Behavior. Nanomaterials 2020, 10, 577. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Guo, Y.; Yang, Z.; Chen, H.; Ren, K.; Weir, M.D.; Chow, L.C.; Reynolds, M.A.; Zhang, F.; Gu, N.; et al. Iron oxide nanoparticle-calcium phosphate cement enhanced the osteogenic activities of stem cells through WNT/beta-catenin signaling. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109955. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhao, Y.; Zhang, F.; Chen, B.; Hu, X.; Weir, M.D.; Schneider, A.; Jia, L.; Gu, N.; Xu, H.H.K. Iron oxide nanoparticles in liquid or powder form enhanced osteogenesis via stem cells on injectable calcium phosphate scaffold. Nanomedicine 2019, 21, 102069. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, H.; Zhao, Y.; Zhang, F.; Li, X.; Wang, L.; Weir, M.D.; Ma, J.; Reynolds, M.A.; Gu, N.; et al. Novel magnetic calcium phosphate-stem cell construct with magnetic field enhances osteogenic differentiation and bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 30–41. [Google Scholar] [CrossRef]
- Zeng, X.B.; Hu, H.; Xie, L.Q.; Lan, F.; Jiang, W.; Wu, Y.; Gu, Z.W. Magnetic responsive hydroxyapatite composite scaffolds construction for bone defect reparation. Int. J. Nanomed. 2012, 7, 3365–3378. [Google Scholar] [CrossRef]
- Tanasa, E.; Zaharia, C.; Hudita, A.; Radu, I.C.; Costache, M.; Galateanu, B. Impact of the magnetic field on 3T3-E1 preosteoblasts inside SMART silk fibroin-based scaffolds decorated with magnetic nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110714. [Google Scholar] [CrossRef]
- Filippi, M.; Dasen, B.; Guerrero, J.; Garello, F.; Isu, G.; Born, G.; Ehrbar, M.; Martin, I.; Scherberich, A. Magnetic nanocomposite hydrogels and static magnetic field stimulate the osteoblastic and vasculogenic profile of adipose-derived cells. Biomaterials 2019, 223, 119468. [Google Scholar] [CrossRef]
- Aldebs, A.I.; Zohora, F.T.; Nosoudi, N.; Singh, S.P.; Ramirez-Vick, J.E. Effect of Pulsed Electromagnetic Fields on Human Mesenchymal Stem Cells Using 3D Magnetic Scaffolds. Bioelectromagnetics 2020, 41, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Wang, D.; Li, Y.; Xie, W.; Wang, X.; Tao, L.; Wei, Y.; Wang, X.; Zhao, L. Effect of nanoheat stimulation mediated by magnetic nanocomposite hydrogel on the osteogenic differentiation of mesenchymal stem cells. Sci. China Life Sci. 2018, 61, 448–456. [Google Scholar] [CrossRef]
- Yuan, Z.; Memarzadeh, K.; Stephen, A.S.; Allaker, R.P.; Brown, R.A.; Huang, J. Development of a 3D Collagen Model for the In Vitro Evaluation of Magnetic-assisted Osteogenesis. Sci. Rep. 2018, 8, 16270. [Google Scholar] [CrossRef]
- Hao, L.; Li, L.; Wang, P.; Wang, Z.; Shi, X.; Guo, M.; Zhang, P. Synergistic osteogenesis promoted by magnetically actuated nano-mechanical stimuli. Nanoscale 2019, 11, 23423–23437. [Google Scholar] [CrossRef]
- Meng, J.; Xiao, B.; Zhang, Y.; Liu, J.; Xue, H.; Lei, J.; Kong, H.; Huang, Y.; Jin, Z.; Gu, N.; et al. Super-paramagnetic responsive nanofibrous scaffolds under static magnetic field enhance osteogenesis for bone repair in vivo. Sci. Rep. 2013, 3, 2655. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sun, J.; Wang, Z.; Zhou, Y.; Lou, Z.; Chen, B.; Wang, P.; Guo, Z.; Tang, H.; Ma, J.; et al. Magnetic Cell-Scaffold Interface Constructed by Superparamagnetic IONP Enhanced Osteogenesis of Adipose-Derived Stem Cells. ACS Appl. Mater. Interfaces 2018, 10, 44279–44289. [Google Scholar] [CrossRef]
- Fernandes, M.M.; Correia, D.M.; Ribeiro, C.; Castro, N.; Correia, V.; Lanceros-Mendez, S. Bioinspired Three-Dimensional Magnetoactive Scaffolds for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 45265–45275. [Google Scholar] [CrossRef]
- Marycz, K.; Alicka, M.; Kornicka-Garbowska, K.; Polnar, J.; Lis-Bartos, A.; Wiglusz, R.J.; Roecken, M.; Nedelec, J.M. Promotion through external magnetic field of osteogenic differentiation potential in adipose-derived mesenchymal stem cells: Design of polyurethane/poly(lactic) acid sponges doped with iron oxide nanoparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1398–1411. [Google Scholar] [CrossRef] [PubMed]
- Aliramaji, S.; Zamanian, A.; Mozafari, M. Super-paramagnetic responsive silk fibroin/chitosan/magnetite scaffolds with tunable pore structures for bone tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 736–744. [Google Scholar] [CrossRef]
- Paun, I.A.; Popescu, R.C.; Calin, B.S.; Mustaciosu, C.C.; Dinescu, M.; Luculescu, C.R. 3D Biomimetic Magnetic Structures for Static Magnetic Field Stimulation of Osteogenesis. Int. J. Mol. Sci. 2018, 19, 495. [Google Scholar] [CrossRef]
- Bock, N.; Riminucci, A.; Dionigi, C.; Russo, A.; Tampieri, A.; Landi, E.; Goranov, V.A.; Marcacci, M.; Dediu, V. A novel route in bone tissue engineering: Magnetic biomimetic scaffolds. Acta Biomater. 2010, 6, 786–796. [Google Scholar] [CrossRef]
- Panseri, S.; Russo, A.; Giavaresi, G.; Sartori, M.; Veronesi, F.; Fini, M.; Salter, D.M.; Ortolani, A.; Strazzari, A.; Visani, A.; et al. Innovative magnetic scaffolds for orthopedic tissue engineering. J. Biomed. Mater. Res. Part A 2012, 100, 2278–2286. [Google Scholar] [CrossRef]
- Luo, C.; Yang, X.; Li, M.; Huang, H.; Kang, Q.; Zhang, X.; Hui, H.; Zhang, X.; Cen, C.; Luo, Y.; et al. A novel strategy for in vivo angiogenesis and osteogenesis: Magnetic micro-movement in a bone scaffold. Artif. Cells Nanomed. Biotechnol. 2018, 46, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Su, J.Y.; Chen, S.H.; Chen, Y.P.; Chen, W.C. Evaluation of Magnetic Nanoparticle-Labeled Chondrocytes Cultivated on a Type II Collagen-Chitosan/Poly(Lactic-co-Glycolic) Acid Biphasic Scaffold. Int. J. Mol. Sci. 2017, 18, 87. [Google Scholar] [CrossRef]
- Huang, J.; Liu, W.; Liang, Y.; Li, L.; Duan, L.; Chen, J.; Zhu, F.; Lai, Y.; Zhu, W.; You, W.; et al. Preparation and biocompatibility of diphasic magnetic nanocomposite scaffold. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 87, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Armstrong, J.P.; Pence, I.J.; Kit-Anan, W.; Puetzer, J.L.; Correia Carreira, S.; Moore, A.C.; Stevens, M.M. Glycosylated superparamagnetic nanoparticle gradients for osteochondral tissue engineering. Biomaterials 2018, 176, 24–33. [Google Scholar] [CrossRef]
- Saldanha, K.J.; Piper, S.L.; Ainslie, K.M.; Kim, H.T.; Majumdar, S. Magnetic resonance imaging of iron oxide labelled stem cells: Applications to tissue engineering based regeneration of the intervertebral disc. Eur. Cell Mater. 2008, 16, 17–25. [Google Scholar] [CrossRef]
- Kaggie, J.D.; Markides, H.; Graves, M.J.; MacKay, J.; Houston, G.; El Haj, A.; Gilbert, F.; Henson, F. Ultra Short Echo Time MRI of Iron-Labelled Mesenchymal Stem Cells in an Ovine Osteochondral Defect Model. Sci. Rep. 2020, 10, 8451. [Google Scholar] [CrossRef]
- Woo, S.L.; Debski, R.E.; Zeminski, J.; Abramowitch, S.D.; Saw, S.S.; Fenwick, J.A. Injury and repair of ligaments and tendons. Annu. Rev. Biomed. Eng. 2000, 2, 83–118. [Google Scholar] [CrossRef]
- Scharf, A.; Holmes, S.; Thoresen, M.; Mumaw, J.; Stumpf, A.; Peroni, J. Superparamagnetic iron oxide nanoparticles as a means to track mesenchymal stem cells in a large animal model of tendon injury. Contrast Media Mol. Imaging 2015, 10, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Kremen, T.J.; Bez, M.; Sheyn, D.; Ben-David, S.; Da, X.; Tawackoli, W.; Wagner, S.; Gazit, D.; Pelled, G. In Vivo Imaging of Exogenous Progenitor Cells in Tendon Regeneration via Superparamagnetic Iron Oxide Particles. Am. J. Sports Med. 2019, 47, 2737–2744. [Google Scholar] [CrossRef] [PubMed]
- Burk, J.; Erbe, I.; Berner, D.; Kacza, J.; Kasper, C.; Pfeiffer, B.; Winter, K.; Brehm, W. Freeze-thaw cycles enhance decellularization of large tendons. Tissue Eng. Part C Methods 2014, 20, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.I.; Rodrigues, M.T.; Carvalho, P.P.; Banobre-Lopez, M.; Paz, E.; Freitas, P.; Gomes, M.E. Exploring the Potential of Starch/Polycaprolactone Aligned Magnetic Responsive Scaffolds for Tendon Regeneration. Adv. Healthc. Mater. 2016, 5, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.I.; Rodrigues, M.T.; Gomes, M.E. Tissue-engineered magnetic cell sheet patches for advanced strategies in tendon regeneration. Acta Biomater. 2017, 63, 110–122. [Google Scholar] [CrossRef]
- Diana, R.; Ardhani, R.; Kristanti, Y.; Santosa, P. Dental pulp stem cells response on the nanotopography of scaffold to regenerate dentin-pulp complex tissue. Regen. Ther. 2020, 15, 243–250. [Google Scholar] [CrossRef]
- Anastasiou, A.D.; Strafford, S.; Thomson, C.L.; Gardy, J.; Edwards, T.J.; Malinowski, M.; Hussain, S.A.; Metzger, N.K.; Hassanpour, A.; Brown, C.T.A.; et al. Exogenous mineralization of hard tissues using photo-absorptive minerals and femto-second lasers; the case of dental enamel. Acta Biomater. 2018, 71, 86–95. [Google Scholar] [CrossRef]
- Li, Y.; Hu, X.; Xia, Y.; Ji, Y.; Ruan, J.; Weir, M.D.; Lin, X.; Nie, Z.; Gu, N.; Masri, R.; et al. Novel magnetic nanoparticle-containing adhesive with greater dentin bond strength and antibacterial and remineralizing capabilities. Dent. Mater. 2018, 34, 1310–1322. [Google Scholar] [CrossRef]
- Sprio, S.; Campodoni, E.; Sandri, M.; Preti, L.; Keppler, T.; Muller, F.A.; Pugno, N.M.; Tampieri, A. A Graded Multifunctional Hybrid Scaffold with Superparamagnetic Ability for Periodontal Regeneration. Int. J. Mol. Sci. 2018, 19, 3604. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, H.; Zhang, F.; Wang, L.; Chen, B.; Reynolds, M.A.; Ma, J.; Schneider, A.; Gu, N.; Xu, H.H.K. Injectable calcium phosphate scaffold with iron oxide nanoparticles to enhance osteogenesis via dental pulp stem cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 423–433. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, Y.; Liu, H.; Zhu, X.; Gu, Y.; Lan, Y.; Tan, J.; Xu, H.; Guo, R. A non-invasive monitoring of USPIO labeled silk fibroin/hydroxyapatite scaffold loaded DPSCs for dental pulp regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109736. [Google Scholar] [CrossRef]
- Zare, S.; Mehrabani, D.; Jalli, R.; Saeedi Moghadam, M.; Manafi, N.; Mehrabani, G.; Jamhiri, I.; Ahadian, S. MRI-Tracking of Dental Pulp Stem Cells In Vitro and In Vivo Using Dextran-Coated Superparamagnetic Iron Oxide Nanoparticles. J. Clin. Med. 2019, 8, 1418. [Google Scholar] [CrossRef]
- Kolagar, T.A.; Farzaneh, M.; Nikkar, N.; Khoshnam, S.E. Human Pluripotent Stem Cells in Neurodegenerative Diseases: Potentials, Advances and Limitations. Curr. Stem Cell Res. Ther. 2020, 15, 102–110. [Google Scholar] [CrossRef]
- De Feo, D.; Merlini, A.; Laterza, C.; Martino, G. Neural stem cell transplantation in central nervous system disorders: From cell replacement to neuroprotection. Curr. Opin. Neurol. 2012, 25, 322–333. [Google Scholar] [CrossRef]
- Liau, M.T.; Amini, F.; Ramasamy, T.S. The therapeutic potential of stem cells and progenitor cells for the treatment of Parkinson’s disease. Tissue Eng. Regen. Med. 2016, 13, 455–464. [Google Scholar] [CrossRef]
- Genc, B.; Bozan, H.R.; Genc, S.; Genc, K. Stem Cell Therapy for Multiple Sclerosis. Adv. Exp. Med. Biol. 2019, 1084, 145–174. [Google Scholar] [CrossRef]
- Polak, P.; Shefi, O. Nanometric agents in the service of neuroscience: Manipulation of neuronal growth and activity using nanoparticles. Nanomedicine 2015, 11, 1467–1479. [Google Scholar] [CrossRef]
- Chen, M.W.; Zhang, X.; Lu, L.J.; Zhang, F.; Duan, X.H.; Zheng, C.S.; Chen, Y.Y.; Shen, J. Monitoring of macrophage recruitment enhanced by Toll-like receptor 4 activation with MR imaging in nerve injury. Muscle Nerve 2018, 58, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Ghanouni, P.; Behera, D.; Xie, J.; Chen, X.; Moseley, M.; Biswal, S. In vivo USPIO magnetic resonance imaging shows that minocycline mitigates macrophage recruitment to a peripheral nerve injury. Mol. Pain 2012, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.N.; Yang, X.; Li, Z.; Li, M.; Shi, S.X.; Wood, K.; Liu, Q.; Fu, Y.; Han, W.; Xu, Y.; et al. Non-invasive tracking of CD4+ T cells with a paramagnetic and fluorescent nanoparticle in brain ischemia. J. Cereb. Blood Flow Metab. 2016, 36, 1464–1476. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, R.P.; Pottler, M.; Cicha, I.; Unterweger, H.; Janko, C.; Alexiou, C. Nanomedicine for neuroprotection. Nanomedicine 2019, 14, 127–130. [Google Scholar] [CrossRef]
- Pickard, M.R.; Jenkins, S.I.; Koller, C.J.; Furness, D.N.; Chari, D.M. Magnetic nanoparticle labeling of astrocytes derived for neural transplantation. Tissue Eng. Part C Methods 2011, 17, 89–99. [Google Scholar] [CrossRef]
- Joris, F.; Valdeperez, D.; Pelaz, B.; Wang, T.; Doak, S.H.; Manshian, B.B.; Soenen, S.J.; Parak, W.J.; De Smedt, S.C.; Raemdonck, K. Choose your cell model wisely: The in vitro nanoneurotoxicity of differentially coated iron oxide nanoparticles for neural cell labeling. Acta Biomater. 2017, 55, 204–213. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nejadnik, H.; Pandit, P.; Lenkov, O.; Lahiji, A.P.; Yerneni, K.; Daldrup-Link, H.E. Ferumoxytol Can Be Used for Quantitative Magnetic Particle Imaging of Transplanted Stem Cells. Mol. Imaging Biol. 2019, 21, 465–472. [Google Scholar] [CrossRef]
- Jasmin; de Souza, G.T.; Louzada, R.A.; Rosado-de-Castro, P.H.; Mendez-Otero, R.; Campos de Carvalho, A.C. Tracking stem cells with superparamagnetic iron oxide nanoparticles: Perspectives and considerations. Int. J. Nanomed. 2017, 12, 779–793. [Google Scholar] [CrossRef]
- Li, K.; Qin, J.; Wang, X.; Xu, Y.; Shen, Z.; Lu, X.; Zhang, G. Magnetic resonance imaging monitoring dual-labeled stem cells for treatment of mouse nerve injury. Cytotherapy 2013, 15, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fan, X.; Jin, G.; Wan, X.; Qiu, R.; Yi, G.; You, Y.; Xu, Q. Treatment of rat with traumatic brain injury and MR tracing in vivo via combined transplantation of bone marrow stromal cells labeled with superparamagnetic iron oxide and Schwann cells. J. Biomed. Nanotechnol. 2014, 10, 205–215. [Google Scholar] [CrossRef]
- Jendelova, P.; Herynek, V.; Urdzikova, L.; Glogarova, K.; Kroupova, J.; Andersson, B.; Bryja, V.; Burian, M.; Hajek, M.; Sykova, E. Magnetic resonance tracking of transplanted bone marrow and embryonic stem cells labeled by iron oxide nanoparticles in rat brain and spinal cord. J. Neurosci. Res. 2004, 76, 232–243. [Google Scholar] [CrossRef]
- Sykova, E.; Jendelova, P. Magnetic resonance tracking of implanted adult and embryonic stem cells in injured brain and spinal cord. Ann. N. Y. Acad. Sci. 2005, 1049, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Sykova, E.; Jendelova, P. Magnetic resonance tracking of transplanted stem cells in rat brain and spinal cord. Neurodegener. Dis. 2006, 3, 62–67. [Google Scholar] [CrossRef]
- Li, S.C.; Tachiki, L.M.; Luo, J.; Dethlefs, B.A.; Chen, Z.; Loudon, W.G. A biological global positioning system: Considerations for tracking stem cell behaviors in the whole body. Stem Cell Rev. Rep. 2010, 6, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Sykova, E.; Jendelova, P.; Herynek, V. MR tracking of stem cells in living recipients. Methods Mol. Biol. 2009, 549, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Morikawa, S.; Morita, M.; Inubushi, T.; Takada, T.; Torii, R.; Tooyama, I. Magnetic resonance imaging using hemagglutinating virus of Japan-envelope vector successfully detects localization of intra-cardially administered microglia in normal mouse brain. Neurosci. Lett. 2006, 395, 42–45. [Google Scholar] [CrossRef]
- Pickard, M.R.; Barraud, P.; Chari, D.M. The transfection of multipotent neural precursor/stem cell transplant populations with magnetic nanoparticles. Biomaterials 2011, 32, 2274–2284. [Google Scholar] [CrossRef]
- Adams, C.F.; Pickard, M.R.; Chari, D.M. Magnetic nanoparticle mediated transfection of neural stem cell suspension cultures is enhanced by applied oscillating magnetic fields. Nanomedicine 2013, 9, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Morano, M.; Wrobel, S.; Fregnan, F.; Ziv-Polat, O.; Shahar, A.; Ratzka, A.; Grothe, C.; Geuna, S.; Haastert-Talini, K. Nanotechnology versus stem cell engineering: In vitro comparison of neurite inductive potentials. Int. J. Nanomed. 2014, 9, 5289–5306. [Google Scholar] [CrossRef]
- Tickle, J.A.; Chari, D.M. Less is more: Investigating the influence of cellular nanoparticle load on transfection outcomes in neural cells. J. Tissue Eng. Regen. Med. 2019, 13, 1732–1737. [Google Scholar] [CrossRef]
- Gwak, S.J.; Koo, H.; Yun, Y.; Yhee, J.Y.; Lee, H.Y.; Yoon, D.H.; Kim, K.; Ha, Y. Multifunctional nanoparticles for gene delivery and spinal cord injury. J. Biomed. Mater. Res. Part A 2015, 103, 3474–3482. [Google Scholar] [CrossRef] [PubMed]
- Marcus, M.; Smith, A.; Maswadeh, A.; Shemesh, Z.; Zak, I.; Motiei, M.; Schori, H.; Margel, S.; Sharoni, A.; Shefi, O. Magnetic Targeting of Growth Factors Using Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 707. [Google Scholar] [CrossRef] [PubMed]
- Pop, N.L.; Nan, A.; Urda-Cimpean, A.E.; Florea, A.; Toma, V.A.; Moldovan, R.; Decea, N.; Mitrea, D.R.; Orasan, R. Chitosan Functionalized Magnetic Nanoparticles to Provide Neural Regeneration and Recovery after Experimental Model Induced Peripheral Nerve Injury. Biomolecules 2021, 11, 676. [Google Scholar] [CrossRef]
- Adams, C.F.; Rai, A.; Sneddon, G.; Yiu, H.H.; Polyak, B.; Chari, D.M. Increasing magnetite contents of polymeric magnetic particles dramatically improves labeling of neural stem cell transplant populations. Nanomedicine 2015, 11, 19–29. [Google Scholar] [CrossRef]
- Jin, Y.; Lee, J.U.; Chung, E.; Yang, K.; Kim, J.; Kim, J.W.; Lee, J.S.; Cho, A.N.; Oh, T.; Lee, J.H.; et al. Magnetic Control of Axon Navigation in Reprogrammed Neurons. Nano Lett. 2019, 19, 6517–6523. [Google Scholar] [CrossRef]
- Shin, J.; Lee, K.M.; Lee, J.H.; Lee, J.; Cha, M. Magnetic manipulation of bacterial magnetic nanoparticle-loaded neurospheres. Integr. Biol. 2014, 6, 532–539. [Google Scholar] [CrossRef]
- Silva, L.H.; Cruz, F.F.; Morales, M.M.; Weiss, D.J.; Rocco, P.R. Magnetic targeting as a strategy to enhance therapeutic effects of mesenchymal stromal cells. Stem Cell Res. Ther. 2017, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, J.J.; Urquia, L.N.; Hurtig, C.V.; Gutierrez, J.; Anderson, C.; Piferi, P.; Federici, T.; Oshinski, J.N.; Boulis, N.M. Magnetic Resonance Imaging-Guided Transplantation of Neural Stem Cells into the Porcine Spinal Cord. Stereotact. Funct. Neurosurg. 2017, 95, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Soto, P.A.; Vence, M.; Pinero, G.M.; Coral, D.F.; Usach, V.; Muraca, D.; Cueto, A.; Roig, A.; van Raap, M.B.F.; Setton-Avruj, C.P. Sciatic Nerve Regeneration After Traumatic Injury Using Magnetic Targeted Adipose-derived Mesenchymal Stem Cells. Acta Biomater. 2021, 130, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Glaser, T.; Bueno, V.B.; Cornejo, D.R.; Petri, D.F.; Ulrich, H. Neuronal adhesion, proliferation and differentiation of embryonic stem cells on hybrid scaffolds made of xanthan and magnetite nanoparticles. Biomed. Mater. 2015, 10, 045002. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Bagher, Z.; Najmoddin, N.; Simorgh, S.; Pezeshki-Modaress, M. Alginate-magnetic short nanofibers 3D composite hydrogel enhances the encapsulated human olfactory mucosa stem cells bioactivity for potential nerve regeneration application. Int. J. Biol. Macromol. 2021, 167, 796–806. [Google Scholar] [CrossRef]
- Kim, J.; Tanner, K. Three-Dimensional Patterning of the ECM Microenvironment Using Magnetic Nanoparticle Self Assembly. Curr. Protoc. Cell Biol. 2016, 70, 25.3.1–25.3.14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, L.; Liu, L.; Luo, B.; Liang, M.; Sun, Z.; Zhu, S.; Quan, X.; Yang, Y.; Ma, T.; et al. Activation of Schwann cells in vitro by magnetic nanocomposites via applied magnetic field. Int. J. Nanomed. 2015, 10, 43–61. [Google Scholar] [CrossRef]
- Tickle, J.A.; Poptani, H.; Taylor, A.; Chari, D.M. Noninvasive imaging of nanoparticle-labeled transplant populations within polymer matrices for neural cell therapy. Nanomedicine 2018, 13, 1333–1348. [Google Scholar] [CrossRef]
- Viventi, S.; Dottori, M. Modelling the dorsal root ganglia using human pluripotent stem cells: A platform to study peripheral neuropathies. Int. J. Biochem. Cell Biol. 2018, 100, 61–68. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, G.; Hou, Y.; Kuang, G.; Jia, Z.; Li, P.; Fan, Y. Effect of nano-hydroxyapatite-coated magnetic nanoparticles on axonal guidance growth of rat dorsal root ganglion neurons. J. Biomed. Mater. Res. Part A 2015, 103, 3066–3071. [Google Scholar] [CrossRef] [PubMed]
- Ziv-Polat, O.; Shahar, A.; Levy, I.; Skaat, H.; Neuman, S.; Fregnan, F.; Geuna, S.; Grothe, C.; Haastert-Talini, K.; Margel, S. The role of neurotrophic factors conjugated to iron oxide nanoparticles in peripheral nerve regeneration: In vitro studies. Biomed. Res. Int. 2014, 2014, 267808. [Google Scholar] [CrossRef] [PubMed]
- Assuncao-Silva, R.C.; Oliveira, C.C.; Ziv-Polat, O.; Gomes, E.D.; Sahar, A.; Sousa, N.; Silva, N.A.; Salgado, A.J. Induction of neurite outgrowth in 3D hydrogel-based environments. Biomed. Mater. 2015, 10, 051001. [Google Scholar] [CrossRef]
- Zuidema, J.M.; Provenza, C.; Caliendo, T.; Dutz, S.; Gilbert, R.J. Magnetic NGF-releasing PLLA/iron oxide nanoparticles direct extending neurites and preferentially guide neurites along aligned electrospun microfibers. ACS Chem. Neurosci. 2015, 6, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.D.L.; Ganguly, D.; Zuidema, J.M.; Cardinal, T.J.; Ziemba, A.M.; Kearns, K.R.; McCarthy, S.M.; Thompson, D.M.; Ramanath, G.; Borca-Tasciuc, D.A.; et al. Injectable, Magnetically Orienting Electrospun Fiber Conduits for Neuron Guidance. ACS Appl. Mater. Interfaces 2019, 11, 356–372. [Google Scholar] [CrossRef] [PubMed]
- Lacko, C.S.; Singh, I.; Wall, M.A.; Garcia, A.R.; Porvasnik, S.L.; Rinaldi, C.; Schmidt, C.E. Magnetic particle templating of hydrogels: Engineering naturally derived hydrogel scaffolds with 3D aligned microarchitecture for nerve repair. J. Neural Eng. 2020, 17, 016057. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, S.; Liu, L.; Ge, J.; Huang, L.; Sun, Z.; Zeng, W.; Huang, J.; Luo, Z. A magnetically responsive nanocomposite scaffold combined with Schwann cells promotes sciatic nerve regeneration upon exposure to magnetic field. Int. J. Nanomed. 2017, 12, 7815–7832. [Google Scholar] [CrossRef]
- Tseng, T.C.; Hsu, S.H. Substrate-mediated nanoparticle/gene delivery to MSC spheroids and their applications in peripheral nerve regeneration. Biomaterials 2014, 35, 2630–2641. [Google Scholar] [CrossRef] [PubMed]
- Kubinova, S.; Sykova, E. Nanotechnology for treatment of stroke and spinal cord injury. Nanomedicine 2010, 5, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Sykova, E.; Jendelova, P. Migration, fate and in vivo imaging of adult stem cells in the CNS. Cell Death Differ. 2007, 14, 1336–1342. [Google Scholar] [CrossRef]
- Struys, T.; Ketkar-Atre, A.; Gervois, P.; Leten, C.; Hilkens, P.; Martens, W.; Bronckaers, A.; Dresselaers, T.; Politis, C.; Lambrichts, I.; et al. Magnetic resonance imaging of human dental pulp stem cells in vitro and in vivo. Cell Transplant. 2013, 22, 1813–1829. [Google Scholar] [CrossRef]
- Stroh, A.; Kressel, J.; Coras, R.; Dreyer, A.Y.; Frohlich, W.; Forschler, A.; Lobsien, D.; Blumcke, I.; Zoubaa, S.; Schlegel, J.; et al. A Safe and Effective Magnetic Labeling Protocol for MRI-Based Tracking of Human Adult Neural Stem Cells. Front. Neurosci. 2019, 13, 1092. [Google Scholar] [CrossRef]
- Kubelick, K.P.; Emelianov, S.Y. A Trimodal Ultrasound, Photoacoustic and Magnetic Resonance Imaging Approach for Longitudinal Post-operative Monitoring of Stem Cells in the Spinal Cord. Ultrasound Med. Biol. 2020, 46, 3468–3474. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Wen, S.; Xiang, Q.; Xiang, X.; Xu, C.; Wan, Y.; Wang, J.; Li, B.; Wan, Y.; et al. Magnetic resonance imaging tracking and assessing repair function of the bone marrow mesenchymal stem cells transplantation in a rat model of spinal cord injury. Oncotarget 2017, 8, 58985–58999. [Google Scholar] [CrossRef][Green Version]
- Kubelick, K.P.; Emelianov, S.Y. In vivo photoacoustic guidance of stem cell injection and delivery for regenerative spinal cord therapies. Neurophotonics 2020, 7, 030501. [Google Scholar] [CrossRef] [PubMed]
- Kyle, S.; Saha, S. Nanotechnology for the detection and therapy of stroke. Adv. Healthc. Mater. 2014, 3, 1703–1720. [Google Scholar] [CrossRef]
- Hudson, J.S.; Chung, T.K.; Prout, B.S.; Nagahama, Y.; Raghavan, M.L.; Hasan, D.M. Iron nanoparticle contrast enhanced microwave imaging for emergent stroke: A pilot study. J. Clin. Neurosci. 2019, 59, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Jang, M.S.; Gao, G.H.; Lee, J.H.; Lee, D.S. pH-Responsive biodegradable polymeric micelles with anchors to interface magnetic nanoparticles for MR imaging in detection of cerebral ischemic area. Nanoscale 2016, 8, 12588–12598. [Google Scholar] [CrossRef]
- Alvarim, L.T.; Nucci, L.P.; Mamani, J.B.; Marti, L.C.; Aguiar, M.F.; Silva, H.R.; Silva, G.S.; Nucci-da-Silva, M.P.; DelBel, E.A.; Gamarra, L.F. Therapeutics with SPION-labeled stem cells for the main diseases related to brain aging: A systematic review. Int. J. Nanomed. 2014, 9, 3749–3770. [Google Scholar] [CrossRef]
- Wen, X.; Wang, Y.; Zhang, F.; Zhang, X.; Lu, L.; Shuai, X.; Shen, J. In vivo monitoring of neural stem cells after transplantation in acute cerebral infarction with dual-modal MR imaging and optical imaging. Biomaterials 2014, 35, 4627–4635. [Google Scholar] [CrossRef] [PubMed]
- Emerich, D.F.; Orive, G.; Borlongan, C. Tales of biomaterials, molecules, and cells for repairing and treating brain dysfunction. Curr. Stem Cell Res. Ther. 2011, 6, 171–189. [Google Scholar] [CrossRef]
- Sharma, H.S.; Menon, P.K.; Lafuente, J.V.; Aguilar, Z.P.; Wang, Y.A.; Muresanu, D.F.; Mossler, H.; Patnaik, R.; Sharma, A. The role of functionalized magnetic iron oxide nanoparticles in the central nervous system injury and repair: New potentials for neuroprotection with Cerebrolysin therapy. J. Nanosci. Nanotechnol. 2014, 14, 577–595. [Google Scholar] [CrossRef]
- Umashankar, A.; Corenblum, M.J.; Ray, S.; Valdez, M.; Yoshimaru, E.S.; Trouard, T.P.; Madhavan, L. Effects of the iron oxide nanoparticle Molday ION Rhodamine B on the viability and regenerative function of neural stem cells: Relevance to clinical translation. Int. J. Nanomed. 2016, 11, 1731–1748. [Google Scholar] [CrossRef]
- Roet, M.; Hescham, S.A.; Jahanshahi, A.; Rutten, B.P.F.; Anikeeva, P.O.; Temel, Y. Progress in neuromodulation of the brain: A role for magnetic nanoparticles? Prog. Neurobiol. 2019, 177, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Menon, P.K.; Sharma, A.; Lafuente, J.V.; Muresanu, D.F.; Aguilar, Z.P.; Wang, Y.A.; Patnaik, R.; Mossler, H.; Sharma, H.S. Intravenous Administration of Functionalized Magnetic Iron Oxide Nanoparticles Does Not Induce CNS Injury in the Rat: Influence of Spinal Cord Trauma and Cerebrolysin Treatment. Int. Rev. Neurobiol. 2017, 137, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Pardue, M.T.; Allen, R.S. Neuroprotective strategies for retinal disease. Prog. Retin. Eye Res. 2018, 65, 50–76. [Google Scholar] [CrossRef] [PubMed]
- Komaromy, A.M.; Koehl, K.L.; Park, S.A. Looking into the future: Gene and cell therapies for glaucoma. Vet. Ophthalmol. 2021, 24 (Suppl. S1), 16–33. [Google Scholar] [CrossRef] [PubMed]
- Zarbin, M.A.; Arlow, T.; Ritch, R. Regenerative nanomedicine for vision restoration. Mayo Clin. Proc. 2013, 88, 1480–1490. [Google Scholar] [CrossRef]
- Naik, S.; Pandey, A.; Lewis, S.A.; Rao, B.S.S.; Mutalik, S. Neuroprotection: A versatile approach to combat glaucoma. Eur. J. Pharmacol. 2020, 881, 173208. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.; Bartlett, C.A.; Cowin, G.; Nicholls, P.K.; Evans, C.W.; Clemons, T.D.; Zdyrko, B.; Luzinov, I.A.; Harvey, A.R.; Iyer, K.S.; et al. In vivo imaging and biodistribution of multimodal polymeric nanoparticles delivered to the optic nerve. Small 2012, 8, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Giannaccini, M.; Usai, A.; Chiellini, F.; Guadagni, V.; Andreazzoli, M.; Ori, M.; Pasqualetti, M.; Dente, L.; Raffa, V. Neurotrophin-conjugated nanoparticles prevent retina damage induced by oxidative stress. Cell. Mol. Life Sci. 2018, 75, 1255–1267. [Google Scholar] [CrossRef]
- Mesentier-Louro, L.A.; Zaverucha-do-Valle, C.; da Silva-Junior, A.J.; Nascimento-Dos-Santos, G.; Gubert, F.; de Figueiredo, A.B.; Torres, A.L.; Paredes, B.D.; Teixeira, C.; Tovar-Moll, F.; et al. Distribution of mesenchymal stem cells and effects on neuronal survival and axon regeneration after optic nerve crush and cell therapy. PLoS ONE 2014, 9, e110722. [Google Scholar] [CrossRef]
- Pita-Thomas, W.; Steketee, M.B.; Moysidis, S.N.; Thakor, K.; Hampton, B.; Goldberg, J.L. Promoting filopodial elongation in neurons by membrane-bound magnetic nanoparticles. Nanomedicine 2015, 11, 559–567. [Google Scholar] [CrossRef][Green Version]
- Chotivichit, A.; Ruangchainikom, M.; Chiewvit, P.; Wongkajornsilp, A.; Sujirattanawimol, K. Chronic spinal cord injury treated with transplanted autologous bone marrow-derived mesenchymal stem cells tracked by magnetic resonance imaging: A case report. J. Med. Case Rep. 2015, 9, 79. [Google Scholar] [CrossRef]
- Kubinova, S. Biomaterials and Magnetic Stem Cell Delivery in the Treatment of Spinal Cord Injury. Neurochem. Res. 2020, 45, 171–179. [Google Scholar] [CrossRef]
- Sykova, E.; Jendelova, P.; Urdzikova, L.; Lesny, P.; Hejcl, A. Bone marrow stem cells and polymer hydrogels—Two strategies for spinal cord injury repair. Cell. Mol. Neurobiol. 2006, 26, 1113–1129. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, W.; Zhang, B.; Wang, B.; Wang, L.; Liu, S.; Chen, B.; Mai, X.; Zang, F. Systematic Intracellular Biocompatibility Assessments of Superparamagnetic Iron Oxide Nanoparticles in Human Umbilical Cord Mesenchyme Stem Cells in Testifying Its Reusability for Inner Cell Tracking by MRI. J. Biomed. Nanotechnol. 2019, 15, 2179–2192. [Google Scholar] [CrossRef]
- Callera, F.; de Melo, C.M. Magnetic resonance tracking of magnetically labeled autologous bone marrow CD34+ cells transplanted into the spinal cord via lumbar puncture technique in patients with chronic spinal cord injury: CD34+ cells’ migration into the injured site. Stem Cells Dev. 2007, 16, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, K.; Li, J.; Liu, Q.; Xie, J. In vivo tracking of neuronal-like cells by magnetic resonance in rabbit models of spinal cord injury. Neural Regen. Res. 2013, 8, 3373–3381. [Google Scholar] [CrossRef] [PubMed]
- Kubelick, K.P.; Emelianov, S.Y. Prussian blue nanocubes as a multimodal contrast agent for image-guided stem cell therapy of the spinal cord. Photoacoustics 2020, 18, 100166. [Google Scholar] [CrossRef]
- Hu, S.L.; Lu, P.G.; Zhang, L.J.; Li, F.; Chen, Z.; Wu, N.; Meng, H.; Lin, J.K.; Feng, H. In vivo magnetic resonance imaging tracking of SPIO-labeled human umbilical cord mesenchymal stem cells. J. Cell. Biochem. 2012, 113, 1005–1012. [Google Scholar] [CrossRef]
- Amemori, T.; Romanyuk, N.; Jendelova, P.; Herynek, V.; Turnovcova, K.; Prochazka, P.; Kapcalova, M.; Cocks, G.; Price, J.; Sykova, E. Human conditionally immortalized neural stem cells improve locomotor function after spinal cord injury in the rat. Stem Cell Res. Ther. 2013, 4, 68. [Google Scholar] [CrossRef]
- Hu, S.L.; Zhang, J.Q.; Hu, X.; Hu, R.; Luo, H.S.; Li, F.; Xia, Y.Z.; Li, J.T.; Lin, J.K.; Zhu, G.; et al. In vitro labeling of human umbilical cord mesenchymal stem cells with superparamagnetic iron oxide nanoparticles. J. Cell. Biochem. 2009, 108, 529–535. [Google Scholar] [CrossRef]
- Willenbrock, S.; Knippenberg, S.; Meier, M.; Hass, R.; Wefstaedt, P.; Nolte, I.; Murua Escobar, H.; Petri, S. In vivo MRI of intraspinally injected SPIO-labelled human CD34+ cells in a transgenic mouse model of ALS. In Vivo 2012, 26, 31–38. [Google Scholar]
- Dunning, M.D.; Kettunen, M.I.; Ffrench Constant, C.; Franklin, R.J.; Brindle, K.M. Magnetic resonance imaging of functional Schwann cell transplants labelled with magnetic microspheres. Neuroimage 2006, 31, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Lepore, A.C.; Walczak, P.; Rao, M.S.; Fischer, I.; Bulte, J.W. MR imaging of lineage-restricted neural precursors following transplantation into the adult spinal cord. Exp. Neurol. 2006, 201, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, Z.J.; Xu, B.; Wu, Q.Z.; Liu, G.; Zhu, H.; Zhong, Q.; Deng, D.Y.; Ai, H.; Yue, Q.; et al. Evaluation of cell tracking effects for transplanted mesenchymal stem cells with jetPEI/Gd-DTPA complexes in animal models of hemorrhagic spinal cord injury. Brain Res. 2011, 1391, 24–35. [Google Scholar] [CrossRef]
- Bulte, J.W.; Zhang, S.; van Gelderen, P.; Herynek, V.; Jordan, E.K.; Duncan, I.D.; Frank, J.A. Neurotransplantation of magnetically labeled oligodendrocyte progenitors: Magnetic resonance tracking of cell migration and myelination. Proc. Natl. Acad. Sci. USA 1999, 96, 15256–15261. [Google Scholar] [CrossRef]
- Sykova, E.; Jendelova, P. In vivo tracking of stem cells in brain and spinal cord injury. Prog. Brain Res. 2007, 161, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, J.J.; Gutierrez, J.; Urquia, L.N.; Hurtig, C.V.; Amador, E.; Grin, N.; Svendsen, C.N.; Federici, T.; Oshinski, J.N.; Boulis, N.M. Ferumoxytol Labeling of Human Neural Progenitor Cells for Diagnostic Cellular Tracking in the Porcine Spinal Cord with Magnetic Resonance Imaging. Stem Cells Transl. Med. 2017, 6, 139–150. [Google Scholar] [CrossRef]
- Won, J.S.; Nam, H.; Lee, H.W.; Hwang, J.Y.; Noh, Y.J.; Nam, D.H.; Lee, S.H.; Joo, K.M. In vivo distribution of U87MG cells injected into the lateral ventricle of rats with spinal cord injury. PLoS ONE 2018, 13, e0202307. [Google Scholar] [CrossRef]
- Cho, H.; Choi, Y.K.; Lee, D.H.; Park, H.J.; Seo, Y.K.; Jung, H.; Kim, S.C.; Kim, S.M.; Park, J.K. Effects of magnetic nanoparticle-incorporated human bone marrow-derived mesenchymal stem cells exposed to pulsed electromagnetic fields on injured rat spinal cord. Biotechnol. Appl. Biochem. 2013, 60, 596–602. [Google Scholar] [CrossRef]
- Tukmachev, D.; Lunov, O.; Zablotskii, V.; Dejneka, A.; Babic, M.; Sykova, E.; Kubinova, S. An effective strategy of magnetic stem cell delivery for spinal cord injury therapy. Nanoscale 2015, 7, 3954–3958. [Google Scholar] [CrossRef]
- Zhang, R.P.; Xu, C.; Liu, Y.; Li, J.D.; Xie, J. Visual bone marrow mesenchymal stem cell transplantation in the repair of spinal cord injury. Neural Regen. Res. 2015, 10, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yu, W.; Zhang, F.; Liu, T.; Li, K.; Lin, M.; Wang, Y.; Zhao, G.; Jiang, J. Fe3O4@Polydopamine-Labeled MSCs Targeting the Spinal Cord to Treat Neuropathic Pain Under the Guidance of a Magnetic Field. Int. J. Nanomed. 2021, 16, 3275–3292. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Tanaka, N.; Nakanishi, K.; Kamei, N.; Hamasaki, T.; Yanada, S.; Mochizuki, Y.; Ochi, M. Magnetic targeting of bone marrow stromal cells into spinal cord: Through cerebrospinal fluid. Neuroreport 2006, 17, 1269–1272. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Tanaka, N.; Nakanishi, K.; Nishida, K.; Hamasaki, T.; Yamada, K.; Ochi, M. Therapeutic effects with magnetic targeting of bone marrow stromal cells in a rat spinal cord injury model. Spine 2011, 36, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Vanecek, V.; Zablotskii, V.; Forostyak, S.; Ruzicka, J.; Herynek, V.; Babic, M.; Jendelova, P.; Kubinova, S.; Dejneka, A.; Sykova, E. Highly efficient magnetic targeting of mesenchymal stem cells in spinal cord injury. Int. J. Nanomed. 2012, 7, 3719–3730. [Google Scholar] [CrossRef]
- Choi, J.I.; Cho, H.T.; Jee, M.K.; Kang, S.K. Core-shell nanoparticle controlled hATSCs neurogenesis for neuropathic pain therapy. Biomaterials 2013, 34, 4956–4970. [Google Scholar] [CrossRef]
- Huang, L.; Xia, B.; Liu, Z.; Cao, Q.; Huang, J.; Luo, Z. Superparamagnetic Iron Oxide Nanoparticle-Mediated Forces Enhance the Migration of Schwann Cells Across the Astrocyte-Schwann Cell Boundary In vitro. Front. Cell. Neurosci. 2017, 11, 83. [Google Scholar] [CrossRef]
- Gao, J.; Xia, B.; Li, S.; Huang, L.; Ma, T.; Shi, X.; Luo, K.; Yang, Y.; Zhao, L.; Zhang, H.; et al. Magnetic Field Promotes Migration of Schwann Cells with Chondroitinase ABC (ChABC)-Loaded Superparamagnetic Nanoparticles Across Astrocyte Boundary in vitro. Int. J. Nanomed. 2020, 15, 315–332. [Google Scholar] [CrossRef]
- Xia, B.; Huang, L.; Zhu, L.; Liu, Z.; Ma, T.; Zhu, S.; Huang, J.; Luo, Z. Manipulation of Schwann cell migration across the astrocyte boundary by polysialyltransferase-loaded superparamagnetic nanoparticles under magnetic field. Int. J. Nanomed. 2016, 11, 6727–6741. [Google Scholar] [CrossRef]
- Barnett, S.C.; Riddell, J.S. Olfactory ensheathing cells (OECs) and the treatment of CNS injury: Advantages and possible caveats. J. Anat. 2004, 204, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Riggio, C.; Nocentini, S.; Catalayud, M.P.; Goya, G.F.; Cuschieri, A.; Raffa, V.; del Rio, J.A. Generation of magnetized olfactory ensheathing cells for regenerative studies in the central and peripheral nervous tissue. Int. J. Mol. Sci. 2013, 14, 10852–10868. [Google Scholar] [CrossRef]
- Sandvig, I.; Hoang, L.; Sardella, T.C.; Barnett, S.C.; Brekken, C.; Tvedt, K.; Berry, M.; Haraldseth, O.; Sandvig, A.; Thuen, M. Labelling of olfactory ensheathing cells with micron-sized particles of iron oxide and detection by MRI. Contrast Media Mol. Imaging 2012, 7, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.H.; Bulte, J.W.; Schweinhardt, P.; Douglas, T.; Trifunovski, A.; Hofstetter, C.; Olson, L.; Spenger, C. In vivo magnetic resonance tracking of olfactory ensheathing glia grafted into the rat spinal cord. Exp. Neurol. 2004, 187, 509–516. [Google Scholar] [CrossRef]
- Delaney, A.M.; Adams, C.F.; Fernandes, A.R.; Al-Shakli, A.F.; Sen, J.; Carwardine, D.R.; Granger, N.; Chari, D.M. A fusion of minicircle DNA and nanoparticle delivery technologies facilitates therapeutic genetic engineering of autologous canine olfactory mucosal cells. Nanoscale 2017, 9, 8560–8566. [Google Scholar] [CrossRef]
- Kong, S.D.; Lee, J.; Ramachandran, S.; Eliceiri, B.P.; Shubayev, V.I.; Lal, R.; Jin, S. Magnetic targeting of nanoparticles across the intact blood-brain barrier. J. Control. Release 2012, 164, 49–57. [Google Scholar] [CrossRef]
- Jeffery, N.D.; McBain, S.C.; Dobson, J.; Chari, D.M. Uptake of systemically administered magnetic nanoparticles (MNPs) in areas of experimental spinal cord injury (SCI). J. Tissue Eng. Regen. Med. 2009, 3, 153–157. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kumar, H.; Jo, M.J.; Kim, J.; Yoon, J.K.; Lee, J.R.; Kang, M.; Choo, Y.W.; Song, S.Y.; Kwon, S.P.; et al. Therapeutic Efficacy-Potentiated and Diseased Organ-Targeting Nanovesicles Derived from Mesenchymal Stem Cells for Spinal Cord Injury Treatment. Nano Lett. 2018, 18, 4965–4975. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Liao, Z.; Wang, C.; Liu, Y.; Feng, S.; Jiang, X.; Chang, J. PEGlated magnetic polymeric liposome anchored with TAT for delivery of drugs across the blood-spinal cord barrier. Biomaterials 2010, 31, 6589–6596. [Google Scholar] [CrossRef]
- Song, H.P.; Yang, J.Y.; Lo, S.L.; Wang, Y.; Fan, W.M.; Tang, X.S.; Xue, J.M.; Wang, S. Gene transfer using self-assembled ternary complexes of cationic magnetic nanoparticles, plasmid DNA and cell-penetrating Tat peptide. Biomaterials 2010, 31, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-H.; Wang, A.-H.; Zhang, C.; Wu, C.-Z.; Hu, L.-L.; Huo, X.-L. Design of site-directed magnetic targeting system in acute spinal cord injury. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2015, 2015, 5813–5816. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Dinda, A.; Vishnubhatla, S.; Anwar, M.F.; Jain, S. A combinatorial approach to modulate microenvironment toward regeneration and repair after spinal cord injury in rats. Neurosci. Lett. 2021, 741, 135500. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.C.; Camara-Torres, M.; Rahimi, K.; Kohler, J.; Moller, M.; De Laporte, L. Nerve Cells Decide to Orient inside an Injectable Hydrogel with Minimal Structural Guidance. Nano Lett. 2017, 17, 3782–3791. [Google Scholar] [CrossRef]
- Pal, A.; Kumar, S.; Jain, S.; Nag, T.C.; Mathur, R. Neuroregenerative Effects of Electromagnetic Field and Magnetic Nanoparticles on Spinal Cord Injury in Rats. J. Nanosci. Nanotechnol. 2018, 18, 6756–6764. [Google Scholar] [CrossRef]
- Pal, A.; Singh, A.; Nag, T.C.; Chattopadhyay, P.; Mathur, R.; Jain, S. Iron oxide nanoparticles and magnetic field exposure promote functional recovery by attenuating free radical-induced damage in rats with spinal cord transection. Int. J. Nanomed. 2013, 8, 2259–2272. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.; Guo, C.; Wang, J.; Ma, J.; Liang, X.; Yang, L.R.; Liu, H.Z. Temperature-responsive magnetite/PEO-PPO-PEO block copolymer nanoparticles for controlled drug targeting delivery. Langmuir 2007, 23, 12669–12676. [Google Scholar] [CrossRef]
- Min, K.J.; Kim, T.H.; Choi, J.W. Magnetic Force-Driven Graphene Patterns to Direct Synaptogenesis of Human Neuronal Cells. Materials 2017, 10, 1151. [Google Scholar] [CrossRef]
- Bowser, D.A.; Moore, M.J. Biofabrication of neural microphysiological systems using magnetic spheroid bioprinting. Biofabrication 2019, 12, 015002. [Google Scholar] [CrossRef]
- Adams, C.F.; Delaney, A.M.; Carwardine, D.R.; Tickle, J.; Granger, N.; Chari, D.M. Nanoparticle-Based Imaging of Clinical Transplant Populations Encapsulated in Protective Polymer Matrices. Macromol. Biosci. 2019, 19, e1800389. [Google Scholar] [CrossRef]
- Duncan, T.; Valenzuela, M. Alzheimer’s disease, dementia, and stem cell therapy. Stem Cell Res. Ther. 2017, 8, 111. [Google Scholar] [CrossRef]
- Larijani, B.; Esfahani, E.N.; Amini, P.; Nikbin, B.; Alimoghaddam, K.; Amiri, S.; Malekzadeh, R.; Yazdi, N.M.; Ghodsi, M.; Dowlati, Y.; et al. Stem cell therapy in treatment of different diseases. Acta Med. Iran. 2012, 50, 79–96. [Google Scholar] [PubMed]
- Karussis, D.; Karageorgiou, C.; Vaknin-Dembinsky, A.; Gowda-Kurkalli, B.; Gomori, J.M.; Kassis, I.; Bulte, J.W.; Petrou, P.; Ben-Hur, T.; Abramsky, O.; et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch. Neurol. 2010, 67, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Shichinohe, H.; Abumiya, T.; Nakayama, N.; Kazumata, K.; Hokari, M.; Hamauchi, S.; Houkin, K. Short-, middle- and long-term safety of superparamagnetic iron oxide-labeled allogeneic bone marrow stromal cell transplantation in rat model of lacunar infarction. Neuropathology 2015, 35, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Millward, J.M.; Schnorr, J.; Taupitz, M.; Wagner, S.; Wuerfel, J.T.; Infante-Duarte, C. Iron oxide magnetic nanoparticles highlight early involvement of the choroid plexus in central nervous system inflammation. ASN Neuro 2013, 5, e00110. [Google Scholar] [CrossRef] [PubMed]
- Aghayan, H.R.; Soleimani, M.; Goodarzi, P.; Norouzi-Javidan, A.; Emami-Razavi, S.H.; Larijani, B.; Arjmand, B. Magnetic resonance imaging of transplanted stem cell fate in stroke. J. Res. Med. Sci. 2014, 19, 465–471. [Google Scholar] [PubMed]
- Chu, K.; Kim, M.; Jeong, S.W.; Kim, S.U.; Yoon, B.W. Human neural stem cells can migrate, differentiate, and integrate after intravenous transplantation in adult rats with transient forebrain ischemia. Neurosci. Lett. 2003, 343, 129–133. [Google Scholar] [CrossRef]
- Kelly, S.; Bliss, T.M.; Shah, A.K.; Sun, G.H.; Ma, M.; Foo, W.C.; Masel, J.; Yenari, M.A.; Weissman, I.L.; Uchida, N.; et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc. Natl. Acad. Sci. USA 2004, 101, 11839–11844. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Vazin, T.; Goodwill, P.W.; Conway, A.; Verma, A.; Saritas, E.U.; Schaffer, D.; Conolly, S.M. Magnetic Particle Imaging tracks the long-term fate of in vivo neural cell implants with high image contrast. Sci. Rep. 2015, 5, 14055. [Google Scholar] [CrossRef]
- Ali, A.A.A.; Shahror, R.A.; Chen, K.Y. Efficient Labeling Of Mesenchymal Stem Cells For High Sensitivity Long-Term MRI Monitoring In Live Mice Brains. Int. J. Nanomed. 2020, 15, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Kim, H.S.; Yoo, D.; Hwang, J.W.; Choi, S.J.; Oh, W.; Chang, J.W.; Na, D.L. Magnetic Resonance Imaging of Ferumoxytol-Labeled Human Mesenchymal Stem Cells in the Mouse Brain. Stem Cell Rev. Rep. 2017, 13, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.L.; Zhang, J.Z.; Lu, L.J.; Mao, J.J.; Cao, M.H.; Mao, X.H.; Zhang, F.; Duan, X.H.; Zheng, C.S.; Zhang, L.M.; et al. Superparamagnetic Iron Oxide Nanoparticles-Complexed Cationic Amylose for In Vivo Magnetic Resonance Imaging Tracking of Transplanted Stem Cells in Stroke. Nanomaterials 2017, 7, 107. [Google Scholar] [CrossRef]
- Lim, S.; Yoon, H.Y.; Jang, H.J.; Song, S.; Kim, W.; Park, J.; Lee, K.E.; Jeon, S.; Lee, S.; Lim, D.K.; et al. Dual-Modal Imaging-Guided Precise Tracking of Bioorthogonally Labeled Mesenchymal Stem Cells in Mouse Brain Stroke. ACS Nano 2019, 13, 10991–11007. [Google Scholar] [CrossRef]
- Gorelik, M.; Orukari, I.; Wang, J.; Galpoththawela, S.; Kim, H.; Levy, M.; Gilad, A.A.; Bar-Shir, A.; Kerr, D.A.; Levchenko, A.; et al. Use of MR cell tracking to evaluate targeting of glial precursor cells to inflammatory tissue by exploiting the very late antigen-4 docking receptor. Radiology 2012, 265, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Chan, J.; Shuter, B.; Tan, L.G.; Chong, M.S.; Ramachandra, D.L.; Dawe, G.S.; Ding, J.; Teoh, S.H.; Beuf, O.; et al. Microgel iron oxide nanoparticles for tracking human fetal mesenchymal stem cells through magnetic resonance imaging. Stem Cells 2009, 27, 1921–1931. [Google Scholar] [CrossRef]
- Shahror, R.A.; Wu, C.C.; Chiang, Y.H.; Chen, K.Y. Tracking Superparamagnetic Iron Oxide-labeled Mesenchymal Stem Cells using MRI after Intranasal Delivery in a Traumatic Brain Injury Murine Model. J. Vis. Exp. 2019, 153, e60450. [Google Scholar] [CrossRef]
- Wu, M.R.; Lee, C.H.; Hsiao, J.K. Bidirectional Enhancement of Cell Proliferation Between Iron Oxide Nanoparticle-Labeled Mesenchymal Stem Cells and Choroid Plexus in a Cell-Based Therapy Model of Ischemic Stroke. Int. J. Nanomed. 2020, 15, 9181–9195. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Chang, K.A. Therapeutic Potential of Magnetic Nanoparticle-Based Human Adipose-Derived Stem Cells in a Mouse Model of Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 654. [Google Scholar] [CrossRef] [PubMed]
- Nucci, L.P.; Silva, H.R.; Giampaoli, V.; Mamani, J.B.; Nucci, M.P.; Gamarra, L.F. Stem cells labeled with superparamagnetic iron oxide nanoparticles in a preclinical model of cerebral ischemia: A systematic review with meta-analysis. Stem Cell Res. Ther. 2015, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.I.; Pickard, M.R.; Furness, D.N.; Yiu, H.H.; Chari, D.M. Differences in magnetic particle uptake by CNS neuroglial subclasses: Implications for neural tissue engineering. Nanomedicine 2013, 8, 951–968. [Google Scholar] [CrossRef]
- Jenkins, S.I.; Pickard, M.R.; Granger, N.; Chari, D.M. Magnetic nanoparticle-mediated gene transfer to oligodendrocyte precursor cell transplant populations is enhanced by magnetofection strategies. ACS Nano 2011, 5, 6527–6538. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, F.; Wang, Y.; Sun, X.; Choi, K.Y.; Liu, D.; Choi, J.S.; Shin, T.H.; Cheon, J.; Niu, G.; et al. Design considerations of iron-based nanoclusters for noninvasive tracking of mesenchymal stem cell homing. ACS Nano 2014, 8, 4403–4414. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.H.; Hsu, S.C.; Wu, S.H.; Hsiao, J.K.; Lin, C.P.; Yao, M.; Huang, D.M. Dextran-coated iron oxide nanoparticle-improved therapeutic effects of human mesenchymal stem cells in a mouse model of Parkinson’s disease. Nanoscale 2018, 10, 2998–3007. [Google Scholar] [CrossRef]
- Schoneborn, H.; Raudzus, F.; Secret, E.; Otten, N.; Michel, A.; Fresnais, J.; Menager, C.; Siaugue, J.M.; Zaehres, H.; Dietzel, I.D.; et al. Novel Tools towards Magnetic Guidance of Neurite Growth: (I) Guidance of Magnetic Nanoparticles into Neurite Extensions of Induced Human Neurons and In Vitro Functionalization with RAS Regulating Proteins. J. Funct. Biomater. 2019, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Raudzus, F.; Schoneborn, H.; Neumann, S.; Secret, E.; Michel, A.; Fresnais, J.; Brylski, O.; Menager, C.; Siaugue, J.M.; Heumann, R. Magnetic spatiotemporal control of SOS1 coupled nanoparticles for guided neurite growth in dopaminergic single cells. Sci. Rep. 2020, 10, 22452. [Google Scholar] [CrossRef]
- Hour, F.Q.; Moghadam, A.J.; Shakeri-Zadeh, A.; Bakhtiyari, M.; Shabani, R.; Mehdizadeh, M. Magnetic targeted delivery of the SPIONs-labeled mesenchymal stem cells derived from human Wharton’s jelly in Alzheimer’s rat models. J. Control. Release 2020, 321, 430–441. [Google Scholar] [CrossRef]
- Kang, M.K.; Kim, T.J.; Kim, Y.J.; Kang, L.; Kim, J.; Lee, N.; Hyeon, T.; Lim, M.S.; Mo, H.J.; Shin, J.H.; et al. Targeted Delivery of Iron Oxide Nanoparticle-Loaded Human Embryonic Stem Cell-Derived Spherical Neural Masses for Treating Intracerebral Hemorrhage. Int. J. Mol. Sci. 2020, 21, 3658. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Xu, K.; Gu, J.; Huang, L.; Zhang, L.; Liu, N.; Kong, J.; Xing, M.; Zhang, L.; et al. Characterization of superparamagnetic iron oxide nanoparticle-induced apoptosis in PC12 cells and mouse hippocampus and striatum. Toxicol. Lett. 2018, 292, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, V.N.; Copeland, C.; Mathew, E.; Newbern, J.; Anderson, T.R.; Lifshitz, J.; Kodibagkar, V.D.; Stabenfeldt, S.E. Sex-Dependent Macromolecule and Nanoparticle Delivery in Experimental Brain Injury. Tissue Eng. Part A 2020, 26, 688–701. [Google Scholar] [CrossRef]
- Tomitaka, A.; Kaushik, A.; Kevadiya, B.D.; Mukadam, I.; Gendelman, H.E.; Khalili, K.; Liu, G.; Nair, M. Surface-engineered multimodal magnetic nanoparticles to manage CNS diseases. Drug Discov. Today 2019, 24, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.H. Targeted Delivery of Erythropoietin Hybridized with Magnetic Nanocarriers for the Treatment of Central Nervous System Injury: A Literature Review. Int. J. Nanomed. 2020, 15, 9683–9701. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.; Kim, C.R.; Le, T.H.; Koo, K.I.; Hwang, C.H. Magnetically guided targeted delivery of erythropoietin using magnetic nanoparticles: Proof of concept. Medicine 2020, 99, e19972. [Google Scholar] [CrossRef] [PubMed]
- Naserzadeh, P.; Hafez, A.A.; Abdorahim, M.; Abdollahifar, M.A.; Shabani, R.; Peirovi, H.; Simchi, A.; Ashtari, K. Curcumin loading potentiates the neuroprotective efficacy of Fe3O4 magnetic nanoparticles in cerebellum cells of schizophrenic rats. Biomed. Pharmacother. 2018, 108, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Pavon, J.J.; Allain, J.P.; Verma, D.; Echeverry-Rendon, M.; Cooper, C.L.; Reece, L.M.; Shetty, A.R.; Tomar, V. In situ Study Unravels Bio-Nanomechanical Behavior in a Magnetic Bacterial Nano-cellulose (MBNC) Hydrogel for Neuro-Endovascular Reconstruction. Macromol. Biosci. 2019, 19, e1800225. [Google Scholar] [CrossRef]
- Echeverry-Rendon, M.; Reece, L.M.; Pastrana, F.; Arias, S.L.; Shetty, A.R.; Pavon, J.J.; Allain, J.P. Bacterial Nanocellulose Magnetically Functionalized for Neuro-Endovascular Treatment. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef]
- Watada, Y.; Yamashita, D.; Toyoda, M.; Tsuchiya, K.; Hida, N.; Tanimoto, A.; Ogawa, K.; Kanzaki, S.; Umezawa, A. Magnetic resonance monitoring of superparamagnetic iron oxide (SPIO)-labeled stem cells transplanted into the inner ear. Neurosci. Res. 2015, 95, 21–26. [Google Scholar] [CrossRef]
- Zou, J.; Ostrovsky, S.; Israel, L.L.; Feng, H.; Kettunen, M.I.; Lellouche, J.M.; Pyykko, I. Efficient penetration of ceric ammonium nitrate oxidant-stabilized gamma-maghemite nanoparticles through the oval and round windows into the rat inner ear as demonstrated by MRI. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Youm, I.; Musazzi, U.M.; Gratton, M.A.; Murowchick, J.B.; Youan, B.C. Label-Free Ferrocene-Loaded Nanocarrier Engineering for In Vivo Cochlear Drug Delivery and Imaging. J. Pharm. Sci. 2016, 105, 3162–3171. [Google Scholar] [CrossRef]
- Ahn, Y.J.; Yun, W.S.; Choi, J.S.; Kim, W.C.; Lee, S.H.; Park, D.J.; Park, J.E.; Key, J.; Seo, Y.J. Biodistribution of poly clustered superparamagnetic iron oxide nanoparticle labeled mesenchymal stem cells in aminoglycoside induced ototoxic mouse model. Biomed. Eng. Lett 2021, 11, 39–53. [Google Scholar] [CrossRef]
- Mead, B.; Berry, M.; Logan, A.; Scott, R.A.; Leadbeater, W.; Scheven, B.A. Stem cell treatment of degenerative eye disease. Stem Cell Res. 2015, 14, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.H.; Tian, Y.; Funderburgh, J.; Pellegrini, G.; Zhang, K.; Goldberg, J.L.; Ali, R.R.; Young, M.; Xie, Y.; Temple, S. Regenerating Eye Tissues to Preserve and Restore Vision. Cell Stem Cell 2018, 22, 834–849. [Google Scholar] [CrossRef]
- Snider, E.J.; Kubelick, K.P.; Tweed, K.; Kim, R.K.; Li, Y.; Gao, K.; Read, A.T.; Emelianov, S.; Ethier, C.R. Improving Stem Cell Delivery to the Trabecular Meshwork Using Magnetic Nanoparticles. Sci. Rep. 2018, 8, 12251. [Google Scholar] [CrossRef]
- Cornell, L.E.; Wehmeyer, J.L.; Johnson, A.J.; Desilva, M.N.; Zamora, D.O. Magnetic Nanoparticles as a Potential Vehicle for Corneal Endothelium Repair. Mil. Med. 2016, 181, 232–239. [Google Scholar] [CrossRef]
- Ito, A.; Hibino, E.; Kobayashi, C.; Terasaki, H.; Kagami, H.; Ueda, M.; Kobayashi, T.; Honda, H. Construction and delivery of tissue-engineered human retinal pigment epithelial cell sheets, using magnetite nanoparticles and magnetic force. Tissue Eng. 2005, 11, 489–496. [Google Scholar] [CrossRef]
- Yun, W.S.; Choi, J.S.; Ju, H.M.; Kim, M.H.; Choi, S.J.; Oh, E.S.; Seo, Y.J.; Key, J. Enhanced Homing Technique of Mesenchymal Stem Cells Using Iron Oxide Nanoparticles by Magnetic Attraction in Olfactory-Injured Mouse Models. Int. J. Mol. Sci. 2018, 19, 1376. [Google Scholar] [CrossRef]
- Lee, S.; Seon, S.; Park, K.; Ryu, J. Vocal Fold Reconstruction Using an Autologous Pedicled Fat Flap in a Rabbit Model. Laryngoscope 2020, 130, 1770–1774. [Google Scholar] [CrossRef]
- Li, L.; Stiadle, J.M.; Lau, H.K.; Zerdoum, A.B.; Jia, X.; Thibeault, S.L.; Kiick, K.L. Tissue engineering-based therapeutic strategies for vocal fold repair and regeneration. Biomaterials 2016, 108, 91–110. [Google Scholar] [CrossRef]
- Ling, C.; Li, Q.; Brown, M.E.; Kishimoto, Y.; Toya, Y.; Devine, E.E.; Choi, K.O.; Nishimoto, K.; Norman, I.G.; Tsegyal, T.; et al. Bioengineered vocal fold mucosa for voice restoration. Sci. Transl. Med. 2015, 7, 314ra187. [Google Scholar] [CrossRef]
- Durr, S.; Bohr, C.; Pottler, M.; Lyer, S.; Friedrich, R.P.; Tietze, R.; Dollinger, M.; Alexiou, C.; Janko, C. Magnetic Tissue Engineering for Voice Rehabilitation—First Steps in a Promising Field. Anticancer. Res. 2016, 36, 3085–3091. [Google Scholar] [PubMed]
- Pottler, M.; Fliedner, A.; Schreiber, E.; Janko, C.; Friedrich, R.P.; Bohr, C.; Dollinger, M.; Alexiou, C.; Durr, S. Impact of Superparamagnetic Iron Oxide Nanoparticles on Vocal Fold Fibroblasts: Cell Behavior and Cellular Iron Kinetics. Nanoscale Res. Lett. 2017, 12, 284. [Google Scholar] [CrossRef][Green Version]
- Pottler, M.; Fliedner, A.; Bergmann, J.; Bui, L.K.; Muhlberger, M.; Braun, C.; Graw, M.; Janko, C.; Friedrich, O.; Alexiou, C.; et al. Magnetic Tissue Engineering of the Vocal Fold Using Superparamagnetic Iron Oxide Nanoparticles. Tissue Eng. Part A 2019, 25, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Chang, F.H.; Chen, C.Y.; Huang, C.Y.; Hu, F.C.; Huang, W.K.; Ju, S.S.; Chen, M.H. Cell therapy for salivary gland regeneration. J. Dent. Res. 2011, 90, 341–346. [Google Scholar] [CrossRef]
- Ferreira, J.N.; Hasan, R.; Urkasemsin, G.; Ng, K.K.; Adine, C.; Muthumariappan, S.; Souza, G.R. A magnetic three-dimensional levitated primary cell culture system for the development of secretory salivary gland-like organoids. J. Tissue Eng. Regen. Med. 2019, 13, 495–508. [Google Scholar] [CrossRef]
- Urkasemsin, G.; Rungarunlert, S.; Ferreira, J.N. Bioprinting Strategies for Secretory Epithelial Organoids. Methods Mol. Biol. 2020, 2140, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Adine, C.; Ng, K.K.; Rungarunlert, S.; Souza, G.R.; Ferreira, J.N. Engineering innervated secretory epithelial organoids by magnetic three-dimensional bioprinting for stimulating epithelial growth in salivary glands. Biomaterials 2018, 180, 52–66. [Google Scholar] [CrossRef]
- Moon, K.H.; Ko, I.K.; Yoo, J.J.; Atala, A. Kidney diseases and tissue engineering. Methods 2016, 99, 112–119. [Google Scholar] [CrossRef]
- Montserrat, N.; Garreta, E.; Izpisua Belmonte, J.C. Regenerative strategies for kidney engineering. FEBS J. 2016, 283, 3303–3324. [Google Scholar] [CrossRef]
- Soriano, M.L.; Rodriguez-Benot, A.; Valcarcel, M. Nanotechnological foundations of a “new” Nephrology. Nefrologia 2018, 38, 368–378. [Google Scholar] [CrossRef]
- Hauger, O.; Delalande, C.; Deminiere, C.; Fouqueray, B.; Ohayon, C.; Garcia, S.; Trillaud, H.; Combe, C.; Grenier, N. Nephrotoxic nephritis and obstructive nephropathy: Evaluation with MR imaging enhanced with ultrasmall superparamagnetic iron oxide-preliminary findings in a rat model. Radiology 2000, 217, 819–826. [Google Scholar] [CrossRef]
- Hauger, O.; Frost, E.E.; van Heeswijk, R.; Deminiere, C.; Xue, R.; Delmas, Y.; Combe, C.; Moonen, C.T.; Grenier, N.; Bulte, J.W. MR evaluation of the glomerular homing of magnetically labeled mesenchymal stem cells in a rat model of nephropathy. Radiology 2006, 238, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.J.; Sung, P.H.; Wallace, C.G.; Yang, C.C.; Chen, K.H.; Shao, P.L.; Chu, Y.C.; Huang, C.R.; Chen, Y.L.; Ko, S.F.; et al. Intravenous administration of iPS-MSC(SPIONs) mobilized into CKD parenchyma and effectively preserved residual renal function in CKD rat. J. Cell. Mol. Med. 2020, 24, 3593–3610. [Google Scholar] [CrossRef] [PubMed]
- Udompap, P.; Kim, D.; Kim, W.R. Current and Future Burden of Chronic Nonmalignant Liver Disease. Clin. Gastroenterol. Hepatol. 2015, 13, 2031–2041. [Google Scholar] [CrossRef]
- Eftekhari, A.; Arjmand, A.; Asheghvatan, A.; Svajdlenkova, H.; Sausa, O.; Abiyev, H.; Ahmadian, E.; Smutok, O.; Khalilov, R.; Kavetskyy, T.; et al. The Potential Application of Magnetic Nanoparticles for Liver Fibrosis Theranostics. Front. Chem. 2021, 9, 674786. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Li, D.; Qian, J.; Li, Z.; Pang, P.; Shan, H. MR tracking of SPIO-labeled mesenchymal stem cells in rats with liver fibrosis could not monitor the cells accurately. Contrast Media Mol. Imaging 2015, 10, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, Y.H.; Mourad, G.M.; Stephanos, W.M.; Omar, S.A.; Mehanna, R.A. Bone Marrow-Derived Mesenchymal Stem Cell Potential Regression of Dysplasia Associating Experimental Liver Fibrosis in Albino Rats. Biomed. Res. Int. 2019, 2019, 5376165. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, Y.; Gao, C.; Zhang, J.; Bao, H.; Wang, Z.; Gong, P. Superparamagnetic iron oxide magnetic nanomaterial-labeled bone marrow mesenchymal stem cells for rat liver repair after hepatectomy. J. Surg. Res. 2014, 191, 290–301. [Google Scholar] [CrossRef]
- Lai, L.; Chen, J.; Wei, X.; Huang, M.; Hu, X.; Yang, R.; Jiang, X.; Shan, H. Transplantation of MSCs Overexpressing HGF into a Rat Model of Liver Fibrosis. Mol. Imaging Biol. 2016, 18, 43–51. [Google Scholar] [CrossRef]
- Kurniawan, D.W.; Booijink, R.; Pater, L.; Wols, I.; Vrynas, A.; Storm, G.; Prakash, J.; Bansal, R. Fibroblast growth factor 2 conjugated superparamagnetic iron oxide nanoparticles (FGF2-SPIONs) ameliorate hepatic stellate cells activation in vitro and acute liver injury in vivo. J. Control. Release 2020, 328, 640–652. [Google Scholar] [CrossRef]
- Ito, A.; Takizawa, Y.; Honda, H.; Hata, K.; Kagami, H.; Ueda, M.; Kobayashi, T. Tissue engineering using magnetite nanoparticles and magnetic force: Heterotypic layers of cocultured hepatocytes and endothelial cells. Tissue Eng. 2004, 10, 833–840. [Google Scholar] [CrossRef]
- Li, H.; Yin, Y.; Xiang, Y.; Liu, H.; Guo, R. A novel 3D printing PCL/GelMA scaffold containing USPIO for MRI-guided bile duct repair. Biomed. Mater. 2020, 15, 045004. [Google Scholar] [CrossRef]
- Tan, S.Y.; Mei Wong, J.L.; Sim, Y.J.; Wong, S.S.; Mohamed Elhassan, S.A.; Tan, S.H.; Ling Lim, G.P.; Rong Tay, N.W.; Annan, N.C.; Bhattamisra, S.K.; et al. Type 1 and 2 diabetes mellitus: A review on current treatment approach and gene therapy as potential intervention. Diabetes Metab. Syndr. 2019, 13, 364–372. [Google Scholar] [CrossRef]
- Wang, P.; Goodwill, P.W.; Pandit, P.; Gaudet, J.; Ross, A.; Wang, J.; Yu, E.; Hensley, D.W.; Doyle, T.C.; Contag, C.H.; et al. Magnetic particle imaging of islet transplantation in the liver and under the kidney capsule in mouse models. Quant. Imaging Med. Surg. 2018, 8, 114–122. [Google Scholar] [CrossRef]
- Juang, J.H.; Wang, J.J.; Shen, C.R.; Chen, C.Y.; Kao, C.W.; Chen, C.L.; Lin, S.H.; Wu, S.T.; Li, W.C.; Tsai, Z.T. Magnetic Resonance Imaging of Transplanted Porcine Neonatal Pancreatic Cell Clusters Labeled with Chitosan-Coated Superparamagnetic Iron Oxide Nanoparticles in Mice. Polymers 2021, 13, 1238. [Google Scholar] [CrossRef]
- Constantinidis, I.; Grant, S.C.; Simpson, N.E.; Oca-Cossio, J.A.; Sweeney, C.A.; Mao, H.; Blackband, S.J.; Sambanis, A. Use of magnetic nanoparticles to monitor alginate-encapsulated betaTC-tet cells. Magn. Reson. Med. 2009, 61, 282–290. [Google Scholar] [CrossRef]
- Zou, C.; Lu, Y.; Teng, X.; Wang, S.; Sun, X.; Huang, F.; Shu, G.; Huang, X.; Guo, H.; Chen, Z.; et al. MRI tracking of autologous pancreatic progenitor-derived insulin-producing cells in monkeys. Sci. Rep. 2017, 7, 2505. [Google Scholar] [CrossRef]
- Toso, C.; Vallee, J.P.; Morel, P.; Ris, F.; Demuylder-Mischler, S.; Lepetit-Coiffe, M.; Marangon, N.; Saudek, F.; James Shapiro, A.M.; Bosco, D.; et al. Clinical magnetic resonance imaging of pancreatic islet grafts after iron nanoparticle labeling. Am. J. Transplant. 2008, 8, 701–706. [Google Scholar] [CrossRef]
- Auer, V.J.; Bucher, J.; Schremmer-Danninger, E.; Paulmurugan, R.; Maechler, P.; Reiser, M.F.; Stangl, M.J.; Berger, F. Non-invasive imaging of ferucarbotran labeled INS-1E cells and rodent islets in vitro and in transplanted diabetic rats. Curr. Pharm. Biotechnol. 2011, 12, 488–496. [Google Scholar] [CrossRef]
- Hwang, J.H.; Noh, Y.W.; Choi, J.H.; Noh, J.R.; Kim, Y.H.; Gang, G.T.; Kim, K.S.; Park, H.S.; Lim, Y.T.; Moon, H.; et al. In vivo imaging of islet transplantation using PLGA nanoparticles containing iron oxide and indocyanine green. Magn. Reson. Med. 2014, 71, 1054–1063. [Google Scholar] [CrossRef]
- Li, X.; Wei, Z.; Wu, L.; Lv, H.; Zhang, Y.; Li, J.; Yao, H.; Zhang, H.; Yang, B.; Xu, X.; et al. Efficacy of Fe3O4@polydopamine nanoparticle-labeled human umbilical cord Wharton’s jelly-derived mesenchymal stem cells in the treatment of streptozotocin-induced diabetes in rats. Biomater. Sci. 2020, 8, 5362–5375. [Google Scholar] [CrossRef]
- Espona-Noguera, A.; Etxebarria-Elezgarai, J.; Saenz Del Burgo, L.; Canibano-Hernandez, A.; Gurruchaga, H.; Blanco, F.J.; Orive, G.; Hernandez, R.M.; Benito-Lopez, F.; Ciriza, J.; et al. Type 1 Diabetes Mellitus reversal via implantation of magnetically purified microencapsulated pseudoislets. Int. J. Pharm. 2019, 560, 65–77. [Google Scholar] [CrossRef]
- Delcassian, D.; Luzhansky, I.; Spanoudaki, V.; Bochenek, M.; McGladrigan, C.; Nguyen, A.; Norcross, S.; Zhu, Y.; Shan, C.S.; Hausser, R.; et al. Magnetic Retrieval of Encapsulated Beta Cell Transplants from Diabetic Mice Using Dual-Function MRI Visible and Retrievable Microcapsules. Adv. Mater. 2020, 32, e1904502. [Google Scholar] [CrossRef]
- Wang, P.; Yigit, M.V.; Medarova, Z.; Wei, L.; Dai, G.; Schuetz, C.; Moore, A. Combined small interfering RNA therapy and in vivo magnetic resonance imaging in islet transplantation. Diabetes 2011, 60, 565–571. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pomposelli, T.; Wang, P.; Takeuchi, K.; Miyake, K.; Ariyoshi, Y.; Watanabe, H.; Chen, X.; Shimizu, A.; Robertson, N.; Yamada, K.; et al. Protection of Pancreatic Islets Using Theranostic Silencing Nanoparticles in a Baboon Model of Islet Transplantation. Diabetes 2020, 69, 2414–2422. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Kim, M.J.; Lee, D.Y. MRI-sensitive contrast agent with anticoagulant activity for surface camouflage of transplanted pancreatic islets. Biomaterials 2017, 138, 121–130. [Google Scholar] [CrossRef]
- Peng, C.; Li, Z.; Yu, X. The Role of Pancreatic Infiltrating Innate Immune Cells in Acute Pancreatitis. Int. J. Med. Sci. 2021, 18, 534–545. [Google Scholar] [CrossRef]
- Dang, S.C.; Zeng, Y.H.; Wang, P.J.; Chen, B.D.; Chen, R.F.; Kumar Singh, A.; Kumar, P.; Feng, S.; Cui, L.; Wang, H.; et al. Clodronate-superparamagnetic iron oxide-containing liposomes attenuate renal injury in rats with severe acute pancreatitis. J. Zhejiang Univ. Sci. B 2014, 15, 556–565. [Google Scholar] [CrossRef]
- Lee, H.J.; An, J.; Doo, S.W.; Kim, J.H.; Choi, S.S.; Lee, S.R.; Park, S.W.; Song, Y.S.; Kim, S.U. Improvement in Spinal Cord Injury-Induced Bladder Fibrosis Using Mesenchymal Stem Cell Transplantation Into the Bladder Wall. Cell Transplant. 2015, 24, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Chun, S.Y.; Lee, J.K.; Lim, H.J.; Bae, J.S.; Chung, H.Y.; Atala, A.; Soker, S.; Yoo, J.J.; Kwon, T.G. Human amniotic fluid stem cell injection therapy for urethral sphincter regeneration in an animal model. BMC Med. 2012, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Won, J.H.; Doo, S.H.; Kim, J.H.; Song, K.Y.; Lee, S.J.; Lim, I.; Chang, K.T.; Song, Y.S.; Kim, S.U. Inhibition of collagen deposit in obstructed rat bladder outlet by transplantation of superparamagnetic iron oxide-labeled human mesenchymal stem cells as monitored by molecular magnetic resonance imaging (MRI). Cell Transplant. 2012, 21, 959–970. [Google Scholar] [CrossRef]
- Yudintceva, N.M.; Bogolyubova, I.O.; Muraviov, A.N.; Sheykhov, M.G.; Vinogradova, T.I.; Sokolovich, E.G.; Samusenko, I.A.; Shevtsov, M.A. Application of the allogenic mesenchymal stem cells in the therapy of the bladder tuberculosis. J. Tissue Eng. Regen. Med. 2018, 12, e1580–e1593. [Google Scholar] [CrossRef]
- Riviere, C.; Lecoeur, C.; Wilhelm, C.; Pechoux, C.; Combrisson, H.; Yiou, R.; Gazeau, F. The MRI assessment of intraurethrally--delivered muscle precursor cells using anionic magnetic nanoparticles. Biomaterials 2009, 30, 6920–6928. [Google Scholar] [CrossRef]
- Sadahide, K.; Teishima, J.; Inoue, S.; Tamura, T.; Kamei, N.; Adachi, N.; Matsubara, A. Endoscopic repair of the urinary bladder with magnetically labeled mesenchymal stem cells: Preliminary report. Regen. Ther. 2019, 10, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, S.; Yang, R.; Rahman, M.; Sequeira, R.C.; Cao, N.; Zhang, Y.; Zhao, W.; Fu, Q. Magnetic targeting of super-paramagnetic iron oxide nanoparticle labeled myogenic-induced adipose-derived stem cells in a rat model of stress urinary incontinence. Nanomedicine 2020, 30, 102281. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Duan, M.; Rahman, M.; Yang, M.; Zhao, W.; Zhou, S.; Gao, G.; Fu, Q. Use of bioactive extracellular matrix fragments as a urethral bulking agent to treat stress urinary incontinence. Acta Biomater. 2020, 117, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Yudintceva, N.M.; Nashchekina, Y.A.; Blinova, M.I.; Orlova, N.V.; Muraviov, A.N.; Vinogradova, T.I.; Sheykhov, M.G.; Shapkova, E.Y.; Emeljannikov, D.V.; Yablonskii, P.K.; et al. Experimental bladder regeneration using a poly-l-lactide/silk fibroin scaffold seeded with nanoparticle-labeled allogenic bone marrow stromal cells. Int. J. Nanomed. 2016, 11, 4521–4533. [Google Scholar] [CrossRef]
- Yudintceva, N.M.; Nashchekina, Y.A.; Mikhailova, N.A.; Vinogradova, T.I.; Yablonsky, P.K.; Gorelova, A.A.; Muraviov, A.N.; Gorelov, A.V.; Samusenko, I.A.; Nikolaev, B.P.; et al. Urethroplasty with a bilayered poly-D,L-lactide-co-epsilon-caprolactone scaffold seeded with allogenic mesenchymal stem cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1010–1021. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, S.; Yang, R.; Zou, Q.; Zhang, K.; Tian, Q.; Zhao, W.; Zong, L.; Fu, Q. Bioengineered bladder patches constructed from multilayered adipose-derived stem cell sheets for bladder regeneration. Acta Biomater. 2019, 85, 131–141. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, R.; Zou, Q.; Zhang, K.; Yin, T.; Zhao, W.; Shapter, J.G.; Gao, G.; Fu, Q. Fabrication of Tissue-Engineered Bionic Urethra Using Cell Sheet Technology and Labeling By Ultrasmall Superparamagnetic Iron Oxide for Full-Thickness Urethral Reconstruction. Theranostics 2017, 7, 2509–2523. [Google Scholar] [CrossRef]
- Ito, A.; Ino, K.; Hayashida, M.; Kobayashi, T.; Matsunuma, H.; Kagami, H.; Ueda, M.; Honda, H. Novel methodology for fabrication of tissue-engineered tubular constructs using magnetite nanoparticles and magnetic force. Tissue Eng. 2005, 11, 1553–1561. [Google Scholar] [CrossRef]
- Ge, L.; Chen, S. Recent Advances in Tissue Adhesives for Clinical Medicine. Polymers 2020, 12, 939. [Google Scholar] [CrossRef]
- Duarte, A.P.; Coelho, J.F.; Bordado, J.C.; Cidade, M.T.; Gil, M.H. Surgical adhesives: Systematic review of the main types and development forecast. Prog. Polym. Sci. 2012, 37, 1031–1050. [Google Scholar] [CrossRef]
- Meddahi-Pelle, A.; Legrand, A.; Marcellan, A.; Louedec, L.; Letourneur, D.; Leibler, L. Organ repair, hemostasis, and in vivo bonding of medical devices by aqueous solutions of nanoparticles. Angew. Chem. Int. Ed. Engl. 2014, 53, 6369–6373. [Google Scholar] [CrossRef] [PubMed]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Nanomaterials for Wound Dressings: An Up-to-Date Overview. Molecules 2020, 25, 2699. [Google Scholar] [CrossRef]
- Naskar, A.; Kim, K.S. Recent Advances in Nanomaterial-Based Wound-Healing Therapeutics. Pharmaceutics 2020, 12, 499. [Google Scholar] [CrossRef]
- Matter, M.T.; Starsich, F.; Galli, M.; Hilber, M.; Schlegel, A.A.; Bertazzo, S.; Pratsinis, S.E.; Herrmann, I.K. Developing a tissue glue by engineering the adhesive and hemostatic properties of metal oxide nanoparticles. Nanoscale 2017, 9, 8418–8426. [Google Scholar] [CrossRef]
- Wang, Z.; Brown, A.; Andre, P.; Brown, S.I.; Florence, G.J.; Cuschieri, A. Magnetic retraction of bowel by intraluminal injectable cyanoacrylate-based magnetic glue. Biomed. Res. Int. 2013, 2013, 526512. [Google Scholar] [CrossRef]
- Pai, B.G.; Kulkarni, A.V.; Jain, S. Study of smart antibacterial PCL-xFe3O4 thin films using mouse NIH-3T3 fibroblast cells in vitro. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 795–804. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Holban, A.M.; Andronescu, E.; Mogosanu, G.D.; Vasile, B.S.; Chifiriuc, M.C.; Lazar, V.; Andrei, E.; Constantinescu, A.; Maniu, H. Anionic polymers and 10 nm Fe(3)O(4)@UA wound dressings support human foetal stem cells normal development and exhibit great antimicrobial properties. Int. J. Pharm. 2014, 463, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Bunea, M.C.; Vasile, E.; Galateanu, B.; Hudita, A.; Serban, M.; Zaharia, C. Silk Fibroin Films Decorated with Magnetic Nanoparticles for Wound Healling Applications. Mater. Plast. 2017, 54, 83–87. [Google Scholar] [CrossRef]
- Anghel, I.; Holban, A.M.; Grumezescu, A.M.; Andronescu, E.; Ficai, A.; Anghel, A.G.; Maganu, M.; Laz, R.V.; Chifiriuc, M.C. Modified wound dressing with phyto-nanostructured coating to prevent staphylococcal and pseudomonal biofilm development. Nanoscale Res. Lett. 2012, 7, 690. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, J.; Wu, Q.; An, Y.; Wang, K.; Xuan, T.; Zhang, J.; Song, W.; He, H.; Song, L.; et al. Mussel-Inspired Surface Immobilization of Heparin on Magnetic Nanoparticles for Enhanced Wound Repair via Sustained Release of a Growth Factor and M2 Macrophage Polarization. ACS Appl. Mater. Interfaces 2021, 13, 2230–2244. [Google Scholar] [CrossRef]
- Li, X.; Wei, Z.; Zhang, W.; Lv, H.; Li, J.; Wu, L.; Zhang, H.; Yang, B.; Zhu, M.; Jiang, J. Anti-Inflammatory Effects of Magnetically Targeted Mesenchymal Stem Cells on Laser-Induced Skin Injuries in Rats. Int. J. Nanomed. 2020, 15, 5645–5659. [Google Scholar] [CrossRef]
- Wu, D.; Kang, L.; Tian, J.; Wu, Y.; Liu, J.; Li, Z.; Wu, X.; Huang, Y.; Gao, B.; Wang, H.; et al. Exosomes Derived from Bone Mesenchymal Stem Cells with the Stimulation of Fe3O4 Nanoparticles and Static Magnetic Field Enhance Wound Healing Through Upregulated miR-21-5p. Int. J. Nanomed. 2020, 15, 7979–7993. [Google Scholar] [CrossRef]
- Heun, Y.; Pogoda, K.; Anton, M.; Pircher, J.; Pfeifer, A.; Woernle, M.; Ribeiro, A.; Kameritsch, P.; Mykhaylyk, O.; Plank, C.; et al. HIF-1alpha Dependent Wound Healing Angiogenesis In Vivo Can Be Controlled by Site-Specific Lentiviral Magnetic Targeting of SHP-2. Mol. Ther. 2017, 25, 1616–1627. [Google Scholar] [CrossRef]
- Ito, A.; Kamihira, M. Tissue engineering using magnetite nanoparticles. Prog. Mol. Biol. Transl. Sci. 2011, 104, 355–395. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, J.; Pang, X.; Zhao, M.; Wang, B.; Yang, L.; Wan, H.; Wu, J.; Fu, S. Magnetic nanoparticle-loaded electrospun polymeric nanofibers for tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 537–543. [Google Scholar] [CrossRef]
- Paun, I.A.; Mustaciosu, C.C.; Mihailescu, M.; Calin, B.S.; Sandu, A.M. Magnetically-driven 2D cells organization on superparamagnetic micromagnets fabricated by laser direct writing. Sci. Rep. 2020, 10, 16418. [Google Scholar] [CrossRef]
- Ngadiman, N.H.; Idris, A.; Irfan, M.; Kurniawan, D.; Yusof, N.M.; Nasiri, R. gamma-Fe2O3 nanoparticles filled polyvinyl alcohol as potential biomaterial for tissue engineering scaffold. J. Mech. Behav. Biomed. Mater. 2015, 49, 90–104. [Google Scholar] [CrossRef]
- Farr, A.C.; Hogan, K.J.; Mikos, A.G. Nanomaterial Additives for Fabrication of Stimuli-Responsive Skeletal Muscle Tissue Engineering Constructs. Adv. Healthc. Mater. 2020, 9, 2000730. [Google Scholar] [CrossRef] [PubMed]
- Odintsov, B.; Chun, J.L.; Mulligan, J.A.; Berry, S.E. 14.1 T whole body MRI for detection of mesoangioblast stem cells in a murine model of Duchenne muscular dystrophy. Magn. Reson. Med. 2011, 66, 1704–1714. [Google Scholar] [CrossRef]
- Qin, J.; Li, K.; Peng, C.; Li, X.; Lin, J.; Ye, K.; Yang, X.; Xie, Q.; Shen, Z.; Jin, Y.; et al. MRI of iron oxide nanoparticle-labeled ADSCs in a model of hindlimb ischemia. Biomaterials 2013, 34, 4914–4925. [Google Scholar] [CrossRef]
- Winkler, T.; von Roth, P.; Schuman, M.R.; Sieland, K.; Stoltenburg-Didinger, G.; Taupitz, M.; Perka, C.; Duda, G.N.; Matziolis, G. In vivo visualization of locally transplanted mesenchymal stem cells in the severely injured muscle in rats. Tissue Eng. Part A 2008, 14, 1149–1160. [Google Scholar] [CrossRef]
- Farini, A.; Villa, C.; Manescu, A.; Fiori, F.; Giuliani, A.; Razini, P.; Sitzia, C.; Del Fraro, G.; Belicchi, M.; Meregalli, M.; et al. Novel insight into stem cell trafficking in dystrophic muscles. Int. J. Nanomed. 2012, 7, 3059–3067. [Google Scholar] [CrossRef][Green Version]
- Plair, A.; Bennington, J.; Williams, J.K.; Parker-Autry, C.; Matthews, C.A.; Badlani, G. Regenerative medicine for anal incontinence: A review of regenerative therapies beyond cells. Int. Urogynecol. J. 2020, 32, 2337–2347. [Google Scholar] [CrossRef]
- Elmi, A.; Kajbafzadeh, A.M.; Oghabian, M.A.; Talab, S.S.; Tourchi, A.; Khoei, S.; Rafie, B.; Esfahani, S.A. Anal sphincter repair with muscle progenitor cell transplantation: Serial assessment with iron oxide-enhanced MRI. AJR Am. J. Roentgenol. 2014, 202, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.Y.; Cheng, W.Y.; Yeh, Y.C.; Lai, C.H.; Hwang, S.M.; Hsiao, C.W.; Huang, C.W.; Chen, M.C.; Sung, H.W. Magnetically directed self-assembly of electrospun superparamagnetic fibrous bundles to form three-dimensional tissues with a highly ordered architecture. Tissue Eng. Part C Methods 2011, 17, 651–661. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Ito, A.; Kato, M.; Kawabe, Y.; Shimizu, K.; Fujita, H.; Nagamori, E.; Kamihira, M. Preparation of artificial skeletal muscle tissues by a magnetic force-based tissue engineering technique. J. Biosci. Bioeng. 2009, 108, 538–543. [Google Scholar] [CrossRef]
- Jia, Y.; Fan, M.; Chen, H.; Miao, Y.; Xing, L.; Jiang, B.; Cheng, Q.; Liu, D.; Bao, W.; Qian, B.; et al. Magnetic hyaluronic acid nanospheres via aqueous Diels-Alder chemistry to deliver dexamethasone for adipose tissue engineering. J. Colloid Interface Sci. 2015, 458, 293–299. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Yue, Y.; Sun, J.; Cui, L. Reconstruction of epidural fat with engineered adipose tissue from adipose derived stem cells and PLGA in the rabbit dorsal laminectomy model. Biomaterials 2012, 33, 6965–6973. [Google Scholar] [CrossRef]
- Tseng, H.; Daquinag, A.C.; Souza, G.R.; Kolonin, M.G. Three-Dimensional Magnetic Levitation Culture System Simulating White Adipose Tissue. Methods Mol. Biol. 2018, 1773, 147–154. [Google Scholar] [CrossRef]
- Daquinag, A.C.; Souza, G.R.; Kolonin, M.G. Adipose tissue engineering in three-dimensional levitation tissue culture system based on magnetic nanoparticles. Tissue Eng. Part C Methods 2013, 19, 336–344. [Google Scholar] [CrossRef]
- Silva, L.H.; da Silva, J.R.; Ferreira, G.A.; Silva, R.C.; Lima, E.C.; Azevedo, R.B.; Oliveira, D.M. Labeling mesenchymal cells with DMSA-coated gold and iron oxide nanoparticles: Assessment of biocompatibility and potential applications. J. Nanobiotechnol. 2016, 14, 59. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Elbaz, N.M.; Sedki, M.; Elgammal, A.; Yacoub, M.H. Magnetic nanoparticles-based drug and gene delivery systems for the treatment of pulmonary diseases. Nanomedicine 2017, 12, 387–402. [Google Scholar] [CrossRef]
- Omlor, A.J.; Nguyen, J.; Bals, R.; Dinh, Q.T. Nanotechnology in respiratory medicine. Respir. Res. 2015, 16, 64. [Google Scholar] [CrossRef]
- Ordidge, K.L.; Duffy, B.A.; Wells, J.A.; Kalber, T.L.; Janes, S.M.; Lythgoe, M.F. Imaging the paediatric lung: What does nanotechnology have to offer? Paediatr. Respir. Rev. 2012, 13, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Azarmi, S.; Roa, W.H.; Lobenberg, R. Targeted delivery of nanoparticles for the treatment of lung diseases. Adv. Drug Deliv. Rev. 2008, 60, 863–875. [Google Scholar] [CrossRef]
- Brandt, Y.; Armijo, L.; Rivera, A.; Plumley, J.; Cook, N.; Smolyakov, G.; Smyth, H.; Osiński, M. Effectiveness of tobramycin conjugated to iron oxide nanoparticles in treating infection in cystic fibrosis. Colloid. Nanocryst. Biomed. Appl. VIII 2013, 8595, 85951C. [Google Scholar]
- Plank, C. Nanomagnetosols: Magnetism opens up new perspectives for targeted aerosol delivery to the lung. Trends Biotechnol. 2008, 26, 59–63. [Google Scholar] [CrossRef]
- Dzamukova, M.R.; Naumenko, E.A.; Lannik, N.I.; Fakhrullin, R.F. Surface-modified magnetic human cells for scaffold-free tissue engineering. Biomater. Sci. 2013, 1, 810–813. [Google Scholar] [CrossRef]
- Gao, Y.; Lim, J.; Teoh, S.H.; Xu, C. Emerging translational research on magnetic nanoparticles for regenerative medicine. Chem. Soc. Rev. 2015, 44, 6306–6329. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Aberg, C.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012, 7, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Janko, C.; Munoz, L.; Chaurio, R.; Maueroder, C.; Berens, C.; Lauber, K.; Herrmann, M. Navigation to the graveyard-induction of various pathways of necrosis and their classification by flow cytometry. Methods Mol. Biol. 2013, 1004, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Poller, J.M.; Zaloga, J.; Schreiber, E.; Unterweger, H.; Janko, C.; Radon, P.; Eberbeck, D.; Trahms, L.; Alexiou, C.; Friedrich, R.P. Selection of potential iron oxide nanoparticles for breast cancer treatment based on in vitro cytotoxicity and cellular uptake. Int. J. Nanomed. 2017, 12, 3207–3220. [Google Scholar] [CrossRef]
- Janko, C.; Pottler, M.; Matuszak, J.; Unterweger, H.; Hornung, A.; Friedrich, R.P.; Alexiou, C. Innovative Toxicological Investigation Methods for Iron Oxide Nanoparticles in Nanomedicine. Chem. Ing. Tech. 2017, 89, 244–251. [Google Scholar] [CrossRef]
- Lugert, S.; Unterweger, H.; Muhlberger, M.; Janko, C.; Draack, S.; Ludwig, F.; Eberbeck, D.; Alexiou, C.; Friedrich, R.P. Cellular effects of paclitaxel-loaded iron oxide nanoparticles on breast cancer using different 2D and 3D cell culture models. Int. J. Nanomed. 2019, 14, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.M.; Kaplan, B.; Kaufman, D. Toxic effects of immunosuppressive drugs: Mechanisms and strategies for controlling them. Clin. Chem. 1996, 42, 1316–1321. [Google Scholar] [CrossRef]
- Barnett, B.P.; Arepally, A.; Stuber, M.; Arifin, D.R.; Kraitchman, D.L.; Bulte, J.W. Synthesis of magnetic resonance-, X-ray- and ultrasound-visible alginate microcapsules for immunoisolation and noninvasive imaging of cellular therapeutics. Nat. Protoc. 2011, 6, 1142–1151. [Google Scholar] [CrossRef]
- Barnett, B.P.; Arepally, A.; Karmarkar, P.V.; Qian, D.; Gilson, W.D.; Walczak, P.; Howland, V.; Lawler, L.; Lauzon, C.; Stuber, M.; et al. Magnetic resonance-guided, real-time targeted delivery and imaging of magnetocapsules immunoprotecting pancreatic islet cells. Nat. Med. 2007, 13, 986–991. [Google Scholar] [CrossRef]
- Köhler, T.; Feoktystov, A.; Petracic, O.; Kentzinger, E.; Bhatnagar-Schöffmann, T.; Feygenson, M.; Nandakumaran, N.; Landers, J.; Wende, H.; Cervellino, A.; et al. Mechanism of magnetization reduction in iron oxide nanoparticles. Nanoscale 2021, 13, 6965–6976. [Google Scholar] [CrossRef]
- Hirota, Y.; Chuzawa, M.; Mishima, F.; Akiyama, Y.; Nishijima, S. Development of Magnetic Drug Delivery System Using Superconducting Bulk Magnet. TEION KOGAKU J. Cryog. Supercond. Soc. Jpn. 2010, 45, 298–303. [Google Scholar] [CrossRef]
- Nacev, A.; Komaee, A.; Sarwar, A.; Probst, R.; Kim, S.; Emmert-Buck, M.; Shapiro, B. Towards Control of Magnetic Fluids in Patients: Directing Therapeutic Nanoparticles to Disease Locations. IEEE Control Syst. Mag. 2012, 32, 32–74. [Google Scholar] [CrossRef]
- Lyer, S.; Tietze, R.; Unterweger, H.; Zaloga, J.; Singh, R.; Matuszak, J.; Poettler, M.; Friedrich, R.P.; Duerr, S.; Cicha, I.; et al. Nanomedical innovation: The SEON-concept for an improved cancer therapy with magnetic nanoparticles. Nanomedicine 2015, 10, 3287–3304. [Google Scholar] [CrossRef] [PubMed]
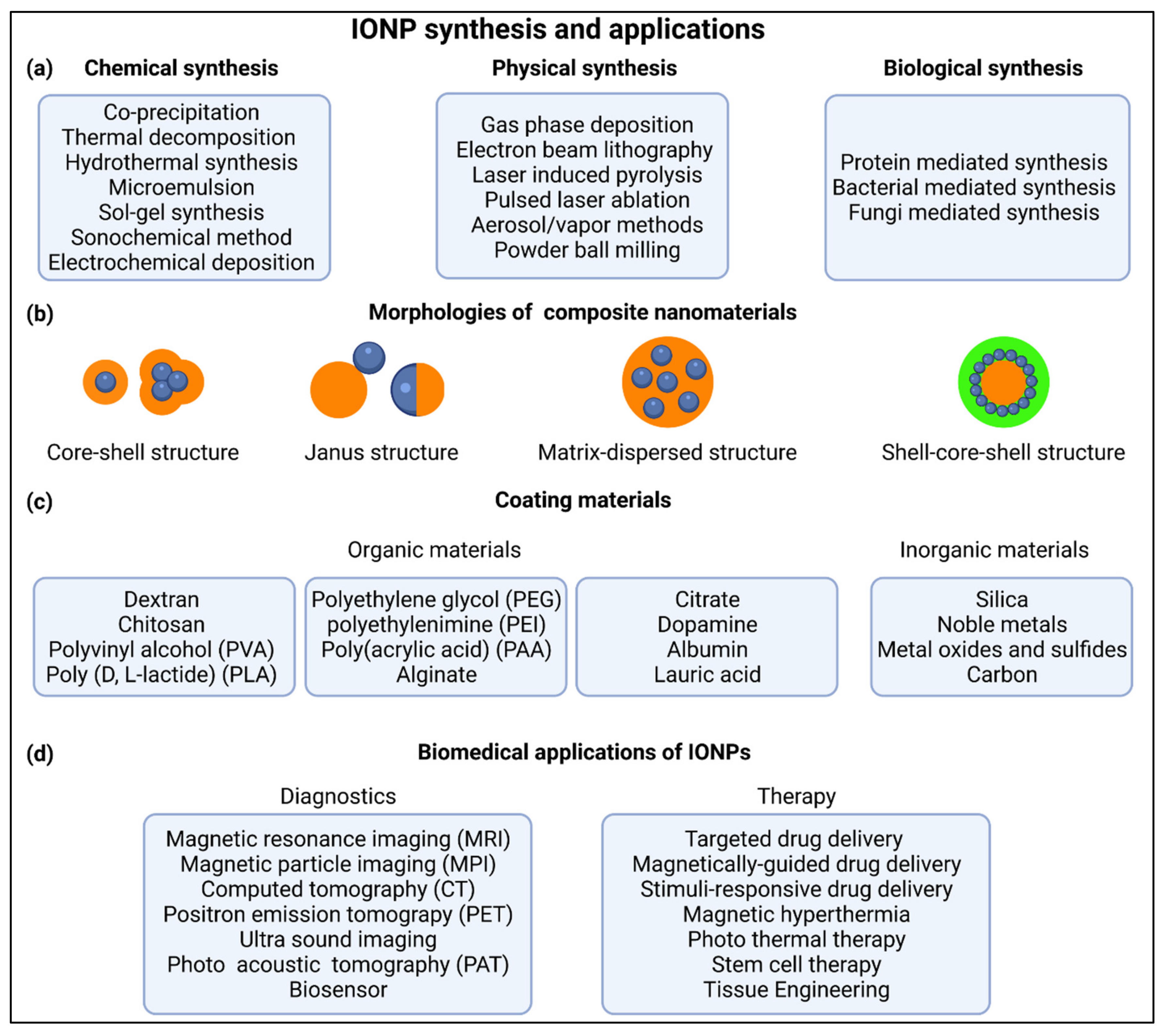

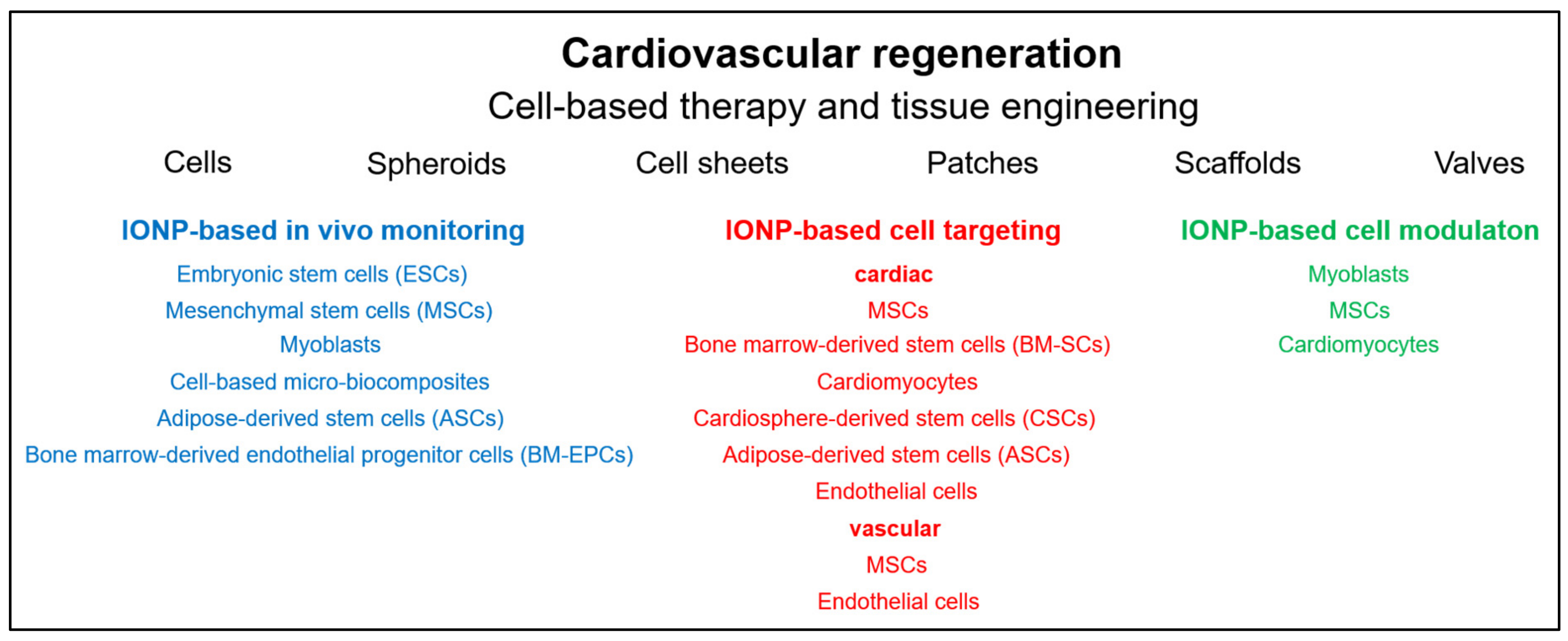
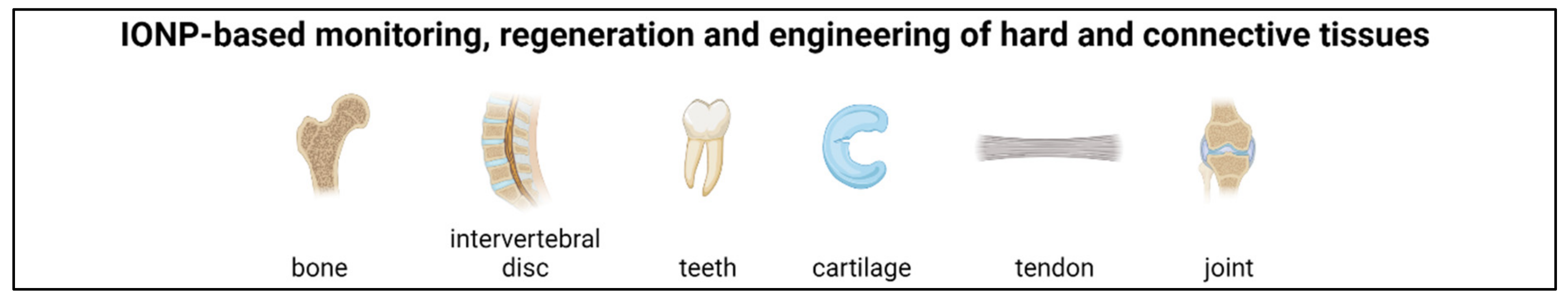
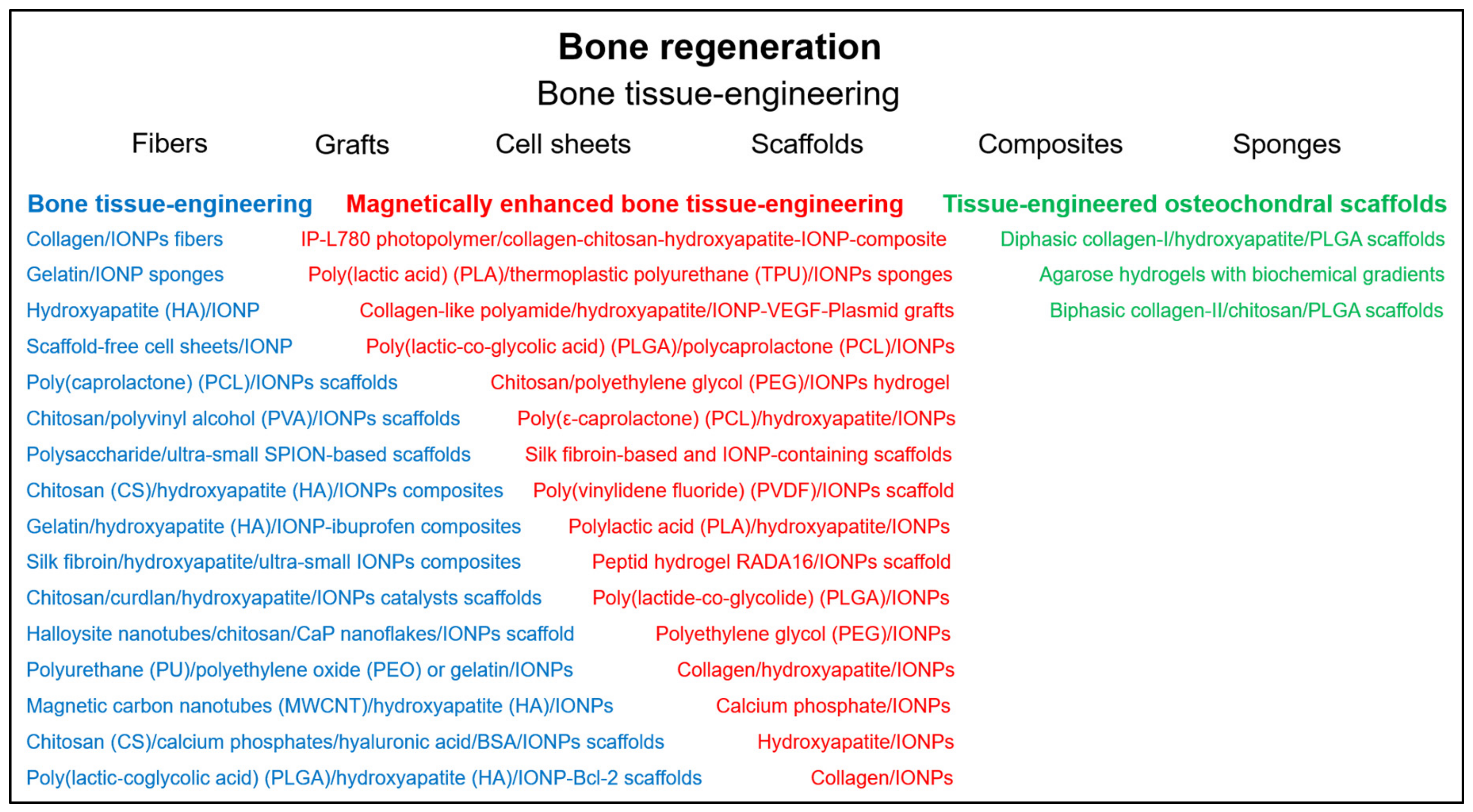
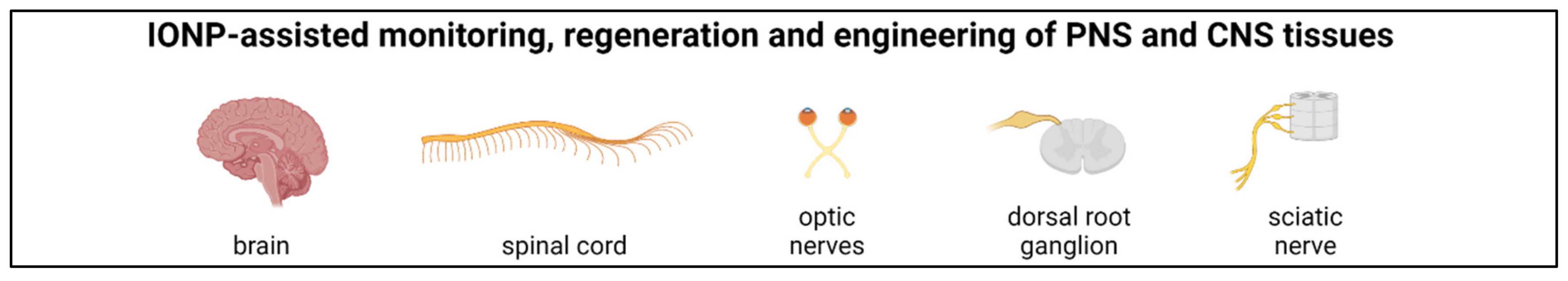
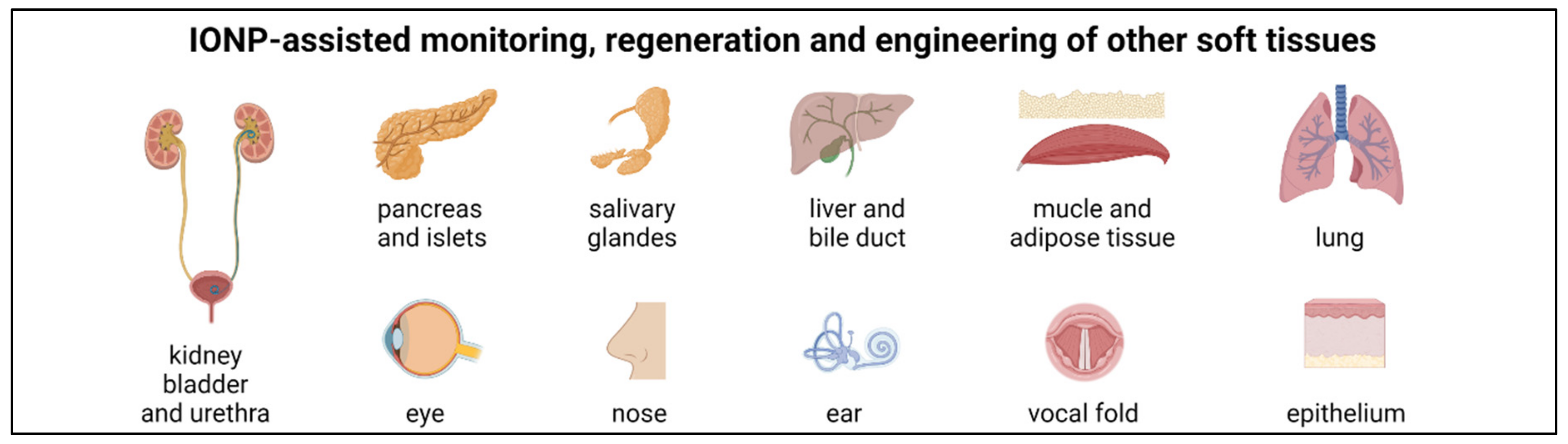
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friedrich, R.P.; Cicha, I.; Alexiou, C. Iron Oxide Nanoparticles in Regenerative Medicine and Tissue Engineering. Nanomaterials 2021, 11, 2337. https://doi.org/10.3390/nano11092337
Friedrich RP, Cicha I, Alexiou C. Iron Oxide Nanoparticles in Regenerative Medicine and Tissue Engineering. Nanomaterials. 2021; 11(9):2337. https://doi.org/10.3390/nano11092337
Chicago/Turabian StyleFriedrich, Ralf P., Iwona Cicha, and Christoph Alexiou. 2021. "Iron Oxide Nanoparticles in Regenerative Medicine and Tissue Engineering" Nanomaterials 11, no. 9: 2337. https://doi.org/10.3390/nano11092337
APA StyleFriedrich, R. P., Cicha, I., & Alexiou, C. (2021). Iron Oxide Nanoparticles in Regenerative Medicine and Tissue Engineering. Nanomaterials, 11(9), 2337. https://doi.org/10.3390/nano11092337