Quantitative Assessment of Trout Fish Spoilage with a Single Nanowire Gas Sensor in a Thermal Gradient
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of SnO2 Nanowires
2.2. Material Characterization
2.3. Fabrication of the Sensor
2.4. Gas Sensor Measurements
2.5. Trout Spoilage Measurements
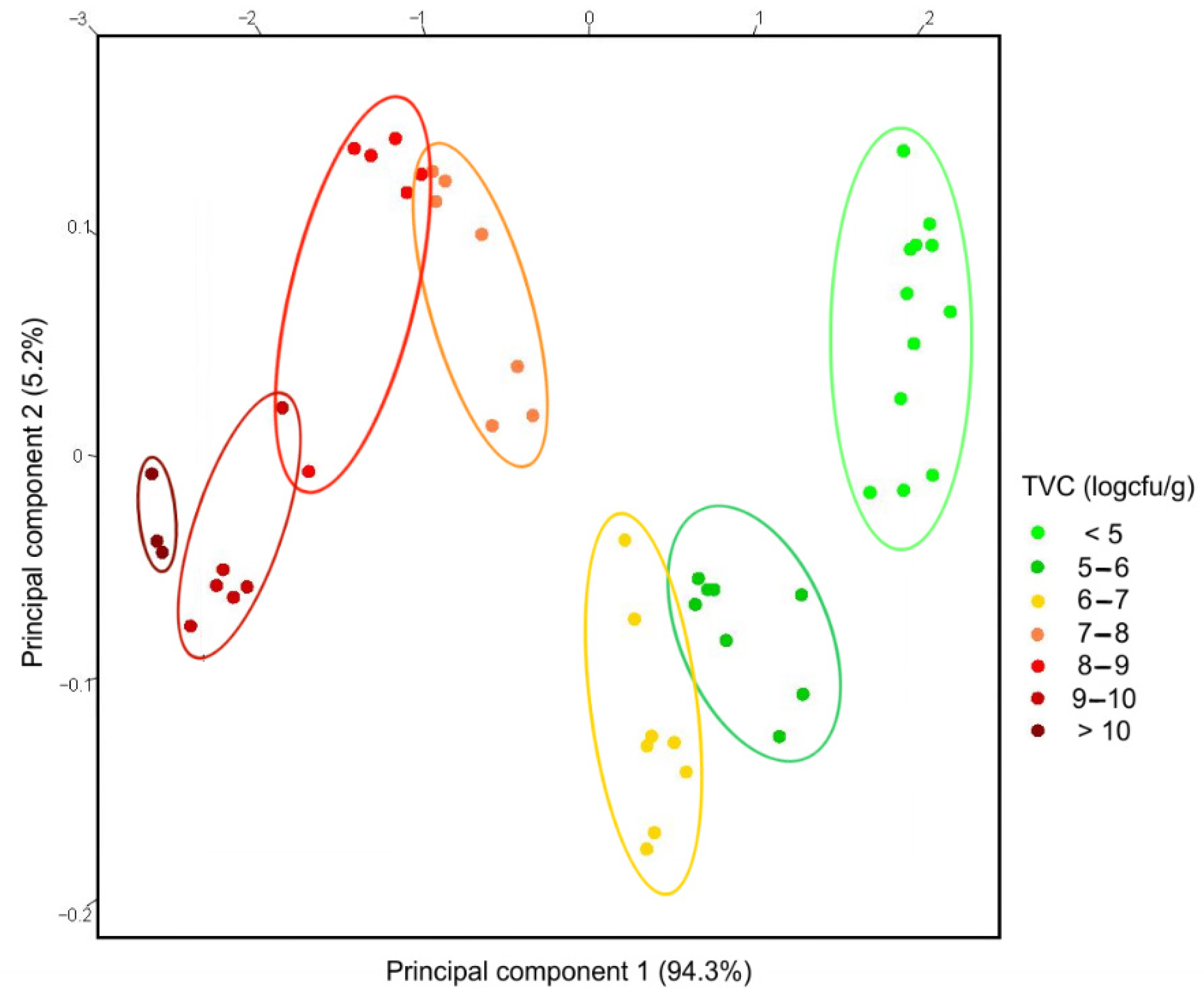
2.6. Multivariate Statistics and Data Mining
3. Results and Discussion
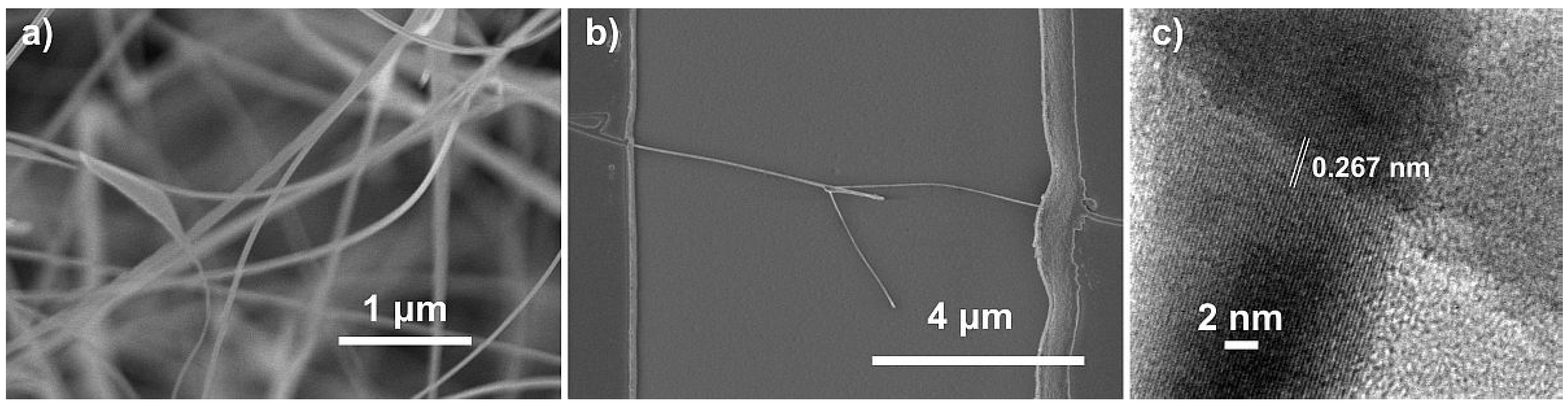
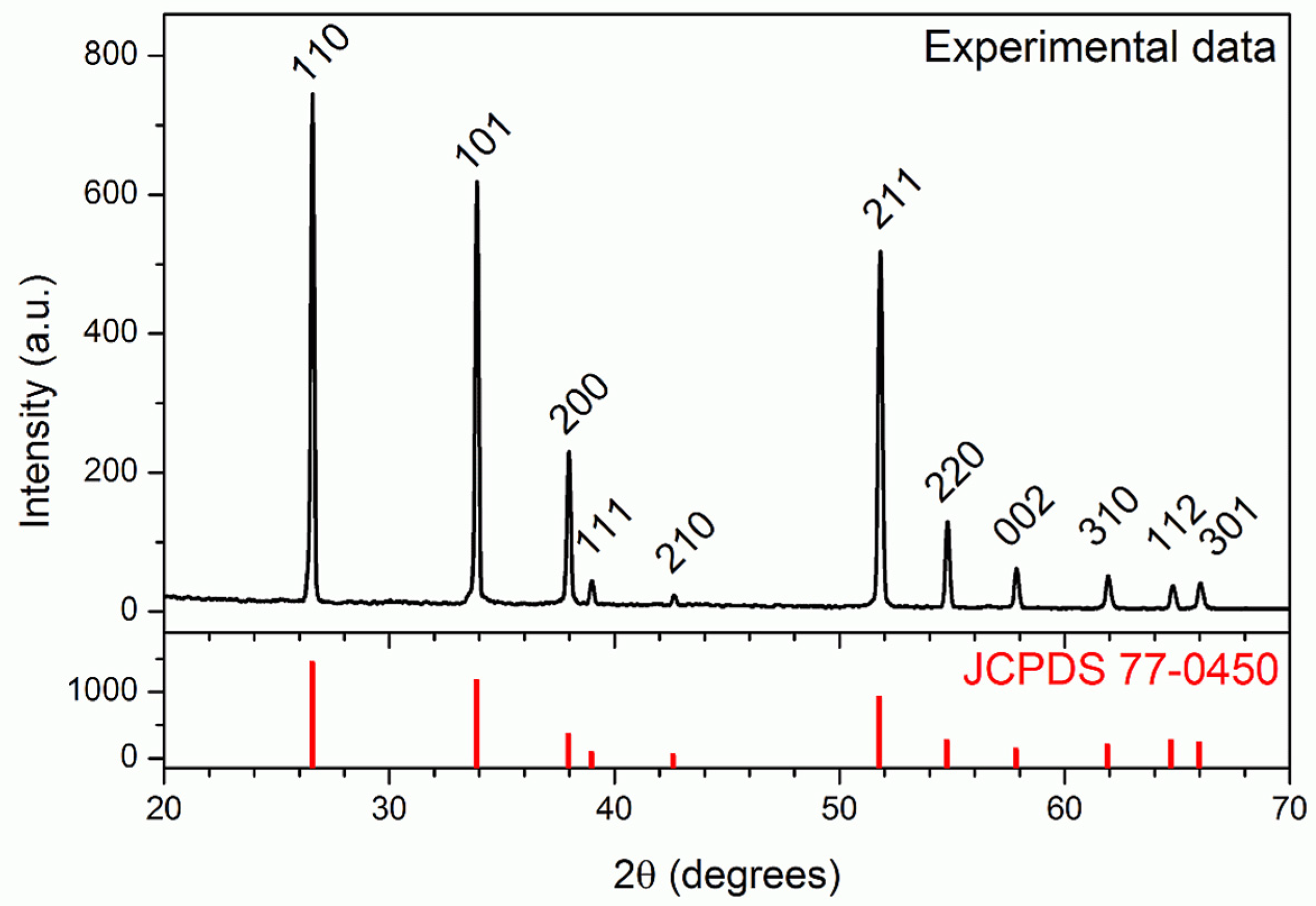
3.1. Characterization of the Nanowires
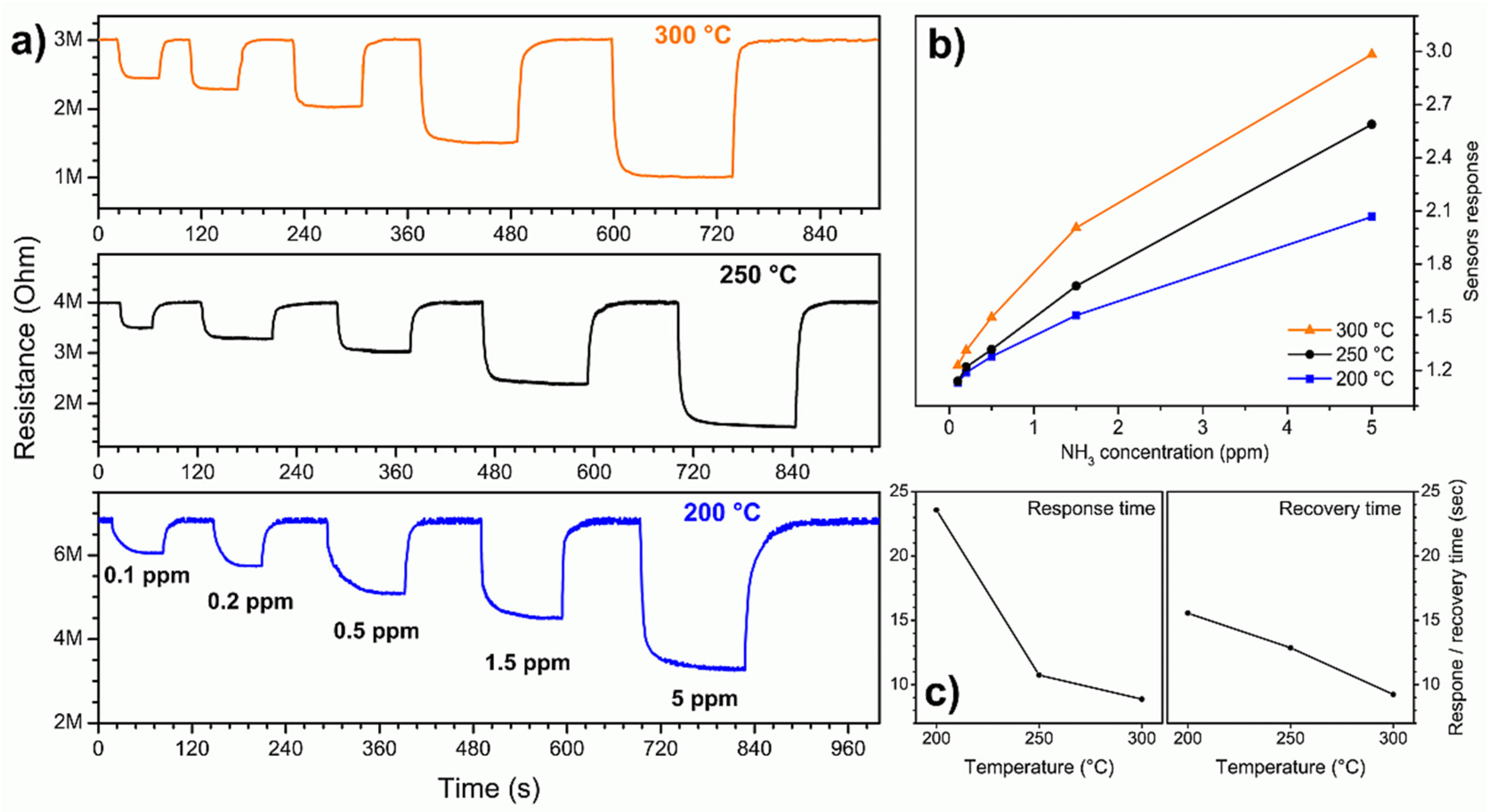
3.2. Ammonia-Sensing Performance
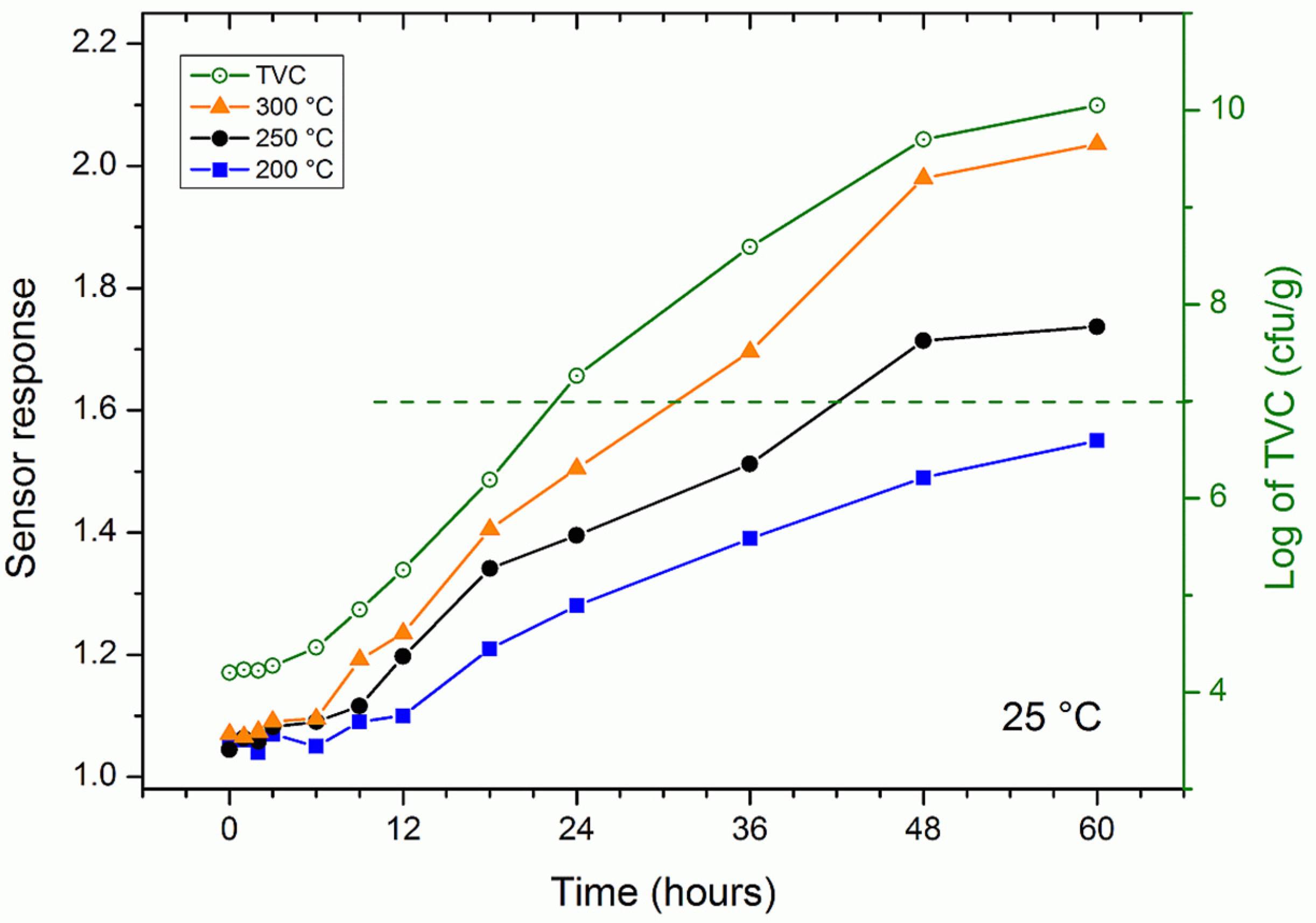
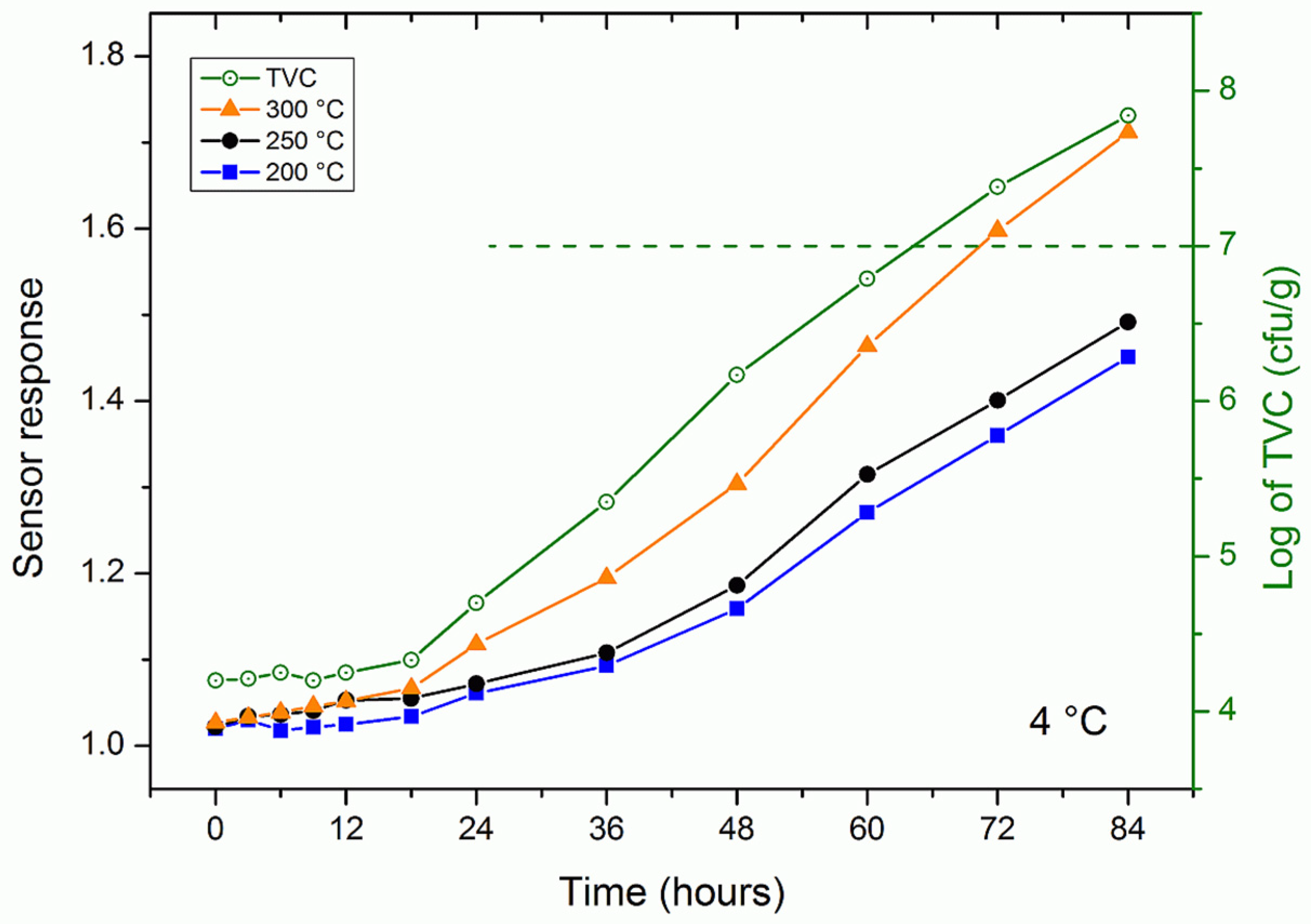
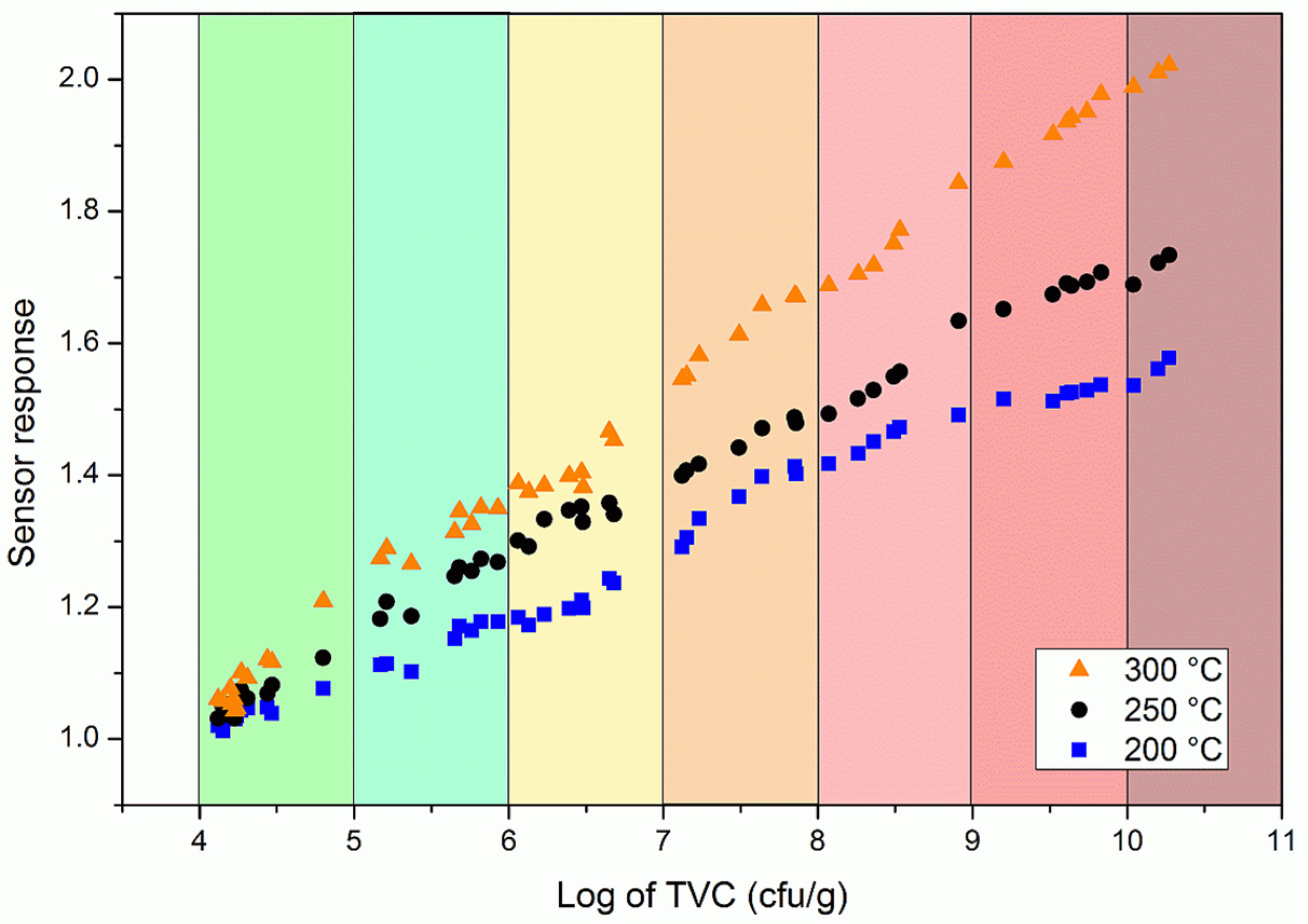
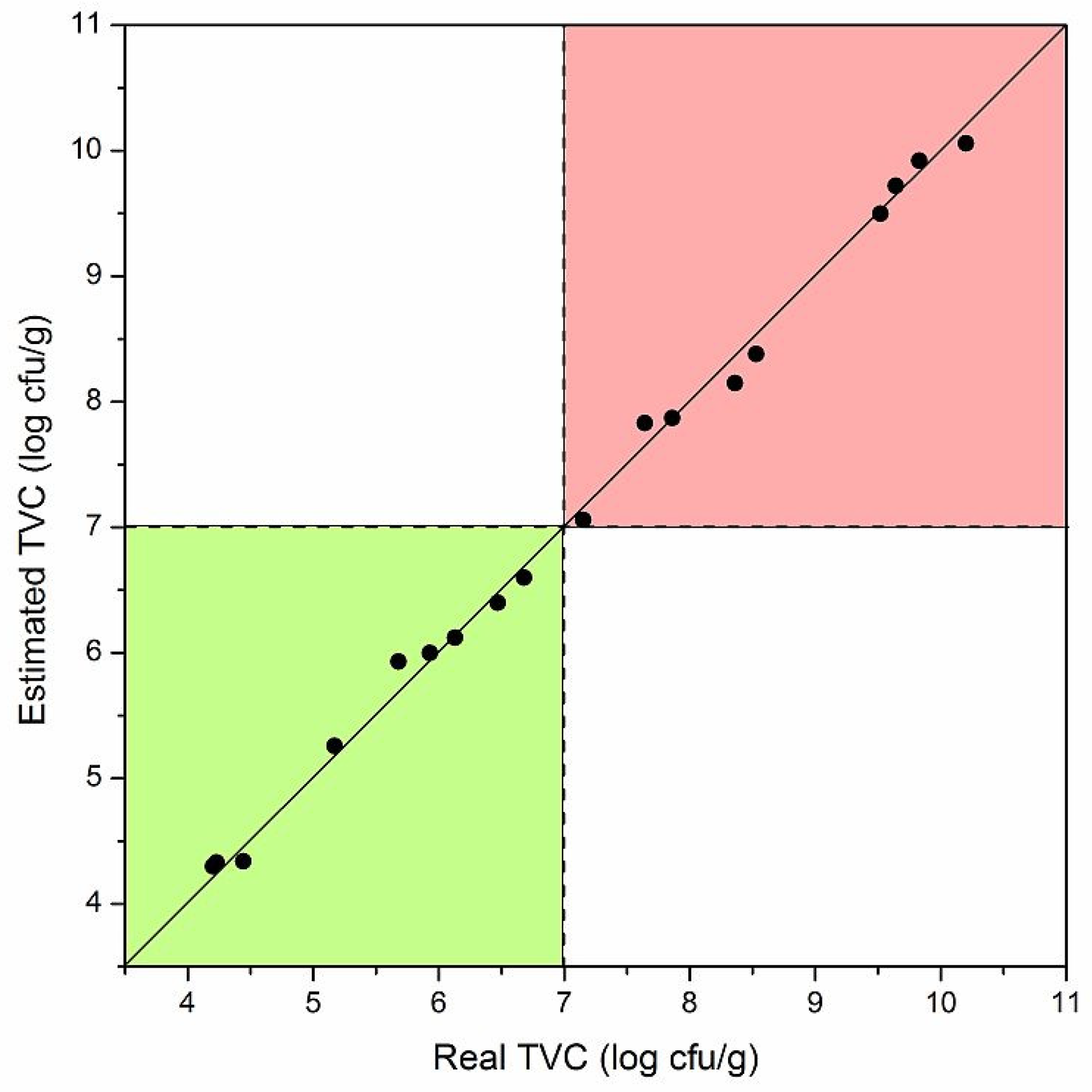
3.3. Trout Fish Spoilage Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scharff, R.L. Economic burden from health losses due to foodborne illness in the United States. J. Food Prot. 2012, 75, 123–131. [Google Scholar] [CrossRef]
- Sundström, K. Cost of Illness for Five Major Foodborne Illnesses and Sequelae in Sweden. Appl. Health Econ. Health Policy 2018, 16, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Boyer, D.; Ramaswami, A. Comparing urban food system characteristics and actions in US and Indian cities from a multi-environmental impact perspective: Toward a streamlined approach. J. Ind. Ecol. 2020, 24, 841–854. [Google Scholar] [CrossRef]
- The State of World Fisheries and Aquaculture 2020: Sustainability in Action; FAO: Rome, Italy, 2020; ISBN 978-92-5-132692-3. [CrossRef]
- Skilbrei, O.T. The importance of escaped farmed rainbow trout (Oncorhynchus mykiss) as a vector for the salmon louse (Lepeophtheirus salmonis) depends on the hydrological conditions in the fjord. Hydrobiologia 2012, 686, 287–297. [Google Scholar] [CrossRef][Green Version]
- FAO Cultured Aquatic Species Information Programme. Oncorhynchus Mykiss. Available online: http://www.fao.org/fishery/culturedspecies/Oncorhynchus_mykiss/en (accessed on 28 April 2021).
- Chytiri, S.; Chouliara, I.; Savvaidis, I.N.; Kontominas, M.G. Microbiological, chemical and sensory assessment of iced whole and filleted aquacultured rainbow trout. Food Microbiol. 2004, 21, 157–165. [Google Scholar] [CrossRef]
- Olafsdóttir, G.; Martinsdóttir, E.; Oehlenschläger, J.; Dalgaard, P.; Jensen, B.; Undeland, I.; Mackie, I.M.; Henehan, G.; Nielsen, J.; Nilseng, H. Methods to evaluate fish freshness in research and industry. Trends Food Sci. Technol. 1997, 8, 258–265. [Google Scholar] [CrossRef]
- Silva, F.; Duarte, A.M.; Mender, S.; Pinto, F.R.; Barroso, S.; Ganhão, R.; Gil, M.M. CATA vs. FCP for a rapid descriptive analysis in sensory characterization of fish. J. Sens. Stud. 2020, 35, e12605. [Google Scholar] [CrossRef]
- Mustafa, F.; Andreescu, S. Chemical and Biological Sensors for Food-Quality Monitoring and Smart Packaging. Foods 2018, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Gram, L.; Huss, H.H. Microbiological spoilage of fish and fish products. Int. J. Food Microbiol. 1996, 33, 121–137. [Google Scholar] [CrossRef]
- Jia, S.; Li, Y.; Zhuang, S.; Sun, X.; Zhang, L.; Shi, J.; Hong, H.; Luo, Y. Biochemical changes induced by dominant bacteria in chill-stored silver carp (Hypophthalmichthys molitrix) and GC-IMS identification of volatile organic compounds. Food Microbiol. 2019, 84, 103248. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Dave, D.; Budge, S.; Brooks, M. Fish spoilage mechanisms and preservation techniques: Review. Am. J. Appl. Sci. 2010, 7, 859–877. [Google Scholar] [CrossRef]
- Dikeman, M.; Devine, C. Encyclopedia of Meat Sciences, 2nd ed.; Academic Press: Amsterdam, The Netherlands, 2004; ISBN 978-0124649705. [Google Scholar]
- Pacquit, A.; Frisby, J.; Diamond, D.; Lau, K.T.; Farrell, A.; Quilty, B.; Diamond, D. Development of a smart packaging for the monitoring of fish spoilage. Food Chem. 2007, 102, 466–470. [Google Scholar] [CrossRef]
- Seyama, T.; Kato, A.; Fujishi, K.; Nagatani, M. A New Detector for Gasous Components Using Semiconductive Thin Films. Anal. Chem. 1962, 34, 1502. [Google Scholar] [CrossRef]
- Korotcenkov, G. Current Trends in Nanomaterials for Metal Oxide-Based Conductometric Gas Sensors: Advantages and Limitations. Part 1: 1D and 2D Nanostructures. Nanomaterials 2020, 10, 1392. [Google Scholar] [CrossRef] [PubMed]
- Akbari-Saatlu, M.; Procek, M.; Mattsson, C.; Thungström, G.; Nilsson, H.-E.; Xiong, W.; Xu, B.; Li, Y.; Radamson, H.H. Silicon Nanowires for Gas Sensing: A Review. Nanomaterials 2020, 10, 2215. [Google Scholar] [CrossRef]
- Kim, D.; Park, C.; Choi, W.; Shin, S.-H.; Jin, B.; Baek, R.-H.; Lee, J.-S. Improved Long-Term Responses of Au-Decorated Si Nanowire FET Sensor for NH3 Detection. IEEE Sens. J. 2020, 20, 2270. [Google Scholar] [CrossRef]
- Pichon, L.; Rogel, R.; Jacques, E.; Salaun, A.C. N-type in-situ doping effect on vapour-liquid-solid silicon nanowire properties for gas sensing applications. Phys. Status Solidi C 2014, 11, 344. [Google Scholar] [CrossRef]
- Pichon, L.; Salaün, A.-C.; Wenga, G.; Rogel, R.; Jacques, E. Ammonia sensors based on suspended silicon nanowires. Proc. Eng. 2014, 87, 1003. [Google Scholar] [CrossRef]
- Jacques, E.; Ni, L.; Salaün, A.C.; Rogel, R.; Pichon, L. Polysilicon Nanowires for chemical sensing applications. Mater. Res. Soc. Symp. Proc. 2012, 1429, 133. [Google Scholar] [CrossRef]
- Selvaraj, B.; Balaguru Rayappan, J.B.; Jayanth Babu, K. Influence of calcination temperature on the growth of electrospun multi-junction ZnO nanowires: A room temperature ammonia sensor. Mater. Sci. Semicon. Proc. 2020, 112, 105006. [Google Scholar] [CrossRef]
- Srinivasan, P.; Rayappan, J.B.B. Growth of Eshelby twisted ZnO nanowires through nanoflakes & nanoflowers: A room temperature ammonia sensor. Sens. Actuat. B Chem 2018, 277, 129. [Google Scholar] [CrossRef]
- Tonezzer, M.; Le, D.T.T.; Iannotta, S.; Hieu, N.V. Selective discrimination of hazardous gases using one single metal oxide resistive sensor. Sensor Actuat. B Chem. 2018, 277, 121–128. [Google Scholar] [CrossRef]
- Krawczyk, M.; Suchorska-Woźniak, P.; Szukiewicz, R.; Kuchowicz, M.; Korbutowicz, R.; Teterycz, H. Morphology of Ga2O3 Nanowires and Their Sensitivity to Volatile Organic Compounds. Nanomaterials 2021, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, T.M.; Van Duy, N.; Hung, C.M.; Hoa, N.D.; Nguyen, H.; Tonezzer, M.; Hieu, N.V. Self-heated Ag-decorated SnO2 nanowires with low power consumption used as a predictive virtual multisensor for H2S-selective sensing. Anal. Chim. Acta 2019, 1069, 108–116. [Google Scholar] [CrossRef]
- Thai, N.X.; Van Duy, N.; Hung, C.M.; Nguyen, H.; Tonezzer, M.; Van Hieu, N.; Hoa, N.D. Prototype edge-grown nanowire sensor array for the real-time monitoring and classification of multiple gases. J. Sci. Adv. Mater. Dev. 2020, 5, 409–416. [Google Scholar] [CrossRef]
- Tonezzer, M. Selective gas sensor based on one single SnO2 nanowire. Sens. Actuators B Chem. 2019, 288, 53–59. [Google Scholar] [CrossRef]
- Tonezzer, M. Detection of mackerel fish spoilage with a gas sensor based on one single SnO2 nanowire. Chemosensors 2021, 9, 1–10. [Google Scholar] [CrossRef]
- D’Amico, A.; Di Natale, C. A contribution on some basic definitions of sensors properties. IEEE Sens. J. 2001, 1, 183–190. [Google Scholar] [CrossRef]
- Thai, N.X.; Tonezzer, M.; Masera, L.; Nguyen, H.; Van Duy, N.; Hoa, N.D. Multi gas sensors using one nanomaterial, temperature gradient, and machine learning algorithms for discrimination of gases and their concentration. Anal. Chim. Acta 2020, 1124, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Koutsoumanis, K. Predictive Modeling of the Shelf Life of Fish under Nonisothermal Conditions. Appl. Environ. Microbiol. 2001, 67, 1821–1829. [Google Scholar] [CrossRef]
- Khoshnoudi-Nia, S.; Moosavi-Nasab, M. Nondestructive Determination of Microbial, Biochemical, and Chemical Changes in Rainbow Trout (Oncorhynchus mykiss) During Refrigerated Storage Using Hyperspectral Imaging Technique. Food Anal. Methods 2019, 12, 1635–1647. [Google Scholar] [CrossRef]
- Sciortino, J.A.; Ravikumar, R. Chapter 5: Fish Quality Assurance. In Fishery Harbour Manual on the Prevention of Pollution; Bay of Bengal Programme: Madras, India, 1999. [Google Scholar]
- Poli, B.M. Total quality in the fisheries chain, Chapter 19.2. In The State of Italian Marine Fisheries and Aquaculture; Cautadella, S., Spagnolo, M., Eds.; Ministero delle Politiche Agricole, Alimentari e Forestali (MiPAAF): Rome, Italy, 2011. [Google Scholar]
- Gui, M.; Song, J.; Zhang, Z.; Hui, P.; Li, P. Biogenic amines formation, nucleotide degradation and TVB-N accumulation of vacuum-packed minced sturgeon (Acipenser schrencki) stored at 4 °C and their relation to microbiological attributes. J. Sci. Food Agric. 2014, 94, 2057–2063. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.E.-D.; Holman, B.W.B.; Giteru, S.G.; Hopkins, D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, S.S.; Tonezzer, M. Selective gas detection and quantification using a resistive sensor based on Pd-decorated soda-lime glass. Sens. Actuat. B Chem. 2021, 335, 129714. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Phil. Trans. R. Soc. A 2016, 374, 20150202. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonezzer, M.; Thai, N.X.; Gasperi, F.; Van Duy, N.; Biasioli, F. Quantitative Assessment of Trout Fish Spoilage with a Single Nanowire Gas Sensor in a Thermal Gradient. Nanomaterials 2021, 11, 1604. https://doi.org/10.3390/nano11061604
Tonezzer M, Thai NX, Gasperi F, Van Duy N, Biasioli F. Quantitative Assessment of Trout Fish Spoilage with a Single Nanowire Gas Sensor in a Thermal Gradient. Nanomaterials. 2021; 11(6):1604. https://doi.org/10.3390/nano11061604
Chicago/Turabian StyleTonezzer, Matteo, Nguyen Xuan Thai, Flavia Gasperi, Nguyen Van Duy, and Franco Biasioli. 2021. "Quantitative Assessment of Trout Fish Spoilage with a Single Nanowire Gas Sensor in a Thermal Gradient" Nanomaterials 11, no. 6: 1604. https://doi.org/10.3390/nano11061604
APA StyleTonezzer, M., Thai, N. X., Gasperi, F., Van Duy, N., & Biasioli, F. (2021). Quantitative Assessment of Trout Fish Spoilage with a Single Nanowire Gas Sensor in a Thermal Gradient. Nanomaterials, 11(6), 1604. https://doi.org/10.3390/nano11061604







