PVDF Composite Membranes with Hydrophobically-Capped CuONPs for Direct-Contact Membrane Distillation
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
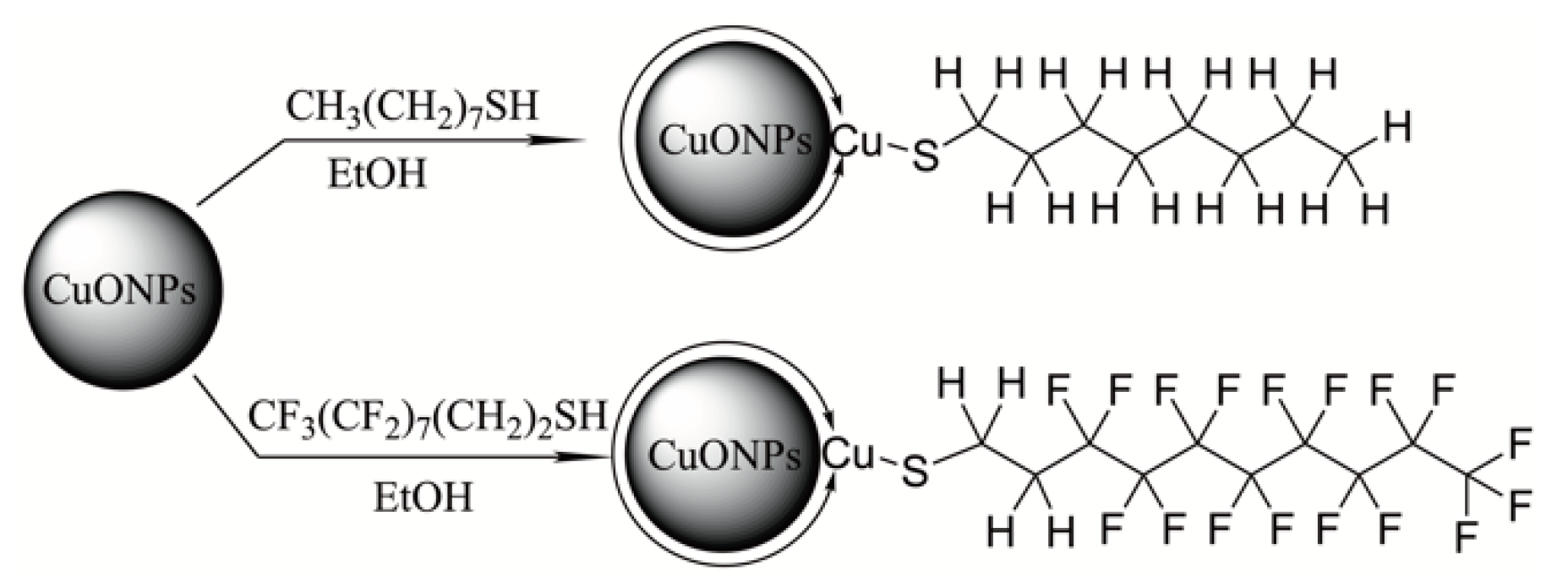
2.2.1. Surface Modification of CuONPs
2.2.2. Preparation of the PVDF Casting Solution Containing CuO Nanoparticles
2.2.3. Preparation of PVDF-CuO Composite Membranes with CuO Nanoparticles
2.2.4. Equipment
ATR-FT-IR
Thermogravimetric Measurements
2.2.5. Characterization of PVDF-CuO@CH and PVDF-CuO@CF Composite Membranes
Scanning Electron Microscopy
Water Contact Angle Measurements
Surface Roughness Determination
Membrane Porosity
Membrane Performance
3. Results and Discussion
3.1. Preparation and Characterization of Hydrophobically Capped CuONPs
3.1.1. ATR-FT-IR Characterization
3.1.2. Thermogravimetry Analysis (TGA)
3.2. Membrane Preparation and Characterization
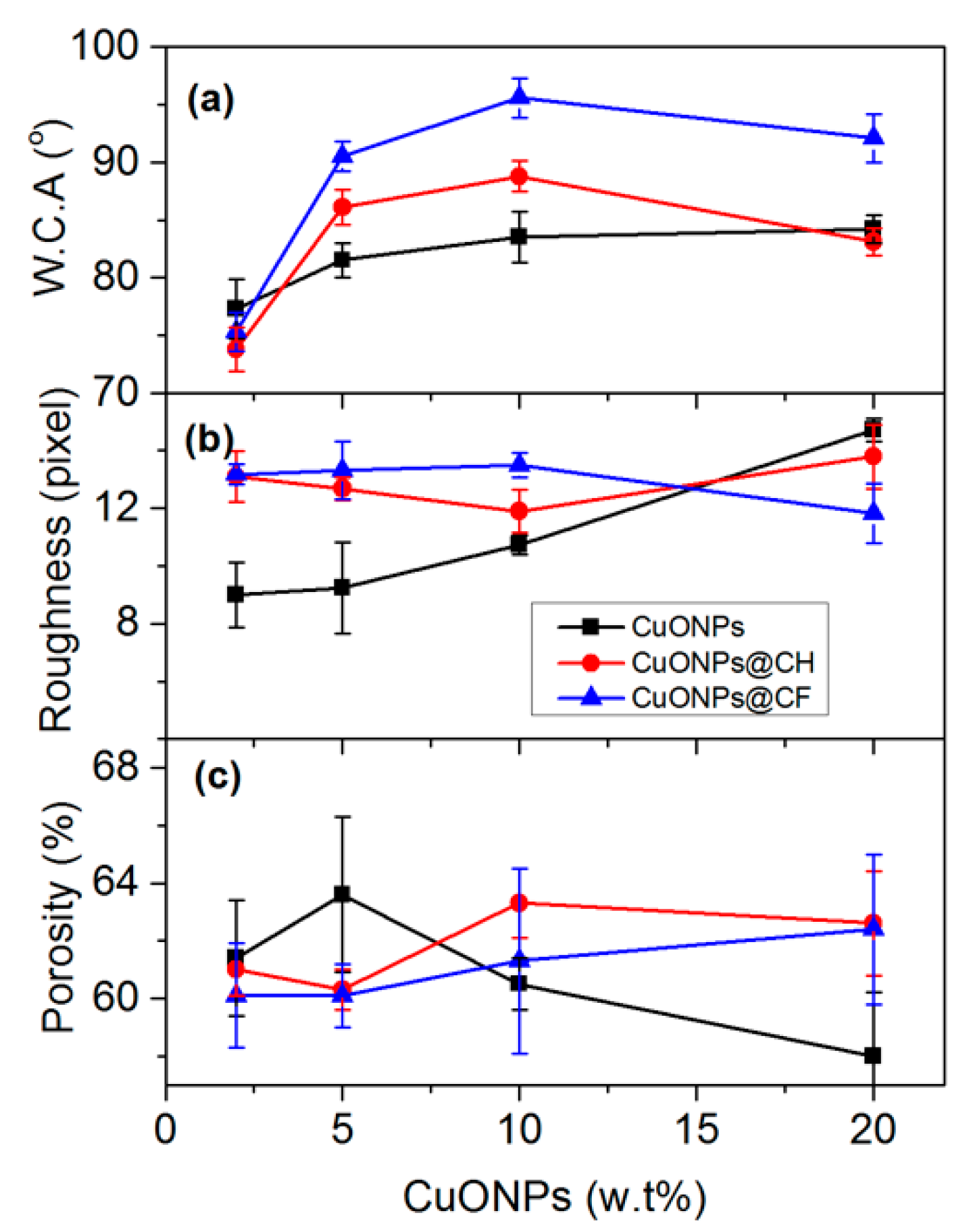
3.2.1. Water Contact Angle, Membrane Roughness and Porosity
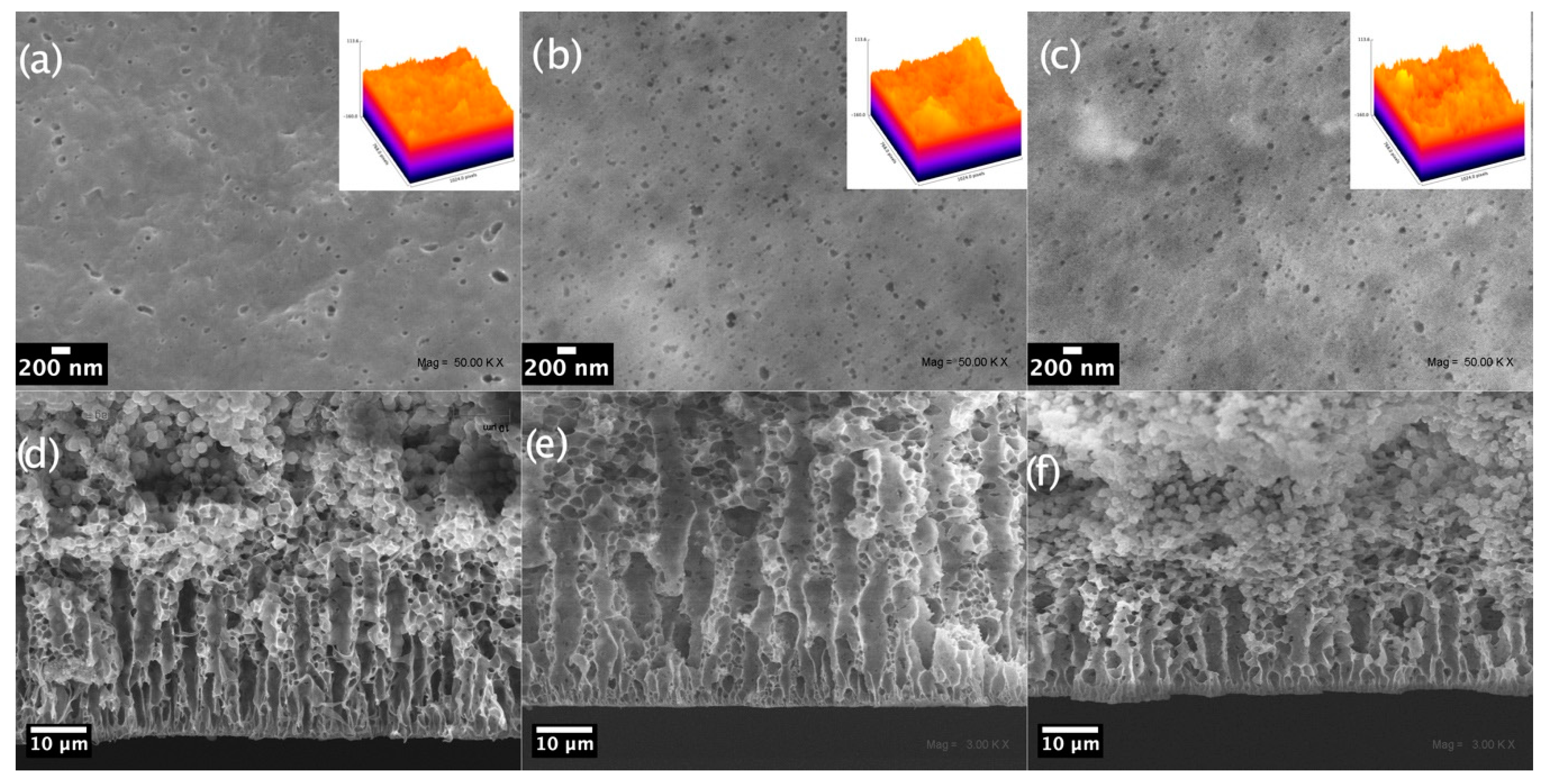
3.2.2. SEM Micrograph Analysis of Membranes
- I.
- In membranes containing CuONPs, the finger-like pore lengths increased when the nanoparticle concentration increased from 2% to 5% but decreased at 10%.
- II.
- In membranes containing CuONPs@CH, finger-like pore lengths were similar at the two lower nanoparticle concentrations but larger at 10%.
- III.
- In membranes containing CuONPs@CF, finger-like pore lengths increased when increasing the nanoparticle concentration.
- (a)
- A casting solution with hydrophilic nanoparticles (naked CuONPs) draws non-solvent molecules (water) to a deeper level into the polymer solution and at a higher rate when the concentration increases from 2% to 5%. The faster the demixing, the larger the finger-like pore layer. At a concentration of 10%, the casting solution increases in viscosity and delayed demixing takes place. Under this regime, a decrease in the finger-like layer thickness occurs as observed here.
- (b)
- A casting solution with hydrophobically capped nanoparticles (CuONPs@CH and CuONPs@CF) has low affinity for the used solvent (DMF) but high affinity for the polymer backbone (-(CF2-CH2)-). Therefore, the casting solution becomes more thermodynamically unstable when increasing the nanoparticle concentration. The more unstable the solution, the higher the demixing rate and the larger the formed finger-like pores [55].
3.2.3. Membranes Performance Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- The United Nations World Water Development Report 2020: Water and Climate Change; United Nations Educational, Scientific and Cultural Organization: Paris, France, 2020; ISBN 9789231003714.
- Mekonnen, M.M.; Hoekstra, A.Y. Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef]
- Boretti, A.; Rosa, L. Reassessing the projections of the World Water Development Report. NPJ Clean Water 2019, 2, 15. [Google Scholar] [CrossRef]
- Cheng, H.-H.; Pien, T.-T.; Lee, Y.-C.; Lu, I.-C.; Whang, L.-M. Effects of copper on biological treatment of NMF- and MDG-containing wastewater from TFT-LCD industry. Chemosphere 2020, 258, 127125. [Google Scholar] [CrossRef] [PubMed]
- Ravi, J.; Othman, M.H.D.; Matsuura, T.; Ro’il Bilad, M.; El-badawy, T.H.; Aziz, F.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. Polymeric membranes for desalination using membrane distillation: A review. Desalination 2020, 490, 114530. [Google Scholar] [CrossRef]
- Ozbey-Unal, B.; Gezmis-Yavuz, E.; Eryildiz, B.; Koseoglu-Imer, D.Y.; Keskinler, B.; Koyuncu, I. Boron removal from geothermal water by nanofiber-based membrane distillation membranes with significantly improved surface hydrophobicity. J. Environ. Chem. Eng. 2020, 8, 104113. [Google Scholar] [CrossRef]
- Thabit, M.S.; Hawari, A.H.; Ammar, M.H.; Zaidi, S.; Zaragoza, G.; Altaee, A. Evaluation of forward osmosis as a pretreatment process for multi stage flash seawater desalination. Desalination 2019, 461, 22–29. [Google Scholar] [CrossRef]
- Delgado-Torres, A.M.; García-Rodríguez, L.; del Moral, M.J. Preliminary assessment of innovative seawater reverse osmosis (SWRO) desalination powered by a hybrid solar photovoltaic (PV)—Tidal range energy system. Desalination 2020, 477, 114247. [Google Scholar] [CrossRef]
- Park, K.; Kim, J.; Yang, D.R.; Hong, S. Towards a low-energy seawater reverse osmosis desalination plant: A review and theoretical analysis for future directions. J. Membr. Sci. 2020, 595, 117607. [Google Scholar] [CrossRef]
- Winter, D.; Koschikowski, J.; Ripperger, S. Desalination using membrane distillation: Flux enhancement by feed water deaeration on spiral-wound modules. J. Membr. Sci. 2012, 423–424, 215–224. [Google Scholar] [CrossRef]
- Boriskina, S.V.; Raza, A.; Zhang, T.; Wang, P.; Zhou, L.; Zhu, J. Nanomaterials for the water-energy nexus. MRS Bull. 2019, 44, 59–66. [Google Scholar] [CrossRef]
- Politano, A.; Argurio, P.; Di Profio, G.; Sanna, V.; Cupolillo, A.; Chakraborty, S.; Arafat, H.A.; Curcio, E. Photothermal Membrane Distillation for Seawater Desalination. Adv. Mater. 2017, 29, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lawal, D.U.; Qasem, N.A.A. Humidification-dehumidification desalination systems driven by thermal-based renewable and low-grade energy sources: A critical review. Renew. Sustain. Energy Rev. 2020, 125, 109817. [Google Scholar] [CrossRef]
- Koschikowski, J.; Wieghaus, M.; Rommel, M. Solar thermal-driven desalination plants based on membrane distillation. Desalination 2003, 156, 295–304. [Google Scholar] [CrossRef]
- Zarzoum, K.; Zhani, K.; Ben Bacha, H.; Koschikowski, J. Experimental parametric study of membrane distillation unit using solar energy. Sol. Energy 2019, 188, 1274–1282. [Google Scholar] [CrossRef]
- Noamani, S.; Niroomand, S.; Rastgar, M.; McDonald, A.; Sadrzadeh, M. Development of a self-sustained model to predict the performance of direct contact membrane distillation. Sep. Purif. Technol. 2021, 263, 118407. [Google Scholar] [CrossRef]
- Li, K.; Hou, D.; Fu, C.; Wang, K.; Wang, J. Fabrication of PVDF nanofibrous hydrophobic composite membranes reinforced with fabric substrates via electrospinning for membrane distillation desalination. J. Environ. Sci. 2019, 75, 277–288. [Google Scholar] [CrossRef]
- Frappa, M.; Del Rio Castillo, A.E.; Macedonio, F.; Politano, A.; Drioli, E.; Bonaccorso, F.; Pellegrini, V.; Gugliuzza, A. A few-layer graphene for advanced composite PVDF membranes dedicated to water desalination: A comparative study. Nanoscale Adv. 2020, 2, 4728–4739. [Google Scholar] [CrossRef]
- Terraza, C.A.; Martin-Trasanco, R.; Saldías, C.; González, M.; Leiva, Á.; Tundidor-Camba, A. Preparation of CuONPs@PVDF/Non-Woven Polyester Composite Membrane: Structural Influence of Nanoparticle Addition. Polymers 2018, 10, 862. [Google Scholar] [CrossRef] [PubMed]
- Ardeshiri, F.; Salehi, S.; Peyravi, M.; Jahanshahi, M.; Amiri, A.; Rad, A.S. PVDF membrane assisted by modified hydrophobic ZnO nanoparticle for membrane distillation. Asia Pac. J. Chem. Eng. 2018, 13, 1–12. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, C.; Lu, X.; Ng, D.; Truong, Y.B.; Xie, Z. Activated carbon enhanced hydrophobic/hydrophilic dual-layer nanofiber composite membranes for high-performance direct contact membrane distillation. Desalination 2018, 446, 59–69. [Google Scholar] [CrossRef]
- Kharraz, J.A.; Farid, M.U.; Khanzada, N.K.; Deka, B.J.; Arafat, H.A.; An, A.K. Macro-corrugated and nano-patterned hierarchically structured superomniphobic membrane for treatment of low surface tension oily wastewater by membrane distillation. Water Res. 2020, 174, 115600. [Google Scholar] [CrossRef] [PubMed]
- Deka, B.J.; Guo, J.; Khanzada, N.K.; An, A.K. Omniphobic re-entrant PVDF membrane with ZnO nanoparticles composite for desalination of low surface tension oily seawater. Water Res. 2019, 165, 114982. [Google Scholar] [CrossRef] [PubMed]
- Nthunya, L.N.; Gutierrez, L.; Nxumalo, E.N.; Verliefde, A.R.; Mhlanga, S.D.; Onyango, M.S. f-MWCNTs/AgNPs-coated superhydrophobic PVDF nanofibre membrane for organic, colloidal, and biofouling mitigation in direct contact membrane distillation. J. Environ. Chem. Eng. 2020, 8, 103654. [Google Scholar] [CrossRef]
- Tijing, L.D.; Woo, Y.C.; Shim, W.G.; He, T.; Choi, J.S.; Kim, S.H.; Shon, H.K. Superhydrophobic nanofiber membrane containing carbon nanotubes for high-performance direct contact membrane distillation. J. Membr. Sci. 2016, 502, 158–170. [Google Scholar] [CrossRef]
- Fahmey, M.S.; El-Aassar, A.H.M.; Abo-Elfadel, M.; Orabi, A.S.; Das, R. Comparative performance evaluations of nanomaterials mixed polysulfone: A scale-up approach through vacuum enhanced direct contact membrane distillation for water desalination. Desalination 2019, 451, 111–116. [Google Scholar] [CrossRef]
- Li, Z.; Rana, D.; Wang, Z.; Matsuura, T.; Lan, C.Q. Synergic effects of hydrophilic and hydrophobic nanoparticles on performance of nanocomposite distillation membranes: An experimental and numerical study. Sep. Purif. Technol. 2018, 202, 45–58. [Google Scholar] [CrossRef]
- Al-Gharabli, S.; Kujawski, W.; Arafat, H.A.; Kujawa, J. Tunable separation via chemical functionalization of polyvinylidenefluoride membranes using piranha reagent. J. Membr. Sci. 2017, 541, 567–579. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, S.K.; Lindström, T.; Hsiao, B.S. Nanocellulose-Enabled Membranes for Water Purification: Perspectives. Adv. Sustain. Syst. 2020, 4, 1900114. [Google Scholar] [CrossRef]
- Wu, W.; Huang, R.; Qi, W.; Su, R.; He, Z. Bioinspired Peptide-Coated Superhydrophilic Poly(vinylidene fluoride) Membrane for Oil/Water Emulsion Separation. Langmuir 2018, 34, 6621–6627. [Google Scholar] [CrossRef]
- Shen, S.; Hao, Y.; Zhang, Y.; Zhang, G.; Zhou, X.; Bai, R.B. Enhancing the Antifouling Properties of Poly(vinylidene fluoride) (PVDF) Membrane through a Novel Blending and Surface-Grafting Modification Approach. ACS Omega 2018, 3, 17403–17415. [Google Scholar] [CrossRef]
- Liang, S.; Kang, Y.; Tiraferri, A.; Giannelis, E.P.; Huang, X.; Elimelech, M. Highly Hydrophilic Polyvinylidene Fluoride (PVDF) Ultrafiltration Membranes via Postfabrication Grafting of Surface-Tailored Silica Nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 6694–6703. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Z.; Mai, W.; Min, C.; Zhou, B.; Shan, M.; Li, Y.; Yang, C.; Wang, Z.; Qian, X. Improved hydrophilicity, permeability, antifouling and mechanical performance of PVDF composite ultrafiltration membranes tailored by oxidized low-dimensional carbon nanomaterials. J. Mater. Chem. A 2013, 1, 3101. [Google Scholar] [CrossRef]
- Zhao, C.; Lv, J.; Xu, X.; Zhang, G.; Yang, Y.; Yang, F. Highly antifouling and antibacterial performance of poly (vinylidene fluoride) ultrafiltration membranes blending with copper oxide and graphene oxide nanofillers for effective wastewater treatment. J. Colloid Interface Sci. 2017, 505, 341–351. [Google Scholar] [CrossRef]
- Ren, G.; Hu, D.; Cheng, E.W.C.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Sri Abirami Saraswathi, M.S.; Rana, D.; Divya, K.; Gowrishankar, S.; Nagendran, A. Versatility of hydrophilic and antifouling PVDF ultrafiltration membranes tailored with polyhexanide coated copper oxide nanoparticles. Polym. Test. 2020, 84, 106367. [Google Scholar] [CrossRef]
- Sri Abirami Saraswathi, M.S.; Rana, D.; Divya, K.; Gowrishankar, S.; Sakthivel, A.; Alwarappan, S.; Nagendran, A. Highly permeable, antifouling and antibacterial poly(ether imide) membranes tailored with poly(hexamethylenebiguanide) coated copper oxide nanoparticles. Mater. Chem. Phys. 2020, 240, 122224. [Google Scholar] [CrossRef]
- Baghbanzadeh, M.; Rana, D.; Matsuura, T.; Lan, C.Q. Effects of hydrophilic CuO nanoparticles on properties and performance of PVDF VMD membranes. Desalination 2015, 369, 75–84. [Google Scholar] [CrossRef]
- Li, J.; Shi, L.; Chen, Y.; Zhang, Y.; Guo, Z.; Su, B.L.; Liu, W. Stable superhydrophobic coatings from thiol-ligand nanocrystals and their application in oil/water separation. J. Mater. Chem. 2012, 22, 9774–9781. [Google Scholar] [CrossRef]
- Wang, B.; Li, J.; Wang, G.; Liang, W.; Zhang, Y.; Shi, L.; Guo, Z.; Liu, W. Methodology for robust superhydrophobic fabrics and sponges from in situ growth of transition metal/metal oxide nanocrystals with thiol modification and their applications in oil/water separation. ACS Appl. Mater. Interfaces 2013, 5, 1827–1839. [Google Scholar] [CrossRef]
- Basu, M.; Sinha, A.K.; Pradhan, M.; Sarkar, S.; Negishi, Y.; Pal, T. Fabrication and functionalization of CuO for tuning superhydrophobic thin film and cotton wool. J. Phys. Chem. C 2011, 115, 20953–20963. [Google Scholar] [CrossRef]
- Guo, J.; Yang, F.; Guo, Z. Fabrication of stable and durable superhydrophobic surface on copper substrates for oil-water separation and ice-over delay. J. Colloid Interface Sci. 2016, 466, 36–43. [Google Scholar] [CrossRef]
- Wen, Q.; Guo, F.; Peng, Y.; Guo, Z. Simple fabrication of superamphiphobic copper surfaces with multilevel structures. Colloids Surf. A Physicochem. Eng. Asp. 2018, 539, 11–17. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Chinga, G.; Johnsen, P.O.; Dougherty, R.; Berli, E.L.; Walter, J. Quantification of the 3D microstructure of SC surfaces. J. Microsc. 2007, 227, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Winter, D. Membrane Distillation A Thermodynamic, Technological and Economic Analysis. Ph.D. Thesis, University of Kaiserslautern, Kaiserslautern, Germany, 2014. [Google Scholar]
- Hagemann, H.; Bill, H.; Sadowski, W.; Walker, E.; François, M. Raman spectra of single crystal CuO. Solid State Commun. 1990, 73, 447–451. [Google Scholar] [CrossRef]
- Kang, G.; Cao, Y. Application and modification of poly(vinylidene fluoride) (PVDF) membranes—A review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Boo, C.; Lee, J.; Elimelech, M. Omniphobic Polyvinylidene Fluoride (PVDF) Membrane for Desalination of Shale Gas Produced Water by Membrane Distillation. Environ. Sci. Technol. 2016, 50, 12275–12282. [Google Scholar] [CrossRef]
- Munirasu, S.; Banat, F.; Durrani, A.A.; Haija, M.A. Intrinsically superhydrophobic PVDF membrane by phase inversion for membrane distillation. Desalination 2017, 417, 77–86. [Google Scholar] [CrossRef]
- Smolders, C.A.; Reuvers, A.J.; Boom, R.M.; Wienk, I.M. Microstructures in phase-inversion membranes. Part 1. Formation of macrovoids. J. Membr. Sci. 1992, 73, 259–275. [Google Scholar] [CrossRef]
- Matsuura, T. Synthetic Membranes and Membrane Separation Processing. Sep. Purif. Methods 1994, 23, 71–73. [Google Scholar] [CrossRef]
- Bakeri, G.; Matsuura, T.; Ismail, A.F. The effect of phase inversion promoters on the structure and performance of polyetherimide hollow fiber membrane using in gas-liquid contacting process. J. Membr. Sci. 2011, 383, 159–169. [Google Scholar] [CrossRef]
- Chen, Z.; Rana, D.; Matsuura, T.; Yang, Y.; Lan, C.Q. Study on the structure and vacuum membrane distillation performance of PVDF composite membranes: I. Influence of blending. Sep. Purif. Technol. 2014, 133, 303–312. [Google Scholar] [CrossRef]
- Fan, H.; Peng, Y. Application of PVDF membranes in desalination and comparison of the VMD and DCMD processes. Chem. Eng. Sci. 2012, 79, 94–102. [Google Scholar] [CrossRef]
- Hou, D.; Dai, G.; Wang, J.; Fan, H.; Zhang, L.; Luan, Z. Preparation and characterization of PVDF/nonwoven fabric flat-sheet composite membranes for desalination through direct contact membrane distillation. Sep. Purif. Technol. 2012, 101, 1–10. [Google Scholar] [CrossRef]
- Noamani, S.; Niroomand, S.; Rastgar, M.; Azhdarzadeh, M.; Sadrzadeh, M. Modeling of Air-Gap Membrane Distillation and Comparative Study with Direct Contact Membrane Distillation. Ind. Eng. Chem. Res. 2020, 59, 21930–21947. [Google Scholar] [CrossRef]
- Noor, I.E.; Coenen, J.; Martin, A.; Dahl, O. Performance assessment of chemical mechanical planarization wastewater treatment in nano-electronics industries using membrane distillation. Sep. Purif. Technol. 2020, 235, 116201. [Google Scholar] [CrossRef]





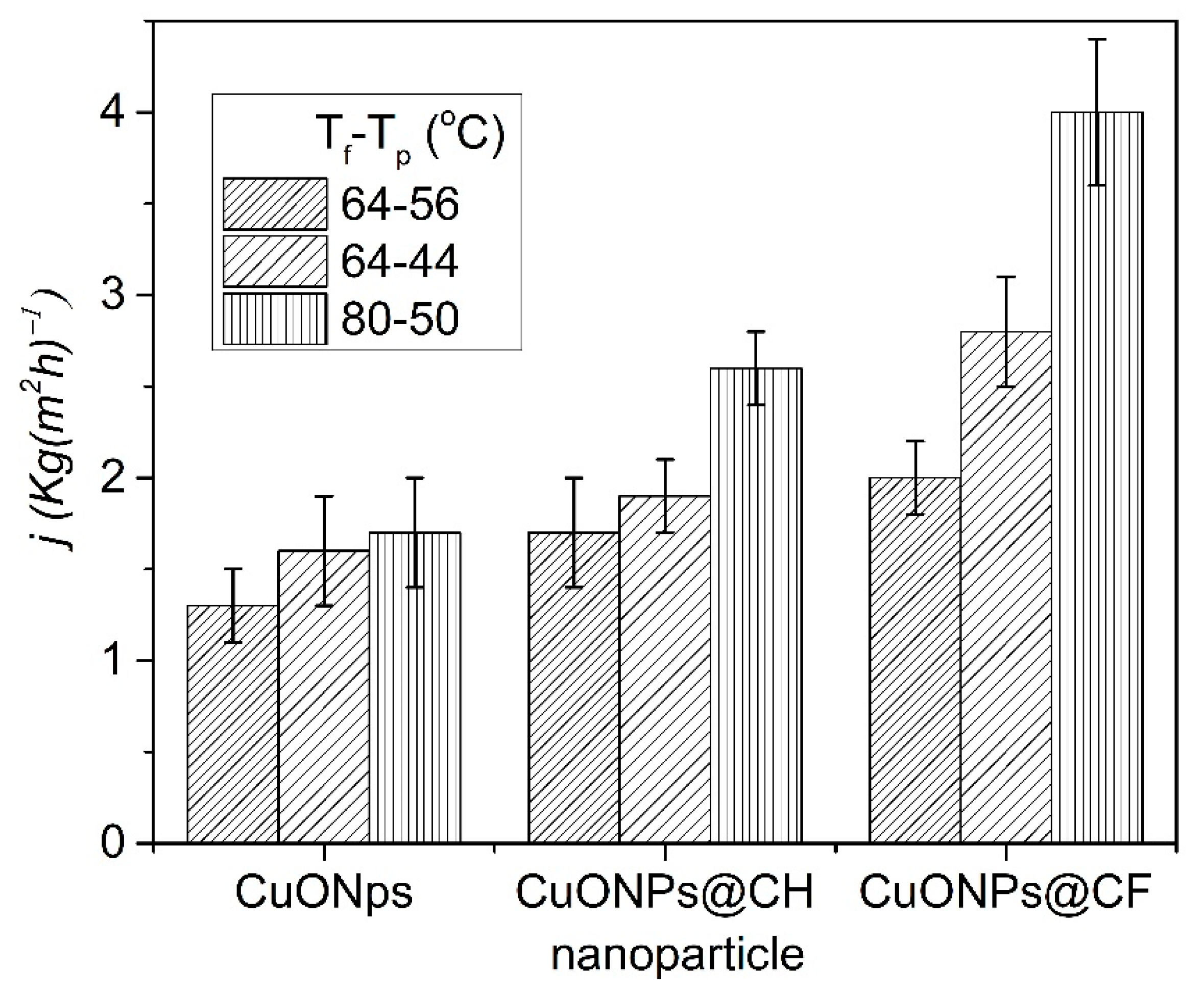
| Membrane | Thickness (μm) | TF–Tp °C | Porosity (%) | WCA (°) | J (kg(m2·h)−1) | Ref. |
|---|---|---|---|---|---|---|
| CuO-PVDF | 20–30 | VMD | 85–90 | 73–90 | (a) 1.25, 2.5, 4.5 | [39] |
| PVDF-NWPET | 200 | 80.5–20 | 49–54 | 75–85 | 47.6 | [57] |
| CuONPs-PVDF-NWPET | 260 | 80–50 | 63.6 | 81.5 | 1.8 | t.w |
| CuONPs@CH-PVDF-NWPET | 250 | 80–50 | 63.3 | 88.8 | 2.7 | t.w |
| CuONPs@CF-PVDF-NWPET | 220 | 80–50 | 61.3 | 95.6 | 3.9 | t.w |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saldías, C.; Terraza, C.A.; Leiva, A.; Koschikowski, J.; Winter, D.; Tundidor-Camba, A.; Martin-Trasanco, R. PVDF Composite Membranes with Hydrophobically-Capped CuONPs for Direct-Contact Membrane Distillation. Nanomaterials 2021, 11, 1497. https://doi.org/10.3390/nano11061497
Saldías C, Terraza CA, Leiva A, Koschikowski J, Winter D, Tundidor-Camba A, Martin-Trasanco R. PVDF Composite Membranes with Hydrophobically-Capped CuONPs for Direct-Contact Membrane Distillation. Nanomaterials. 2021; 11(6):1497. https://doi.org/10.3390/nano11061497
Chicago/Turabian StyleSaldías, César, Claudio A. Terraza, Angel Leiva, Joachim Koschikowski, Daniel Winter, Alain Tundidor-Camba, and Rudy Martin-Trasanco. 2021. "PVDF Composite Membranes with Hydrophobically-Capped CuONPs for Direct-Contact Membrane Distillation" Nanomaterials 11, no. 6: 1497. https://doi.org/10.3390/nano11061497
APA StyleSaldías, C., Terraza, C. A., Leiva, A., Koschikowski, J., Winter, D., Tundidor-Camba, A., & Martin-Trasanco, R. (2021). PVDF Composite Membranes with Hydrophobically-Capped CuONPs for Direct-Contact Membrane Distillation. Nanomaterials, 11(6), 1497. https://doi.org/10.3390/nano11061497





