Enhancing Lithium and Sodium Storage Properties of TiO2(B) Nanobelts by Doping with Nickel and Zinc
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthetic Procedure
2.2. Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
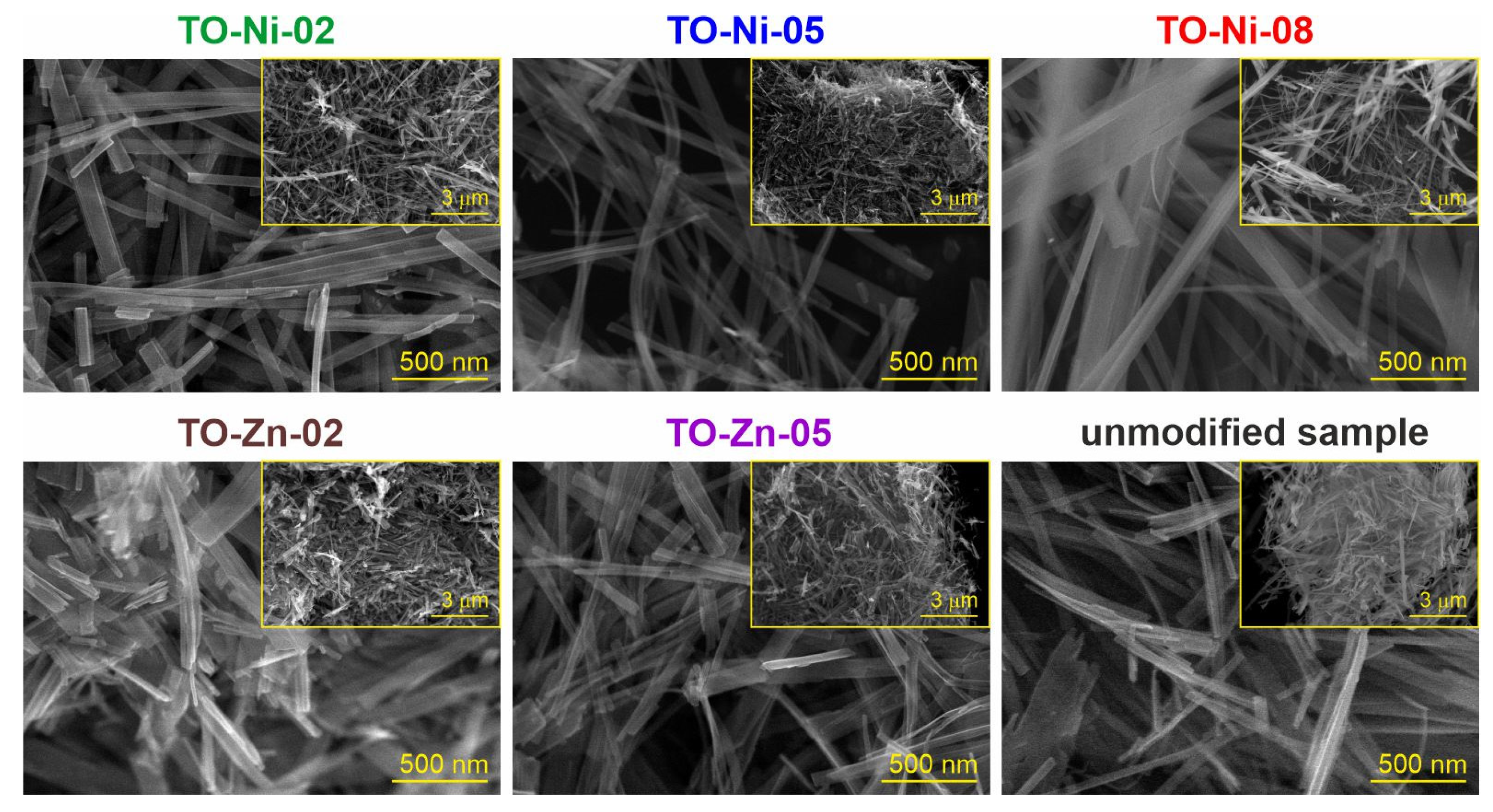
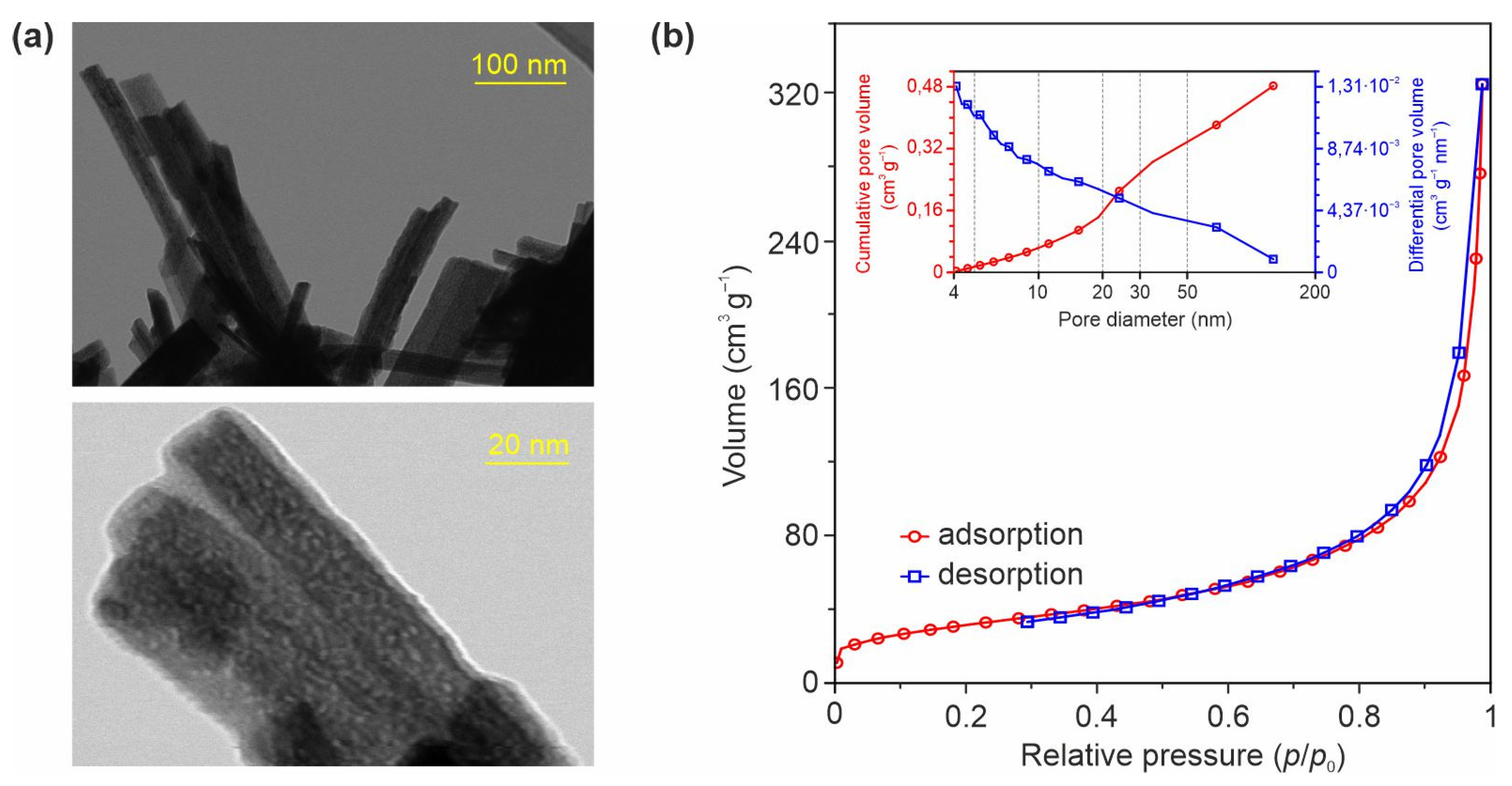
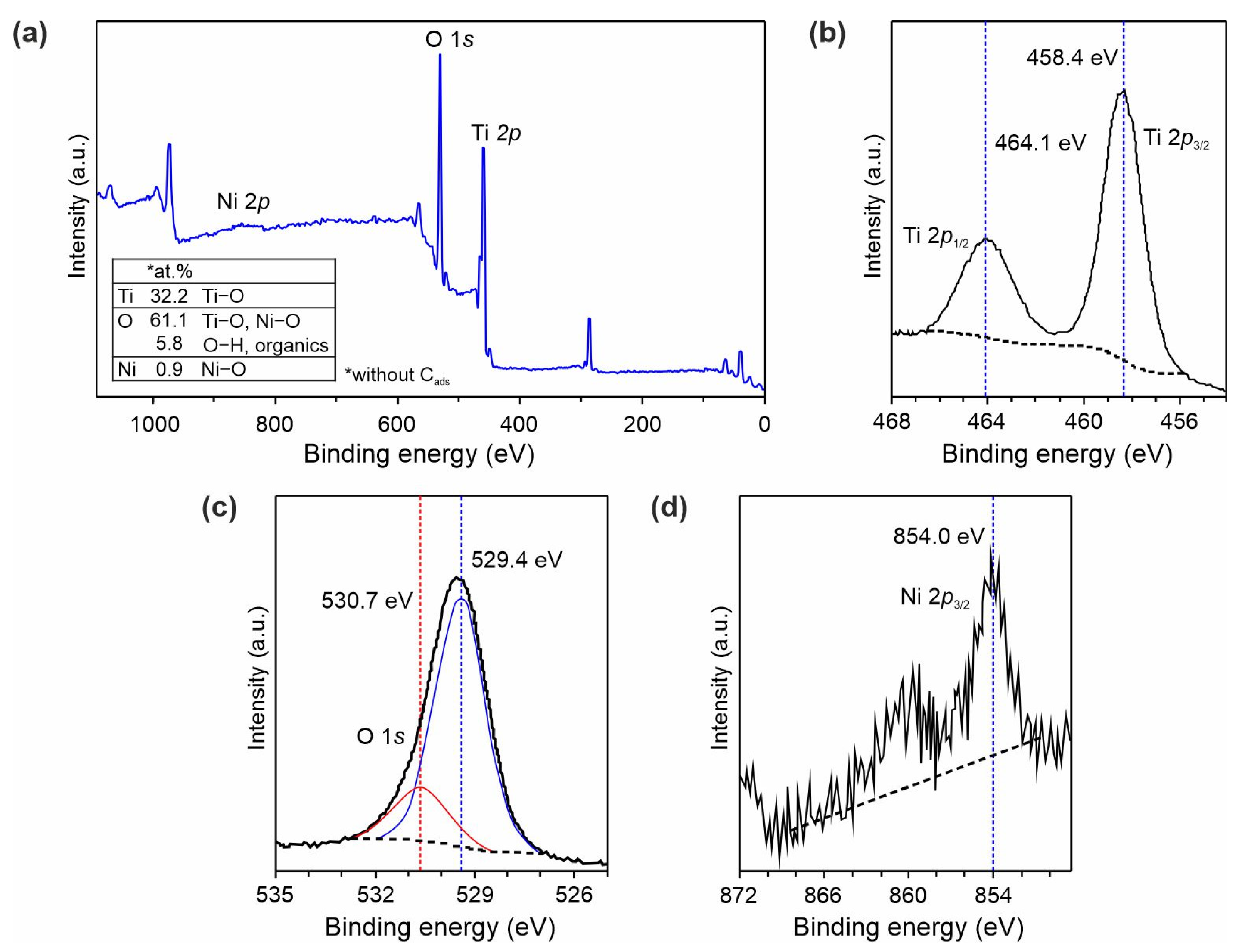
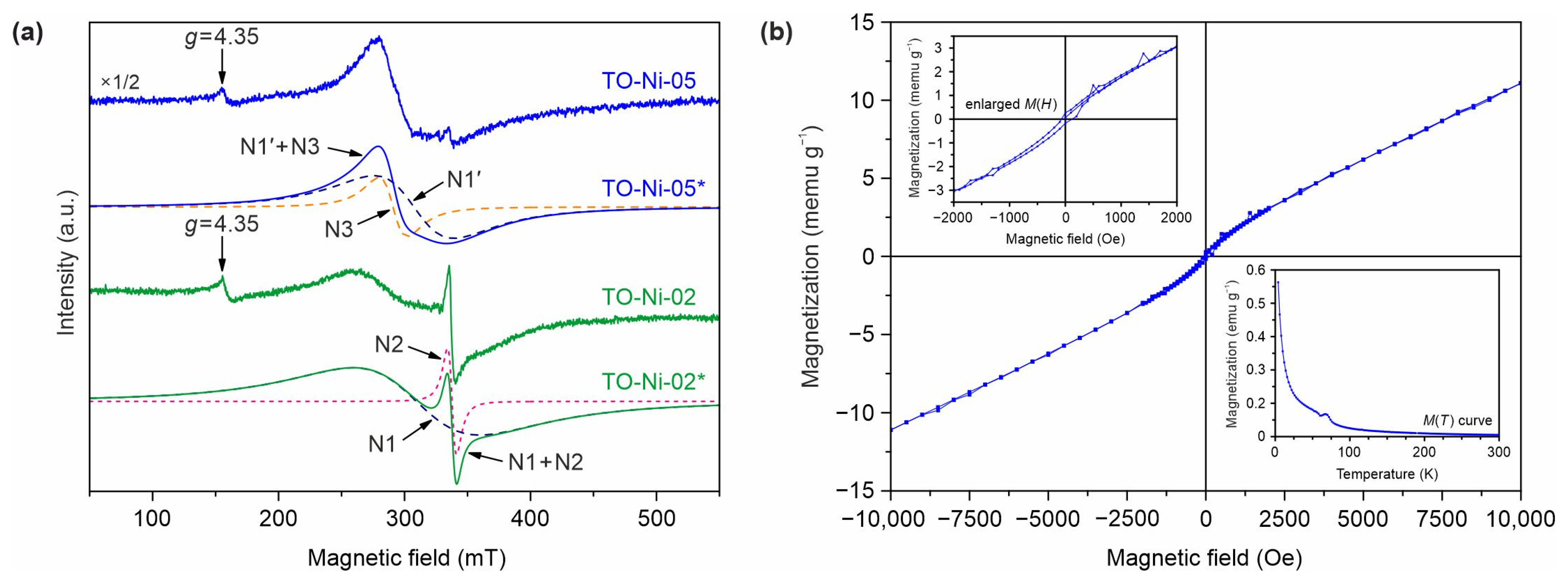
3.1. Structure, Morphology, and Electronic Properties of Ni- and Zn-Doped TiO2(B) Nanobelts
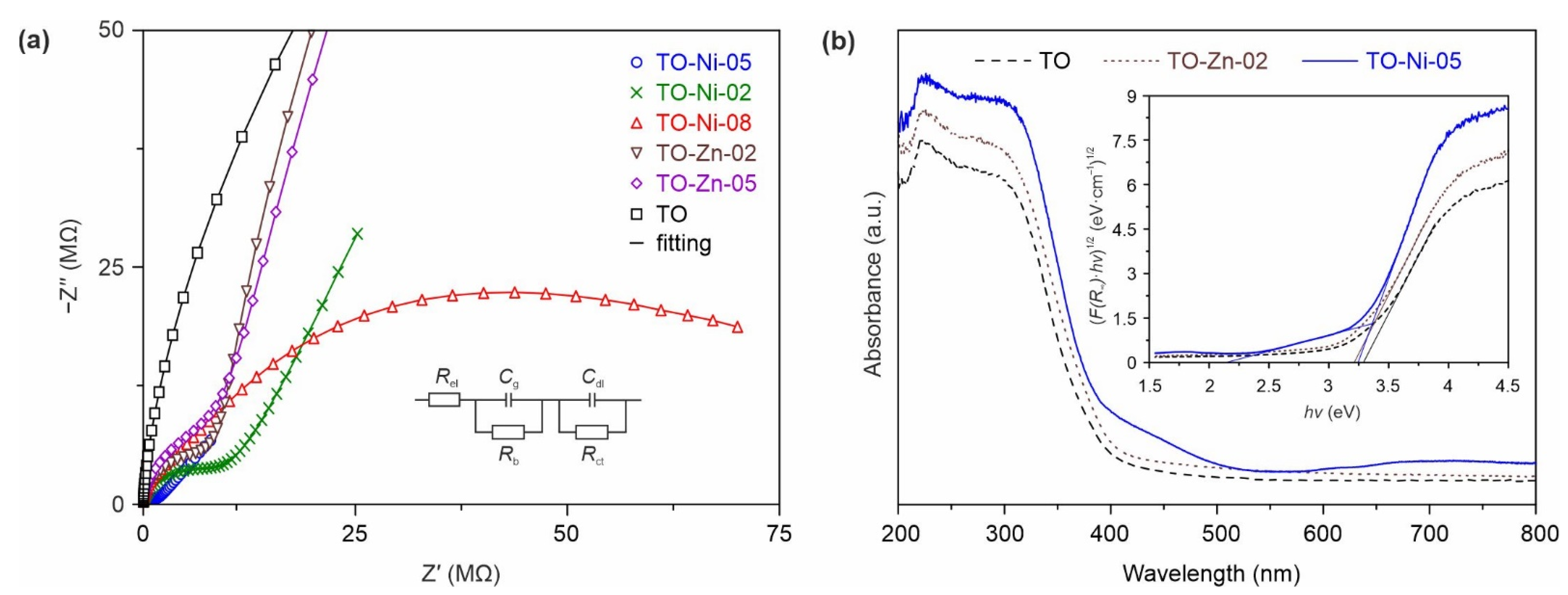
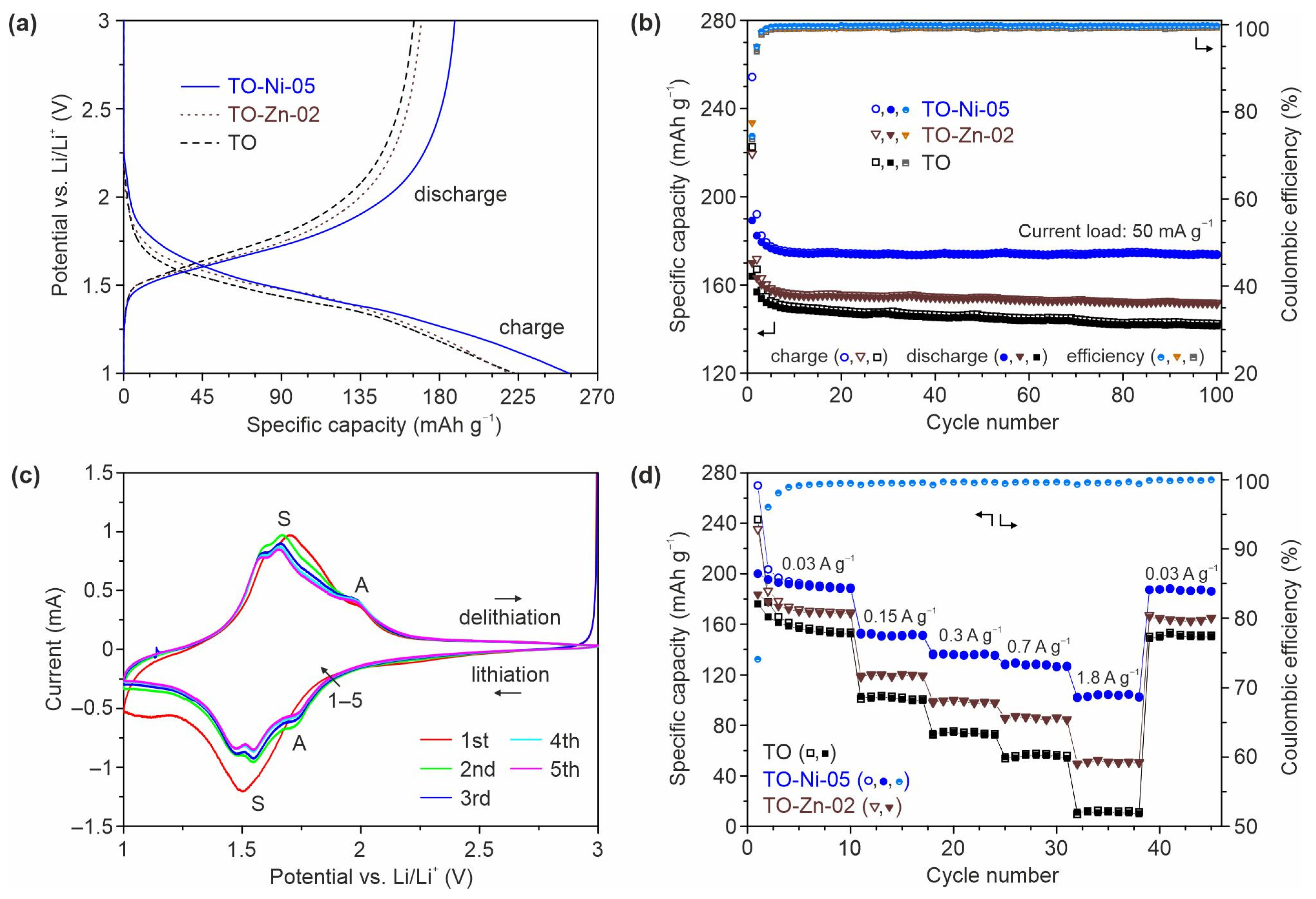
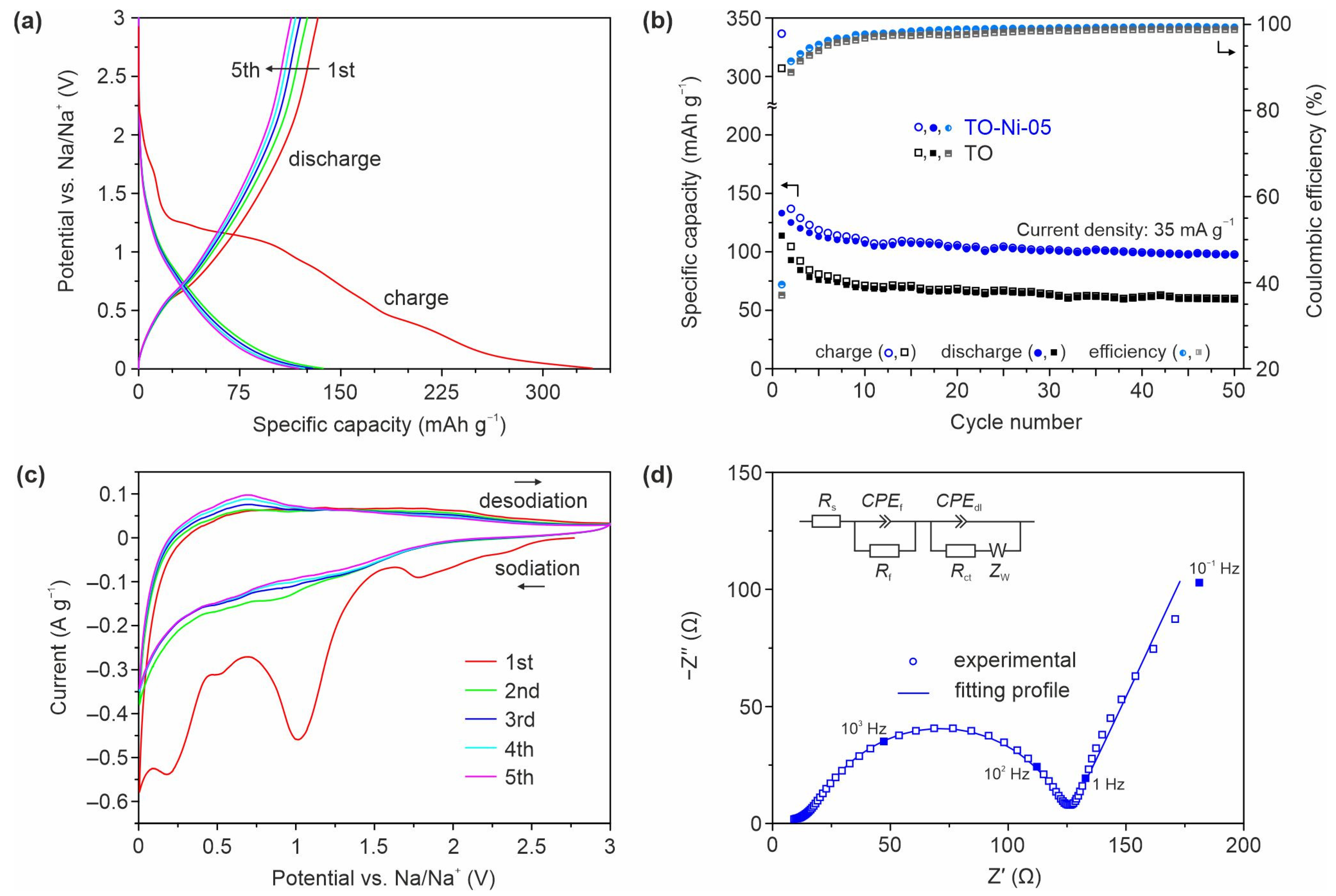
3.2. Electrochemical Performance of Ni- and Zn-Doped TiO2(B) Nanobelts in Lithium and Sodium Batteries
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fehse, M.; Ventosa, E. Is TiO2(B) the future of titanium-based battery materials? Chempluschem 2015, 80, 785–795. [Google Scholar] [CrossRef]
- Münster, P.; Diehl, M.; Frerichs, J.E.; Börner, M.; Hansen, M.R.; Winter, M.; Niehoff, P. Effect of Li plating during formation of lithium ion batteries on their cycling performance and thermal safety. J. Power Sources 2021, 484, 229306. [Google Scholar] [CrossRef]
- Ivanishchev, A.V.; Ivanishcheva, I.A. Ion transport in lithium electrochemical systems: Problems and solutions. Rus. J. Electrochem. 2020, 56, 907–928. [Google Scholar] [CrossRef]
- Ushakov, A.V.; Makhov, S.V.; Gridina, N.A.; Ivanishchev, A.V.; Gamayunova, I.M. Rechargeable lithium-ion system based on lithium-vanadium(III) phosphate and lithium titanate and the peculiarity of it functioning. Mon. Chem. Chem. Mon. 2019, 150, 499–509. [Google Scholar] [CrossRef]
- Stenina, I.A.; Shaydullin, R.R.; Desyatov, A.V.; Kulova, T.L.; Yaroslavtsev, A.B. Effect of carbon and N-doped carbon nanomaterials on the electrochemical performance of lithium titanate-based composites. Electrochim. Acta 2020, 364, 137330. [Google Scholar] [CrossRef]
- Yan, X.; Wang, Z.; He, M.; Hou, Z.; Xia, T.; Liu, G.; Chen, X. TiO2 nanomaterials as anode materials for lithium-ion rechargeable batteries. Energy Technol. 2015, 3, 801–814. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Yu, L.; Wu, N.-L.; Huang, H.; Wei, M. TiO2-B nanowires via topological conversion with enhanced lithium-ion intercalation properties. J. Mater. Chem. A 2019, 7, 3842–3847. [Google Scholar] [CrossRef]
- Vazquez-Santos, M.B.; Tartaj, P.; Morales, E.; Amarilla, J.M. TiO2 nanostructures as anode materials for Li/Na-ion batteries. Chem. Rec. 2018, 18, 1178–1191. [Google Scholar] [CrossRef] [PubMed]
- Panduwinata, D.; Gale, J.D. A first principles investigation of lithium intercalation in TiO2-B. J. Mater. Chem. 2009, 19, 3931. [Google Scholar] [CrossRef] [Green Version]
- Okumura, T.; Fukutsuka, T.; Yanagihara, A.; Orikasa, Y.; Arai, H.; Ogumi, Z.; Uchimoto, Y. Electronic and local structural changes with lithium-ion insertion in TiO2-B: X-ray absorption spectroscopy study. J. Mater. Chem. 2011, 21, 15369–15377. [Google Scholar] [CrossRef]
- Zukalová, M.; Kalbáč, M.; Kavan, L.; Exnar, I.; Graetzel, M.; Kalbáč, M.; Kavan, L.; Exnar, I.; Graetzel, M. Pseudocapacitive lithium storage in TiO2(B). Chem. Mater. 2005, 17, 1248–1255. [Google Scholar] [CrossRef]
- Hasa, I.; Mariyappan, S.; Saurel, D.; Adelhelm, P.; Koposov, A.Y.; Masquelier, C.; Croguennec, L.; Casas-Cabanas, M. Challenges of today for Na-based batteries of the future: From materials to cell metrics. J. Power Sources 2021, 482, 228872. [Google Scholar] [CrossRef]
- Skundin, A.M.; Kulova, T.L.; Yaroslavtsev, A.B. Sodium-ion batteries (a review). Rus. J. Electrochem. 2018, 54, 113–152. [Google Scholar] [CrossRef]
- Hou, H.; Qiu, X.; Wei, W.; Zhang, Y.; Ji, X. Carbon anode materials for advanced sodium-ion batteries. Adv. Energy Mater. 2017, 7, 1602898. [Google Scholar] [CrossRef]
- Górka, J.; Vix-Guterl, C.; Matei Ghimbeu, C. Recent progress in design of biomass-derived hard carbons for sodium ion batteries. C J. Carbon Res. 2016, 2, 24. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhu, W.; Guerfi, A.; Kim, C.; Zaghib, K. Roles of Ti in electrode materials for sodium-ion batteries. Front. Energy Res. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Liu, Y.; Wu, X.; Wang, J.; Fu, L.; Zhu, Y.; Wu, Y.; Liu, X. Advances of TiO2 as negative electrode materials for sodium-ion batteries. Adv. Mater. Technol. 2018, 3, 1800004. [Google Scholar] [CrossRef]
- Dawson, J.A.; Robertson, J. Improved calculation of Li and Na intercalation properties in anatase, rutile, and TiO2(B). J. Phys. Chem. C 2016, 120, 22910–22917. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhao, F.; Li, J.; Li, Y.; McLeod, J.A.; Liu, L. Influence of crystal phase on TiO2 nanowire anodes in sodium ion batteries. J. Mater. Chem. A 2017, 5, 20005–20013. [Google Scholar] [CrossRef]
- Kang, S.H.; Jo, Y.N.; Prasanna, K.; Santhoshkumar, P.; Joe, Y.C.; Vediappan, K.; Gnanamuthu, R.; Lee, C.W. Bandgap tuned and oxygen vacant TiO2−x anode materials with enhanced electrochemical properties for lithium ion batteries. J. Ind. Eng. Chem. 2019, 71, 177–183. [Google Scholar] [CrossRef]
- Xie, F.; Zhu, J.; Li, Y.; Shen, D.; Abate, A.; Wei, M. TiO2-B as an electron transporting material for highly efficient perovskite solar cells. J. Power Sources 2019, 415, 8–14. [Google Scholar] [CrossRef]
- Huang, J.P.; Yuan, D.D.; Zhang, H.Z.; Cao, Y.L.; Li, G.R.; Yang, H.X.; Gao, X.P. Electrochemical sodium storage of TiO2(B) nanotubes for sodium ion batteries. RSC Adv. 2013, 3, 12593. [Google Scholar] [CrossRef]
- Agostini, M.; Brutti, S.; Navarra, M.A.; Panero, S.; Reale, P.; Matic, A.; Scrosati, B. A high-power and fast charging Li-ion battery with outstanding cycle-life. Sci. Rep. 2017, 7, 1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannuzzi, R.; Manca, M.; De Marco, L.; Belviso, M.R.; Cannavale, A.; Sibillano, T.; Giannini, C.; Cozzoli, P.D.; Gigli, G. Ultrathin TiO2(B) nanorods with superior lithium-ion storage performance. ACS Appl. Mater. Interfaces 2014, 6, 1933–1943. [Google Scholar] [CrossRef]
- Mason, C.W.; Yeo, I.; Saravanan, K.; Balaya, P. Interconnected nanofibrous titanium dioxide bronze: An emerging lithium ion anode material for high rate performance. RSC Adv. 2013, 3, 2935. [Google Scholar] [CrossRef]
- Pineda-Aguilar, N.; Garza-Tovar, L.L.; Sánchez-Cervantes, E.M.; Sánchez-Domínguez, M. Preparation of TiO2–(B) by microemulsion mediated hydrothermal method: Effect of the precursor and its electrochemical performance. J. Mater. Sci. Mater. Electron. 2018, 29, 15464–15479. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.K.; Chung, K.Y.; Jung, H.-G.; Kim, H.; Mun, J.; Choi, W. Electrochemical investigations on TiO2-B nanowires as a promising high capacity anode for sodium-ion batteries. Electrochim. Acta 2016, 200, 21–28. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, D.; Wei, Y.; Zhu, K.; Zhao, Y.; Bian, X.; Du, F.; Liu, B.; Gao, Y.; Chen, G. Competition between insertion of Li+ and Mg2+: An example of TiO2-B nanowires for Mg rechargeable batteries and Li+/Mg2+ hybrid-ion batteries. J. Power Sources 2017, 346, 134–142. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Yang, C.; Wei, Q.; Wei, M. Hierarchical TiO2-B composed of nanosheets with exposed {010} facets as a high-performance anode for lithium ion batteries. J. Power Sources 2018, 392, 226–231. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, T.; Mu, Q. Electrochemical performance of W-doped anatase TiO2 nanoparticles as an electrode material for lithium-ion batteries. J. Mater. Chem. 2011, 21, 6006. [Google Scholar] [CrossRef]
- Zhang, W.; Gong, Y.; Mellott, N.P.; Liu, D.; Li, J. Synthesis of nickel doped anatase titanate as high performance anode materials for lithium ion batteries. J. Power Sources 2015, 276, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Anh, L.T.; Rai, A.K.; Thi, T.V.; Gim, J.; Kim, S.; Shin, E.-C.; Lee, J.-S.; Kim, J. Improving the electrochemical performance of anatase titanium dioxide by vanadium doping as an anode material for lithium-ion batteries. J. Power Sources 2013, 243, 891–898. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, H.; Liu, Y.; Zhao, F.; Li, J.; He, X. Defective TiO2-graphene heterostructures enabling in-situ electrocatalyst evolution for lithium-sulfur batteries. J. Energy Chem. 2021, 62, 508–515. [Google Scholar] [CrossRef]
- Kyeremateng, N.A.; Vacandio, F.; Sougrati, M.-T.; Martinez, H.; Jumas, J.-C.; Knauth, P.; Djenizian, T. Effect of Sn-doping on the electrochemical behaviour of TiO2 nanotubes as potential negative electrode materials for 3D Li-ion micro batteries. J. Power Sources 2013, 224, 269–277. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Sinebryukhov, S.L.; Zheleznov, V.V.; Opra, D.P.; Voit, E.I.; Modin, E.B.; Sokolov, A.A.; Ustinov, A.Y.; Sergienko, V.I. Effect of Hf-doping on electrochemical performance of anatase TiO2 as an anode material for lithium storage. R. Soc. Open Sci. 2018, 5, 171811. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ding, Z.; Foster, C.W.; Banks, C.E.; Qiu, X.; Ji, X. Oxygen vacancies evoked blue TiO2(B) nanobelts with efficiency enhancement in sodium storage behaviors. Adv. Funct. Mater. 2017, 27, 1700856. [Google Scholar] [CrossRef]
- Lei, Y.; Li, J.; Wang, Z.; Sun, J.; Chen, F.; Liu, H.; Ma, X.; Liu, Z. Atomic-scale investigation of a new phase transformation process in TiO2 nanofibers. Nanoscale 2017, 9, 4601–4609. [Google Scholar] [CrossRef] [Green Version]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Ni, J.; Fu, S.; Wu, C.; Zhao, Y.; Maier, J.; Yu, Y.; Li, L. Superior sodium storage in Na2Ti3O7 nanotube arrays through surface engineering. Adv. Energy Mater. 2016, 6, 1502568. [Google Scholar] [CrossRef]
- Ganesh, I.; Gupta, A.K.; Kumar, P.P.; Sekhar, P.S.C.; Radha, K.; Padmanabham, G.; Sundararajan, G. Preparation and characterization of Ni-doped materials for photocurrent and photocatalytic applications. Sci. World J. 2012, 2012, 127326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- She, H.; Zhou, H.; Li, L.; Wang, L.; Huang, J.; Wang, Q. Nickel-doped excess oxygen defect titanium dioxide for efficient selective photocatalytic oxidation of benzyl alcohol. ACS Sustain. Chem. Eng. 2018, 6, 11939–11948. [Google Scholar] [CrossRef]
- Arif Sher Shah, M.S.; Zhang, K.; Park, A.R.; Kim, K.S.; Park, N.-G.; Park, J.H.; Yoo, P.J. Single-step solvothermal synthesis of mesoporous Ag–TiO2–reduced graphene oxide ternary composites with enhanced photocatalytic activity. Nanoscale 2013, 5, 5093. [Google Scholar] [CrossRef] [Green Version]
- Bakhmutov, V.I. Strategies for solid-state NMR studies of materials: From diamagnetic to paramagnetic porous solids. Chem. Rev. 2011, 111, 530–562. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Gao, H.; Kong, H.; Yang, P.; Zhang, W.; Chu, J. Influence of transition metal doping on the structural, optical, and magnetic properties of TiO2 films deposited on Si substrates by a sol–gel process. Nanoscale Res. Lett. 2013, 8, 533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bashir, A.; Bashir, F.; Sultan, M.; Mubeen, M.; Iqbal, A.; Akhter, Z. Influence of nickel and lanthanum ions co-doping on photocatalytic properties of TiO2 for effective degradation of reactive yellow 145 in the visible region. J. Sol. Gel Sci. Technol. 2020, 93, 438–451. [Google Scholar] [CrossRef]
- Biju, V.; Abdul Khadar, M. DC conductivity of consolidated nanoparticles of NiO. Mater. Res. Bull. 2001, 36, 21–33. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Li, J.; Li, L.; Lin, S. First-principles study on transition metal-doped anatase TiO2. Nanoscale Res. Lett. 2014, 9, 46. [Google Scholar] [CrossRef] [Green Version]
- Smirnova, N.; Petrik, I.; Vorobets, V.; Kolbasov, G.; Eremenko, A. Sol-gel synthesis, photo- and electrocatalytic properties of mesoporous TiO2 modified with transition metal ions. Nanoscale Res. Lett. 2017, 12, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodbeck, C.M.; Bukrey, R.R. Model calculations for the coordination of Fe3+ and Mn2+ ions in oxide glasses. Phys. Rev. B 1981, 24, 2334–2342. [Google Scholar] [CrossRef]
- Martos, M.; Julián, B.; Dehouli, H.; Gourier, D.; Cordoncillo, E.; Escribano, P. Synthesis and characterization of Ti1−2xNbxNixO2−x/2 solid solutions. J. Solid State Chem. 2007, 180, 679–687. [Google Scholar] [CrossRef]
- Weil, J.; Bolton, J.R. Electron Paramagnetic Resonance: Elementary Theory and Practical Applications; Wiley-Interscience: Hoboken, NJ, USA, 2007; ISBN 9780471754961. [Google Scholar]
- Rane, K.S.; Mhalsiker, R.; Yin, S.; Sato, T.; Cho, K.; Dunbar, E.; Biswas, P. Visible light-sensitive yellow TiO2−xNx and Fe–N co-doped Ti1−yFeyO2−xNx anatase photocatalysts. J. Solid State Chem. 2006, 179, 3033–3044. [Google Scholar] [CrossRef]
- Ran, Y.; Seung-li, S.; Han, J.; Joo, K.; Sung, C. Ferromagnetic properties of Ni-doped rutile TiO2–δ. J. Korean Phys. Soc. 2007, 50, 638. [Google Scholar] [CrossRef]
- Coey, J.M.D.; Venkatesan, M.; Fitzgerald, C.B. Donor impurity band exchange in dilute ferromagnetic oxides. Nat. Mater. 2005, 4, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, X.; Wei, X.; Dai, J.; Li, W. Ferromagnetic property of Co and Ni doped TiO2 nanoparticles. J. Nanomater. 2015, 2015, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Dakhel, A.A.; Hamad, H.; Jaafar, A. Investigation to the structural, optical, and magnetic properties of synthesized Ni-doped anatase nanoparticles: Essential role of treatment in hydrogen on long-range ferromagnetic order. J. Supercond. Nov. Magn. 2019, 32, 253–260. [Google Scholar] [CrossRef]
- Beuvier, T.; Richard-Plouet, M.; Mancini-Le Granvalet, M.; Brousse, T.; Crosnier, O.; Brohan, L. TiO2(B) nanoribbons as negative electrode material for lithium ion batteries with high rate performance. Inorg. Chem. 2010, 49, 8457–8464. [Google Scholar] [CrossRef]
- Dylla, A.G.; Henkelman, G.; Stevenson, K.J. Lithium insertion in nanostructured TiO2(B) architectures. Acc. Chem. Res. 2013, 46, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-S.; Kim, R.-H.; Baek, S.-W.; Lee, K.-S.; Park, K.; Benayad, A. Is Li4Ti5O12 a solid-electrolyte-interphase-free electrode material in Li-ion batteries? Reactivity between the Li4Ti5O12 electrode and electrolyte. J. Mater. Chem. A 2014, 2, 631–636. [Google Scholar] [CrossRef]
- Brutti, S.; Gentili, V.; Reale, P.; Carbone, L.; Panero, S. Mitigation of the irreversible capacity and electrolyte decomposition in a LiNi0.5Mn1.5O4/nano-TiO2 Li-ion battery. J. Power Sources 2011, 196, 9792–9799. [Google Scholar] [CrossRef]
- Brutti, S.; Gentili, V.; Menard, H.; Scrosati, B.; Bruce, P.G. TiO2-(B) nanotubes as anodes for lithium batteries: Origin and mitigation of irreversible capacity. Adv. Energy Mater. 2012, 2, 322–327. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, Y.; Dai, X.; Yan, X.; Huang, B.; Li, J. Electrochemical performance of ZnO-coated Li4Ti5O12 composite electrodes for lithium-ion batteries with the voltage ranging from 3 to 0.01 V. R. Soc. Open Sci. 2018, 5, 180762. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Fang, J.; Xia, Y.; Tao, X.; Gan, Y.; Du, J.; Zhu, W.; Zhang, W. Construction of sheet–belt hybrid nanostructures from one-dimensional mesoporous TiO2(B) nanobelts and graphene sheets for advanced lithium-ion batteries. J. Mater. Chem. A 2013, 1, 2495. [Google Scholar] [CrossRef]
- Chen, C.; Hu, X.; Zhang, B.; Miao, L.; Huang, Y. Architectural design and phase engineering of N/B-codoped TiO2(B)/anatase nanotube assemblies for high-rate and long-life lithium storage. J. Mater. Chem. A 2015, 3, 22591–22598. [Google Scholar] [CrossRef]
- Etacheri, V.; Kuo, Y.; Van der Ven, A.; Bartlett, B.M. Mesoporous TiO2–B microflowers composed of (10) facet-exposed nanosheets for fast reversible lithium-ion storage. J. Mater. Chem. A 2013, 1, 12028. [Google Scholar] [CrossRef]
- Wang, J.-F.; Zhang, J.-J.; He, D.-N. Flower-like TiO2-B particles wrapped by graphene with different contents as an anode material for lithium-ion batteries. Nano Struct. Nano Objects 2018, 15, 216–223. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, Y.; Zhu, K.; Gao, Y.; Zhang, D.; Chen, G.; Wang, C.; Wei, Y. Enhanced electrochemical properties of TiO2(B) nanoribbons using the styrene butadiene rubber and sodium carboxyl methyl cellulose water binder. J. Power Sources 2014, 246, 95–102. [Google Scholar] [CrossRef]
- Shin, K.; Kim, H.J.; Choi, J.-M.; Choi, Y.-M.; Song, M.S.; Park, J.H. Controlled synthesis of skein shaped TiO2–B nanotube cluster particles with outstanding rate capability. Chem. Commun. 2013, 49, 2326. [Google Scholar] [CrossRef] [PubMed]
- Wessel, C.; Zhao, L.; Urban, S.; Ostermann, R.; Djerdj, I.; Smarsly, B.M.; Chen, L.; Hu, Y.-S.; Sallard, S. Ionic-liquid synthesis route of TiO2(B) nanoparticles for functionalized materials. Chem. A Eur. J. 2011, 17, 775–779. [Google Scholar] [CrossRef]
- Yang, Y.; Liao, S.; Shi, W.; Wu, Y.; Zhang, R.; Leng, S. Nitrogen-doped TiO2(B) nanorods as high-performance anode materials for rechargeable sodium-ion batteries. RSC Adv. 2017, 7, 10885–10890. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.; Pan, L. A new sodium storage mechanism of TiO2 for sodium ion batteries. Inorg. Chem. Front. 2016, 3, 464–468. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, M.; Wen, L.; Wang, C.; Zhao, H.; Mi, Y.; Liang, L.; Fu, Q.; Wu, M.; Lei, Y. Highly ordered three-dimensional Ni-TiO2 nanoarrays as sodium ion battery anodes. Chem. Mater. 2015, 27, 4274–4280. [Google Scholar] [CrossRef]
- Wu, L.; Bresser, D.; Buchholz, D.; Passerini, S. Nanocrystalline TiO2(B) as anode material for sodium-ion batteries. J. Electrochem. Soc. 2015, 162, A3052–A3058. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Zhao, F.; Du, G.; Porter, S.; Liu, Y.; Zhang, P.; Cheng, Z.; Liu, H.K.; Huang, Z. Boron-doped anatase TiO2 as a high-performance anode material for sodium-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 16009–16015. [Google Scholar] [CrossRef]
- Yang, Y.; Ji, X.; Jing, M.; Hou, H.; Zhu, Y.; Fang, L.; Yang, X.; Chen, Q.; Banks, C.E. Carbon dots supported upon N-doped TiO2 nanorods applied into sodium and lithium ion batteries. J. Mater. Chem. A 2015, 3, 5648–5655. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-N.; Su, J.; Lv, X.-Y.; Long, Y.-F.; Yu, H.; Huang, R.-R.; Xie, Y.-C.; Wen, Y.-X. Zn2+ doped TiO2/C with enhanced sodium-ion storage properties. Ceram. Int. 2017, 43, 10326–10332. [Google Scholar] [CrossRef]
- He, H.; Wang, H.; Sun, D.; Shao, M.; Huang, X.; Tang, Y. N-doped rutile TiO2/C with significantly enhanced Na storage capacity for Na-ion batteries. Electrochim. Acta 2017, 236, 43–52. [Google Scholar] [CrossRef]
- Nie, S.; Liu, L.; Liu, J.; Xie, J.; Zhang, Y.; Xia, J.; Yan, H.; Yuan, Y.; Wang, X. Nitrogen-doped TiO2–C composite nanofibers with high-capacity and long-cycle life as anode materials for sodium-ion batteries. Nano Micro Lett. 2018, 10, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opra, D.P.; Gnedenkov, S.V.; Sinebryukhov, S.L.; Gerasimenko, A.V.; Ziatdinov, A.M.; Sokolov, A.A.; Podgorbunsky, A.B.; Ustinov, A.Y.; Kuryavyi, V.G.; Mayorov, V.Y.; et al. Enhancing Lithium and Sodium Storage Properties of TiO2(B) Nanobelts by Doping with Nickel and Zinc. Nanomaterials 2021, 11, 1703. https://doi.org/10.3390/nano11071703
Opra DP, Gnedenkov SV, Sinebryukhov SL, Gerasimenko AV, Ziatdinov AM, Sokolov AA, Podgorbunsky AB, Ustinov AY, Kuryavyi VG, Mayorov VY, et al. Enhancing Lithium and Sodium Storage Properties of TiO2(B) Nanobelts by Doping with Nickel and Zinc. Nanomaterials. 2021; 11(7):1703. https://doi.org/10.3390/nano11071703
Chicago/Turabian StyleOpra, Denis P., Sergey V. Gnedenkov, Sergey L. Sinebryukhov, Andrey V. Gerasimenko, Albert M. Ziatdinov, Alexander A. Sokolov, Anatoly B. Podgorbunsky, Alexander Yu. Ustinov, Valery G. Kuryavyi, Vitaly Yu. Mayorov, and et al. 2021. "Enhancing Lithium and Sodium Storage Properties of TiO2(B) Nanobelts by Doping with Nickel and Zinc" Nanomaterials 11, no. 7: 1703. https://doi.org/10.3390/nano11071703
APA StyleOpra, D. P., Gnedenkov, S. V., Sinebryukhov, S. L., Gerasimenko, A. V., Ziatdinov, A. M., Sokolov, A. A., Podgorbunsky, A. B., Ustinov, A. Y., Kuryavyi, V. G., Mayorov, V. Y., Tkachenko, I. A., & Sergienko, V. I. (2021). Enhancing Lithium and Sodium Storage Properties of TiO2(B) Nanobelts by Doping with Nickel and Zinc. Nanomaterials, 11(7), 1703. https://doi.org/10.3390/nano11071703





