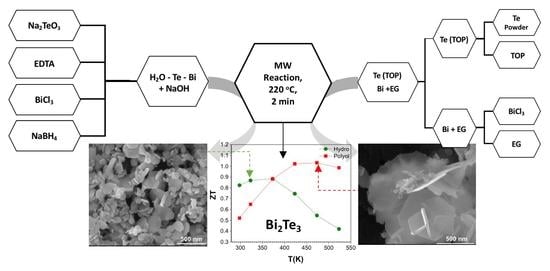
Minute-Made, High-Efficiency Nanostructured Bi2Te3 via High-Throughput Green Solution Chemical Synthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Processing of Thermoelectric Materials
2.1.1. Synthesis by Hydrothermal Method
2.1.2. Synthesis by Polyol Method
2.2. Consolidation of the Powders via Spark Plasma Sintering
2.3. Structural and Morphological Characterization
2.4. Electronic and Thermal Transport Property Measurements
2.5. X-ray Photoelectron Spectroscopy
3. Results and Discussion
3.1. Structural Analysis
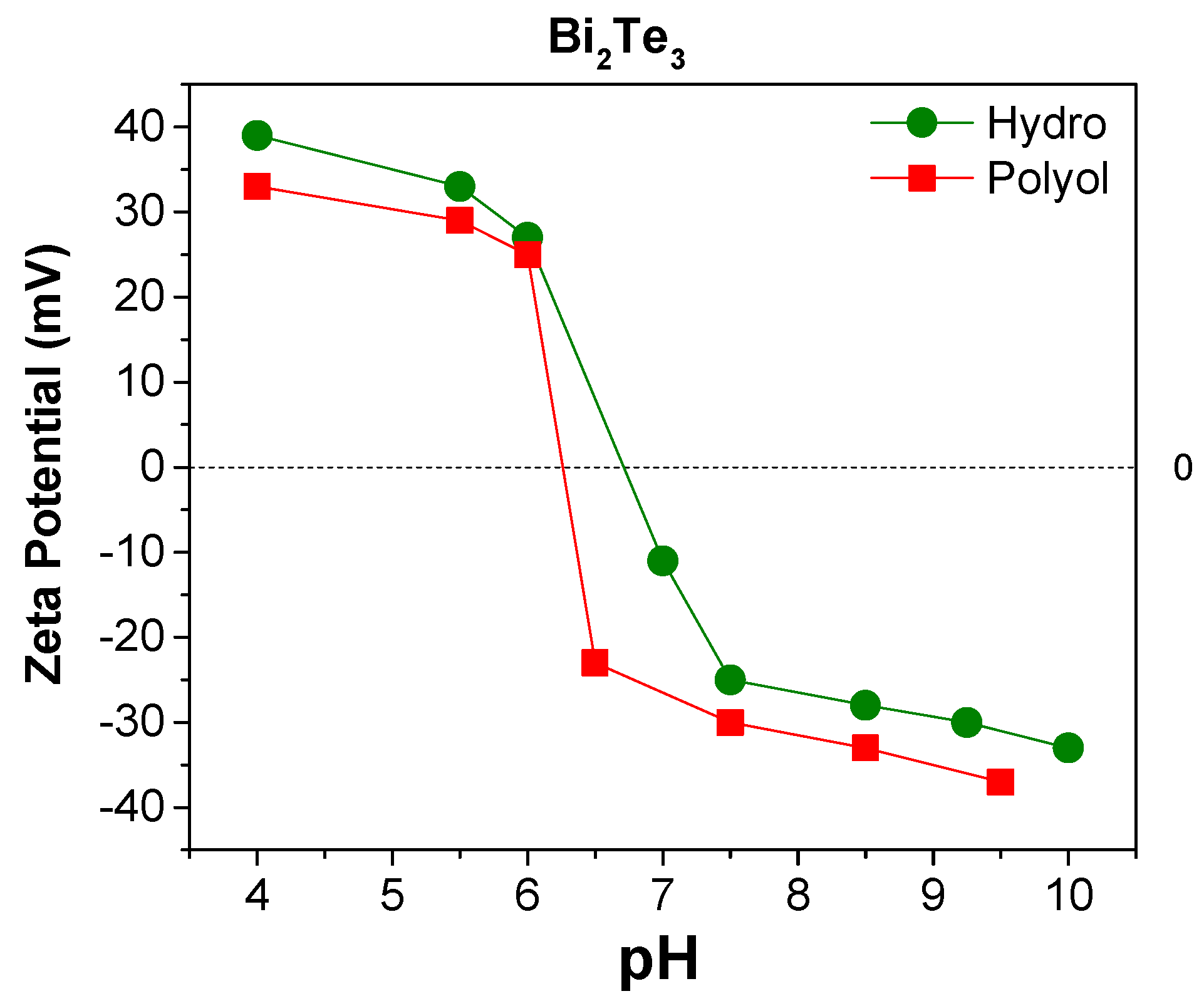
3.2. Surface Analysis
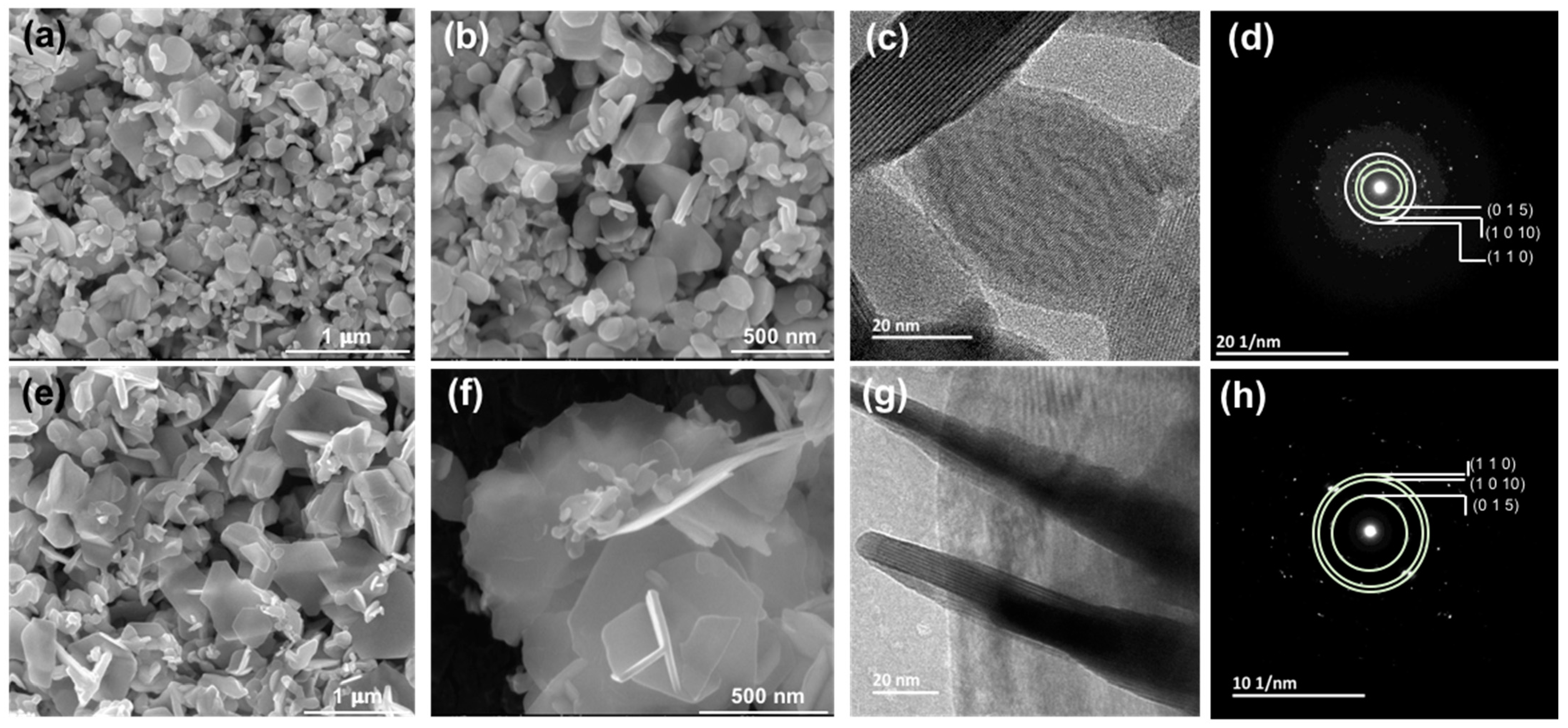
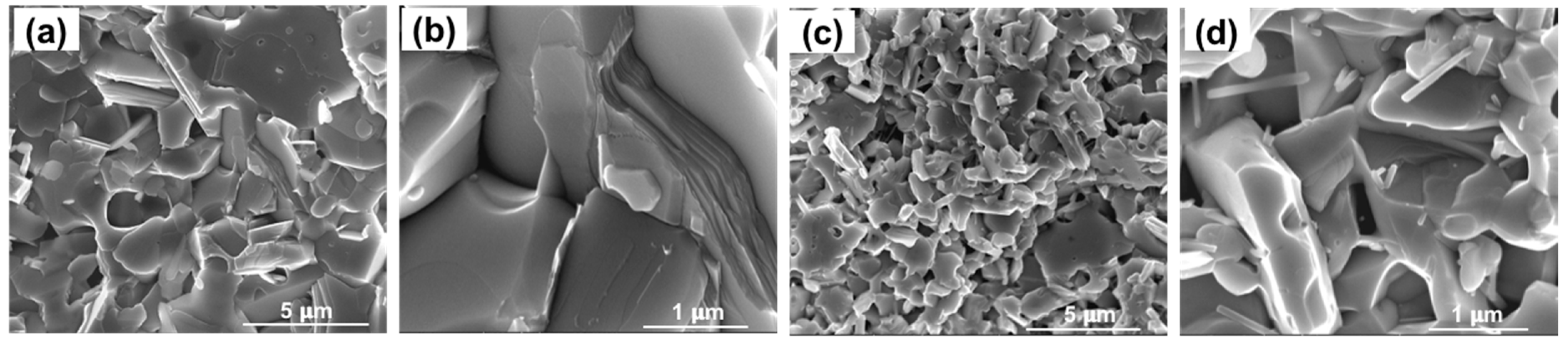
3.3. Microstructure Analysis
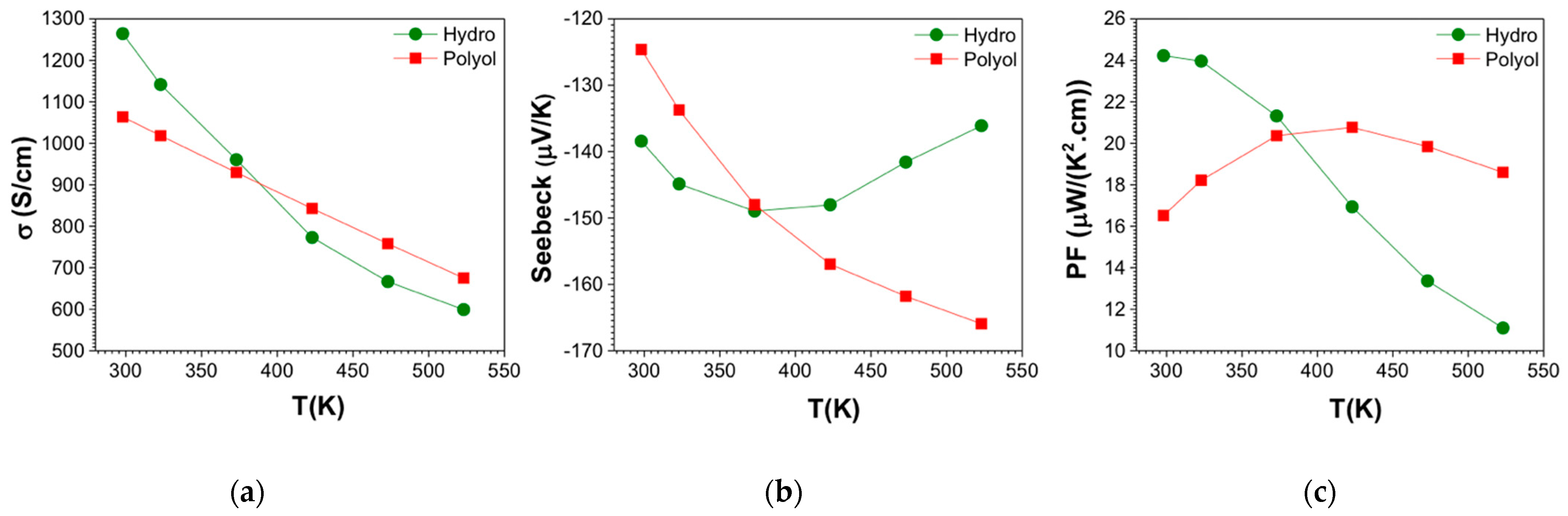
3.4. Electronic Transport Property Evaluation
3.5. Thermal Conductivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Shatkin, J.A. Nanomaterials Risks and Benefits (NATO Science for Peace and Security Studies). Risk Anal. 2009, 29, 1192–1195. [Google Scholar] [CrossRef]
- Petsagkourakis, I.; Tybrandt, K.; Crispin, X.; Ohkubo, I.; Satoh, N.; Mori, T. Thermoelectric materials and applications for energy harvesting power generation. Sci. Technol. Adv. Mater. 2018, 19, 836–862. [Google Scholar] [CrossRef]
- Sajid, M.; Hassan, I.; Rahman, A. An overview of cooling of thermoelectric devices. Renew. Sustain. Energy Rev. 2017, 78, 15–22. [Google Scholar] [CrossRef]
- Ovik, R.; Long, B.D.; Barma, M.C.; Riaz, M.; Sabri, M.F.M.; Said, S.M.; Saidur, R. A review on nanostructures of high-temperature thermoelectric materials for waste heat recovery. Renew. Sustain. Energy Rev. 2016, 64, 635–659. [Google Scholar] [CrossRef]
- Huen, P.; Daoud, W.A. Advances in hybrid solar photovoltaic and thermoelectric generators. Renew. Sustain. Energy Rev. 2017, 72, 1295–1302. [Google Scholar] [CrossRef]
- Jaziri, N.; Boughamoura, A.; Müller, J.; Mezghani, B.; Tounsi, F.; Ismail, M. A comprehensive review of Thermoelectric Generators: Technologies and common applications. Energy Rep. 2020, 6, 264–287. [Google Scholar] [CrossRef]
- Yusuf, A.; Bayhan, N.; Tiryaki, H.; Hamawandi, B.; Toprak, M.S.; Ballikaya, S. Multi-objective optimization of concentrated Photovoltaic-Thermoelectric hybrid system via non-dominated sorting genetic algorithm (NSGA II). Energy Convers. Manag. 2021, 236, 114065. [Google Scholar] [CrossRef]
- Champier, D. Thermoelectric generators: A review of applications. Energy Convers. Manag. 2017, 140, 167–181. [Google Scholar] [CrossRef]
- Snyder, G.J.; Toberer, E.S. Complex TE meterials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef]
- Polozine, A.; Sirotinskaya, S.; Schaeffer, L. History of development of thermoelectric materials for electric power generation and criteria of their quality. Mater. Res. 2014, 17, 1260–1267. [Google Scholar] [CrossRef]
- Ioffe, A.F. Semiconductor thermoelements and thermoelec. Phys. Today 1959, 12, 42. [Google Scholar] [CrossRef]
- Nakamura, Y.; Isogawa, M.; Ueda, T.; Yamasaka, S.; Matsui, H.; Kikkawa, J.; Ikeuchi, S.; Oyake, T.; Hori, T.; Shiomi, J.; et al. Anomalous reduction of thermal conductivity in coherent nanocrystal architecture for silicon thermoelectric material. Nano Energy 2015, 12, 845–851. [Google Scholar] [CrossRef]
- Rowe, D.M.; Shukla, V.S.; Savvides, N. Phonon scattering at grain boundaries in heavily doped fine-grained silicon-germanium alloys. Nature 1981, 290, 765–766. [Google Scholar] [CrossRef]
- Szczech, J.R.; Higgins, J.M.; Jin, S. Enhancement of the thermoelectric properties in nanoscale and nanostructured materials. J. Mater. Chem. 2011, 21, 4037–4055. [Google Scholar] [CrossRef]
- Nomura, M.; Kage, Y.; Müller, D.; Moser, D.; Paul, O. Electrical and thermal properties of polycrystalline Si thin films with phononic crystal nanopatterning for thermoelectric applications. Appl. Phys. Lett. 2015, 106, 1–5. [Google Scholar] [CrossRef]
- CRC Handbook of Thermoelectrics; CRC Press: Boca Raton, FL, USA, 2018.
- Harman, T.C.; Taylor, P.J.; Walsh, M.P.; LaForge, B.E. Quantum dot superlattice thermoelectric materials and devices. Science 2002, 297, 2229–2232. [Google Scholar] [CrossRef]
- Venkatasubramanian, R.; Siivola, E.; Colpitts, T.; O’Quinn, B. Thin-film thermoelectric devices with high room-temperature figures of merit. Nature 2001, 413, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Hamawandi, B.; Noroozi, M.; Jayakumar, G.; Ergül, A.; Zahmatkesh, K.; Toprak, M.S.; Radamson, H.H. Electrical properties of sub-100 nm SiGe nanowires. J. Semicond. 2016, 37, 102001. [Google Scholar] [CrossRef]
- Hicks, L.; Dresselhaus, M.S. Thermoelectric figure of merit of a one-dimensional conductor. Phys. Rev. B 1993, 47, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Harman, T.C.; Spears, D.L.; Manfra, M.J. High thermoelectric figures of merit in PbTe quantum wells. J. Electron. Mater. 1996, 25, 1121–1127. [Google Scholar] [CrossRef]
- Biswas, K.; He, J.; Zhang, Q.; Wang, G.; Uher, C.; Dravid, V.P.; Kanatzidis, M.G. Strained endotaxial nanostructures with high thermoelectric figure of merit. Nat. Chem. 2011, 3, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Hochbaum, A.I.; Chen, R.; Delgado, R.D.; Liang, W.; Garnett, E.C.; Najarian, M.; Majumdar, A.; Yang, P. Enhanced thermoelectric performance of rough silicon nanowires. Nature 2008, 451, 163–167. [Google Scholar] [CrossRef]
- Son, J.H.; Oh, M.W.; Kim, B.S.; Park, S.D.; Min, B.K.; Kim, M.H.; Lee, H.W. Effect of ball milling time on the thermoelectric properties of p-type (Bi,Sb)2Te3. J. Alloys Compd. 2013, 566, 168–174. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, C.; Miao, L.; Lin, H.; Gao, J.; Wang, X.; Chen, J.; Wu, S.; Li, X.; Cai, H. Cost effective synthesis of p-type Zn-doped MgAgSb by planetary ball-milling with enhanced thermoelectric properties. RSC Adv. 2018, 8, 35353–35359. [Google Scholar] [CrossRef]
- Sun, T.; Zhao, X.B.; Zhu, T.J.; Tu, J.P. Aqueous chemical reduction synthesis of Bi2Te3 nanowires with surfactant assistance. Mater. Lett. 2006, 60, 2534–2537. [Google Scholar] [CrossRef]
- Foos, E.E.; Stroud, R.M.; Berry, A.D. Synthesis and Characterization of Nanocrystalline Bismuth Telluride. Nano Lett. 2001, 1, 693–695. [Google Scholar] [CrossRef]
- Giani, A.; Pascal-Delannoy, F.; Boyer, A.; Foucaran, A.; Gschwind, M.; Ancey, P. Elaboration of Bi2Te3 by metal organic chemical vapor deposition. Thin Solid Film. 1997, 303, 1–3. [Google Scholar] [CrossRef]
- Venkatasubramanian, R.; Colpitts, T.; Watko, E.; Lamvik, M.; El-Masry, N. MOCVD of Bi2Te3, Sb2Te3 and their superlattice structures for thin-film thermoelectric applications. J. Cryst. Growth 1997, 170, 817–821. [Google Scholar] [CrossRef]
- Longo, M.; Cecchi, S.; Selmo, S.; Fanciulli, M.; Wiemer, C.; Mdm, L. MOCVD growth and thermal analysis of Sb2Te3 thin films and nanowires. In Proceedings of the 2015 1st Workshop on Nanotechnology in Instrumentation and Measurement (NANOFIM), Lecce, Italy, 24–25 July 2015; pp. 24–25. [Google Scholar]
- Lee, J.S.; Brittman, S.; Yu, D.; Park, H. Supporting Information for “ Vapor-Liquid-Solid and Vapor-Solid Growth of Phase-Change Sb2Te3 Nanowires and Sb2Te3/GeTe Nanowire Heterostructures. J. Am. Chem. Soc. 2008, 130, 1–8. [Google Scholar]
- Park, D.; Park, S.; Jeong, K.; Jeong, H.S.; Song, J.Y.; Cho, M.H. Thermal and Electrical Conduction of Single-crystal Bi2Te3Nanostructures grown using a one step process. Sci. Rep. 2016, 6, 13–15. [Google Scholar] [CrossRef]
- Kim, C.; Kim, D.H.; Han, Y.S.; Chung, J.S.; Kim, H. Fabrication of antimony telluride nanoparticles using a brief chemical synthetic process under atmospheric conditions. J. Alloys Compd. 2011, 509, 609–613. [Google Scholar] [CrossRef]
- Gupta, S.; Neeleshwar, S.; Kumar, V.; Chen, Y.Y. Synthesis of bismuth telluride nanostructures by refluxing method. Adv. Mater. Lett. 2012, 3, 50–54. [Google Scholar] [CrossRef]
- Pelz, U.; Kaspar, K.; Schmidt, S.; Dold, M.; Jägle, M.; Pfaadt, A.; Hillebrecht, H. An aqueous-chemistry approach to nano-bismuth telluride and nano-antimony telluride as thermoelectric materials. J. Electron. Mater. 2012, 41, 1851–1857. [Google Scholar] [CrossRef]
- Yang, H.Q.; Miao, L.; Liu, C.Y.; Li, C.; Honda, S.; Iwamoto, Y.; Huang, R.; Tanemura, S. A Facile Surfactant-Assisted Reflux Method for the Synthesis of Single-Crystalline Sb2Te3 Nanostructures with Enhanced Thermoelectric Performance. ACS Appl. Mater. Interfaces 2015, 7, 14263–14271. [Google Scholar] [CrossRef]
- Sapp, S.A.; Lakshmi, B.B.; Martin, C.R. Template synthesis of bismuth telluride nano wires. Adv. Mater. 1999, 11, 402–404. [Google Scholar] [CrossRef]
- Pinisetty, D.; Gupta, M.; Karki, A.B.; Young, D.P.; Devireddy, R.V. Fabrication and characterization of electrodeposited antimony telluride crystalline nanowires and nanotubes. J. Mater. Chem. 2011, 21, 4098–4107. [Google Scholar] [CrossRef]
- Dhak, D.; Pramanik, P. Characterization of nanocrystalline bismuth telluride (Bi2Te3) synthesized by a novel approach through aqueous precursor method. J. Am. Ceram. Soc. 2006, 89, 534–537. [Google Scholar] [CrossRef]
- Saleemi, M.; Toprak, M.S.; Li, S.; Johnsson, M.; Muhammed, M. Synthesis, processing, and thermoelectric properties of bulk nanostructured bismuth telluride (Bi2Te3). J. Mater. Chem. 2012, 22, 725–730. [Google Scholar] [CrossRef]
- Zhao, X.B.; Ji, X.H.; Zhang, Y.H.; Lu, B.H. Effect of solvent on the microstructures of nanostructured Bi2Te3 prepared by solvothermal synthesis. J. Alloys Compd. 2004, 368, 349–352. [Google Scholar] [CrossRef]
- Deng, Y.; Wei, G.; Liu, J.; Zhou, X.; Wu, J.; Nan, C. Solvothermal preparation and characterization of nanocrystalline SnTe powder with different morphologies. Xiyou Jinshu Cailiao Yu Gongcheng/Rare Met. Mater. Eng. 2002, 31, 42. [Google Scholar]
- Deng, Y.; Nan, C.W.; Wei, G.D.; Guo, L.; Lin, Y.H. Organic-assisted growth of bismuth telluride nanocrystals. Chem. Phys. Lett. 2003, 374, 410–415. [Google Scholar] [CrossRef]
- Jin, R.; Liu, J.; Li, G. Facile solvothermal synthesis, growth mechanism and thermoelectric property of flower-like Bi2Te3. Cryst. Res. Technol. 2014, 49, 460–466. [Google Scholar] [CrossRef]
- Stavila, V.; Robinson, D.B.; Hekmaty, M.A.; Nishimoto, R.; Medlin, D.L.; Zhu, S.; Tritt, T.M.; Sharma, P.A. Wet-chemical synthesis and consolidation of stoichiometric bismuth telluride nanoparticles for improving the thermoelectric figure-of-merit. ACS Appl. Mater. Interfaces 2013, 5, 6678–6686. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ren, Z.; Cao, G.; Ren, W.; Deng, K.; Zhong, Y. Fabrication and characterization of Bi2Te3 nanoplates via a simple solvothermal process. Phys. B Condens. Matter 2009, 404, 4029–4033. [Google Scholar] [CrossRef]
- Sun, S.; Peng, J.; Jin, R.; Song, S.; Zhu, P.; Xing, Y. Template-free solvothermal synthesis and enhanced thermoelectric performance of Sb2Te3 nanosheets. J. Alloys Compd. 2013, 558, 6–10. [Google Scholar] [CrossRef]
- Im, H.J.; Koo, B.; Kim, M.S.; Lee, J.E. Solvothermal synthesis of Sb2Te3 nanoplates under various synthetic conditions and their thermoelectric properties. Appl. Surf. Sci. 2019, 475, 510–514. [Google Scholar] [CrossRef]
- Wang, W.; Poudel, B.; Yang, J.; Wang, D.Z.; Ren, Z.F. High-yield synthesis of single-crystalline antimony telluride hexagonal nanoplates using a solvothermal approach. J. Am. Chem. Soc. 2005, 127, 13792–13793. [Google Scholar] [CrossRef]
- Kim, H.J.; Han, M.K.; Kim, H.Y.; Lee, W.; Kim, S.J. Morphology controlled synthesis of nanostructured Bi2Te3. Bull. Korean Chem. Soc. 2012, 33, 3977–3980. [Google Scholar] [CrossRef][Green Version]
- Giri, L.; Mallick, G.; Jackson, A.C.; Griep, M.H.; Karna, S.P. Synthesis and characterization of high-purity, single phase hexagonal Bi2Te3 nanostructures. RSC Adv. 2015, 5, 24930–24935. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, Z.; Ren, W.; Cao, G.; Deng, K.; Zhong, Y. Hydrothermal synthesis of single-crystalline Bi2Te3 nanoplates. Mater. Lett. 2008, 62, 4273–4276. [Google Scholar] [CrossRef]
- Cao, Y.Q.; Zhu, T.J.; Zhao, X.B. Thermoelectric Bi2Te3 nanotubes synthesized by low-temperature aqueous chemical method. J. Alloys Compd. 2008, 449, 109–112. [Google Scholar] [CrossRef]
- Shi, W.; Yu, J.; Wang, H.; Zhang, H. Hydrothermal synthesis of single-crystalline antimony telluride nanobelts. J. Am. Chem. Soc. 2006, 128, 16490–16491. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.H.; Zhu, Y.J.; Cheng, G.F.; Ruan, Y.J. Sb2Te3 nanobelts and nanosheets: Hydrothermal synthesis, morphology evolution and thermoelectric properties. J. Alloys Compd. 2013, 550, 164–168. [Google Scholar] [CrossRef]
- Yuan, Q.L.; Nie, Q.L.; Huo, D.X. Preparation and characterization of the antimony telluride hexagonal nanoplates. Curr. Appl. Phys. 2009, 9, 224–226. [Google Scholar] [CrossRef]
- Dharmaiah, P.; Hong, S.J. Hydrothermal method for the synthesis of Sb2Te3, and Bi0.5Sb1.5Te3 nanoplates and their thermoelectric properties. Int. J. Appl. Ceram. Technol. 2018, 15, 132–139. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, G.; Mi, J.; Han, F.; Wang, Z.; Ge, C. Hydrothermal synthesis and thermoelectric properties of nanostructured Bi0.5Sb1.5Te3 compounds. Mater. Res. Bull. 2011, 46, 760–764. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, F.Q.; Chen, H.; Zhu, L.; Yu, H.J.; Jian, X.Y. Synthesis and characterization of Bi2Te3 nanotubes by a hydrothermal method. J. Alloys Compd. 2010, 492, 50–53. [Google Scholar] [CrossRef]
- Novaconi, S.; Vlazan, P.; Malaescu, I.; Badea, I.; Grozescu, I.; Sfirloaga, P. Doped Bi2Te3 nano-structured semiconductors obtained by ultrasonically assisted hydrothermal method. Cent. Eur. J. Chem. 2013, 11, 1599–1605. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Zhu, T.J.; Zhao, X.B.; Tu, J.P.; Cao, G.S. Sonochemical synthesis of nanocrystalline Bi2Te3 thermoelectric compounds. Mater. Lett. 2005, 59, 2886–2888. [Google Scholar] [CrossRef]
- Zhou, B.; Zhao, Y.; Pu, L.; Zhu, J.J. Microwave-assisted synthesis of nanocrystalline Bi2Te3. Mater. Chem. Phys. 2006, 96, 192–196. [Google Scholar] [CrossRef]
- Dong, G.H.; Zhu, Y.J.; Chen, L.D. Microwave-assisted rapid synthesis of Sb2Te3 nanosheets and thermoelectric properties of bulk samples prepared by spark plasma sintering. J. Mater. Chem. 2010, 20, 1976–1981. [Google Scholar] [CrossRef]
- Hamawandi, B.; Ballikaya, S.; Batili, H.; Roosmark, V.; Orlovská, M.; Yusuf, A.; Johnsson, M.; Szukiewicz, R.; Kuchowicz, M.; Toprak, M.S. Facile solution synthesis, processing and characterization of n-and p-type binary and ternary Bi-Sb tellurides. Appl. Sci. 2020, 10, 1178. [Google Scholar] [CrossRef]
- Hamawandi, B.; Mansouri, H.; Ballikaya, S.; Demirci, Y.; Orlovská, M.; Bolghanabadi, N.; Sajjadi, S.A.; Toprak, M.S. A Comparative Study on the Thermoelectric Properties of Bismuth Chalcogenide Alloys Synthesized through Mechanochemical Alloying and Microwave-Assisted Solution Synthesis Routes. Front. Mater. 2020, 7, 1–13. [Google Scholar] [CrossRef]
- Pradhan, S.; Das, R.; Bhar, R.; Bandyopadhyay, R.; Pramanik, P. A simple fast microwave-assisted synthesis of thermoelectric bismuth telluride nanoparticles from homogeneous reaction-mixture. J. Nanopart. Res. 2017, 19, 69. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Speth, T.F.; Varma, R.S. Microwave-assisted green synthesis of silver nanostructures. Acc. Chem. Res. 2011, 44, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Gou, L.F.; Chipara, M.; Zaleski, J.M. Convenient, rapid synthesis of Ag nanowires. Chem. Mater. 2007, 19, 4378. [Google Scholar] [CrossRef]
- Hamawandi, B.; Ballikaya, S.; Råsander, M.; Halim, J.; Vinciguerra, L.; Rosen, J.; Johnsson, M.; Toprak, M. Composition tuning of nanostructured binary copper selenides through rapid chemical synthesis and their thermoelectric property evaluation. Nanomaterials 2020, 10, 854. [Google Scholar] [CrossRef]
- Demirci, Y.; Yusuf, A.; Hamawandi, B.; Toprak, M.S.; Ballikaya, S. The Effect of Crystal Mismatch on the Thermoelectric Performance Enhancement of Nano Cu2Se. Front. Mater. 2021, 7, 1–9. [Google Scholar] [CrossRef]
- Cintas, P.; Veronesi, P.; Leonelli, C.; Keglevich, G.; Mucsi, Z.; Radoiu, M.; de la Hoz, A.; Prieto, P.; Wada, Y.; Mochizuki, D.; et al. Microwave chemistry: History, development and legacy. In Microwave Chemistry; De Gruyter: Berlin, Germany, 2017; pp. 1–17. [Google Scholar] [CrossRef]
- Köseoglu, Y.; Yildiz, H.; Yilgin, R. Synthesis, characterization and superparamagnetic resonance studies of ZnFe2O4 nanoparticles. J. Nanosci. Nanotechnol. 2012, 12, 2261–2269. [Google Scholar] [CrossRef] [PubMed]
- Kasapoǧlu, N.; Baykal, A.; Köseoǧlu, Y.; Toprak, M.S. Microwave-assisted combustion synthesis of CoFe2O4 with urea, and its magnetic characterization. Scr. Mater. 2007, 57, 441–444. [Google Scholar] [CrossRef]
- Nehru, L.C.; Swaminathan, V.; Sanjeeviraja, C. Rapid synthesis of nanocrystalline ZnO by a microwave-assisted combustion method. Powder Technol. 2012, 226, 29–33. [Google Scholar] [CrossRef]
- Qiu, G.; Dharmarathna, S.; Zhang, Y.; Opembe, N.; Huang, H.; Suib, S.L. Facile microwave-assisted hydrothermal synthesis of CuO nanomaterials and their catalytic and electrochemical properties. J. Phys. Chem. C 2012, 116, 468–477. [Google Scholar] [CrossRef]
- Nehru, L.C.; Sanjeeviraja, C. Rapid synthesis of nanocrystalline SnO2 by a microwave-assisted combustion method. J. Adv. Ceram. 2014, 3, 171–176. [Google Scholar] [CrossRef]
- Pires, F.I.; Joanni, E.; Savu, R.; Zaghete, M.A.; Longo, E.; Varela, J.A. Microwave-assisted hydrothermal synthesis of nanocrystalline SnO powders. Mater. Lett. 2008, 62, 239–242. [Google Scholar] [CrossRef]
- Gao, F.; Lu, Q.; Komarneni, S. Interface reaction for the self-assembly of silver nanocrystals under microwave-assisted solvothermal conditions. Chem. Mater. 2005, 17, 856–860. [Google Scholar] [CrossRef]
- Liao, X.H.; Chen, N.Y.; Xu, S.; Yang, S.B.; Zhu, J.J. A microwave assisted heating method for the preparation of copper sulfide nanorods. J. Cryst. Growth 2003, 252, 593–598. [Google Scholar] [CrossRef]
- Qiu, G.; Dharmarathna, S.; Genuino, H.; Zhang, Y.; Huang, H.; Suib, S.L. Facile microwave-refluxing synthesis and catalytic properties of vanadium pentoxide nanomaterials. ACS Catal. 2011, 1, 1702–1709. [Google Scholar] [CrossRef]
- Kolhatkar, G.; Ambriz-Vargas, F.; Thomas, R.; Ruediger, A. Microwave-Assisted Hydrothermal Synthesis of BiFexCr1−xO3 Ferroelectric Thin Films. Cryst. Growth Des. 2017, 17, 5697–5703. [Google Scholar] [CrossRef]
- Vaseashta, A.; Reisfeld, R.; Mihailescu, I.N. Green nanotechnologies for responsible manufacturing. Mater. Res. Soc. Symp. Proc. 2008, 1106, 15–28. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Jiang, Y.Q.; Li, J.; Feng, Z.W.; Xu, G.Q.; Shi, X.; Ding, Q.J.; Li, W.; Ma, C.H.; Yu, B. Ethylene Glycol: A Green Solvent for Visible Light-Promoted Aerobic Transition Metal-Free Cascade Sulfonation/Cyclization Reaction. Adv. Synth. Catal. 2020, 362, 2609–2614. [Google Scholar] [CrossRef]
- Zeynizadeh, B.; Setamdideh, D. Water as a green solvent for fast and efficient reduction of carbonyl compounds with NaBH4 under microwave irradiation. J. Chin. Chem. Soc. 2005, 52, 1179–1184. [Google Scholar] [CrossRef]
- Akerlof, G.C.; Oshry, H.I. The Dielectric Constant of Water at High Temperatures and in Equilibrium with its Vapor. J. Am. Chem. Soc. 1950, 72, 2844–2847. [Google Scholar] [CrossRef]
- Kim, C.; Kim, D.H.; Han, Y.S.; Chung, J.S.; Park, S.H.; Kim, H. Fabrication of bismuth telluride nanoparticles using a chemical synthetic process and their thermoelectric evaluations. Powder Technol. 2011, 214, 463–468. [Google Scholar] [CrossRef]
- Mote, V.; Purushotham, Y.; Dole, B. Williamson-Hall analysis in estimation of lattice strain in nanometer-sized ZnO particles. J. Theor. Appl. Phys. 2012, 6, 2–9. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.D.; Gao, H.; Wang, L.J.; Li, M.; Shi, X.L.; Hong, M.; Wang, H.; Zou, J.; Chen, Z.G. High Porosity in Nanostructured n-Type Bi2Te3 Obtaining Ultralow Lattice Thermal Conductivity. ACS Appl. Mater. Interfaces 2019, 11, 31237–31244. [Google Scholar] [CrossRef]
- Du, Y.; Cai, K.F.; Li, H.; An, B.J. The influence of sintering temperature on the microstructure and thermoelectric properties of n-Type Bi2Te3−xSex nanomaterials. J. Electron. Mater. 2011, 40, 518–522. [Google Scholar] [CrossRef]
- Du, Y.; Li, J.; Xu, J.; Eklund, P. Thermoelectric properties of reduced graphene oxide/Bi2Te3 nanocomposites. Energies 2019, 12, 2430. [Google Scholar] [CrossRef]
- Crist, B.V. Handbooks of Monochromatic XPS Spectra Volume 1-The Elements and Native Oxides. Handb. Elem. Nativ. Oxides 1999, 1, 1–87. [Google Scholar]
- Diouf, S.; Molinari, A. Densification mechanisms in spark plasma sintering: Effect of particle size and pressure. Powder Technol. 2012, 221, 220–227. [Google Scholar] [CrossRef]
- Domoratsky, K.V.; Kudzin, A.Y.; Sadovskaya, L.Y.; Sokolyanskii, G.C. Doping influence on the physical properties of Bi2TeO5 single crystals. Ferroelectrics 1998, 214, 191–197. [Google Scholar] [CrossRef]
- Zhang, C.; Peng, Z.; Li, Z.; Yu, L.; Khor, K.A.; Xiong, Q. Controlled growth of bismuth antimony telluride BixSb2−xTe3 nanoplatelets and their bulk thermoelectric nanocomposites. Nano Energy 2015, 15, 688–696. [Google Scholar] [CrossRef]
- Scheele, M.; Oeschler, N.; Veremchuk, I.; Reinsberg, K.G.; Kreuziger, A.M.; Kornowski, A.; Broekaert, J.; Klinke, C.; Weller, H. ZT enhancement in solution-grown Sb(2−x)BixTe 3 nanoplatelets. ACS Nano 2010, 4, 4283–4291. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Dyck, J.S.; Hernandez, B.M.; Burda, C. Enhancing thermoelectric performance of ternary nanocrystals through adjusting carrier concentration. J. Am. Chem. Soc. 2010, 132, 4982–4983. [Google Scholar] [CrossRef] [PubMed]
- Poudel, B.; Hao, Q.; Ma, Y.; Lan, Y.; Minnich, A.; Yu, B.; Yan, X.; Wang, D.; Muto, A.; Vashaee, D.; et al. High-thermoelectric performance of nanostructured bismuth antimony telluride bulk alloys. Science 2008, 320, 634–638. [Google Scholar] [CrossRef]
- Goldsmid, H.J.; Sharp, J.W. Estimation of the thermal band gap of a semiconductor from Seebeck measurements. J. Electron. Mater. 1999, 28, 869–872. [Google Scholar] [CrossRef]
- Goldsmid, H.J.; Sheard, A.R.; Wright, D.A. The performance of bismuth telluride thermojunctions. Br. J. Appl. Phys. 1958, 9, 365–370. [Google Scholar] [CrossRef]
- Mishra, S.K.; Satpathy, S.; Jepsen, O. Electronic structure and thermoelectric properties of bismuth telluride and bismuth selenide. J. Phys. Condens. Matter 1997, 9, 461–470. [Google Scholar] [CrossRef]
- Goltsman, B.; Kudinov, B.A.; Smirnov, I. Thermoelectric Semiconductor Materials Based on Bi2Te3; Defense Technical Information Center: Ft. Belvoir, VA, USA, 1973.
- Lang, X.Y.; Zheng, W.T.; Jiang, Q. Finite-Size Effect on Band Structure and Photoluminescence of Semiconductor Nanocrystals. IEEE Trans. Nanotechnol. 2008, 7, 5–9. [Google Scholar] [CrossRef]
- Taniguchi, T.; Terada, T.; Komatsubara, Y.; Ishibe, T.; Konoike, K.; Sanada, A.; Naruse, N.; Mera, Y.; Nakamura, Y. Phonon transport in the nano-system of Si and SiGe films with Ge nanodots and approach to ultralow thermal conductivity. Nanoscale 2021, 13, 4971–4977. [Google Scholar] [CrossRef]
- Imamuddin, M.; Dupre, A. Thermoelectric properties of p-type Bi2Te3–Sb2Te3–Sb2Se3 alloys and n-type Bi2Te3–Bi2Se3 alloys in the temperature range 300 to 600 K. Phys. Status Solidi 1972, 10, 415–424. [Google Scholar] [CrossRef]
- Han, M.K.; Jin, Y.; Lee, D.H.; Kim, S.J. Thermoelectric properties of Bi2Te3: CuI and the effect of its doping with Pb atoms. Materials 2017, 10, 1235. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Poudel, B.; Ma, Y.; Liu, W.S.; Joshi, G.; Wang, H.; Lan, Y.; Wang, D.; Chen, G.; Ren, Z.F. Experimental studies on anisotropic thermoelectric properties and structures of n-type Bi2Te2.7Se0.3. Nano Lett. 2010, 10, 3373–3378. [Google Scholar] [CrossRef] [PubMed]





| Tsint | thold | Psint | dbulk | dPellet | ρ (%) | |
|---|---|---|---|---|---|---|
| (°C) | (min) | (MPa) | (g/cm3) | (g/cm3) | ||
| Hydro-Bi2Te3 | 400 | 1 | 50 | 7.86 | 7.051 | 89 |
| Polyol-Bi2Te3 | 400 | 1 | 50 | 7.86 | 6.13 | 78 |
| Samples | Fraction [at%] | Assigned to [92] | iep (pH) | |
|---|---|---|---|---|
| Polyol-Bi2Te3 | 39.6 | C | C–O, C–C, | 6.30 |
| O–C=O | ||||
| 10.48 | Bi | Bi2O3 | ||
| 25.86 | Te | Te met (8.66%), TeO2 (17.2%) | ||
| 22.29 | O | |||
| 1.77 | S | |||
| Hydro-Bi2Te3 | 61.53 | C | C–O/C–N, C–C, O–C=O | 6.80 |
| 5.43 | Bi | Bi met (0.6%), Bi2O3 (4.8%) | ||
| 13.21 | Te | Te met (5.3%), TeO2 (7.91%) | ||
| 16.24 | O | |||
| 3.59 | N | |||
| Sample | T | κtot | S | σ | PF | ZT |
|---|---|---|---|---|---|---|
| (K) | (W/(m·K)) | (µV/K) | (S/m) | (µW/(K2·cm)) | ||
| Hydro-Bi2Te3 | 298 | 0.92 | −138 | 126,421 | 24.22 | 0.8 |
| 373 | 0.9 | −149 | 96,048 | 21.31 | 0.88 | |
| 473 | 1.16 | −142 | 66,600 | 13.36 | 0.54 | |
| Polyol-Bi2Te3 | 298 | 0.95 | −125 | 106,356 | 16.51 | 0.52 |
| 373 | 0.86 | −148 | 92,964 | 20.36 | 0.88 | |
| 473 | 0.9 | −162 | 75,802 | 19.84 | 1.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamawandi, B.; Batili, H.; Paul, M.; Ballikaya, S.; Kilic, N.I.; Szukiewicz, R.; Kuchowicz, M.; Johnsson, M.; Toprak, M.S. Minute-Made, High-Efficiency Nanostructured Bi2Te3 via High-Throughput Green Solution Chemical Synthesis. Nanomaterials 2021, 11, 2053. https://doi.org/10.3390/nano11082053
Hamawandi B, Batili H, Paul M, Ballikaya S, Kilic NI, Szukiewicz R, Kuchowicz M, Johnsson M, Toprak MS. Minute-Made, High-Efficiency Nanostructured Bi2Te3 via High-Throughput Green Solution Chemical Synthesis. Nanomaterials. 2021; 11(8):2053. https://doi.org/10.3390/nano11082053
Chicago/Turabian StyleHamawandi, Bejan, Hazal Batili, Moon Paul, Sedat Ballikaya, Nuzhet I. Kilic, Rafal Szukiewicz, Maciej Kuchowicz, Mats Johnsson, and Muhammet S. Toprak. 2021. "Minute-Made, High-Efficiency Nanostructured Bi2Te3 via High-Throughput Green Solution Chemical Synthesis" Nanomaterials 11, no. 8: 2053. https://doi.org/10.3390/nano11082053
APA StyleHamawandi, B., Batili, H., Paul, M., Ballikaya, S., Kilic, N. I., Szukiewicz, R., Kuchowicz, M., Johnsson, M., & Toprak, M. S. (2021). Minute-Made, High-Efficiency Nanostructured Bi2Te3 via High-Throughput Green Solution Chemical Synthesis. Nanomaterials, 11(8), 2053. https://doi.org/10.3390/nano11082053