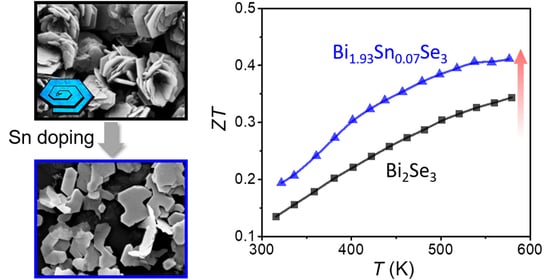
Enhanced Thermoelectric Performance of n-Type Bi2Se3 Nanosheets through Sn Doping
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solvents
2.2. Synthesis of Bi2Se3
2.3. Synthesis of Bi2−xMxSe3
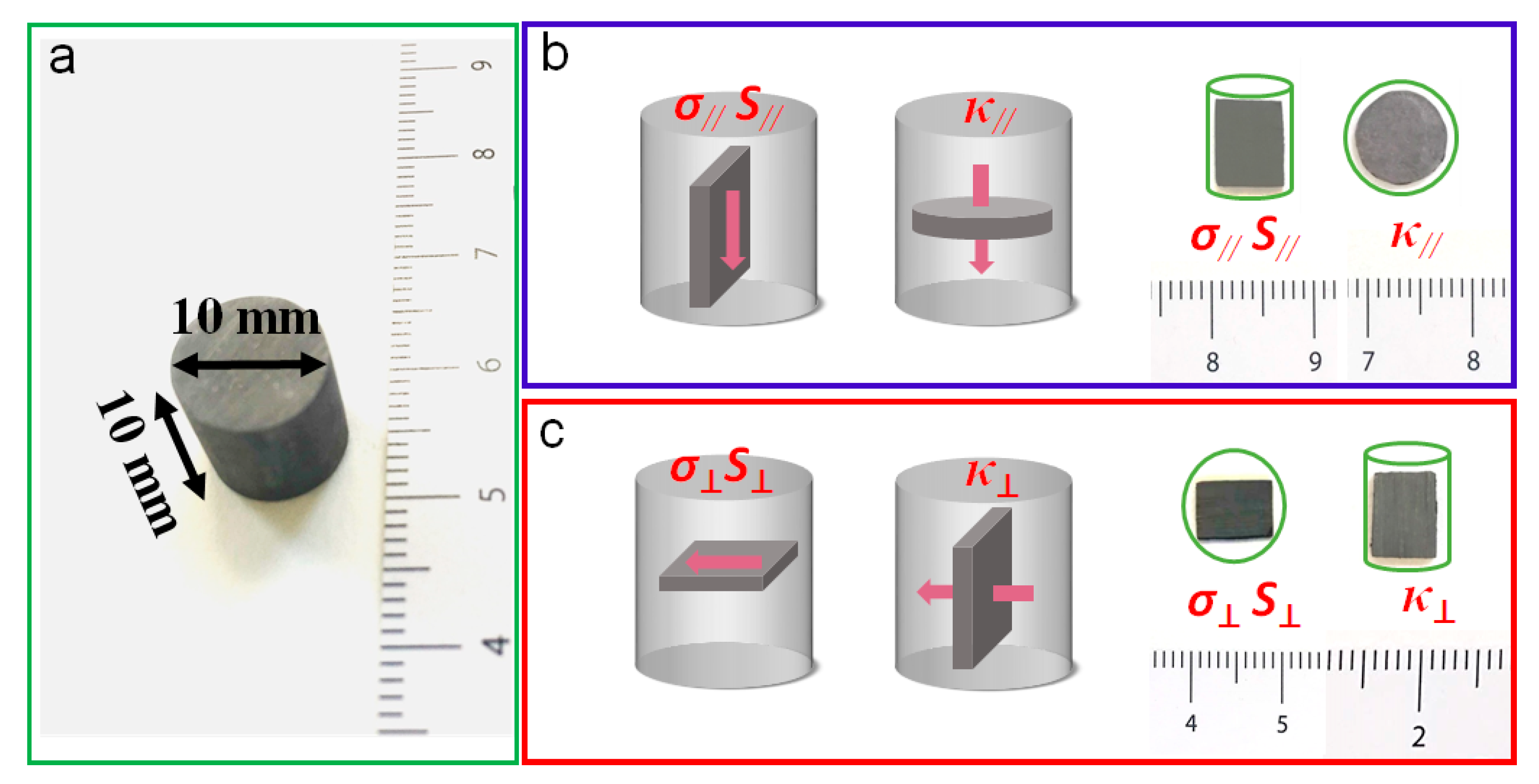
2.4. Nanomaterial Consolidation
2.5. Structural and Chemical Characterization
2.6. Performance Characterization of Bulk Nanomaterial
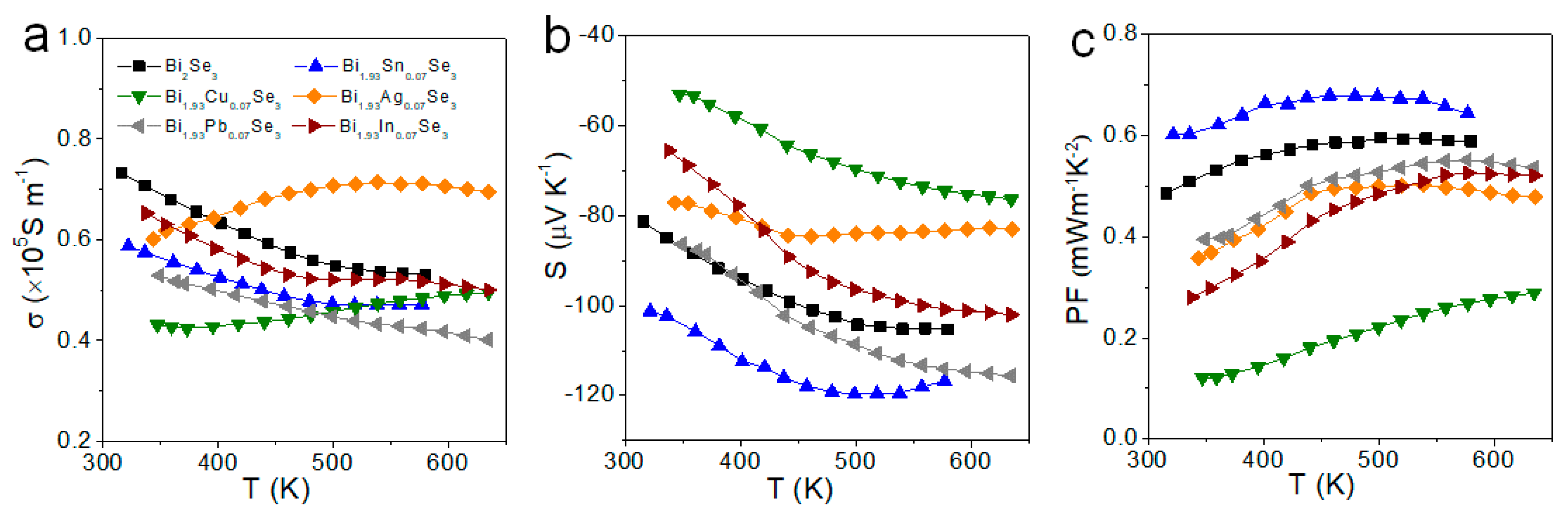
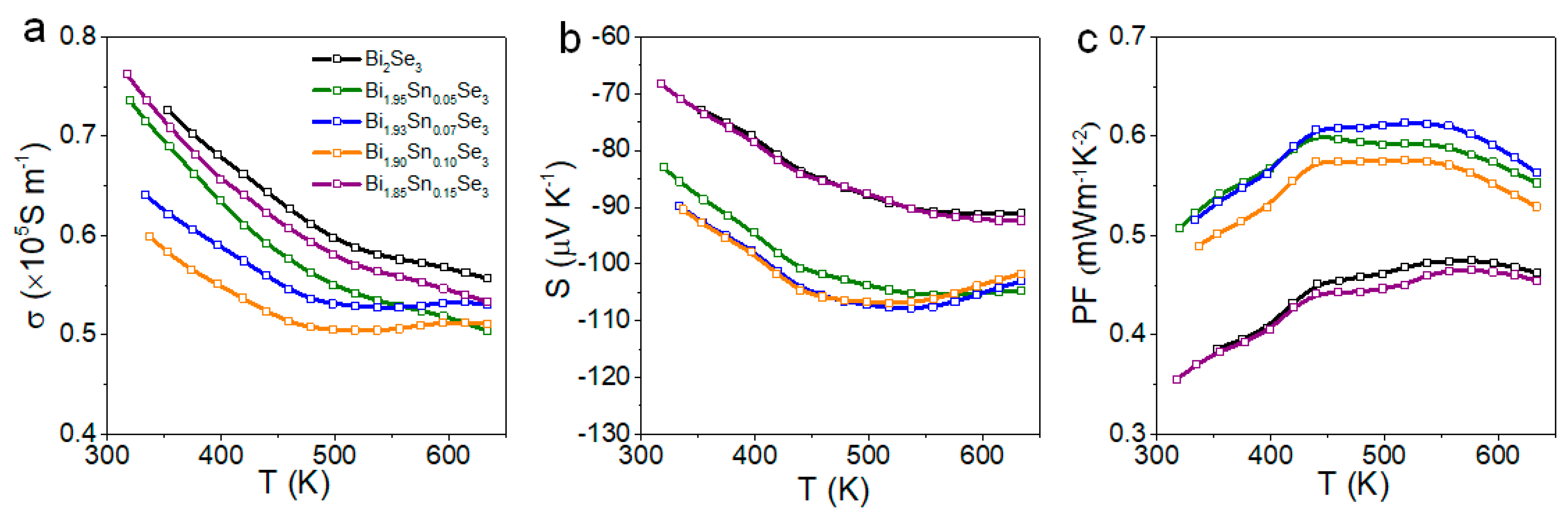
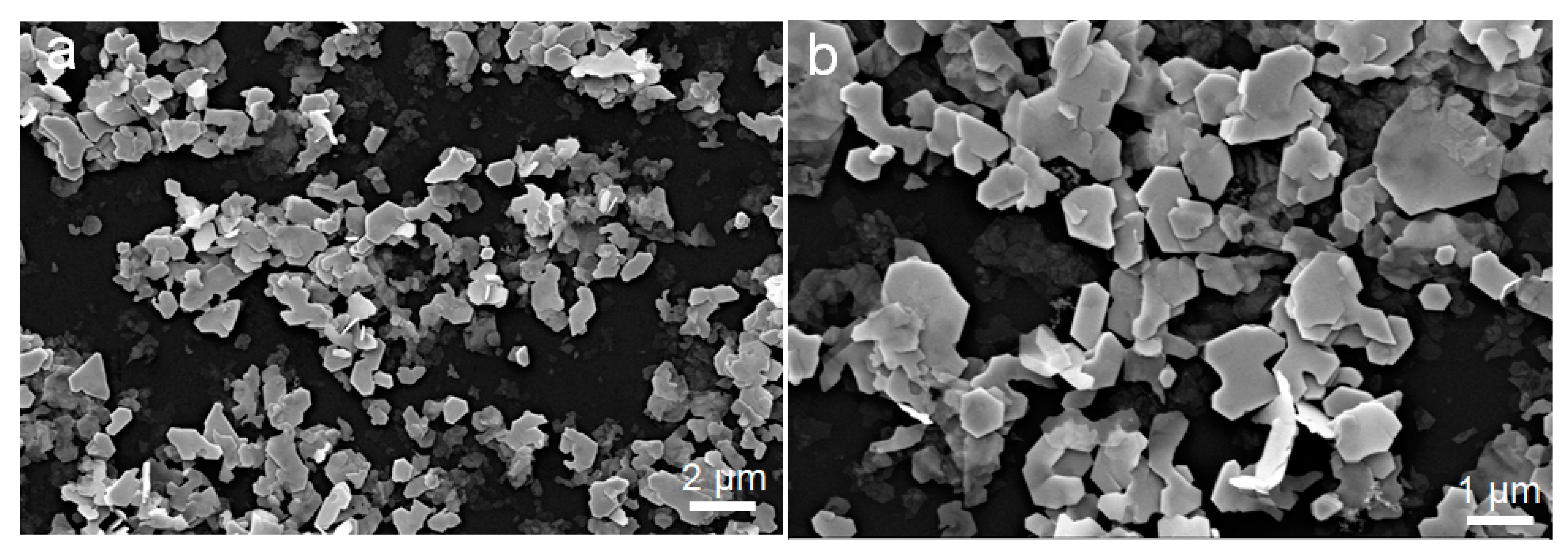
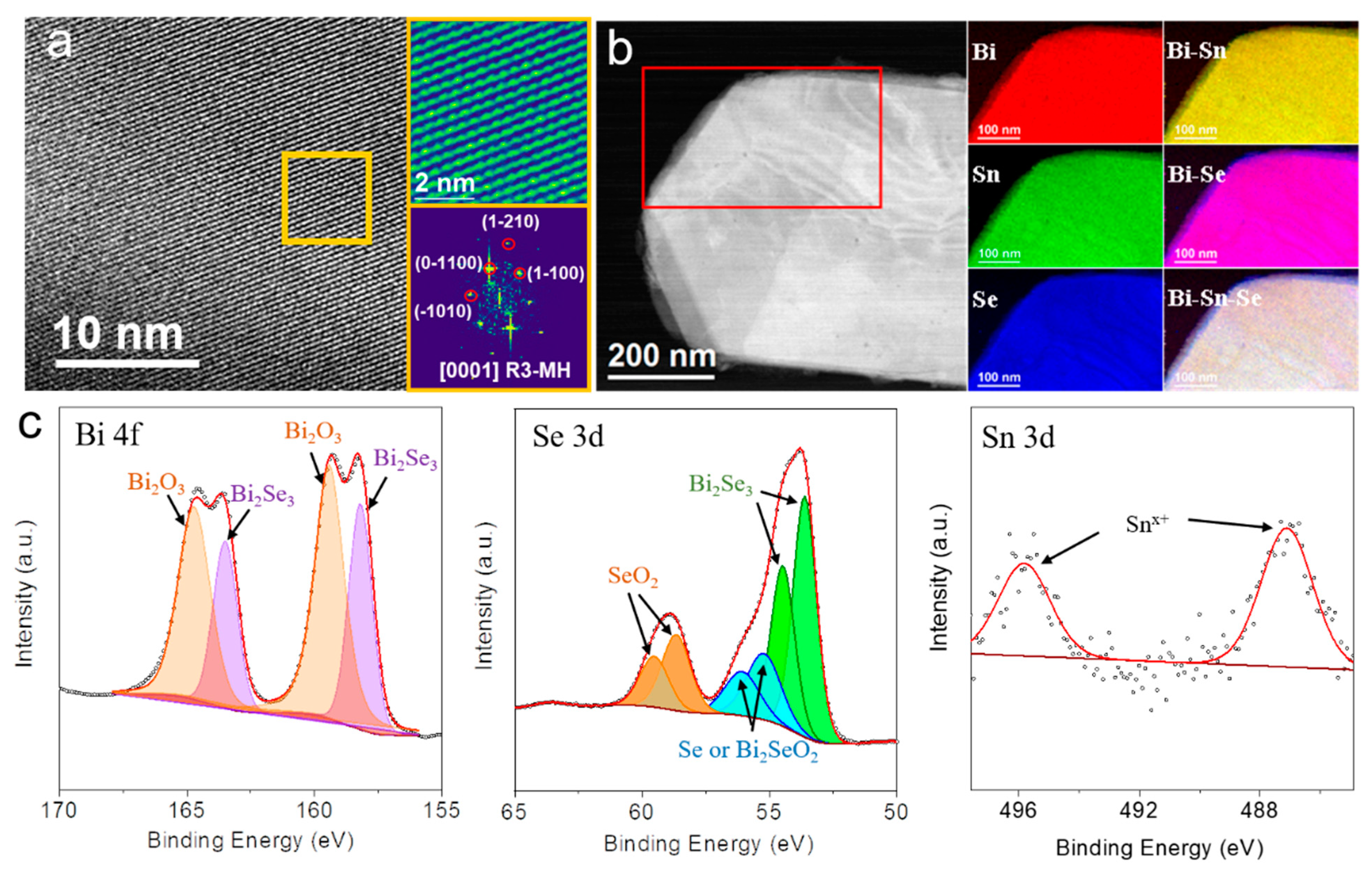
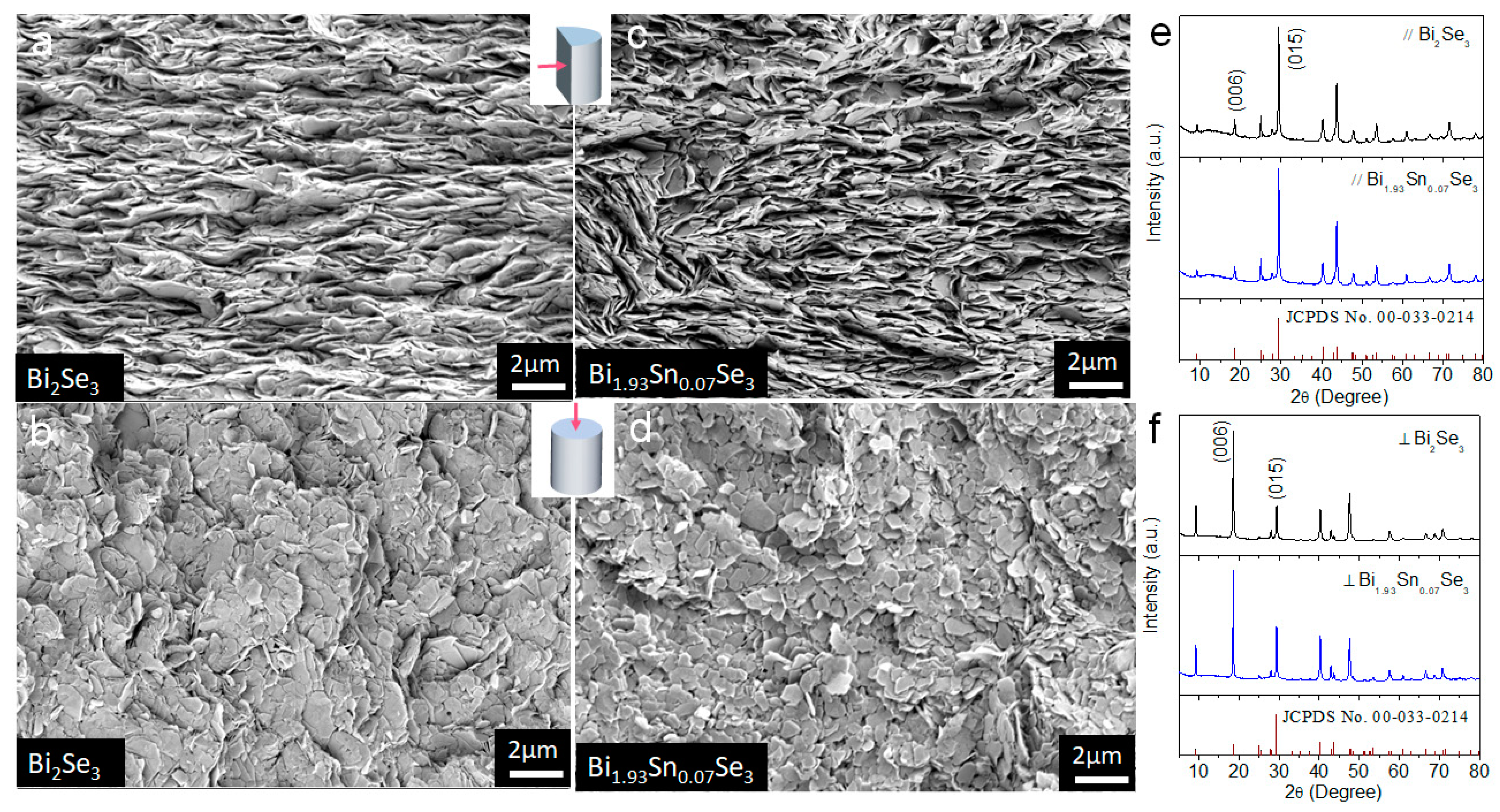
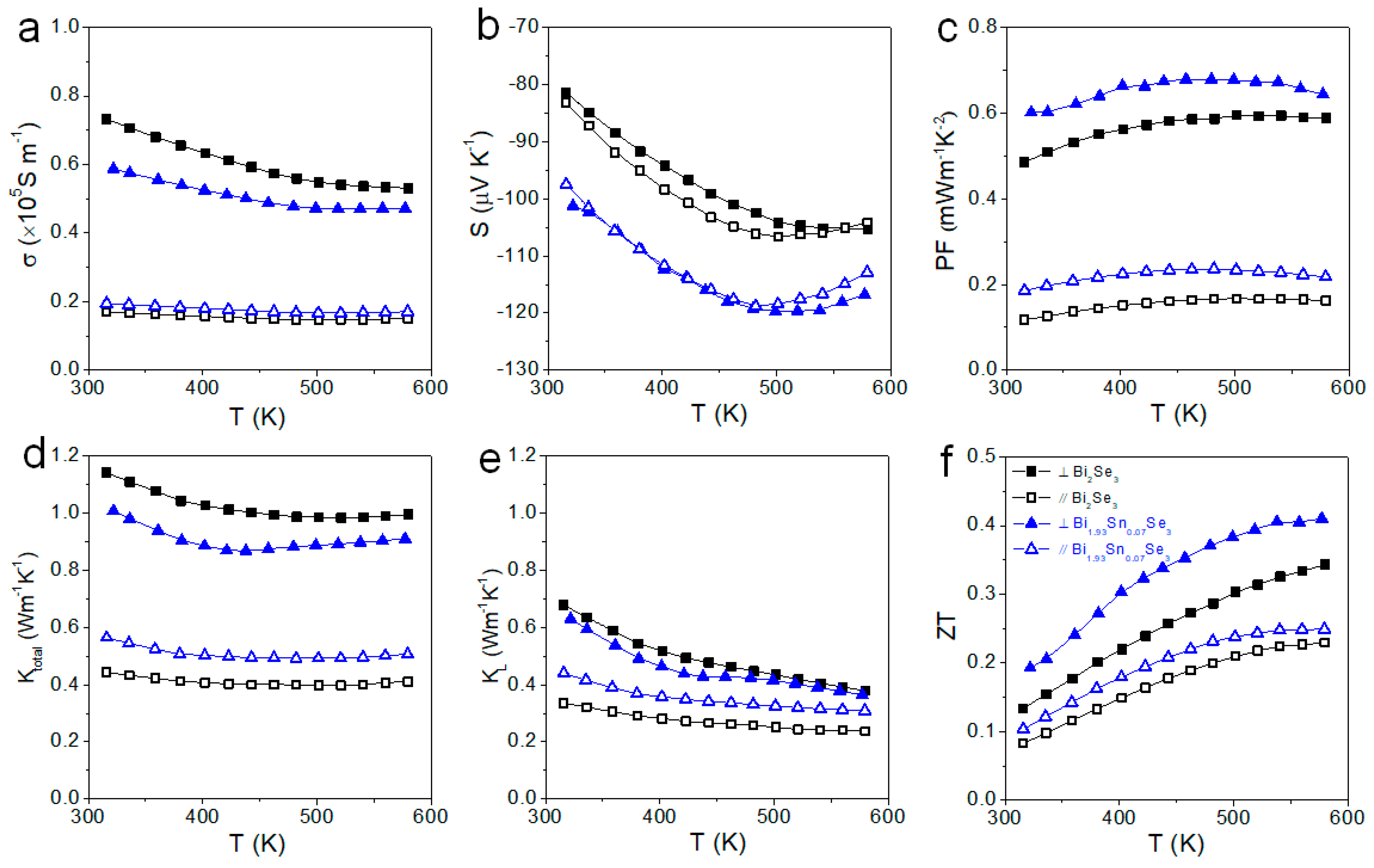
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Zhang, Y.; Lim, K.H.; Ibáñez, M.; Ortega, S.; Li, M.; David, J.; Martí-Sànchez, S.; Ng, K.M.; Arbiol, J.; et al. High Thermoelectric Performance in Crystallographically Textured n type Bi2Te3-xSex Produced from Asymmetric Colloidal Nanocrystals. ACS Nano 2018, 12, 7174–7184. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, Y.; Ortega, S.; Ibáñez, M.; Lim, K.H.; Grau-Carbonell, A.; Martí-Sánchez, S.; Ng, K.M.; Arbiol, J.; Kovalenko, M.V.; et al. Crystallographically Textured Nanomaterials Produced from the Liquid Phase Sintering of BixSb2-xTe3 Nanocrystal Building Blocks. Nano Lett. 2018, 18, 2557–2563. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, Y.; Lim, K.H.; Xing, C.; Li, M.; Zhang, T.; Tang, P.; Arbiol, J.; Llorca, J.; Ng, K.M.; et al. Tin Diselenide Molecular Precursor for Solution-Processable Thermoelectric Materials. Angew. Chem. 2018, 130, 17309–17314. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Zhang, Y.; Han, X.; Zhang, T.; Zuo, Y.; Xie, C.; Xiao, K.; Arbiol, J.; Llorca, J.; et al. Effect of the Annealing Atmosphere on Crystal Phase and Thermoelectric Properties of Copper Sulfide. ACS Nano 2021, 15, 4967–4978. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Y.; Zhang, Y.; Zuo, Y.; Li, J.; Lim, K.H.; Cadavid, D.; Ng, K.M.; Cabot, A. Crystallographically textured SnSe nanomaterials produced from the liquid phase sintering of nanocrystals. Dalt. Trans. 2019, 48, 3641–3647. [Google Scholar] [CrossRef] [PubMed]
- Ortega, S.; Ibáñez, M.; Liu, Y.; Zhang, Y.; Kovalenko, M.V.; Cadavid, D.; Cabot, A. Bottom-up engineering of thermoelectric nanomaterials and devices from solution-processed nanoparticle building blocks. Chem. Soc. Rev. 2017, 46, 3510–3528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibañez, M.; Hasler, R.; Genc, A.; Liu, Y.; Kuster, B.; Schuster, M.; Dobrozhan, O.; Cadavid, D.; Arbiol, J.; Cabot, A.; et al. Ligand-mediated band engineering in bottom-up assembled SnTe nanocomposites for thermoelectric energy conversion. J. Am. Chem. Soc. 2019, 141, 8025–8029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Xing, C.; Liu, Y.; Li, M.; Xiao, K.; Guardia, P.; Lee, S.; Han, X.; Ostovari Moghaddam, A.; Josep Roa, J.; et al. Influence of copper telluride nanodomains on the transport properties of n-type bismuth telluride. Chem. Eng. J. 2021, 418, 129374. [Google Scholar] [CrossRef]
- Ibáñez, M.; Genç, A.; Hasler, R.; Liu, Y.; Dobrozhan, O.; Nazarenko, O.; De La Mata, M.; Arbiol, J.; Cabot, A.; Kovalenko, M.V. Tuning transport properties in thermoelectric nanocomposites through inorganic ligands and heterostructured building blocks. ACS Nano 2019, 13, 6572–6580. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Sun, Y.; Yang, J.; Duan, B.; Wu, L.; Zhang, W.; Yang, J. High thermoelectric performance in Te-free (Bi,Sb)2Se3: Via structural transition induced band convergence and chemical bond softening. Energy Environ. Sci. 2016, 9, 3436–3447. [Google Scholar] [CrossRef]
- Min, Y.; Park, G.; Kim, B.; Giri, A.; Zeng, J.; Roh, J.W.; Kim, S.I.; Lee, K.H.; Jeong, U. Synthesis of Multishell Nanoplates by Consecutive Epitaxial Growth of Bi2Se3 and Bi2Te3 Nanoplates and Enhanced Thermoelectric Properties. ACS Nano 2015, 9, 6843–6853. [Google Scholar] [CrossRef]
- Min, Y.; Roh, J.W.; Yang, H.; Park, M.; Kim, S.I.; Hwang, S.; Lee, S.M.; Lee, K.H.; Jeong, U. Surfactant-free scalable synthesis of Bi2Te3 and Bi2Se3 nanoflakes and enhanced thermoelectric properties of Their Nanocomposites. Adv. Mater. 2013, 25, 1425–1429. [Google Scholar] [CrossRef]
- Liu, W.; Lukas, K.C.; McEnaney, K.; Lee, S.; Zhang, Q.; Opeil, C.P.; Chen, G.; Ren, Z. Studies on the Bi2Te3-Bi2Se3-Bi2S3 system for mid-temperature thermoelectric energy conversion. Energy Environ. Sci. 2013, 6, 552–560. [Google Scholar] [CrossRef]
- Le, P.H.; Wu, K.H.; Luo, C.W.; Leu, J. Growth and characterization of topological insulator Bi2Se3 thin films on SrTiO3 using pulsed laser deposition. Thin Solid Films 2013, 534, 659–665. [Google Scholar] [CrossRef]
- Morin, S.A.; Forticaux, A.; Bierman, M.J.; Jin, S. Screw Dislocation-Driven Growth of 2D Nanoplates. Nano Lett. 2011, 11, 4449–4455. [Google Scholar] [CrossRef]
- Janíček, P.; Drašar, Č.; Beneš, L.; Lošták, P. Thermoelectric properties of Tl-doped Bi2Se3 single crystals. Cryst. Res. Technol. 2009, 44. [Google Scholar] [CrossRef]
- Cui, H.; Liu, H.; Li, X.; Wang, J.; Han, F.; Zhang, X.; Boughton, R.I. Synthesis of Bi2Se3 thermoelectric nanosheets and nanotubes through hydrothermal co-reduction method. J. Solid State Chem. 2004, 177, 4001–4006. [Google Scholar] [CrossRef]
- Bauer, C.; Veremchuk, I.; Kunze, C.; Benad, A.; Dzhagan, V.M.; Haubold, D.; Pohl, D.; Schierning, G.; Nielsch, K.; Lesnyak, V.; et al. Heterostructured Bismuth Telluride Selenide Nanosheets for Enhanced Thermoelectric Performance. Small Sci. 2021, 1, 2000021. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, Z.; Soni, A.; Zhao, Y.; Xiong, Y.; Peng, B.; Wang, J.; Dresselhaus, M.S.; Xiong, Q. Raman spectroscopy of few-quintuple layer topological insulator Bi2Se3 nanoplatelets. Nano Lett. 2011, 11, 2407–2414. [Google Scholar] [CrossRef]
- Kadel, K.; Kumari, L.; Li, W.Z.; Huang, J.Y.; Provencio, P.P. Synthesis and Thermoelectric Properties of Bi2Se3 Nanostructures. Nanoscale Res. Lett. 2011, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Cheng, H.; Gao, S.; Liu, Q.; Sun, Z.; Xiao, C.; Wu, C.; Wei, S.; Xie, Y. Atomically thick bismuth selenide freestanding single layers achieving enhanced thermoelectric energy harvesting. J. Am. Chem. Soc. 2012, 134, 20294–20297. [Google Scholar] [CrossRef]
- Pei, Y.; Wang, H.; Snyder, G.J. Band engineering of thermoelectric materials. Adv. Mater. 2012, 24, 6125–6135. [Google Scholar] [CrossRef]
- Zhuang, A.; Li, J.J.; Wang, Y.C.; Wen, X.; Lin, Y.; Xiang, B.; Wang, X.; Zeng, J. Screw-dislocation-driven bidirectional spiral growth of Bi2Se3 nanoplates. Angew. Chem. 2014, 53, 6425–6429. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; We, J.H.; Cho, B.J. A wearable thermoelectric generator fabricated on a glass fabric. Energy Environ. Sci. 2014, 7, 1959–1965. [Google Scholar] [CrossRef]
- Saeed, Y.; Singh, N.; Schwingenschlögl, U. Thickness and strain effects on the thermoelectric transport in nanostructured Bi2Se3. Appl. Phys. Lett. 2014, 104, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Wang, X. Mechanisms in the solution growth of free-standing two-dimensional inorganic nanomaterials. Nanoscale 2014, 6, 6398–6414. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhang, G.; Zhang, X.; Ji, J.; Li, G.; Zhao, X. Recent development and application of thermoelectric generator and cooler. Appl. Energy 2015, 143, 1–25. [Google Scholar] [CrossRef]
- Hong, M.; Chen, Z.G.; Yang, L.; Han, G.; Zou, J. Enhanced Thermoelectric Performance of Ultrathin Bi2Se3 Nanosheets through Thickness Control. Adv. Electron. Mater. 2015, 1, 1–9. [Google Scholar] [CrossRef]
- Gayner, C.; Amouyal, Y. Energy Filtering of Charge Carriers: Current Trends, Challenges, and Prospects for Thermoelectric Materials. Adv. Funct. Mater. 2020, 30, 1901789. [Google Scholar] [CrossRef]
- Green, A.J.; Dey, S.; An, Y.Q.; O’Brien, B.; O’Mullane, S.; Thiel, B.; Diebold, A.C. Surface oxidation of the topological insulator Bi2Se3. J. Vac. Sci. Technol. A Vacuum Surfaces Films 2016, 34, 061403. [Google Scholar] [CrossRef] [Green Version]
- Das, L.; Guleria, A.; Adhikari, S. Aqueous phase one-pot green synthesis of SnSe nanosheets in a protein matrix: Negligible cytotoxicity and room temperature emission in the visible region. RSC Adv. 2015, 5, 61390–61397. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Chen, Z.; Lin, S.; Zheng, L.; Li, W.; Pei, Y. Single Parabolic Band Behavior of Thermoelectric P-Type CuGaTe2. J. Mater. Chem. C 2015, 4, 209–214. [Google Scholar] [CrossRef]
- Luo, Z.Z.; Cai, S.; Hao, S.; Bailey, T.P.; Hu, X.; Hanus, R.; Ma, R.; Tan, G.; Chica, D.G.; Snyder, G.J.; et al. Ultralow Thermal Conductivity and High-Temperature Thermoelectric Performance in n-Type K2.5Bi8.5Se14. Chem. Mater. 2019, 31, 5943–5952. [Google Scholar] [CrossRef]
- Samanta, M.; Pal, K.; Pal, P.; Waghmare, U.V.; Biswas, K. Localized Vibrations of Bi Bilayer Leading to Ultralow Lattice Thermal Conductivity and High Thermoelectric Performance in Weak Topological Insulator n-Type BiSe. J. Am. Chem. Soc. 2018, 140, 5866–5872. [Google Scholar] [CrossRef]
- Jia, F.; Liu, Y.Y.; Zhang, Y.F.; Shu, X.; Chen, L.; Wu, L.M. Bi8Se7: Delocalized Interlayer π-Bond Interactions Enhancing Carrier Mobility and Thermoelectric Performance near Room Temperature. J. Am. Chem. Soc. 2020, 142, 12536–12543. [Google Scholar] [CrossRef] [PubMed]
- Zong, P.A.; Zhang, P.; Yin, S.; Huang, Y.; Wang, Y.; Wan, C. Fabrication and Characterization of a Hybrid Bi2Se3/Organic Superlattice for Thermoelectric Energy Conversion. Adv. Electron. Mater. 2019, 5, 1–8. [Google Scholar] [CrossRef]
- Dun, C.; Hewitt, C.A.; Huang, H.; Xu, J.; Zhou, C.; Huang, W.; Cui, Y.; Zhou, W.; Jiang, Q.; Carroll, D.L. Flexible n-type thermoelectric films based on Cu-doped Bi2Se3 nanoplate and Polyvinylidene Fluoride composite with decoupled Seebeck coefficient and electrical conductivity. Nano Energy 2015, 18, 306–314. [Google Scholar] [CrossRef]


| Materials | nH [1019 cm−3] | μH [cm2 V−1 S−1] | m*/m0 |
|---|---|---|---|
| Bi2Se3 | 1.77 | 178.6 | 0.27 |
| Bi1.93Sn0.07Se3 | 1.34 | 239.3 | 0.29 |
| ZT at Room Temperature | ZTmax | |||||||
|---|---|---|---|---|---|---|---|---|
| Materials | σ (104S/m) | S (μV/K) | κ (W/mK) | ZT | σ (104 S/m) | S (μV/K) | κ (W/mK) | ZT |
| K2.5Bi8.5Se14 [33] | 0.4 | −100 | 0.6 | 0.05 | 1.8 | −160 | 0.38 | 1 (873 K) |
| Bi0.7Sb0.3Se [34] | 4.5 | −138 | 0.5 | 0.45 | 4.2 | −160 | 0.7 | 0.8 (425 k) |
| Bi5.6Sb2.4Se7 [35] | 4.8 | −168.5 | 0.73 | 0.55 (300 K) | ||||
| Bi2Se3 [28] | 2 | −120 | 0.5 | 0.17 | 1.95 | −155 | 0.4 | 0.48 (427 K) |
| Bi2Se3 [21] | 1.78 | −90 | 0.42 | 0.1 | 3 | −120.7 | 0.49 | 0.35 (400 K) |
| Bi2Se3 HA0.11DMSO0.06 [36] | 14.8 | −80 | 1.52 | 0.187 | ||||
| Bi2Se3 [12] | 2.5 | −60 | 0.55 | 0.05 | 2 | −100 | 0.6 | 0.18 (480 K) |
| Cu0.1Bi2Se3 [37] | 1.46 | −84 | 0.32 | 0.1 (290 K) | ||||
| Bi2Se3 [20] | 0.212 | −115 | 0.75 | 0.011 | 0.6755 | −150 | 0.83 | 0.096 (523 K) |
| This work | 5.87 | −101 | 1 | 0.195 | 4.72 | −116 | 0.91 | 0.41 (577 K) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Zhang, Y.; Zhang, T.; Zuo, Y.; Xiao, K.; Arbiol, J.; Llorca, J.; Liu, Y.; Cabot, A. Enhanced Thermoelectric Performance of n-Type Bi2Se3 Nanosheets through Sn Doping. Nanomaterials 2021, 11, 1827. https://doi.org/10.3390/nano11071827
Li M, Zhang Y, Zhang T, Zuo Y, Xiao K, Arbiol J, Llorca J, Liu Y, Cabot A. Enhanced Thermoelectric Performance of n-Type Bi2Se3 Nanosheets through Sn Doping. Nanomaterials. 2021; 11(7):1827. https://doi.org/10.3390/nano11071827
Chicago/Turabian StyleLi, Mengyao, Yu Zhang, Ting Zhang, Yong Zuo, Ke Xiao, Jordi Arbiol, Jordi Llorca, Yu Liu, and Andreu Cabot. 2021. "Enhanced Thermoelectric Performance of n-Type Bi2Se3 Nanosheets through Sn Doping" Nanomaterials 11, no. 7: 1827. https://doi.org/10.3390/nano11071827
APA StyleLi, M., Zhang, Y., Zhang, T., Zuo, Y., Xiao, K., Arbiol, J., Llorca, J., Liu, Y., & Cabot, A. (2021). Enhanced Thermoelectric Performance of n-Type Bi2Se3 Nanosheets through Sn Doping. Nanomaterials, 11(7), 1827. https://doi.org/10.3390/nano11071827