Metal Oxide-Related Dendritic Structures: Self-Assembly and Applications for Sensor, Catalysis, Energy Conversion and Beyond
Abstract
:1. Introduction
2. Synthesis of Dendritic Metal Oxides
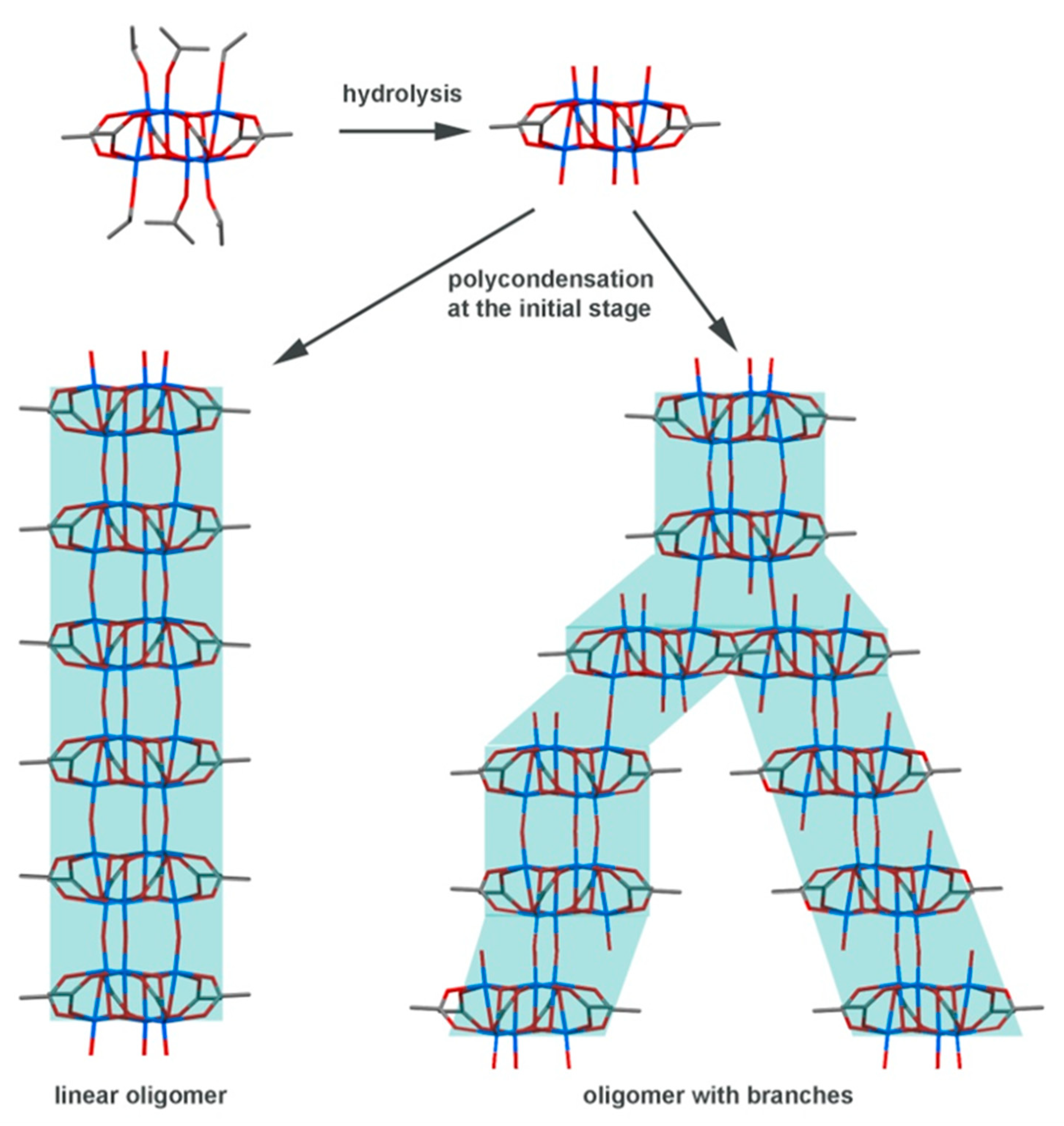
2.1. Sol‒Gel Method
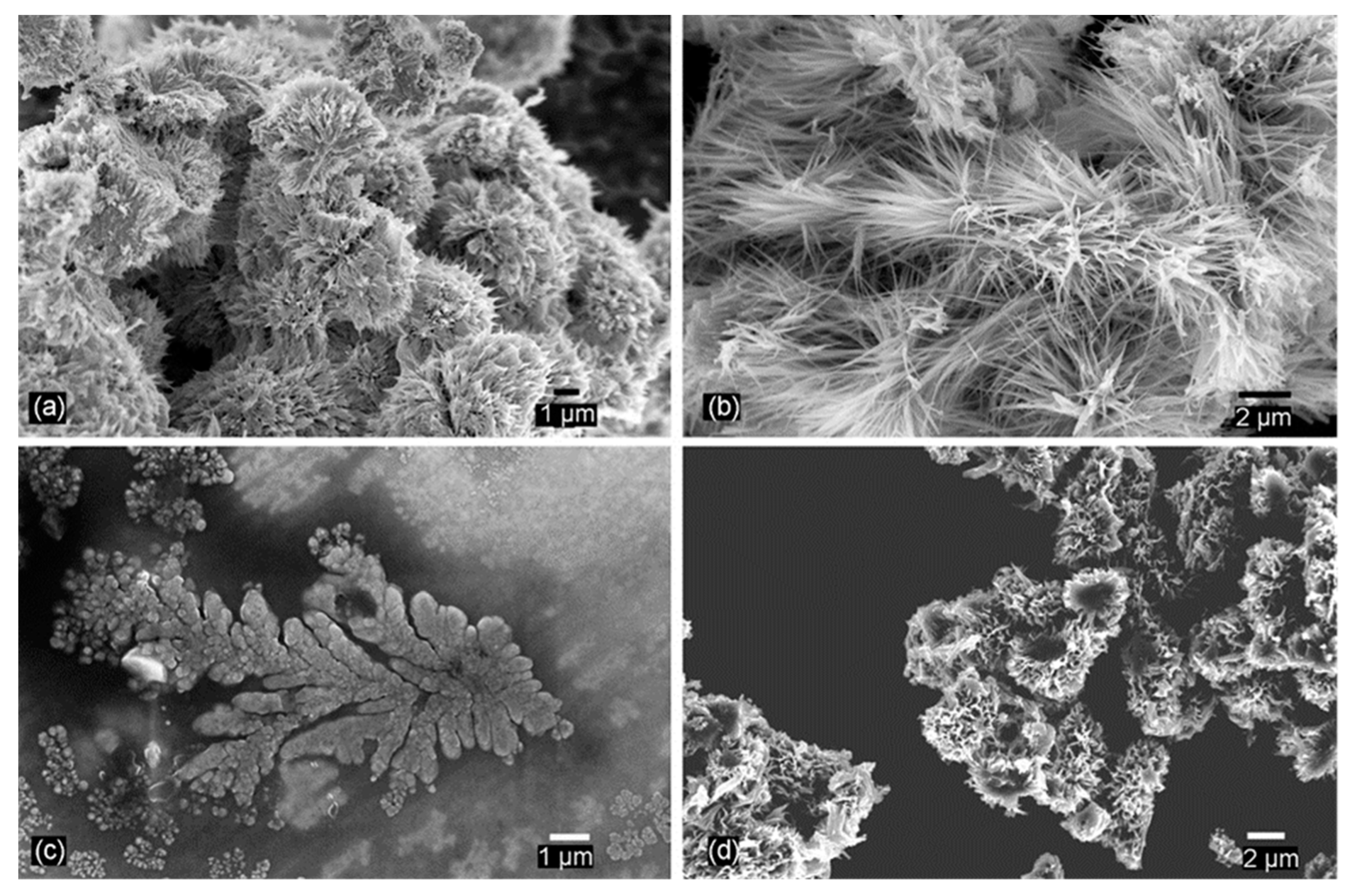
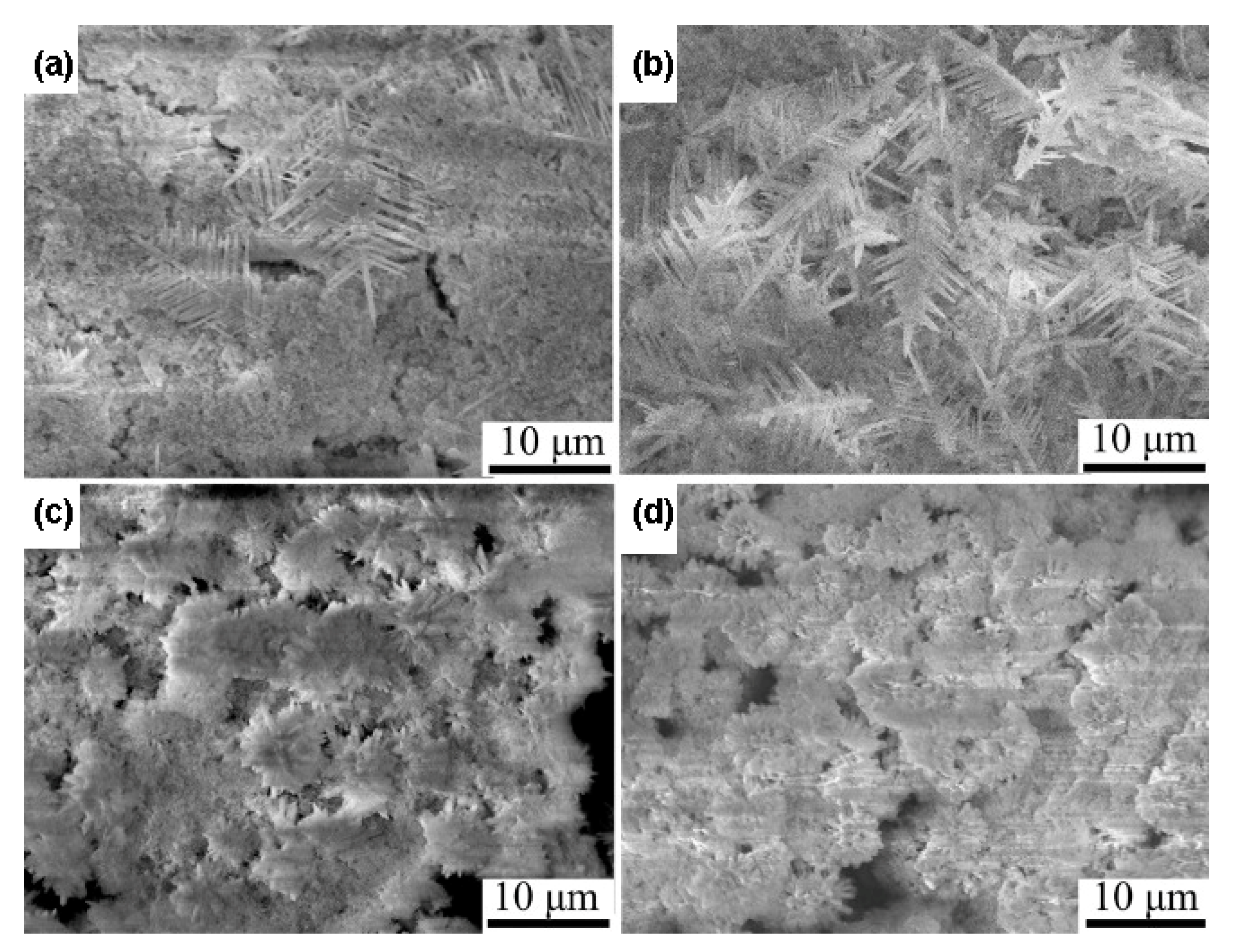
2.2. Hydrothermal and Solvothermal Methods
2.3. Electrochemical Synthesis
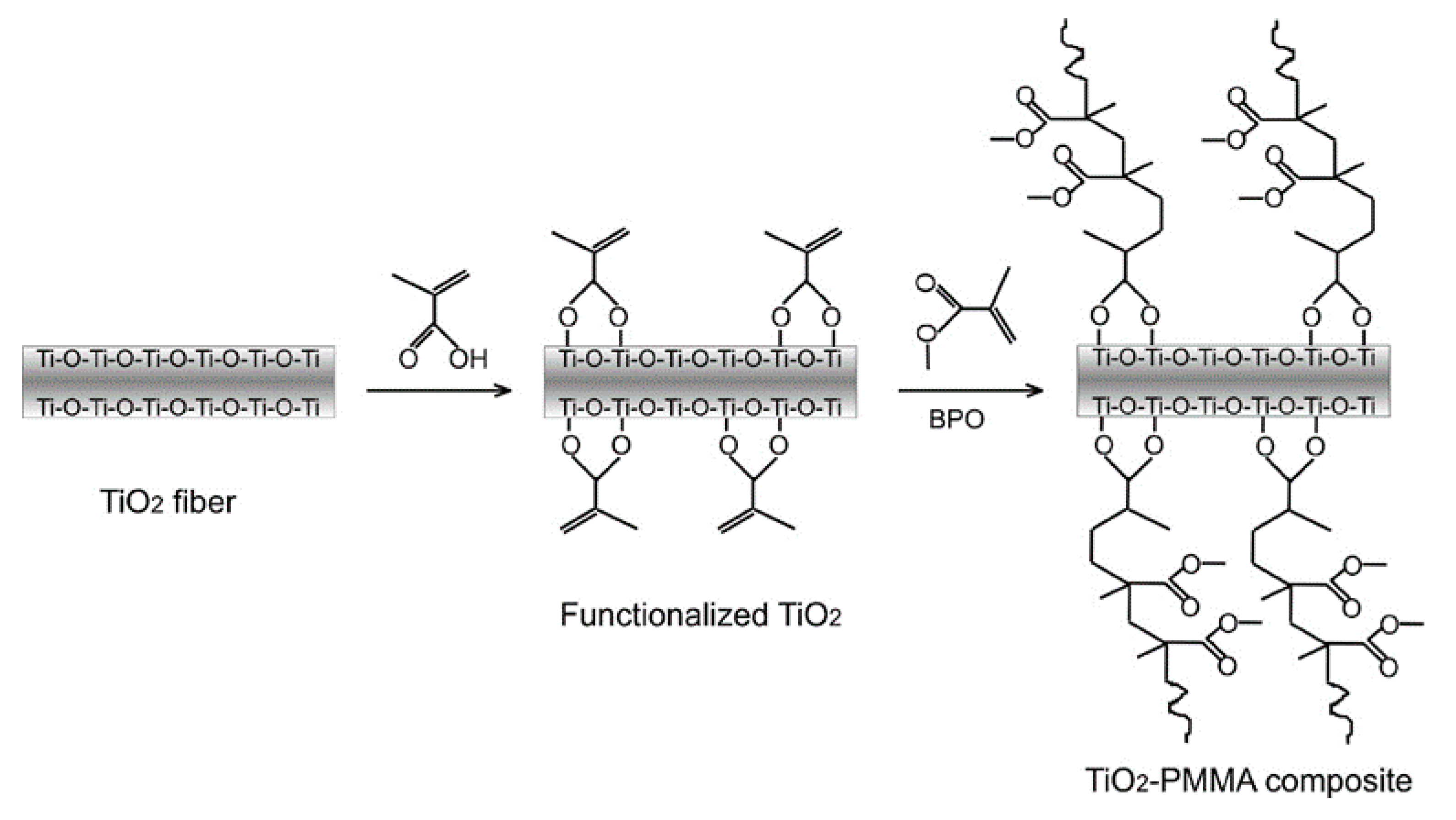
2.4. Organic‒Inorganic Hybrid materials
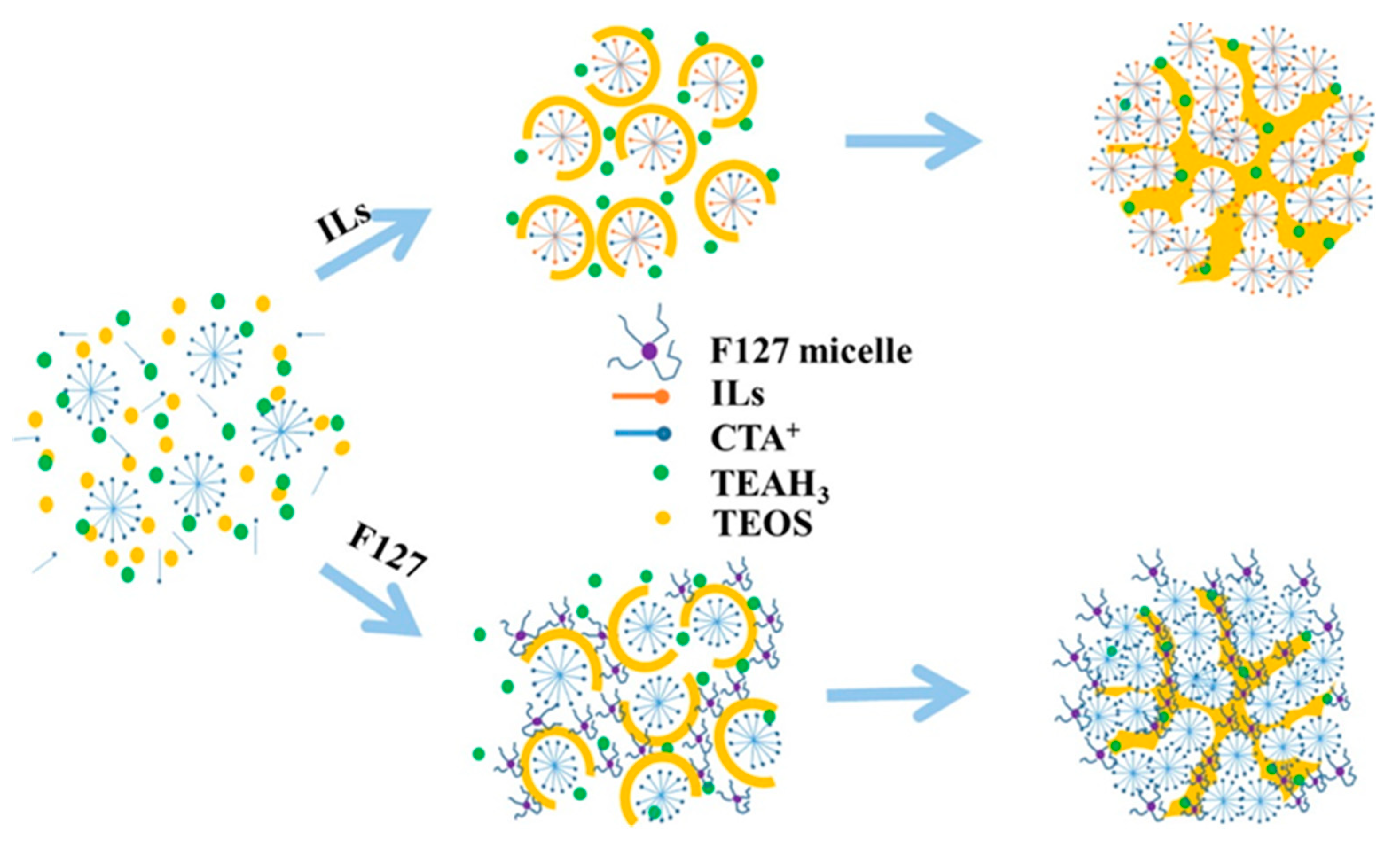
2.5. Supramolecular Dendrimers
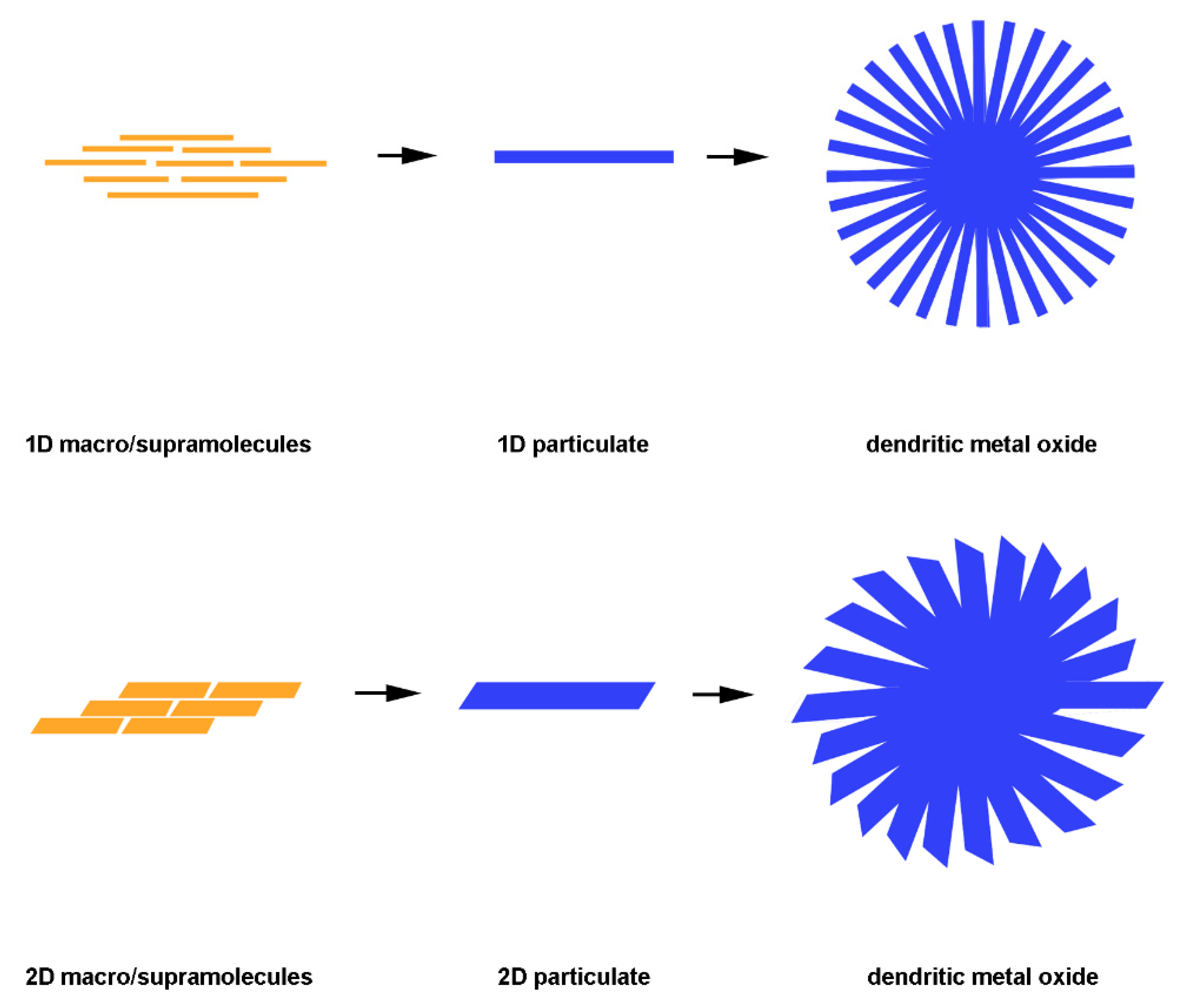
3. Dendritic Structure Formation Mechanism
3.1. Molecular Recognition for Dendritic Mesopore Formation
3.2. Sol‒Gel Anisotropic-Assembly
3.3. Hydrothermal Reactions
4. Applications
4.1. Conductometer Sensors
4.2. Energy Conversion and Storages
4.3. Catalysis
4.4. Drug Delivery
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seo, Y.; Manivannan, S.; Kang, I.; Lee, S.-W.; Kim, K. Gold dendrites Co-deposited with M13 virus as a biosensor platform for nitrite ions. Biosens. Bioelectron. 2017, 94, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.-W.; Lv, J.-J.; Liu, L.; Wang, A.-J.; Feng, J.-J.; Xu, Q.-Q. Amino acid-assisted fabrication of uniform dendrite-like PtAu porous nanoclusters as highly efficient electrocatalyst for methanol oxidation and oxygen reduction reactions. Int. J. Hydrog. Energ. 2017, 42, 2104–2115. [Google Scholar] [CrossRef]
- Buhleier, E.; Wehner, W.; Vögtle, F. “Cascade”- and “Nonskid-Chain-like” Syntheses of Molecular Cavity Topologies. Synthesis 1978, 1978, 155–158. [Google Scholar] [CrossRef]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef] [Green Version]
- Ishiwari, F.; Shoji, Y.; Fukushima, T. Supramolecular scaffolds enabling the controlled assembly of functional molecular units. Chem. Sci. 2018, 9, 2028–2041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, M.E.; Green, O.; Gnaim, S.; Shabat, D. Dendritic, Oligomeric, and Polymeric Self-Immolative Molecular Amplification. Chem. Rev. 2016, 116, 1309–1352. [Google Scholar] [CrossRef]
- Wang, D.; Tong, G.; Dong, R.; Zhou, Y.; Shen, J.; Zhu, X. Self-assembly of supramolecularly engineered polymers and their biomedical applications. Chem. Commun. 2014, 50, 11994–12017. [Google Scholar] [CrossRef]
- Ariga, K.; Nakanishi, T.; Hill, J.P. Self-assembled microstructures of functional molecules. Curr. Opin. Colloid Interface Sci. 2007, 12, 106–120. [Google Scholar] [CrossRef]
- Astruc, D.; Chardac, F. Dendritic Catalysts and Dendrimers in Catalysis. Chem. Rev. 2001, 101, 2991–3024. [Google Scholar] [CrossRef]
- Allabashi, R.; Arkas, M.; Hörmann, G.; Tsiourvas, D. Removal of some organic pollutants in water employing ceramic membranes impregnated with cross-linked silylated dendritic and cyclodextrin polymers. Water Res. 2007, 41, 476–486. [Google Scholar] [CrossRef]
- Gasparotto, A.; Barreca, D.; Maccato, C.; Tondello, E. Manufacturing of inorganic nanomaterials: Concepts and perspectives. Nanoscale 2012, 4, 2813–2825. [Google Scholar] [CrossRef] [PubMed]
- Tabacchi, G. Supramolecular Organization in Confined Nanospaces. ChemPhysChem 2018, 19, 1249–1297. [Google Scholar] [CrossRef]
- Bekermann, D.; Barreca, D.; Gasparotto, A.; Maccato, C. Multi-component oxide nanosystems by Chemical Vapor Deposition and related routes: Challenges and perspectives. CrystEngComm 2012, 14, 6347–6358. [Google Scholar] [CrossRef]
- Wei, H.; Xu, Q.; Li, A.; Wan, T.; Huang, Y.; Cui, D.; Pan, D.; Dong, B.; Wei, R.; Naik, N.; et al. Dendritic core-shell copper-nickel alloy@metal oxide for efficient non-enzymatic glucose detection. Sens. Actuators B Chem. 2021, 337, 129687. [Google Scholar] [CrossRef]
- Darband, G.B.; Aliofkhazraei, M.; Shanmugam, S. Recent advances in methods and technologies for enhancing bubble detachment during electrochemical water splitting. Renew. Sust. Energ. Rev 2019, 114, 109300. [Google Scholar] [CrossRef]
- Lei, Q.; Guo, J.; Noureddine, A.; Wang, A.; Wuttke, S.; Brinker, C.J.; Zhu, W. Sol–Gel-Based Advanced Porous Silica Materials for Biomedical Applications. Adv. Funct. Mater. 2020, 30, 1909539. [Google Scholar] [CrossRef]
- González, B.; Ruiz-Hernández, E.; Feito, M.J.; López de Laorden, C.; Arcos, D.; Ramírez-Santillán, C.; Matesanz, C.; Portolés, M.T.; Vallet-Regí, M. Covalently bonded dendrimer-maghemite nanosystems: Nonviral vectors for in vitrogene magnetofection. J. Mater. Chem. 2011, 21, 4598–4604. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, D.; Jin, G.; Tan, B.H.; He, C. Facile Layer-by-Layer Self-Assembly toward Enantiomeric Poly(lactide) Stereocomplex Coated Magnetite Nanocarrier for Highly Tunable Drug Deliveries. ACS Appl. Mater. Interface 2016, 8, 1842–1853. [Google Scholar] [CrossRef]
- Kundu, B.; Eltohamy, M.; Yadavalli, V.K.; Kundu, S.C.; Kim, H.-W. Biomimetic Designing of Functional Silk Nanotopography Using Self-assembly. ACS Appl. Mater. Interface 2016, 8, 28458–28467. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.-S.; Nong, H.N.; Reier, T.; Gliech, M.; Strasser, P. Oxide-supported Ir nanodendrites with high activity and durability for the oxygen evolution reaction in acid PEM water electrolyzers. Chem. Sci. 2015, 6, 3321–3328. [Google Scholar] [CrossRef] [Green Version]
- Lamaka, S.V.; Shchukin, D.G.; Andreeva, D.V.; Zheludkevich, M.L.; Möhwald, H.; Ferreira, M.G.S. Sol-Gel/Polyelectrolyte Active Corrosion Protection System. Adv. Funct. Mater. 2008, 18, 3137–3147. [Google Scholar] [CrossRef]
- Roussi, E.; Tsetsekou, A.; Tsiourvas, D.; Karantonis, A. Novel hybrid organo-silicate corrosion resistant coatings based on hyperbranched polymers. Surf. Coat. Technol. 2011, 205, 3235–3244. [Google Scholar] [CrossRef]
- Tian, Z.; Shi, H.; Liu, F.; Xu, S.; Han, E.-H. Inhibiting effect of 8-hydroxyquinoline on the corrosion of silane-based sol–gel coatings on AA 2024-T3. Prog. Org. Coat. 2015, 82, 81–90. [Google Scholar] [CrossRef]
- Wanka, R.; Koc, J.; Clarke, J.; Hunsucker, K.Z.; Swain, G.W.; Aldred, N.; Finlay, J.A.; Clare, A.S.; Rosenhahn, A. Sol–Gel-Based Hybrid Materials as Antifouling and Fouling-Release Coatings for Marine Applications. ACS Appl. Mater. Interface 2020, 12, 53286–53296. [Google Scholar] [CrossRef]
- Angelos, S.; Johansson, E.; Stoddart, J.F.; Zink, J.I. Mesostructured Silica Supports for Functional Materials and Molecular Machines. Adv. Funct. Mater. 2007, 17, 2261–2271. [Google Scholar] [CrossRef]
- Li, C.; Li, Q.; Kaneti, Y.V.; Hou, D.; Yamauchi, Y.; Mai, Y. Self-assembly of block copolymers towards mesoporous materials for energy storage and conversion systems. Chem. Soc. Rev. 2020, 49, 4681–4736. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Wu, Z.; Wang, M.; Luo, J.; Torun, H.; Hu, P.; Yang, C.; Grundmann, M.; Liu, X.; et al. Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature. Mater. Horiz. 2019, 6, 470–506. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Wan, Q. Gas Sensors Based on Semiconducting Metal Oxide One-Dimensional Nanostructures. Sensors 2009, 9, 9903–9924. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski, T.; Gębicki, J. Emerging strategies for enhancing detection of explosives by artificial olfaction. Microchem. J. 2021, 164, 106025. [Google Scholar] [CrossRef]
- Yang, B.-X.; Tseng, C.-Y.; Chiang, A.S.-T.; Liu, C.-L. A sol–gel titanium–silicon oxide/organic hybrid dielectric for low-voltage organic thin film transistors. J. Mater. Chem. C 2015, 3, 968–972. [Google Scholar] [CrossRef]
- Huang, H.; Liang, B.; Liu, Z.; Wang, X.; Chen, D.; Shen, G. Metal oxide nanowire transistors. J. Mater. Chem. 2012, 22, 13428–13445. [Google Scholar] [CrossRef]
- Acton, O.; Ting Ii, G.G.; Ma, H.; Hutchins, D.; Wang, Y.; Purushothaman, B.; Anthony, J.E.; Jen, A.K.Y. π-σ-Phosphonic acid organic monolayer–amorphous sol–gel hafnium oxide hybrid dielectric for low-voltage organic transistors on plastic. J. Mater. Chem. 2009, 19, 7929–7936. [Google Scholar] [CrossRef]
- Acton, B.O.; Ting, G.G.; Shamberger, P.J.; Ohuchi, F.S.; Ma, H.; Jen, A.K.Y. Dielectric Surface-Controlled Low-Voltage Organic Transistors via n-Alkyl Phosphonic Acid Self-Assembled Monolayers on High-k Metal Oxide. ACS Appl. Mater. Interface 2010, 2, 511–520. [Google Scholar] [CrossRef]
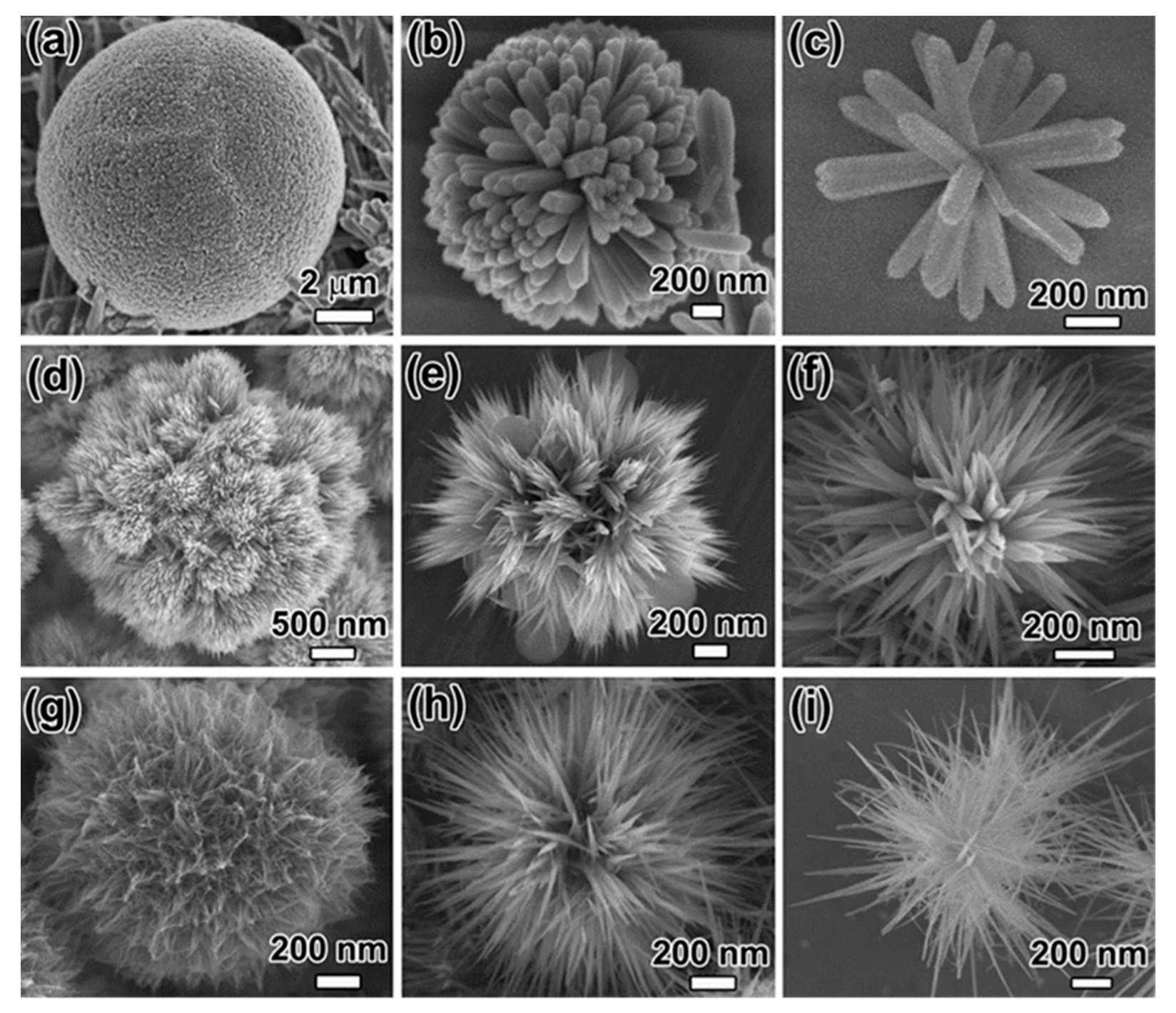
- Hao, P.; Peng, B.; Shan, B.-Q.; Yang, T.-Q.; Zhang, K. Comprehensive understanding of the synthesis and formation mechanism of dendritic mesoporous silica nanospheres. Nanoscale Adv. 2020, 2, 1792–1810. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Qiao, S.Z. Dendritic Silica Particles with Center-Radial Pore Channels: Promising Platforms for Catalysis and Biomedical Applications. Small 2015, 11, 392–413. [Google Scholar] [CrossRef] [PubMed]
- Alfredsson, V.; Keung, M.; Monnier, A.; Stucky, G.D.; Unger, K.K.; Schüth, F. High-resolution transmission electron microscopy of mesoporous MCM-41 type materials. J. Chem. Soc. Chem. Commun. 1994, 8, 921–922. [Google Scholar] [CrossRef]
- Dislich, H.; Hinz, P. History and principles of the sol-gel process, and some new multicomponent oxide coatings. J. Non-Cryst. Solids 1982, 48, 11–16. [Google Scholar] [CrossRef]
- Uhlmann, D.R.; Teowee, G. Sol-Gel Science and Technology: Current State and Future Prospects. J. Sol. Gel Sci. Technol. 1998, 13, 153–162. [Google Scholar] [CrossRef]
- Gesser, H.D.; Goswami, P.C. Aerogels and related porous materials. Chem. Rev. 1989, 89, 765–788. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The Sol-Gel Process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Sui, R.; Charpentier, P. Synthesis of metal oxide nanostructures by direct sol–gel chemistry in supercritical fluids. Chem. Rev. 2012, 112, 3057–3082. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: New York, NY, USA, 1990. [Google Scholar]
- Sanchez, C.; Livage, J.; Henry, M.; Babonneau, F. Chemical modification of alkoxide precursors. J. Non-Cryst. Solids 1988, 100, 65–76. [Google Scholar] [CrossRef]
- Gash, A.E.; Tillotson, T.M.; Satcher, J.H.; Poco, J.F.; Hrubesh, L.W.; Simpson, R.L. Use of Epoxides in the Sol−Gel Synthesis of Porous Iron(III) Oxide Monoliths from Fe(III) Salts. Chem. Mater. 2001, 13, 999–1007. [Google Scholar] [CrossRef]
- Benad, A.; Jürries, F.; Vetter, B.; Klemmed, B.; Hübner, R.; Leyens, C.; Eychmüller, A. Mechanical Properties of Metal Oxide Aerogels. Chem. Mater. 2018, 30, 145–152. [Google Scholar] [CrossRef]
- Sui, R.; Rizkalla, A.S.; Charpentier, P.A. Formation of titania nanofibers: A direct sol-gel route in supercritical CO2. Langmuir 2005, 21, 6150–6153. [Google Scholar] [CrossRef] [PubMed]
- Sui, R.; Deering, C.E.; Prinsloo, R.; Lavery, C.B.; Chou, N.; Marriott, R.A. Sol–Gel Synthesis of 2-Dimensional TiO2: Self-Assembly of Ti–Oxoalkoxy–Acetate Complexes by Carboxylate Ligand Directed Condensation. Faraday Discuss. 2021, 227, 125–140. [Google Scholar] [CrossRef]
- Sui, R.; Lavery, C.B.; Li, D.; Deering, C.E.; Chou, N.; Dowling, N.I.; Marriott, R.A. Improving low-temperature CS2 conversion for the Claus process by using La(III)-doped nanofibrous TiO2 xerogel. Appl. Catal. B 2019, 241, 217–226. [Google Scholar] [CrossRef]
- Lucky, R.A.; Charpentier, P.A. N-doped ZrO2/TiO2 bimetallic materials synthesized in supercritical CO2: Morphology and photocatalytic activity. Appl. Catal. B 2010, 96, 516–523. [Google Scholar] [CrossRef]
- Sui, R.; Lavery, C.B.; Deering, C.E.; Prinsloo, R.; Li, D.; Chou, N.; Lesage, K.L.; Marriott, R.A. Improved carbon disulfide conversion: Modification of an alumina Claus catalyst by deposition of transition metal oxides. Appl. Catal. A Gen. 2020, 604, 117773. [Google Scholar] [CrossRef]
- Singh, S.; Singh, A.; Wan, M.; Yadav, R.R.; Tandon, P.; Rasool, S.S.A.; Yadav, B.C. Fabrication of self-assembled hierarchical flowerlike zinc stannate thin film and its application as liquefied petroleum gas sensor. Sens. Actuators B Chem. 2014, 205, 102–110. [Google Scholar] [CrossRef]
- Souza, R.L.; Faria, E.L.P.; Figueiredo, R.T.; Fricks, A.T.; Zanin, G.M.; Santos, O.A.A.; Lima, Á.S.; Soares, C.M.F. Use of polyethylene glycol in the process of sol–gel encapsulation of Burkholderia cepacia lipase. J. Therm. Anal. Calorim. 2014, 117, 301–306. [Google Scholar] [CrossRef]
- Islam, S.; Bidin, N.; Riaz, S.; Naseem, S. Self-assembled hierarchical phenolphthalein encapsulated silica nanoparticles: Structural, optical and sensing response. Sens. Actuators A Phys. 2017, 266, 111–121. [Google Scholar] [CrossRef]
- Ibanez, A.; Maximov, S.; Guiu, A.; Chaillout, C.; Baldeck, P.L. Controlled Nanocrystallization of Organic Molecules in Sol-Gel Glasses. Adv. Mater. 1998, 10, 1540–1543. [Google Scholar] [CrossRef]
- Gracheva, I.E.; Olchowik, G.; Gareev, K.G.; Moshnikov, V.A.; Kuznetsov, V.V.; Olchowik, J.M. Investigations of nanocomposite magnetic materials based on the oxides of iron, nickel, cobalt and silicon dioxide. J. Phys. Chem. Solids 2013, 74, 656–663. [Google Scholar] [CrossRef]
- Sutar, P.; Suresh, V.M.; Jayaramulu, K.; Hazra, A.; Maji, T.K. Binder driven self-assembly of metal-organic cubes towards functional hydrogels. Nat. Commun. 2018, 9, 3587. [Google Scholar] [CrossRef]
- Yoshimura, K.B.A.M. Handbook of Hydrothermal Technology; Cambridge University Press: Norwich, NY, USA, 2008. [Google Scholar]
- Xiong, D.; Zhang, Q.; Du, Z.; Verma, S.K.; Li, H.; Zhao, X. Low temperature hydrothermal synthesis mechanism and thermal stability of p-type CuMnO2 nanocrystals. New J. Chem. 2016, 40, 6498–6504. [Google Scholar] [CrossRef]
- O’Hare, D. Hydrothermal Synthesis. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Oxford, UK, 2001; pp. 3989–3992. [Google Scholar]
- Yin, H.; Wada, Y.; Kitamura, T.; Kambe, S.; Murasawa, S.; Mori, H.; Sakata, T.; Yanagida, S. Hydrothermal synthesis of nanosized anatase and rutile TiO2 using amorphous phase TiO2. J. Mater. Chem. 2001, 11, 1694–1703. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, Q.; Du, L.; Jiang, C.; Piao, L. Progress in the synthesis and applications of hierarchical flower-like TiO2 nanostructures. Particuology 2014, 15, 61–70. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Qin, L.; Zhao, P.; Liu, F.; Chuai, X.; Sun, P.; Liang, X.; Gao, Y.; Sun, Y.; et al. Preparation and gas sensing properties of hierarchical leaf-like SnO2 materials. Sens. Actuators B Chem. 2018, 255, 2944–2951. [Google Scholar] [CrossRef]
- Murugavel, R.; Choudhury, A.; Walawalkar, M.G.; Pothiraja, R.; Rao, C.N.R. Metal Complexes of Organophosphate Esters and Open-Framework Metal Phosphates: Synthesis, Structure, Transformations, and Applications. Chem. Rev. 2008, 108, 3549–3655. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, X.; Han, L.; Asahina, S.; Xu, D.; Cao, Y.; Yao, Y.; Che, S. Optically Active Chiral CuO “Nanoflowers”. J. Am. Chem. Soc. 2014, 136, 7193–7196. [Google Scholar] [CrossRef]
- Janene, F.; Dhaouadi, H.; Etteyeb, N.; Touati, F. Flower-like cuprous oxide: Hydrothermal synthesis, optical, and electrochemical properties. Ionics 2015, 21, 477–485. [Google Scholar] [CrossRef]
- Qiu, J.; Weng, B.; Zhao, L.; Chang, C.; Shi, Z.; Li, X.; Kim, H.-K.; Hwang, Y.-H. Synthesis and Characterization of Flower-Like Bundles of ZnO Nanosheets by a Surfactant-Free Hydrothermal Process. J. Nanomater. 2014, 2014, 281461. [Google Scholar] [CrossRef] [Green Version]
- Kale, R.B.; Hsu, Y.-J.; Lin, Y.-F.; Lu, S.-Y. Hydrothermal synthesis, characterizations and photoluminescence study of single crystalline hexagonal ZnO nanorods with three dimensional flowerlike microstructures. Superlattices Microstruct. 2014, 69, 239–252. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, W.; Zou, G.; Yu, W.; Qian, Y. Precursor-Induced Hydrothermal Synthesis of Flowerlike Cupped-End Microrod Bundles of ZnO. J. Phys. Chem. B 2005, 109, 1361–1363. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Y.; Zeng, W. Hydrothermal synthesis of hierarchical flower-like ZnO nanostructure and its enhanced ethanol gas-sensing properties. Appl. Surf. Sci. 2018, 427, 281–287. [Google Scholar] [CrossRef]
- Yang, L.-X.; Zhu, Y.-J.; Li, L.; Zhang, L.; Tong, H.; Wang, W.-W.; Cheng, G.-F.; Zhu, J. A Facile Hydrothermal Route to Flower-Like Cobalt Hydroxide and Oxide. Eur. J. Inorg. Chem. 2006, 2006, 4787–4792. [Google Scholar] [CrossRef]
- Xu, H.; Zeng, M.; Li, J.; Tong, X. Facile hydrothermal synthesis of flower-like Co-doped NiO hierarchical nanosheets as anode materials for lithium-ion batteries. RSC Adv. 2015, 5, 91493–91499. [Google Scholar] [CrossRef]
- Dong, W.; Zhao, G.; Song, B.; Xu, G.; Zhou, J.; Han, G. Surfactant-free fabrication of CaTiO3 butterfly-like dendrite via a simple one-step hydrothermal route. CrystEngComm 2012, 14, 6990–6997. [Google Scholar] [CrossRef]
- Jadhav, H.S.; Pawar, S.M.; Jadhav, A.H.; Thorat, G.M.; Seo, J.G. Hierarchical Mesoporous 3D Flower-like CuCo2O4/NF for High-Performance Electrochemical Energy Storage. Sci. Rep. 2016, 6, 31120. [Google Scholar] [CrossRef] [PubMed]
- Thirumalairajan, S.; Girija, K.; Ganesh, V.; Mangalaraj, D.; Viswanathan, C.; Ponpandian, N. Novel Synthesis of LaFeO3 Nanostructure Dendrites: A Systematic Investigation of Growth Mechanism, Properties, and Biosensing for Highly Selective Determination of Neurotransmitter Compounds. Cryst. Growth Des. 2013, 13, 291–302. [Google Scholar] [CrossRef]
- Ensikat, H.J.D.-K.P.; Neinhuis, C.; Barthlott, W. Beilstein Superhydrophobicity in perfection: The outstanding properties of the lotus leaf. J. Nanotechnol. 2011, 2, 152–161. [Google Scholar]
- Shin, S.; Seo, J.; Han, H.; Kang, S.; Kim, H.; Lee, T. Bio-Inspired Extreme Wetting Surfaces for Biomedical Applications. Materials 2016, 9, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darmanin, T.; de Givenchy, E.T.; Amigoni, S.; Guittard, F. Superhydrophobic Surfaces by Electrochemical Processes. Adv. Mater. 2013, 25, 1378–1394. [Google Scholar] [CrossRef] [PubMed]
- Jeun, J.-H.; Kim, D.-H.; Hong, S.-H. Synthesis of porous SnO2 foams on SiO2/Si substrate by electrochemical deposition and their gas sensing properties. Sens. Actuators B Chem. 2012, 161, 784–790. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Cong, H.-P.; Yu, S.-H. Self-assembly of functionalized inorganic–organic hybrids. Curr. Opin. Colloid Interface Sci. 2009, 14, 71–80. [Google Scholar] [CrossRef]
- Toksoz, S.; Acar, H.; Guler, M.O. Self-assembled one-dimensional soft nanostructures. Soft Matter 2010, 6, 5839–5849. [Google Scholar] [CrossRef]
- Charpentier, P.A.; Li, X.; Sui, R. Study of the Sol-Gel Reaction Mechanism in Supercritical CO2 for the Formation of SiO2 Nanocomposites. Langmuir 2009, 25, 3748–3754. [Google Scholar] [CrossRef]
- Zhao, S.; Malfait, W.J.; Jeong, E.; Fischer, B.; Zhang, Y.; Xu, H.; Angelica, E.; Risen, W.M.; Suggs, J.W.; Koebel, M.M. Facile One-Pot Synthesis of Mechanically Robust Biopolymer–Silica Nanocomposite Aerogel by Cogelation of Silicic Acid with Chitosan in Aqueous Media. ACS Sustain. Chem. Eng. 2016, 4, 5674–5683. [Google Scholar] [CrossRef]
- Qu, L.; Hu, H.; Yu, J.; Yu, X.; Liu, J.; Xu, Y.; Zhang, Q. High-Yield Synthesis of Janus Dendritic Mesoporous Silica@Resorcinol–Formaldehyde Nanoparticles: A Competing Growth Mechanism. Langmuir 2017, 33, 5269–5274. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.M.; Sui, R.; Charpentier, P.A.; Rizkalla, A.S. Synthesis of TiO2-PMMA nanocomposite: Using methacrylic acid as a coupling agent. Langmuir 2007, 23, 3988–3995. [Google Scholar] [CrossRef] [PubMed]
- Katir, N.; Brahmi, Y.; Majoral, J.P.; Bousmina, M.; El Kadib, A. Ternary cooperative assembly—polymeric condensation of photoactive viologen, phosphonate-terminated dendrimers and crystalline anatase nanoparticles. Chem. Commun. 2015, 51, 17716–17719. [Google Scholar] [CrossRef] [PubMed]
- Brahmi, Y.; Katir, N.; Hameau, A.; Essoumhi, A.; Essassi, E.M.; Caminade, A.-M.; Bousmina, M.; Majoral, J.-P.; El Kadib, A. Hierarchically porous nanostructures through phosphonate–metal alkoxide condensation and growth using functionalized dendrimeric building blocks. Chem. Commun. 2011, 47, 8626–8628. [Google Scholar] [CrossRef]
- Fréchet, J.M.J. Dendrimers and supramolecular chemistry. Proc. Natl. Acad. Sci. USA 2002, 99, 4782. [Google Scholar] [CrossRef] [Green Version]
- Lyu, Z.; Ding, L.; Tintaru, A.; Peng, L. Self-Assembling Supramolecular Dendrimers for Biomedical Applications: Lessons Learned from Poly(amidoamine) Dendrimers. Acc. Chem. Res. 2020, 53, 2936–2949. [Google Scholar] [CrossRef]
- He, H.; Chen, S.; Tong, X.; An, Z.; Ma, M.; Wang, X.; Wang, X. Self-Assembly of a Strong Polyhedral Oligomeric Silsesquioxane Core-Based Aspartate Derivative Dendrimer Supramolecular Gelator in Different Polarity Solvents. Langmuir 2017, 33, 13332–13342. [Google Scholar] [CrossRef]
- Scott, R.W.J.; Wilson, O.M.; Crooks, R.M. Synthesis, Characterization, and Applications of Dendrimer-Encapsulated Nanoparticles. J. Phys. Chem. B 2005, 109, 692–704. [Google Scholar] [CrossRef]
- Fajalia, A.I.; Tsianou, M. Self-assembly control via molecular recognition: Effect of cyclodextrins on surfactant micelle structure and interactions determined by SANS. Colloid. Surf. A Physicochem. Eng. 2015, 480, 91–104. [Google Scholar] [CrossRef]
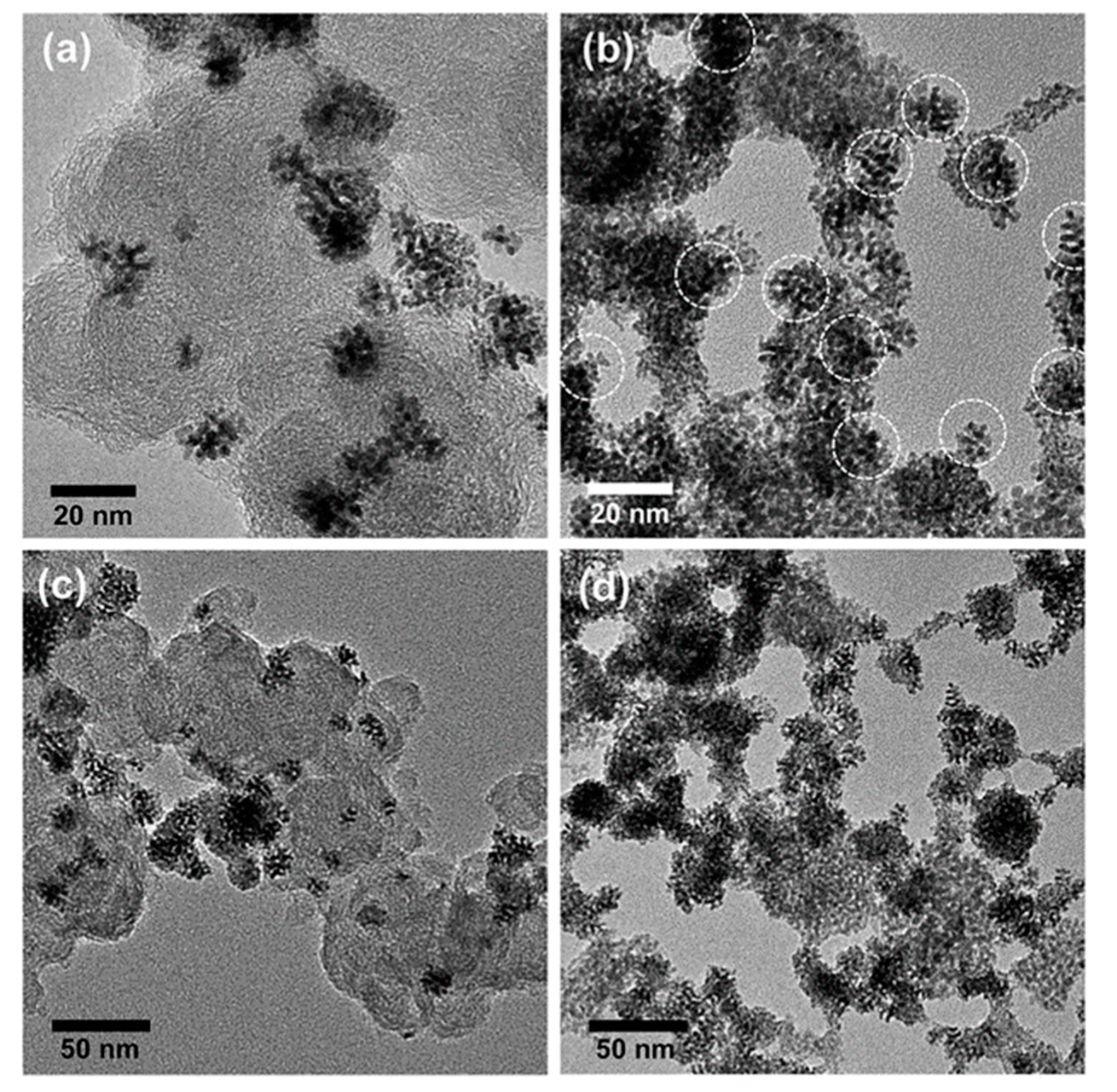
- Yu, Y.-J.; Xing, J.-L.; Pang, J.-L.; Jiang, S.-H.; Lam, K.-F.; Yang, T.-Q.; Xue, Q.-S.; Zhang, K.; Wu, P. Facile Synthesis of Size Controllable Dendritic Mesoporous Silica Nanoparticles. ACS Appl. Mater. Interface 2014, 6, 22655–22665. [Google Scholar] [CrossRef]
- Hong, Y.; Zhan, Q.; Zheng, Y.; Pu, C.; Zhao, H.; Lan, M. Hydrophilic phytic acid-functionalized magnetic dendritic mesoporous silica nanospheres with immobilized Ti4+: A dual-purpose affinity material for highly efficient enrichment of glycopeptides/phosphopeptides. Talanta 2019, 197, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Sui, R.; Rizkalla, A.S.; Charpentier, P.A. FTIR study on the formation of TiO2 nanostructures in supercritical CO2. J. Phys. Chem. B 2006, 110, 16212–16218. [Google Scholar] [CrossRef]
- Sui, R.; Thangadurai, V.; Berlinguette, C.P. Simple protocol for generating TiO2 nanofibers in organic media. Chem. Mater. 2008, 20, 7022–7030. [Google Scholar] [CrossRef]
- Sui, R.; Charpentier, P.A.; Marriott, R.A. Synthesizing 1D and 2D metal oxide nanostructures: Using metal acetate complexes as building blocks. Nanoscale 2020, 12, 17971–17981. [Google Scholar] [CrossRef]
- Witten, T.A.; Sander, L.M. Diffusion-limited aggregation. Phys. Rev. B 1983, 27, 5686–5697. [Google Scholar] [CrossRef] [Green Version]
- Abu-Much, R.; Meridor, U.; Frydman, A.; Gedanken, A. Formation of a Three-Dimensional Microstructure of Fe3O4−Poly(vinyl alcohol) Composite by Evaporating the Hydrosol under a Magnetic Field. J. Phys. Chem. B 2006, 110, 8194–8203. [Google Scholar] [CrossRef]
- Wei-Hsiu, H.; Ming-Hao, H.; Shih-Shou, L. Enhanced photoluminescence of Alq3 via patterned array silver dendritic nanostructures. In Proceedings of the SPIE photonics Europe, Nanophotonics IV, Brussels, Belgium, 16–19 April 2012. [Google Scholar]
- Johnston, K.P. New Directions in Supercritical Fluid Science and Technology. In Supercritical Science and Technology; Johnston, K.P., Penninger, J.M.L., Eds.; American Chemical Society: Washington, DC, USA, 1989; pp. 1–12. [Google Scholar]
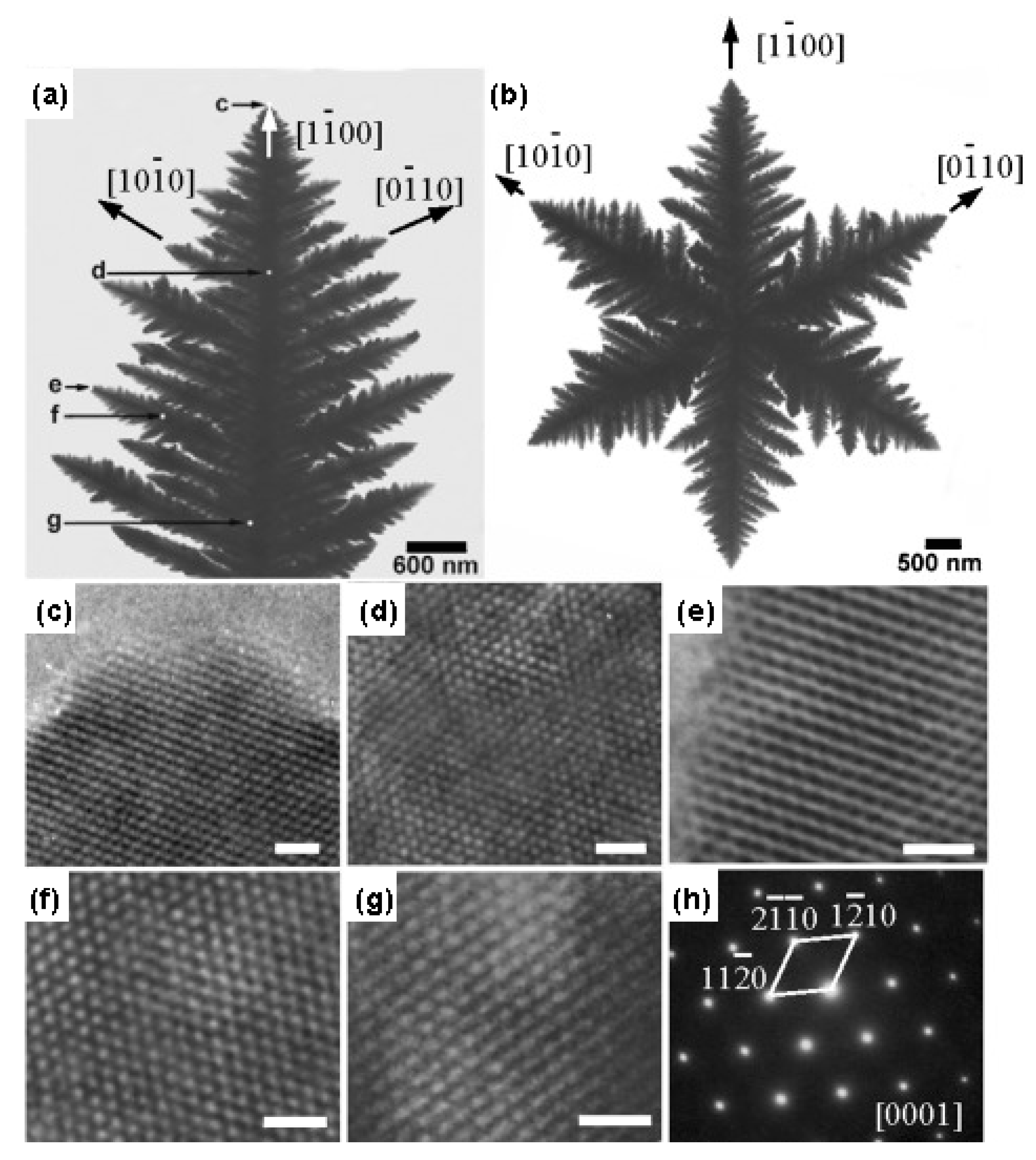
- Cao, M.; Liu, T.; Gao, S.; Sun, G.; Wu, X.; Hu, C.; Wang, Z.L. Single-Crystal Dendritic Micro-Pines of Magnetic α-Fe2O3: Large-Scale Synthesis, Formation Mechanism, and Properties. Angew. Chem. Int. Ed. 2005, 44, 4197–4201. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Xie, Y.-T.; Mak, W.C.; Cheung, K.Y.; Li, X.-Y.; Renneberg, R.; Yang, S. Dendritic Nanostructures of Silver: Facile Synthesis, Structural Characterizations, and Sensing Applications. Langmuir 2006, 22, 4836–4842. [Google Scholar] [CrossRef]
- Sun, Z.; Kim, J.H.; Zhao, Y.; Bijarbooneh, F.; Malgras, V.; Lee, Y.; Kang, Y.-M.; Dou, S.X. Rational Design of 3D Dendritic TiO2 Nanostructures with Favorable Architectures. J. Am. Chem. Soc. 2011, 133, 19314–19317. [Google Scholar] [CrossRef] [PubMed]
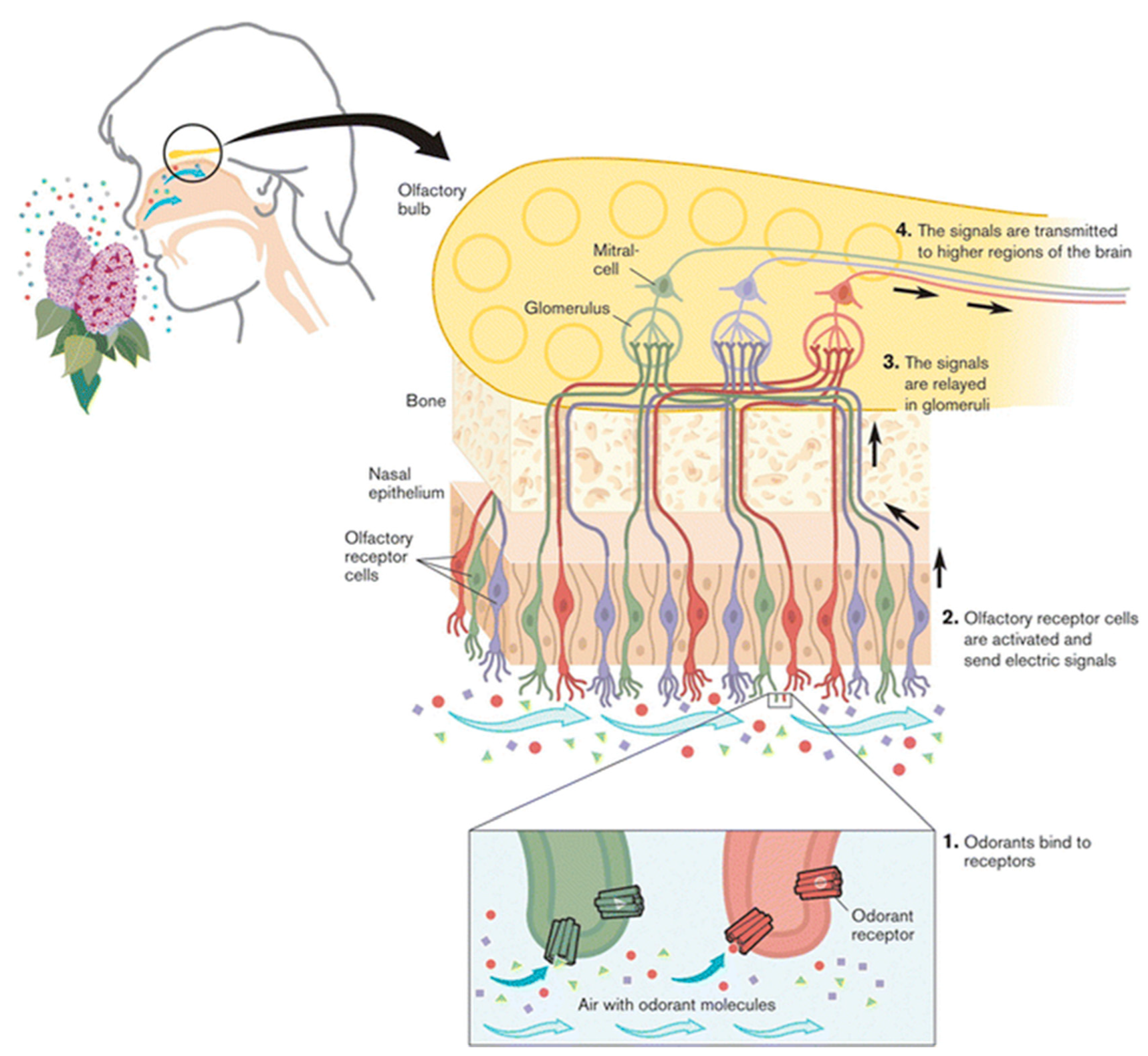
- Sarafoleanu, C.; Mella, C.; Georgescu, M.; Perederco, C. The importance of the olfactory sense in the human behavior and evolution. J. Med. Life 2009, 2, 196–198. [Google Scholar] [PubMed]
- Ropp, R.C. Chapter 3—Group 16 (O, S, Se, Te) Alkaline Earth Compounds. In Encyclopedia of the Alkaline Earth Compounds; Ropp, R.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 105–197. [Google Scholar]
- Carannante, I.; Marasco, A. Olfactory Sensory Neurons to Odor Stimuli: Mathematical Modeling of the Response. In Encyclopedia of Computational Neuroscience; Jaeger, D., Jung, R., Eds.; Springer: New York, NY, USA, 2018; pp. 1–12. [Google Scholar]
- Sun, W.; Qi, X.; Zhang, Y.; Yang, H.; Gao, H.; Chen, Y.; Sun, Z. Electrochemical DNA biosensor for the detection of Listeria monocytogenes with dendritic nanogold and electrochemical reduced graphene modified carbon ionic liquid electrode. Electrochim. Acta 2012, 85, 145–151. [Google Scholar] [CrossRef]
- Hashkavayi, A.B.; Raoof, J.B. Design an aptasensor based on structure-switching aptamer on dendritic gold nanostructures/Fe3O4@SiO2/DABCO modified screen printed electrode for highly selective detection of epirubicin. Biosens. Bioelectron. 2017, 91, 650–657. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Z.; Zhang, H.; Luo, L.; Yao, S. Selective and sensitive molecularly imprinted sol–gel film-based electrochemical sensor combining mercaptoacetic acid-modified PbS nanoparticles with Fe3O4@Au–multi-walled carbon nanotubes–chitosan. J. Solid State Electrochem. 2012, 16, 857–867. [Google Scholar] [CrossRef]
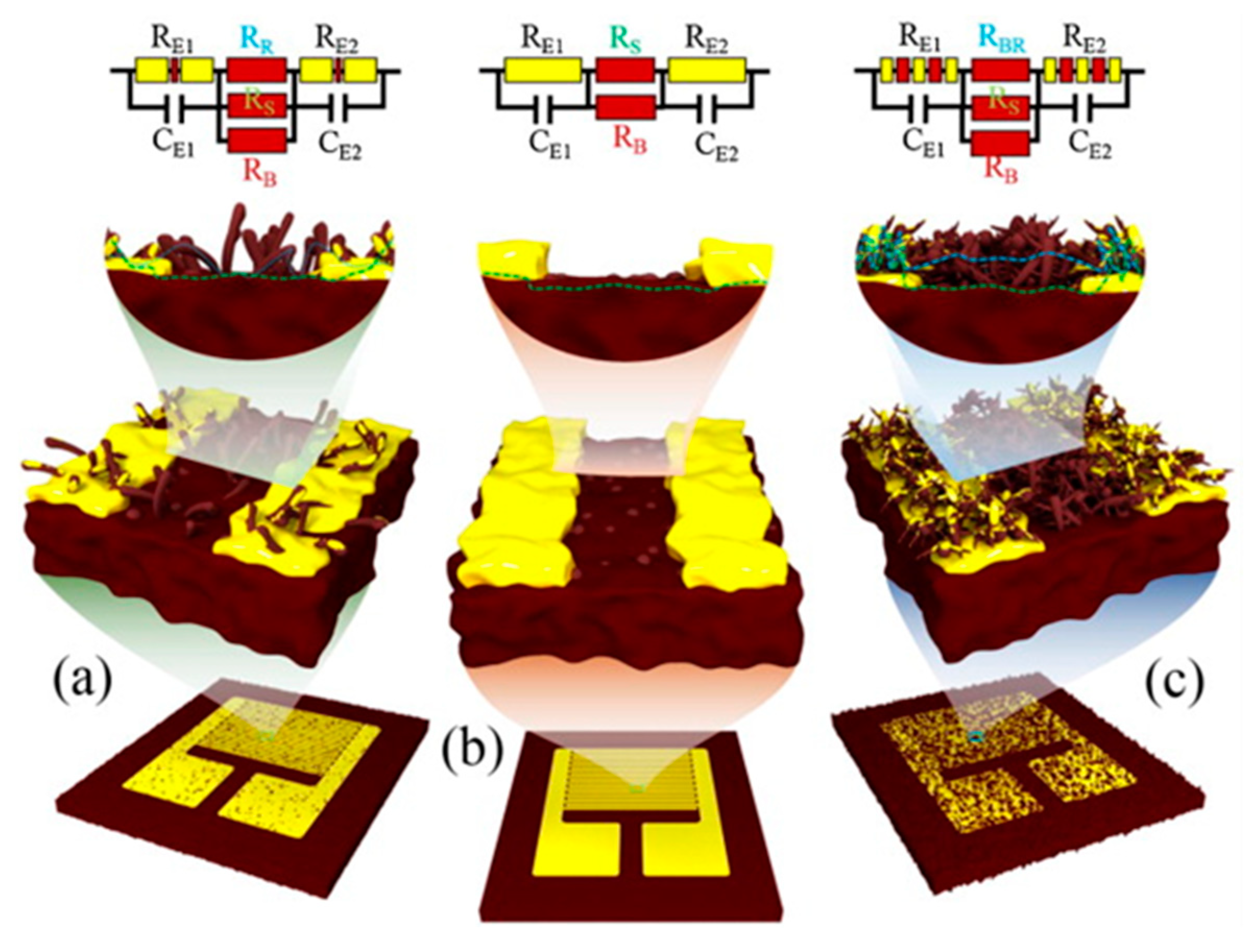
- Firtat, B.; Moldovan, C.; Brasoveanu, C.; Muscalu, G.; Gartner, M.; Zaharescu, M.; Chesler, P.; Hornoiu, C.; Mihaiu, S.; Vladut, C.; et al. Miniaturised MOX based sensors for pollutant and explosive gases detection. Sens. Actuators B Chem. 2017, 249, 647–655. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, S.S.; Kim, H.W. Resistance-based H2S gas sensors using metal oxide nanostructures: A review of recent advances. J. Hazard. Mater. 2018, 357, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Shen, Y.; Zhou, P.; Li, G.; Han, C.; Wei, D.; Zhong, X.; Zhang, Y.; Ao, Y. Influence of Synthesis Conditions on Microstructure and NO2 Sensing Properties of WO3 Porous Films Synthesized by Non-Hydrolytic Sol–Gel Method. Nanomaterials 2019, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Dutt, M.; Ratan, A.; Tomar, M.; Gupta, V.; Singh, V. Mesoporous metal oxide–α-Fe2O3 nanocomposites for sensing formaldehyde and ethanol at room temperature. J. Phys. Chem. Solids 2020, 145, 109536. [Google Scholar] [CrossRef]
- Lu, S.; Hu, W.; Hu, X. CuS2 sheets: A hidden anode material with a high capacity for sodium-ion batteries. J. Mater. Chem. C 2021, 9, 1387–1395. [Google Scholar] [CrossRef]
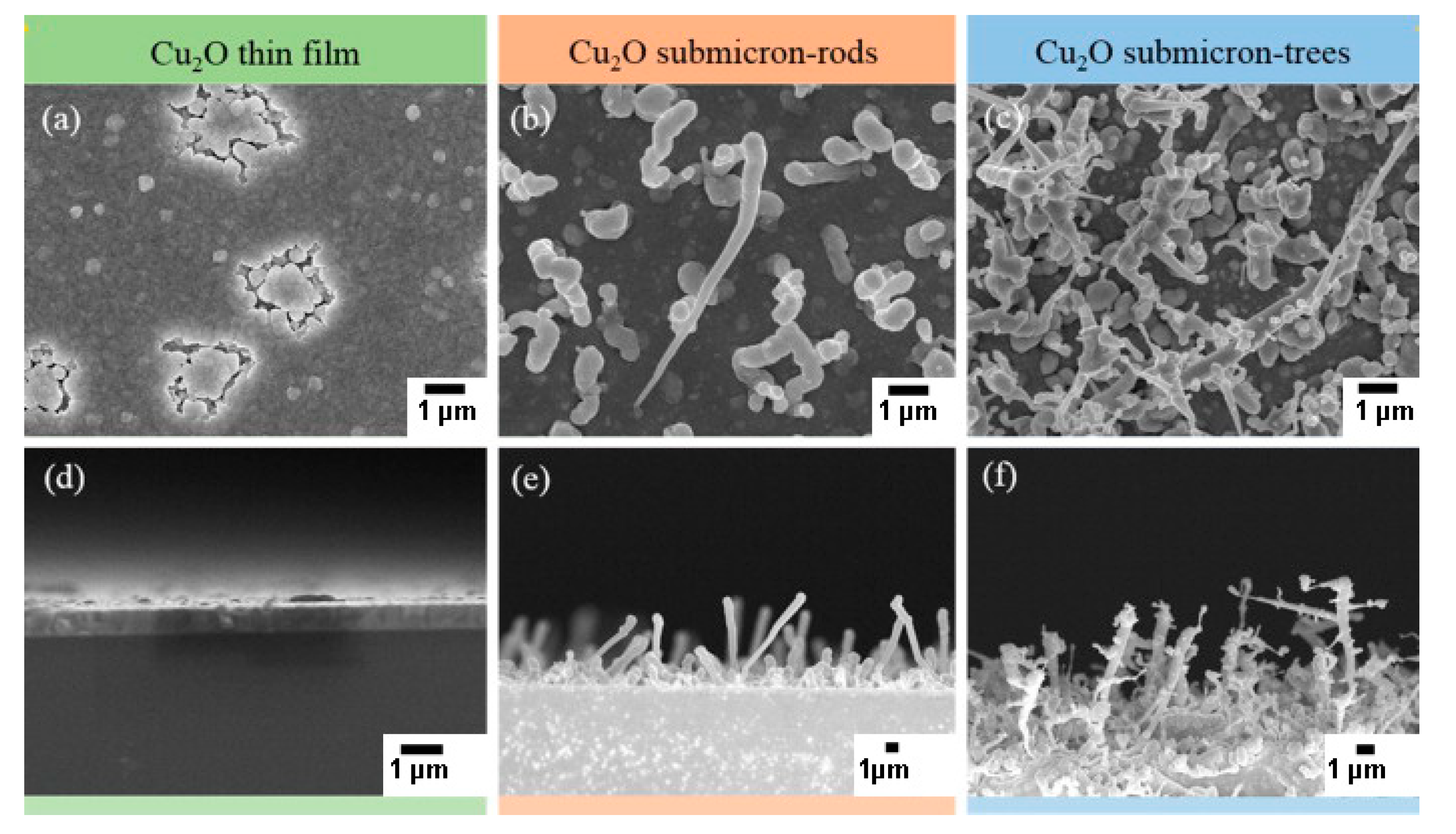
- Hien, V.X.; You, J.-L.; Jo, K.-M.; Kim, S.-Y.; Lee, J.-H.; Kim, J.-J.; Heo, Y.-W. H2S-sensing properties of Cu2O submicron-sized rods and trees synthesized by radio-frequency magnetron sputtering. Sens. Actuators B Chem. 2014, 202, 330–338. [Google Scholar] [CrossRef]
- Comini, E. Metal oxides nanowires chemical/gas sensors: Recent advances. Mater. Today Adv. 2020, 7, 100099. [Google Scholar] [CrossRef]
- Martínez-Ferrero, E.; Franc, G.; Mazères, S.; Turrin, C.-O.; Boissière, C.; Caminade, A.-M.; Majoral, J.-P.; Sanchez, C. Optical Properties of Hybrid Dendritic–Mesoporous Titania Nanocomposite Films. Chem. Eur. J. 2008, 14, 7658–7669. [Google Scholar] [CrossRef]
- Pan, A.; Wu, Y.; Yan, K.; Yu, Y.; Jurow, M.J.; Ren, B.; Zhang, C.; Ding, S.; He, L.; Liu, Y. Stable Luminous Nanocomposites of Confined Mn2+-Doped Lead Halide Perovskite Nanocrystals in Mesoporous Silica Nanospheres as Orange Fluorophores. Inorg. Chem. 2019, 58, 3950–3958. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Li, L.; Nordlund, D.; Chen, H.; Fan, L.; Zhang, B.; Sheng, X.; Daniel, Q.; Sun, L. Dendritic core-shell nickel-iron-copper metal/metal oxide electrode for efficient electrocatalytic water oxidation. Nat. Commun. 2018, 9, 381. [Google Scholar] [CrossRef] [Green Version]
- Delmondo, L.; Salvador, G.P.; Muñoz-Tabares, J.A.; Sacco, A.; Garino, N.; Castellino, M.; Gerosa, M.; Massaglia, G.; Chiodoni, A.; Quaglio, M. Nanostructured MnxOy for oxygen reduction reaction (ORR) catalysts. Appl. Surf. Sci. 2016, 388, 631–639. [Google Scholar] [CrossRef]
- Maitra, S.; Mitra, R.; Nath, T.K. Investigation of electrochemical performance of MgNiO2 prepared by sol-gel synthesis route for aqueous-based supercapacitor application. Curr. Appl. Phys. 2020, 20, 628–637. [Google Scholar] [CrossRef]
- Mohd Abdah, M.A.A.; Azman, N.H.N.; Kulandaivalu, S.; Sulaiman, Y. Review of the use of transition-metal-oxide and conducting polymer-based fibres for high-performance supercapacitors. Mater. Des. 2020, 186, 108199. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, S.; Zhang, Q.; Cao, M.; Wang, Y.; Gu, Y.; Xu, X. Thermoreversible and Self-Protective Sol–Gel Transition Electrolytes for All-Printed Transferable Microsupercapacitors as Safer Micro-Energy Storage Devices. ACS Appl. Mater. Interface 2020, 12, 41819–41831. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.S.; Dubal, D.P.; Deonikar, V.G.; Tamboli, M.S.; Ambekar, J.D.; Gomez-Romero, P.; Kolekar, S.S.; Kale, B.B.; Patil, D.R. Fern-like rGO/BiVO4 Hybrid Nanostructures for High-Energy Symmetric Supercapacitor. ACS Appl. Mater. Interface 2016, 8, 31602–31610. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Duh, J.-G. Aqueous sol–gel synthesized anatase TiO2 nanoplates with high-rate capabilities for lithium-ion and sodium-ion batteries. RSC Adv. 2016, 6, 37160–37166. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, F.; Chen, C.; Shi, L.; Yuan, S.; Sun, L.; Zhu, J. Self-Assembly of PEI/SiO2 on Polyethylene Separators for Li-Ion Batteries with Enhanced Rate Capability. ACS Appl. Mater. Interface 2015, 7, 3314–3322. [Google Scholar] [CrossRef]
- Duan, M.; Meng, Y.; Zhang, H.; Zhao, G.; Hu, J.; Ren, G.; Zhu, F. Facile synthesis of graphene-like carbon-coated Ni3S2 nanoparticles self-assembled on 3D dendritic nanostructure as high-performance anode materials of sodium-ion batteries. Ionics 2020, 26, 4511–4522. [Google Scholar] [CrossRef]
- Wei, T.; Li, Q.; Yang, G.; Wang, C. High-rate and durable aqueous zinc ion battery using dendritic V10O24·12H2O cathode material with large interlamellar spacing. Electrochim. Acta 2018, 287, 60–67. [Google Scholar] [CrossRef]
- Ye, B.; Huang, L.; Hou, Y.; Jiang, R.; Sun, L.; Yu, Z.; Zhang, B.; Huang, Y.; Zhang, Y. Pt (111) quantum dot decorated flower-like αFe2O3 thin film nanosheets as a highly efficient bifunctional electrocatalyst for overall water splitting. J. Mater. Chem. A 2019, 7, 11379–11386. [Google Scholar] [CrossRef]
- Du, X.; Zhao, C.; Luan, Y.; Zhang, C.; Jaroniec, M.; Huang, H.; Zhang, X.; Qiao, S.-Z. Dendritic porous yolk@ordered mesoporous shell structured heterogeneous nanocatalysts with enhanced stability. J. Mater. Chem. A 2017, 5, 21560–21569. [Google Scholar] [CrossRef]
- Xue, X.-L.; Lang, W.-Z.; Yan, X.; Guo, Y.-J. Dispersed Vanadium in Three-Dimensional Dendritic Mesoporous Silica Nanospheres: Active and Stable Catalysts for the Oxidative Dehydrogenation of Propane in the Presence of CO2. ACS Appl. Mater. Interface 2017, 9, 15408–15423. [Google Scholar] [CrossRef]
- Beshkar, F.; Amiri, O.; Salavati-Niasari, M.; Beshkar, F. Novel dendrite-like CuCr2O4 photocatalyst prepared by a simple route in order to remove of Azo Dye in textile and dyeing wastewater. J. Mater. Sci. Mater. Electron. 2015, 26, 8182–8192. [Google Scholar] [CrossRef]
- Yin, Z.; Zheng, H.; Ma, D.; Bao, X. Porous Palladium Nanoflowers that Have Enhanced Methanol Electro-Oxidation Activity. J. Phys. Chem. C 2009, 113, 1001–1005. [Google Scholar] [CrossRef]
- Bannat, I.; Wessels, K.; Oekermann, T.; Rathousky, J.; Bahnemann, D.; Wark, M. Improving the Photocatalytic Performance of Mesoporous Titania Films by Modification with Gold Nanostructures. Chem. Mater. 2009, 21, 1645–1653. [Google Scholar] [CrossRef]
- Maity, A.; Polshettiwar, V. Dendritic Fibrous Nanosilica for Catalysis, Energy Harvesting, Carbon Dioxide Mitigation, Drug Delivery, and Sensing. ChemSusChem 2017, 10, 3866–3913. [Google Scholar] [CrossRef]
- Gai, S.; Yang, P.; Ma, P.A.; Wang, D.; Li, C.; Li, X.; Niu, N.; Lin, J. Fibrous-structured magnetic and mesoporous Fe3O4/silica microspheres: Synthesis and intracellular doxorubicin delivery. J. Mater. Chem. 2011, 21, 16420–16426. [Google Scholar] [CrossRef]
- Fotouhi, A.; Auger, D.J.; Propp, K.; Longo, S.; Wild, M. A review on electric vehicle battery modelling: From Lithium-ion toward Lithium–Sulphur. Renew. Sustain. Energy Rev. 2016, 56, 1008–1021. [Google Scholar] [CrossRef] [Green Version]



| Methods | Advantages | Disadvantages | Scalability | Cost |
|---|---|---|---|---|
| Sol‒gel | mild synthesis conditions, low reaction temperature | long reaction time, organic solvents | high | low |
| Hydro-/solvothermal | short reaction time | anti-corrosive and pressure vessels, organic solvents, and surfactants | high | high |
| Electrochemical | involving less chemicals | energy intensive | relatively low | high |
| Supramolecular self-assembly | mild synthesis conditions, low reaction temperature | organic solvents and surfactants | high | low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sui, R.; Charpentier, P.A.; Marriott, R.A. Metal Oxide-Related Dendritic Structures: Self-Assembly and Applications for Sensor, Catalysis, Energy Conversion and Beyond. Nanomaterials 2021, 11, 1686. https://doi.org/10.3390/nano11071686
Sui R, Charpentier PA, Marriott RA. Metal Oxide-Related Dendritic Structures: Self-Assembly and Applications for Sensor, Catalysis, Energy Conversion and Beyond. Nanomaterials. 2021; 11(7):1686. https://doi.org/10.3390/nano11071686
Chicago/Turabian StyleSui, Ruohong, Paul A. Charpentier, and Robert A. Marriott. 2021. "Metal Oxide-Related Dendritic Structures: Self-Assembly and Applications for Sensor, Catalysis, Energy Conversion and Beyond" Nanomaterials 11, no. 7: 1686. https://doi.org/10.3390/nano11071686
APA StyleSui, R., Charpentier, P. A., & Marriott, R. A. (2021). Metal Oxide-Related Dendritic Structures: Self-Assembly and Applications for Sensor, Catalysis, Energy Conversion and Beyond. Nanomaterials, 11(7), 1686. https://doi.org/10.3390/nano11071686






