Structural Insight into La0.5Ca0.5Mn0.5Co0.5O3 Decomposition in the Methane Combustion Process
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, L.; Fan, Y.; Bellettre, J.; Yue, J.; Luo, L. A review on catalytic methane combustion at low temperatures: Catalysts, mechanisms, reaction conditions and reactor designs. Renew. Sustain. Energy Rev. 2020, 119, 109589. [Google Scholar] [CrossRef]
- Choudhary, T.; Banerjee, S.; Choudhary, V. Catalysts for combustion of methane and lower alkanes. Appl. Catal. A Gen. 2002, 234, 1–23. [Google Scholar] [CrossRef]
- Li, D.; Nakagawa, Y.; Tomishige, K. Methane reforming to synthesis gas over Ni catalysts modified with noble metals. Appl. Catal. A Gen. 2011, 408, 1–24. [Google Scholar] [CrossRef]
- Giebeler, L.; Kießling, D.; Wendt, G. LaMnO3 Perovskite Supported Noble Metal Catalysts for the Total Oxidation of Methane. Chem. Eng. Technol. 2007, 30, 889–894. [Google Scholar] [CrossRef]
- Murata, K.; Ohyama, J.; Yamamoto, Y.; Arai, S.; Satsuma, A. Methane Combustion over Pd/Al2O3 Catalysts in the Presence of Water: Effects of Pd Particle Size and Alumina Crystalline Phase. ACS Catal. 2020, 10, 8149–8156. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Y. Nanostructured perovskite oxides as promising substitutes of noble metals catalysts for catalytic combustion of methane. Chin. Chem. Lett. 2018, 29, 252–260. [Google Scholar] [CrossRef]
- Barbato, P.S.; Di Sarli, V.; Landi, G.; Di Benedetto, A. High pressure methane catalytic combustion over novel partially coated LaMnO3-based monoliths. Chem. Eng. J. 2015, 259, 381–390. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D.; Can, F.; Courtois, X.; Batiot-Dupeyrat, C.; Laassiri, S.; Alamdari, H. Perovskites as Substitutes of Noble Metals for Heterogeneous Catalysis: Dream or Reality. Chem. Rev. 2014, 114, 10292–10368. [Google Scholar] [CrossRef]
- Mazo, G.N.; Shlyakhtin, O.A.; Loktev, A.S.; Dedov, A.G. Methane oxidation catalysts based on the perovskite-like complex oxides of cobalt and nickel. Russ. Chem. Bull. 2019, 68, 1949–1953. [Google Scholar] [CrossRef]
- Zhu, J.; Li, H.; Zhong, L.; Xiao, P.; Xu, X.; Yang, X.; Zhao, Z.; Li, J. Perovskite Oxides: Preparation, Characterizations, and Applications in Heterogeneous Catalysis. ACS Catal. 2014, 4, 2917–2940. [Google Scholar] [CrossRef]
- McDonald, C.; Ni, C.; Maguire, P.; Connor, P.; Irvine, J.T.S.; Mariotti, D.; Svrcek, V. Nanostructured Perovskite Solar Cells. Nanomaterials 2019, 9, 1481. [Google Scholar] [CrossRef] [Green Version]
- Izyumov, Y.; Skryabin, Y. Double exchange model and the unique properties of the manganites. Uspekhi Fizicheskih Nauk. 2001, 171, 121–148. [Google Scholar] [CrossRef]
- Grabowska-Musiał, E. Selected perovskite oxides: Characterization, preparation and photocatalytic properties—A review. Appl. Catal. B Environ. 2016, 186, 97–126. [Google Scholar] [CrossRef]
- Ansari, M.I.H.; Qurashi, A.; Nazeeruddin, M.K. Frontiers, opportunities, and challenges in perovskite solar cells: A critical review. J. Photochem. Photobiol. C Photochem. Rev. 2018, 35, 1–24. [Google Scholar] [CrossRef]
- Yin, Y.; Li, Q. A review on all-perovskite multiferroic tunnel junctions. J. Mater. 2017, 3, 245–254. [Google Scholar] [CrossRef]
- Niu, G.; Guo, X.; Wang, L. Review of recent progress in chemical stability of perovskite solar cells. J. Mater. Chem. A 2015, 3, 8970–8980. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent Advances in the Catalytic Oxidation of Volatile Organic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, G.; Wang, L.; Irvine, J. Inorganic perovskite photocatalysts for solar energy utilization. Chem. Soc. Rev. 2016, 45, 5951–5984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, S.; Karthik, S.; Thakur, U.K.; Shankar, K. A review on photocatalytic CO2 reduction using perovskite oxide nanomaterials. Nanotechnology 2018, 29, 052001. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, Y.; Niu, X.; Yuan, F.; Fu, H. Preparation of La-Mn-O Perovskite Catalyst by Microwave Irradiation Method and its Application to Methane Combustion. Catal. Lett. 2010, 135, 152–158. [Google Scholar] [CrossRef]
- Sihaib, Z.; Puleo, F.; Pantaleo, G.; La Parola, V.; Valverde, J.L.; Gil, S.; Liotta, L.F.; Giroir-Fendler, A. The Effect of Citric Acid Concentration on the Properties of LaMnO3 as a Catalyst for Hydrocarbon Oxidation. Catalysts 2019, 9, 226. [Google Scholar] [CrossRef] [Green Version]
- Si, W.; Wang, Y.; Peng, Y.; Li, J. Selective Dissolution of A-Site Cations in ABO3 Perovskites: A New Path to High-Performance Catalysts. Angew. Chem. Int. Ed. 2015, 54, 7954–7957. [Google Scholar] [CrossRef] [PubMed]
- Bashan, V.; Ust, Y. Perovskite catalysts for methane combustion: Applications, design, effects for reactivity and partial oxidation. Int. J. Energy Res. 2019, 43, 7755–7789. [Google Scholar] [CrossRef]
- Ruocco, C.; de Caprariis, B.; Palma, V.; Petrullo, A.; Ricca, A.; Scarsella, M.; De Filippis, P. Methane dry reforming on Ru perovskites, AZrRuO3: Influence of preparation method and substitution of A cation with alkaline earth metals. J. CO2 Util. 2019, 30, 222–231. [Google Scholar] [CrossRef]
- Ulrich, V.; Froese, C.; Moroz, B.; Pyrjaev, P.; Gerasimov, E.; Sinev, I.; Cuenya, B.R.; Muhler, M.; Bukhtiyarov, V.; Grünert, W. Three-way catalysis with supported gold catalysts: Poisoning effects of hydrocarbons. Appl. Catal. B Environ. 2018, 237, 1021–1032. [Google Scholar] [CrossRef]
- Grünbacher, M.; Tarjomannejad, A.; Nezhad, P.D.K.; Praty, C.; Ploner, K.; Mohammadi, A.; Niaei, A.; Klötzer, B.; Schwarz, S.; Bernardi, J.; et al. Promotion of La(Cu0.7Mn0.3)0.98M0.02O3−δ (M = Pd, Pt, Ru and Rh) perovskite catalysts by noble metals for the reduction of NO by CO. J. Catal. 2019, 379, 18–32. [Google Scholar] [CrossRef]
- Polo-Garzon, F.; Fung, V.; Liu, X.; Hood, Z.D.; Bickel, E.E.; Bai, L.; Tian, H.; Foo, G.S.; Chi, M.; Jiang, D.-E.; et al. Understanding the Impact of Surface Reconstruction of Perovskite Catalysts on CH4 Activation and Combustion. ACS Catal. 2018, 8, 10306–10315. [Google Scholar] [CrossRef]
- Isupova, L.A.; Gerasimov, E.Y.; Prosvirin, I.P. LaMn1−xFexO3 (x = 0–1) Perovskites in Methane and Carbon Monoxide Oxi-dation Reactions. Kinet. Catal. 2021, 62, 146–154. [Google Scholar] [CrossRef]
- Ciambelli, P.; Cimino, S.; De Rossi, S.; Faticanti, M.; Lisi, L.; Minelli, G.; Pettiti, I.; Porta, P.; Russo, G.; Turco, M. AMnO3 (A = La, Nd, Sm) and Sm1−xSrxMnO3 perovskites as combustion catalysts: Structural, redox and catalytic properties. Appl. Catal. B Environ. 2000, 24, 243–253. [Google Scholar] [CrossRef]
- Huang, J.; Teng, Z.; Kang, R.; Bin, F.; Wei, X.; Hao, Q.; Hui, K.N.; Hui, K.S.; Dou, B. Study on activity, stability limit and reaction mechanism of CO self-sustained combustion over the LaMnO3, La0.9Ce0.1MnO3 and La0.9Sr0.1MnO3 perovskite catalysts using sugar agent. Fuel 2021, 292, 120289. [Google Scholar] [CrossRef]
- Lisi, L.; Bagnascob, G.; Ciambellic, P.; De Rossi, S.; Portad, P.; Russob, G.; Turcob, M. Perovskite-Type Oxides: II. Redox Properties of LaMn1−xCuxO3 and LaCo1−xCuxO3 and Methane Catalytic Combustion. J. Solid State Chem. 1999, 146, 176–183. [Google Scholar] [CrossRef]
- Isupova, L.A.; Gerasimov, E.Y.; Zaikovskii, V.I.; Tsybulya, S.V. Effect of the reaction medium on the structure of the La1−xCax MnO3 (x = 0–1) solid solutions prepared by the pechini method. Kinet. Catal. 2011, 52, 104–110. [Google Scholar] [CrossRef]
- Gerasimov, E.Y.; Rogov, V.A.; Prosvirin, I.P.; Isupova, L.A.; Tsybulya, S.V. Microstructural Changes in La0.5Ca0.5Mn0.5Fe0.5O3 Solid Solutions under the Influence of Catalytic Reaction of Methane Combustion. Catalysts 2019, 9, 563. [Google Scholar] [CrossRef] [Green Version]
- Vashook, V.; Franke, D.; Zosel, J.; Vasylechko, L.; Schmidt, M.; Güth, U. Electrical conductivity and oxygen nonstoichiometry in the double B mixed La0.6Ca0.4Mn1−xCoxO3−δ perovskite system. J. Alloys Compd. 2009, 487, 577–584. [Google Scholar] [CrossRef]
- Gerasimov, E.; Isupova, L.; Tsybulya, S. Microstructural features of the La1−xCaxFeO3−δ solid solutions prepared via Pechini route. Mater. Res. Bull. 2015, 70, 291–295. [Google Scholar] [CrossRef]
- Gerasimov, E.; Kulikovskaya, N.; Chuvilin, A.; Isupova, L.; Tsybulya, S. Microstructural Changes in La1−xCaxCoO3−δ Solid Solutions Under the Influence of Catalytic Reaction of Methane Combustion. Top. Catal. 2016, 59, 1354–1360. [Google Scholar] [CrossRef]
- Isupova, L.; Yakovleva, I.; Sutormina, E.; Gerasimov, E.; Rogov, V. Catalytic activity in methane oxidation of La1−xCaxCoO3−δ (x = 0–1) perovskites prepared by mechanochemical route. Adv. Mater. Sci. 2018, 3. [Google Scholar] [CrossRef]
- Mishra, A.; Li, T.; Li, F.; Santiso, E. Oxygen Vacancy Creation Energy in Mn-Containing Perovskites: An Effective Indicator for Chemical Looping with Oxygen Uncoupling. Chem. Mater. 2018, 31, 689–698. [Google Scholar] [CrossRef]
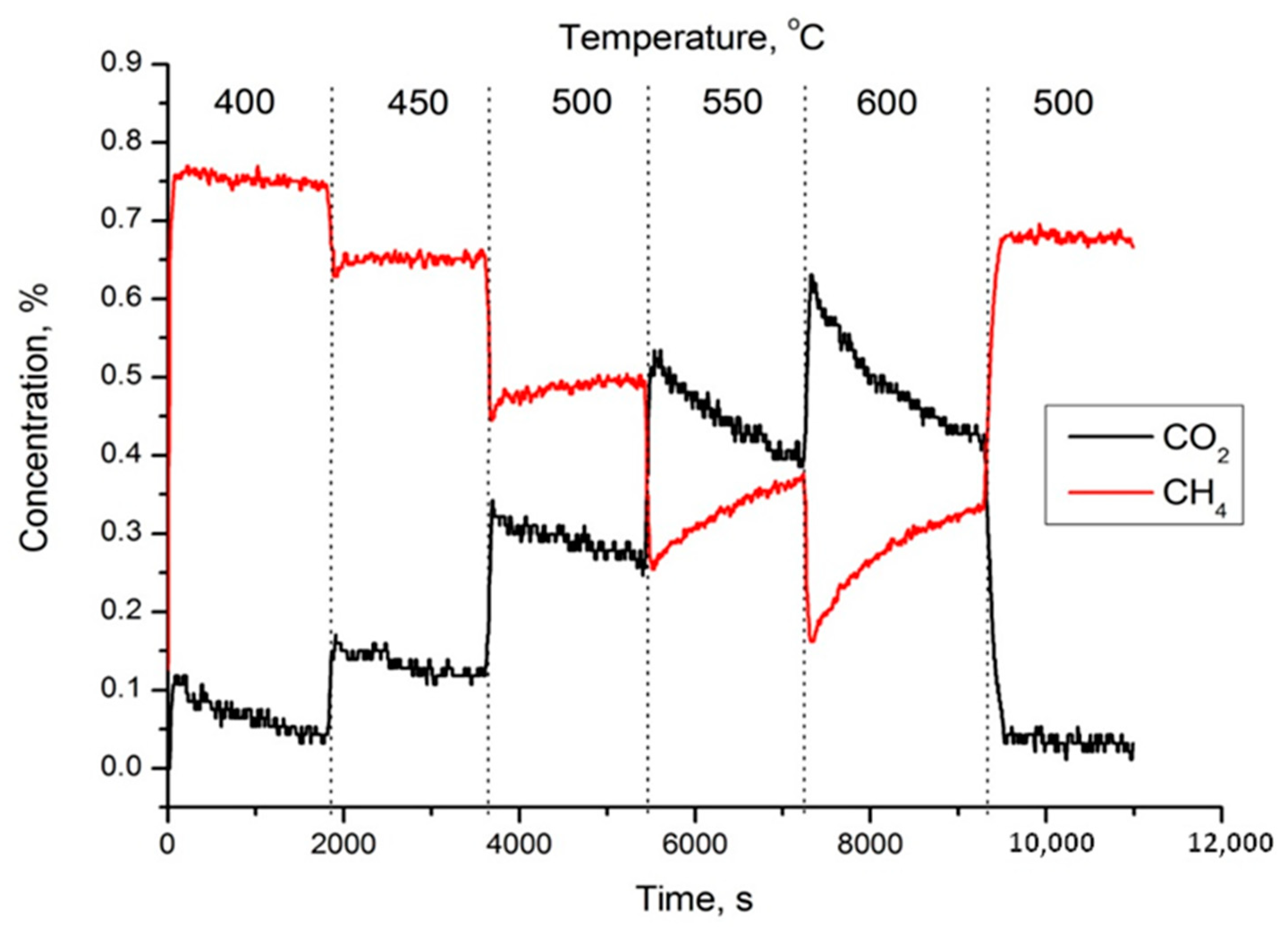
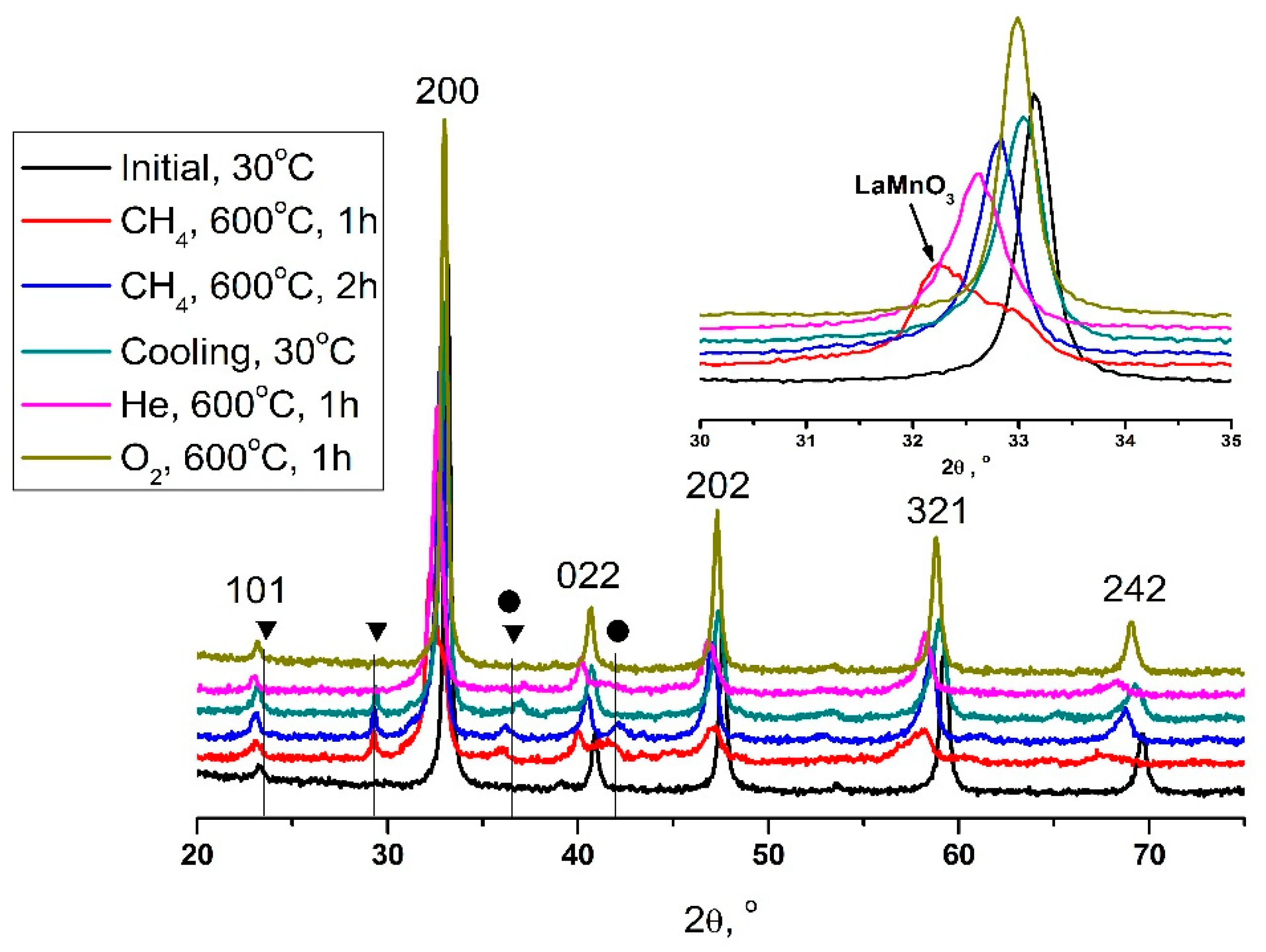
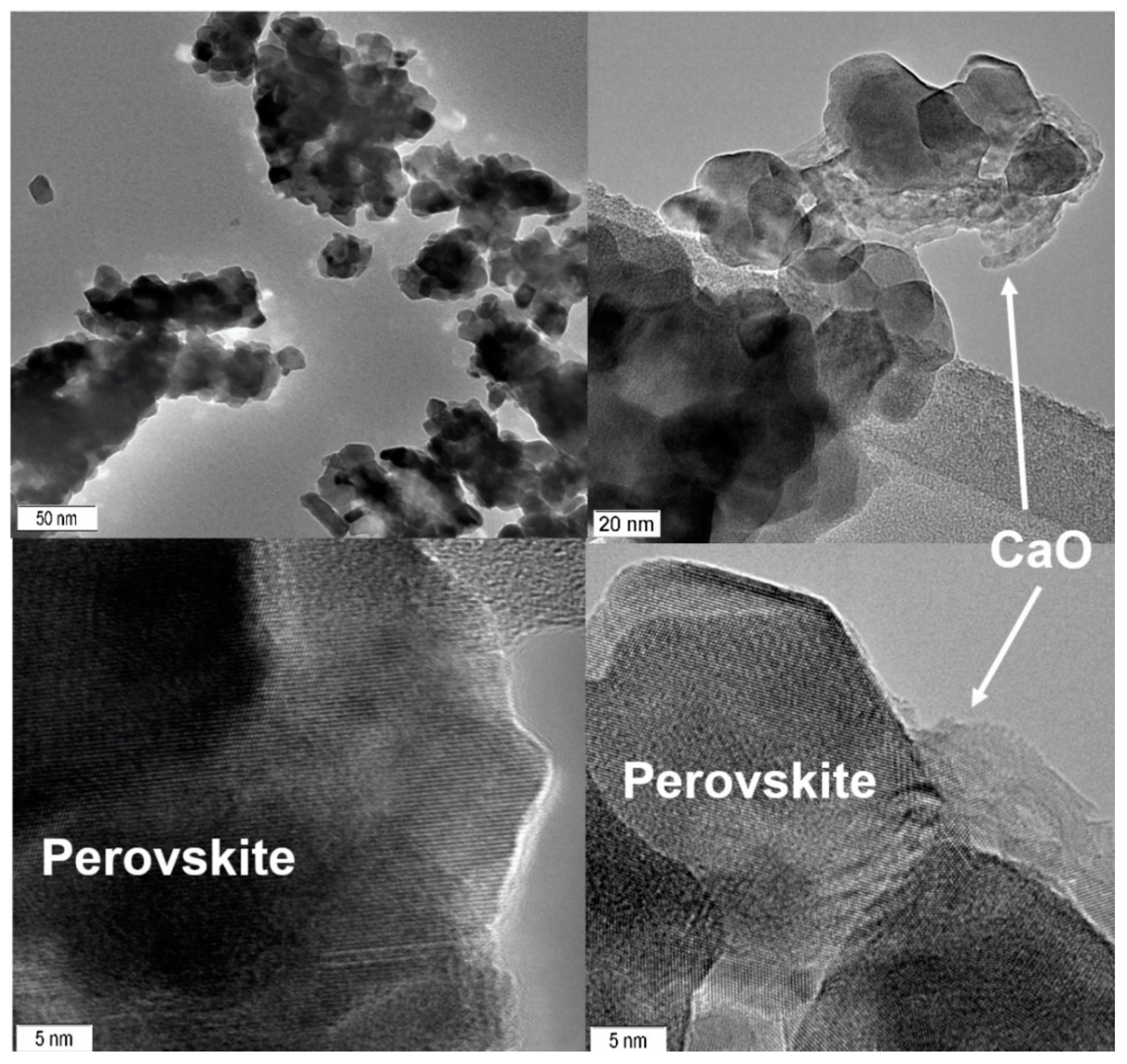
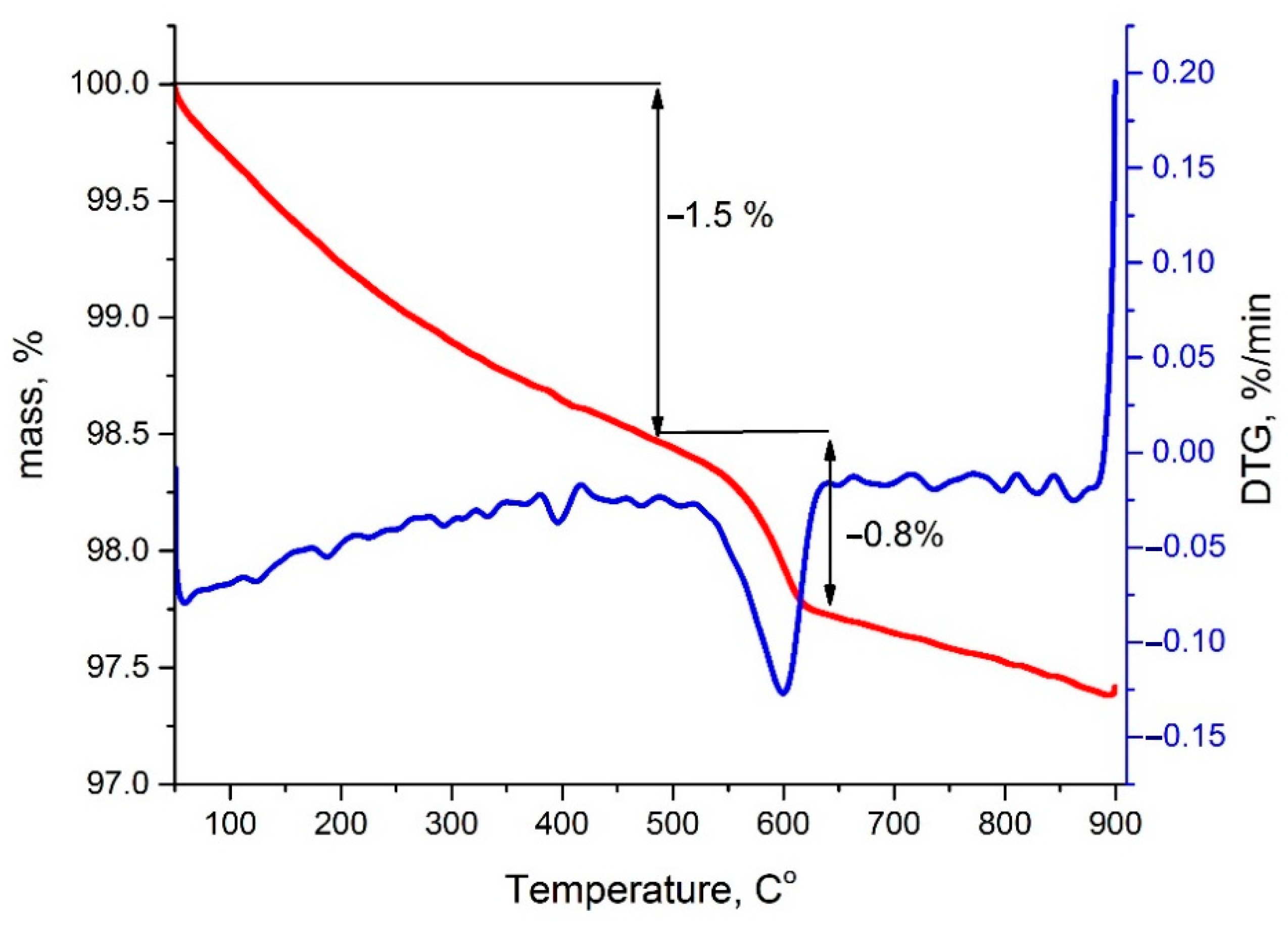
| Temperature, °C | Perovskite | CoO-Co3O4 | Calcite |
|---|---|---|---|
| 25 (initial) | 100 | 0 | 0 |
| 600 (1 h) | 84.5 | 5.9 | 9.6 |
| 600 (2 h) | 90.3 | 2.1 | 7.7 |
| 25 (cooling) | 91.1 | 1.5 (Co3O4) | 7.4 |
| Temperature, °C | a, Å | b, Å | c, Å | Cr. Size, Å |
|---|---|---|---|---|
| 25 (initial) | 5.391(1) | 7.616(3) | 5.419(3) | 270 |
| 600 (1 h) | 5.467(6) | 8.030(9) | 5.483(7) | 120 |
| 600 (2 h) | 5.453(5) | 7.704(5) | 5.515(2) | 170 |
| 25 (cooling) | 5.478(4) | 7.652(3) | 5.412(6) | 220 |
| 600 (He treatment) | 5.478(3) | 7.773(7) | 5.503(6) | 150 |
| 600 (O2 treatment) | 5.438(2) | 7.691(2) | 5.436(4) | 260 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolaeva, O.; Kapishnikov, A.; Gerasimov, E. Structural Insight into La0.5Ca0.5Mn0.5Co0.5O3 Decomposition in the Methane Combustion Process. Nanomaterials 2021, 11, 2283. https://doi.org/10.3390/nano11092283
Nikolaeva O, Kapishnikov A, Gerasimov E. Structural Insight into La0.5Ca0.5Mn0.5Co0.5O3 Decomposition in the Methane Combustion Process. Nanomaterials. 2021; 11(9):2283. https://doi.org/10.3390/nano11092283
Chicago/Turabian StyleNikolaeva, Olga, Aleksandr Kapishnikov, and Evgeny Gerasimov. 2021. "Structural Insight into La0.5Ca0.5Mn0.5Co0.5O3 Decomposition in the Methane Combustion Process" Nanomaterials 11, no. 9: 2283. https://doi.org/10.3390/nano11092283
APA StyleNikolaeva, O., Kapishnikov, A., & Gerasimov, E. (2021). Structural Insight into La0.5Ca0.5Mn0.5Co0.5O3 Decomposition in the Methane Combustion Process. Nanomaterials, 11(9), 2283. https://doi.org/10.3390/nano11092283







