Detection of Silver Nanoparticles in Seawater Using Surface-Enhanced Raman Scattering
Abstract
1. Introduction
2. Materials and Methods
2.1. Silver Nanoparticles (AgNPs)
2.2. Synthesis and Functionalization of Gold Nanostars (AuNSs)
2.3. Nanoparticles Characterization
2.4. Surface-Enhanced Raman Spectroscopy
3. Results
3.1. Physicochemical Characterization of AgNPs in Artificial Seawater
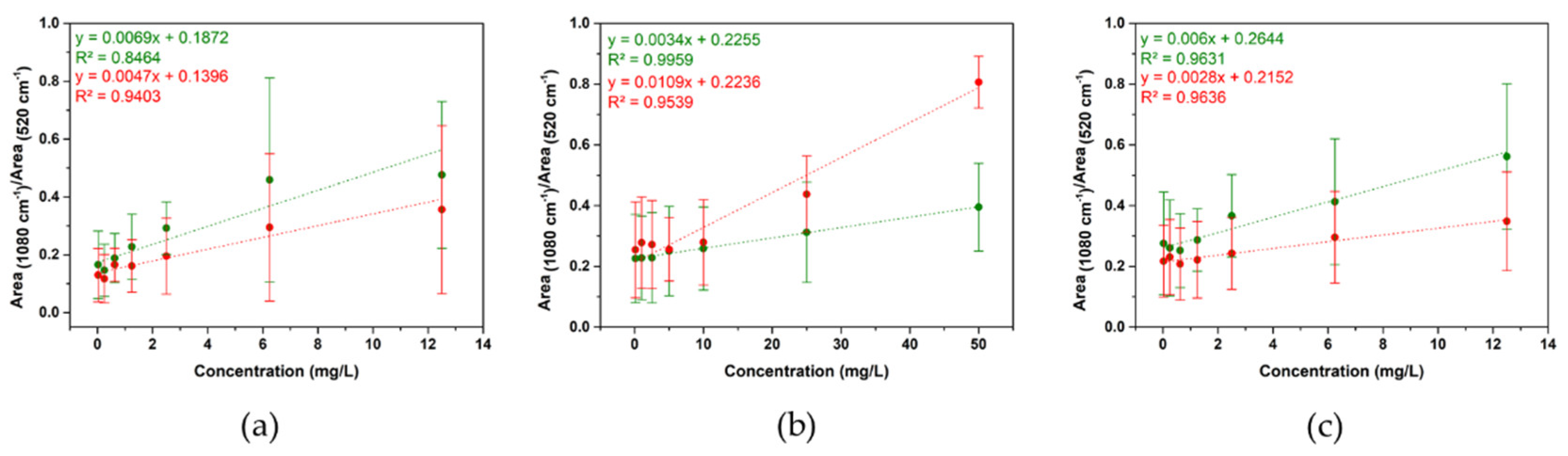
3.2. Detection of AgNPs in Artificial Seawater Using SERS
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Auría-Soro, C.; Nesma, T.; Juanes-Velasco, P.; Landeira-Viñuela, A.; Fidalgo-Gomez, H.; Acebes-Fernandez, V.; Gongora, R.; Parra, M.J.A.; Manzano-Roman, R.; Fuentes, M. Interactions of nanoparticles and biosystems: Microenvironment of nanoparticles and biomolecules in nanomedicine. Nanomaterials 2019, 9, 1365. [Google Scholar] [CrossRef]
- Sarfraz, J.; Gulin-Sarfraz, T.; Nilsen-Nygaard, J.; Pettersen, M.K. Nanocomposites for food packaging applications: An overview. Nanomaterials 2021, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Thorling, C.A.; Liang, X.; Bridle, K.R.; Grice, J.E.; Zhu, Y.; Crawford, D.H.G.; Xu, Z.P.; Liu, X.; Roberts, M.S. Diagnostic imaging and therapeutic application of nanoparticles targeting the liver. J. Mater. Chem. B 2015, 3, 939–958. [Google Scholar] [CrossRef]
- Otsuka, H.; Nagasaki, Y.; Kataoka, K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv. Drug Deliv. Rev. 2003, 55, 403–419. [Google Scholar] [CrossRef]
- Fabrega, J.; Luoma, S.N.; Tyler, C.R.; Galloway, T.S.; Lead, J.R. Silver nanoparticles: Behaviour and effects in the aquatic environment. Environ. Int. 2011, 37, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Benn, T.M.; Westerhoff, P. Nanoparticle silver released into water from commercially available sock fabrics. Environ. Sci. Technol. 2008, 42, 4133–4139. [Google Scholar] [CrossRef] [PubMed]
- Geranio, L.; Heuberger, M.; Nowack, B. The behavior of silver nanotextiles during washing. Environ. Sci. Technol. 2009, 43, 8113–8118. [Google Scholar] [CrossRef]
- Tortella, G.R.; Rubilar, O.; Durán, N.; Diez, M.C.; Martínez, M.; Parada, J.; Seabra, A.B. Silver nanoparticles: Toxicity in model organisms as an overview of its hazard for human health and the environment. J. Hazard. Mater. 2020, 390, 121974. [Google Scholar] [CrossRef]
- Ricardo, A.I.C.; Fariñas, N.R.; Bernardo, F.J.G.; Martín-Doimeadios, R.C.R.; Ríos, Á. Screening-confirmation strategy for nanomaterials involving spectroscopic analytical techniques and its application to the control of silver nanoparticles in pastry samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 246, 119015. [Google Scholar] [CrossRef] [PubMed]
- Culková, E.; Lukáčová-Chomisteková, Z.; Bellová, R.; Melicherčíková, D.; Durdiak, J.; Rievaj, M.; Vojs, M.; Tomčík, P. Voltammetric detection of silver in commercial products on boron doped diamond electrode: Stripping at lowered potential in the presence of thiosulfate ions. Mon. Chem. 2020, 151, 1009–1017. [Google Scholar] [CrossRef]
- Laborda, F.; Bolea, E.; Cepriá, G.; Gómez, M.T.; Jiménez, M.S.; Pérez-Arantegui, J.; Castillo, J.R. Detection, characterization and quantification of inorganic engineered nanomaterials: A review of techniques and methodological approaches for the analysis of complex samples. Anal. Chim. Acta 2016, 904, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, K.N.M.; Peters, R.J.B.; Klumpp, E.; Bohme, S.; van der Ploeg, M.; Ritsema, C.; Geissen, V. Silver nanoparticles in soil: Aqueous extraction combined with single-particle ICP-MS for detection and characterization. Environ. Nanotechnol. Monit. Manag. 2017, 7, 24–33. [Google Scholar] [CrossRef]
- Yang, Y.; Long, C.L.; Li, H.P.; Wang, Q.; Yang, Z.G. Analysis of silver and gold nanoparticles in environmental water using single particle-inductively coupled plasma-mass spectrometry. Sci. Total Environ. 2016, 563–564, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Ramos, K.; Gómez-Gómez, M.M.; Cámara, C.; Ramos, L. Silver speciation and characterization of nanoparticles released from plastic food containers by single particle ICPMS. Talanta 2016, 151, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Asamoah, B.O.; Kanyathare, B.; Roussey, M.; Peiponen, K.E. A prototype of a portable optical sensor for the detection of transparent and translucent microplastics in freshwater. Chemosphere 2019, 231, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, C.; Ji, Y.; Tian, Y.; Wei, H.; Li, C.; Li, Z.; Zhu, T.; Sun, Q.; Man, B.; et al. 3D Ultrasensitive Polymers-Plasmonic Hybrid Flexible Platform for In-Situ Detection. Polymers 2020, 12, 392. [Google Scholar] [CrossRef] [PubMed]
- Willner, M.R.; Vikesland, P.J. Nanomaterial enabled sensors for environmental contaminants. J. Nanobiotechnol. 2018, 16, 1–16. [Google Scholar] [CrossRef]
- Demirel, G.; Usta, H.; Yilmaz, M.; Celik, M.; Alidagi, H.A.; Buyukserin, F. Surface-enhanced Raman spectroscopy (SERS): An adventure from plasmonic metals to organic semiconductors as SERS platforms. J. Mater. Chem. C 2018, 6, 5314–5335. [Google Scholar] [CrossRef]
- Hankus, M.E.; Li, H.; Gibson, G.J.; Cullum, B.M. Surface-enhanced Raman scattering-based nanoprobe for high-resolution, non-scanning chemical imaging. Anal. Chem. 2006, 78, 7535–7546. [Google Scholar] [CrossRef] [PubMed]
- Culha, M.; Cullum, B.; Lavrik, N.; Klutse, C.K. Surface-enhanced Raman scattering as an emerging characterization and detection technique. J. Nanotechnol. 2012, 2012. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Z.; Xing, B.; Mukherjee, A.; Musante, C.; White, J.C.; He, L. Analysis of silver nanoparticles in antimicrobial products using surface-enhanced raman spectroscopy (SERS). Environ. Sci. Technol. 2015, 49, 4317–4324. [Google Scholar] [CrossRef]
- Shi, W.; Han, Y.; Guo, C.; Su, W.; Zhao, X.; Zha, S.; Wang, Y.; Liu, G. Ocean acidification increases the accumulation of titanium dioxide nanoparticles (nTiO2) in edible bivalve mollusks and poses a potential threat to seafood safety. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, J.; Ko, H. Transparent and Flexible Surface-Enhanced Raman Scattering (SERS) Sensors Based on Gold Nanostar Arrays Embedded in Silicon Rubber Film. ACS Appl. Mater. Interfaces 2017, 9, 44088–44095. [Google Scholar] [CrossRef]
- Rodríguez-Lorenzo, L.; Garrido-Maestu, A.; Bhunia, A.K.; Espiña, B.; Prado, M.; Diéguez, L.; Abalde-Cela, S. Gold Nanostars for the Detection of Foodborne Pathogens via Surface-Enhanced Raman Scattering Combined with Microfluidics. ACS Appl. Nano Mater. 2019, 2, 6081–6086. [Google Scholar] [CrossRef]
- Rodríguez-Lorenzo, L.; Álvarez-Puebla, R.A.; Pastoriza-Santos, I.; Mazzucco, S.; Stéphan, O.; Kociak, M.; Liz-Marzán, L.M.; García de Abajo, F.J. Zeptomol Detection Through Controlled Ultrasensitive Surface-Enhanced Raman Scattering. J. Am. Chem. Soc. 2009, 131, 4616–4618. [Google Scholar] [CrossRef]
- Kim, K.; Kim, K.L.; Shin, D.; Choi, J.Y.; Shin, K.S. Surface-enhanced Raman scattering of 4-aminobenzenethiol on Ag and Au: PH dependence of b 2-type bands. J. Phys. Chem. C 2012, 116, 4774–4779. [Google Scholar] [CrossRef]
- Wu, D.Y.; Liu, X.M.; Huang, Y.F.; Ren, B.; Xu, X.; Tian, Z.Q. Surface catalytic coupling reaction of p-mercaptoaniline linking to silver nanostructures responsible for abnormal SERS enhancement: A DFT study. J. Phys. Chem. C 2009, 113, 18212–18222. [Google Scholar] [CrossRef]
- Rycenga, M.; Camargo, P.H.C.; Li, W.; Moran, C.H.; Xia, Y. Understanding the SERS Effects of Single Silver Nanoparticles and Their Dimers, One at a Time. J. Phys. Chem. Lett. 2010, 1, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Bonyani, M. Characterization and optical studies of PVP-capped silver nanoparticles. J. Nanostruct. Chem. 2017, 7, 37–46. [Google Scholar] [CrossRef]
- Tejamaya, M.; Römer, I.; Merrifield, R.C.; Lead, J.R. Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environ. Sci. Technol. 2012, 46, 7011–7017. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Lamana, J.; Slaveykova, V.I. Silver nanoparticle behaviour in lake water depends on their surface coating. Sci. Total Environ. 2016, 573, 946–953. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, S.; Xu, G.; Liu, R.; Xu, A.; Chen, S.; Wu, L. Effects of ionic strength on physicochemical properties and toxicity of silver nanoparticles. Sci. Total Environ. 2019, 647, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.F.; Blake, T.A.; Bernacki, B.E.; Johnson, T.J. Design Considerations for a Portable Raman Probe Spectrometer for Field Forensics. Int. J. Spectrosc. 2012, 2012, 1–15. [Google Scholar] [CrossRef][Green Version]
- Barone, G.; Bersani, D.; Crupi, V.; Longo, F.; Longobardo, U.; Lottici, P.P.; Aliatis, I.; Majolino, D.; Mazzoleni, P.; Raneri, S.; et al. A portable versus micro-Raman equipment comparison for gemmological purposes: The case of sapphires and their imitations. J. Raman Spectrosc. 2014, 45, 1309–1317. [Google Scholar] [CrossRef]
- Moore, M.M.; Kanekar, S.G.; Dhamija, R. Ethylene Glycol Toxicity: Chemistry, Pathogenesis, and Imaging. Radiol. Case Rep. 2008, 3, 122. [Google Scholar] [CrossRef] [PubMed]
- Enüstün, B.V.; Turkevich, J. Coagulation of Colloidal Gold. J. Am. Chem. Soc. 1963, 85, 3317–3328. [Google Scholar] [CrossRef]
- Slistan-Grijalva, A.; Herrera-Urbina, R.; Rivas-Silva, J.F.; Ávalos-Borja, M.; Castillón-Barraza, F.F.; Posada-Amarillas, A. Synthesis of silver nanoparticles in a polyvinylpyrrolidone (PVP) paste, and their optical properties in a film and in ethylene glycol. Mater. Res. Bull. 2008, 43, 90–96. [Google Scholar] [CrossRef]
- Pastoriza-Santos, I.; Hamanaka, Y.; Fukuta, K.; Nakamura, A.; Liz-MarzáN, L.M. Anisotropic Silver Nanoparticles: Synthesis and Optical Properties. In Low-Dimensional Systems: Theory, Preparation, and Some Applications; Springer: Dordrecht, The Netherlands, 2003; pp. 65–75. [Google Scholar]
- Moore, T.L.; Rodriguez-Lorenzo, L.; Hirsch, V.; Balog, S.; Urban, D.; Jud, C.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015, 44, 6287–6305. [Google Scholar] [CrossRef] [PubMed]
- Vanderkooy, A.; Chen, Y.; Gonzaga, F.; Brook, M.A. Silica Shell/Gold Core Nanoparticles: Correlating Shell Thickness with the Plasmonic Red Shift upon Aggregation. ACS Appl. Mater. Interfaces 2011, 3, 3942–3947. [Google Scholar] [CrossRef]
- Pazos-perez, N.; Guerrini, L.; Alvarez-puebla, R.A. Plasmon Tunability of Gold Nanostars at the Tip Apexes. ACS Omega 2018, 12, 17173–17179. [Google Scholar] [CrossRef]
- Rodríguez-Lorenzo, L.; Álvarez-Puebla, R.A.; De Abajo, F.J.G.; Liz-Marzán, L.M. Surface enhanced Raman scattering using star-shaped gold colloidal nanoparticles. J. Phys. Chem. C 2010, 114, 7336–7340. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, X.; Fan, Q.; Zhang, X.; Zheng, J. Charge transfer between metal nanoparticles interconnected with a functionalized molecule probed by surface-enhanced Raman spectroscopy. Angew. Chem. Int. Ed. 2006, 45, 3970–3973. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Choi, J.; Lee, H.B.; Shin, K.S.; Kim, K.; Choi, J.; Lee, H.B.; Shin, K.S. Effects of size of Ag nanoparticles and the excitation wavelength Raman scattering of 4-aminobenzenethiol sandwiched between Ag nanopartic. J. Chem. Phys. 2011, 28, 124705. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Li, X. Optimal Size of Gold Nanoparticles for Surface-Enhanced Raman Spectroscopy under Different Conditions. J. Nanomater. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- McGillicuddy, E.; Murray, I.; Kavanagh, S.; Morrison, L.; Fogarty, A.; Cormican, M.; Dockery, P.; Prendergast, M.; Rowan, N.; Morris, D. Silver nanoparticles in the environment: Sources, detection and ecotoxicology. Sci. Total Environ. 2017, 575, 231–246. [Google Scholar] [CrossRef]
- Nguyen, T.H.D.; Zhou, P.; Mustapha, A.; Lin, M. Use of aminothiophenol as an indicator for the analysis of silver nanoparticles in consumer products by surface-enhanced Raman spectroscopy. Analyst 2016, 141, 5382–5389. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pérez-Jiménez, A.I.; Lyu, D.; Lu, Z.; Liu, G.; Ren, B. Surface-enhanced Raman spectroscopy: Benefits, trade-offs and future developments. Chem. Sci. 2020, 11, 4563–4577. [Google Scholar] [CrossRef]
- Asamoah, B.O.; Uurasjärvi, E.; Räty, J.; Koistinen, A.; Roussey, M.; Peiponen, K.-E. Towards the Development of Portable and In Situ Optical Devices for Detection of Micro-and Nanoplastics in Water: A Review on the Current Status. Polymers 2021, 13, 730. [Google Scholar] [CrossRef]
- He, L.; Özdemir, Ş.K.; Zhu, J.; Kim, W.; Yang, L. Detecting single viruses and nanoparticles using whispering gallery microlasers. Nat. Nanotechnol. 2011, 6, 428–432. [Google Scholar] [CrossRef]
- Pabortsava, K.; Lampitt, R.S. High concentrations of plastic hidden beneath the surface of the Atlantic Ocean. Nat. Commun. 2020, 11, 4073. [Google Scholar] [CrossRef]
- Bianchin, J.N.; Martendal, E.; Carasek, E. Determination of Trace Silver in Water Samples by Online Column Preconcentration Flame Atomic Absorption Spectrometry Using Termite Digestion Product. J. Autom. Methods Manag. Chem. 2011, 2011, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chao, J.; Liu, R.; Tan, Z.; Yin, Y.; Wu, Y.; Jiang, G. Cloud Point Extraction as an Advantageous Preconcentration Approach for Analysis of Trace Silver Nanoparticles in Environmental Waters. Anal. Chem. 2009, 81, 6496–6502. [Google Scholar] [CrossRef]
- Khaksar, M.; Jolley, D.F.; Sekine, R.; Vasilev, K.; Johannessen, B.; Donner, E.; Lombi, E. In Situ Chemical Transformations of Silver Nanoparticles along the Water–Sediment Continuum. Environ. Sci. Technol. 2015, 49, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Jiang, T.; Zhao, Q.; Yin, H. Charge-transfer contributions in surface-enhanced Raman scattering from Ag, Ag2S and Ag2Se substrates. J. Raman Spectrosc. 2012, 43, 1191–1195. [Google Scholar] [CrossRef]
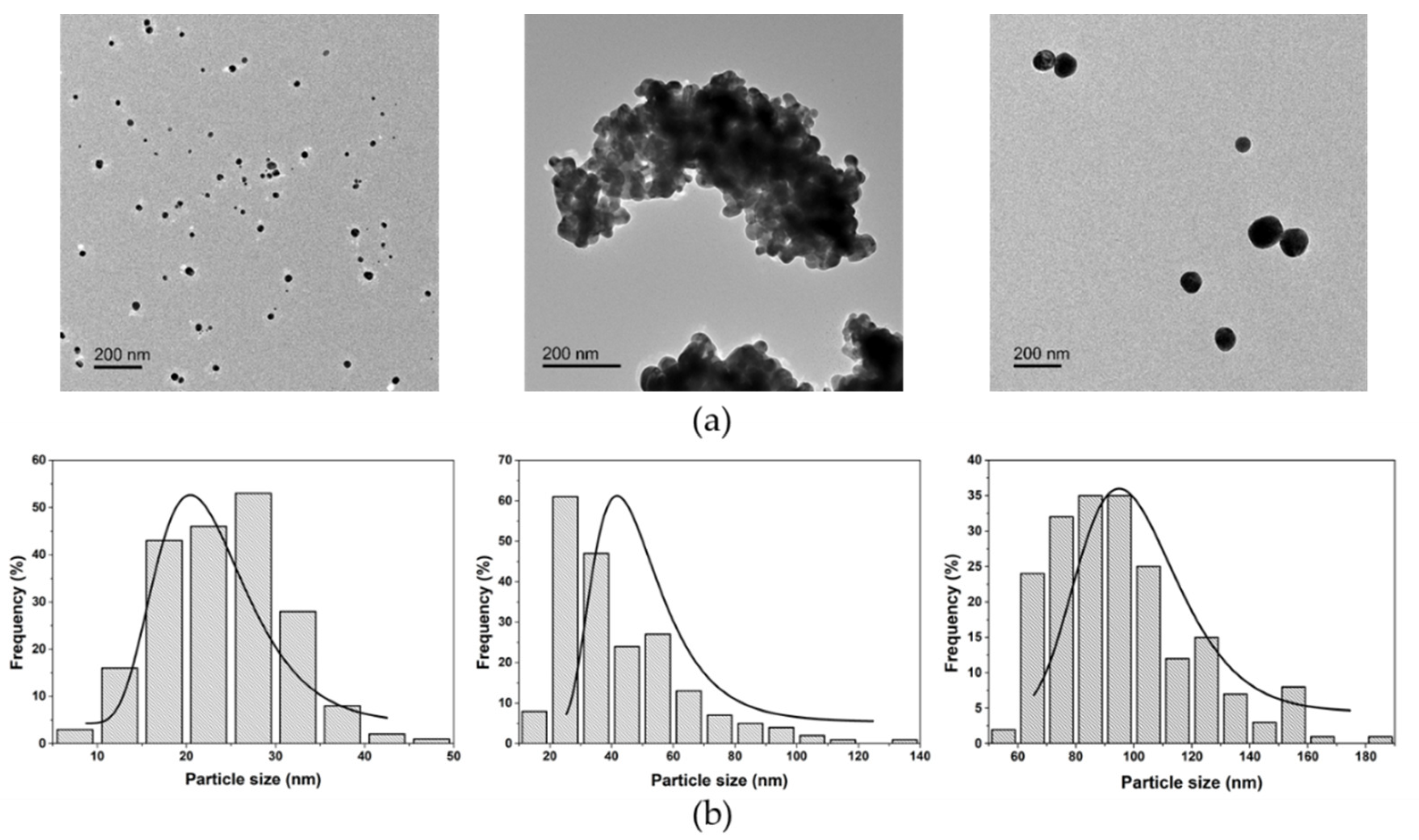


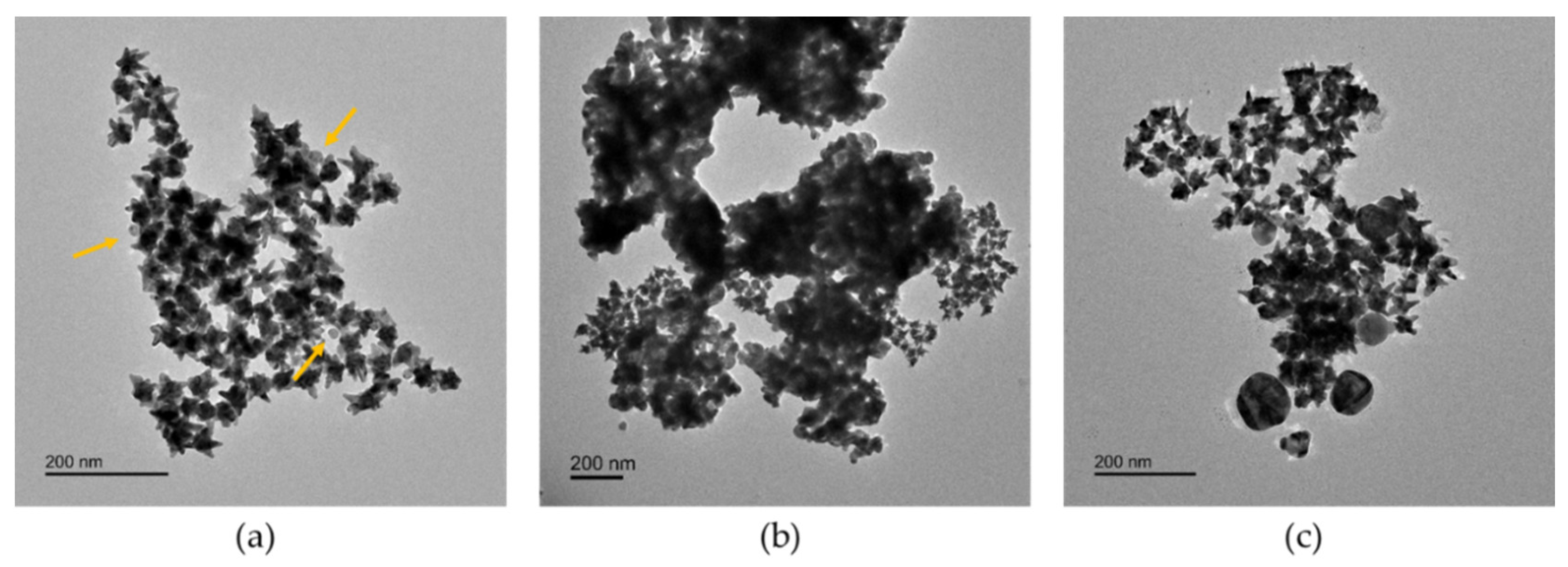
| Ultrapure Water | Artificial Seawater | |||||
|---|---|---|---|---|---|---|
| PVP-15nmAg NPs | PVP-100nmAg NPs | PVP-50–80nmAg NPs | PVP-15nmAg NPs | PVP-100nmAg NPs | PVP-50–80nmAg NPs | |
| Hydrodynamic diameter 1 (nm) | 49 ± 3 | 139 ± 2 | 618 ± 83 | 47 ± 2 | 97 ± 1 | 1348 ± 407 |
| PDI 2 (%) | 57 ± 7 | 20 ± 2 | 146 ± 11 | 36 ± 3 | 27 ± 4 | 78 ± 12 |
| Z potential 3 (mV) | −24 ± 5 | −21 ± 12 | −83 ± 6 | −9 ± 16 | −6 ± 10 | −5 ± 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quarato, M.; Pinheiro, I.; Vieira, A.; Espiña, B.; Rodriguez-Lorenzo, L. Detection of Silver Nanoparticles in Seawater Using Surface-Enhanced Raman Scattering. Nanomaterials 2021, 11, 1711. https://doi.org/10.3390/nano11071711
Quarato M, Pinheiro I, Vieira A, Espiña B, Rodriguez-Lorenzo L. Detection of Silver Nanoparticles in Seawater Using Surface-Enhanced Raman Scattering. Nanomaterials. 2021; 11(7):1711. https://doi.org/10.3390/nano11071711
Chicago/Turabian StyleQuarato, Monica, Ivone Pinheiro, Ana Vieira, Begoña Espiña, and Laura Rodriguez-Lorenzo. 2021. "Detection of Silver Nanoparticles in Seawater Using Surface-Enhanced Raman Scattering" Nanomaterials 11, no. 7: 1711. https://doi.org/10.3390/nano11071711
APA StyleQuarato, M., Pinheiro, I., Vieira, A., Espiña, B., & Rodriguez-Lorenzo, L. (2021). Detection of Silver Nanoparticles in Seawater Using Surface-Enhanced Raman Scattering. Nanomaterials, 11(7), 1711. https://doi.org/10.3390/nano11071711







