Controlled pDNA Release in Gemini Cationic Lipoplexes by Femtosecond Laser Irradiation of Gold Nanostars
Abstract
1. Introduction
2. Materials and Methods
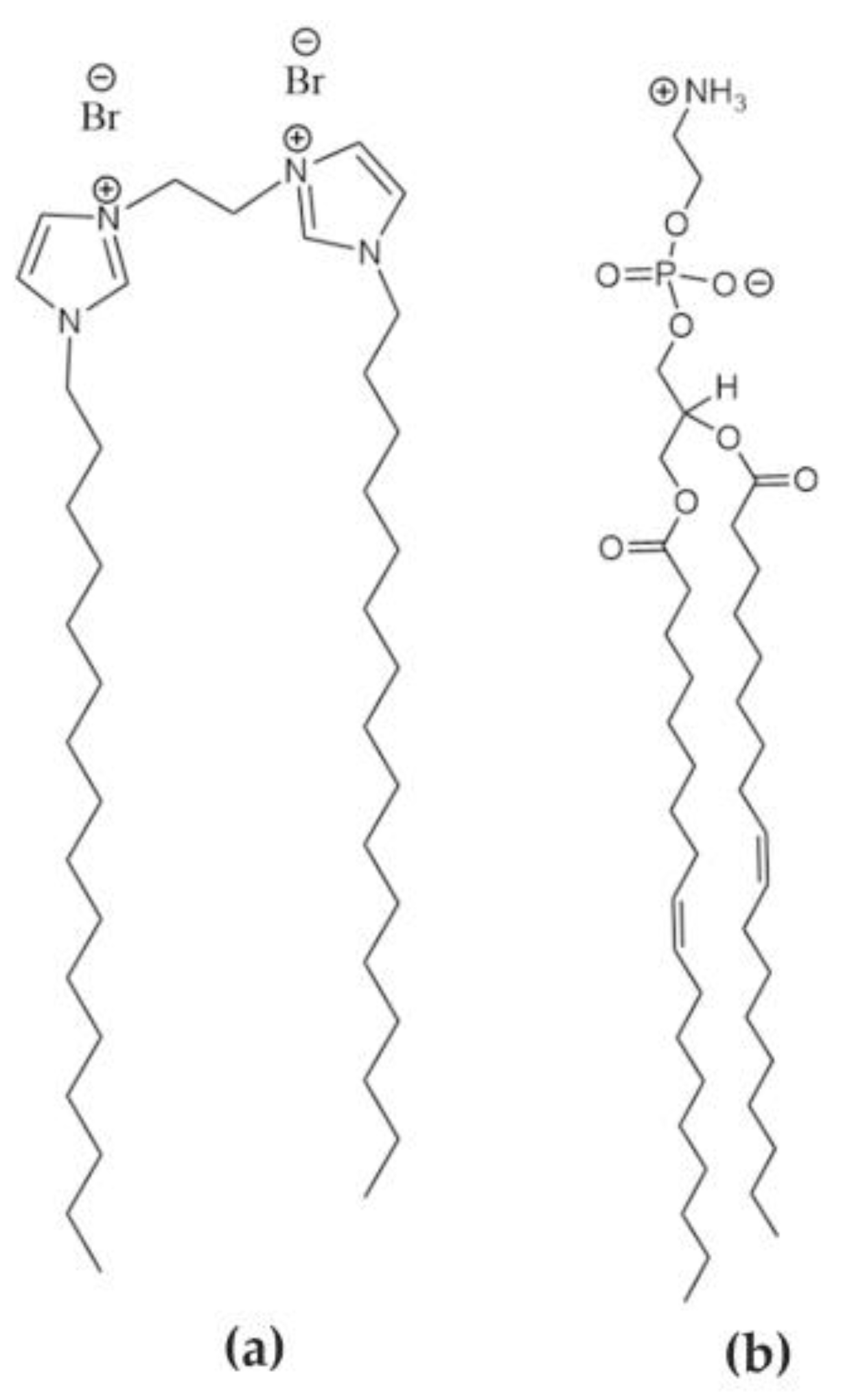
2.1. Materials
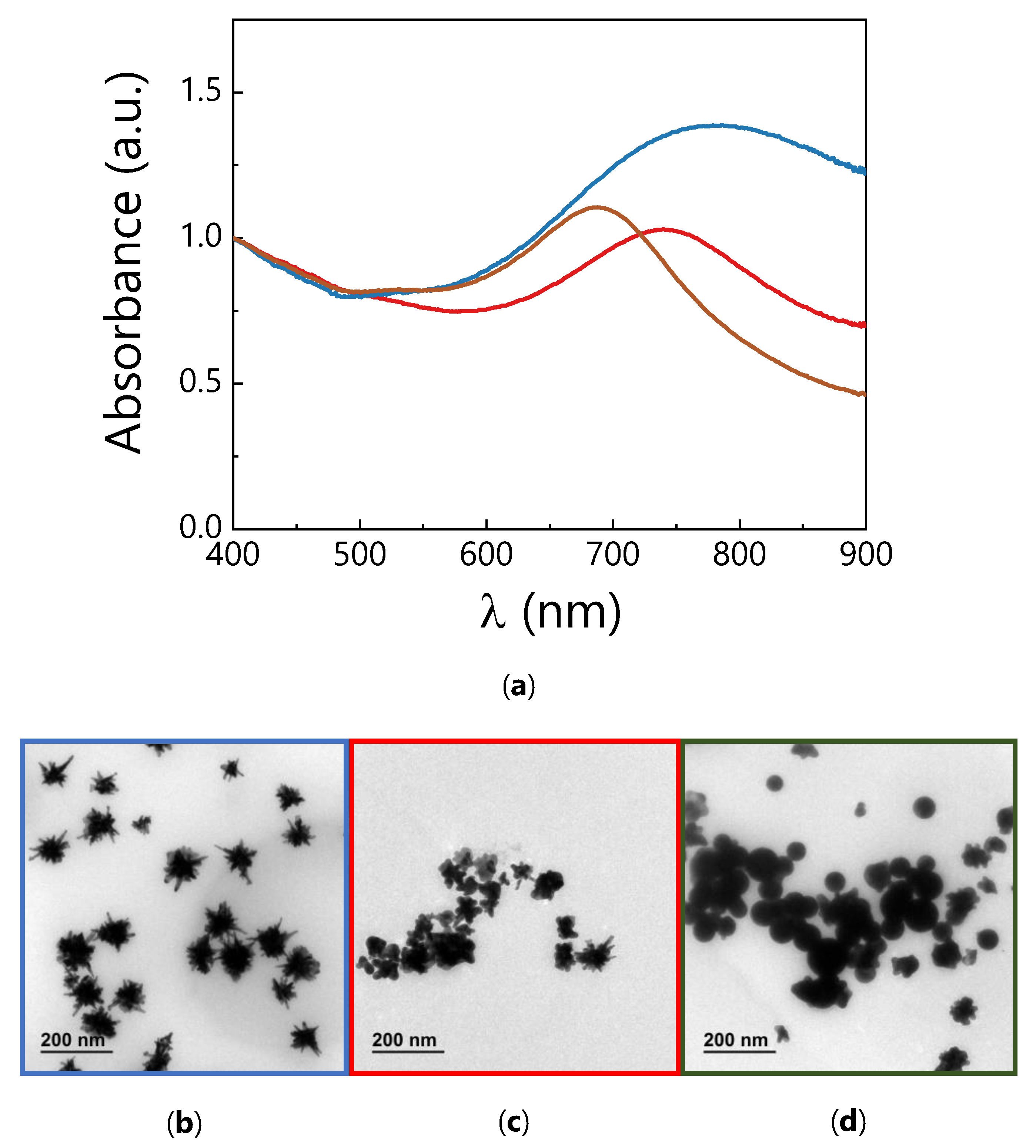
2.2. Optical Characterization
2.3. AuNSs Synthesis
2.4. Preparation of Lipoplex-AuNS System
2.5. ζ–Potential Measurements
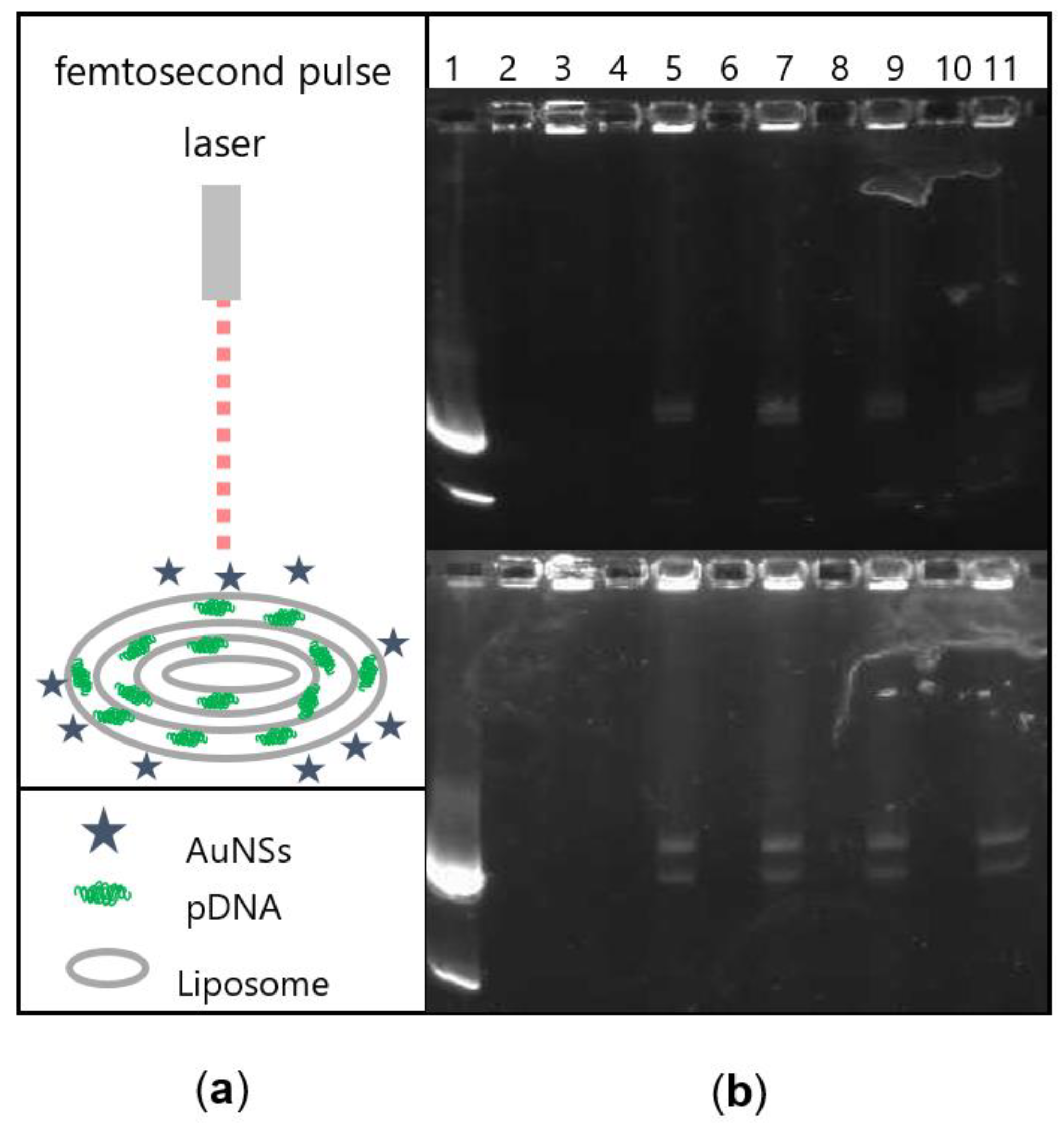
2.6. Femtosecond Laser Irradiation
2.7. Agarose Gel Electrophoresis
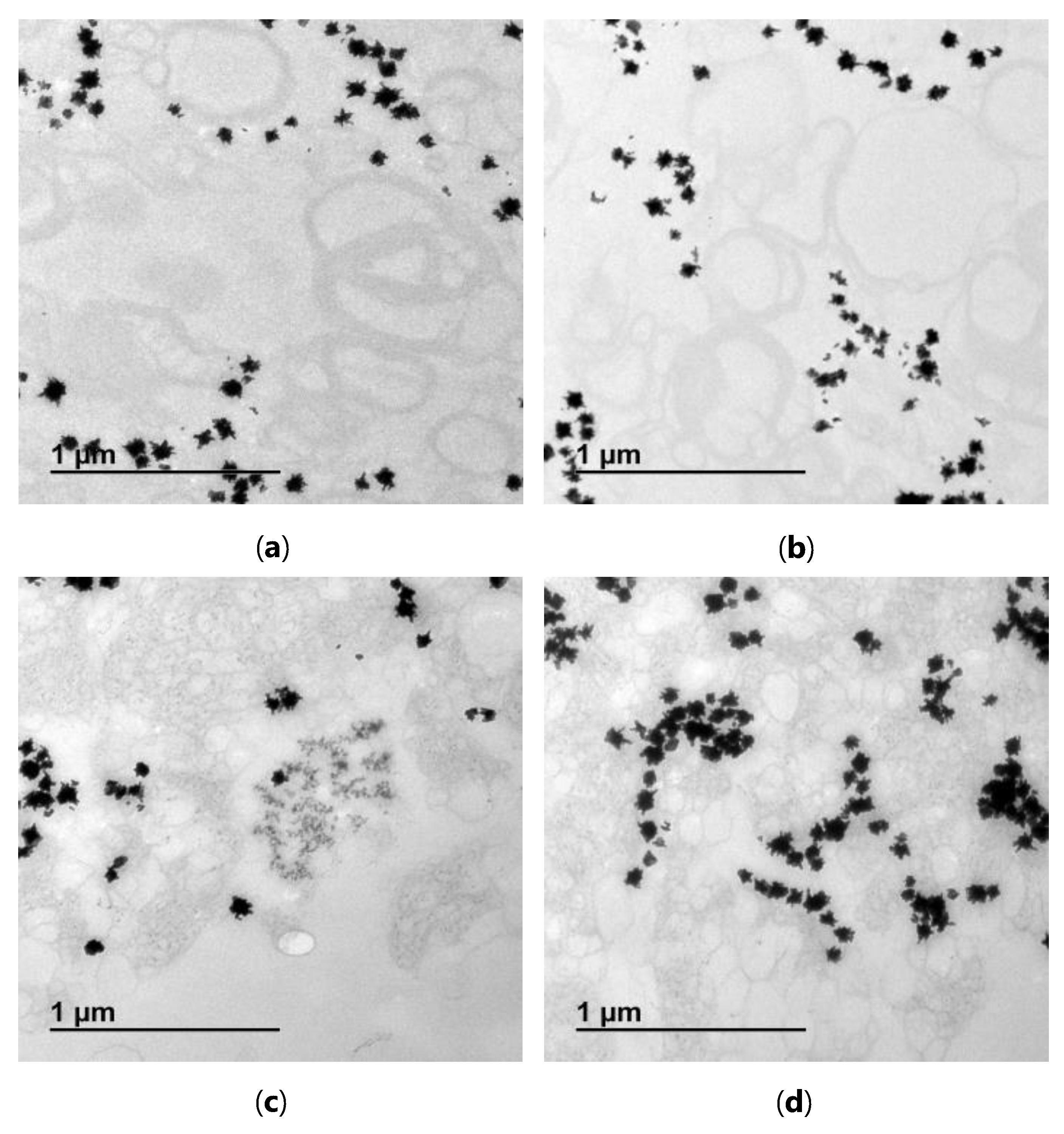
2.8. Transmission Electron Microscopy
3. Results and Discussion
3.1. Synthesis and Characterization of AuNSs
3.2. Formation and Characterization of the Lipoplex-AuNS System
3.3. Femtosecond Laser Irradiation of the Lipoplex-AuNS System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ponti, F.; Campolungo, M.; Melchiori, C.; Bono, N.; Candiani, G. Cationic lipids for gene delivery: Many players, one goal. Chem. Phys. Lipids 2021, 235. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, K. Viral Vectors in Gene Therapy. Diseases 2018, 6, 42. [Google Scholar] [CrossRef]
- Nayak, S.; Herzog, R.W. Progress and prospects: Immune responses to viral vectors. Gene Ther. 2010, 17, 295–304. [Google Scholar] [CrossRef]
- Jones, C.H.; Chen, C.K.; Ravikrishnan, A.; Rane, S.; Pfeifer, B.A. Overcoming nonviral gene delivery barriers: Perspective and future. Mol. Pharm. 2013, 10, 4082–4098. [Google Scholar] [CrossRef]
- Donkuru, M.; Badea, I.; Wettig, S.; Verrall, R.; Elsabahy, M.; Foldvari, M. Advancing nonviral gene delivery: Lipid- and surfactant-based nanoparticle design strategies. Nanomedicine 2010, 5, 1103–1127. [Google Scholar] [CrossRef]
- Ahmed, T.; Kamel, A.O.; Wettig, S.D. Interactions between DNA and gemini surfactant: Impact on gene therapy: Part I. Nanomedicine 2016, 11, 289–306. [Google Scholar] [CrossRef]
- Sharma, V.D.; Lees, J.; Hoffman, N.E.; Brailoiu, E.; Madesh, M.; Wunder, S.L.; Ilies, M.A. Modulation of pyridinium cationic lipid-DNA complex properties by pyridinium gemini surfactants and its impact on lipoplex transfection properties. Mol. Pharm. 2014, 11, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Llizo, A.; Li, P.; Wang, C.X.; Guo, Y.Y.; Ao, M.Q.; Bai, L.L.; Wang, C.; Yang, Y.L.; Xu, G.Y. High transfection efficiency of homogeneous DNA nanoparticles induced by imidazolium gemini surfactant as nonviral vector. J. Phys. Chem. C 2013, 117, 26573–26581. [Google Scholar] [CrossRef]
- Kumar, K.; Barrán-Berdón, A.L.; Datta, S.; Muñoz-Úbeda, M.; Aicart-Ramos, C.; Kondaiah, P.; Junquera, E.; Bhattacharya, S.; Aicart, E. A delocalizable cationic headgroup together with an oligo-oxyethylene spacer in gemini cationic lipids improves their biological activity as vectors of plasmid DNA. J. Mater. Chem. B 2015, 3, 1495–1506. [Google Scholar] [CrossRef]
- Misra, S.K.; Muñoz-Úbeda, M.; Datta, S.; Barrán-Berdón, A.L.; Aicart-Ramos, C.; Castro-Hartmann, P.; Kondaiah, P.; Junquera, E.; Bhattacharya, S.; Aicart, E. Effects of a delocalizable cation on the headgroup of gemini lipids on the lipoplex-type nano-aggregates directly formed from plasmid DNA. Biomacromolecules 2013, 14, 3951–3963. [Google Scholar] [CrossRef]
- Dass, C.R. Lipoplex-mediated delivery of nucleic acids: Factors affecting in vivo transfection. J. Mol. Med. 2004, 82, 579–591. [Google Scholar] [CrossRef]
- Junquera, E.; Aicart, E. Recent progress in gene therapy to deliver nucleic acids with multivalent cationic vectors. Adv. Colloid Interface Sci. 2016, 233, 161–175. [Google Scholar] [CrossRef]
- Zuhorn, I.S.; Engberts, J.; Hoekstra, D. Gene delivery by cationic lipid vectors: Overcoming cellular barriers. Eur. Biophys. J. 2007, 36, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Verrneulen, L.M.P.; Brans, T.; De Smedt, S.C.; Remaut, K.; Braeckmans, K. Methodologies to investigate intracellular barriers for nucleic acid delivery in non-viral gene therapy. Nano Today 2018, 21, 74–90. [Google Scholar] [CrossRef]
- Gilleron, J.; Querbes, W.; Zeigerer, A.; Borodovsky, A.; Marsico, G.; Schubert, U.; Manygoats, K.; Seifert, S.; Andree, C.; Stoter, M.; et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 2013, 31, 638-U102. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Kang, P.M. Recent advances in nanocarrier-assisted therapeutics delivery systems. Pharmaceutics 2020, 12, 837. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Hirata, T.; Yuba, E.; Harada, A.; Kono, K. Light-activatable transfection system using hybrid vectors composed of thermosensitive dendron lipids and gold nanorods. Pharmaceutics 2020, 12, 239. [Google Scholar] [CrossRef]
- Huang, X.H.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Singhana, B.; Slattery, P.; Chen, A.; Wallace, M.; Melancon, M.P. Light-activatable gold nanoshells for drug delivery applications. AAPS PharmSciTech 2014, 15, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Perez-Hernandez, M.; del Pino, P.; Mitchell, S.G.; Moros, M.; Stepien, G.; Pelaz, B.; Parak, W.J.; Galvez, E.M.; Pardo, J.; de la Fuente, J.M. Dissecting the molecular mechanism of apoptosis during photothermal therapy using gold nanoprisms. ACS Nano 2015, 9, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Martinez, A.; Barbosa, S.; Pastoriza-Santos, I.; Liz-Marzan, L.M. Nanostars shine bright for you Colloidal synthesis, properties and applications of branched metallic nanoparticles. Curr. Opin. Colloid Interface Sci. 2011, 16, 118–127. [Google Scholar] [CrossRef]
- Espinosa, A.; Silva, A.K.A.; Sanchez-Iglesias, A.; Grzelczak, M.; Pechoux, C.; Desboeufs, K.; Liz-Marzan, L.M.; Wilhelm, C. Cancer cell internalization of gold nanostars impacts their photothermal efficiency in vitro and in vivo: Toward a plasmonic thermal fingerprint in tumoral environment. Adv. Healthc. Mater. 2016, 5, 1040–1048. [Google Scholar] [CrossRef]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Mancini, M.C.; Nie, S.M. Second window for in vivo imaging. Nat. Nanotechnol. 2009, 4, 710–711. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.F.; Chang, S.H.G.; Cheng, F.Y.; Shanmugam, V.; Cheng, Y.S.; Su, C.H.; Yeh, C.S. Au nanorod design as light-absorber in the first and second biological near-infrared windows for in vivo photothermal therapy. ACS Nano 2013, 7, 5330–5342. [Google Scholar] [CrossRef] [PubMed]
- Ahijado-Guzman, R.; Sanchez-Arribas, N.; Martinez-Negro, M.; Gonzalez-Rubio, G.; Santiago-Varela, M.; Pardo, M.; Pineiro, A.; Lopez-Montero, I.; Junquera, E.; Guerrero-Martinez, A. Intercellular trafficking of gold nanostars in uveal melanoma cells for plasmonic photothermal therapy. Nanomaterials 2020, 10, 590. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rubio, G.; Guerrero-Martinez, A.; Liz-Marzan, L.M. Reshaping, fragmentation, and assembly of gold nanoparticles assisted by pulse lasers. Acc. Chem. Res. 2016, 49, 678–686. [Google Scholar] [CrossRef]
- Yuan, H.K.; Khoury, C.G.; Hwang, H.; Wilson, C.M.; Grant, G.A.; Vo-Dinh, T. Gold nanostars: Surfactant-free synthesis, 3D modelling, and two-photon photoluminescence imaging. Nanotechnology 2012, 23, 9. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Úbeda, M.; Misra, S.K.; Barrán-Berdón, A.L.; Datta, S.; Aicart-Ramos, C.; Castro-Hartmann, P.; Kondaiah, P.; Junquera, E.; Bhattacharya, S.; Aicart, E. How does the spacer length of cationic gemini lipids influence the lipoplex formation with plasmid DNA? Physicochemical and biochemical characterizations and their relevance in gene therapy. Biomacromolecules 2012, 13, 3926–3937. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Úbeda, M.; Misra, S.K.; Barrán-Berdón, A.L.; Aicart-Ramos, C.; Sierra, M.B.; Biswas, J.; Kondaiah, P.; Junquera, E.; Bhattacharya, S.; Aicart, E. Why is less cationic lipid required to prepare lipoplexes from plasmid DNA than linear DNA in gene therapy? J. Am. Chem. Soc. 2011, 133, 18014–18017. [Google Scholar] [CrossRef]
- Pelaz, B.; del Pino, P.; Maffre, P.; Hartmann, R.; Gallego, M.; Rivera-Fernandez, S.; de la Fuente, J.M.; Nienhaus, G.U.; Parak, W.J. Surface functionalization of nanoparticles with polyethylene glycol: Effects on protein adsorption and cellular uptake. ACS Nano 2015, 9, 6996–7008. [Google Scholar] [CrossRef] [PubMed]
- Ahijado-Guzman, R.; Gonzalez-Rubio, G.; Izquierdo, J.G.; Banares, L.; Lopez-Montero, I.; Calzado-Martin, A.; Calleja, M.; Tardajos, G.; Guerrero-Martinez, A. Intracellular pH-induced tip-to-tip assembly of gold nanorods for enhanced plasmonic photothermal therapy. ACS Omega 2016, 1, 388–395. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Arribas, N.; Díaz-Núñez, P.; Osío Barcina, J.; Aicart, E.; Junquera, E.; Guerrero-Martínez, A. Controlled pDNA Release in Gemini Cationic Lipoplexes by Femtosecond Laser Irradiation of Gold Nanostars. Nanomaterials 2021, 11, 1498. https://doi.org/10.3390/nano11061498
Sánchez-Arribas N, Díaz-Núñez P, Osío Barcina J, Aicart E, Junquera E, Guerrero-Martínez A. Controlled pDNA Release in Gemini Cationic Lipoplexes by Femtosecond Laser Irradiation of Gold Nanostars. Nanomaterials. 2021; 11(6):1498. https://doi.org/10.3390/nano11061498
Chicago/Turabian StyleSánchez-Arribas, Natalia, Pablo Díaz-Núñez, José Osío Barcina, Emilio Aicart, Elena Junquera, and Andrés Guerrero-Martínez. 2021. "Controlled pDNA Release in Gemini Cationic Lipoplexes by Femtosecond Laser Irradiation of Gold Nanostars" Nanomaterials 11, no. 6: 1498. https://doi.org/10.3390/nano11061498
APA StyleSánchez-Arribas, N., Díaz-Núñez, P., Osío Barcina, J., Aicart, E., Junquera, E., & Guerrero-Martínez, A. (2021). Controlled pDNA Release in Gemini Cationic Lipoplexes by Femtosecond Laser Irradiation of Gold Nanostars. Nanomaterials, 11(6), 1498. https://doi.org/10.3390/nano11061498






