Impact of Structural Functionalization, Pore Size, and Presence of Extra-Framework Ions on the Capture of Gaseous I2 by MOF Materials
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lenzen, M. Life cycle energy and greenhouse gas emissions of nuclear energy: A review. Energy Convers. Manag. 2008, 49, 2178–2199. [Google Scholar] [CrossRef]
- Cohen, B.L. High-level radioactive waste from light-water reactors. Rev. Mod. Phys. 1977, 49, 1–20. [Google Scholar] [CrossRef]
- Küpper, F.C.; Feiters, M.C.; Olofsson, B.; Kaiho, T.; Yanagida, S.; Zimmermann, M.B.; Carpentier, L.J.; Luther, G.W.; Lu, Z.; Jonsson, M.; et al. Commemorating Two Centuries of Iodine Research: An Interdisciplinary Overview of Current Research. Angew. Chem. Int. Ed. 2011, 50, 11598–11620. [Google Scholar] [CrossRef]
- Haefner, D.; Tranter, T. Methods of Gas Phase Capture of Iodine from Fuel Reprocessing Off-Gas: A Literature Survey; Report INL/EXT-07-12299, 16; Idaho National Laboratory: Idaho Falls, ID, USA, 2007.
- Mnasri, N.; Charnay, C.; de Ménorval, L.C.; Moussaoui, Y.; Elaloui, E.; Zajac, J. Silver nanoparticle-containing submicron-in-size mesoporous silica-based systems for iodine entrapment and immobilization from gas phase. Microporous Mesoporous Mater. 2014, 196, 305–313. [Google Scholar] [CrossRef]
- Massasso, G.; Long, J.; Haines, J.; Devautour-Vinot, S.; Maurin, G.; Grandjean, A.; Onida, B.; Donnadieu, B.; Larionova, J.; Guérin, C.; et al. Iodine Capture by Hofmann-Type Clathrate NiII(pz)[NiII(CN)4]. Inorg. Chem. 2014, 53, 4269–4271. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal–Organic Frameworks in Biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef]
- Czaja, A.U.; Trukhan, N.; Müller, U. Industrial applications of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1284–1293. [Google Scholar] [CrossRef]
- Lee, J.Y.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal-Organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Canivet, J.; Fateeva, A.; Guo, Y.; Coasne, B.; Farrusseng, D. Water adsorption in MOFs: Fundamentals and applications. Chem. Soc. Rev. 2014, 43, 5594–5617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramaswamy, P.; Wong, N.E.; Shimizu, G.K.H. MOFs as proton conductors—challenges and opportunities. Chem. Soc. Rev. 2014, 43, 5913–5932. [Google Scholar] [CrossRef]
- Getman, R.B.; Bae, Y.S.; Wilmer, C.E.; Snurr, R.Q. Review and Analysis of Molecular Simulations of Methane, Hydrogen, and Acetylene Storage in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 703–723. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.H.; Long, J.R. Carbon Dioxide Capture in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
- Suh, M.P.; Park, H.J.; Prasad, T.K.; Lim, D.W. Hydrogen Storage in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 782–835. [Google Scholar] [CrossRef]
- Li, J.R.; Sculley, J.; Zhou, H.C. Metal–Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Kim, K.H. Green synthesis of metal-organic frameworks: A state-of-the-art review of potential environmental and medical applications. Coord. Chem. Rev. 2020, 420, 213407. [Google Scholar] [CrossRef]
- Zhang, S.; Xia, W.; Yang, Q.; Kaneti, Y.V.; Xu, X.; Alshehri, S.M.; Ahamad, T.; Hossain, S.A.; Na, J.; Tang, J.; et al. Core-shell motif construction: Highly graphitic nitrogen-doped porous carbon electrocatalysts using MOF-derived carbon@COF heterostructures as sacrificial templates. Chem. Eng. J. 2020, 396, 125154. [Google Scholar] [CrossRef]
- Gumilar, G.; Kaneti, Y.V.; Henzie, J.; Chatterjee, S.; Na, J.; Yuliarto, B.; Nugraha, N.; Patah, A.; Bhaumik, A.; Yamauchi, Y. General synthesis of hierarchical sheet/plate-like M-BDC (M=Cu, Mn, Ni and Zr) metal-organic frameworks fpr electrochemical non-enzymatic glucose sensing. Chem. Sci. 2020, 11, 3644–3655. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Kaneti, Y.V.; Bando, Y.; Lin, J.; Liu, C.; Li, J.; Yamauchi, Y. Metal-Organic framework-derived one-dimensional porous or hollow carbon-based nanofibres for energy storage and conversion. Mater. Horiz. 2018, 5, 394. [Google Scholar] [CrossRef] [Green Version]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef]
- Lu, W.; Wei, Z.; Gu, Z.Y.; Liu, T.F.; Park, J.; Park, J.; Tian, J.; Zhang, M.; Zhang, Q.; Gentle, T., III; et al. Tuning the structure and function of metal–organic frameworks via linker design. Chem. Soc. Rev. 2014, 43, 5561–5593. [Google Scholar] [CrossRef]
- Nouar, F.; Breeze, M.I.; Campo, B.C.; Vimont, A.; Clet, G.; Daturi, M.; Devic, T.; Walton, R.I.; Serre, C. Tuning the properties of the UiO-66 metal organic framework by Ce substitution. Chem. Comm. 2015, 51, 14458–14461. [Google Scholar] [CrossRef]
- Devic, T.; Serre, C. High valence 3p and transition metal based MOFs. Chem. Soc. Rev. 2014, 43, 6097–6115. [Google Scholar] [CrossRef]
- Zhai, Q.G.; Bu, X.H.; Mao, C.Y.; Zhao, X.; Feng, P.Y. Systematic and Dramatic Tuning on Gas Sorption Performance in Heterometallic Metal–Organic Frameworks. J. Am. Chem. Soc. 2016, 138, 2524–2527. [Google Scholar] [CrossRef]
- Xiong, Y.; Fan, Y.Z.; Damasceno Borges, D.; Chen, C.X.; Wei, Z.W.; Wang, H.P.; Pan, M.; Jiang, J.J.; Maurin, G.; Su, C.Y. Ligand and Metal Effects on the Stability and Adsorption Properties of an Isoreticular Series of MOFs Based on T-Shaped Ligands and Paddle-Wheel Secondary Building Units. Chem. Eur. J. 2016, 22, 16147–16156. [Google Scholar] [CrossRef] [PubMed]
- Yot, P.; Yang, K.; Guillerm, V.; Ragon, F.; Dmitriev, V.; Parisiades, P.; Elkaim, E.; Devic, T.; Horcajada, P.; Serre, C.; et al. Impact of the Metal Centre and Functionalization on the Mechanical Behaviour of MIL-53 Metal–Organic Frameworks. Eur. J. Inorg. Chem. 2016, 27, 4424–4429. [Google Scholar] [CrossRef] [Green Version]
- Tanabe, K.K.; Cohen, S.M. Postsynthetic modification of metal–organic frameworks—A progress report. Chem. Soc. Rev. 2011, 40, 498–519. [Google Scholar] [CrossRef] [PubMed]
- Dau, P.V.; Tanabe, K.K.; Cohen, S.M. Functional group effects on metal–organic framework topology. Chem. Comm. 2012, 48, 9370–9372. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic Design of Pore Size and Functionality in Isoreticular MOFs and Their Application in Methane Storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Cohen, S.M. Modulating Metal−Organic Frameworks to Breathe: A Postsynthetic Covalent Modification Approach. J. Am. Chem. Soc. 2009, 131, 16675–16677. [Google Scholar] [CrossRef]
- He, Y.; Li, B.; O’Keeffe, M.; Chen, B. Multifunctional metal–organic frameworks constructed from meta-benzenedicarboxylate units. Chem. Soc. Rev. 2014, 43, 5618–5656. [Google Scholar] [CrossRef] [PubMed]
- Devic, T.; Horcajada, P.; Serre, C.; Salles, F.; Maurin, G.; Moulin, B.; Heurtaux, D.; Clet, G.; Vimont, A.; Greneche, J.M.; et al. Functionalization in flexible porous solids: Effects on the pore opening and the host-guest interactions. J. Am. Chem. Soc. 2010, 132, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Salles, F.; Wuttke, S.; Devic, T.; Heurtaux, D.; Maurin, G.; Vimont, A.; Daturi, M.; David, O.; Magnier, E.; et al. How Linker’s Modification Controls Swelling Properties of Highly Flexible Iron(III) Dicarboxylates MIL-88. J. Am. Chem. Soc. 2011, 133, 17839–17847. [Google Scholar] [CrossRef] [PubMed]
- Sava, D.F.; Garino, T.J.; Nenoff, T.M. Iodine Confinement into Metal–Organic Frameworks (MOFs): Low-Temperature Sintering Glasses To Form Novel Glass Composite Material (GCM) Alternative Waste Forms. Ind. Eng. Chem. Res. 2012, 51, 614–620. [Google Scholar] [CrossRef]
- Banerjee, D.; Simon, C.M.; Plonka, A.M.; Motkuri, R.K.; Liu, J.; Chen, X.; Smit, B.; Parise, J.B.; Haranczyk, M.; Thallapally, P.K. Metal–organic framework with optimally selective xenon adsorption and separation. Nat. Comm. 2016, 7, 11831. [Google Scholar] [CrossRef]
- Riley, B.J.; Vienna, J.D.; Strachan, D.M.; McCloy, J.S.; Jerden, J.L., Jr. Materials and processes for the effective capture and immobilization of radioiodine: A review. J. Nucl. Mater. 2016, 470, 307–326. [Google Scholar] [CrossRef] [Green Version]
- Munn, A.S.; Millange, F.; Frigoli, M.; Guillou, N.; Falaise, C.; Stevenson, V.; Volkringer, C.; Loiseau, T.; Cibin, G.; Walton, R.I. Iodine sequestration by thiol-modified MIL-53(Al). CrystEngComm 2016, 18, 8108–8114. [Google Scholar] [CrossRef] [Green Version]
- Mao, P.; Qi, B.; Liu, Y.; Zhao, L.; Jiao, Y.; Zhang, Y.; Jiang, Z.; Li, Q.; Wang, J.; Chen, S.; et al. AgII doped MIL-101 and its adsorption of iodine with high speed in solution. J. Solid State Chem. 2016, 237, 274–283. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Yang, J.; Wang, S.; Li, J. Reversible flexible structural changes in multidimensional MOFs by guest molecules (I2, NH3) and thermal stimulation. J. Solid State Chem. 2015, 226, 114–119. [Google Scholar] [CrossRef]
- Abney, C.W.; Taylor-Pashow, M.L.; Russell, S.R.; Chen, Y.; Samantaray, R.; Lockard, J.V.; Lin, W. Topotactic Transformations of Metal–Organic Frameworks to Highly Porous and Stable Inorganic Sorbents for Efficient Radionuclide Sequestration. Chem. Mater. 2014, 26, 5231–5243. [Google Scholar] [CrossRef]
- Shi, P.F.; Hu, H.C.; Zhang, Z.Y.; Xiong, G.; Zhao, B. Heterometal–organic frameworks as highly sensitive and highly selective luminescent probes to detect I− ions in aqueous solutions. Chem. Commun. 2015, 51, 3985–3988. [Google Scholar] [CrossRef] [PubMed]
- Düren, T.; Bae, Y.S.; Snurr, R.Q. Using molecular simulation to characterise metal–organic frameworks for adsorption applications. Chem. Soc. Rev. 2009, 38, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Basdogan, Y.; Keskin, S. Simulation and modelling of MOFs for hydrogen storage. CrystEngComm 2015, 17, 261–275. [Google Scholar] [CrossRef] [Green Version]
- Trousselet, F.; Archereau, A.; Boutin, A.; Coudert, F.X. Heterometallic Metal–Organic Frameworks of MOF-5 and UiO-66 Families: Insight from Computational Chemistry. J. Phys. Chem. C 2016, 120, 24885–24894. [Google Scholar] [CrossRef] [Green Version]
- Taddei, M.; Tiana, D.; Casati, N.; van Bokhoven, J.A.; Smit, B.; Ranocchiari, M. Mixed-linker UiO-66: Structure–property relationships revealed by a combination of high-resolution powder X-ray diffraction and density functional theory calculations. Phys. Chem. Chem. Phys. 2017, 19, 1551–1559. [Google Scholar] [CrossRef] [Green Version]
- Sava, D.F.; Chapman, K.W.; Rodriguez, M.A.; Greathouse, J.A.; Crozier, P.S.; Zhao, H.; Chupas, P.J.; Nenoff, T.M. Competitive I2 Sorption by Cu-BTC from Humid Gas Streams. Chem. Mater. 2013, 25, 2591–2596. [Google Scholar] [CrossRef]
- Ghose, S.K.; Li, Y.; Yakovenko, A.; Dooryhee, E.; Ehm, L.; Ecker, L.E.; Dippel, A.C.; Halder, G.J.; Strachan, D.M.; Thallapally, P.K. Understanding the Adsorption Mechanism of Xe and Kr in a Metal–Organic Framework from X-ray Structural Analysis and First-Principles Calculations. J. Phys. Chem. Lett. 2015, 6, 1790–1794. [Google Scholar] [CrossRef]
- Ryan, P.; Farha, O.K.; Broadbelt, L.; Snurr, R.Q. Computational screening of metal-organic frameworks for xenon/krypton separation. AIChE J. 2011, 57, 1759–1766. [Google Scholar] [CrossRef]
- Sikora, B.J.; Wilmer, C.E.; Greenfield, M.L.; Snurr, R.Q. Thermodynamic analysis of Xe/Kr selectivity in over 137 000 hypothetical metal–organic frameworks. Chem. Sci. 2012, 3, 2217–2223. [Google Scholar] [CrossRef]
- Maurin, G. Role of molecular simulations in the structure exploration of Metal-Organic Frameworks: Illustrations through recent advances in the field. C. R. Chim. 2016, 19, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Hughes, J.T.; Bennett, T.D.; Cheetham, A.K.; Navrotsky, A. Thermochemistry of Zeolitic Imidazolate Frameworks of Varying Porosity. J. Am. Chem. Soc. 2013, 135, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Sava, D.F.; Rodriguez, M.A.; Chapman, K.W.; Chupas, P.J.; Greathouse, J.A.; Crozier, P.S.; Nenoff, T.M. Capture of Volatile Iodine, a Gaseous Fission Product, by Zeolitic Imidazolate Framework-8. J. Am. Chem. Soc. 2011, 133, 12398–12401. [Google Scholar] [CrossRef] [PubMed]
- Falaise, C.; Volkringer, C.; Facqueur, J.; Bousquet, T.; Gasnot, L.; Loiseau, T. Capture of iodine in highly stable metal–organic frameworks: A systematic study. Chem. Commun. 2013, 49, 10320–10322. [Google Scholar] [CrossRef] [PubMed]
- Assfour, B.; Assaad, T.; Odeh, A. In silico screening of metal organic framework for iodine capture and storage. Chem. Phys. Lett. 2014, 610–611, 45–49. [Google Scholar] [CrossRef]
- Yuan, S.; Qin, J.S.; Zou, L.F.; Chen, Y.P.; Wang, X.; Zhang, Q.; Zhou, H.C. Thermodynamically Guided Synthesis of Mixed-Linker Zr-MOFs with Enhanced Tunability. J. Am. Chem. Soc. 2016, 138, 6636–6642. [Google Scholar] [CrossRef]
- Lan, Y.; Tong, M.; Yang, Q.; Zhong, C. Computational screening of covalent organic frameworks for the capture of radioactive iodine and methyl iodide. CrystEngComm 2017, 19, 4920–4926. [Google Scholar] [CrossRef]
- Jin, K.; Lee, B.; Park, J. Metal-Organic frameworks as a versatile platform for radionuclide management. Coord. Chem. Rev. 2020, 424, 213473. [Google Scholar]
- Horcajada, P.; Chevreau, H.; Heurtaux, D.; Benyettou, F.; Salles, F.; Devic, T.; Garcia-Marquez, A.; Yu, C.; Lavrard, H.; Dutson, C.L.; et al. Extended and functionalized porous iron(III) tri- or dicarboxylates with MIL-100/101 topologies. Chem. Commun. 2014, 50, 6872–6874. [Google Scholar] [CrossRef]
- Chevreau, H.; Permyakova, A.; Nouar, F.; Fabry, P.; Livage, C.; Ragon, F.; Garcia-Marquez, A.; Devic, T.; Steunou, N.; Serre, C.; et al. Synthesis of the biocompatible and highly stable MIL-127(Fe): From large scale synthesis to particle size control. CrystEngComm 2016, 18, 4094–4101. [Google Scholar] [CrossRef]
- Yang, E.; Ding, Q.R.; Kang, Y.; Wang, F. Synthesis and characterization of homo- and heterometallic metal–organic frameworks based on 1,2,4,5-benzenetetracarboxylate ligand. Inorg. Chem. Commun. 2013, 36, 195–198. [Google Scholar] [CrossRef]
- Yang, Q.Y.; Wiersum, A.D.; Llewellyn, P.L.; Guillerm, V.; Serre, C.; Maurin, G. Functionalizing porous zirconium terephthalate UiO-66(Zr) for natural gas upgrading: A computational exploration. Chem. Commun. 2011, 47, 9603–9605. [Google Scholar] [CrossRef]
- Nandanwar, S.U.; Coldsnow, K.; Utgikar, V.; Sabharwall, P.; Aston, D.E. Capture of harmful radioactive contaminants from off-gas stream using solid sorbents for clean environment—A review. Chem. Eng. J. 2016, 306, 369–381. [Google Scholar] [CrossRef] [Green Version]
- Rappe, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A.; Skiff, W.M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Salles, F.; Maurin, G.; Serre, C.; Llewellyn, P.L.; Knöfel, C.; Choi, H.J.; Filinchuk, Y.; Oliviero, L.; Vimont, A.; Long, J.R.; et al. Multistep N2 breathing in the Metal-Organic Framework Co(1,4-benzenedipyrazolate). J. Am. Chem. Soc. 2010, 132, 13782–13788. [Google Scholar] [CrossRef]
- Dassault Systèmes. BIOVIA Materials Studio; Dassault Systèmes: San Diego, CA, USA, 2020. [Google Scholar]
- Chebbi, M.; Chibani, S.; Paul, J.F.; Cantrel, L.; Badawi, M. Evaluation of volatile iodine trapping in presence of contaminants: A periodic DFT study on cation exchanged-faujasite. Microporous Mesoporous Mater. 2017, 239, 111–122. [Google Scholar] [CrossRef]
- Sava-Gallis, D.F.; Ermanoski, I.; Greathouse, J.A.; Chapman, K.W.; Nenoff, T.M. Iodine gas adsorption in nanoporous materials: A combined experimental-modeling study. Int. Eng. Chem. Res. 2017, 56, 2331–2338. [Google Scholar] [CrossRef]
- Chibani, S.; Chiter, F.; Cantrel, L.; Paul, J.F. Capture of iodine species in MIL-53(Al), MIL-120(Al) and HKUST-1(Cu) periodic DFT and ab initio Molecular Dynamics studies. J. Phys. Chem. C 2017, 121, 25283–25291. [Google Scholar] [CrossRef]
- Chen, P.; He, X.; Pang, M.; Dong, X.; Zhao, S.; Zhang, W. Iodine capture using Zr-based Metal-Organic Frameworks (Zr-MOFs): Adsorption performance and mechanism. Appl. Mater. Interf. 2020, 12, 20429–20439. [Google Scholar] [CrossRef] [PubMed]
- Düren, T.; Millange, F.; Férey, G.; Walton, K.S.; Snurr, R.Q. Calculating Geometric Surface Areas as a Characterization Tool for Metal−Organic Frameworks. J. Phys. Chem. C 2007, 111, 15350–15356. [Google Scholar] [CrossRef]
- Wang, L.; Chen, P.; Dong, X.; Zhang, W.; Zhao, S.; Xiao, S.; Ouyang, Y. Porous MOF-808@PVDF beads for removal of iodine from gas streams. RSC Adv. 2020, 10, 44679. [Google Scholar] [CrossRef]
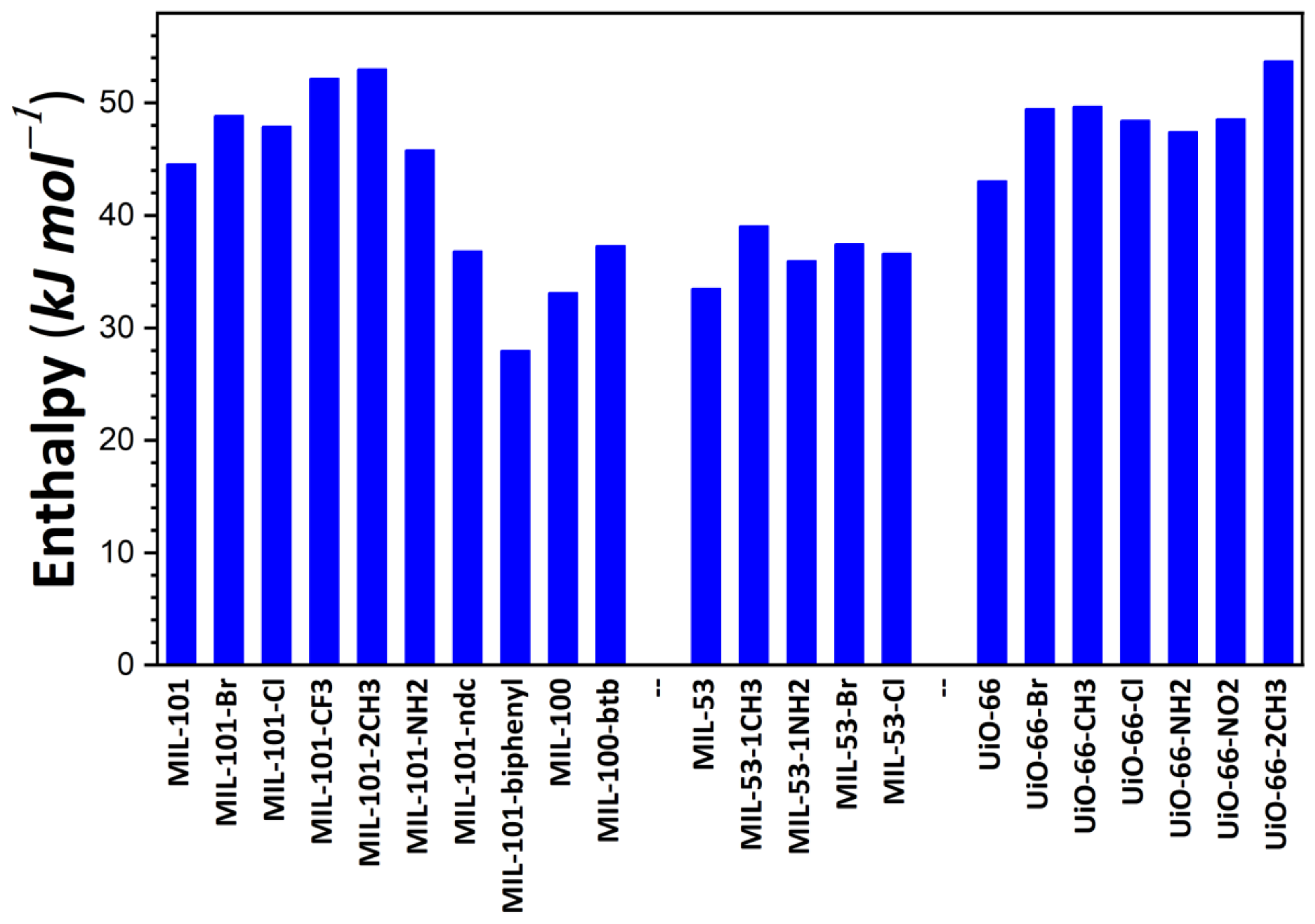
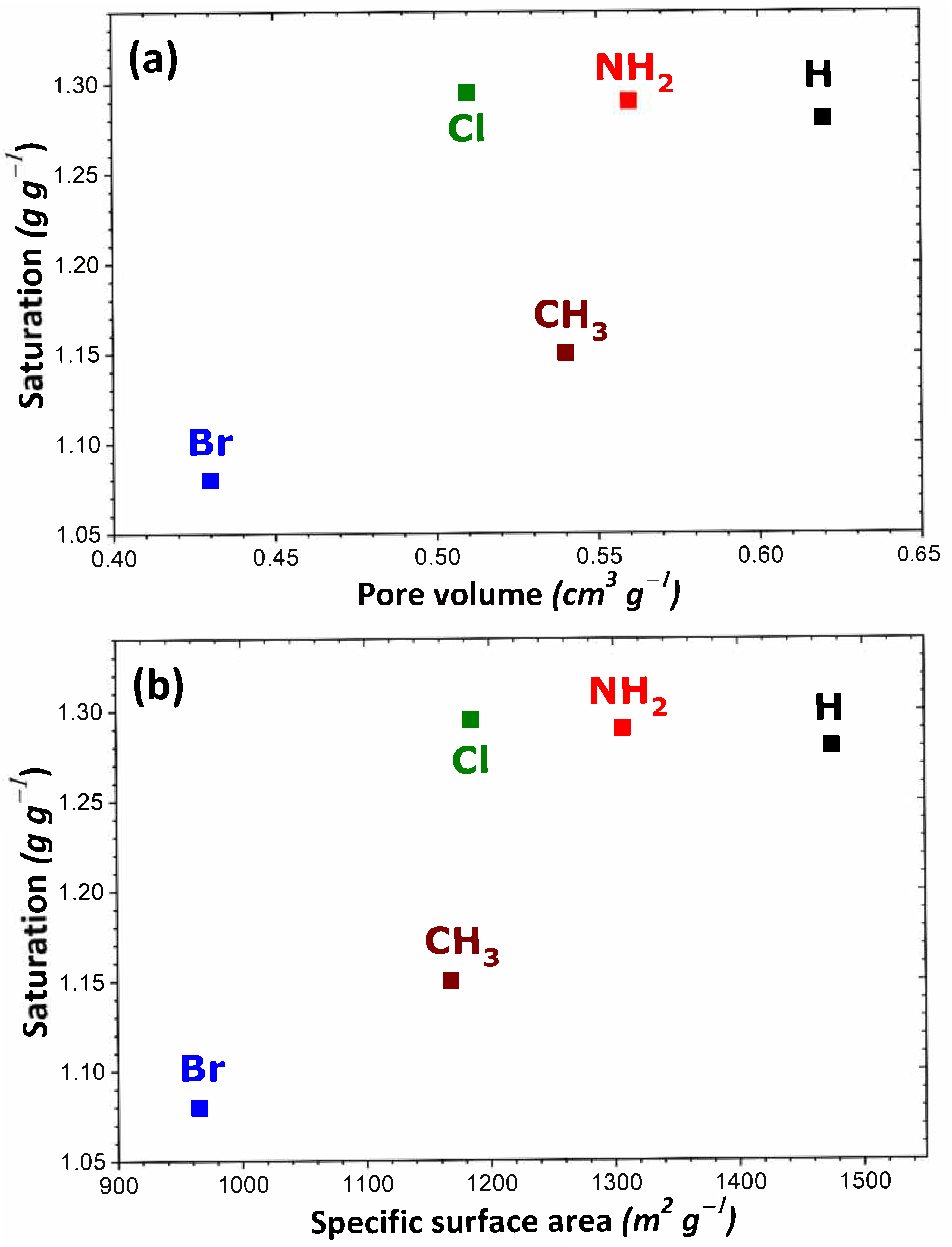

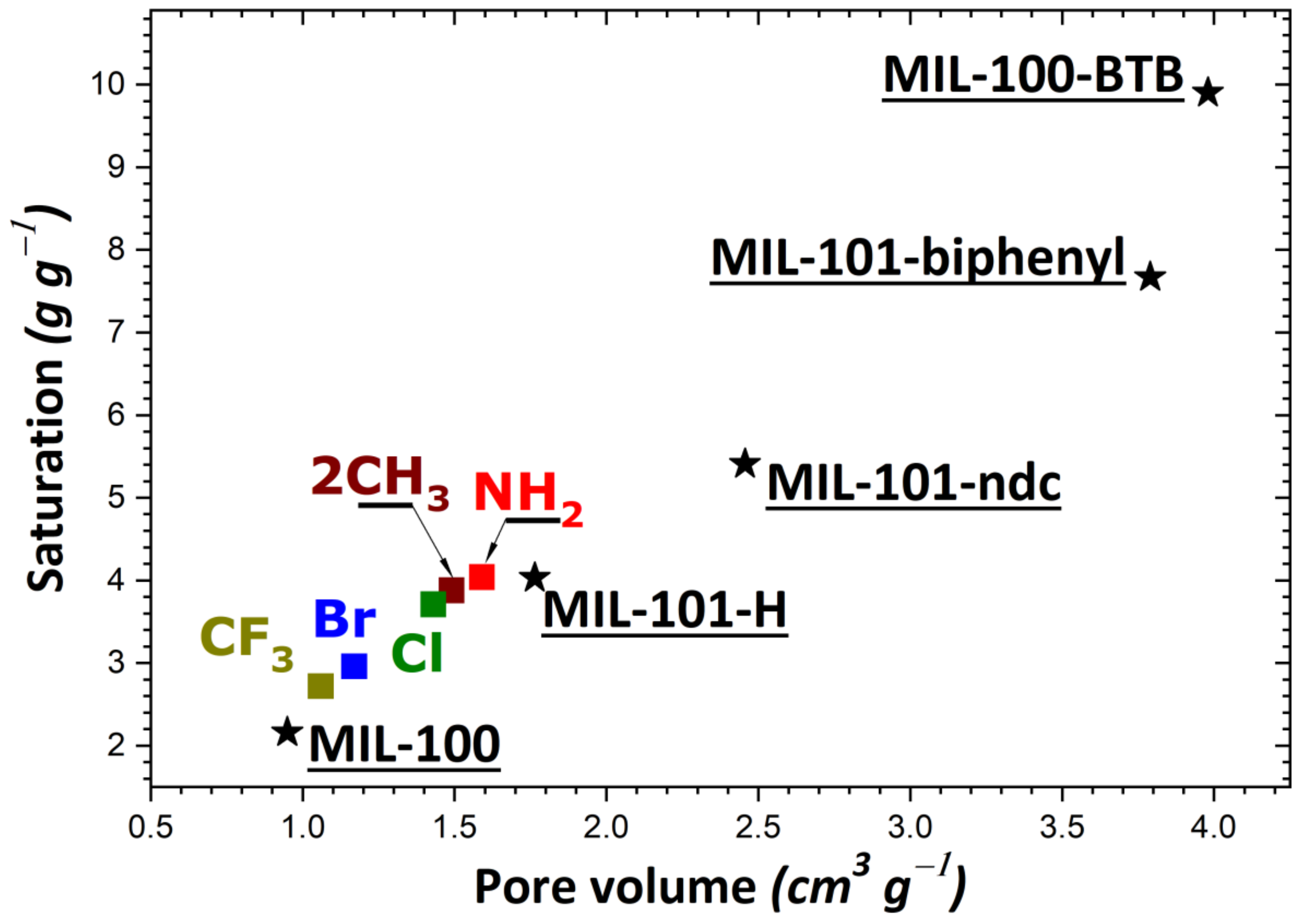
| Sample | Enthalpy (kJ/mol) | I2 Amount Adsorbed at 1 bar (g/g) | ||
|---|---|---|---|---|
| 300 K | 353 K | 300 K | 353 K | |
| MIL-53-Br | 37.5 | 37.3 | 0.97 | 0.73 |
| UiO-66-CH3 | 49.7 | 49.7 | 0.57 | 0.54 |
| UiO-66-2CH3 | 53.7 | 53.6 | 0.60 | 0.45 |
| MIL-100-BTB | 37.2 | 36.7 | 2.30 | 0.80 |
| MIL-101-CF3 | 52.2 | 51.9 | 1.68 | 0.50 |
| MIL-101-2CH3 | 53.1 | 53.4 | 2.67 | 0.72 |
| MIL-101-biphenyl | 27.9 | 26.1 | 2.60 | 0.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salles, F.; Zajac, J. Impact of Structural Functionalization, Pore Size, and Presence of Extra-Framework Ions on the Capture of Gaseous I2 by MOF Materials. Nanomaterials 2021, 11, 2245. https://doi.org/10.3390/nano11092245
Salles F, Zajac J. Impact of Structural Functionalization, Pore Size, and Presence of Extra-Framework Ions on the Capture of Gaseous I2 by MOF Materials. Nanomaterials. 2021; 11(9):2245. https://doi.org/10.3390/nano11092245
Chicago/Turabian StyleSalles, Fabrice, and Jerzy Zajac. 2021. "Impact of Structural Functionalization, Pore Size, and Presence of Extra-Framework Ions on the Capture of Gaseous I2 by MOF Materials" Nanomaterials 11, no. 9: 2245. https://doi.org/10.3390/nano11092245
APA StyleSalles, F., & Zajac, J. (2021). Impact of Structural Functionalization, Pore Size, and Presence of Extra-Framework Ions on the Capture of Gaseous I2 by MOF Materials. Nanomaterials, 11(9), 2245. https://doi.org/10.3390/nano11092245







