Synthesis and Characterization of PdAgNi/C Trimetallic Nanoparticles for Ethanol Electrooxidation
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Physical Characterization
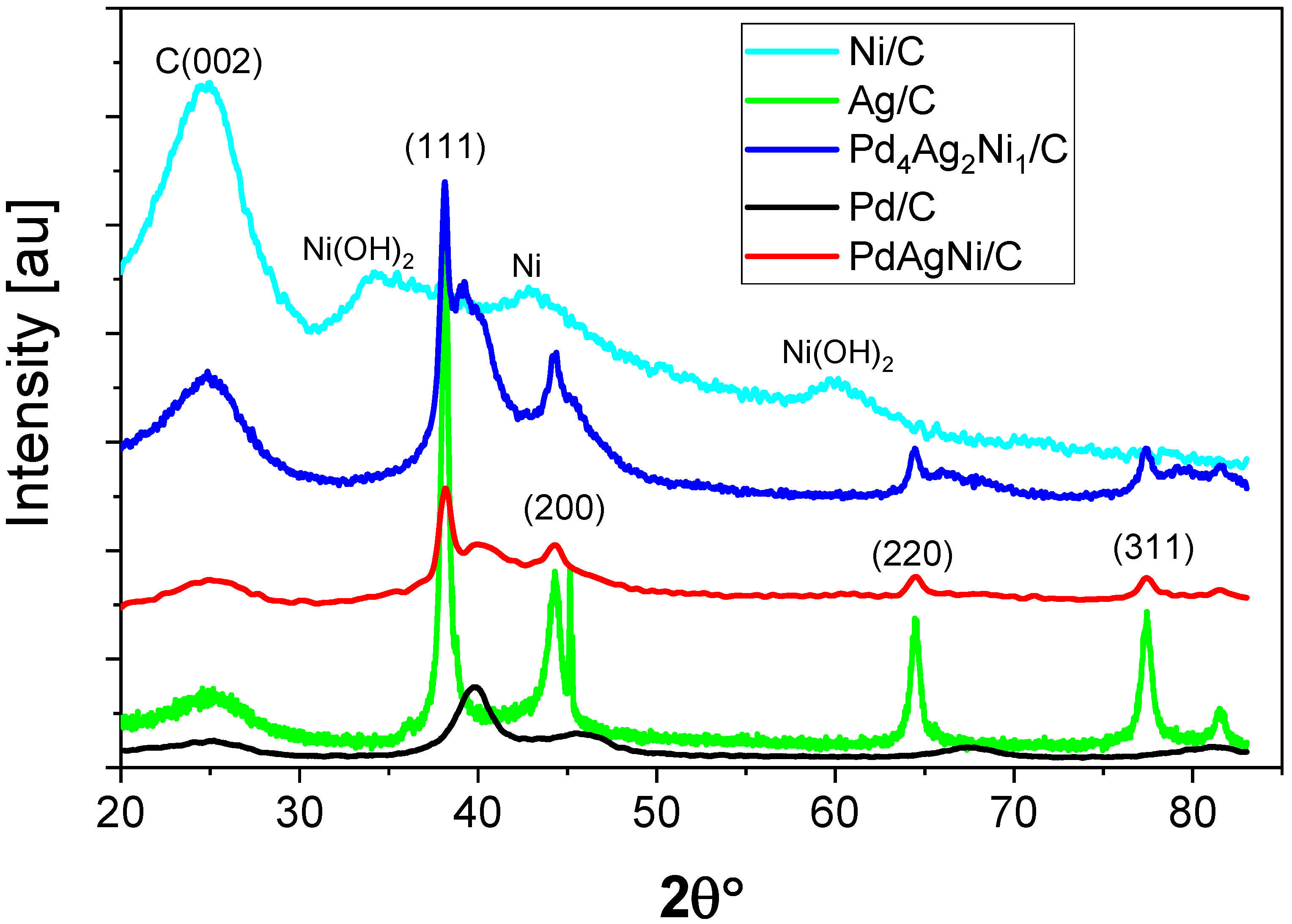
X-ray Diffraction (XRD)
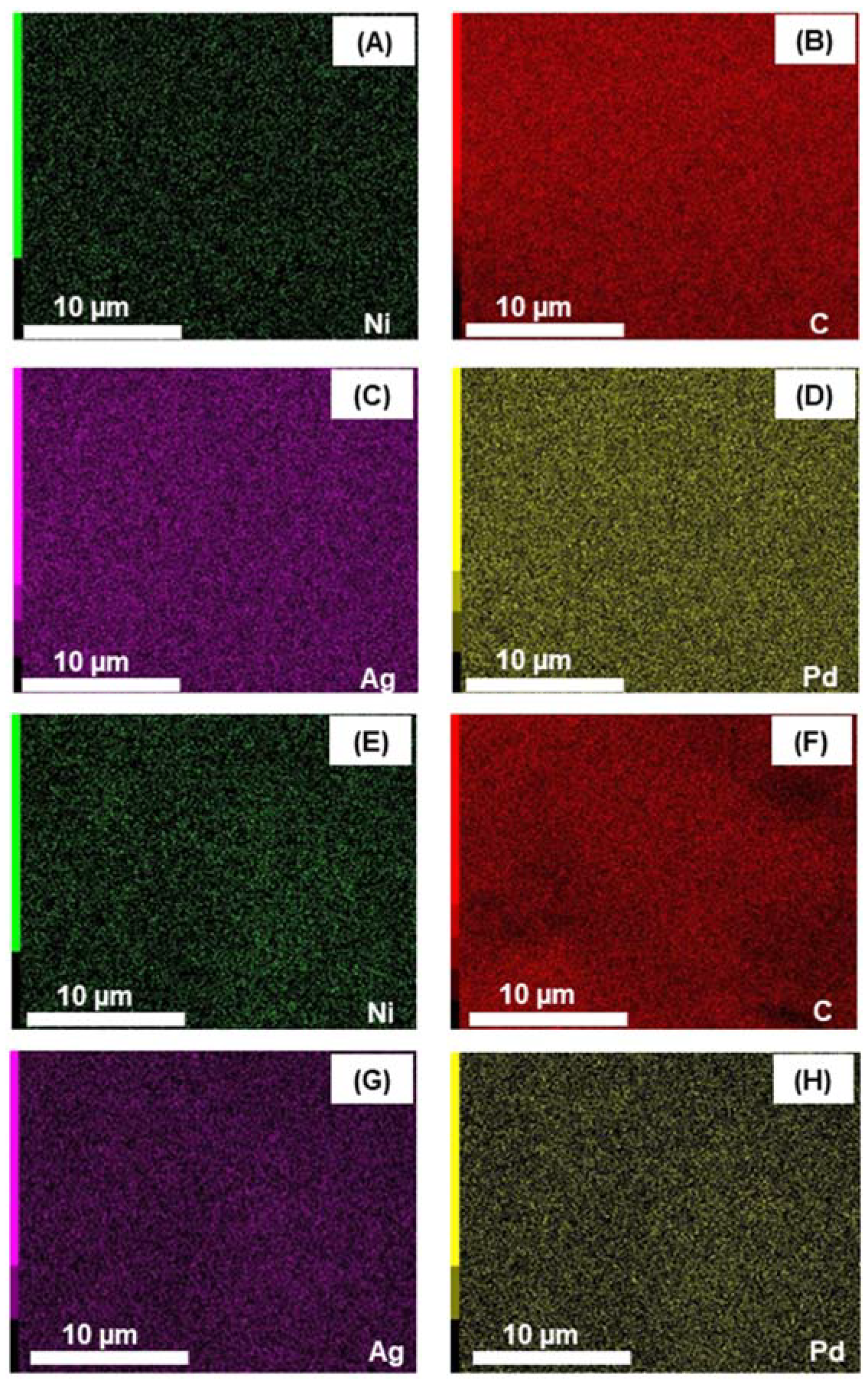
3.2. Energy Dispersive X-ray Spectroscopy (EDX)
3.3. Transmission Electron Microscopy (TEM)
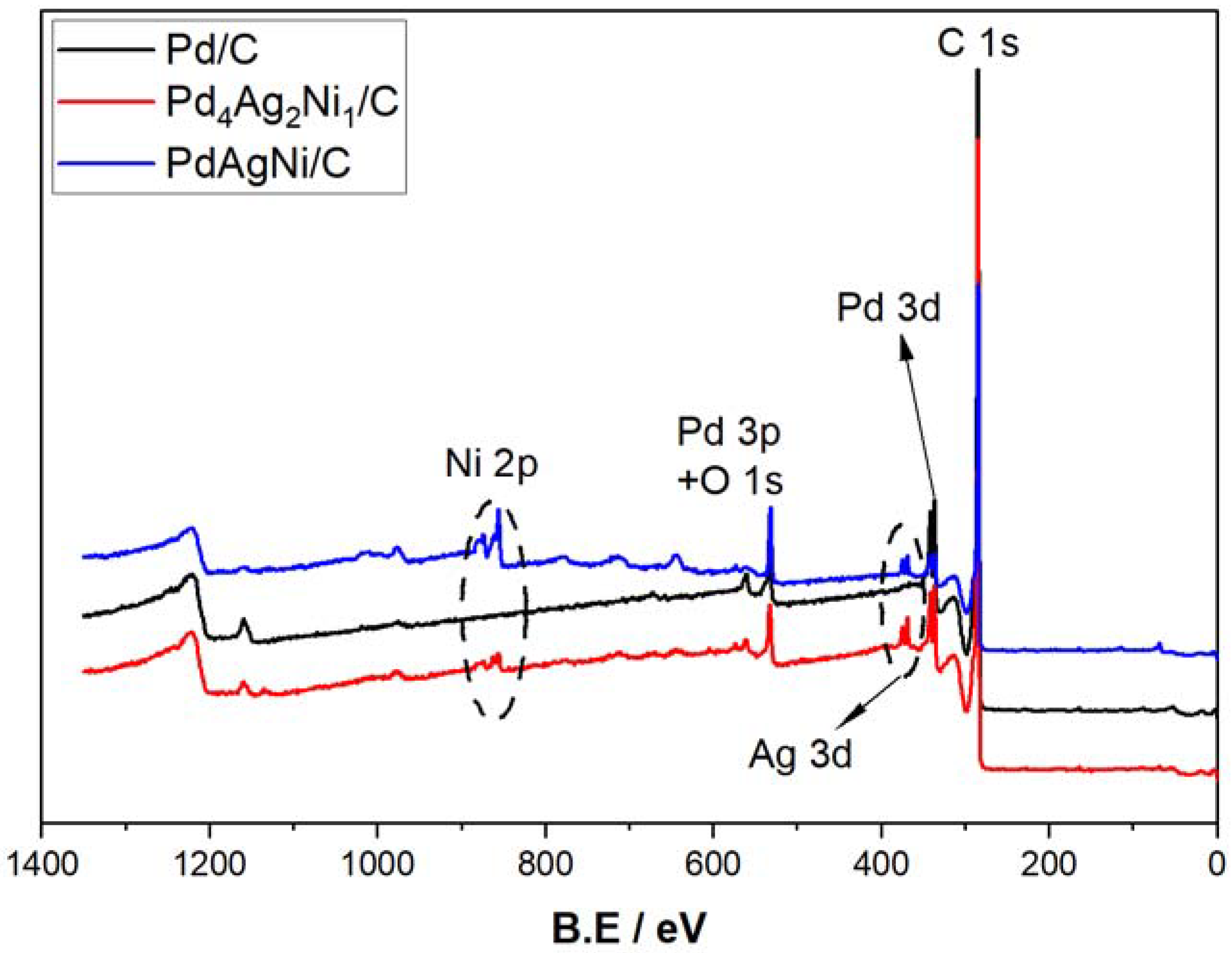
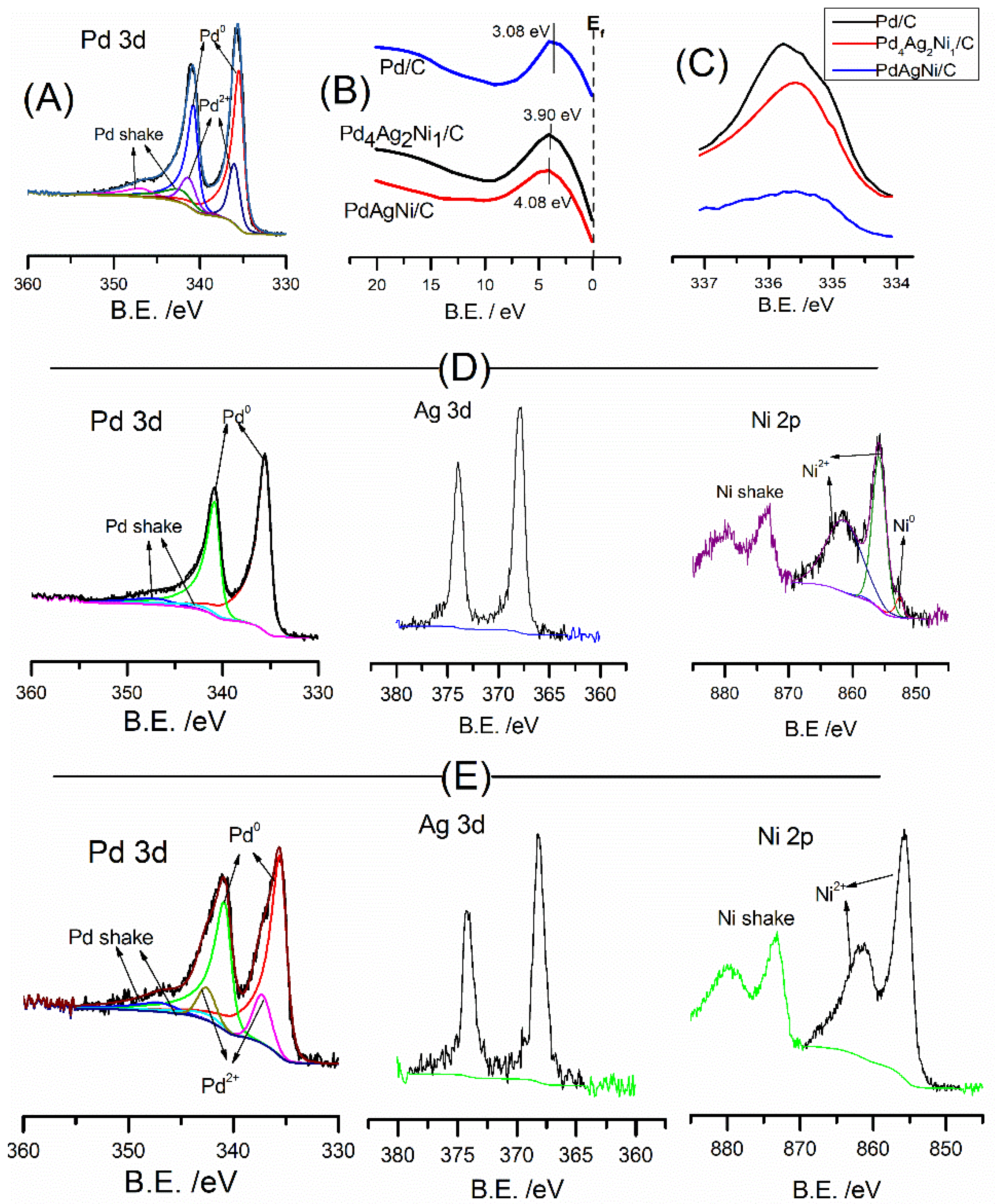
3.4. X-ray Photoelectron Spectroscopy (XPS)
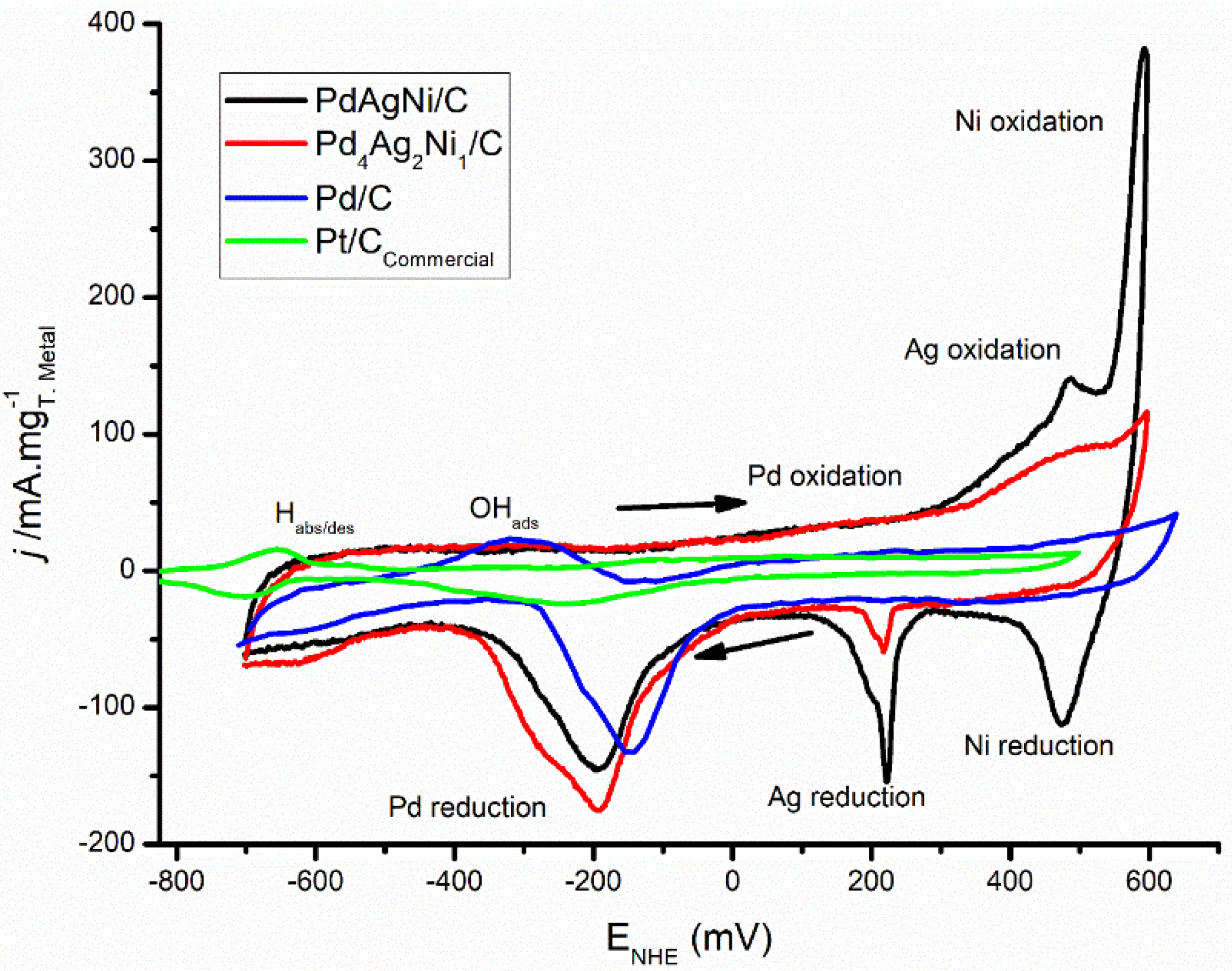
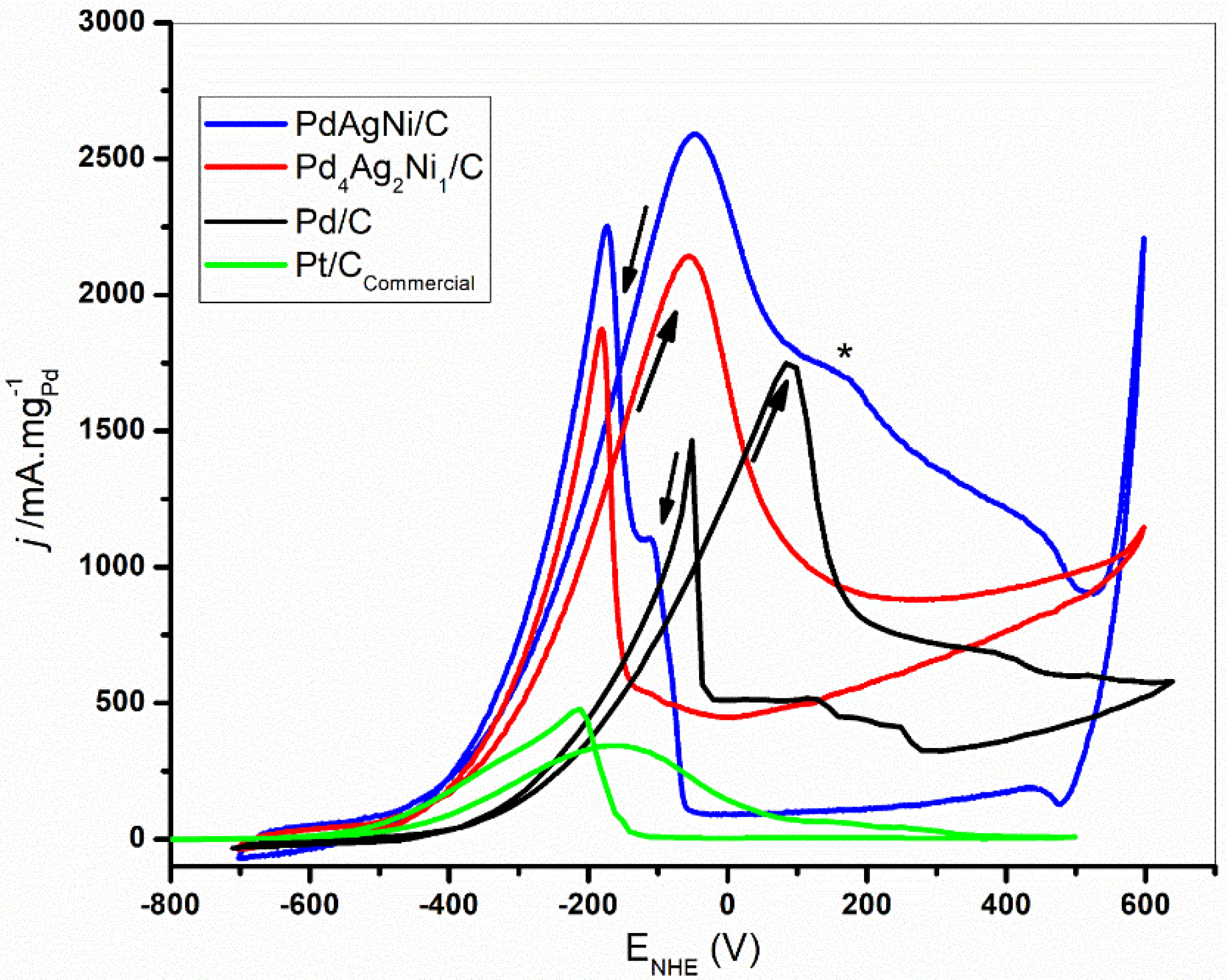
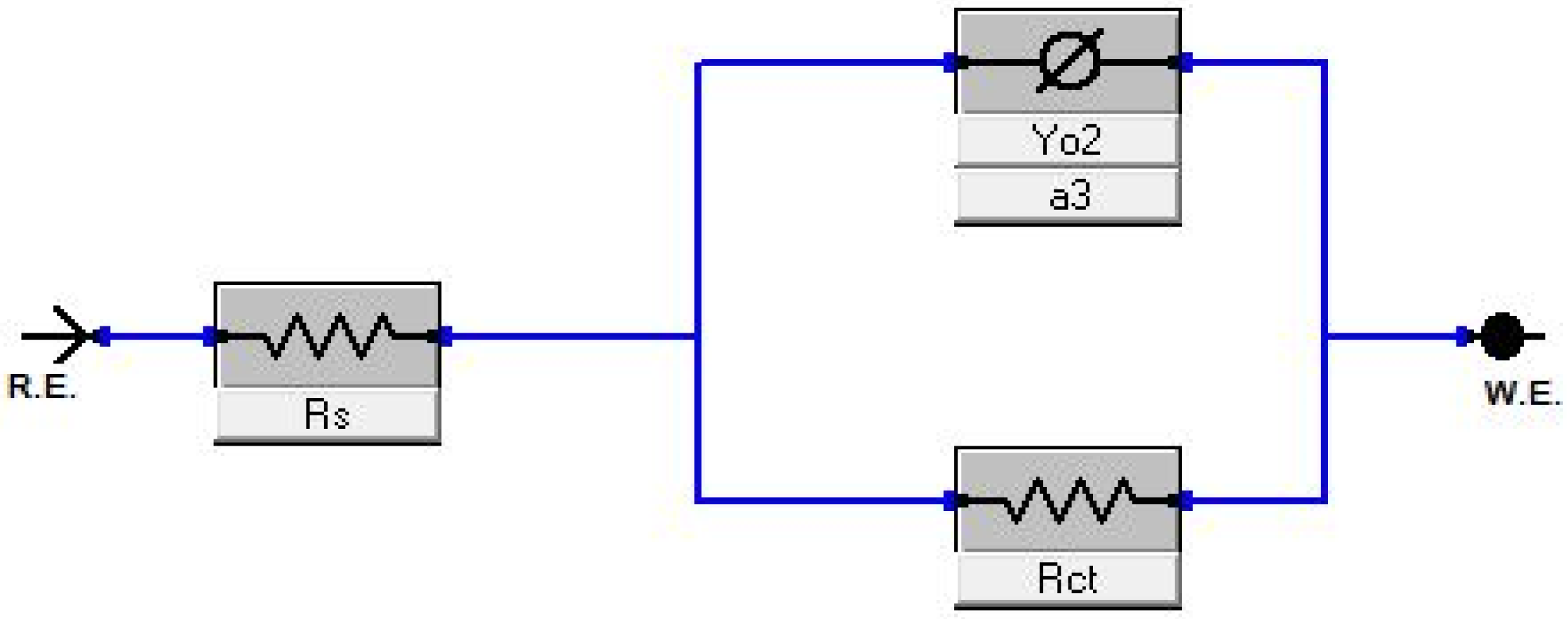
3.5. Electrochemical Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katsounaros, I.; Koper, M.T.M. Electrocatalysis for the hydrogen economy, In Electrochemical Science for a Sustainable Society; A Tribut. to John O’M Bockris; Springer International Publishing: Basel, Switzerland, 2017; pp. 23–50. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Strmcnik, D.; Lopes, P.; Markovic, N.M. Energy and fuels from electrochemical interfaces. Nat. Mater. 2017, 16, 57–69. [Google Scholar] [CrossRef]
- O’Hayre, R.; Cha, S.-W.; Colella, W.; Prinz, F.B. Prinz, Fuel Cell Fundamentals; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Friedl, J.; Stimming, U. Model catalyst studies on hydrogen and ethanol oxidation for fuel cells. Electrochim. Acta 2013, 101, 41–58. [Google Scholar] [CrossRef] [Green Version]
- Badwal, S.; Giddey, S.; Kulkarni, A.; Goel, J.; Basu, S. Direct ethanol fuel cells for transport and stationary applications—A comprehensive review. Appl. Energy 2015, 145, 80–103. [Google Scholar] [CrossRef]
- Bianchini, C.; Shen, P.K. Palladium-Based Electrocatalysts for Alcohol Oxidation in Half Cells and in Direct Alcohol Fuel Cells. Chem. Rev. 2009, 109, 4183–4206. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Zhao, T.; Li, Y. Carbon-neutral sustainable energy technology: Direct ethanol fuel cells. Renew. Sustain. Energy Rev. 2015, 50, 1462–1468. [Google Scholar] [CrossRef]
- O’Hayre, R.; Cha, S.; Colella, W.; Prinz, F.B. Chapter 3: Fuel Cell Reaction Kinetics. In Fuel Cell Fundam; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 77–116. [Google Scholar] [CrossRef]
- O’Hayre, F.B.P.R.; Cha, S.; Colella, W.; O’Hayre, R.; Cha, S.-W.; Colella, W.; Prinz, F.B. Chapter 8: Overview of Fuel Cell Types. In Fuel Cell Fundam; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 269–302. [Google Scholar] [CrossRef]
- Guo, J.; Chen, R.; Zhu, F.-C.; Sun, S.-G.; Villullas, H.D.L.M. New understandings of ethanol oxidation reaction mechanism on Pd/C and Pd2Ru/C catalysts in alkaline direct ethanol fuel cells. Appl. Catal. B Environ. 2018, 224, 602–611. [Google Scholar] [CrossRef] [Green Version]
- Kamarudin, M.; Kamarudin, S.; Masdar, M.S.; Daud, W.R.W. Review: Direct ethanol fuel cells. Int. J. Hydrog. Energy 2013, 38, 9438–9453. [Google Scholar] [CrossRef]
- Antolini, E. Palladium in fuel cell catalysis. Energy Environ. Sci. 2009, 2, 915–931. [Google Scholar] [CrossRef]
- Antolini, E.; Gonzalez, E. Alkaline direct alcohol fuel cells. J. Power Sources 2010, 195, 3431–3450. [Google Scholar] [CrossRef]
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion exchange membranes for alkaline fuel cells: A review. J. Membr. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Brouzgou, A.; Podias, A.; Tsiakaras, P. PEMFCs and AEMFCs directly fed with ethanol: A current status comparative review. J. Appl. Electrochem. 2013, 43, 119–136. [Google Scholar] [CrossRef]
- Sverdrup, H.U.; Ragnarsdottir, K.V. A system dynamics model for platinum group metal supply, market price, depletion of extractable amounts, ore grade, recycling and stocks-in-use. Resour. Conserv. Recycl. 2016, 114, 130–152. [Google Scholar] [CrossRef]
- Khan, K.; Köseoğlu, S.D. Is palladium price in bubble? Resour. Policy 2020, 68, 101780. [Google Scholar] [CrossRef]
- Hagelüken, C. Markets for the catalyst metals platinum, palladium and rhodium. Metall 2006, 60, 31–42. [Google Scholar]
- Company news. Solder. Surf. Mt. Technol. 2012, 24, 433–434. [CrossRef]
- Platinum, V.S. Palladium: A Key Factor Holding Back Platinum Demand and Price|Seeking Alpha. Available online: https://seekingalpha.com/article/4270121-platinum-vs-palladium-key-factor-holding-back-platinum-demand-and-price (accessed on 24 January 2021).
- Ma, L.; Chu, D.; Chen, R. Comparison of ethanol electro-oxidation on Pt/C and Pd/C catalysts in alkaline media. Int. J. Hydrog. Energy 2012, 37, 11185–11194. [Google Scholar] [CrossRef]
- Amin, R.; Hameed, R.A.; El-Khatib, K.; Youssef, M.E. Electrocatalytic activity of nanostructured Ni and Pd–Ni on Vulcan XC-72R carbon black for methanol oxidation in alkaline medium. Int. J. Hydrog. Energy 2014, 39, 2026–2041. [Google Scholar] [CrossRef]
- Sheikh, A.M.; Silva, E.L.; Moares, L.; Antonini, L.M.; Abellah, M.Y.; Malfatti, C.F. Pd-based Catalysts for Ethanol Oxidation in Alkaline Electrolyte. Am. J. Min. Metall. 2014, 2, 64–69. [Google Scholar] [CrossRef]
- Obradović, M.; Stančić, Z.; Lacnjevac, U.; Radmilovic, V.; Gavrilovic-Wohlmuther, A.; Gojković, S. Electrochemical oxidation of ethanol on palladium-nickel nanocatalyst in alkaline media. Appl. Catal. B Environ. 2016, 189, 110–118. [Google Scholar] [CrossRef]
- Jongsomjit, S.; Sombatmankhong, K.; Prapainainar, P. Effect of acid functionalised carbon supports for Pd–Ni–Sn catalyst on ethanol oxidation reaction. RSC Adv. 2015, 5, 61298–61308. [Google Scholar] [CrossRef]
- Moraes, L.; Matos, B.; Radtke, C.; Santiago, E.; Fonseca, F.C.; Amico, S.; Malfatti, C. Synthesis and performance of palladium-based electrocatalysts in alkaline direct ethanol fuel cell. Int. J. Hydrog. Energy 2016, 41, 6457–6468. [Google Scholar] [CrossRef]
- Zhang, Z.; Xin, L.; Sun, K.; Li, W. Pd–Ni electrocatalysts for efficient ethanol oxidation reaction in alkaline electrolyte. Int. J. Hydrog. Energy 2011, 36, 12686–12697. [Google Scholar] [CrossRef]
- Hassaninejad-Darzi, S.K.; Gholami-Esfidvajani, M. Electrocatalytic oxidation of ethanol using modified nickel phosphate nanoparticles and multi-walled carbon nanotubes paste electrode in alkaline media for fuel cell. Int. J. Hydrog. Energy 2016, 41, 20085–20099. [Google Scholar] [CrossRef]
- Feng, Y.; Bin, D.; Yan, B.; Du, Y.; Majima, T.; Zhou, W. Porous bimetallic PdNi catalyst with high electrocatalytic activity for ethanol electrooxidation. J. Colloid Interface Sci. 2017, 493, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.-G.; Kim, S.-M.; Kim, J.-W.; Lee, S.-Y. Composition-tuned porous Pd-Ag bimetallic dendrites for the enhancement of ethanol oxidation reactions. J. Alloy. Compd. 2016, 688, 447–453. [Google Scholar] [CrossRef]
- Li, L.; Chen, M.; Huang, G.; Yang, N.; Zhang, L.; Wang, H.; Liu, Y.; Wang, W.; Gao, J. A green method to prepare Pd–Ag nanoparticles supported on reduced graphene oxide and their electrochemical catalysis of methanol and ethanol oxidation. J. Power Sources 2014, 263, 13–21. [Google Scholar] [CrossRef]
- Oliveira, M.; Rego, R.; Fernandes, L.S.G.; Tavares, P. Evaluation of the catalytic activity of Pd–Ag alloys on ethanol oxidation and oxygen reduction reactions in alkaline medium. J. Power Sources 2011, 196, 6092–6098. [Google Scholar] [CrossRef]
- Qi, J.; Benipal, N.; Liang, C.; Li, W. PdAg/CNT catalyzed alcohol oxidation reaction for high-performance anion exchange membrane direct alcohol fuel cell (alcohol = methanol, ethanol, ethylene glycol and glycerol). Appl. Catal. B Environ. 2016, 199, 494–503. [Google Scholar] [CrossRef] [Green Version]
- Lam, B.T.X.; Chiku, M.; Higuchi, E.; Inoue, H. Preparation of PdAg and PdAu nanoparticle-loaded carbon black catalysts and their electrocatalytic activity for the glycerol oxidation reaction in alkaline medium. J. Power Sources 2015, 297, 149–157. [Google Scholar] [CrossRef]
- Hu, S.; Munoz, F.; Noborikawa, J.; Haan, J.; Scudiero, L.; Ha, S. Carbon supported Pd-based bimetallic and trimetallic catalyst for formic acid electrochemical oxidation. Appl. Catal. B Environ. 2016, 180, 758–765. [Google Scholar] [CrossRef]
- Beyhan, S.; Léger, J.-M.; Kadırgan, F. Understanding the influence of Ni, Co, Rh and Pd addition to PtSn/C catalyst for the oxidation of ethanol by in situ Fourier transform infrared spectroscopy. Appl. Catal. B Environ. 2014, 144, 66–74. [Google Scholar] [CrossRef]
- Shang, C.; Hong, W.; Wang, J.; Wang, E. Carbon supported trimetallic nickel-palladium-gold hollow nanoparticles with superior catalytic activity for methanol electrooxidation electrooxidation. J. Power Sources. 2015, 85, 12–15. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, D.; Kumar, A.; Al-Muhtaseb, A.; Pathania, D.; Naushad, M.; Mola, G.T. Revolution from monometallic to trimetallic nanoparticle composites, various synthesis methods and their applications: A review. Mater. Sci. Eng. C 2017, 71, 1216–1230. [Google Scholar] [CrossRef]
- Ulas, B.; Caglar, A.; Sahin, O.; Kivrak, H. Composition dependent activity of PdAgNi alloy catalysts for formic acid electrooxidation. J. Colloid Interface Sci. 2018, 532, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhao, T.; Xu, J.; Li, Y. High performance of a carbon supported ternary PdIrNi catalyst for ethanol electro-oxidation in anion-exchange membrane direct ethanol fuel cells. Energy Environ. Sci. 2011, 4, 1428–1433. [Google Scholar] [CrossRef]
- Su, P.-C.; Chen, H.-S.; Chen, T.-Y.; Liu, C.-W.; Lee, C.-H.; Lee, J.-F.; Chan, T.-S.; Wang, K.-W. Enhancement of electrochemical properties of Pd/C catalysts toward ethanol oxidation reaction in alkaline solution through Ni and Au alloying. Int. J. Hydrog. Energy 2013, 38, 4474–4482. [Google Scholar] [CrossRef]
- Zhu, F.; Wang, M.; He, Y.; Ma, G.; Zhang, Z.; Wang, X. A comparative study of elemental additives (Ni, Co and Ag) on electrocatalytic activity improvement of PdSn-based catalysts for ethanol and formic acid electro-oxidation. Electrochim. Acta 2014, 148, 291–301. [Google Scholar] [CrossRef]
- Lv, H.; Sun, L.; Zou, L.; Xu, D.; Yao, H.; Liu, B. Electrode for proton exchange membrane fuel cells: A review. Chem. Sci. 2019, 10, 1986–1993. [Google Scholar] [CrossRef] [Green Version]
- Gebre, S.H.; Sendeku, M.G. Trimetallic nanostructures and their applications in electrocatalytic energy conversions. J. Energy Chem. 2022, 65, 329–351. [Google Scholar] [CrossRef]
- Zhu, W.; Ke, J.; Wang, S.-B.; Ren, J.; Wang, H.-H.; Zhou, Z.-Y.; Si, R.; Zhang, Y.-W.; Yan, C.-H. Shaping Single-Crystalline Trimetallic Pt–Pd–Rh Nanocrystals toward High-Efficiency C–C Splitting of Ethanol in Conversion to CO2. ACS Catal. 2015, 5, 1995–2008. [Google Scholar] [CrossRef]
- Nandenha, J.; De Souza, R.; Assumpção, M.; Spinacé, E.V.; Neto, A.O. Preparation of PdAu/C-Sb2O5·SnO2 electrocatalysts by borohydride reduction process for direct formic acid fuel cell. Ionics 2013, 19, 1207–1213. [Google Scholar] [CrossRef]
- Neto, A.O.; Tusi, M.M.; Polanco, N.S.D.O.; da Silva, S.G.; dos Santos, M.C.; Spinacé, E.V. PdBi/C electrocatalysts for ethanol electro-oxidation in alkaline medium. Int. J. Hydrog. Energy 2011, 36, 10522–10526. [Google Scholar] [CrossRef]
- Assumpção, M.; da Silva, S.; De Souza, R.; Buzzo, G.; Spinacé, E.; Santos, M.; Neto, A.; Silva, J. Investigation of PdIr/C electrocatalysts as anode on the performance of direct ammonia fuel cell. J. Power Sources 2014, 268, 129–136. [Google Scholar] [CrossRef]
- Antoniassi, R.; Silva, J.C.; Neto, A.O.; Spinacé, E. Synthesis of Pt+SnO2/C electrocatalysts containing Pt nanoparticles with preferential (100) orientation for direct ethanol fuel cell. Appl. Catal. B Environ. 2017, 218, 91–100. [Google Scholar] [CrossRef]
- Naresh, N.; Wasim, F.G.S.; Ladewig, B.P.; Neergat, M. Removal of surfactant and capping agent from Pd nanocubes (Pd-NCs) using tert-butylamine: Its effect on electrochemical characteristics. J. Mater. Chem. A 2013, 1, 8553–8559. [Google Scholar] [CrossRef]
- Leng, Y. X-ray Spectroscopy for Elemental Analysis. In Materials Characterization; John Wiley & Sons, Ltd.: Chichester, UK, 2008; pp. 171–196. [Google Scholar] [CrossRef]
- Meng, Y. A Sustainable Approach to Fabricating Ag Nanoparticles/PVA Hybrid Nanofiber and Its Catalytic Activity. Nanomaterials 2015, 5, 1124–1135. [Google Scholar] [CrossRef] [Green Version]
- Ramulifho, T.; Ozoemena, K.I.; Modibedi, R.M.; Jafta, C.; Mathe, M. Fast microwave-assisted solvothermal synthesis of metal nanoparticles (Pd, Ni, Sn) supported on sulfonated MWCNTs: Pd-based bimetallic catalysts for ethanol oxidation in alkaline medium. Electrochim. Acta 2012, 59, 310–320. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Wen, D.; Oschatz, M.; Holzschuh, M.; Liu, W.; Herrmann, A.-K.; Simon, F.; Kaskel, S.; Eychmüller, A. Kinetically Controlled Synthesis of PdNi Bimetallic Porous Nanostructures with Enhanced Electrocatalytic Activity. Small 2015, 11, 1430–1434. [Google Scholar] [CrossRef] [Green Version]
- Dutta, A.; Datta, J. Energy efficient role of Ni/NiO in PdNi nano catalyst used in alkaline DEFC. J. Mater. Chem. A 2014, 2, 3237–3250. [Google Scholar] [CrossRef]
- Dutta, A.; Datta, J. Outstanding Catalyst Performance of PdAuNi Nanoparticles for the Anodic Reaction in an Alkaline Direct Ethanol (with Anion-Exchange Membrane) Fuel Cell. J. Phys. Chem. C 2012, 116, 25677–25688. [Google Scholar] [CrossRef]
- Armenta-González, A.J.; Carrera-Cerritos, R.; Guerra-Balcázar, M.; Arriaga, L.G.; Ledesma-García, J. Comparative study of carbon-supported Pd and PdAg catalysts synthesised by the polyol process and reverse micelles methods. J. Appl. Electrochem. 2014, 45, 33–41. [Google Scholar] [CrossRef]
- Chen, L.Y.; Chen, N.; Hou, Y.; Wang, Z.C.; Lv, S.H.; Fujita, T.; Jiang, J.H.; Hirata, A.; Chen, M.W. Geometrically Controlled Nanoporous PdAu Bimetallic Catalysts with Tunable Pd/Au Ratio for Direct Ethanol Fuel Cells. ACS Catal. 2013, 3, 1220–1230. [Google Scholar] [CrossRef]
- Zalineeva, A.; Serov, A.; Padilla, M.; Martinez, U.; Artyushkova, K.; Baranton, S.; Coutanceau, C.; Atanassov, P. Nano-structured Pd-Sn catalysts for alcohol electro-oxidation in alkaline medium. Electrochem. Commun. 2015, 57, 48–51. [Google Scholar] [CrossRef]
- Adekoya, J.; Dare, E.; Mesubi, M.; Nejo, A.A.; Swart, H.; Revaprasadu, N. Synthesis of polyol based Ag/Pd nanocomposites for applications in catalysis. Results Phys. 2014, 4, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yi, Q.; Deng, Z.; Zhou, X.; Nie, H. Excellent Electroactivity of Ternary Pd–Ag–Sn Nanocatalysts for Ethanol Oxidation. Catal. Lett. 2018, 148, 1190–1201. [Google Scholar] [CrossRef]
- Wei, Y.-C.; Liu, C.-W.; Kang, W.-D.; Lai, C.-M.; Tsai, L.-D.; Wang, K.-W. Electro-catalytic activity enhancement of Pd–Ni electrocatalysts for the ethanol electro-oxidation in alkaline medium: The promotional effect of CeO2 addition. J. Electroanal. Chem. 2011, 660, 64–70. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, K.; Cai, W.-B. Enhanced Electrocatalysis of Ethanol on Dealloyed Pd-Ni-P Film in Alkaline Media: An Infrared Spectroelectrochemical Investigation. Electrochim. Acta 2015, 162, 100–107. [Google Scholar] [CrossRef]
- Pan, B.; Chen, F.; Kou, B.; Wang, J.; Tang, Q.; Guo, L.; Wang, Q.; Li, Z.; Bian, W.; Wang, J. Unexpectedly high stability and surface reconstruction of PdAuAg nanoparticles for formate oxidation electrocatalysis. Nanoscale 2020, 12, 11659–11671. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-M.; Lin, Z.-J.; Jia, T.-T.; Cai, Z.-M.; Huang, X.-L.; Jiang, Y.-Q.; Chen, X.; Chen, G.-N. A facile synthesis of palladium nanoparticles supported on functional carbon nanotubes and its novel catalysis for ethanol electrooxidation. Anal. Chim. Acta 2009, 650, 54–58. [Google Scholar] [CrossRef]
- Correa, P.D.S.; Da Silva, E.L.; Da Silva, R.F.; Radtke, C.; Moreno, B.; Chinarro, E.; Malfatti, C. Effect of decreasing platinum amount in Pt–Sn–Ni alloys supported on carbon as electrocatalysts for ethanol electrooxidation. Int. J. Hydrog. Energy 2012, 37, 9314–9323. [Google Scholar] [CrossRef]
- Habibi, E.; Bidad, E.; Feizbakhsh, A.; Fazli, M. Comparative electrooxidation of C1–C4 alcohols on Pd|CC nanoparticle anode catalyst in alkaline medium. Int. J. Hydrog. Energy 2014, 39, 18416–18423. [Google Scholar] [CrossRef]
- Feng, Y.-Y.; Liu, Z.-H.; Xu, Y.; Wang, P.; Wang, W.-H.; Kong, D.-S. Highly active PdAu alloy catalysts for ethanol electro-oxidation. J. Power Sources 2013, 232, 99–105. [Google Scholar] [CrossRef]



| Catalyst | C (mg) | PdCl2 (mg) | NiCl2 (mg) | AgNO3 (mg) | Metal Wt. % |
|---|---|---|---|---|---|
| Pd/C | 132 | 30 | 12 | ||
| PdAgNi/C | 132 | 11.7 | 8.5 | 11.2 | 12 |
| Pd4Ag2Ni1/C | 132 | 18.3 | 3.4 | 8.8 | 12 |
| Ag/C | 132 | 40 | 12 | ||
| Ni/C | 132 | 28.3 | 12 |
| Catalyst | Acc. Voltage | Pd | Ni | Ag | Tot. Metal Wt. % | |||
|---|---|---|---|---|---|---|---|---|
| Wt. % * | At. % ** | Wt. % | At. % | Wt. % | At. % | |||
| Pd4Ag2Ni1/C | 10 kV | 7.89 | 1.02 | 1.64 | 0.38 | 1.98 | 0.25 | 11.51 |
| 20 kV | 8.28 | 1.09 | 1.10 | 0.26 | 3.26 | 0.42 | 12.46 | |
| PdAgNi/C | 10 kV | 6.47 | 0.88 | 7.68 | 1.90 | 3.39 | 0.46 | 17.54 |
| 20 kV | 6.92 | 0.94 | 5.04 | 1.24 | 4.10 | 0.55 | 16.06 | |
| Catalyst | C At. % | O at. % | Pd at. % | Ag at. % | Ni at. % | Pd:Ag:Ni Ratio | ||
|---|---|---|---|---|---|---|---|---|
| Pd0 | Pd2+ | Ni0 | Ni2+ | |||||
| Pd/C | 96.13 | 1.78 | 1.63 | 0.45 | ||||
| Pd4Ag2Ni1/C | 93.91 | 3.14 | 1.59 | 0 | 0.4 | 0.05 | 0.73 | 4:1:2 |
| PdAgNi/C | 92.15 | 4.69 | 0.64 | 0.12 | 0.32 | 0 | 2.01 | 2.7: 1:6.7 |
| Catalyst | ECSA (cm2/mg) | Eonset (mV) vs. NHE | jp (A/mgPd) | Ref. |
|---|---|---|---|---|
| Pd/C | 1350 | −390 | 1.8 | This work |
| PdAgNi/C | 1500 | −500 | 2.7 | |
| Pd4Ag2Ni1/C | 1618 | −500 | 2.3 | |
| Pd/C | 549 | −150 | 0.5 | [54] |
| Pd83Ni17 | 375 | −250 | 1.1 | |
| PdNi | 209 | −260 | 1.45 | [29] |
| Pd | 135 | −209 | 0.8 | |
| Pd1Au1/C | 1320 | −260 | 12 | [68] |
| Pd/C | −260 | 0.75 | ||
| Pd2Sn2Ag1/C | 243 | −360 | 0.8 | [42] |
| Pd2Sn2Ni1/C | 209 | −330 | 0.4 | |
| Pd2Sn2Co1/C | 212 | −300 | 0.35 | |
| PdAgCu | 506 | −360 | 4.56 | [43] |
| Pd/C | −350 | 1.47 | [21] | |
| Pt/C | −400 | 0.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsheikh, A.; McGregor, J. Synthesis and Characterization of PdAgNi/C Trimetallic Nanoparticles for Ethanol Electrooxidation. Nanomaterials 2021, 11, 2244. https://doi.org/10.3390/nano11092244
Elsheikh A, McGregor J. Synthesis and Characterization of PdAgNi/C Trimetallic Nanoparticles for Ethanol Electrooxidation. Nanomaterials. 2021; 11(9):2244. https://doi.org/10.3390/nano11092244
Chicago/Turabian StyleElsheikh, Ahmed, and James McGregor. 2021. "Synthesis and Characterization of PdAgNi/C Trimetallic Nanoparticles for Ethanol Electrooxidation" Nanomaterials 11, no. 9: 2244. https://doi.org/10.3390/nano11092244
APA StyleElsheikh, A., & McGregor, J. (2021). Synthesis and Characterization of PdAgNi/C Trimetallic Nanoparticles for Ethanol Electrooxidation. Nanomaterials, 11(9), 2244. https://doi.org/10.3390/nano11092244







