Modulation of Cell Behavior by 3D Biocompatible Hydrogel Microscaffolds with Precise Configuration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation of Hydrogel
2.2. Fabrication of Different Microstructures
2.3. Cell Culture
2.4. Laser Scanning Confocal Microscope (LSCM) and Scanning Electron Microscopy (SEM) Characterization
2.5. Mechanical Performance
3. Results and Discussion
3.1. Processing Performance of Hydrogel-Based Photoresist
3.2. Mechanical Properties
3.3. Design and Fabrication of Microstructures by TPP
3.4. Modulation of Cell Behavior on Different Microstructures
3.5. Cell Behavior on a Series of 3D Microscaffolds
3.5.1. The Effect of Porosity of 3D Microscaffolds on F-Actin
3.5.2. The Influence of 3D Microscaffold on Cell Distribution
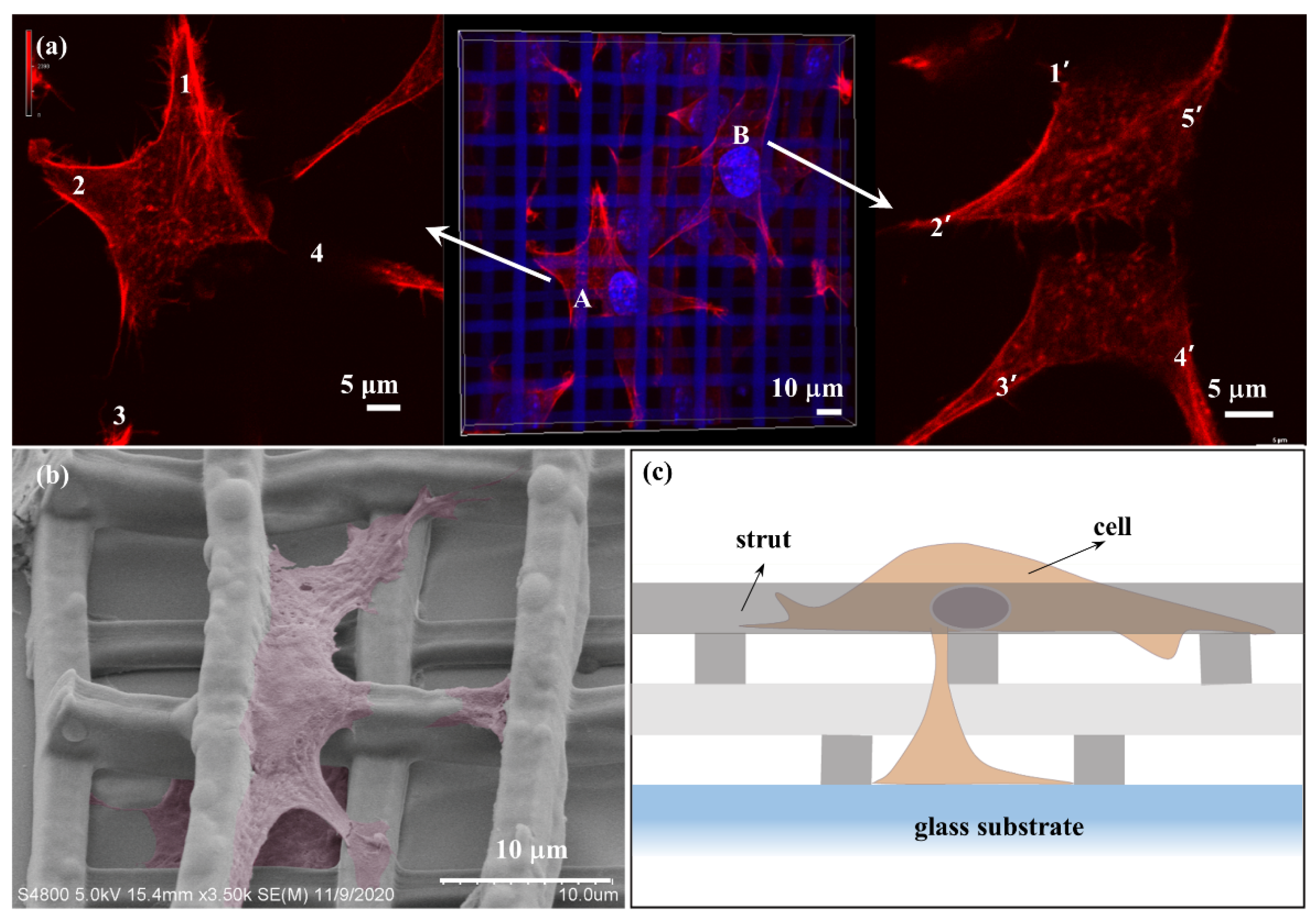
3.6. Cell–Cell and Cell–Scaffold Interactions on 3D Microscaffold
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stappenbeck, T.S.; Miyoshi, H. The Role of Stromal Stem Cells in Tissue Regeneration and Wound Repair. Science 2009, 324, 1666–1669. [Google Scholar] [CrossRef] [Green Version]
- Scotti, C.; Tonnarelli, B.; Papadimitropoulos, A.; Scherberich, A.; Schaeren, S.; Schauerte, A.; Lopez-Rios, J.; Zeller, R.; Barbero, A.; Martin, I. Recapitulation of Endochondral Bone Formation using Human Adult Mesenchymal Stem Cells as a Paradigm for Developmental Engineering. Proc. Natl. Acad. Sci. USA 2010, 107, 7251–7256. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.G.; Dan, B.; Cheng, C.; Ma, S.; Liu, Y.; Cheng, K. Bone Morphogenetic Protein Enhances the Osteogenic Differentiation of Human Dermal-derived CD105+ Fibroblast Cells through the Smad and MAPK Pathways. Int. J. Mol. Med. 2019, 43, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Romana-Ouza, B.; Chen, L.; Leonardo, T.R.; Chen, Z.; DiPietro, L.A. Dermal Fibroblast Phagocytosis of Apoptotic Cells: A Novel Pathway for Wound Resolution. FASEB J. 2021, 35, e21443. [Google Scholar]
- Raimondi, M.T.; Eaton, S.M.; Laganà, M.; Aprile, V.; Nava, M.M.; Cerullo, G.; Osellame, R. Three-Dimensional Structural Niches Engineered via Two-Photon Laser Polymerization Promote Stem Cell Homing. Acta Biomater. 2013, 9, 4579–4584. [Google Scholar] [CrossRef]
- Führmann, T.; Hillen, L.M.; Montzka, K.; Wöltje, M.; Brook, G.A. Cell-Cell Interactions of Human Neural Progenitor-Derived Astrocytes Within a Microstructured 3D-scaffold. Biomaterials 2010, 31, 7705–7715. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Miller, A.L.; Park, S.; George, M.N.; Waletzki, B.E.; Xu, H.; Terzic, A.; Lu, L. Two-Dimensional Black Phosphorus and Graphene Oxide Nanosheets Synergistically Enhance Cell Proliferation and Osteogenesis on 3D Printed Scaffolds. ACS Appl. Mater. Interfaces 2019, 11, 23558–23572. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.P.; Chavali, M.S. Recent Advances in Biomaterials for 3D Scaffolds: A Review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Li, J.Y.; Li, X.J.; Luo, T.; Wang, R.; Liu, C.; Chen, S.; Li, D.; Yue, J.; Cheng, S.H.; Sun, D. Development of a Magnetic Microrobot for Carrying and Delivering Targeted Cells. Sci. Robot. 2018, 3, eaat8829. [Google Scholar] [CrossRef] [Green Version]
- Kolanthai, E.; Sindu, P.A.; Khajuria, D.K.; Veerla, S.C.; Kuppuswamy, D.; Catalani, L.H.; Mahapatra, D.R. Graphene Oxide—A Tool for the Preparation of Chemically Crosslinking Free Alginate–Chitosan–Collagen Scaffolds for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2018, 10, 12441–12452. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Xun, X.; Zhang, W.; Xu, Y.; Gu, D. Three-Dimensional Porous Scaffolds with Biomimetic Microarchitecture and Bioactivity for Cartilage Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 36359–36370. [Google Scholar] [CrossRef]
- Jiang, S.M.; Lyu, C.; Zhao, P.; Li, W.; Kong, W.; Huang, C.; Genin, G.M.; Du, Y. Cryoprotectant Enables Structural Control of Porous Scaffolds for Exploration of Cellular Mechano-Responsiveness in 3D. Nat. Commun. 2019, 10, 3491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nava, M.M.; Raimondi, M.T.; Credi, C.; De Marco, C.; Turri, S.; Cerullo, G.; Osellame, R. Interactions between Structural and Chemical Biomimetism in Synthetic Stem Cell Niches. Biomed Mater. 2015, 10, 015012. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-B.; Liu, Y.L.; Wang, W.-J.; Zhang, H.-W.; Qin, Y.; Guo, S.; Zhang, X.-W.; Fu, L.; Huang, W.-H. Biomimetic Graphene-Based 3D Scaffold for Long-Term Cell Culture and Real-Time Electrochemical Monitoring. Anal. Chem. 2018, 90, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Naka, R.; Fujita, M.; Hara, M. Nylon Mesh-Based 3D Scaffolds for the Adherent Culture of Neural Stem/Progenitor Cells. J. Biosci. Bioeng. 2021, 131, 442–452. [Google Scholar] [CrossRef]
- Ahn, G.; Park, J.H.; Kang, T.; Lee, J.W.; Kang, H.W.; Cho, D.W. Effect of Pore Architecture on Oxygen Diffusion in 3D Scaffolds for Tissue Engineering. J. Biomech. Eng. 2010, 132, 104506. [Google Scholar] [CrossRef]
- Lewis, P.L.; Green, R.M.; Shah, R.N. 3D-Printed Gelatin Scaffolds of Differing Pore Geometry Modulate Hepatocyte Function and Gene Expression. Acta Biomater. 2018, 69, 63–70. [Google Scholar] [CrossRef]
- Barba, A.; Maazouz, Y.; Diez-Escudero, A.; Rappe, K.; Espanol, M.; Montufar, E.B.; Öhman-Mägi, C.; Persson, C.; Fontecha, P.; Manzanares, M.C.; et al. Osteogenesis by Foamed and 3D-printed Nanostructured Calcium Phosphate Scaffolds: Effect of Pore Architecture. Acta Biomater. 2018, 79, 135–147. [Google Scholar] [CrossRef]
- Neves, S.C.; Mota, C.; Longoni, A.; Barrias, C.C.; Granja, P.L.; Moroni, L. Additive Manufactured Polymeric 3D Scaffolds with Tailored Surface Topography Influence Mesenchymal Stromal Cells Activity. Biofabrication 2016, 8, 025012. [Google Scholar] [CrossRef]
- Chen, H.; Huang, X.; Zhang, M.; Damanik, F.; Baker, M.B.; Leferink, A.; Yuan, H. Tailoring Surface Nanoroughness of Electrospun Scaffolds for Skeletal Tissue Engineering. Acta Biomater. 2017, 59, 82–93. [Google Scholar] [CrossRef]
- Roh, H.S.; Jung, S.C.; Kook, M.S.; Kim, B.H. In Vitro Study of 3D PLGA/n-HAp/β-TCP Composite Scaffolds with Etched Oxygen Plasma Surface Modification in Bone Tissue Engineering. Appl. Surf. Sci. 2016, 388, 321–330. [Google Scholar] [CrossRef]
- Ren, X.; Yang, Q.; Yang, D.; Liang, Y.; Dong, J.; Ren, Y.; Lu, X.; Xue, L.; Li, L.; Xu, L. High-Strength Double Network Hydrogels as Potential Materials for Artificial 3D Scaffold of Cell Migration in Vitro. Colloid Surf. A 2018, 549, 50–57. [Google Scholar] [CrossRef]
- Gao, F.; Xu, Z.; Liang, Q.; Liu, B.; Li, H.; Wu, Y.; Zhang, Y.; Lin, Z.; Wu, M.; Ruan, C.; et al. Direct 3D Printing of High Strength Biohybrid Gradient Hydrogel Scaffolds for Efficient Repair of Osteochondral Defect. Adv. Funct. Mater. 2018, 28, 1706644. [Google Scholar] [CrossRef]
- Paolo, P.; Sonia, M.; Matteo, C.; Roberto, F.; Federico, M.; Fabio, D.E.; Paolo, D.I.; Slava, K.; Sigurd, S.; Mauro, C. Photolithography of 3D Scaffolds for Artificial Tissue. Mater. Sci. Forum 2016, 879, 1519–1523. [Google Scholar]
- Hsieh, T.M.; Ng, C.W.B.; Narayanan, K.; Wan, A.C.; Ying, J.Y. Three-Dimensional Microstructured Tissue Scaffolds Fabricated by Two-Photon Laser Scanning Photolithography. Biomaterials 2010, 31, 7648–7652. [Google Scholar] [CrossRef]
- Yu, H.; Liu, J.; Zhao, Y.Y.; Jin, F.; Dong, X.Z.; Zhao, Z.S.; Duan, X.M.; Zheng, M.L. Biocompatible Three-Dimensional Hydrogel Cell Scaffold Fabricated by Sodium Hyaluronate and Chitosan Assisted Two-Photon Polymerization. ACS Appl. Bio. Mater. 2019, 2, 3077–3083. [Google Scholar] [CrossRef]
- Mandal, B.B.; Kundu, S.C. Cell Proliferation and Migration in Silk Fibroin 3D scaffolds-Science Direct. Biomaterials 2009, 30, 2956–2965. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, Y.J. Fabrication of Strontium-Substituted Hydroxyapatite Scaffolds Using 3D Printing for Enhanced Bone Regeneration. J. Mater. Sci. 2021, 56, 1673–1684. [Google Scholar] [CrossRef]
- Melissinaki, V.; Gill, A.A.; Ortega, I.; Vamvakaki, M.; Ranella, A.; Haycock, J.W.; Fotakis, C.; Farsari, M.; Claeyssens, F. Direct Laser Writing of 3D Scaffolds for Neural Tissue Engineering Applications. Biofabrication 2013, 3, 045005. [Google Scholar] [CrossRef]
- Xing, J.F.; Zheng, M.L.; Duan, X.M. Two-photon Polymerization Microfabrication of Hydrogels: An Advanced 3D Printing Technology for Tissue Engineering and Drug Delivery. Chem. Soc. Rev. 2015, 44, 5031–5039. [Google Scholar] [CrossRef] [Green Version]
- Kawata, S.; Sun, H.B.; Tanaka, T.; Takada, K. Finer Features for Functional Microdevices. Nature 2001, 412, 697–698. [Google Scholar] [CrossRef]
- Gao, W.; Chao, H. Ionic Carbazole-Based Water-Soluble Two-Photon Photoinitiator and the Fabrication of Biocompatible 3D Hydrogel Scaffold. ACS Appl. Mater. Interfaces 2021, 13, 27796–27805. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Zhang, Y.L.; Zheng, Y.C.; Zhang, W.C.; Liu, J.; Jin, F.; Dong, X.Z.; Liu, Y.H.; Li, S.J.; Zheng, M.L. Three-dimensional Luneburg Lens at Optical Frequencies. Laser Photonics Rev. 2016, 10, 665–672. [Google Scholar] [CrossRef]
- Zheng, C.; Jin, F.; Zhao, Y.; Zheng, M.; Liu, J.; Dong, X.; Xiong, Z.; Xia, Y.; Duan, X. Light-driven Micron-scale 3D Hydrogel Actuator Produced by Two-photon Polymerization Microfabrication. Sens. Actuators B Chem. 2020, 304, 127345. [Google Scholar] [CrossRef]
- Zheng, Y.C.; Zhao, Y.Y.; Zheng, M.L.; Chen, S.L.; Liu, J.; Jin, F.; Dong, X.Z.; Zhao, Z.S.; Duan, X.M. Cucurbit[7]uril-carbazole Two-Photon Photoinitiators for the Fabrication of Biocompatible Three-dimensional Hydrogel Scaffolds by Laser Direct Writing in Aqueous Solutions. ACS Appl. Mater. Interfaces 2019, 11, 1782–1789. [Google Scholar] [CrossRef]
- Wei, S.; Liu, J.; Zhao, Y.; Zhang, T.; Zheng, M.; Jin, F.; Dong, X.; Xing, J.; Duan, X. Protein-Based 3D Microstructures with Controllable Morphology and pH-Responsive Properties. ACS Appl. Mater. Interfaces 2017, 9, 42247–42257. [Google Scholar] [CrossRef]
- Zhang, W.C.; Zheng, M.L. Research Progress of Two-Photon Initiator with High Efficiency and Preparation of Hydrogel Microstructure in Aqueous Phase. Chin. J. Las. 2021, 48, 0202007. [Google Scholar] [CrossRef]
- Park, S.; Kim, G.H.; Jeon, Y.C.; Koh, Y.; Kim, W. 3D Polycaprolactone Scaffolds with Controlled Pore Structure Using a Rapid Prototyping System. J. Mater. Sci. Mater. Med. 2009, 20, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Meka, S.R.K.; Chacko, L.A.; Ravi, A.; Chatterjee, K.; Ananthanarayanan, V. Role of Microtubules in Osteogenic Differentiation of Mesenchymal Stem Cells on 3D Nanofibrous Scaffolds. ACS Biomater.-Sci. Eng. 2017, 3, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Zhang, K.; Wu, J.; Wong, D.S.H.; Feng, Q.; Bian, L.; Zhang, A.P. Optical µ-Printing of Cellular-Scale Microscaffold Arrays for 3D Cell Culture. Sci. Rep. 2017, 7, 8880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, K.; Sun, Y.; Luo, Z.P. Flexibility of type I collagen and mechanical property of connective tissue. Biorheology 2004, 41, 239–246. [Google Scholar]
- Pan, L.; Zan, L.; Foster, F.S. Ultrasonic and viscoelastic properties of skin under transverse mechanical stress in vitro. Ultrasound. Med. Biol. 1998, 24, 995–1007. [Google Scholar] [CrossRef]
- Santos, E.; Hernández, R.M.; Pedraz, J.L.; Orive, G. Novel Advances in the Design of Three-Dimensional Bio-Scaffolds to Control Cell Fate: Translation from 2D to 3D. Trends Biotechnol. 2012, 30, 331–341. [Google Scholar] [CrossRef]
- Liu, P.; Chen, W.; Liu, C.; Tian, M.; Liu, P. A novel poly (vinyl alcohol)/poly (ethylene glycol) scaffold for tissue engineering with a unique bimodal open-celled structure fabricated using supercritical fluid foaming. Sci. Rep. 2019, 9, 9534. [Google Scholar] [CrossRef]
- Xiong, S.; Gao, H.; Qin, L.; Jia, Y.; Gao, M.; Ren, L. Microgrooved Collagen-Based Corneal Scaffold for Promoting Collective Cell Migration and Antifibrosis. RSC Adv. 2019, 9, 29463–29473. [Google Scholar] [CrossRef] [Green Version]
- Matschegewski, C.; Staehlke, S.; Loeffler, R.; Lange, R.; Chai, F.; Kern, D.P.; Beck, U.; Nebe, B.J. Cell Architecture-Cell Function Dependencies on Titanium Arrays with Regular Geometry. Biomaterials 2010, 31, 5729–5740. [Google Scholar] [CrossRef] [PubMed]
- Albuschies, J.; Vogel, V. The Role of Filopodia in the Recognition of Nanotopographies. Sci. Rep. 2013, 3, 1658. [Google Scholar] [CrossRef] [Green Version]
- Bornschlgl, T.; Romero, S.; Vestergaard, C.L.; Joanny, J.F.; Van Nhieu, G.T.; Bassereau, P. Filopodial Retraction Force is Generated by Cortical Actin Dynamics and Controlled by Reversible Tethering at the Tip. Proc. Natl. Acad. Sci. USA 2013, 110, 18928–18933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunami, H.; Yokota, I.; Igarashi, Y. Influence of the Pattern Size of Micropatterned Scaffolds on Cell Morphology, Proliferation, Migration and F-actin Expression. Biomater. Sci. 2014, 2, 399–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, M.C.; Sinha, R.; Mota, C.; Moroni, L. Improving Cell Distribution on 3D Additive Manufactured Scaffolds through Engineered Seeding Media Density and Viscosity. Acta Biomater. 2020, 101, 183–195. [Google Scholar] [CrossRef]
- Sachar, A.; Strom, T.A.; San Miguel, S.; Serrano, M.J.; Svoboda, K.K.; Liu, X. Cell-Matrix and Cell-Cell Interactions of Human Gingival Fibroblasts on Three-Dimensional Nanofibrous Gelatin Scaffolds. J. Tissue Eng. Regen. Med. 2014, 8, 862–873. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.-C.; Zheng, M.-L.; Liu, J.; Jin, F.; Dong, X.-Z.; Guo, M.; Li, T. Modulation of Cell Behavior by 3D Biocompatible Hydrogel Microscaffolds with Precise Configuration. Nanomaterials 2021, 11, 2325. https://doi.org/10.3390/nano11092325
Zhang W-C, Zheng M-L, Liu J, Jin F, Dong X-Z, Guo M, Li T. Modulation of Cell Behavior by 3D Biocompatible Hydrogel Microscaffolds with Precise Configuration. Nanomaterials. 2021; 11(9):2325. https://doi.org/10.3390/nano11092325
Chicago/Turabian StyleZhang, Wei-Cai, Mei-Ling Zheng, Jie Liu, Feng Jin, Xian-Zi Dong, Min Guo, and Teng Li. 2021. "Modulation of Cell Behavior by 3D Biocompatible Hydrogel Microscaffolds with Precise Configuration" Nanomaterials 11, no. 9: 2325. https://doi.org/10.3390/nano11092325
APA StyleZhang, W.-C., Zheng, M.-L., Liu, J., Jin, F., Dong, X.-Z., Guo, M., & Li, T. (2021). Modulation of Cell Behavior by 3D Biocompatible Hydrogel Microscaffolds with Precise Configuration. Nanomaterials, 11(9), 2325. https://doi.org/10.3390/nano11092325





