Effectiveness of Epoxy Coating Modified with Yttrium Oxide Loaded with Imidazole on the Corrosion Protection of Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Loading of Corrosion Inhibitor into Y2O3 Nanoparticles
2.3. Coating Formulation of Steel Substrate
- i.
- Reference coating (contained only Y2O3);
- ii.
- Modified coating (contained Y2O3/IMD).
2.4. Characterization of Nanoparticles and Coating Samples
3. Results and Discussion
3.1. Structural and Morphological Characterization
Additional Physico-Chemical Characterization
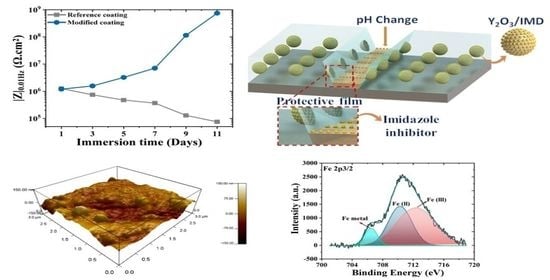
3.2. Corrosion Resistance of Coated Steel Samples
3.2.1. Electrochemical Impedance Spectroscopy (EIS)
3.2.2. XPS Analysis
3.3. Corrosion Inhibition Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montemor, M.F. Functional and smart coatings for corrosion protection: A review of recent advances. Surf. Coat. Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Lyon, S.B.; Bingham, R.; Mills, D.J. Advances in corrosion protection by organic coatings: What we know and what we would like to know. Prog. Org. Coat. 2017, 102, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Kartsonakis, I.A.; Athanasopoulou, E.; Snihirova, D.; Martins, B.; Koklioti, M.A.; Montemor, M.F.; Kordas, G.; Charitidis, C.A. Multifunctional epoxy coatings combining a mixture of traps and inhibitor loaded nanocontainers for corrosion protection of AA2024-T3. Corros. Sci. 2014, 85, 147–159. [Google Scholar] [CrossRef]
- Nawaz, M.; Yusuf, N.; Habib, S.; Shakoor, R.A.; Ubaid, F.; Ahmad, Z.; Kahraman, R.; Mansour, S.; Gao, W. Development and properties of polymeric nanocomposite coatings. Polymers 2019, 11, 852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawaz, M.; Habib, S.; Khan, A.; Shakoor, R.A.; Kahraman, R. Cellulose microfibers (CMFs) as a smart carrier for autonomous self-healing in epoxy coatings. New J. Chem. 2020, 44, 5702–5710. [Google Scholar] [CrossRef]
- Barnhart, J. Chromium chemistry and implications for environmental fate and toxicity. Soil Sediment Contam. 1997, 6, 561–568. [Google Scholar] [CrossRef]
- Hughes, A.E.; Cole, I.S.; Muster, T.H.; Varley, R.J. Designing green, self-healing coatings for metal protection. NPG Asia Mater. 2010, 2, 143–151. [Google Scholar] [CrossRef]
- Wan, H.; Song, D.; Li, X.; Zhang, D.; Gao, J.; Du, C. Effect of zinc phosphate on the corrosion behavior of waterborne acrylic coating/metal interface. Materials 2017, 10, 654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montemor, M.F. Hybrid Nanocontainer-Based Smart Self-Healing Composite Coatings for the Protection of Metallic Assets; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9781782422839. [Google Scholar]
- Morozov, Y.; Calado, L.M.; Shakoor, R.A.; Raj, R.; Kahraman, R.; Taryba, M.G.; Montemor, M.F. Epoxy coatings modified with a new cerium phosphate inhibitor for smart corrosion protection of steel. Corros. Sci. 2019, 159, 108128. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Shchukin, D.G.; Yasakau, K.A.; Möhwald, H.; Ferreira, M.G.S. Anticorrosion coatings with self-healing effect based on nanocontainers impregnated with corrosion inhibitor. Chem. Mater. 2007, 19, 402–411. [Google Scholar] [CrossRef]
- Zea, C.; Barranco-García, R.; Alcántara, J.; Simancas, J.; Morcillo, M.; de la Fuente, D. pH-dependent release of environmentally friendly corrosion inhibitor from mesoporous silica nanoreservoirs. Microporous Mesoporous Mater. 2018, 255, 166–173. [Google Scholar] [CrossRef]
- Raj, R.; Morozov, Y.; Calado, L.M.; Taryba, M.G.; Kahraman, R.; Shakoor, R.A.; Montemor, M.F. Calcium carbonate particles loaded with triethanolamine and polyethylenimine for enhanced corrosion protection of epoxy coated steel. Corros. Sci. 2020, 167, 108548. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Liu, J.; Ge, Y.; Yan, X.; Sun, Y.; Wu, J.; Zhang, P. GO-modified double-walled polyurea microcapsules/epoxy composites for marine anticorrosive self-healing coating. Mater. Des. 2020, 189, 108547. [Google Scholar] [CrossRef]
- Wen, J.; Lei, J.; Chen, J.; Gou, J.; Li, Y.; Li, L. An intelligent coating based on pH-sensitive hybrid hydrogel for corrosion protection of mild steel. Chem. Eng. J. 2020, 392, 123742. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Zheludkevich, M.; Yasakau, K.; Lamaka, S.; Ferreira, M.G.S.; Möhwald, H. Layer-by-layer assembled nanocontainers for self-healing corrosion protection. Adv. Mater. 2006, 18, 1672–1678. [Google Scholar] [CrossRef]
- Khan, A.; Ubaid, F.; Fayyad, E.M.; Ahmad, Z.; Shakoor, R.A.; Montemor, M.F.; Kahraman, R.; Mansour, S.; Hassan, M.K.; Hasan, A.; et al. Synthesis and properties of polyelectrolyte multilayered microcapsules reinforced smart coatings. J. Mater. Sci. 2019, 54, 12079–12094. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Lind, J.U.; Trogler, W.C. Synthesis of hollow silica and titania nanospheres. Chem. Mater. 2008, 20, 2875–2877. [Google Scholar] [CrossRef]
- Ge, T.; Zhao, W.; Wu, X.; Wu, Y.; Shen, L.; Ci, X.; He, Y. Design alternate epoxy-reduced graphene oxide/epoxy-zinc multilayer coatings for achieving long-term corrosion resistance for Cu. Mater. Des. 2020, 186, 108299. [Google Scholar] [CrossRef]
- Chenan, A.; Ramya, S.; George, R.P.; Kamachi Mudali, U. Hollow mesoporous zirconia nanocontainers for storing and controlled releasing of corrosion inhibitors. Ceram. Int. 2014, 40, 10457–10463. [Google Scholar] [CrossRef]
- Borisova, D.; Möhwald, H.; Shchukin, D.G. Mesoporous silica nanoparticles for active corrosion protection. ACS Nano 2011, 5, 1939–1946. [Google Scholar] [CrossRef]
- Nawaz, M.; Shakoor, R.A.; Kahraman, R.; Montemor, M.F. Cerium oxide loaded with gum Arabic as environmentally friendly anti-corrosion additive for protection of coated steel. Mater. Des. 2020, 198, 109361. [Google Scholar] [CrossRef]
- Li, Y.; Lin, X.; Wang, Y.; Luo, J.; Sun, W. Preparation and characterization of porous yttrium oxide powders with high specific surface area. J. Rare Earths 2006, 24, 34–38. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Pandurangan, M.; Veerappan, M.; Kim, D.H.; Sreekanth, T.V.M.; Shim, J. Green synthesis, characterization and anticancer activity of yttrium oxide nanoparticles. Mater. Lett. 2018, 216, 58–62. [Google Scholar] [CrossRef]
- Qiao, J.; Zhang, M.; Zhang, X.; Zhang, K.; Zhang, S. Effect of yttrium oxide nanoparticles on corrosion resistance of chromium-free dacromet coating. Coat. Paint. Electroplat. 2016, 41, 178–181. [Google Scholar]
- Yan, T.; Zhang, S.; Feng, L.; Qiang, Y.; Lu, L.; Fu, D.; Wen, Y.; Chen, J.; Li, W.; Tan, B. Investigation of imidazole derivatives as corrosion inhibitors of copper in sulfuric acid: Combination of experimental and theoretical researches. J. Taiwan Inst. Chem. Eng. 2020, 106, 118–129. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Ghaemi, M.; Nozad Golikand, A.; Yousefi, T.; Jangju, E. Yttrium Oxide Nanoparticles Prepared by Heat Treatment of Cathodically Grown Yttrium Hydroxide. ISRN Ceram. 2011, 2011, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Viveka, S.; Prabhuswamy, M.; Dinesha, D.; Lokanath, N.K.; Nagaraja, G.K. Synthesis, crystal structure, and characterization of new 2,4,5-triphenyl imidazole: 4,5-diphenyl-2-(3,4,5-trimethoxyphenyl)-1 h-imidazole. Mol. Cryst. Liq. Cryst. 2014, 588, 83–94. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Dahryn Trivedi, A.B.; Gunin Saikia, G.N. Physical and Structural Characterization of Biofield Treated Imidazole Derivatives. Nat. Prod. Chem. Res. 2015, 3, 1000187. [Google Scholar] [CrossRef]
- Wong, C.P.; Miller, P.J. Vibrational spectroscopic studies of alane. J. Energ. Mater. 2005, 23, 169–181. [Google Scholar] [CrossRef]
- Khan, A.; Hassanein, A.; Habib, S.; Nawaz, M.; Shakoor, R.A.; Kahraman, R. Hybrid Halloysite Nanotubes as Smart Carriers for Corrosion Protection. ACS Appl. Mater. Interfaces 2020, 12, 37571–37584. [Google Scholar] [CrossRef]
- Behzadnasab, M.; Mirabedini, S.M.; Kabiri, K.; Jamali, S. Corrosion performance of epoxy coatings containing silane treated ZrO2 nanoparticles on mild steel in 3.5% NaCl solution. Corros. Sci. 2011, 53, 89–98. [Google Scholar] [CrossRef]
- Taghavikish, M.; Dutta, N.K.; Choudhury, N.R. Emerging corrosion inhibitors for interfacial coating. Coatings 2017, 7, 217. [Google Scholar] [CrossRef] [Green Version]
- Njoku, D.I.; Cui, M.; Xiao, H.; Shang, B.; Li, Y. Understanding the anticorrosive protective mechanisms of modified epoxy coatings with improved barrier, active and self-healing functionalities: EIS and spectroscopic techniques. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Hosseini, M.; Aboutalebi, K. Electrochemical evaluation of corrosion protection performance of epoxy/polyaniline-imidazole modified ZnO nanocomposite coating on mild steel. Prog. Color Colorants Coat. 2017, 10, 181–192. [Google Scholar]
- Otmacic Curkovic, H.; Stupnisek-Lisac, E.; Takenouti, H. The influence of pH value on the efficiency of imidazole based corrosion inhibitors of copper. Corros. Sci. 2010, 52, 398–405. [Google Scholar] [CrossRef]
- Finšgar, M. The first X-ray photoelectron spectroscopy surface analysis of 4-methyl-2-phenyl-imidazole adsorbed on copper. Anal. Methods 2015, 7, 6496–6503. [Google Scholar] [CrossRef]
- Barr, T.L.; Seal, S. Nature of the use of adventitious carbon as a binding energy standard. J. Vac. Sci. Technol. A Vac. Surf. Film. 1995, 13, 1239–1246. [Google Scholar] [CrossRef]
- Safaei, Z.; Shiroudi, A.; Zahedi, E.; Sillanpää, M. Atmospheric oxidation reactions of imidazole initiated by hydroxyl radicals: Kinetics and mechanism of reactions and atmospheric implications. Phys. Chem. Chem. Phys. 2019, 21, 8445–8456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hantsche, H. High resolution XPS of organic polymers, the scienta ESCA300 database. By G. Beamson and D. Briggs, Wiley, Chichester 1992, 295 pp., hardcover, £ 65.00, ISBN 0-471-93592-1. Adv. Mater. 1993, 5, 778. [Google Scholar] [CrossRef]
- Miller, D.J.; Biesinger, M.C.; McIntyre, N.S. Interactions of CO2 and CO at fractional atmosphere pressures with iron and iron oxide surfaces: One possible mechanism for surface contamination? Surf. Interface Anal. 2002, 33, 299–305. [Google Scholar] [CrossRef]
- Fraoua, K.; Aeiyach, S.; Aubard, J.; Delamar, M.; Lacaze, P.C.; Ferreira, C.A. XPS and SERS evidence for iron nitride species responsible for the strong adhesion of polypyrrole to iron or steel treated with nitric acid. J. Adhes. Sci. Technol. 1999, 13, 517–522. [Google Scholar] [CrossRef]
- Gröning, P.; Nowak, S.; Schlapbach, L. Surface modifications of nitrogen-plasma-treated stainless steels. Appl. Surf. Sci. 1993, 64, 265–273. [Google Scholar] [CrossRef]
- Nardeli, J.V.; Fugivara, C.S.; Taryba, M.; Montemor, M.F.; Benedetti, A.V. Self-healing ability based on hydrogen bonds in organic coatings for corrosion protection of AA1200. Corros. Sci. 2020, 177, 108984. [Google Scholar] [CrossRef]
- Eduok, U.; Faye, O.; Szpunar, J. Corrosion inhibition of X70 sheets by a film-forming imidazole derivative at acidic pH. RSC Adv. 2016, 6, 108777–108790. [Google Scholar] [CrossRef]
- Abdallah, M.; Megahed, H.E.; Sobhi, M. Ni2+ cation and imidazole as corrosion inhibitors for carbon steel in sulfuric acid solutions. Mon. Fur Chem. 2010, 141, 1287–1295. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Kumar, A.; Liu, W.; Songsong, C.; Lin, Y. Electrochemical, surface and quantum chemical studies of novel imidazole derivatives as corrosion inhibitors for J55 steel in sweet corrosive environment. J. Alloys Compd. 2017, 712, 121–133. [Google Scholar] [CrossRef]
- Pire, M.; Lorthioir, C.; Oikonomou, E.K.; Norvez, S.; Iliopoulos, I.; Le Rossignol, B.; Leibler, L. Imidazole-accelerated crosslinking of epoxidized natural rubber by dicarboxylic acids: A mechanistic investigation using NMR spectroscopy. Polym. Chem. 2012, 3, 946–953. [Google Scholar] [CrossRef]
- Shao, L.; Sang, Y.; Huang, J. Imidazole-based hyper-cross-linked polymers derived porous carbons for CO2 capture. Microporous Mesoporous Mater. 2019, 275, 131–138. [Google Scholar] [CrossRef]
- Mendes, J.O.; Da Silva, E.C.; Rocha, A.B. On the nature of inhibition performance of imidazole on iron surface. Corros. Sci. 2012, 57, 254–259. [Google Scholar] [CrossRef]











| Immersion Time (Days) | Faradic Resistance (Rct (Ω.cm2) | ||
|---|---|---|---|
| Reference Coating | Modified Coating | I.E (%) | |
| 1 | 3.630 ± 0.7 × 105 | 1.411 ± 0.6 × 106 | - |
| 3 | 5.211 ± 0.7 × 104 | 4.344 ± 0.4 × 105 | 16.5 |
| 5 | 8.311 ± 1.2 × 104 | 7.475 ± 1.4 × 105 | 51.4 |
| 7 | 4.855 ± 1.5 × 104 | 4.966 ± 1.1 × 106 | 92.6 |
| 9 | 2.859 ± 0.2 × 104 | 4.264 ± 0.3 × 107 | 99.1 |
| 11 | 2.856 ±0.1 × 104 | 2.678 ± 0.2 × 108 | 99.8 |
| Sample | Surface Chemical Composition, Atomic % | |||
|---|---|---|---|---|
| C | O | N | Fe | |
| Modified coating surface | 56.9 | 25.6 | 2.9 | 55.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawaz, M.; Naeem, N.; Kahraman, R.; Montemor, M.F.; Haider, W.; Shakoor, R.A. Effectiveness of Epoxy Coating Modified with Yttrium Oxide Loaded with Imidazole on the Corrosion Protection of Steel. Nanomaterials 2021, 11, 2291. https://doi.org/10.3390/nano11092291
Nawaz M, Naeem N, Kahraman R, Montemor MF, Haider W, Shakoor RA. Effectiveness of Epoxy Coating Modified with Yttrium Oxide Loaded with Imidazole on the Corrosion Protection of Steel. Nanomaterials. 2021; 11(9):2291. https://doi.org/10.3390/nano11092291
Chicago/Turabian StyleNawaz, Muddasir, Nazal Naeem, Ramazan Kahraman, M. F. Montemor, W. Haider, and R. A. Shakoor. 2021. "Effectiveness of Epoxy Coating Modified with Yttrium Oxide Loaded with Imidazole on the Corrosion Protection of Steel" Nanomaterials 11, no. 9: 2291. https://doi.org/10.3390/nano11092291
APA StyleNawaz, M., Naeem, N., Kahraman, R., Montemor, M. F., Haider, W., & Shakoor, R. A. (2021). Effectiveness of Epoxy Coating Modified with Yttrium Oxide Loaded with Imidazole on the Corrosion Protection of Steel. Nanomaterials, 11(9), 2291. https://doi.org/10.3390/nano11092291