The Multifunctionally Graded System for a Controlled Size Effect on Iron Oxide–Gold Based Core-Shell Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Superparamagnetic Fe3O4 NPs
2.3. Preparation of Fe3O4@Au NPs
2.4. Hybridization of Double-Stranded Oligonucleotides (dsDNA)
2.5. Preparation of F Fe3O4@Au NPs-dsDNA and DOX-Intercalated Fe3O4@Au NPs-dsDNA
2.6. Characterization of the NPs
2.7. Molecule Release by Diffusion and under HFMF
2.8. In Vitro Cytotoxicity Assay of Fe3O4@Au NPs-dsDNA and Fe3O4@Au NPs-dsDNA/DOX
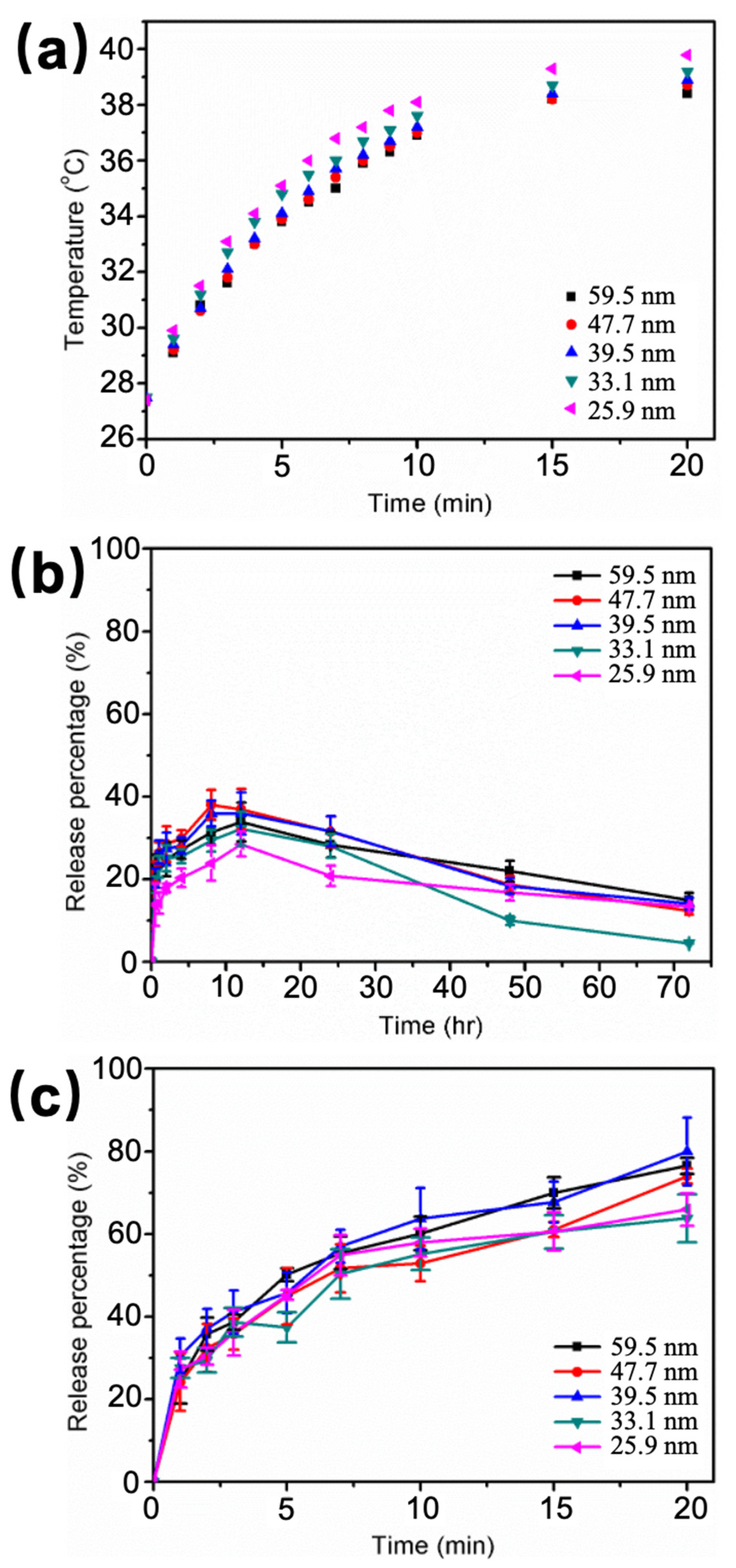
2.9. Size Effects on Molecule Delivery under HFMF
2.10. Target Molecule Delivery under HFMF
3. Results and Discussion
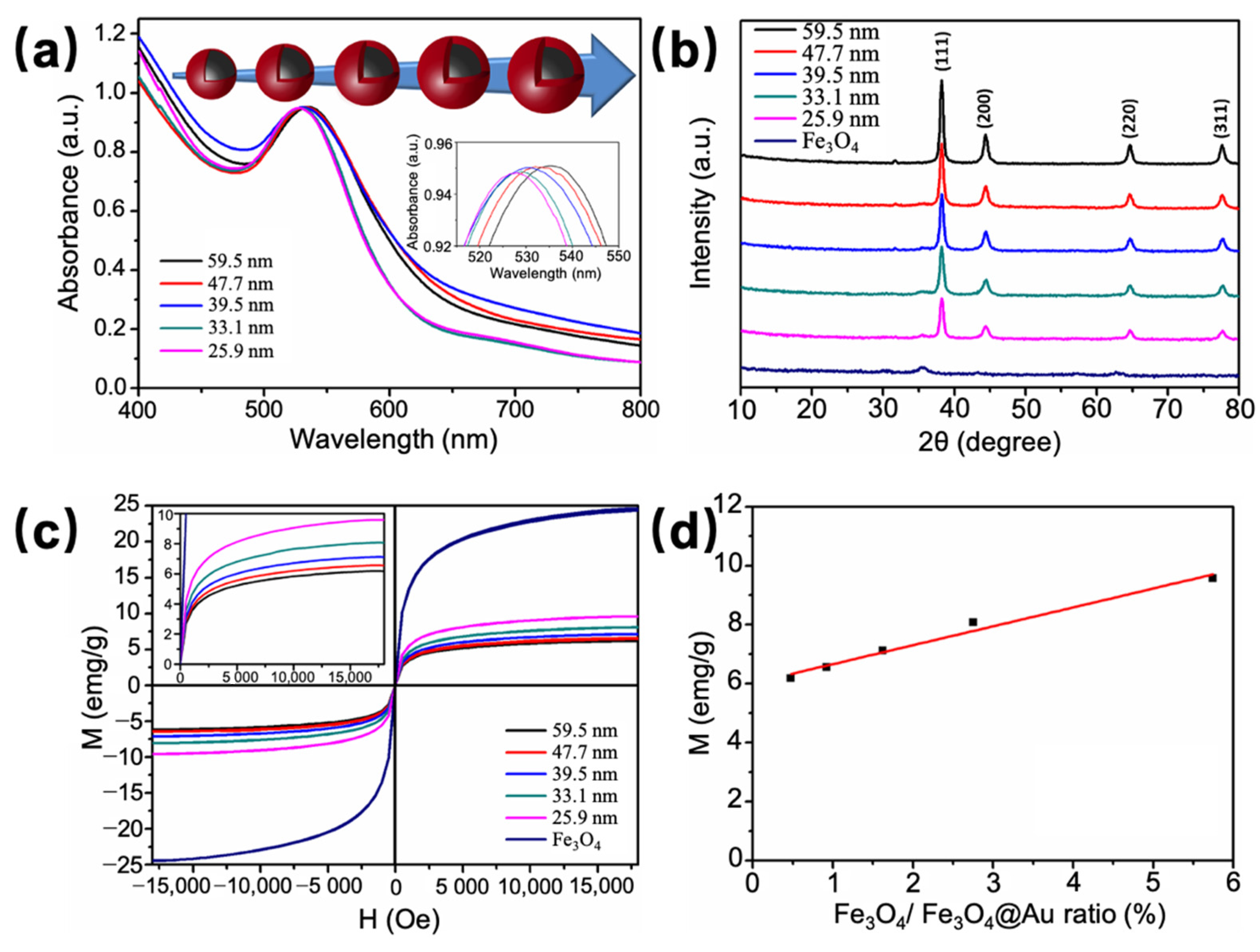
3.1. Synthesis and Characterization of Fe3O4@Au NPs
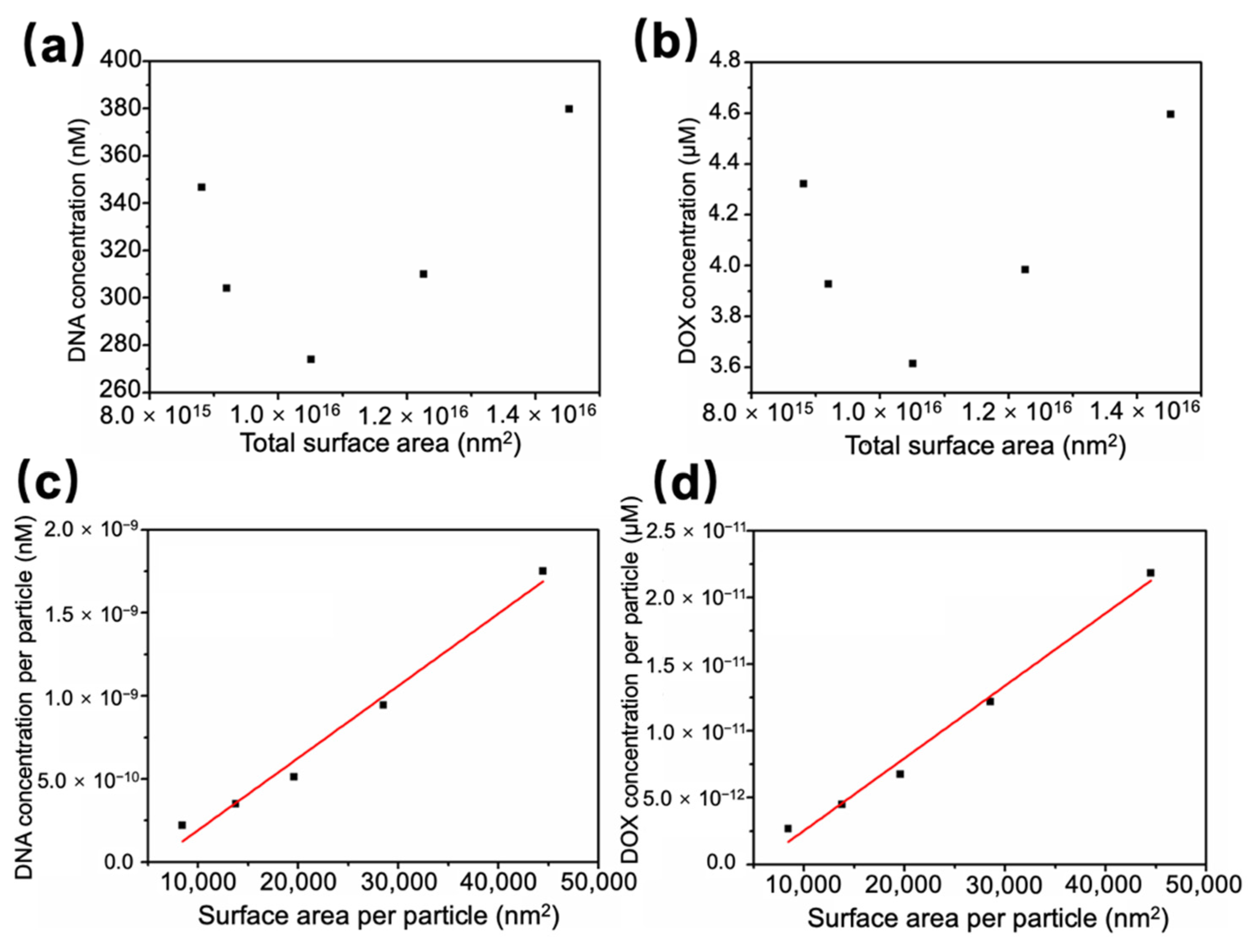
3.2. Quantitative Analysis on Molecule Loading and Sensing Capacity
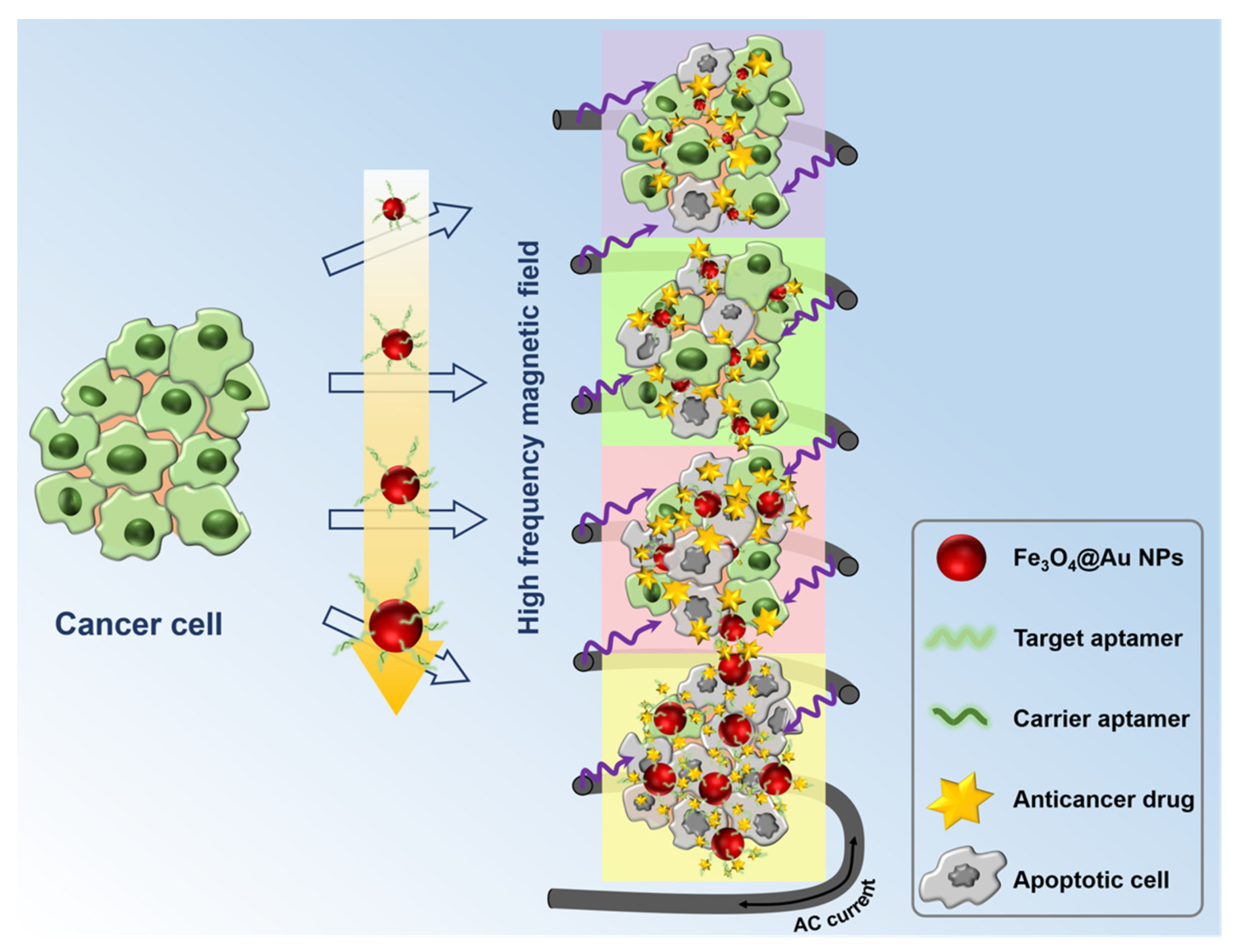
3.3. Capabilities of the Multifunctional Molecule Delivery System
3.4. Application of Release Actuator for Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piorecka, K.; Smith, D.; Kurjata, J.; Stanczyk, M.; Stanczyk, W.A. Synthetic Routes to Nanoconjugates of Anthracyclines. Bioorg. Chem. 2020, 96, 103617. [Google Scholar] [CrossRef]
- Doshi, N.; Swiston, A.J.; Gilbert, J.B.; Alcaraz, M.L.; Cohen, R.E.; Rubner, M.F.; Mitragotri, S. Cell-Based Drug Delivery Devices Using Phagocytosis-Resistant Backpacks. Adv. Mater. 2011, 23, H105–H109. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered Nanoparticles for Drug Delivery in Cancer Therapy. Angew. Chemie Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef] [PubMed]
- Piorecka, K.; Kurjata, J.; Stanczyk, M.; Stanczyk, W.A. Synthetic Routes to Nanomaterials Containing Anthracyclines: Noncovalent Systems. Biomater. Sci. 2018, 6, 2552–2565. [Google Scholar] [CrossRef] [PubMed]
- Spicer, C.D.; Jumeaux, C.; Gupta, B.; Stevens, M.M. Peptide and Protein Nanoparticle Conjugates: Versatile Platforms for Biomedical Applications. Chem. Soc. Rev. 2018, 47, 3574–3620. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.; Faig, A.; Abdelhamid, D.; Uhrich, K. Sugar-Based Amphiphilic Polymers for Biomedical Applications: From Nanocarriers to Therapeutics. Acc. Chem. Res. 2014, 47, 2867–2877. [Google Scholar] [CrossRef]
- Luo, G.F.; Chen, W.H.; Lei, Q.; Qiu, W.X.; Liu, Y.X.; Cheng, Y.J.; Zhang, X.Z. A Triple-Collaborative Strategy for High-Performance Tumor Therapy by Multifunctional Mesoporous Silica-Coated Gold Nanorods. Adv. Funct. Mater. 2016, 26, 4339–4350. [Google Scholar] [CrossRef]
- Ashton, S.; Song, Y.H.; Nolan, J.; Cadogan, E.; Murray, J.; Odedra, R.; Foster, J.; Hall, P.A.; Low, S.; Taylor, P.; et al. Aurora Kinase Inhibitor Nanoparticles Target Tumors with Favorable Therapeutic Index in Vivo. Sci. Transl. Med. 2016, 8, ra17–ra325. [Google Scholar] [CrossRef]
- Kumar, A.; Huo, S.; Zhang, X.; Liu, J.; Tan, A.; Li, S.; Jin, S.; Xue, X.; Zhao, Y.; Ji, T.; et al. Neuropilin-1-Targeted Gold Nanoparticles Enhance Therapeutic Efficacy of Platinum(IV) Drug for Prostate Cancer Treatment. ACS Nano 2014, 8, 4205–4220. [Google Scholar] [CrossRef]
- Sugumaran, P.J.; Liu, X.L.; Herng, T.S.; Peng, E.; Ding, J. GO-Functionalized Large Magnetic Iron Oxide Nanoparticles with Enhanced Colloidal Stability and Hyperthermia Performance. ACS Appl. Mater. Interfaces 2019, 11, 22703–22713. [Google Scholar] [CrossRef]
- Bauer, I.; Knölker, H.J. Iron Catalysis in Organic Synthesis. Chem. Rev. 2015, 115, 3170–3387. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Chen, G.; Liang, C.; Xu, J.; Dong, Z.; Han, X.; Wang, C.; Liu, Z. Iron Nanoparticles for Low-Power Local Magnetic Hyperthermia in Combination with Immune Checkpoint Blockade for Systemic Antitumor Therapy. Nano Lett. 2019, 19, 4287–4296. [Google Scholar] [CrossRef]
- Park, J.H.; Von Maltzahn, G.; Zhang, L.; Schwartz, M.P.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Magnetic Iron Oxide Nanoworms for Tumor Targeting and Imaging. Adv. Mater. 2008, 20, 1630–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.W.; Hua, M.Y.; Liu, H.L.; Huang, C.Y.; Wei, K.C. Potential of Magnetic Nanoparticles for Targeted Drug Delivery. Nanotechnol. Sci. Appl. 2012, 5, 73–86. [Google Scholar] [PubMed] [Green Version]
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Bañobre-López, M.; Martins, P.; Lanceros-Mendez, S. Advances in Magnetic Nanoparticles for Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1–35. [Google Scholar] [CrossRef]
- Jeon, S.; Park, B.C.; Lim, S.; Yoon, H.Y.; Jeon, Y.S.; Kim, B.S.; Kim, Y.K.; Kim, K. Heat-Generating Iron Oxide Multigranule Nanoclusters for Enhancing Hyperthermic Efficacy in Tumor Treatment. ACS Appl. Mater. Interfaces 2020, 12, 33483–33491. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, R.; Wang, S.; Ding, L.; Li, J.; Luo, Y.; Wang, X.; Shen, M.; Shi, X. Multifunctional Fe3O4 @ Au Core/Shell Nanostars: A Unique Platform for Multimode Imaging and Photothermal Therapy of Tumors. Sci. Rep. 2016, 6, 10–21. [Google Scholar] [CrossRef]
- Li, W.P.; Liao, P.Y.; Su, C.H.; Yeh, C.S. Formation of Oligonucleotide-Gated Silica Shell-Coated Fe3O4-Au Core-Shell Nanotrisoctahedra for Magnetically Targeted and near-Infrared Light-Responsive Theranostic Platform. J. Am. Chem. Soc. 2014, 136, 10062–10075. [Google Scholar] [CrossRef]
- Khan, M.S.; Vishakante, G.D.; Siddaramaiah, H. Gold Nanoparticles: A Paradigm Shift in Biomedical Applications. Adv. Colloid Interface Sci. 2013, 199–200, 44–58. [Google Scholar] [CrossRef]
- Kwon, S.P.; Jeon, S.; Lee, S.H.; Yoon, H.Y.; Ryu, J.H.; Choi, D.; Kim, J.Y.; Kim, J.; Park, J.H.; Kim, D.E.; et al. Thrombin-Activatable Fluorescent Peptide Incorporated Gold Nanoparticles for Dual Optical/Computed Tomography Thrombus Imaging. Biomaterials 2018, 150, 125–136. [Google Scholar] [CrossRef]
- Liu, J.; Xiong, Z.; Zhang, J.; Peng, C.; Klajnert-Maculewicz, B.; Shen, M.; Shi, X. Zwitterionic Gadolinium(III)-Complexed Dendrimer-Entrapped Gold Nanoparticles for Enhanced Computed Tomography/Magnetic Resonance Imaging of Lung Cancer Metastasis. ACS Appl. Mater. Interfaces 2019, 11, 15212–15221. [Google Scholar] [CrossRef]
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in Biomaterials for Drug Delivery. Adv. Mater. 2018, 30, 1–29. [Google Scholar] [CrossRef]
- Ding, L.; Ren, J.; Zhang, D.; Li, Y.; Huang, X.; Ji, J.; Hu, Q.; Wang, H.; Ni, Y.; Hou, Y. The TLR3 Agonist Inhibit Drug Efflux and Sequentially Consolidates Low-Dose Cisplatin-Based Chemoimmunotherapy While Reducing Side Effects. Mol. Cancer Ther. 2017, 16, 1068–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sondermann, P.; Szymkowski, D.E. Harnessing Fc Receptor Biology in the Design of Therapeutic Antibodies. Curr. Opin. Immunol. 2016, 40, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Kara, S.; Amon, L.; Lühr, J.J.; Nimmerjahn, F.; Dudziak, D.; Lux, A. Impact of Plasma Membrane Domains on IgG Fc Receptor Function. Front. Immunol. 2020, 11, 1320. [Google Scholar] [CrossRef]
- Adams, G.P.; Weiner, L.M. Monoclonal Antibody Therapy of Cancer. Nat. Biotechnol. 2005, 23, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Mairal, T.; Cengiz Özalp, V.; Lozano Sánchez, P.; Mir, M.; Katakis, I.; O’Sullivan, C.K. Aptamers: Molecular Tools for Analytical Applications. Anal. Bioanal. Chem. 2008, 390, 989–1007. [Google Scholar] [CrossRef]
- Moutsiopoulou, A.; Broyles, D.; Dikici, E.; Daunert, S.; Deo, S.K. Molecular Aptamer Beacons and Their Applications in Sensing, Imaging, and Diagnostics. Small 2019, 15, 1–18. [Google Scholar] [CrossRef]
- Goddard, Z.R.; Marín, M.J.; Russell, D.A.; Searcey, M. Active Targeting of Gold Nanoparticles as Cancer Therapeutics. Chem. Soc. Rev. 2020, 8774–8789. [Google Scholar] [CrossRef]
- Iliuk, A.B.; Hu, L.; Tao, W.A. Aptamer in Bioanalytical Applications. Anal. Chem. 2011, 83, 4440–4452. [Google Scholar] [CrossRef] [Green Version]
- Song, K.M.; Lee, S.; Ban, C. Aptamers and Their Biological Applications. Sensors 2012, 12, 612–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Liu, X.; Liu, Z.; Pu, F.; Ren, J.; Qu, X. Near-Infrared Light-Triggered, Targeted Drug Delivery to Cancer Cells by Aptamer Gated Nanovehicles. Adv. Mater. 2012, 24, 2890–2895. [Google Scholar] [CrossRef]
- Pestourie, C.; Tavitian, B.; Duconge, F. Aptamers against Extracellular Targets for in Vivo Applications. Biochimie 2005, 87, 921–930. [Google Scholar] [CrossRef]
- Chimene, D.; Alge, D.L.; Gaharwar, A.K. Two-Dimensional Nanomaterials for Biomedical Applications: Emerging Trends and Future Prospects. Adv. Mater. 2015, 27, 7261–7284. [Google Scholar] [CrossRef]
- Dhall, A.; Self, W. Cerium Oxide Nanoparticles: A Brief Review of Their Synthesis Methods and Biomedical Applications. Antioxidants 2018, 7, 97. [Google Scholar] [CrossRef] [Green Version]
- Jun, Y.W.; Huh, Y.M.; Choi, J.S.; Lee, J.H.; Song, H.T.; Kim, S.; Yoon, S.; Kim, K.S.; Shin, J.S.; Suh, J.S.; et al. Nanoscale Size Effect of Magnetic Nanocrystals and Their Utilization for Cancer Diagnosis via Magnetic Resonance Imaging. J. Am. Chem. Soc. 2005, 127, 5732–5733. [Google Scholar] [CrossRef]
- Jiang, W.; Kim, B.Y.S.; Rutka, J.T.; Chan, W.C.W. Nanoparticle-Mediated Cellular Response Is Size-Dependent. Nat. Nanotechnol. 2008, 3, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fu, L.; Xin, J.; Wang, S.; Yao, C.; Zhang, Z.; Wang, J. Photoacoustic Response Induced by Nanoparticle-Mediated Photothermal Bubbles beyond the Thermal Expansion for Potential Theranostics. J. Biomed. Opt. 2018, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Li, K.; Cai, X.; Liu, B.; Wei, Y.; Deng, K.; Xie, Z.; Wu, Z.; Ma, P.; Hou, Z.; et al. A Hollow-Structured CuS@Cu2S@Au Nanohybrid: Synergistically Enhanced Photothermal Efficiency and Photoswitchable Targeting Effect for Cancer Theranostics. Adv. Mater. 2017, 29, 1–9. [Google Scholar] [CrossRef]
- Wu, C.S.; Lee, C.C.; Wu, C.T.; Yang, Y.S.; Ko, F.H. Size-Modulated Catalytic Activity of Enzyme-Nanoparticle Conjugates: A Combined Kinetic and Theoretical Study. Chem. Commun. 2011, 47, 7446–7448. [Google Scholar] [CrossRef]
- Kim, D.; Jeong, Y.Y.; Jon, S. A Drug-Loaded Aptamer - Gold Nanoparticle Bioconjugate for Combined Ct Imaging and Therapy of Prostate Cancer. ACS Nano 2010, 4, 3689–3696. [Google Scholar] [CrossRef] [PubMed]
- Sjøgren, C.E.; Briley-Sæbø, K.; Hanson, M.; Johansson, C. Magnetic Characterization of Iron Oxides for Magnetic Resonance Imaging. Magn. Reson. Med. 1994, 31, 268–272. [Google Scholar] [CrossRef]
- Agudelo, D.; Bourassa, P.; Bérubé, G.; Tajmir-Riahi, H.A. Intercalation of Antitumor Drug Doxorubicin and Its Analogue by DNA Duplex: Structural Features and Biological Implications. Int. J. Biol. Macromol. 2014, 66, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Putnam, D.; Zelikin, A.N.; Izumrudov, V.A.; Langer, R. Polyhistidine-PEG: DNA Nanocomposites for Gene Delivery. Biomaterials 2003, 24, 4425–4433. [Google Scholar] [CrossRef]
- Yoo, H.S.; Park, T.G. In Vitro and in Vivo Anti-Tumor Activities of Nanoparticles Based on Doxorubicin-PLGA Conjugates. Am. Chem. Soc. Polym. Prepr. Div. Polym. Chem. 2000, 41, 992–993. [Google Scholar] [CrossRef]
- Chatterjee, D.K.; Diagaradjane, P.; Krishnan, S. Nanoparticle-Mediated Hyperthermia in Cancer Therapy. Ther. Deliv. 2011, 2, 1001–1014. [Google Scholar] [CrossRef] [Green Version]
- Storm, F.K. Clinical Hyperthermia and Chemotherapy. Radiol. Clin. North Am. 1989, 27, 621–627. [Google Scholar]
- Kishimoto, M.; Yanagihara, H.; Kita, E. Dependences of Specific Loss Power on Magnetic Field and Frequency in Elongated Platelet γ-Fe2O3 Particles Using Hysteresis-Loss Heating. IEEE Trans. Magn. 2013, 49, 4756–4760. [Google Scholar] [CrossRef]
- Hua, X.; Zhou, Z.; Yuan, L.; Liu, S. Selective Collection and Detection of MCF-7 Breast Cancer Cells Using Aptamer-Functionalized Magnetic Beads and Quantum Dots Based Nano-Bio-Probes. Anal. Chim. Acta 2013, 788, 135–140. [Google Scholar] [CrossRef] [PubMed]

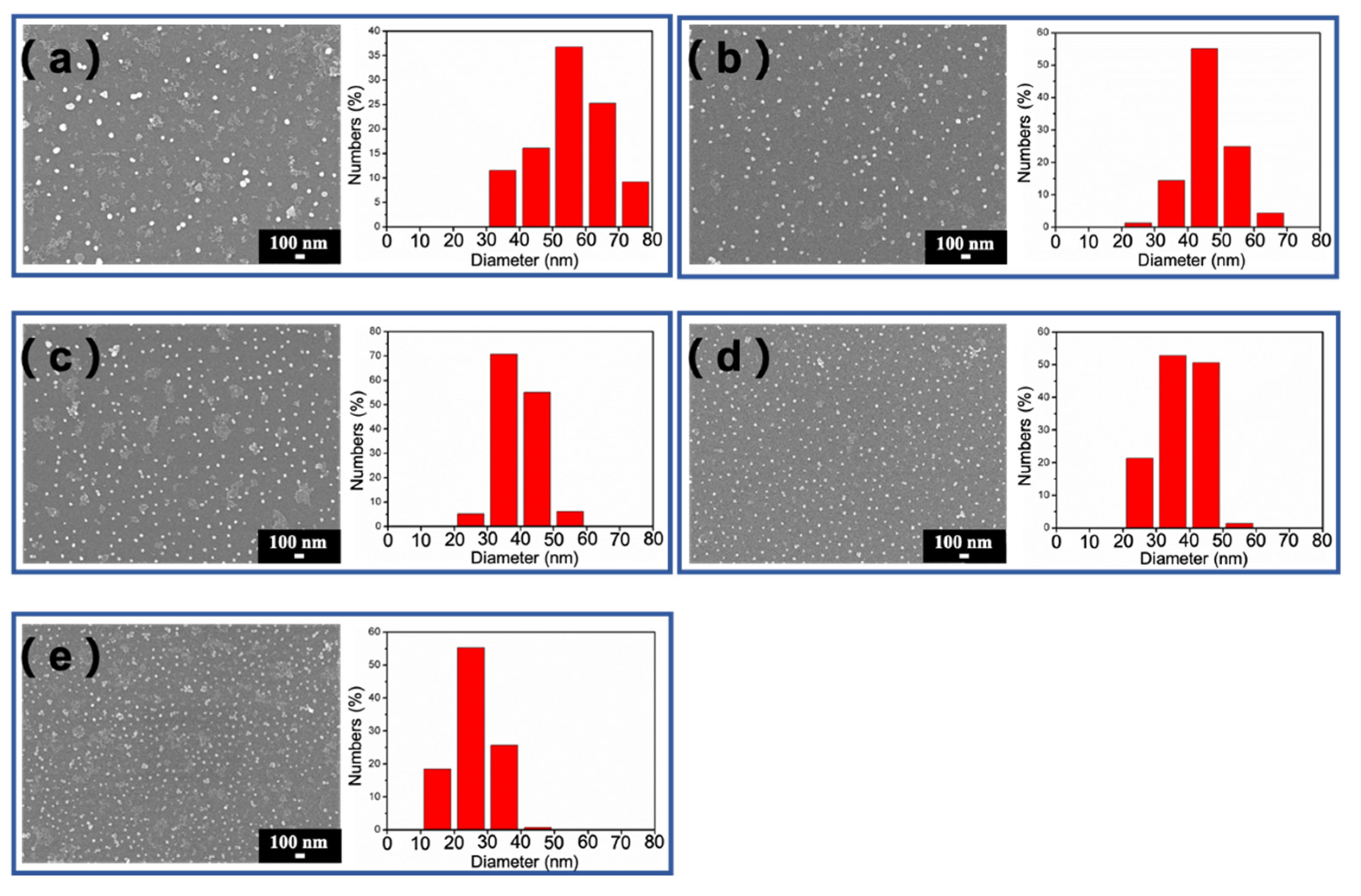

| Particle Size (nm) | Fe3O4 (mL) | C6H5Na3O7 (μL) | NH2OH·HCl (μL) | HAuCl4 (mL) |
|---|---|---|---|---|
| 25.9 | 1 | 1600 | 68 | 2 |
| 33.1 | 1 | 1400 | 80 | 2 |
| 39.5 | 1 | 1000 | 100 | 2 |
| 47.7 | 1 | 800 | 100 | 2 |
| 59.5 | 1 | 600 | 100 | 2 |
| Zeta Potential (mV) | |||
|---|---|---|---|
| Diameter (nm) | Fe3O4@Au | Fe3O4@Au-dsDNA | Fe3O4@Au-dsDNA/DOX |
| 25.9 | −44.3 | −49.3 | −26.0 |
| 33.1 | −40.2 | −45.4 | −29.5 |
| 39.5 | −37.4 | −39.4 | −33.1 |
| 47.7 | −36.7 | −40.5 | −30.7 |
| 59.5 | −36.5 | −46.0 | −29.0 |
| Diameter (nm) | SLP Value (W/g) |
|---|---|
| 25.9 | 149.0 |
| 33.1 | 127.4 |
| 39.5 | 120.3 |
| 47.7 | 112.5 |
| 59.5 | 91.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, B.-W.; Chu, C.-Y.; Lin, C.-C.; Ko, F.-H. The Multifunctionally Graded System for a Controlled Size Effect on Iron Oxide–Gold Based Core-Shell Nanoparticles. Nanomaterials 2021, 11, 1695. https://doi.org/10.3390/nano11071695
Du B-W, Chu C-Y, Lin C-C, Ko F-H. The Multifunctionally Graded System for a Controlled Size Effect on Iron Oxide–Gold Based Core-Shell Nanoparticles. Nanomaterials. 2021; 11(7):1695. https://doi.org/10.3390/nano11071695
Chicago/Turabian StyleDu, Bo-Wei, Chih-Yuan Chu, Ching-Chang Lin, and Fu-Hsiang Ko. 2021. "The Multifunctionally Graded System for a Controlled Size Effect on Iron Oxide–Gold Based Core-Shell Nanoparticles" Nanomaterials 11, no. 7: 1695. https://doi.org/10.3390/nano11071695
APA StyleDu, B.-W., Chu, C.-Y., Lin, C.-C., & Ko, F.-H. (2021). The Multifunctionally Graded System for a Controlled Size Effect on Iron Oxide–Gold Based Core-Shell Nanoparticles. Nanomaterials, 11(7), 1695. https://doi.org/10.3390/nano11071695







