Conformal Self-Assembly of Nanospheres for Light-Enhanced Airtightness Monitoring and Room-Temperature Gas Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polystyrene Nanospheres
2.2. Flat and Curved Substrates
2.3. Plasma Treatment of the Substrates
2.4. Conformal Self-Assembly of the PS Nanospheres
2.5. Fabrication of the WS2-Decorated Gas Sensor
2.6. Characterization
2.7. Performance of the Gas Sensor
2.8. Performance of the Pressure Sensor
3. Results and Discussion
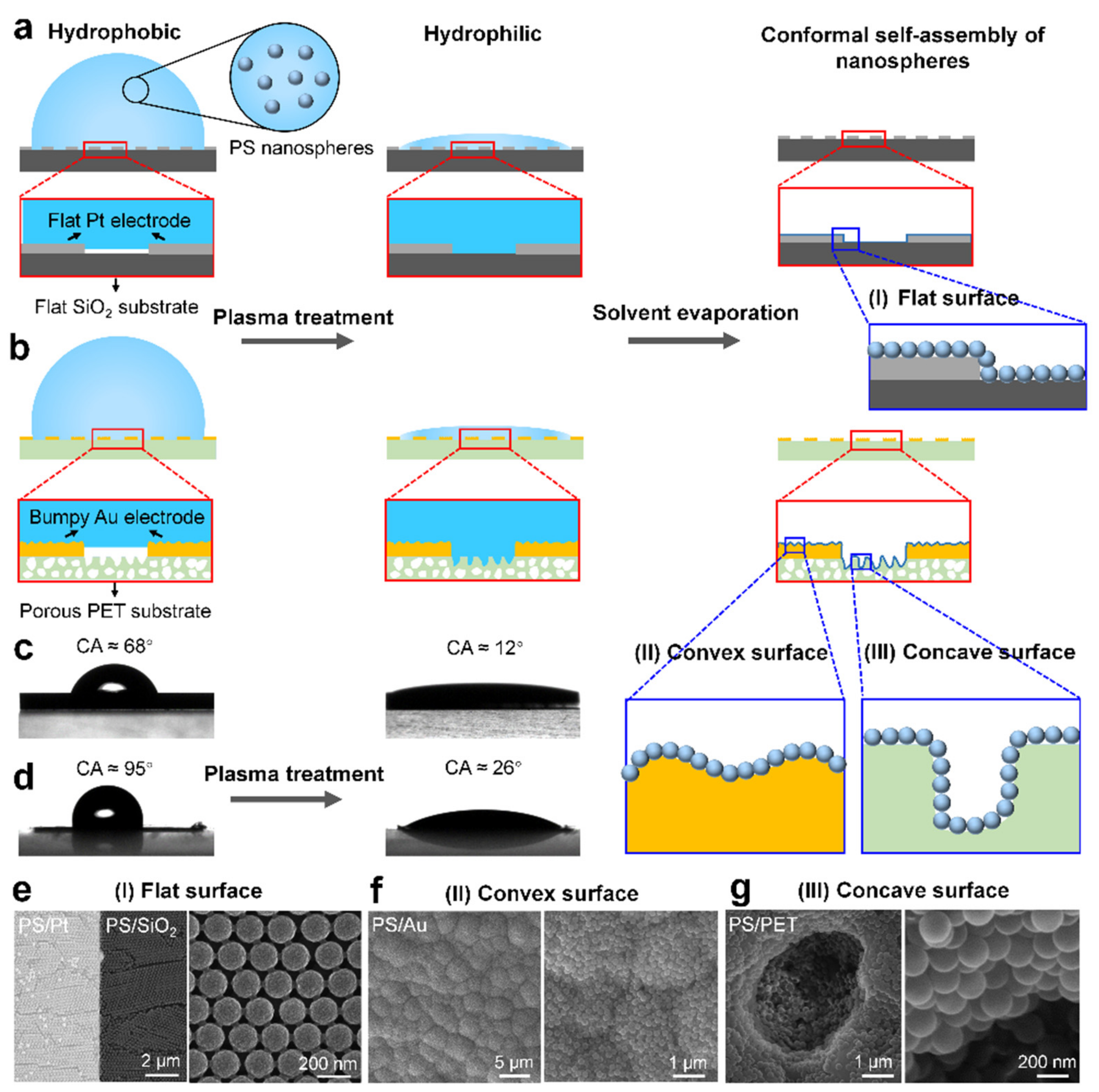
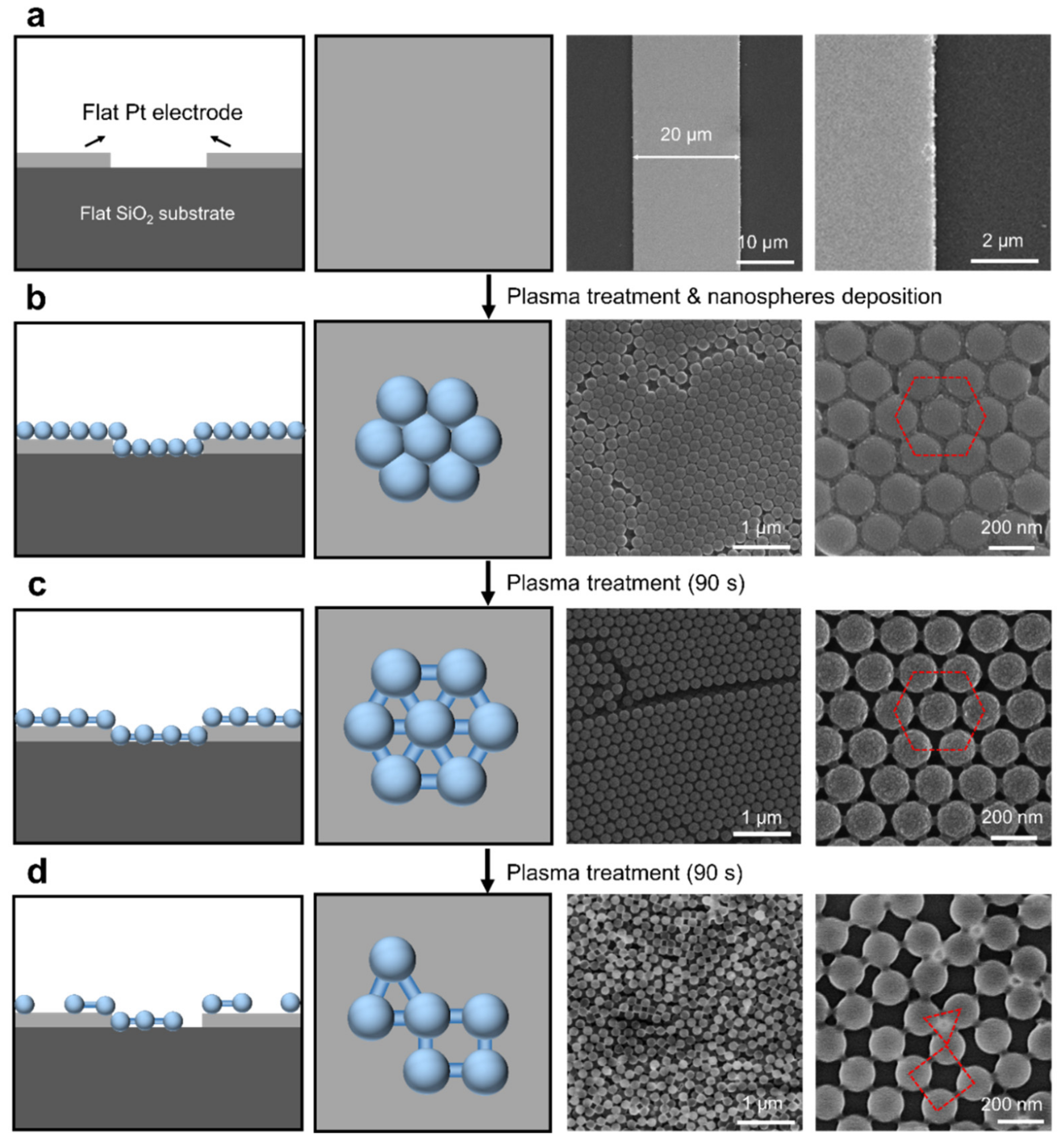
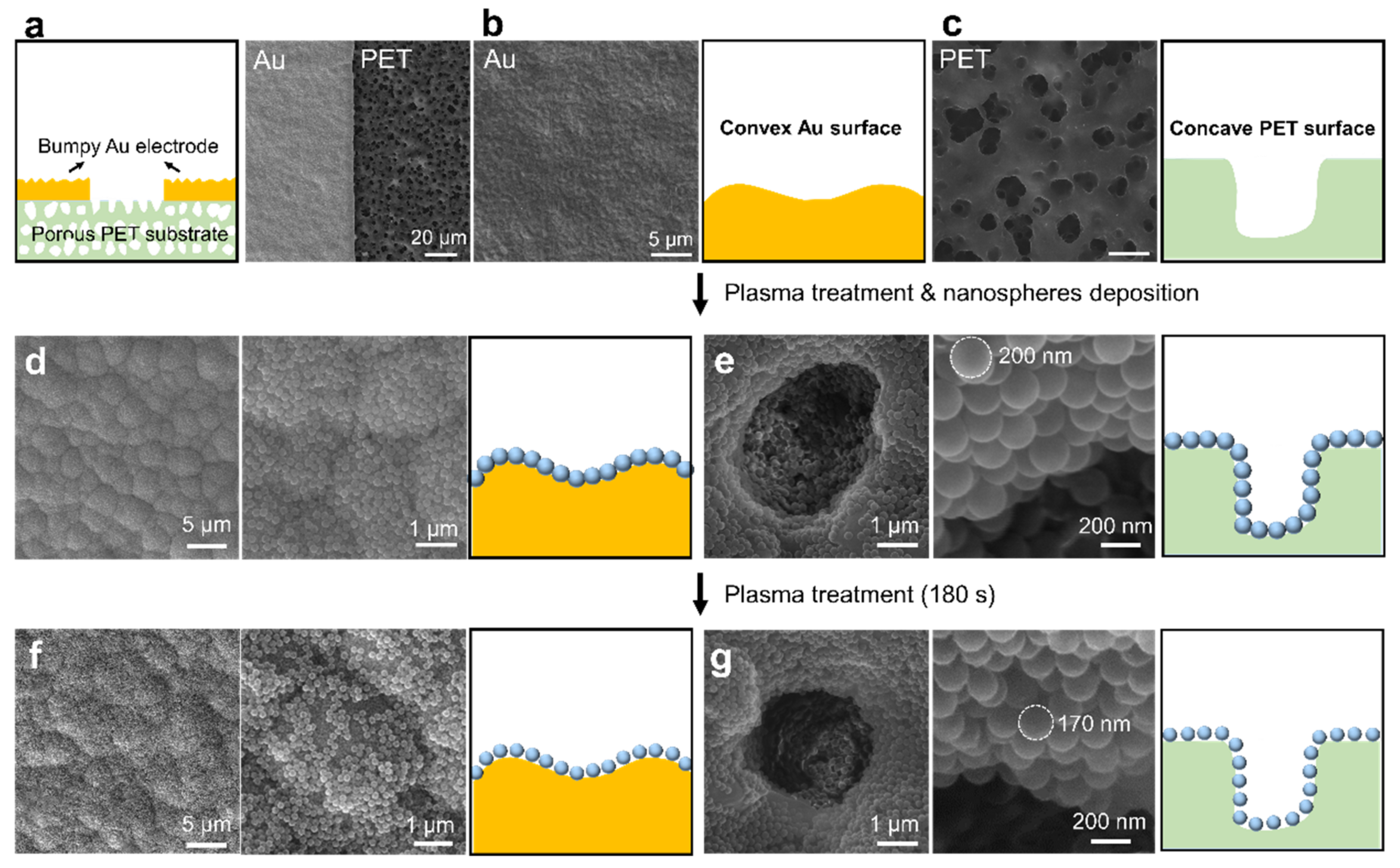
3.1. Conformal Assembly of Nanospheres on Flat and Curved Substrates
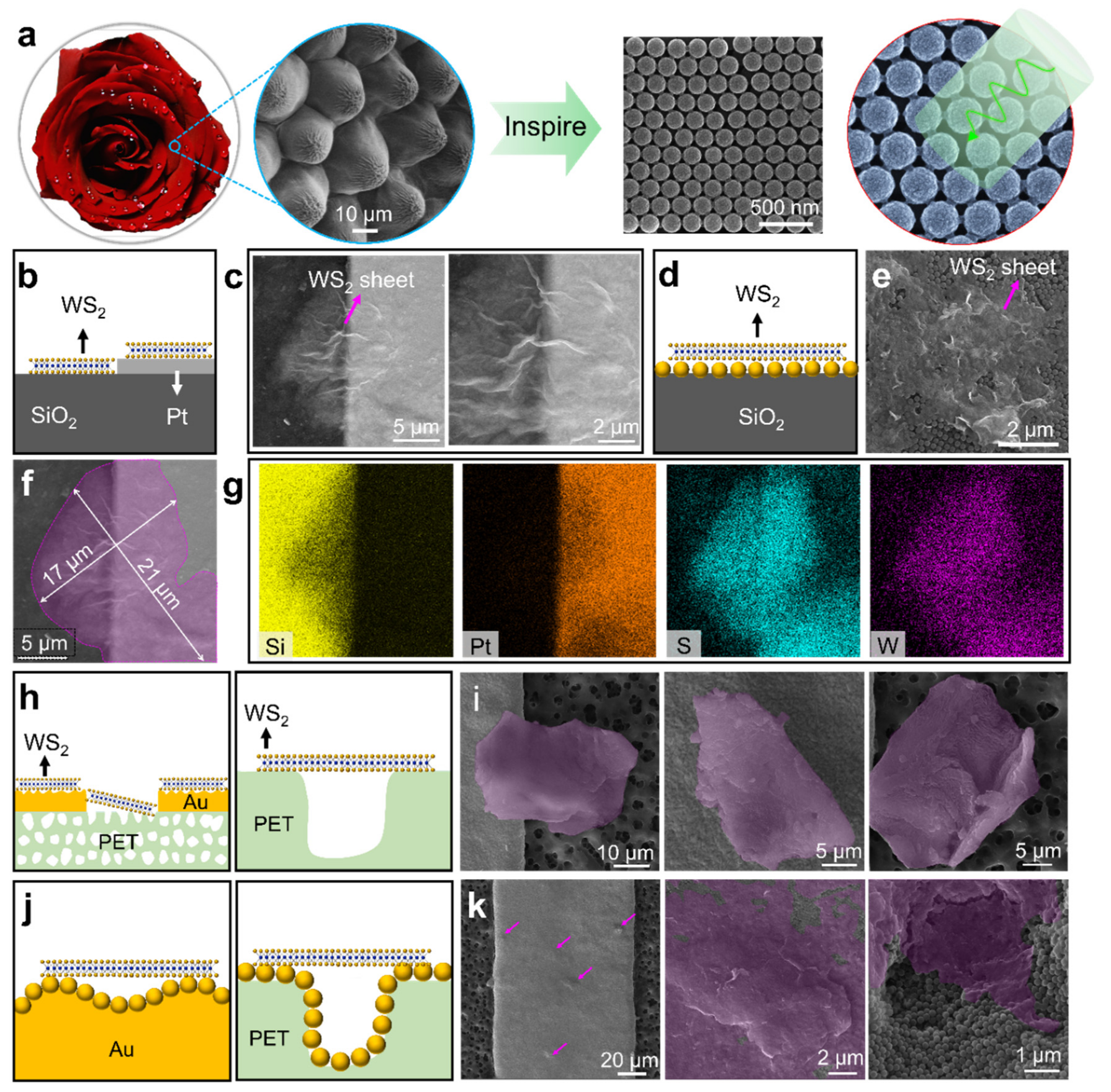
3.2. Decoration of WS2 Sheets on the Conformal Nanospheres
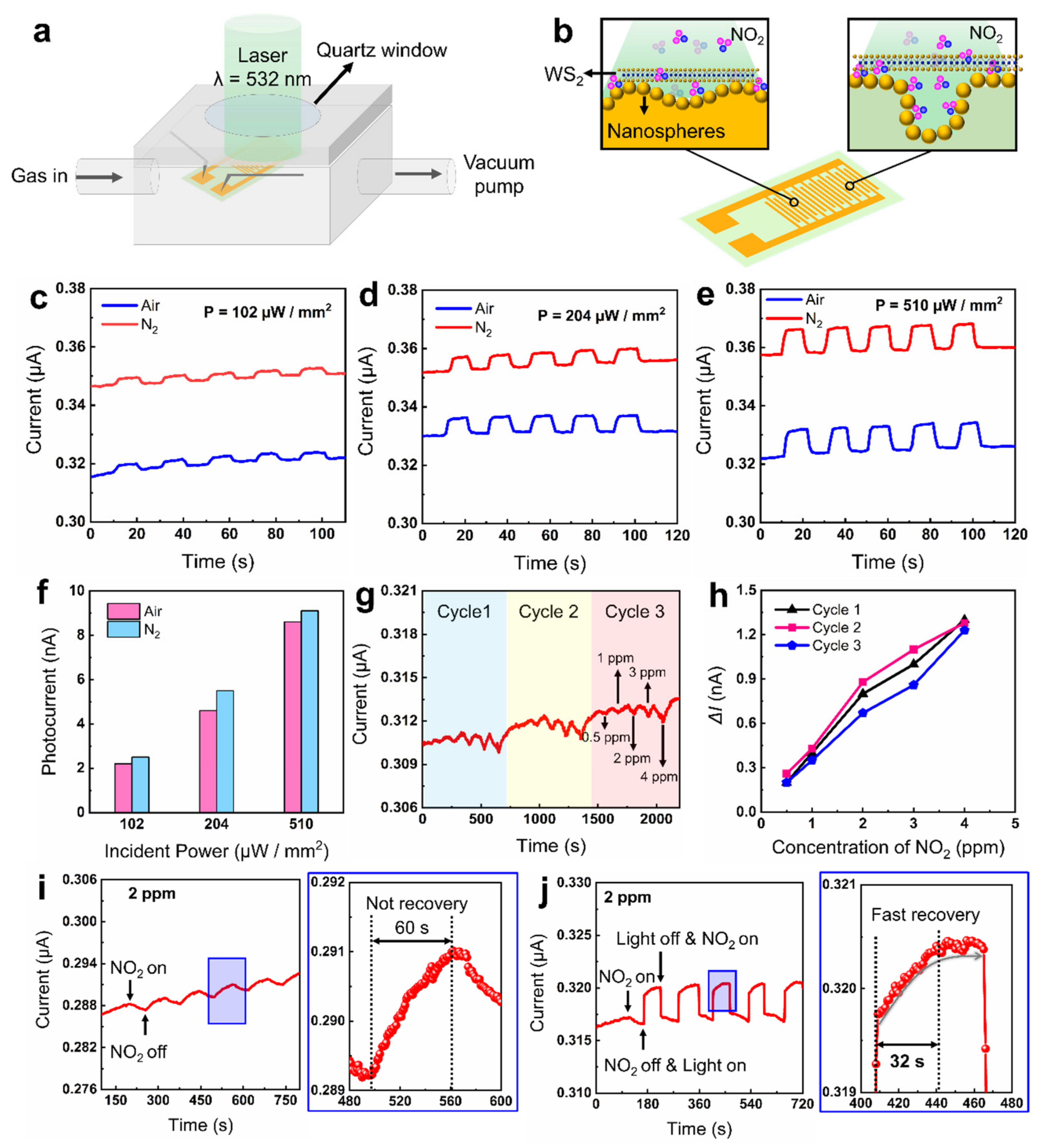
3.3. Light-Enhanced Room-Temperature Gas Sensing
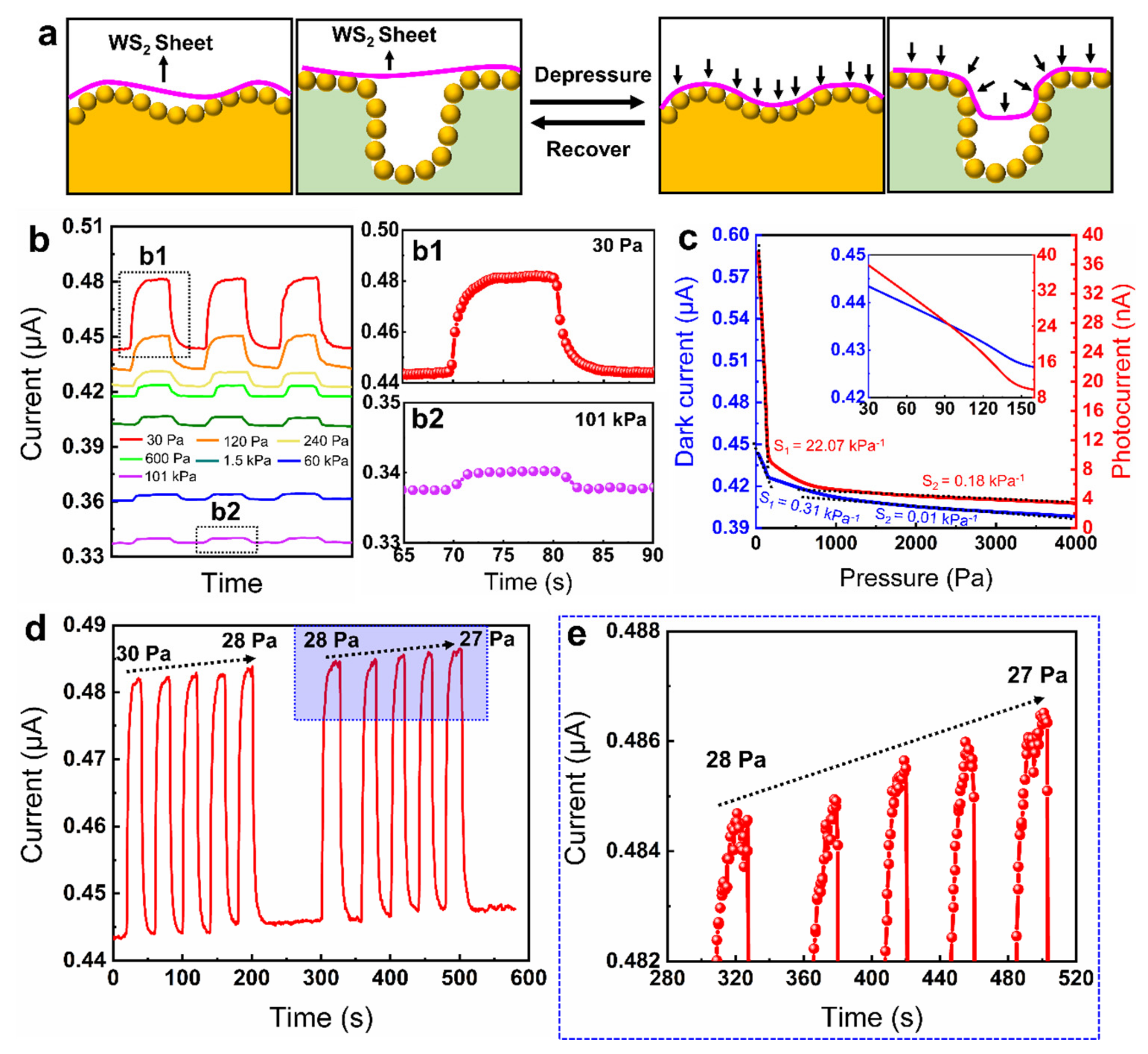
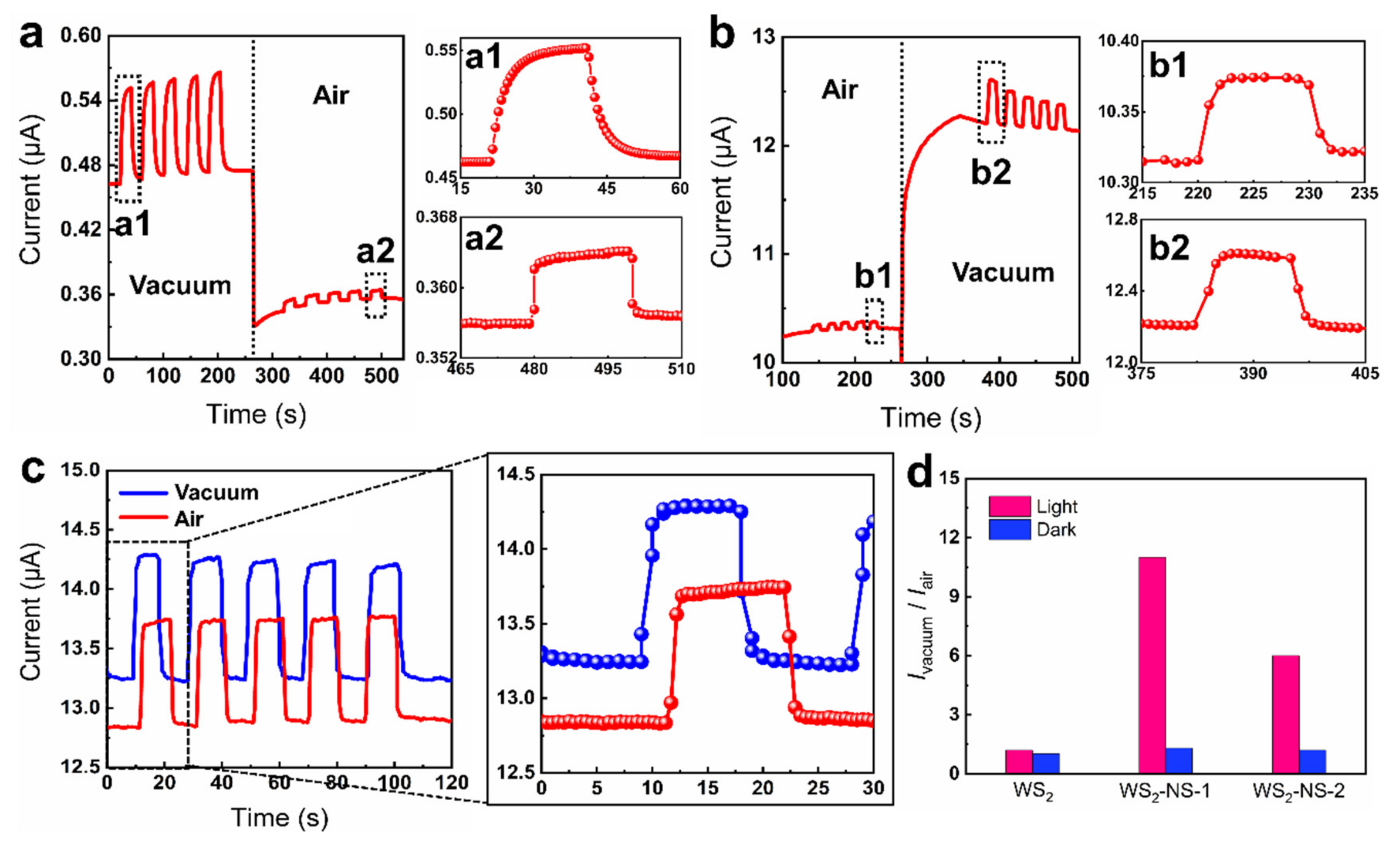
3.4. Light-Enhanced Airtightness Monitoring
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Su, B.; Tian, Y.; Jiang, L. Bioinspired Interfaces with Superwettability: From Materials to Chemistry. J. Am. Chem. Soc. 2016, 138, 1727–1748. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Huang, Z.; Sett, S.; Oh, J.; Cha, H.; Li, L.; Feng, L.; Wu, Y.; Zhao, C.; Orejon, D.; et al. Atmosphere-Mediated Superhydrophobicity of Rationally Designed Micro/Nanostructured Surfaces. ACS Nano 2019, 13, 4160–4173. [Google Scholar] [CrossRef] [Green Version]
- Rim, Y.S.; Bae, S.-H.; Chen, H.; Yang, J.L.; Kim, J.; Andrews, A.; Weiss, P.; Yang, Y.; Tseng, H.-R. Printable Ultrathin Metal Oxide Semiconductor-Based Conformal Biosensors. ACS Nano 2015, 9, 12174–12181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Li, H.; Miller, R.; Malissa, H.; Jamali, S.; Boehme, C.; Grossman, J.C.; Ren, S. Freestanding Organic Charge-Transfer Conformal Electronics. Nano Lett. 2018, 18, 4346–4354. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tang, L.; Luo, W.; Shen, J.; Zhou, D.; Feng, S.; Wei, X.; Shi, H. Light Trapping in Conformal Graphene/Silicon Nanoholes for High-Performance Photodetectors. ACS Appl. Mater. Interfaces 2019, 11, 30421–30429. [Google Scholar] [CrossRef]
- Kwon, Y.W.; Park, J.; Kim, T.; Kang, S.H.; Kim, H.; Shin, J.; Jeon, S.; Hong, S.W. Flexible Near-Field Nanopatterning with Ultrathin, Conformal Phase Masks on Nonplanar Substrates for Biomimetic Hierarchical Photonic Structures. ACS Nano 2016, 10, 4609–4617. [Google Scholar] [CrossRef]
- Ran, L.; Qiu, S.; Zhai, P.; Li, Z.; Gao, J.; Zhang, X.; Zhang, B.; Wang, C.; Sun, L.; Hou, J. Conformal Macroporous Inverse Opal Oxynitride-Based Photoanode for Robust Photoelectrochemical Water Splitting. J. Am. Chem. Soc. 2021, 143, 7402–7413. [Google Scholar] [CrossRef]
- Sun, J.; Litchinitser, N.M. Toward Practical, Subwavelength, Visible-Light Photolithography with Hyperlens. ACS Nano 2018, 12, 542–548. [Google Scholar] [CrossRef]
- Gilboa, T.; Zvuloni, E.; Zrehen, A.; Squires, A.H.; Meller, A. Automated, Ultra-Fast Laser-Drilling of Nanometer Scale Pores and Nanopore Arrays in Aqueous Solutions. Adv. Funct. Mater. 2020, 30, 1900642. [Google Scholar] [CrossRef]
- Zhou, L.; Fu, J.; He, Y. A Review of 3D Printing Technologies for Soft Polymer Materials. Adv. Funct. Mater. 2020, 30, 2000187. [Google Scholar] [CrossRef]
- Yin, X.-T.; Lv, P.; Li, J.; Jafari, A.; Wu, F.-Y.; Wang, Q.; Dastan, D.; Shi, Z.; Yu, S.; Garmestani, H. Nanostructured tungsten trioxide prepared at various growth temperatures for sensing applications. J. Alloys Compd. 2020, 825, 154105. [Google Scholar] [CrossRef]
- Jafari, A.; Alam, M.H.; Dastan, D.; Ziakhodadadian, S.; Shi, Z.; Garmestani, H.; Weidenbach, A.S.; Ţălu, Ş. Statistical, morphological, and corrosion behavior of PECVD derived cobalt oxide thin films. J. Mater. Sci. Mater. Electron. 2019, 30, 21185–21198. [Google Scholar] [CrossRef]
- Tan, Y.; Hu, B.; Song, J.; Chu, Z.; Wu, W. Bioinspired Multiscale Wrinkling Patterns on Curved Substrates: An Overview. Nano-Micro Lett. 2020, 12, 101. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Hakimi, N.; Perez, R.; Waldman, S.; Kozinski, J.A.; Hwang, D.K. Microarchitecture for a Three-Dimensional Wrinkled Surface Platform. Adv. Mater. 2015, 27, 1880–1886. [Google Scholar] [CrossRef]
- Tan, Y.; Hu, B.; Chu, Z.; Wu, W. Bioinspired Superhydrophobic Papillae with Tunable Adhesive Force and Ultralarge Liquid Capacity for Microdroplet Manipulation. Adv. Funct. Mater. 2019, 29, 1900266. [Google Scholar] [CrossRef]
- Liu, D.; Aleisa, R.; Cai, Z.; Li, Y.; Yin, Y. Self-assembly of superstructures at all scales. Matter 2021, 4, 927–941. [Google Scholar] [CrossRef]
- Yu, Y.; Ng, C.; König, T.A.F.; Fery, A. Tackling the Scalability Challenge in Plasmonics by Wrinkle-Assisted Colloidal Self-Assembly. Langmuir 2019, 35, 8629–8645. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Labib, M.; Chen, H.; Wei, M.; Poudineh, M.; Green, B.J.; Duong, B.; Das, J.; Ahmed, S.; et al. Peptide-functionalized nanostructured microarchitectures enable rapid mechanotransductive differentiation. ACS Appl. Mater. Interfaces 2019, 11, 41030–41037. [Google Scholar] [CrossRef]
- Brasse, Y.; Gupta, V.; Schollbach, H.C.T.; Karg, M.; König, T.A.F.; Fery, A. Mechanotunable Plasmonic Properties of Colloidal Assemblies. Adv. Mater. Interfaces 2020, 7, 1901678. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Sun, N.; Stogin, B.B.; Wang, J.; Huang, Y.; Wong, T.-S. Ultra-antireflective synthetic brochosomes. Nat. Commun. 2017, 8, 1285. [Google Scholar] [CrossRef]
- Alenezy, E.K.; Sabri, Y.M.; Kandjani, A.E.; Korcoban, D.; Rashid, S.S.A.A.H.; Ippolito, S.J.; Bhargava, S.K. Low-Temperature Hydrogen Sensor: Enhanced Performance Enabled through Photoactive Pd-Decorated TiO2 Colloidal Crystals. ACS Sens. 2020, 5, 3902–3914. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, X.; Wang, J.; Tao, L.; Long, M.; Liang, S.-J.; Ang, L.K.; Shu, C.; Tsang, H.K.; Xu, J.-B. Synergistic Effects of Plasmonics and Electron Trapping in Graphene Short-Wave Infrared Photodetectors with Ultrahigh Responsivity. ACS Nano 2017, 11, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zheng, Y.; Zhang, D.; Han, C.; Cheng, A.; Shen, J.; Zeng, G.; Zhang, H. A novel and facile-to-synthesize three-dimensional honeycomb-like nano-Fe3O4@C composite: Electromagnetic wave absorption with wide bandwidth. Carbon 2020, 169, 118–128. [Google Scholar] [CrossRef]
- Shin, D.; Huang, T.; Neibloom, D.; Bevan, M.A.; Frechette, J. Multifunctional Liquid Marble Compound Lenses. ACS Appl. Mater. Interfaces 2019, 11, 34478–34486. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, J.; Li, R.; Li, B.; Mei, X.; Sun, X. Fabrication of Hierarchical Micro/Nano Compound Eyes. ACS Appl. Mater. Interfaces 2019, 11, 34507–34516. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Mayorga-Martinez, C.C.; Pané, S.; Zhang, L.; Pumera, M. Magnetically Driven Micro and Nanorobots. Chem. Rev. 2021, 121, 4999–5041. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhou, L.; Yang, J.; Du, P.; Xu, L.; Wang, J. Highly Sensitive and Fast Optoelectronic Room-Temperature NO2 Gas Sensor Based on ZnO Nanorod-Assembled Macro-/Mesoporous Film. ACS Appl. Electron. Mater. 2020, 2, 580–589. [Google Scholar] [CrossRef]
- Kumar, R.; Goel, N.; Kumar, M. UV-Activated MoS2 Based Fast and Reversible NO2 Sensor at Room Temperature. ACS Sens. 2017, 2, 1744–1752. [Google Scholar] [CrossRef]
- Ko, K.Y.; Park, K.; Lee, S.; Kim, Y.; Woo, W.J.; Kim, D.; Song, J.-G.; Park, J. Recovery Improvement for Large-Area Tungsten Diselenide Gas Sensors. ACS Appl. Mater. Interfaces 2018, 10, 23910–23917. [Google Scholar] [CrossRef]
- Kwon, K.C.; Suh, J.M.; Lee, T.H.; Choi, K.S.; Hong, K.T.; Song, Y.G.; Shim, Y.-S.; Shokouhimehr, M.; Kang, C.-Y.; Kim, S.Y.; et al. SnS2 Nanograins on Porous SiO2 Nanorods Template for Highly Sensitive NO2 Sensor at Room Temperature with Excellent Recovery. ACS Sens. 2019, 4, 678–686. [Google Scholar] [CrossRef]
- Raghu, A.V.; Karuppanan, K.K.; Nampoothiri, J.; Pullithadathil, B. Wearable, Flexible Ethanol Gas Sensor Based on TiO2 Nanoparticles-Grafted 2D-Titanium Carbide Nanosheets. ACS Appl. Nano Mater. 2019, 2, 1152–1163. [Google Scholar] [CrossRef]
- Han, L.; Wang, D.; Lu, Y.; Jiang, T.; Liu, B.; Lin, Y. Visible-Light-Assisted HCHO Gas Sensing Based on Fe-Doped Flowerlike ZnO at Room Temperature. J. Phys. Chem. C 2011, 115, 22939–22944. [Google Scholar] [CrossRef]
- Nie, S.; Dastan, D.; Li, J.; Zhou, W.-D.; Wu, S.-S.; Zhou, Y.-W.; Yin, X.-T. Gas-sensing selectivity of n-ZnO/p-Co3O4 sensors for homogeneous reducing gas. J. Phys. Chem. Solids 2021, 150, 109864. [Google Scholar] [CrossRef]
- Yin, X.-T.; Dastan, D.; Wu, F.-Y.; Li, J. Facile Synthesis of SnO2/LaFeO3−XNX Composite: Photocatalytic Activity and Gas Sensing Performance. Nanomaterials 2019, 9, 1163. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.-D.; Dastan, D.; Yin, X.-T.; Nie, S.; Wu, S.; Wang, Q.; Li, J. Optimization of gas sensing properties of n-SnO2/p-xCuO sensors for homogenous gases and the sensing mechanism. J. Mater. Sci. Mater. Electron. 2020, 31, 18412–18426. [Google Scholar] [CrossRef]
- Zhou, W.-D.; Dastan, D.; Li, J.; Yin, X.-T.; Wang, Q. Discriminable Sensing Response Behavior to Homogeneous Gases Based on n-ZnO/p-NiO Composites. Nanomaterials 2020, 10, 785. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.-T.; Wu, S.-S.; Dastan, D.; Nie, S.; Liu, Y.; Li, Z.-G.; Zhou, Y.-W.; Li, J.; Faik, A.; Shan, K.; et al. Sensing selectivity of SnO2-Mn3O4 nanocomposite sensors for the detection of H2 and CO gases. Surfaces Interfaces 2021, 25, 101190. [Google Scholar] [CrossRef]
- Yin, X.-T.; Li, J.; Dastan, D.; Zhou, W.-D.; Garmestani, H.; Alamgir, F.M. Ultra-high selectivity of H2 over CO with a p-n nanojunction based gas sensors and its mechanism. Sens. Actuators B Chem. 2020, 319, 128330. [Google Scholar] [CrossRef]
- Yin, X.-T.; Zhou, W.-D.; Li, J.; Wang, Q.; Wu, F.-Y.; Dastan, D.; Wang, D.; Garmestani, H.; Wang, X.-M.; Ţălu, Ş. A highly sensitivity and selectivity Pt-SnO2 nanoparticles for sensing applications at extremely low level hydrogen gas detection. J. Alloys Compd. 2019, 805, 229–236. [Google Scholar] [CrossRef]
- Yin, X.-T.; Zhou, W.-D.; Li, J.; Lv, P.; Wang, Q.; Wang, D.; Wu, F.-Y.; Dastan, D.; Garmestani, H.; Shi, Z.; et al. Tin dioxide nanoparticles with high sensitivity and selectivity for gas sensors at sub-ppm level of hydrogen gas detection. J. Mater. Sci. Mater. Electron. 2019, 30, 14687–14694. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Liu, J.; Peng, Z.; Zhou, J.; Zhang, H.; Li, Y. Optoelectronic Gas Sensor Based on Few-Layered InSe Nanosheets for NO2 Detection with Ultrahigh Antihumidity Ability. Anal. Chem. 2020, 92, 11277–11287. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Xu, Y.; Zheng, L.; Yang, C.; Pinna, N.; Liu, X.; Zhang, J. MoS2 Van der Waals p–n Junctions Enabling Highly Selective Room-Temperature NO2 Sensor. Adv. Funct. Mater. 2020, 30, 2000435. [Google Scholar] [CrossRef]
- Kim, S.J.; Koh, H.-J.; Ren, C.E.; Kwon, O.; Maleski, K.; Cho, S.; Anasori, B.; Kim, C.-K.; Choi, Y.-K.; Kim, J.; et al. Metallic Ti3C2Tx MXene Gas Sensors with Ultrahigh Signal-to-Noise Ratio. ACS Nano 2018, 12, 986–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H.; Zheng, K.; Xie, Z.; Yu, J.; Zhu, X.; Ren, H.; Wang, Z.; Zhang, F.; Li, X.; Tao, L.-Q.; et al. Tellurene Nanoflake-Based NO2 Sensors with Superior Sensitivity and a Sub-Parts-per-Billion Detection Limit. ACS Appl. Mater. Interfaces 2020, 12, 47704–47713. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wen, R.; Zhai, J.; Wang, Z.L. Enhanced NO2 gas sensing of a single-layer MoS2 by photogating and piezo-phototronic effects. Sci. Bull. 2019, 64, 128–135. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Liu, X.; Zhang, J.; Kumar, M. Room-Temperature Gas Sensors Under Photoactivation: From Metal Oxides to 2D Materials. Nano-Micro Lett. 2020, 12, 164. [Google Scholar] [CrossRef]
- Yan, X.; Wu, Y.; Li, R.; Shi, C.; Moro, R.; Ma, Y.; Ma, L. High-Performance UV-Assisted NO2 Sensor Based on Chemical Vapor Deposition Graphene at Room Temperature. ACS Omega 2019, 4, 14179–14187. [Google Scholar] [CrossRef] [Green Version]
- Wu, E.; Xie, Y.; Yuan, B.; Zhang, H.; Hu, X.; Liu, J.; Zhang, D. Ultrasensitive and Fully Reversible NO2 Gas Sensing Based on p-Type MoTe2 under Ultraviolet Illumination. ACS Sens. 2018, 3, 1719–1726. [Google Scholar] [CrossRef]
- Mai, H.D.; Jeong, S.; Nguyen, T.K.; Youn, J.-S.; Ahn, S.; Park, C.-M.; Jeon, K.-J. Pd Nanocluster/Monolayer MoS2 Heterojunctions for Light-Induced Room-Temperature Hydrogen Sensing. ACS Appl. Mater. Interfaces 2021, 13, 14644–14652. [Google Scholar] [CrossRef]
- Ding, J.; Feng, A.; Li, X.; Ding, S.; Liu, L.; Ren, W. Properties, preparation, and application of tungsten disulfide: A review. J. Phys. D Appl. Phys. 2021, 54, 173002. [Google Scholar] [CrossRef]
- Polyakov, A.Y.; Kozlov, D.A.; Lebedev, V.A.; Chumakov, R.G.; Frolov, A.; Yashina, L.V.; Rumyantseva, M.N.; Goodilin, E. Gold Decoration and Photoresistive Response to Nitrogen Dioxide of WS2 Nanotubes. Chem. A Eur. J. 2018, 24, 18952–18962. [Google Scholar] [CrossRef]
- Huo, N.; Yang, S.; Wei, Z.; Li, S.-S.; Xia, J.-B.; Li, J. Photoresponsive and Gas Sensing Field-Effect Transistors based on Multilayer WS2 Nanoflakes. Sci. Rep. 2015, 4, 5209. [Google Scholar] [CrossRef] [Green Version]
- Paolucci, V.; Emamjomeh, S.M.; Ottaviano, L.; Cantalini, C. Near Room Temperature Light-Activated WS2-Decorated rGO as NO2 Gas Sensor. Sensors 2019, 19, 2617. [Google Scholar] [CrossRef] [Green Version]
- Gu, D.; Li, X.; Wang, H.; Li, M.; Xi, Y.; Chen, Y.; Wang, J.; Rumyntseva, M.N.; Gaskov, A.M. Light enhanced VOCs sensing of WS2 microflakes based chemiresistive sensors powered by triboelectronic nangenerators. Sens. Actuators B Chem. 2018, 256, 992–1000. [Google Scholar] [CrossRef]
- Banik, M.; Mukherjee, R. Fabrication of Ordered 2D Colloidal Crystals on Flat and Patterned Substrates by Spin Coating. ACS Omega 2018, 3, 13422–13432. [Google Scholar] [CrossRef]
- Jeon, H.; Kim, D.J.; Park, M.S.; Ryu, D.Y.; Kim, J.H. Amphiphilic Graft Copolymer Nanospheres: From Colloidal Self-Assembly to CO2 Capture Membranes. ACS Appl. Mater. Interfaces 2016, 8, 9454–9461. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Y.; Li, M.; Zheng, Y.; Shen, W.; Jiang, L. The Structural Color of Red Rose Petals and Their Duplicates. Langmuir 2010, 26, 14885–14888. [Google Scholar] [CrossRef]
- Chen, A.; Qian, J.; Chen, Y.; Lu, X.; Wang, F.; Tang, Z. Enhanced sunlight photocatalytic activity of porous TiO2 hierarchical nanosheets derived from petal template. Powder Technol. 2013, 249, 71–76. [Google Scholar] [CrossRef]
- Hünig, R.; Mertens, A.; Stephan, M.; Schulz, A.; Richter, B.; Hetterich, M.; Powalla, M.; Lemmer, U.; Colsmann, A.; Gomard, G. Flower Power: Exploiting Plants’ Epidermal Structures for Enhanced Light Harvesting in Thin-Film Solar Cells. Adv. Opt. Mater. 2016, 4, 1487–1493. [Google Scholar] [CrossRef]
- Chou, S.-Y.; Yu, C.-C.; Yen, Y.-T.; Lin, K.-T.; Chen, H.-L.; Su, W.-F. Romantic Story or Raman Scattering? Rose Petals as Ecofriendly, Low-Cost Substrates for Ultrasensitive Surface-Enhanced Raman Scattering. Anal. Chem. 2015, 87, 6017–6024. [Google Scholar] [CrossRef]
- Xu, B.-B.; Zhang, Y.-L.; Zhang, W.-Y.; Liu, X.-Q.; Wang, J.-N.; Zhang, X.-L.; Zhang, D.-D.; Jiang, H.; Zhang, R.; Sun, H.-B. Silver-Coated Rose Petal: Green, Facile, Low-Cost and Sustainable Fabrication of a SERS Substrate with Unique Superhydrophobicity and High Efficiency. Adv. Opt. Mater. 2013, 1, 56–60. [Google Scholar] [CrossRef]
- Zhao, W.; Ghorannevis, Z.; Chu, L.; Toh, M.; Kloc, C.; Tan, P.-H.; Eda, G. Evolution of Electronic Structure in Atomically Thin Sheets of WS2 and WSe2. ACS Nano 2013, 7, 791–797. [Google Scholar] [CrossRef] [Green Version]
- Ramasubramaniam, A. Large excitonic effects in monolayers of molybdenum and tungsten dichalcogenides. Phys. Rev. B 2012, 86, 115409. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Tan, Y.; Zhang, R.; Kang, Y.; Zeng, G.; Zhao, X.; Jiang, T. Conformal Self-Assembly of Nanospheres for Light-Enhanced Airtightness Monitoring and Room-Temperature Gas Sensing. Nanomaterials 2021, 11, 1829. https://doi.org/10.3390/nano11071829
Liu Q, Tan Y, Zhang R, Kang Y, Zeng G, Zhao X, Jiang T. Conformal Self-Assembly of Nanospheres for Light-Enhanced Airtightness Monitoring and Room-Temperature Gas Sensing. Nanomaterials. 2021; 11(7):1829. https://doi.org/10.3390/nano11071829
Chicago/Turabian StyleLiu, Qirui, Yinlong Tan, Renyan Zhang, Yan Kang, Ganying Zeng, Xiaoming Zhao, and Tian Jiang. 2021. "Conformal Self-Assembly of Nanospheres for Light-Enhanced Airtightness Monitoring and Room-Temperature Gas Sensing" Nanomaterials 11, no. 7: 1829. https://doi.org/10.3390/nano11071829
APA StyleLiu, Q., Tan, Y., Zhang, R., Kang, Y., Zeng, G., Zhao, X., & Jiang, T. (2021). Conformal Self-Assembly of Nanospheres for Light-Enhanced Airtightness Monitoring and Room-Temperature Gas Sensing. Nanomaterials, 11(7), 1829. https://doi.org/10.3390/nano11071829





