Effect of the Nanostructured Zn/Cu Electrocatalyst Morphology on the Electrochemical Reduction of CO2 to Value-Added Chemicals
Abstract
:1. Introduction
2. Experimental Section
2.1. Electrode Preparation (Electrodeposition of Zn/Cu Electrodes)
2.2. Characterization of Electrodes
2.3. Electrochemical Reduction of CO2
3. Results and Discussion
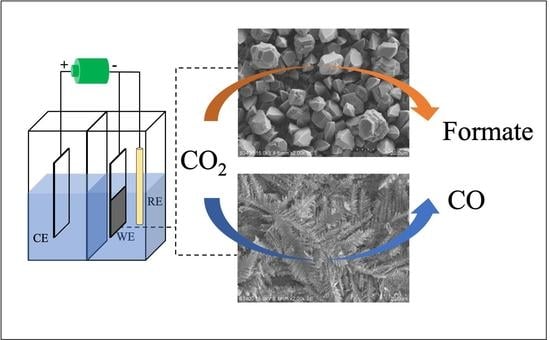
3.1. Electrocatalyst Characterization
3.2. Electrocatalytic Performances in CO2RR
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Choi, J.; Kim, M.J.; Ahn, S.H.; Choi, I.; Jang, J.H.; Ham, Y.S.; Kim, J.J.; Kim, S.-K. Electrochemical CO2 reduction to CO on dendritic Ag–Cu electrocatalysts prepared by electrodeposition. Chem. Eng. J. 2016, 299, 37–44. [Google Scholar] [CrossRef]
- Krause, R.; Reinisch, D.; Reller, C.; Eckert, H.; Hartmann, D.; Taroata, D.; Wiesner-Fleischer, K.; Bulan, A.; Lueken, A.; Schmid, G. Industrial application aspects of the electrochemical reduction of CO2 to CO in aqueous electrolyte. Chem. Ing. Tech. 2020, 92, 53–61. [Google Scholar] [CrossRef]
- Abdinejad, M.; Dao, C.; Deng, B.; Dinic, F.; Voznyy, O.; Zhang, X.-A.; Kraatz, H.-B. Electrocatalytic reduction of CO2 to CH4 and CO in aqueous solution using pyridine-porphyrins immobilized onto carbon nanotubes. ACS Sustain. Chem. Eng. 2020, 8, 9549–9557. [Google Scholar] [CrossRef]
- de Brito, J.F.; Irikura, K.; Terzi, C.M.; Nakagaki, S.; Zanoni, M.V.B. The great performance of TiO2 nanotubes electrodes modified by copper (II) porphyrin in the reduction of carbon dioxide to alcohol. J. CO2 Util. 2020, 41, 101261. [Google Scholar] [CrossRef]
- Weststrate, C.; Van De Loosdrecht, J.; Niemantsverdriet, J. Spectroscopic insights into cobalt-catalyzed Fischer-Tropsch synthesis: A review of the carbon monoxide interaction with single crystalline surfaces of cobalt. J. Catal. 2016, 342, 1–16. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Chu, W.; Fongarland, P. Advances in the development of novel cobalt Fischer−Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels. Chem. Rev. 2007, 107, 1692–1744. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y. Electrochemical CO2 Reduction on Metal Electrodes. In Modern Aspects of Electrochemistry; Vayenas, C.G., White, R.E., Gamboa-Aldeco, M.E., Eds.; Springer: New York, NY, USA, 2008; pp. 89–189. [Google Scholar]
- Guo, W.; Shim, K.; Ngome, F.O.O.; Moon, Y.H.; Choi, S.-Y.; Kim, Y.-T. Highly active coral-like porous silver for electrochemical reduction of CO2 to CO. J. CO2 Util. 2020, 41, 101242. [Google Scholar] [CrossRef]
- Sarno, M.; Ponticorvo, E.; Piotto, S.; Nardiello, A.M.; De Pasquale, S.; Funicello, N. AuAg/ZnO nanocatalyst for CO2 valorization and H2 and CO electrochemical production. J. CO2 Util. 2020, 39, 101179. [Google Scholar] [CrossRef]
- Nguyen, D.L.T.; Jee, M.S.; Won, D.H.; Jung, H.; Oh, H.-S.; Min, B.K.; Hwang, Y.J. Selective CO2 Reduction on Zinc Electrocatalyst: The Effect of Zinc Oxidation State Induced by Pretreatment Environment. ACS Sustain. Chem. Eng. 2017, 5, 11377–11386. [Google Scholar] [CrossRef]
- Jiang, K.; Huang, Y.; Zeng, G.; Toma, F.M.; Goddard, W.A., III; Bell, A.T. Effects of surface roughness on the electrochemical reduction of CO2 over Cu. ACS Energy Lett. 2020, 5, 1206–1214. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.; Zhang, X.; Mu, K.; Ma, X.; Jiao, M.; Wang, Z.; Limpachanangkul, P.; Chalermsinsuwan, B.; Gao, Y.; Li, Y. Recent Progress of Sn-Based Derivative Catalysts for Electrochemical Reduction of CO2. Energy Technol. 2021, 9, 2000799. [Google Scholar] [CrossRef]
- Xiao, J.; Gao, M.-R.; Liu, S.; Luo, J.-L. Hexagonal Zn nanoplates enclosed by Zn (100) and Zn (002) facets for highly selective CO2 electroreduction to CO. ACS Appl. Mater. Interfaces 2020, 12, 31431–31438. [Google Scholar] [CrossRef]
- Quan, F.; Zhong, D.; Song, H.; Jia, F.; Zhang, L. A highly efficient zinc catalyst for selective electroreduction of carbon dioxide in aqueous NaCl solution. J. Mater. Chem. A 2015, 3, 16409–16413. [Google Scholar] [CrossRef]
- Wu, J.; Risalvato, F.G.; Ke, F.-S.; Pellechia, P.; Zhou, X.-D. Electrochemical reduction of carbon dioxide I. Effects of the electrolyte on the selectivity and activity with Sn electrode. J. Electrochem. Soc. 2012, 159, F353. [Google Scholar] [CrossRef]
- Lv, W.; Zhang, R.; Gao, P.; Lei, L. Studies on the faradaic efficiency for electrochemical reduction of carbon dioxide to formate on tin electrode. J. Power Sources 2014, 253, 276–281. [Google Scholar] [CrossRef]
- Hsieh, Y.-C.; Senanayake, S.D.; Zhang, Y.; Xu, W.; Polyansky, D.E. Effect of chloride anions on the synthesis and enhanced catalytic activity of silver nanocoral electrodes for CO2 electroreduction. ACS Catal. 2015, 5, 5349–5356. [Google Scholar] [CrossRef]
- Jiang, X.; Cai, F.; Gao, D.; Dong, J.; Miao, S.; Wang, G.; Bao, X. Electrocatalytic reduction of carbon dioxide over reduced nanoporous zinc oxide. Electrochem. Commun. 2016, 68, 67–70. [Google Scholar] [CrossRef]
- Rosen, J.; Hutchings, G.S.; Lu, Q.; Forest, R.V.; Moore, A.; Jiao, F. Electrodeposited Zn Dendrites with Enhanced CO Selectivity for Electrocatalytic CO2 Reduction. ACS Catal. 2015, 5, 4586–4591. [Google Scholar] [CrossRef]
- Won da, H.; Shin, H.; Koh, J.; Chung, J.; Lee, H.S.; Kim, H.; Woo, S.I. Highly Efficient, Selective, and Stable CO2 Electroreduction on a Hexagonal Zn Catalyst. Angew. Chem. Int. Ed. Engl. 2016, 55, 9297–9300. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Garcia, P.; Schlegel, N.; Zanetti, A.; Cedeno Lopez, A.; Galvez-Vazquez, M.J.; Dutta, A.; Rahaman, M.; Broekmann, P. Selective Electrochemical Reduction of CO2 to CO on Zn-Based Foams Produced by Cu(2+) and Template-Assisted Electrodeposition. ACS Appl. Mater. Interfaces 2018, 10, 31355–31365. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Q.; Qin, X.; Gu, M.; Tao, R.; Lee, B.P.; Zhang, L.; Yao, Y.; Li, T.; Shao, M. Tuning structural and compositional effects in Pd–Au nanowires for highly selective and active CO2 electrochemical reduction reaction. Adv. Energy Mater. 2018, 8, 1802238. [Google Scholar] [CrossRef]
- Kim, J.-H.; Woo, H.; Yun, S.-W.; Jung, H.-W.; Back, S.; Jung, Y.; Kim, Y.-T. Highly active and selective Au thin layer on Cu polycrystalline surface prepared by galvanic displacement for the electrochemical reduction of CO2 to CO. Appl. Catal. B Environ. 2017, 213, 211–215. [Google Scholar] [CrossRef]
- Guo, W.; Shim, K.; Kim, Y.-T. Ag layer deposited on Zn by physical vapor deposition with enhanced CO selectivity for electrochemical CO2 reduction. Appl. Surf. Sci. 2020, 526, 146651. [Google Scholar] [CrossRef]
- Keerthiga, G.; Chetty, R. Electrochemical Reduction of Carbon Dioxide on Zinc-Modified Copper Electrodes. J. Electrochem. Soc. 2017, 164, H164–H169. [Google Scholar] [CrossRef]
- Clark, D.; Wood, D.; Erb, U. Industrial applications of electrodeposited nanocrystals. Nanostruct. Mater. 1997, 9, 755–758. [Google Scholar] [CrossRef]
- Ren, D.; Ang, B.S.-H.; Yeo, B.S. Tuning the selectivity of carbon dioxide electroreduction toward ethanol on oxide-derived Cu x Zn catalysts. ACS Catal. 2016, 6, 8239–8247. [Google Scholar] [CrossRef]
- Yoo, J.S.; Christensen, R.; Vegge, T.; Norskov, J.K.; Studt, F. Theoretical Insight into the Trends that Guide the Electrochemical Reduction of Carbon Dioxide to Formic Acid. ChemSusChem 2016, 9, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, K.P.; Hatsukade, T.; Cave, E.R.; Abram, D.N.; Kibsgaard, J.; Jaramillo, T.F. Electrocatalytic conversion of carbon dioxide to methane and methanol on transition metal surfaces. J. Am. Chem. Soc. 2014, 136, 14107–14113. [Google Scholar] [CrossRef] [PubMed]
- Low, Q.H.; Loo, N.W.X.; Calle-Vallejo, F.; Yeo, B.S. Enhanced Electroreduction of Carbon Dioxide to Methanol Using Zinc Dendrites Pulse-Deposited on Silver Foam. Angew. Chem. Int. Ed. 2019, 58, 2256–2260. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Meng, X.; Li, P.; Chang, K.; Zhou, W.; Wang, X.; Zhang, X.; Jevasuwan, W.; Fukata, N.; Wang, D. Cation vacancy-initiated CO2 photoreduction over ZnS for efficient formate production. ACS Energy Lett. 2019, 4, 1387–1393. [Google Scholar] [CrossRef]
- Ren, D.; Wong, N.T.; Handoko, A.D.; Huang, Y.; Yeo, B.S. Mechanistic Insights into the Enhanced Activity and Stability of Agglomerated Cu Nanocrystals for the Electrochemical Reduction of Carbon Dioxide to n-Propanol. J. Phys. Chem. Lett. 2016, 7, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, K.P.; Cave, E.R.; Abram, D.N.; Jaramillo, T.F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 2012, 5, 7050–7059. [Google Scholar] [CrossRef]
- Hatsukade, T.; Kuhl, K.P.; Cave, E.R.; Abram, D.N.; Jaramillo, T.F. Insights into the electrocatalytic reduction of CO2 on metallic silver surfaces. Phys. Chem. Chem. Phys. 2014, 16, 13814–13819. [Google Scholar] [CrossRef] [PubMed]









| Electrocatalyst | Percentage by Weight | |
|---|---|---|
| Zn (%) | Cu (%) | |
| Zn/Cu–Na60 | 74.9 | 25.1 |
| Zn/Cu–Na200 | 92.9 | 7.1 |
| ZnCu/Cu–Na60 | 72.5 | 27.5 |
| ZnCu/Cu–Na200 | 93.1 | 6.9 |
| Zn/Cu–H60 | 96.0 | 4.0 |
| Zn/Cu–H200 | 98.1 | 1.9 |
| ZnCu/Cu–H60 | 86.1 | 13.9 |
| ZnCu/Cu–H200 | 88.8 | 11.2 |
| Electrocatalyst | Production Rate (μmol/min) | CO/H2 Production Rate Ratio | Average Electrochemical Charge Passing per Minute (C/min) | |||
|---|---|---|---|---|---|---|
| CO | H2 | Formate | n-Propanol | |||
| Zn foil | 0.87 | 0.21 | 0.07 | 0.04 | 4.1 | 0.15 |
| Cu foil | 0.96 | 2.21 | 0.48 | 0.15 | 0.4 | 0.45 |
| Zn/Cu–Na60 | 2.93 | 0.92 | 0.13 | 0.06 | 3.2 | 0.39 |
| Zn/Cu–Na200 | 3.61 | 0.77 | 0.09 | 0.06 | 4.7 | 0.42 |
| ZnCu/Cu–Na60 | 3.28 | 0.93 | 0.05 | 0.04 | 3.5 | 0.40 |
| ZnCu/Cu–Na200 | 4.72 | 1.29 | 0.06 | 0.03 | 3.7 | 0.56 |
| Zn/Cu–H60 | 0.65 | 0.45 | 0.36 | - | 1.5 | 0.13 |
| Zn/Cu–H200 | 1.01 | 0.54 | 0.36 | - | 1.8 | 0.21 |
| ZnCu/Cu–H60 | 3.21 | 0.89 | 0.13 | 0.02 | 3.6 | 0.41 |
| ZnCu/Cu–H200 | 4.14 | 1.63 | 0.16 | 0.02 | 2.5 | 0.63 |
| Zn Electrocatalyst | Electrolyte | Potential (vs. Ag/AgCl) | CO Faradaic Efficiency (%) | Ref. |
|---|---|---|---|---|
| Zn/Cu–Na200 | 0.1 M KHCO3 | −1.60 | 83.2 | This work |
| ZnCu/Cu–Na200 | 0.1 M KHCO3 | −1.60 | 81.5 | This work |
| Zn dendrite | 0.5 M NaHCO3 | −1.72 | 79 | [33] |
| Reduced nanoporous ZnO | 0.25 M K2SO4 | −1.66 | 92 | [18] |
| Dendrite PD–Zn/Ag foam | 0.1 M KHCO3 | −1.80 | 76.4 | [30] |
| Nano–Zn | 0.5 M NaHCO3 | −1.47 | 57 | [14] |
| Entry | Potential (V) vs. Ag/AgCl | Production Rate (μmol/min) | CO/H2 Production Rate Ratio | |||
|---|---|---|---|---|---|---|
| CO | H2 | Formate | n-Propanol | |||
| 1 | −1.4 | 1.08 | 0.48 | - | 0.02 | 2.3 |
| 2 | −1.6 | 3.61 | 0.77 | 0.09 | 0.06 | 4.7 |
| 3 | −1.8 | 6.98 | 2.95 | 0.10 | 0.06 | 2.4 |
| 4 | −2.0 | 7.97 | 10.18 | 0.19 | 0.06 | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinthong, P.; Klongklaew, P.; Praserthdam, P.; Panpranot, J. Effect of the Nanostructured Zn/Cu Electrocatalyst Morphology on the Electrochemical Reduction of CO2 to Value-Added Chemicals. Nanomaterials 2021, 11, 1671. https://doi.org/10.3390/nano11071671
Pinthong P, Klongklaew P, Praserthdam P, Panpranot J. Effect of the Nanostructured Zn/Cu Electrocatalyst Morphology on the Electrochemical Reduction of CO2 to Value-Added Chemicals. Nanomaterials. 2021; 11(7):1671. https://doi.org/10.3390/nano11071671
Chicago/Turabian StylePinthong, Piriya, Phongsathon Klongklaew, Piyasan Praserthdam, and Joongjai Panpranot. 2021. "Effect of the Nanostructured Zn/Cu Electrocatalyst Morphology on the Electrochemical Reduction of CO2 to Value-Added Chemicals" Nanomaterials 11, no. 7: 1671. https://doi.org/10.3390/nano11071671
APA StylePinthong, P., Klongklaew, P., Praserthdam, P., & Panpranot, J. (2021). Effect of the Nanostructured Zn/Cu Electrocatalyst Morphology on the Electrochemical Reduction of CO2 to Value-Added Chemicals. Nanomaterials, 11(7), 1671. https://doi.org/10.3390/nano11071671