Using Graphene-Based Composite Materials to Boost Anti-Corrosion and Infrared-Stealth Performance of Epoxy Coatings
Abstract
1. Introduction
2. Experimental Details
2.1. Graphene Nanosheet (GN) Fabrication
2.2. Preparation of Composite Coatings
2.3. Characterization
3. Results and Discussion
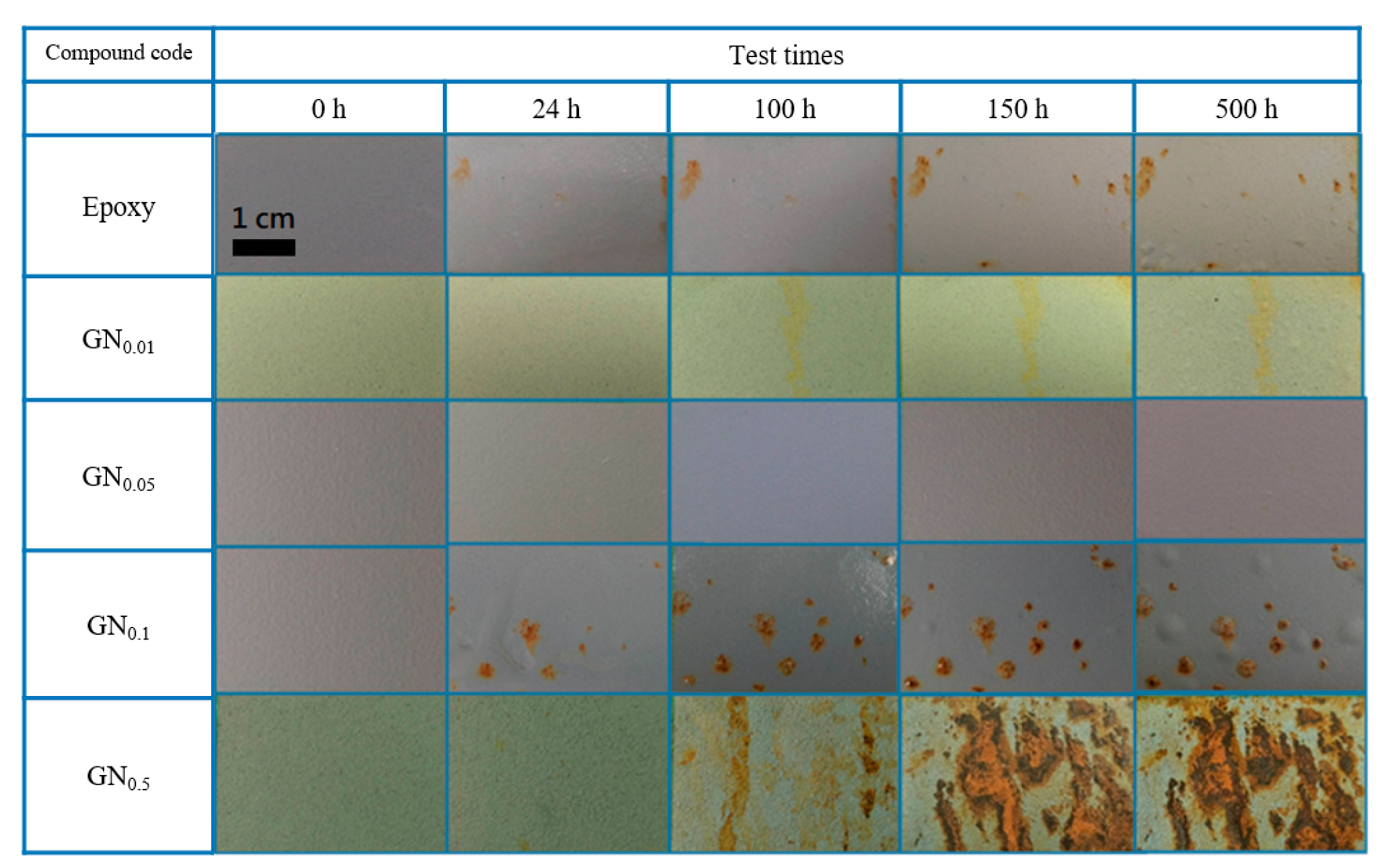
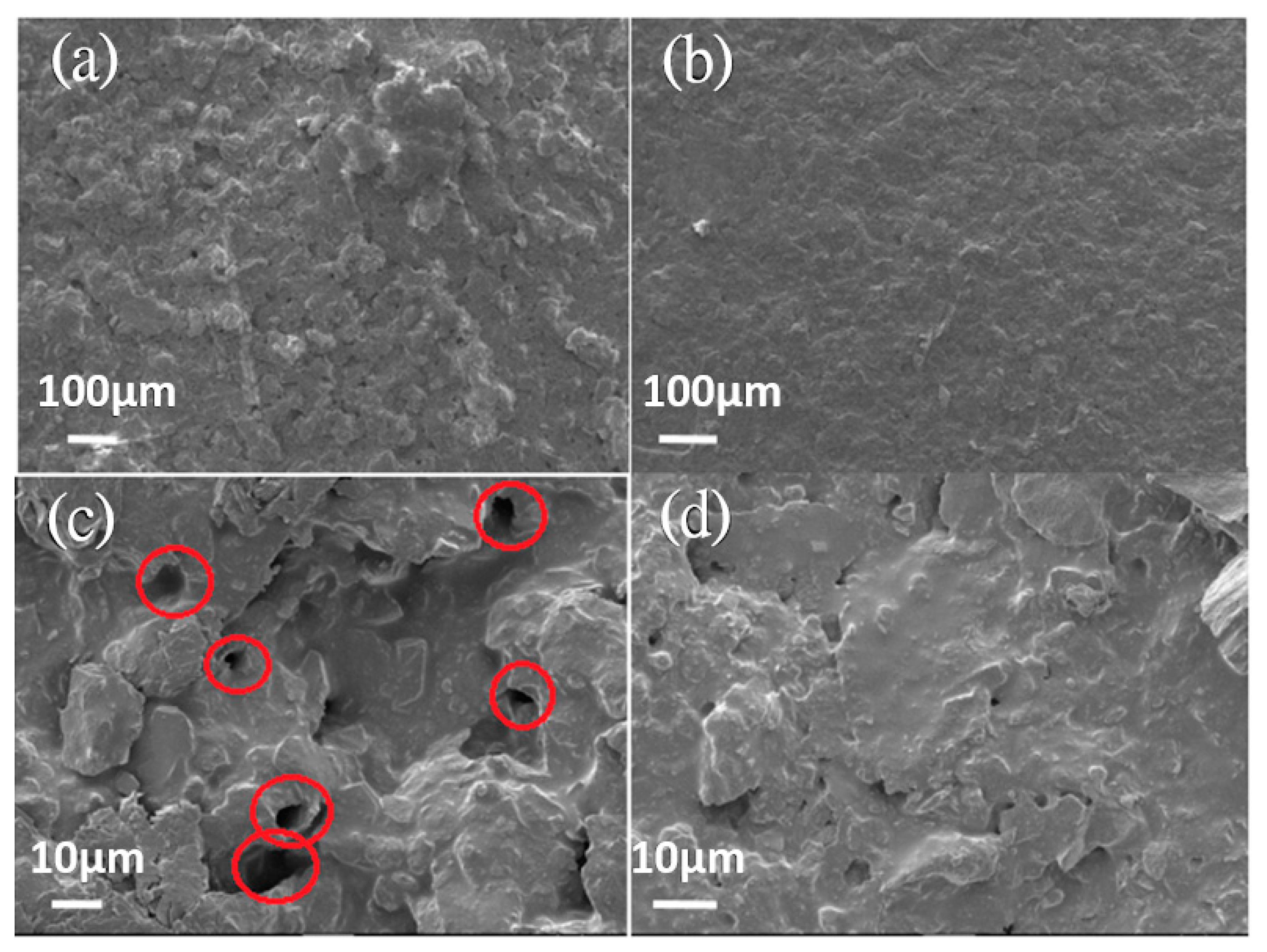
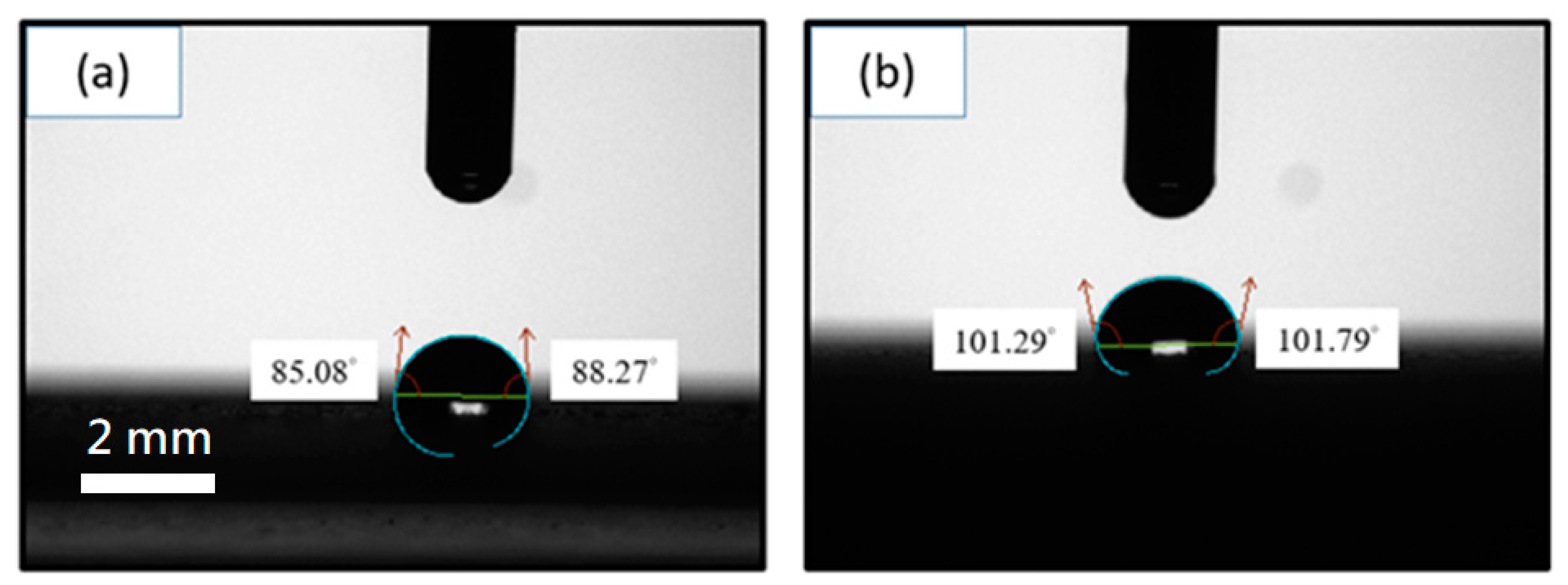
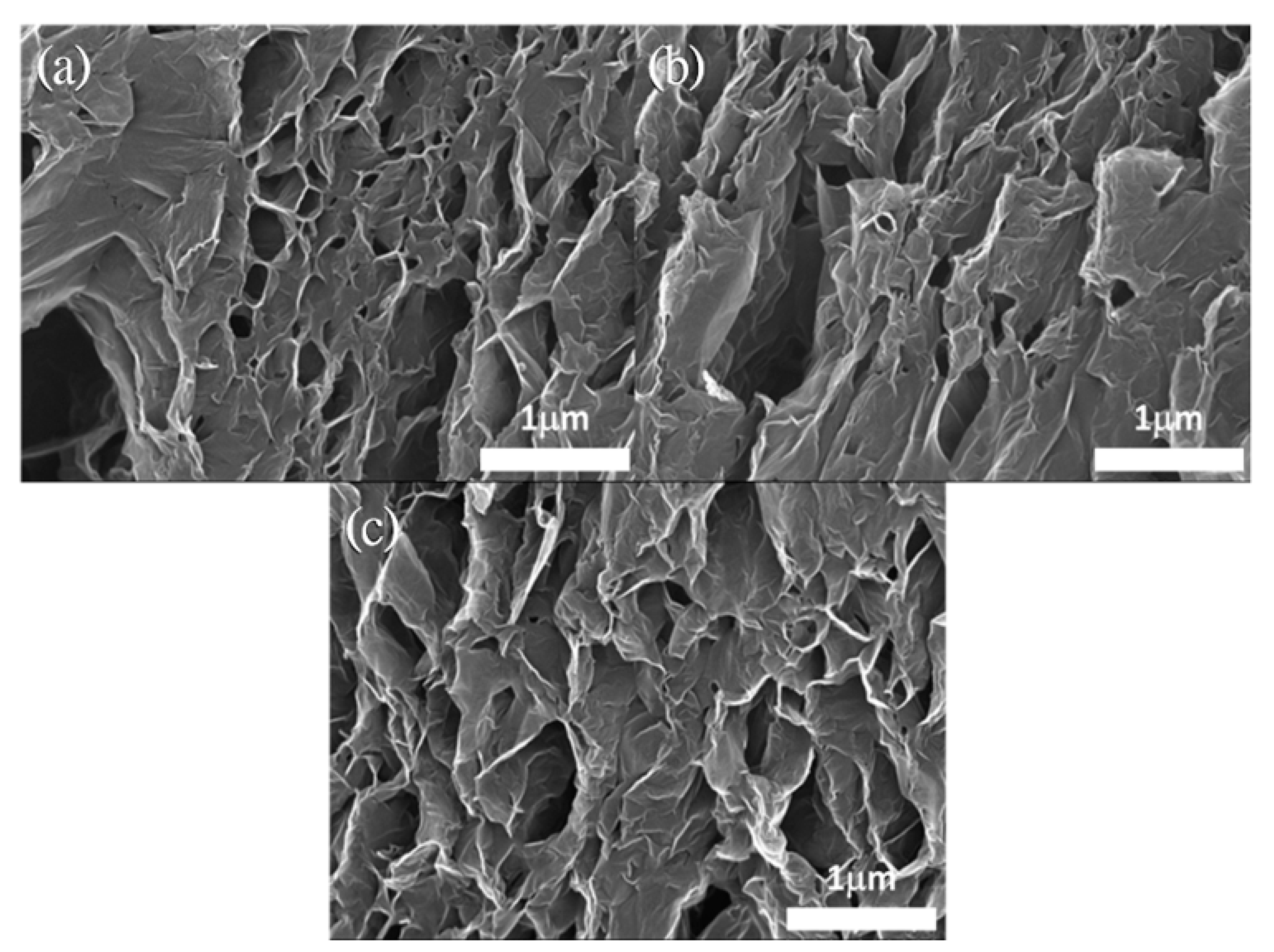
3.1. Corrosion Resistance of GN-Filled Composite Coatings
3.2. Anti-Corrosion and IR-Camouflage Performances of Composite Coatings with Al-GN Fillers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dhoke, S.K.; Khanna, A.S.; Sinha, T.J.M. Effect of nano-ZnO particles on the corrosion behavior of alkyd-based waterborne coatings. Prog. Org. Coat. 2009, 64, 371–382. [Google Scholar] [CrossRef]
- Nematollahi, M.; Heidarian, M.; Peikari, M.; Kassiriha, S.M. Comparison between the effect of nanoglass flake and montmorillonite organoclay on corrosion performance of epoxy coating. Corros. Sci. 2010, 52, 1809–1817. [Google Scholar] [CrossRef]
- Golru, S.S.; Attar, M.M.; Ramezanzadeh, B. Studying the influence of nano-Al2O3 particles on the corrosion performance and hydrolytic degradation resistance of an epoxy/polyamide coating on AA-1050. Prog. Org. Coat. 2014, 77, 1391–1399. [Google Scholar] [CrossRef]
- Sari, M.G.; Ramezanzadeh, B.; Shahbazi, M.; Pakdel, A.S. Influence of nanoclay particles modification by polyester-amide hyperbranched polymer on the corrosion protective performance of the epoxy nanocomposite. Corros. Sci. 2015, 92, 162–172. [Google Scholar] [CrossRef]
- Matin, E.; Attar, M.M.; Ramezanzadeh, B. Investigation of corrosion protection properties of an epoxy nanocomposite loaded with polysiloxane surface modified nanosilica particles on the steel substrate. Prog. Org. Coat. 2015, 78, 395–403. [Google Scholar] [CrossRef]
- Chang, C.H.; Huang, T.C.; Peng, C.W.; Yeh, T.C.; Lu, H.I.; Hung, W.I.; Weng, C.J.; Yang, T.I.; Yeh, J.M. Novel anticorrosion coatings prepared from polyaniline/graphene composites. Carbon 2012, 50, 5044–5051. [Google Scholar] [CrossRef]
- Li, J.; Cui, J.; Yang, J.; Ma, Y.; Qiu, H.; Yang, J. Silanized graphene oxide reinforced organofunctional silane composite coatings for corrosion protection. Prog. Org. Coat. 2016, 99, 443–451. [Google Scholar] [CrossRef]
- Yu, Y.H.; Lin, Y.Y.; Lin, C.H.; Chana, C.C.; Huang, Y.C. High-performance polystyrene/graphene-based nanocomposites with excellent anti-corrosion properties. Polym. Chem. 2014, 5, 535–550. [Google Scholar] [CrossRef]
- Chang, K.C.; Ji, W.F.; Lai, M.C.; Hsiao, Y.R.; Hsu, C.H.; Chuang, T.L.; Wei, H.; Yeh, J.M.; Liu, W.R. Synergistic effects of hydrophobicity and gas barrier properties on the anticorrosion property of PMMA nanocomposite coatings embedded with graphene nanosheets. Polym. Chem. 2014, 5, 1049–1056. [Google Scholar] [CrossRef]
- Jiang, F.; Zhao, W.; Wu, Y.; Dong, J.; Zhou, K.; Lu, G.; Pu, J. Anti-corrosion behaviors of epoxy composite coatings enhanced via graphene oxide with different aspect ratios. Prog. Org. Coat. 2019, 127, 70–79. [Google Scholar] [CrossRef]
- Liu, S.; Gu, L.; Zhao, H.; Chen, J.; Yu, H. Corrosion resistance of graphene-reinforced waterborne epoxy coatings. J. Mater. Sci. Technol. 2016, 32, 425–431. [Google Scholar] [CrossRef]
- Tsai, P.Y.; Chen, T.E.; Lee, Y.L. Development and characterization of anticorrosion and antifriction properties for high performance polyurethane/graphene composite coatings. Coatings 2018, 8, 250. [Google Scholar] [CrossRef]
- Bunch, J.S.; Verbridge, S.S.; Alden, J.S.; van der Zande, A.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Impermeable atomic membranes from graphene sheets. Nano Lett. 2008, 8, 2458–2462. [Google Scholar] [CrossRef]
- Berry, V. Impermeability of graphene and its applications. Carbon 2013, 62, 1–10. [Google Scholar] [CrossRef]
- Miao, M.; Nardelli, M.B.; Wang, Q.; Liu, Y. First principles study of the permeability of graphene to hydrogen atoms. Phys. Chem. Chem. Phys. 2013, 15, 16132–16137. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.Z.; Yang, Q.; Kuang, W.J.; Stebunov, Y.V.; Xiong, W.Q.; Yu, J.; Nair, R.R.; Katsnelson, M.I.; Yuan, S.J.; Grigorieva, I.V.; et al. Limits on gas impermeability of graphene. Nature 2020, 579, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Gao, L.; Tian, J.; Cao, H.; Wu, W.; Yu, Q. Atomic-scale investigation of graphene grown on Cu foil and the effects of thermal annealing. ACS Nano 2011, 5, 3607–3613. [Google Scholar] [CrossRef]
- Chen, S.; Brown, L.; Levendorf, M.; Cai, W.; Ju, S.Y.; Edgeworth, J. Oxidation resistance of graphene-coated Cu and Cu/Ni alloy. ACS Nano 2011, 5, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K. Graphene: Corrosion-inhibiting coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Gadhamshetty, V.; Mukherjee, R.; Chen, Z. Passivation of microbial corrosion using a graphene coating. Carbon 2013, 56, 45–49. [Google Scholar] [CrossRef]
- Pu, N.W.; Shi, G.N.; Liu, Y.M.; Sun, X.; Chang, J.K.; Sun, C.L.; Ger, M.D.; Chen, C.Y.; Wang, P.C.; Peng, Y.Y.; et al. Graphene Grown on Stainless Steel as a High-Performance and Ecofriendly Anti-corrosion Coating for bipolar plates. J. Power Sources 2015, 282, 248–256. [Google Scholar] [CrossRef]
- Yu, Z.; Di, H.; Ma, Y.; He, Y.; Liang, L.; Lv, L.; Ran, X.; Pan, Y.; Luo, Z. Preparation of graphene oxide modified by titanium dioxide to enhance the anti-corrosion performance of epoxy coatings. Surf. Coat. Technol. 2015, 276, 471–478. [Google Scholar] [CrossRef]
- Yu, Z.; Di, H.; Ma, Y.; Lv, L.; Pan, Y.; Zhang, C.; He, Y. Fabrication of graphene oxide-alumina hybrids to reinforce the anticorrosion performance of composite epoxy coatings. Appl. Surf. Sci. 2015, 351, 986–996. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, S.; Zhao, Z.; Zhou, Y.; Li, W. Preparation and corrosion protection of VB2 modified trimer aniline-reduced graphene oxide (VTA-rGO) coatings. Prog. Org. Coat. 2019, 132, 95–99. [Google Scholar] [CrossRef]
- Zheng, H.; Guo, M.; Shao, Y.; Wang, Y.; Liu, B.; Meng, G. Graphene oxide–poly(urea–formaldehyde) composites for corrosion protection of mild steel. Corros. Sci. 2018, 139, 1–12. [Google Scholar] [CrossRef]
- Wang, F.; Mao, J. Nacre-like graphene oxide/waterborne styrene butadiene rubber composite and its reusable anti-corrosion behavior on Al-2024. Prog. Org. Coat. 2019, 132, 191–200. [Google Scholar] [CrossRef]
- Vaezi, S.P.M.R.; Rashidi, A.; Bagherzadeh, M.R. Exploring corrosion protection properties of solvent based epoxy-graphene oxide nanocomposite coatings on mild steel. Corros. Sci. 2017, 115, 78–92. [Google Scholar]
- Qiu, S.; Li, W.; Zheng, W.; Zhao, H.; Wang, L. Synergistic effect of polypyrrole-intercalated graphene for enhanced corrosion protection of aqueous coating in 3.5% NaCl solution. ACS Appl. Mater. Interfaces 2017, 9, 34294–34304. [Google Scholar] [CrossRef]
- Yan, X.; Wang, L.; Qian, X. Preparation and characterization of low infrared emissive aluminum/waterborne acrylic coatings. Coatings 2020, 10, 35. [Google Scholar] [CrossRef]
- Sabato, A.G.; Molin, S.; Javed, H.; Zanchi, E.; Boccaccini, A.R.; Smeacetto, F. In-situ Cu-doped MnCo-spinel coatings for solid oxide cell interconnects processed by electrophoretic deposition. Ceram. Int. 2019, 25, 19148–19157. [Google Scholar] [CrossRef]
- Liu, Y.F.; Xie, J.L.; Luo, M.; Jian, S.; Peng, B.; Deng, L.J. The synthesis and characterization of Al/Co3O4 magnetic composite pigments with low infrared emissivity and low lightness. Infrared Phys. Technol. 2017, 83, 88–93. [Google Scholar] [CrossRef]
- Maile, F.J.; Pfaff, G.; Reynders, P. Effect pigments—past, present and future. Prog. Org. Coat. 2005, 54, 150–163. [Google Scholar] [CrossRef]
- Gunde, M.K.; Kunaver, M. Infrared reflection–absorption spectra of metal-effect coatings. Appl. Spectrosc. 2003, 57, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Schniepp, H.C.; Li, J.L.; McAllister, M.J.; Sai, H.; Herrera-Alonso, M.; Adamson, D.H.; Prud’homme, R.K.; Car, R.; Saville, D.A.; Aksay, I.A. Functionalized Single Graphene Sheets Derived from Splitting Graphite Oxide. J. Phys. Chem. B 2006, 110, 8535–8559. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Pu, N.W.; Wu, P.J.; Peng, Y.Y.; Shih, C.N.; Chen, C.Y.; Liu, Y.M.; Ger, M.D. Performance improvement of lithium ion batteries using magnetite–graphene nanocomposite anode materials synthesized by a microwave-assisted method. Microelectron. Eng. 2015, 138, 47–51. [Google Scholar] [CrossRef]
- Shi, J.N.; Ger, M.D.; Liu, Y.M.; Fan, Y.C.; Wen, N.T.; Lin, C.K.; Pu, N.W. Improving the thermal conductivity and shape-stabilization of phase change materials using nanographite additives. Carbon 2013, 51, 365–372. [Google Scholar] [CrossRef]
- Chen, C.Y.; Pu, N.W.; Liu, Y.M.; Huang, S.Y.; Wu, C.H.; Ger, M.D.; Gong, Y.J.; Chou, Y.C. Remarkable microwave absorption performance of graphene at a very low loading ratio. Compos. Part B-Eng. 2017, 114, 395–403. [Google Scholar] [CrossRef]
- Chen, C.Y.; Pu, N.W.; Liu, Y.M.; Chen, L.H.; Wu, C.H.; Cheng, T.Y.; Lin, M.H.; Ger, M.D.; Gong, Y.J.; Peng, Y.Y.; et al. Microwave absorption properties of holey graphene/silicone rubber composites. Compos. Part B-Eng. 2018, 135, 119–128. [Google Scholar] [CrossRef]
- Peng, Y.Y.; Liu, Y.M.; Chang, J.K.; Wu, C.H.; Ger, M.D.; Pu, N.W.; Chang, C.L. A facile approach to produce holey graphene and its application in supercapacitors. Carbon 2015, 81, 347–356. [Google Scholar] [CrossRef]
- Sun, C.L.; Su, J.S.; Tang, J.H.; Lin, M.C.; Wu, J.J.; Pu, N.W.; Shi, G.N.; Ger, M.D. Investigation of the adsorption of size-selected Pt colloidal nanoparticles on high-surface-area graphene powders for methanol oxidation reaction. J. Taiwan Inst. Chem. Eng. 2014, 45, 1025–1030. [Google Scholar] [CrossRef]
- Conradi, M.; Kocijan, A.; Kek-Merl, D.; Zorko, M. Mechanical and anticorrosion properties of nanosilica-filled epoxy-resin composite coatings. Prog. Org. Coat. 2014, 292, 432–437. [Google Scholar] [CrossRef]
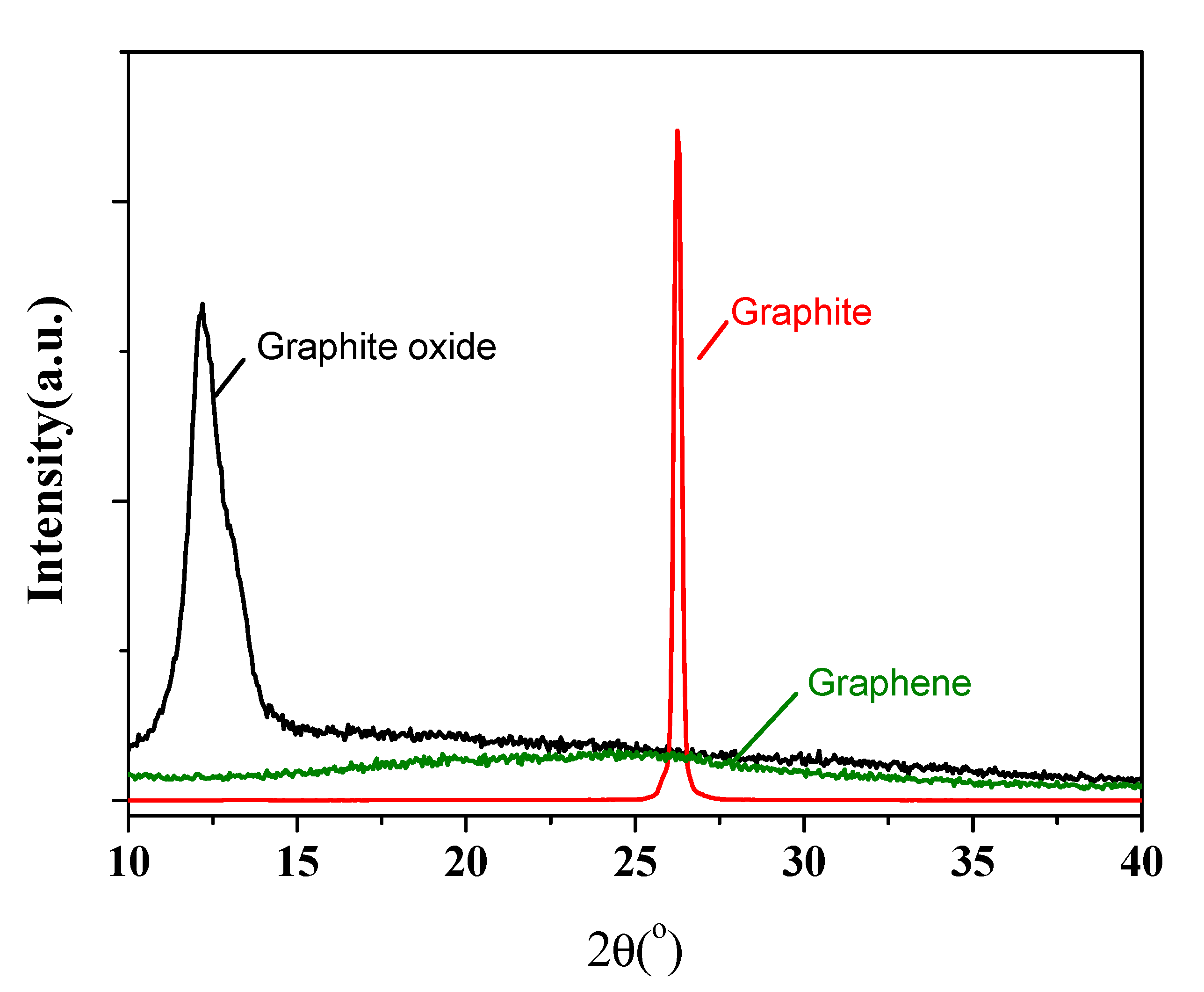
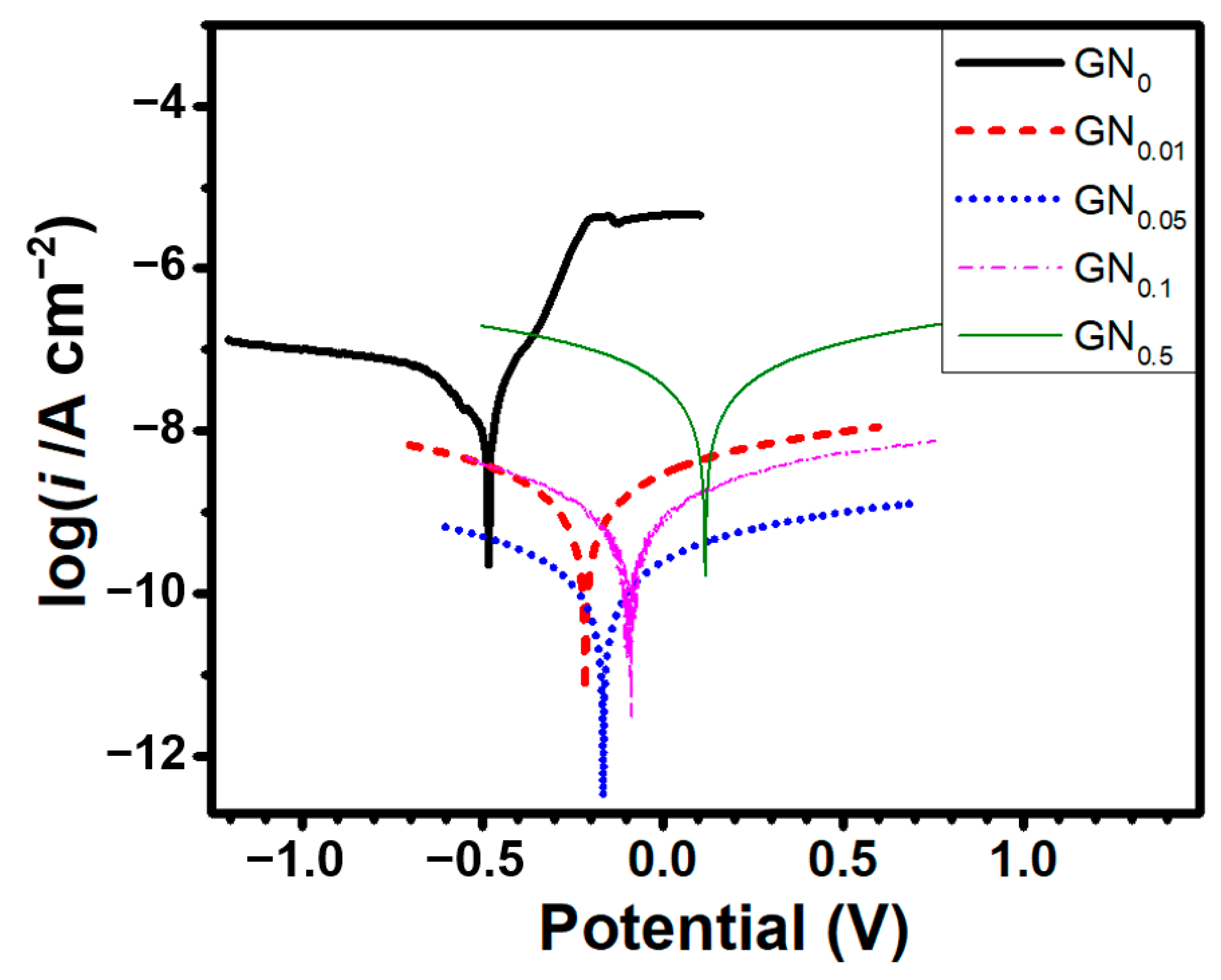
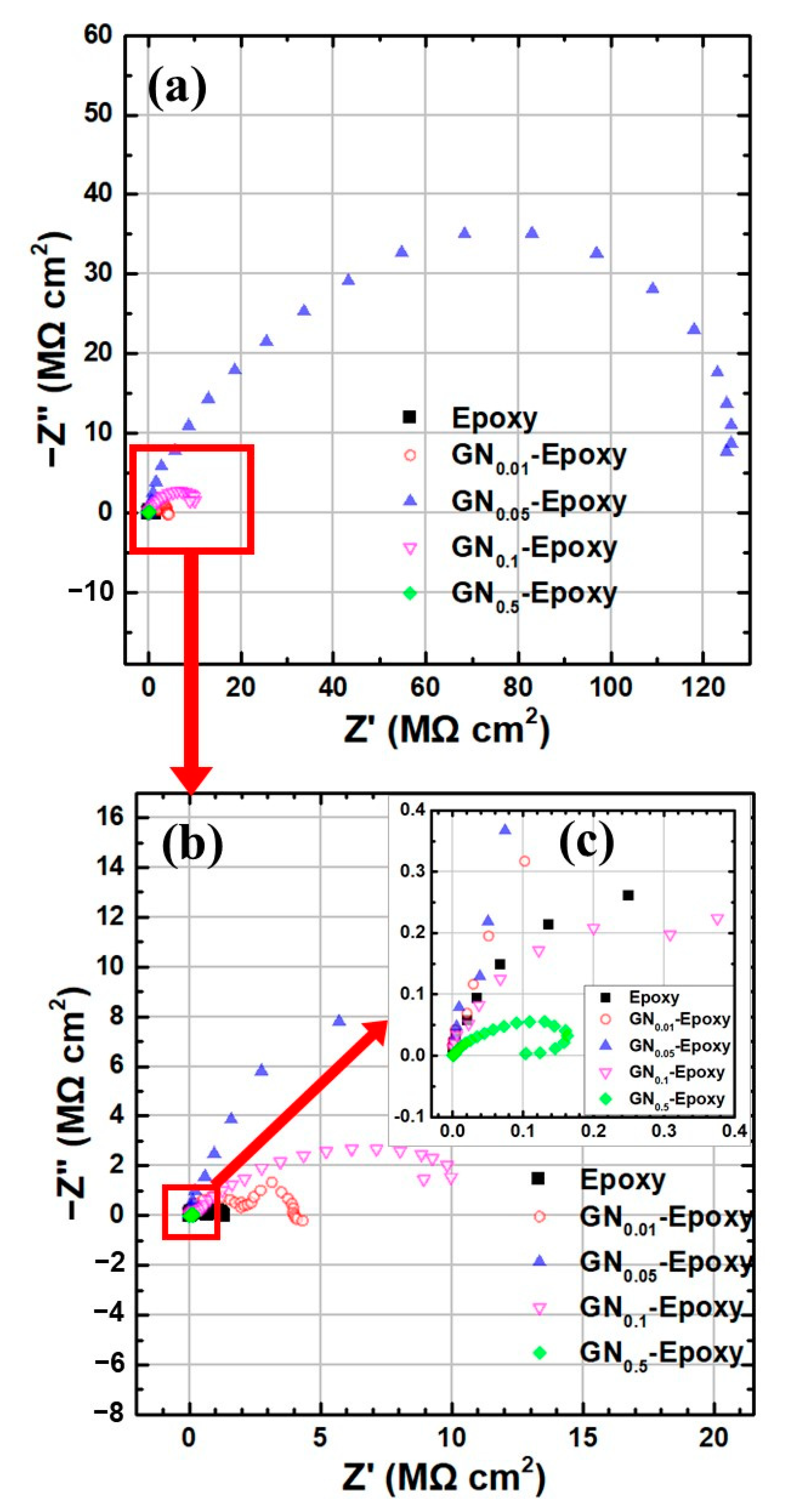
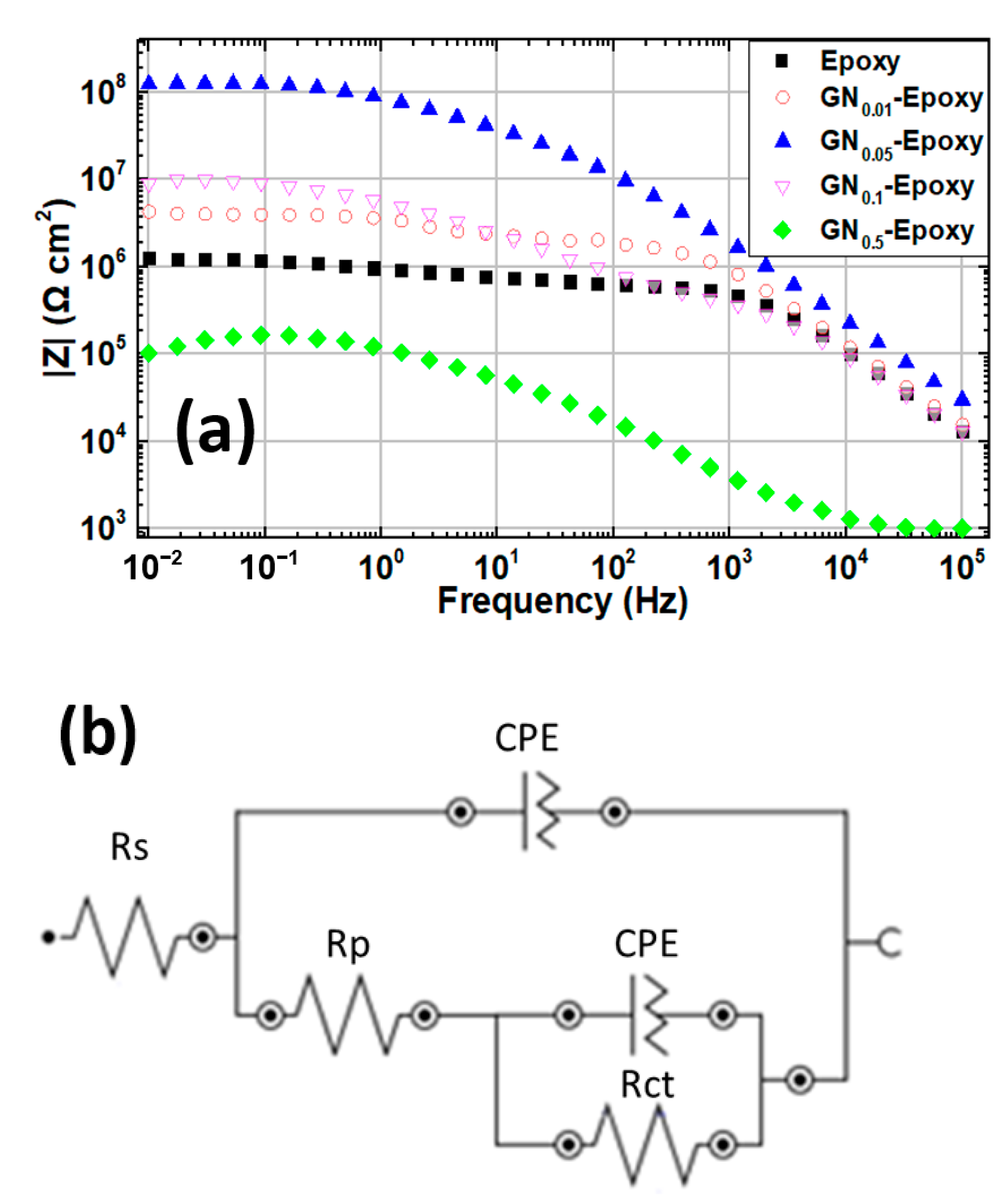
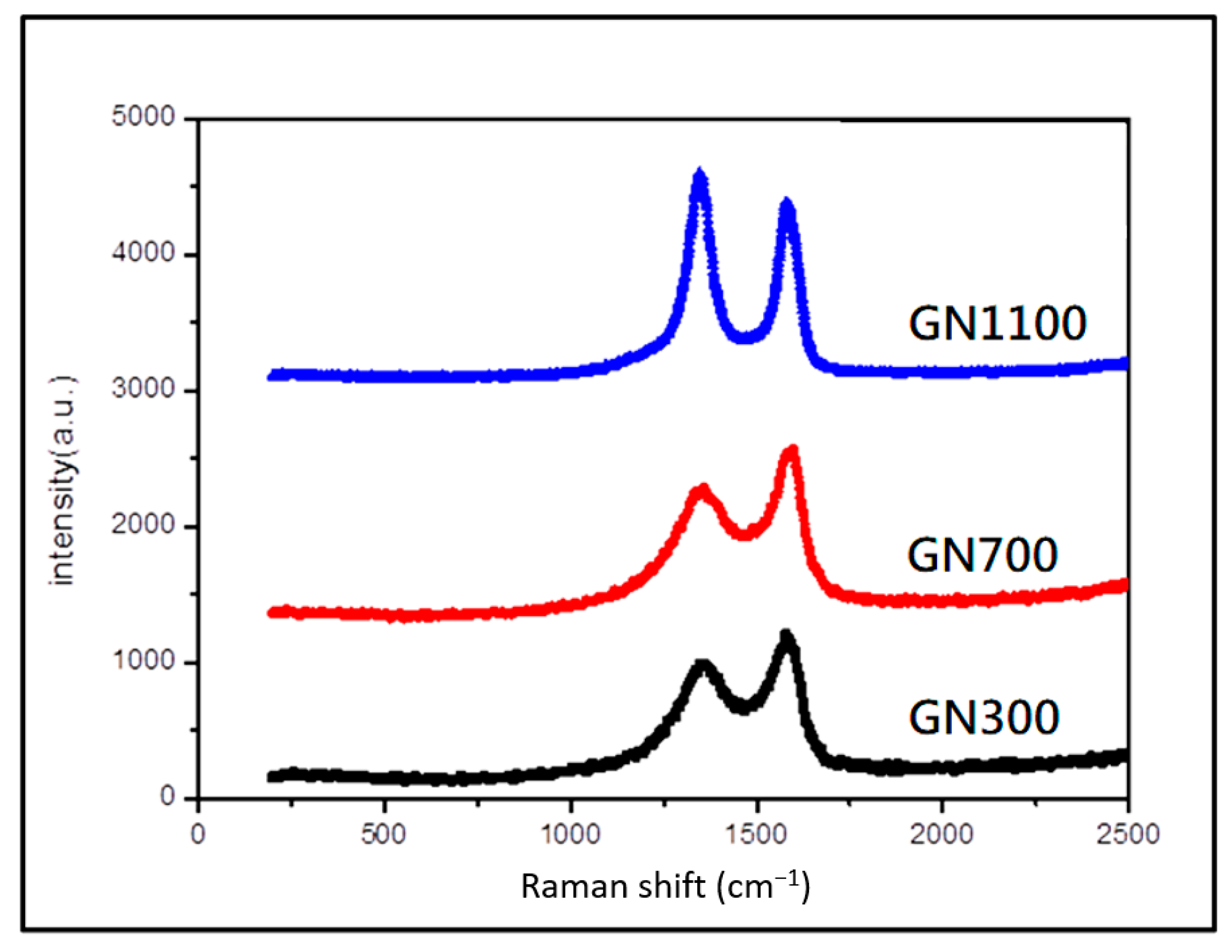
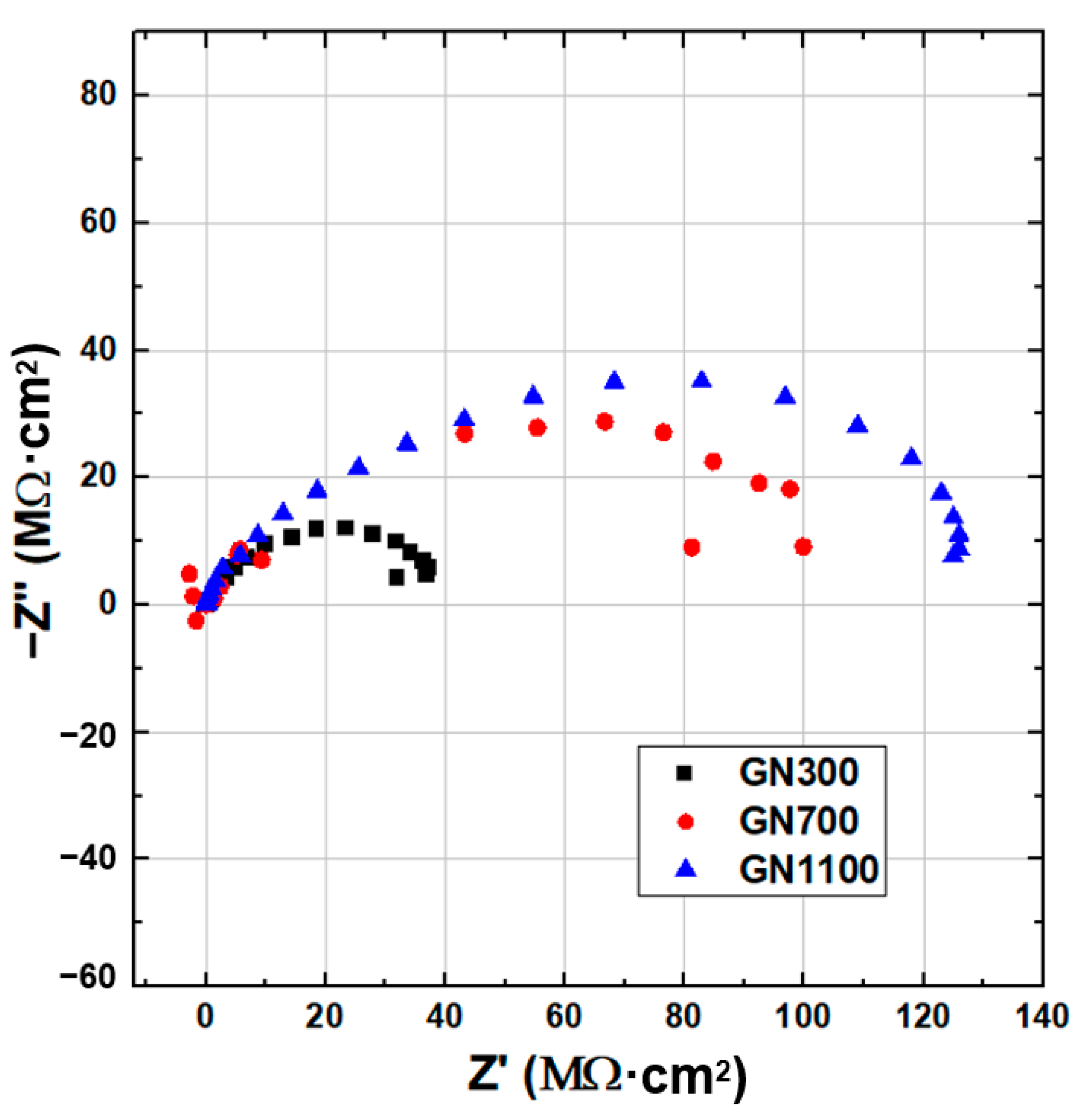
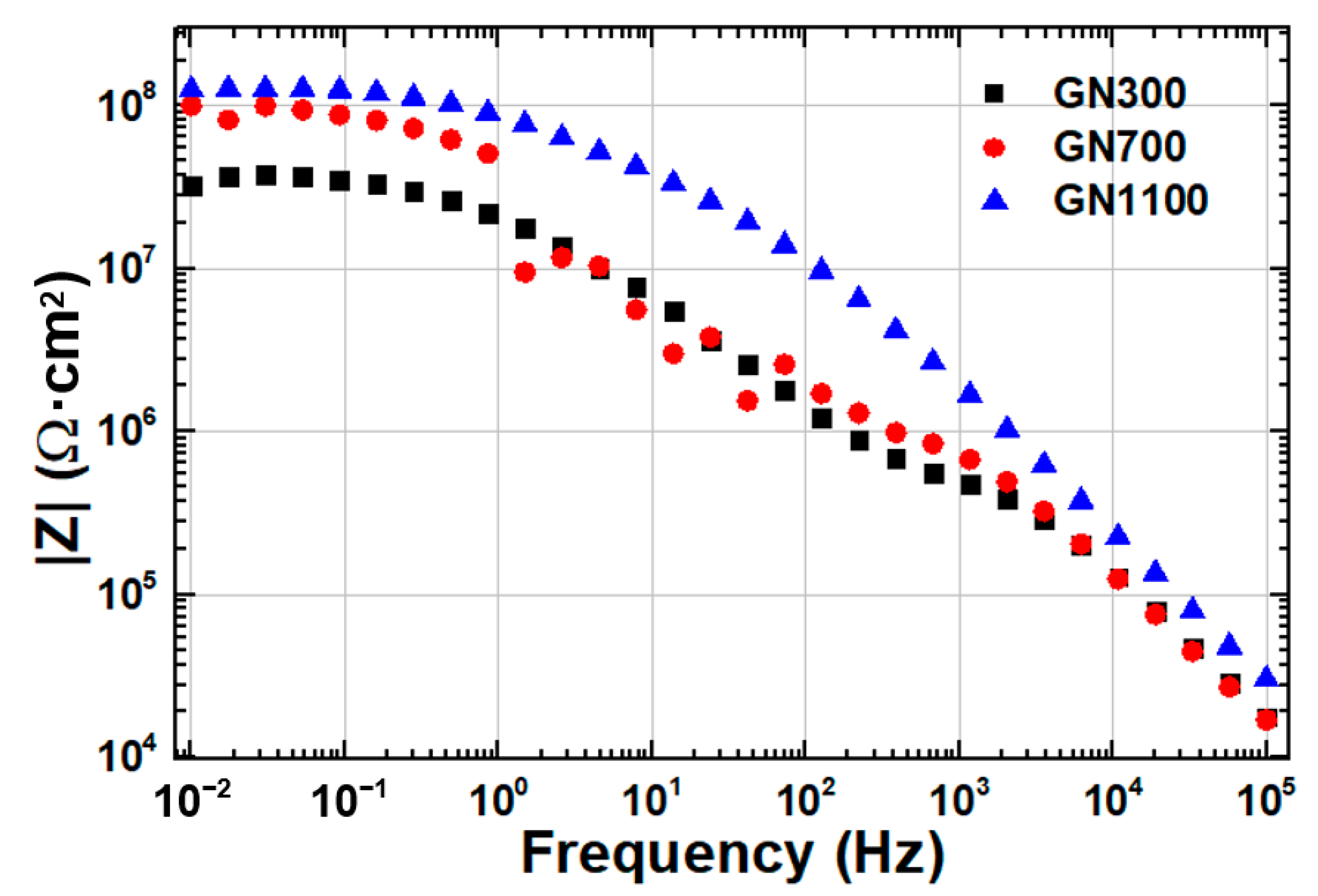
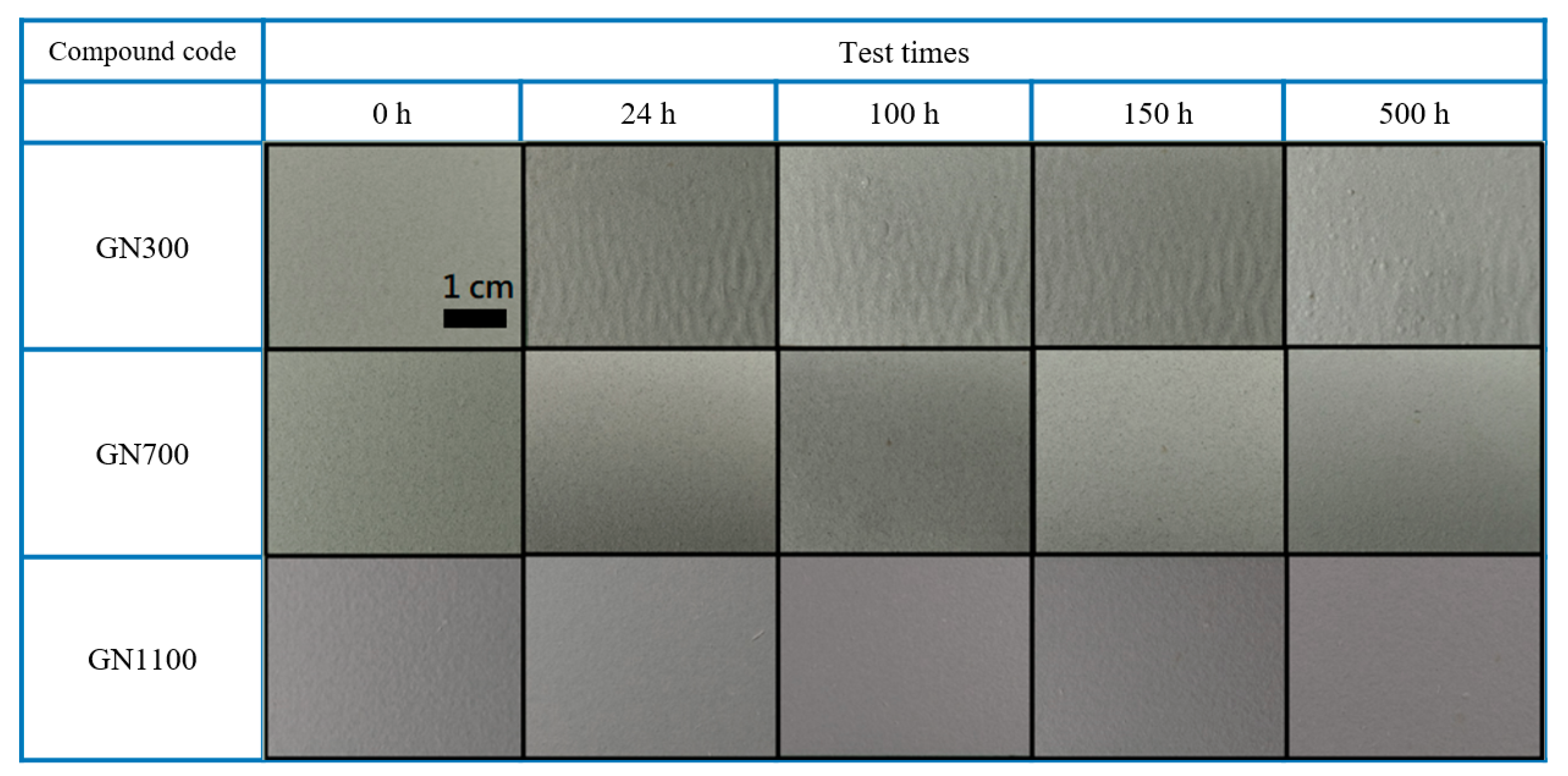
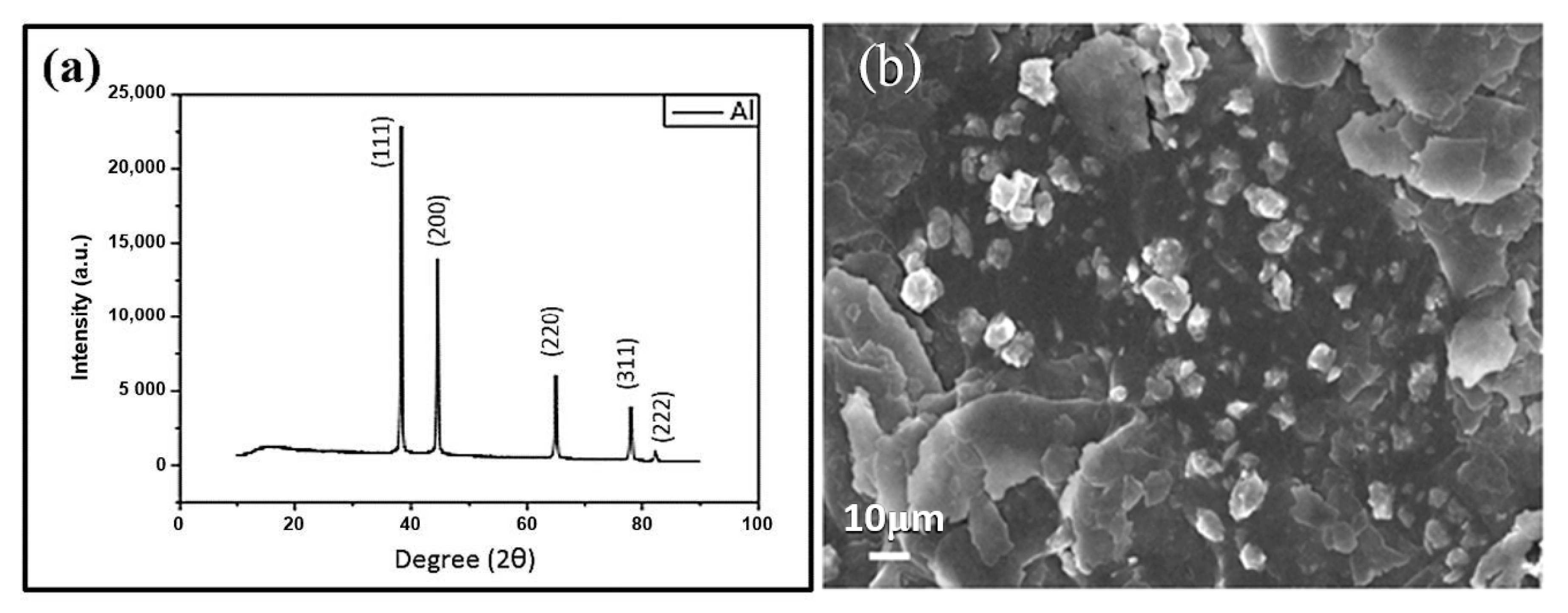
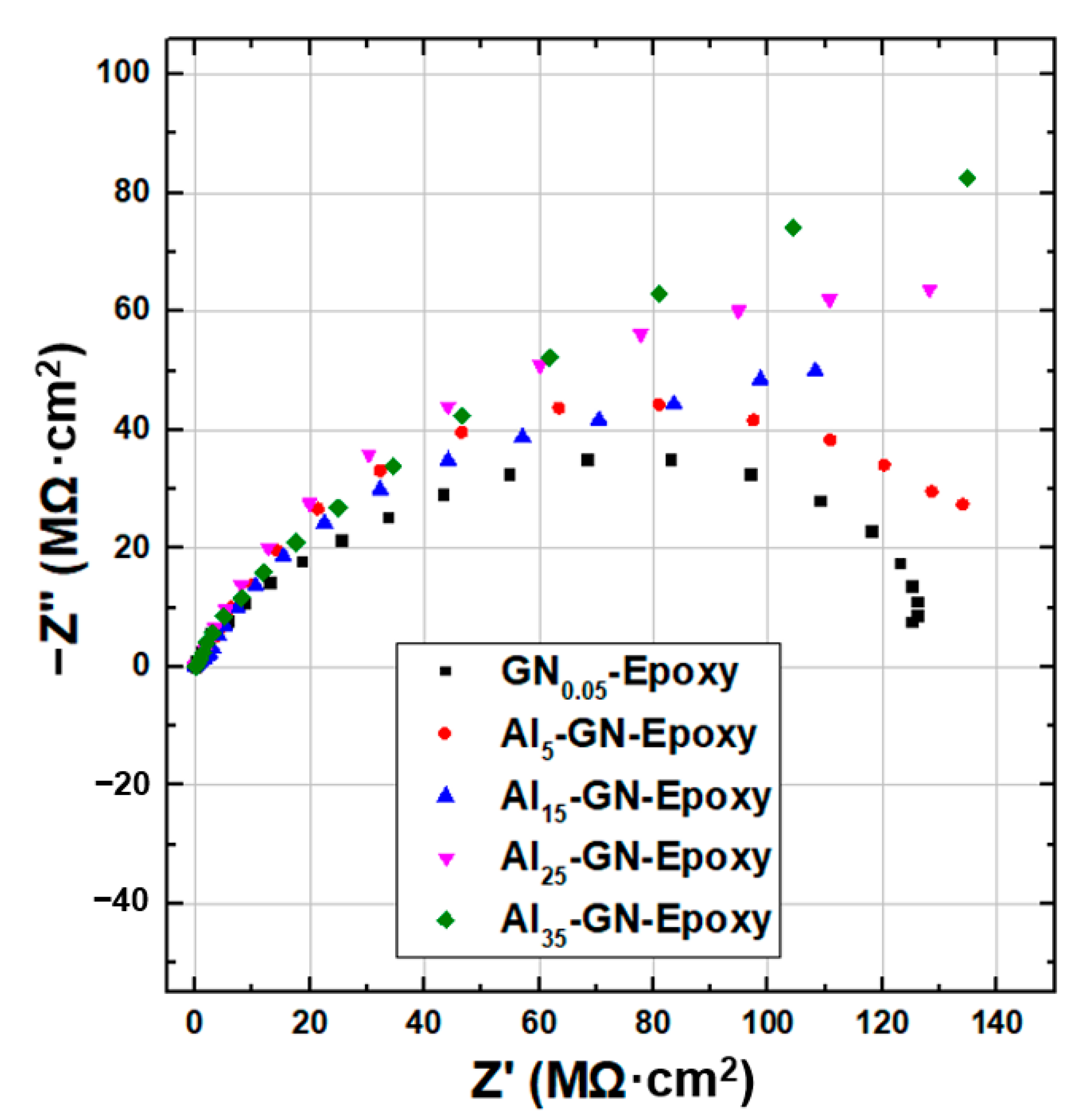
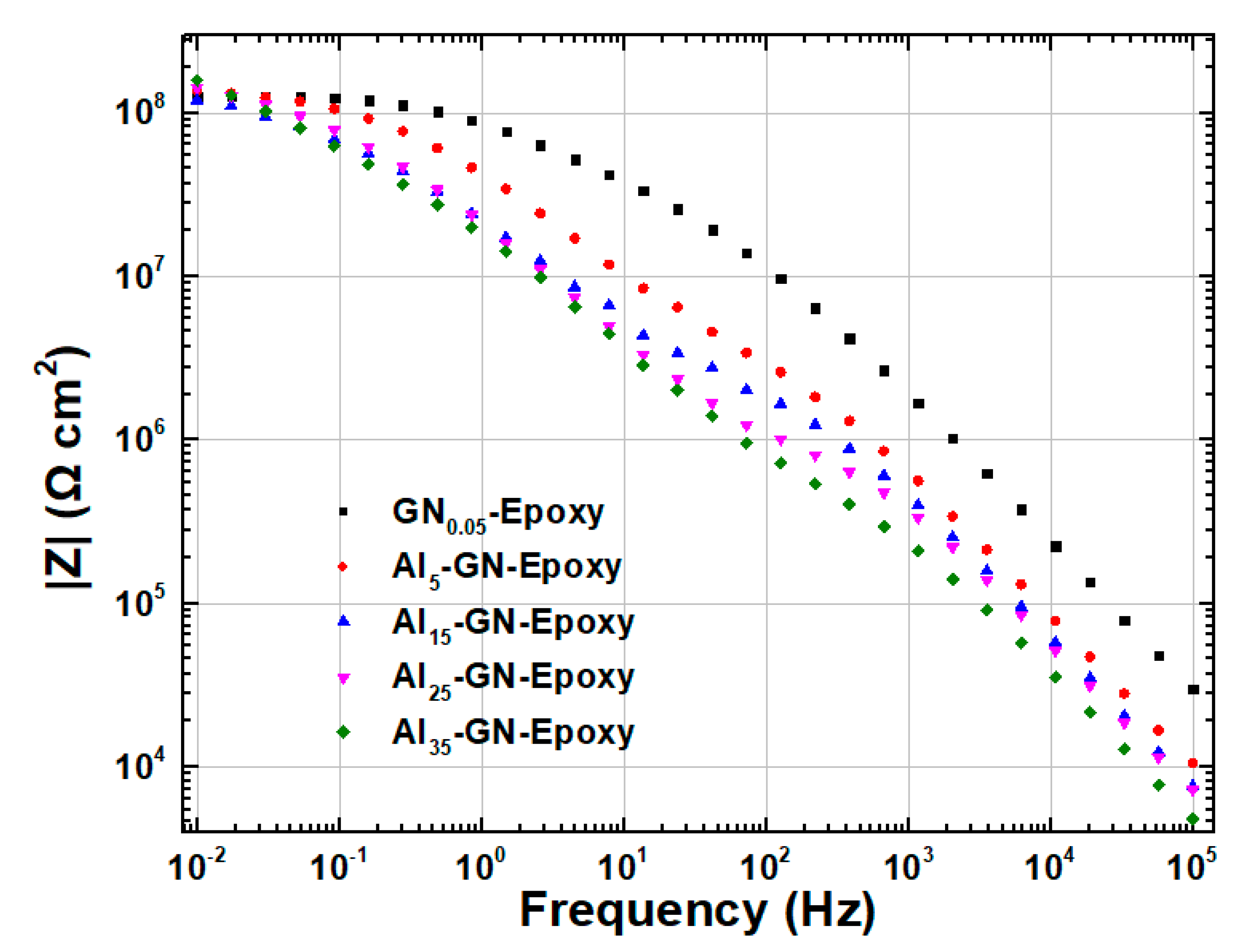
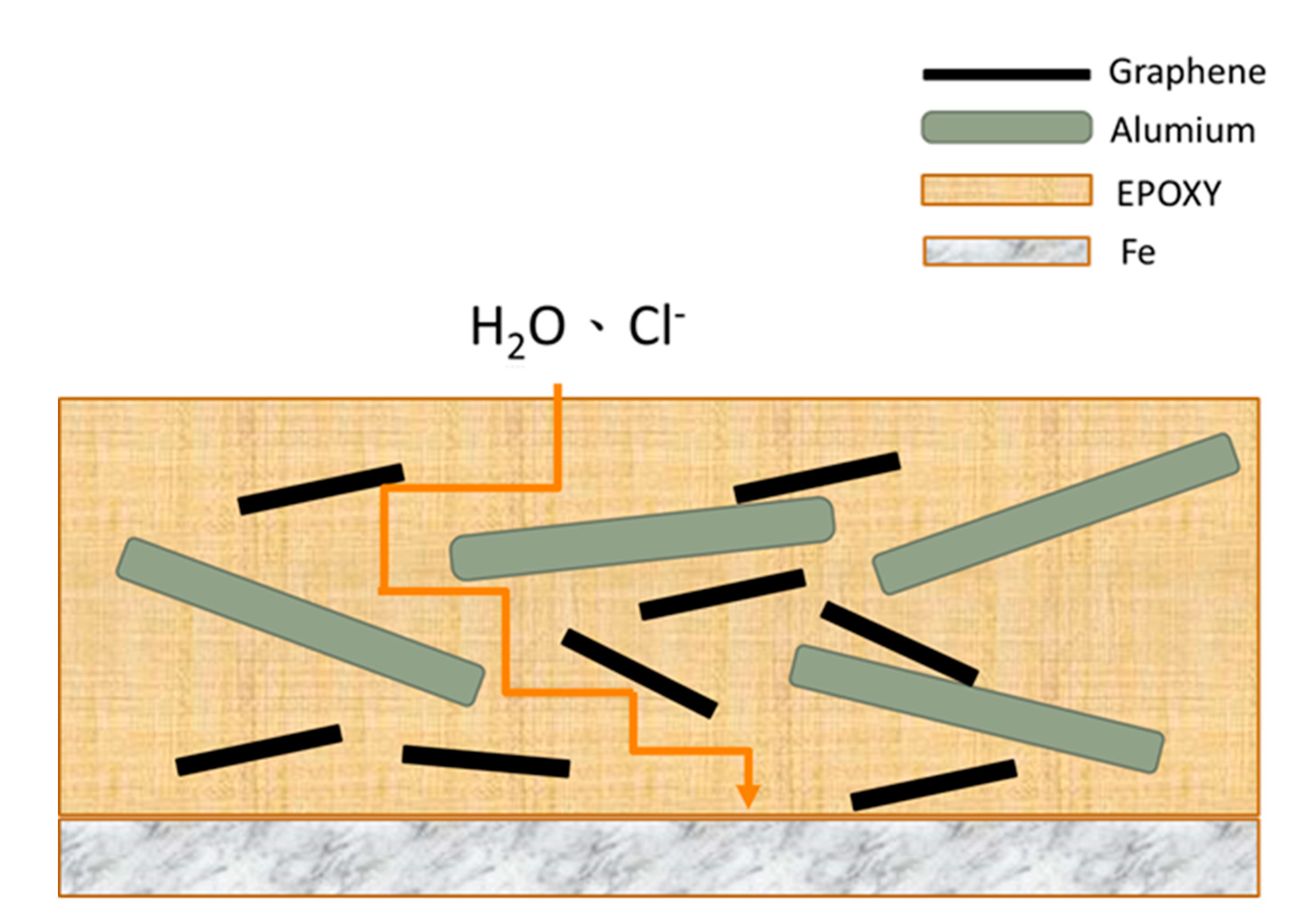
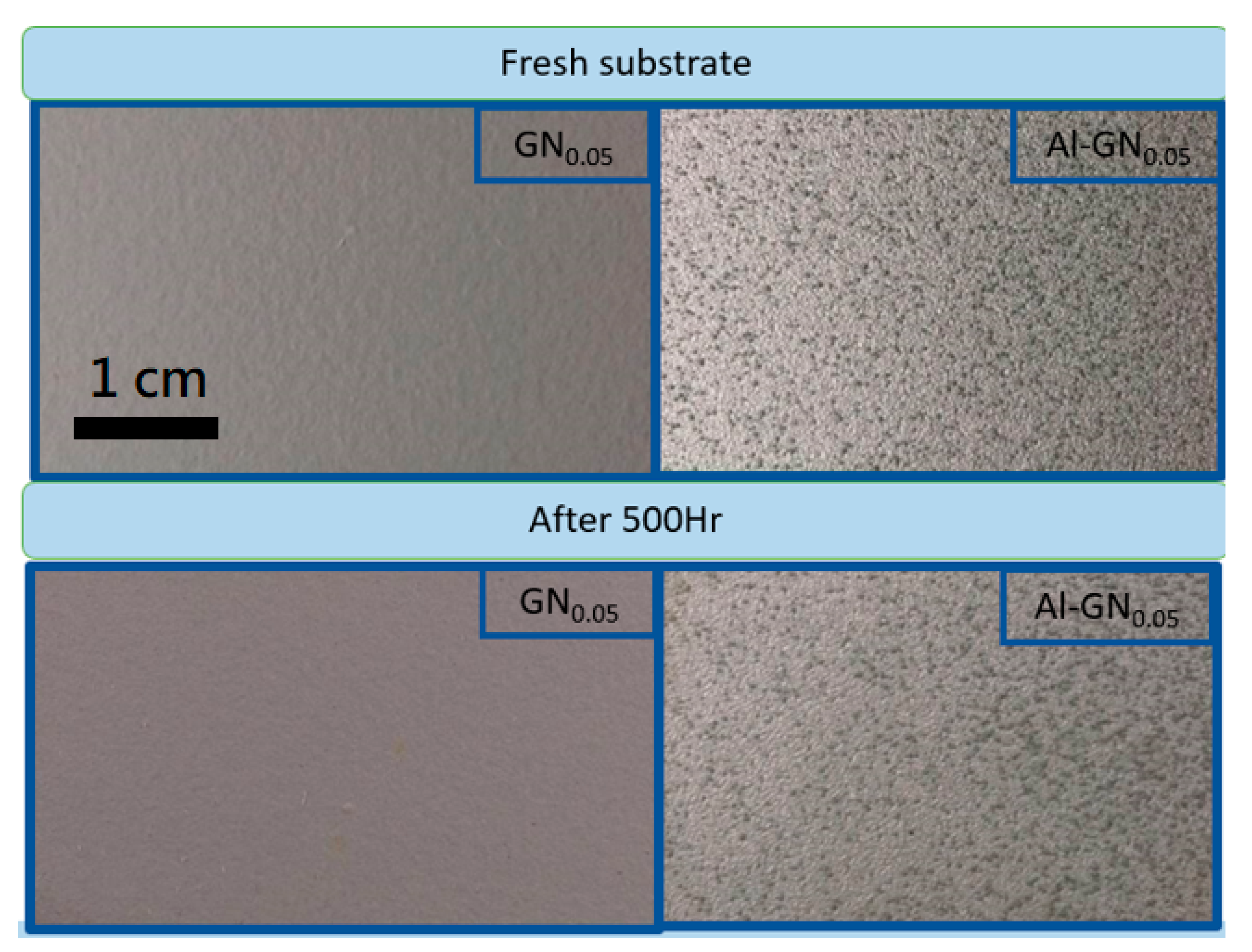
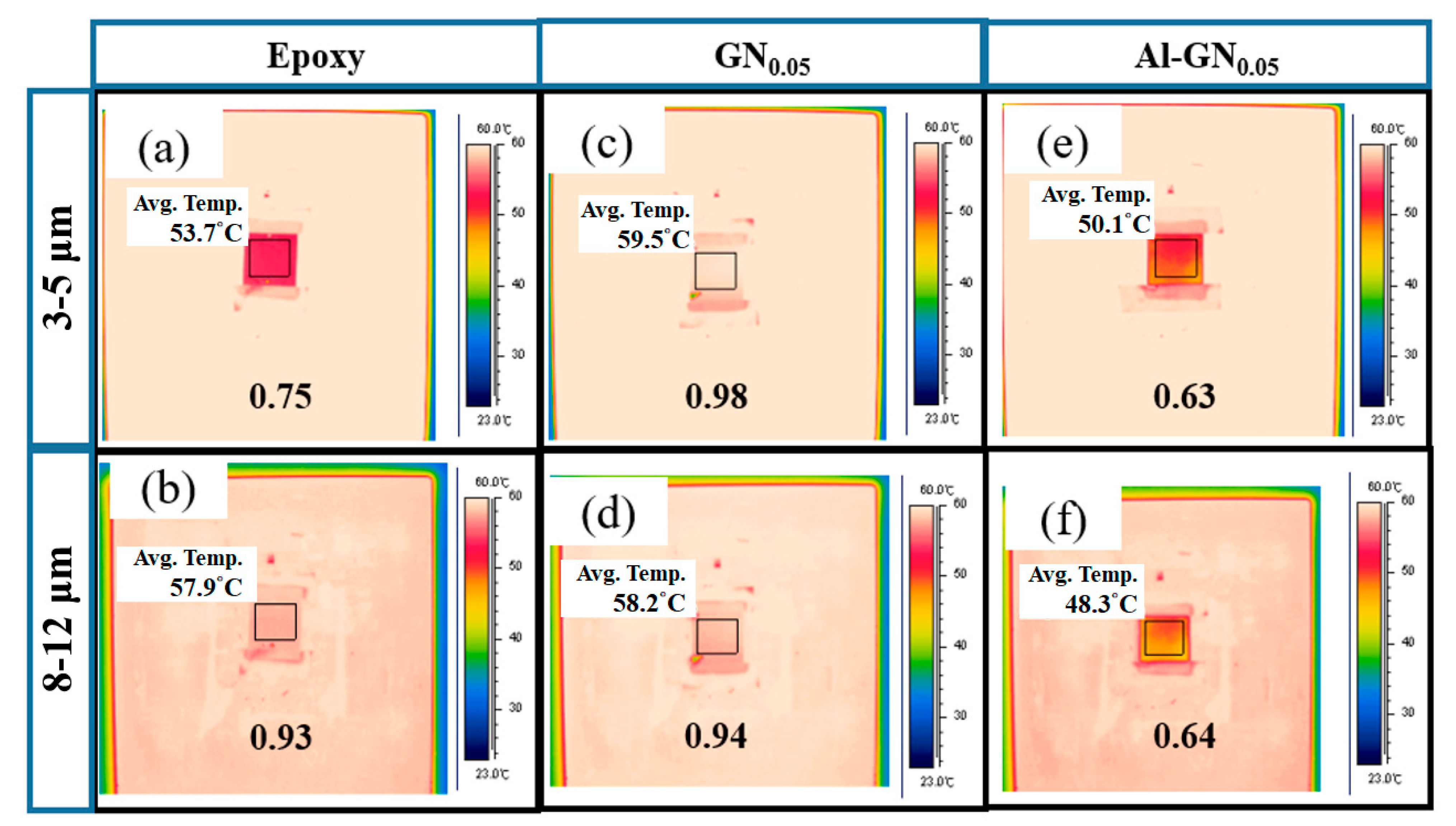
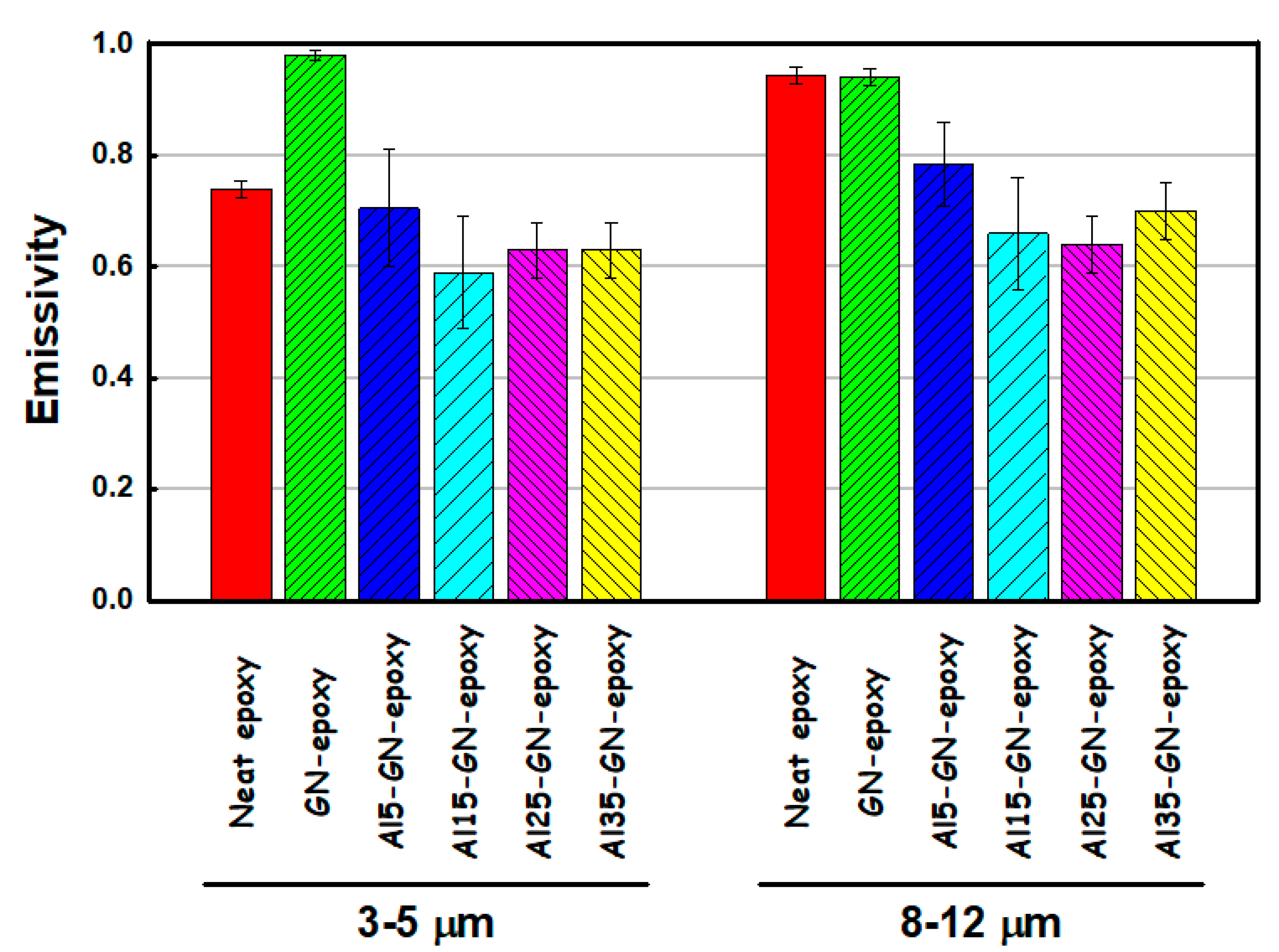
| Sample | Ecorr (V) | icorr (A/cm2) | βc (V/Dec) | βa (V/Dec) | vcorr (mm/Year) |
|---|---|---|---|---|---|
| GN0 | −0.48 | 1.02 × 10−7 | −0.20 | 0.096 | 1.19 × 10−3 |
| GN0.01 | −0.21 | 3.89 × 10−10 | −0.121 | 0.117 | 4.52 × 10−6 |
| GN0.05 | −0.16 | 4.57 × 10−11 | −0.114 | 0.115 | 5.31 × 10−7 |
| GN0.1 | −0.09 | 2.69 × 10−10 | −0.111 | 0.112 | 3.13 × 10−6 |
| GN0.5 | 0.12 | 8.12 × 10−9 | −0.115 | 0.117 | 9.44 × 10−5 |
| Sample | Rs (Ω·cm2) | Rp (Ω·cm2) | Rct (Ω·cm2) |
|---|---|---|---|
| epoxy | 0.01 | 5.2 × 105 | 8.6 × 105 |
| GN0.01 | 0.01 | 1.9 × 106 | 2.3 × 106 |
| GN0.05 | 0.01 | 2.5 × 107 | 1.1 × 108 |
| GN0.1 | 0.01 | 1.1 × 107 | 1.3 × 107 |
| GN0.5 | 0.01 | 3.6 × 103 | 2.3 × 105 |
| Reduction Temperature | Sample Weight (mg) | O (at.%) |
|---|---|---|
| GO (not reduced) | 2.5 | 26.4 |
| 300 °C | 2.5 | 13.2 |
| 700 °C | 2.5 | 10.0 |
| 1100 °C | 2.4 | 5.6 |
| Sample | Rs (Ω·cm2) | Rp (Ω·cm2) | Rct (Ω·cm2) |
|---|---|---|---|
| GN300-epoxy | 0.01 | 3.8 × 105 | 4.2 × 107 |
| GN700-epoxy | 0.01 | 1.3 × 106 | 9.0 × 107 |
| GN1100-epoxy | 0.01 | 2.5 × 107 | 1.1 × 108 |
| Sample | Rs (Ω·cm2) | Rp (Ω·cm2) | Rct (Ω·cm2) |
|---|---|---|---|
| GN0.05-epoxy | 0.01 | 2.47 × 107 | 1.14 × 108 |
| Al5-GN0.05-epoxy | 0.01 | 4.05 × 106 | 1.48 × 108 |
| Al15-GN0.05-epoxy | 0.01 | 3.54 × 107 | 1.60 × 108 |
| Al25-GN0.05-epoxy | 0.01 | 1.01 × 106 | 1.67 × 108 |
| Al35-GN0.05-epoxy | 0.01 | 1.27 × 106 | 2.49 × 108 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youh, M.-J.; Huang, Y.-R.; Peng, C.-H.; Lin, M.-H.; Chen, T.-Y.; Chen, C.-Y.; Liu, Y.-M.; Pu, N.-W.; Liu, B.-Y.; Chou, C.-H.; et al. Using Graphene-Based Composite Materials to Boost Anti-Corrosion and Infrared-Stealth Performance of Epoxy Coatings. Nanomaterials 2021, 11, 1603. https://doi.org/10.3390/nano11061603
Youh M-J, Huang Y-R, Peng C-H, Lin M-H, Chen T-Y, Chen C-Y, Liu Y-M, Pu N-W, Liu B-Y, Chou C-H, et al. Using Graphene-Based Composite Materials to Boost Anti-Corrosion and Infrared-Stealth Performance of Epoxy Coatings. Nanomaterials. 2021; 11(6):1603. https://doi.org/10.3390/nano11061603
Chicago/Turabian StyleYouh, Meng-Jey, Yu-Ren Huang, Cheng-Hsiung Peng, Ming-Hsien Lin, Ting-Yu Chen, Chun-Yu Chen, Yih-Ming Liu, Nen-Wen Pu, Bo-Yi Liu, Chen-Han Chou, and et al. 2021. "Using Graphene-Based Composite Materials to Boost Anti-Corrosion and Infrared-Stealth Performance of Epoxy Coatings" Nanomaterials 11, no. 6: 1603. https://doi.org/10.3390/nano11061603
APA StyleYouh, M.-J., Huang, Y.-R., Peng, C.-H., Lin, M.-H., Chen, T.-Y., Chen, C.-Y., Liu, Y.-M., Pu, N.-W., Liu, B.-Y., Chou, C.-H., Hou, K.-H., & Ger, M.-D. (2021). Using Graphene-Based Composite Materials to Boost Anti-Corrosion and Infrared-Stealth Performance of Epoxy Coatings. Nanomaterials, 11(6), 1603. https://doi.org/10.3390/nano11061603






