Globular Aggregates Stemming from the Self-Assembly of an Amphiphilic N-Annulated Perylene Bisimide in Aqueous Media
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthetic Details and Characterization
3. Results
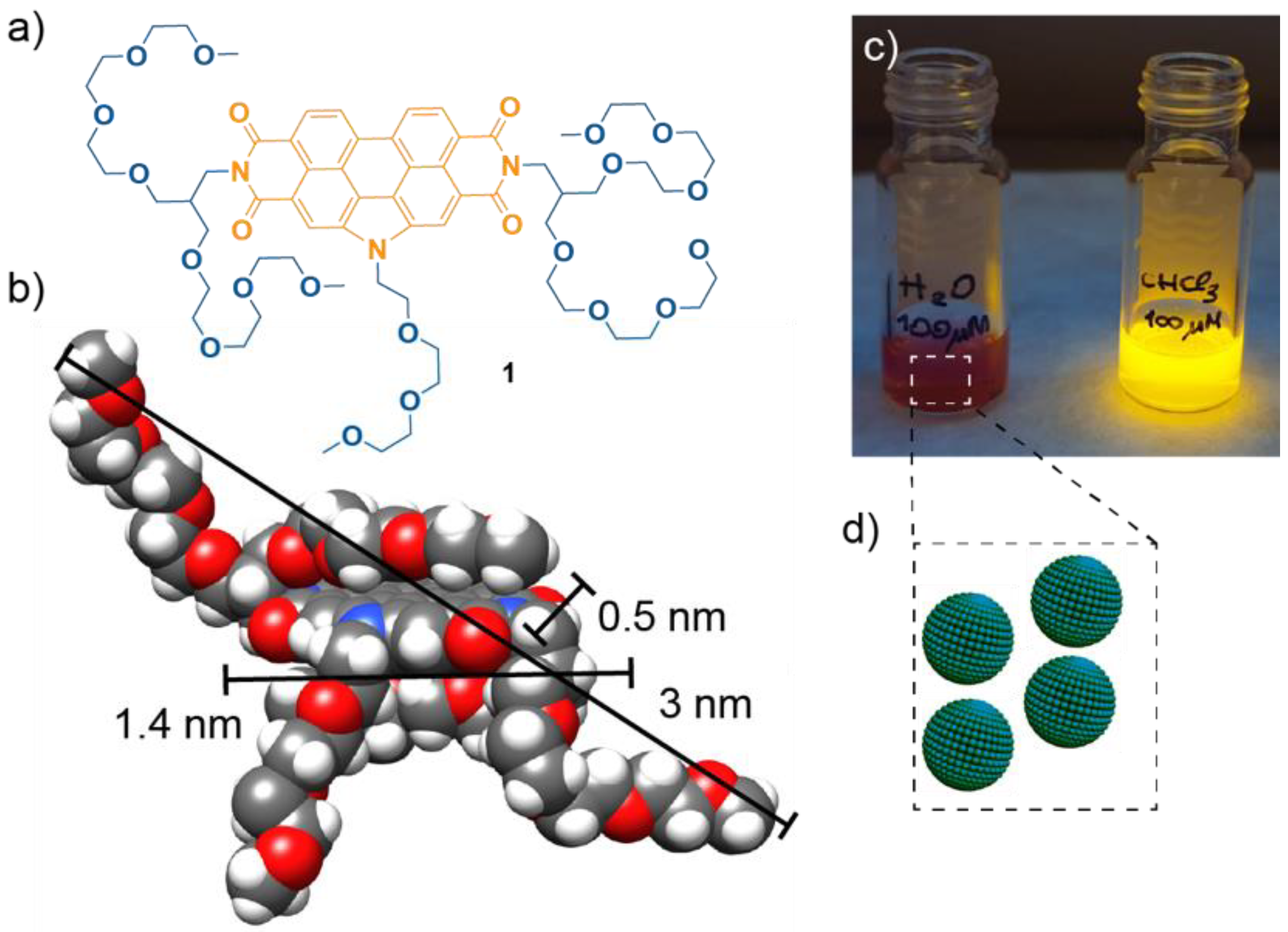
3.1. Synthesis of the Amphiphilic N-annulated PBI 1
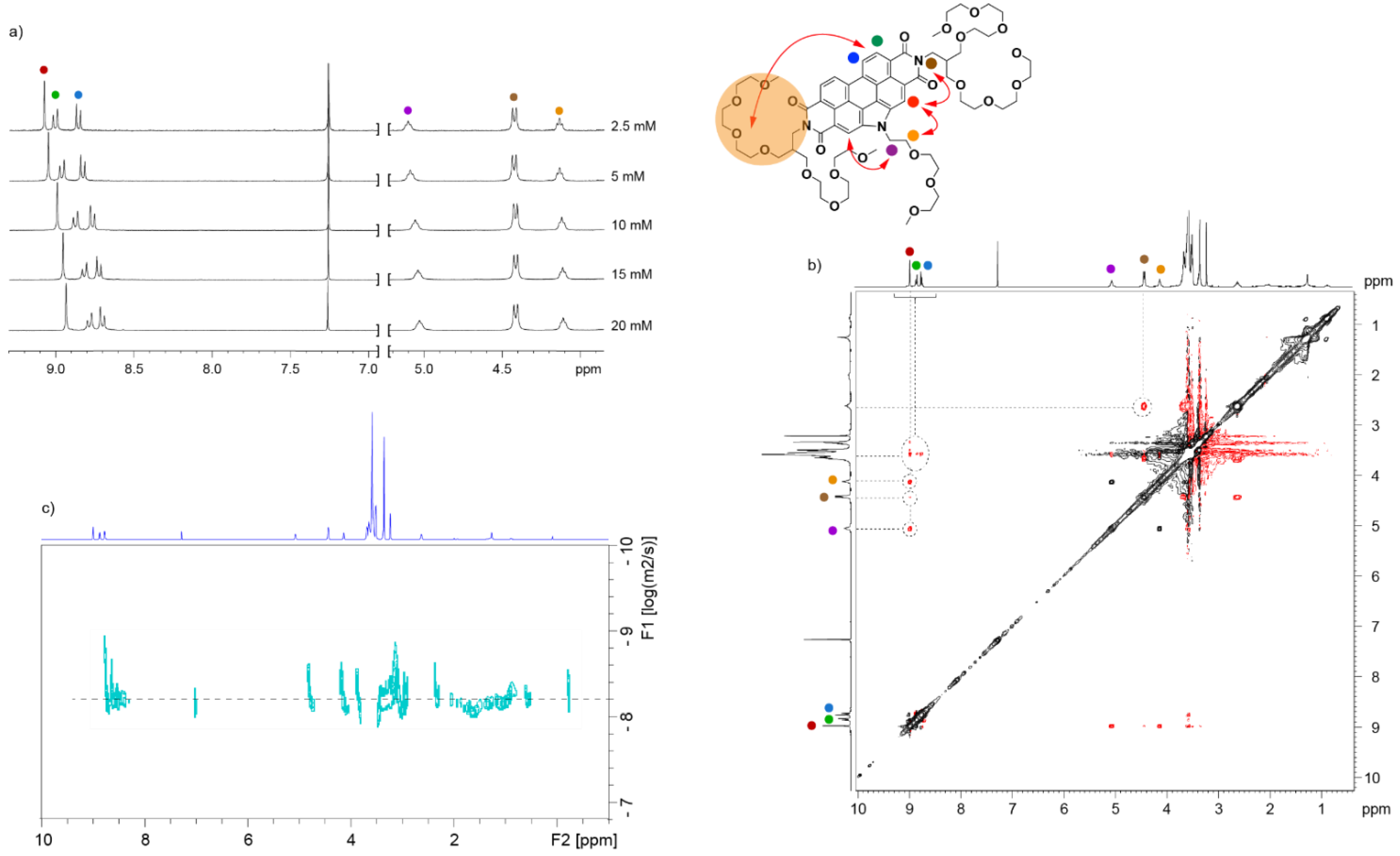
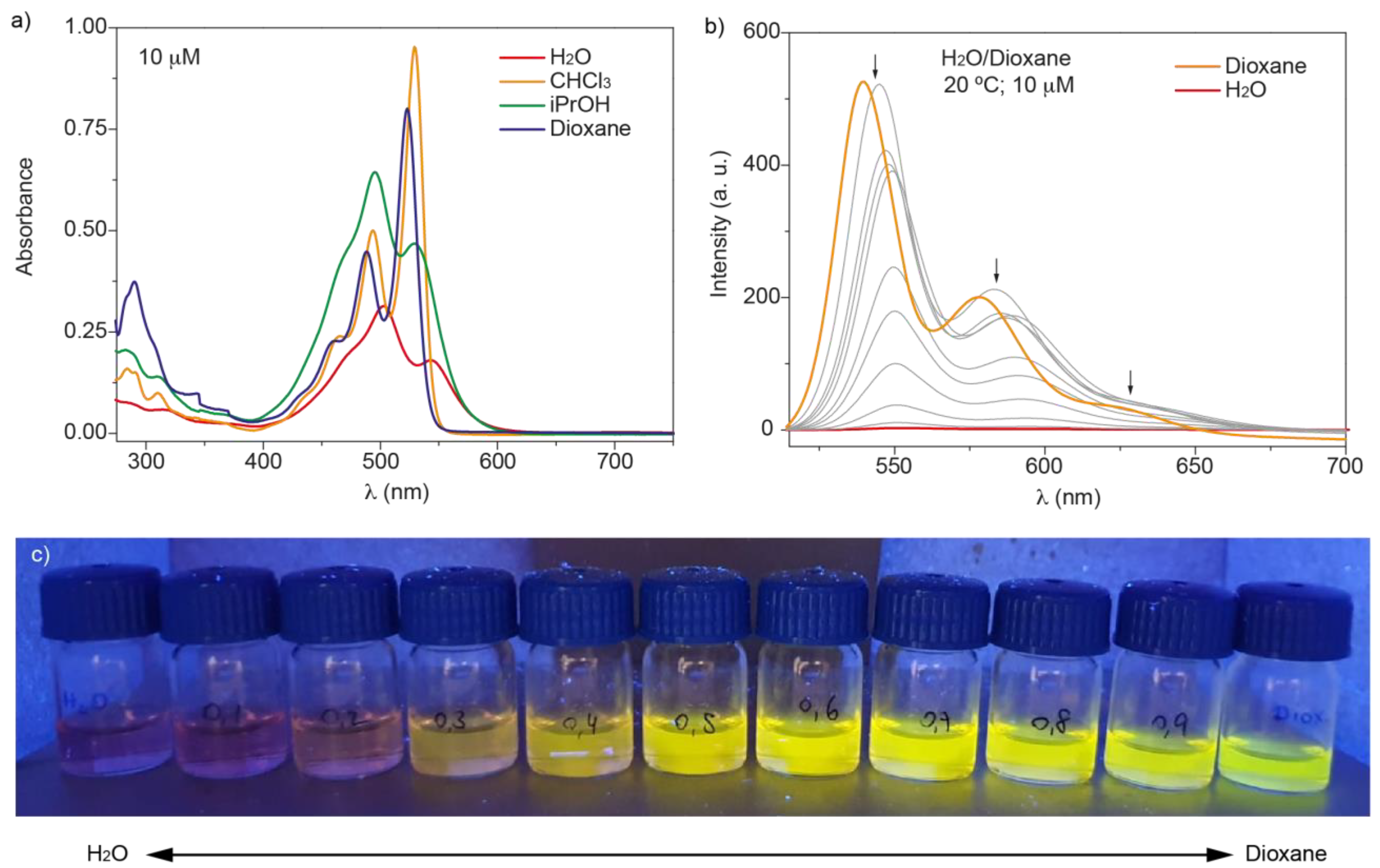
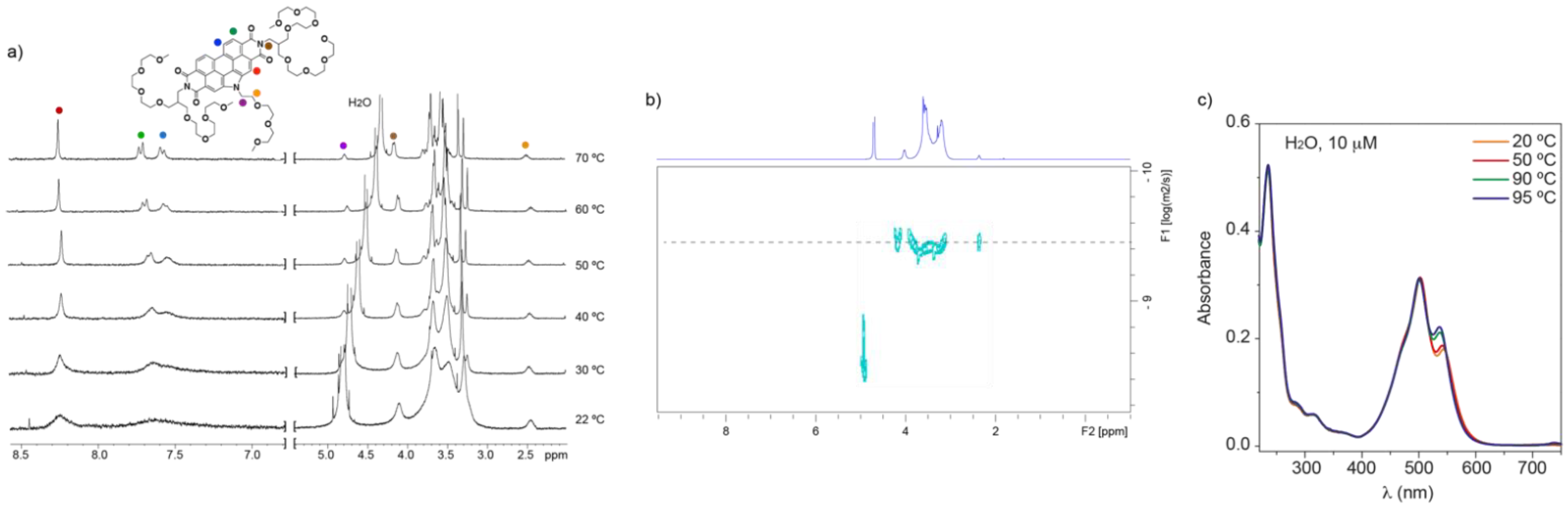
3.2. Self-Assembly of N-annulated PBI 1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Oshovsky, G.V.; Reinhoudt, D.N.; Verboom, W. Supramolecular Chemistry in Water. Angew. Chem. Int. Ed. 2007, 46, 2366–2393. [Google Scholar] [CrossRef]
- Krieg, E.; Bastings, M.M.C.; Besenius, P.; Rybtchinski, B. Supramolecular Polymers in Aqueous Media. Chem. Rev. 2016, 116, 2414–2477. [Google Scholar] [CrossRef]
- Krieg, E.; Niazov-Elkan, A.; Cohen, E.; Tsarfati, Y.; Rybtchinski, B. Noncovalent Aqua Materials Based on Perylene Diimides. Acc. Chem. Res. 2019, 52, 2634–2646. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Presselt, M. Progress and development in structural and optoelectronic tunability of supramolecular nonbonded fullerene assemblies. J. Mater. Chem. C 2019, 7, 6194–6216. [Google Scholar] [CrossRef]
- Kim, Y.J.; Schaller, R.D.; Fry, H.C. Control of Shell Morphology in p–n Heterostructured Water-Processable Semiconductor Colloids: Toward Extremely Efficient Charge Separation. Small 2019, 15, 1803563. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Mynar, J.L.; Yoshida, M.; Lee, E.; Lee, M.; Okuro, K.; Kinbara, K.; Aida, T. High-water-content modulable hydrogels by mixing clay and a dendritic molecular binder. Nature 2010, 463, 339–343. [Google Scholar] [CrossRef]
- Niazov-Elkan, A.; Weissman, H.; Dutta, S.; Cohen, S.R.; Iron, M.A.; Pinkas, I.; Bendikov, T.; Rybtchinski, B. Self-Assembled Hybrid Materials Based on Organic Nanocrystals and Carbon Nanotubes. Adv. Mater. 2018, 30, 1705027. [Google Scholar] [CrossRef]
- Edelbrock, A.N.; Clemons, T.D.; Chin, S.M.; Roan, J.J.W.; Bruckner, E.P.; Álvarez, Z.; Edelbrock, J.F.; Wek, K.S.; Stupp, S.I. Superstructured Biomaterials Formed by Exchange Dynamics and Host–Guest Interactions in Supramolecular Polymers. Adv. Sci. 2021, 8, 2004042. [Google Scholar] [CrossRef]
- Bakker, M.H.; Lee, C.C.; Meijer, E.W.; Dankers, P.Y.W.; Albertazzi, L. Multicomponent Supramolecular Polymers as a Modular Platform for Intracellular Delivery. ACS Nano 2016, 10, 1845–1852. [Google Scholar] [CrossRef] [PubMed]
- Straßburger, D.; Stergiou, N.; Urschbach, M.; Yurugi, H.; Spitzer, D.; Schollmeyer, D.; Schmitt, D.; Besenius, P. Mannose-Decorated Multicomponent Supramolecular Polymers Trigger Effective Uptake into Antigen-Presenting Cells. ChemBioChem 2018, 19, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, C.; La Manna, P.; De Rosa, M.; Soriente, A.; Talotta, C.; Neri, P. Supramolecular Catalysis with Self-Assembled Capsules and Cages: What Happens in Confined Spaces. ChemcatChem 2021, 13, 1638–1658. [Google Scholar] [CrossRef]
- Aliprandi, A.; Mauro, M.; De Cola, L. Controlling and imaging biomimetic self-assembly. Nat. Chem. 2016, 8, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Bäumer, N.; Kartha, K.K.; Palakkal, J.P.; Fernández, G. Morphology Control in Metallosupramolecular Assemblies through Solvent-induced Steric Demand. Soft Matter 2020, 16, 6834–6840. [Google Scholar] [CrossRef] [PubMed]
- Helmers, I.; Shen, B.; Kartha, K.K.; Albuquerque, R.Q.; Lee, M.; Fernández, G. Impact of Positional Isomerism on Pathway Complexity in Aqueous Media. Angew. Chem. Int. Ed. 2020, 59, 5675–5682. [Google Scholar] [CrossRef]
- Helmers, I.; Ghosh, G.; Albuquerque, R.Q.; Fernández, G. Pathway and Length Control of Supramolecular Polymers in Aqueous Media via a Hydrogen Bonding Lock. Angew. Chem. Int. Ed. 2020, 59, 4368–4376. [Google Scholar]
- Waybrant, B.; Pearce, T.R.; Kokkoli, E. Effect of Polyethylene Glycol, Alkyl, and Oligonucleotide Spacers on the Binding, Secondary Structure, and Self-Assembly of Fractalkine Binding FKN-S2 Aptamer-Amphiphiles. Langmuir 2014, 30, 7465–7474. [Google Scholar] [CrossRef]
- Arias-Barros, S.I.; Cid, A.; García-Río, L.; Mejuto, J.C.; Morales, J. Influence of polyethylene glycols on percolative phenomena in AOT microemulsions. Colloid Polym. Sci. 2010, 288, 217–221. [Google Scholar] [CrossRef]
- Fernández, G.; García, F.; Sánchez, L. Morphological changes in the self-assembly of a radial oligo-phenylene ethynylene amphiphilic system. Chem. Comm. 2008, 48, 6567–6569. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-K.; Lee, E.; Lim, Y.; Lee, M. Supramolecular Capsules with Gated Pores from an Amphiphilic Rod Assembly. Angew. Chem. Int. Ed. 2008, 47, 4662–4666. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-K.; Lee, E.; Jeong, Y.-H.; Lee, J.-K.; Zin, W.C.; Lee, M. Two-Dimensional Assembly of Rod Amphiphiles into Planar Networks. J. Am. Chem. Soc. 2007, 129, 6082–6083. [Google Scholar] [CrossRef]
- Kim, Y.; Li, W.; Shin, S.; Lee, M. Development of Toroidal Nanostructures by Self-Assembly: Rational Designs and Applications. Acc. Chem. Res. 2013, 46, 2888–2897. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.P.; Jin, W.; Kosaka, A.; Fukushima, T.; Ichihara, H.; Shimomura, T.; Ito, K.; Hashizume, T.; Ishii, N.; Aida, T. Self-Assembled Hexa-peri-hexabenzocoronene Graphitic Nanotube. Science 2004, 304, 1481–1483. [Google Scholar] [CrossRef] [PubMed]
- Würthner, F.; Saha-Möller, C.R.; Fimmel, B.; Ogi, S.; Leowanawat, P.; Schmidt, D. Perylene Bisimide Dye Assemblies as Archetype Functional Supramolecular Materials. Chem. Rev. 2016, 116, 962–1052. [Google Scholar] [CrossRef]
- Görl, D.; Würthner, F. Entropically Driven Self-Assembly of Bolaamphiphilic Perylene Dyes in Water. Angew. Chem. Int. Ed. 2016, 55, 12094–12098. [Google Scholar] [CrossRef] [PubMed]
- Syamala, P.P.N.; Soberats, B.; Görl, D.; Gekle, S.; Würthner, F. Thermodynamic insights into the entropically driven self-assembly of amphiphilic dyes in water. Chem. Sci. 2019, 10, 9358–9366. [Google Scholar] [CrossRef]
- Syamala, P.P.N.; Würthner, F. Modulation of the Self-Assembly of p-Amphiphiles in Water from Enthalpy- to Entropy-Driven by Enwrapping Substituents. Chem. Eur. J. 2020, 26, 8426–8434. [Google Scholar] [CrossRef]
- Gupta, R.K.; Pathak, S.K.; Pradhan, B.; Rao, D.S.S.; Prasad, S.K.; Achalkumar, A.S. Self-assembly of luminescent N-annulated perylene tetraesters into fluid columnar phases. Soft Matter 2015, 11, 3629–3636. [Google Scholar] [CrossRef]
- Gupta, R.K.; Rao, D.S.S.; Prasad, S.K.; Achalkumar, A.S. Columnar Self-Assembly of Electron-Deficient Dendronized Bay-Annulated Perylene Bisimides. Chem. Eur. J. 2018, 24, 3566–3575. [Google Scholar] [CrossRef]
- Buendía, J.; García, F.; Yélamos, B.; Sánchez, L. Transfer and amplification of chirality in Phe-based C3-symmetric non-ionic amphiphiles. Chem. Comm. 2016, 52, 8830–8833. [Google Scholar] [CrossRef]
- Greciano, E.E.; Calbo, J.; Ortí, E.; Sánchez, L. N-Annulated Perylene Bisimides to Bias the Differentiation of Metastable Supramolecular Assemblies into J- and H-Aggregates. Angew. Chem. Int. Ed. 2020, 59, 17517–17524. [Google Scholar] [CrossRef]
- Greciano, E.E.; Sánchez, L. Seeded Supramolecular Polymerization in a Three-Domain Self-Assembly of an N-Annulated Perylenetetracarboxamide. Chem. Eur. J. 2016, 22, 13724–13730. [Google Scholar] [CrossRef]
- Greciano, E.E.; Alsina, S.; Ghosh, G.; Fernández, G.; Sánchez, L. Alkyl Bridge Length to Bias the Kinetics and Stability of Consecutive Supramolecular Polymerizations. Small Methods 2020, 4, 1900715. [Google Scholar] [CrossRef]
- Rest, C.; Philips, D.S.; Dennebacke, T.; Sutar, P.; Sampedro, A.; Droste, J.; Stepanenko, V.; Hansen, M.R.; Albuquerque, R.Q.; Fernández, G. Tuning Aqueous Supramolecular Polymerization by an Acid-Responsive Conformational Switch. Chem. Eur. J. 2020, 26, 10005–10013. [Google Scholar] [CrossRef] [PubMed]
- Würthner, F.; Kaiser, T.E.; Saha-Möller, C.R. J-Aggregates: From Serendipitous Discovery to Supramolecular Engineering of Functional Dye Materials. Angew. Chem. Int. Ed. 2011, 50, 3376–3410. [Google Scholar] [CrossRef] [PubMed]
- Dünnebacke, T.; Kartha, K.K.; Wiest, J.M.; Albuquerque, R.Q.; Fernández, G. Solvent-controlled E/Z isomerization vs. [2 + 2] photocycloaddition mediated by supramolecular polymerization. Chem. Sci. 2020, 11, 10405–10413. [Google Scholar] [CrossRef]
- Gottlieb, H.; Kotlyar, V.; Nudelman, A. The chemical shift of D2O has been corrected by considering the effect of temperature on this solvent, please, see: NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef]
- Smith, G.D.; Bedrov, D. Roles of Enthalpy, Entropy, and Hydrogen Bonding in the Lower Critical Solution Temperature Behavior of Poly(ethylene oxide)/Water Solutions. J. Phys. Chem. B 2003, 107, 3095–3097. [Google Scholar] [CrossRef]
- García, F.; Sánchez, L. Dendronized Triangular Oligo (phenylene ethynylene) Amphiphiles: Nanofibrillar Self-Assembly and Dye Encapsulation. Chem. Eur. J. 2010, 16, 3138–3146. [Google Scholar] [CrossRef]
- Korevaar, P.A.; Schaefer, C.; de Greef, T.F.A.; Meijer, E.W. Controlling Chemical Self-Assembly by Solvent-Dependent Dynamics. J. Am. Chem. Soc. 2012, 134, 13482–13491. [Google Scholar] [CrossRef]
- De Greef, T.F.A.; Smulders, M.M.J.; Wolffs, M.; Schenning, A.P.H.J.; Sijbesma, R.P.; Meijer, E.W. Supramolecular polymerization. Chem. Rev. 2009, 109, 5687–5754. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, M.A.; Greciano, E.E.; Cuéllar, J.; Valpuesta, J.M.; Sánchez, L. Globular Aggregates Stemming from the Self-Assembly of an Amphiphilic N-Annulated Perylene Bisimide in Aqueous Media. Nanomaterials 2021, 11, 1457. https://doi.org/10.3390/nano11061457
Martínez MA, Greciano EE, Cuéllar J, Valpuesta JM, Sánchez L. Globular Aggregates Stemming from the Self-Assembly of an Amphiphilic N-Annulated Perylene Bisimide in Aqueous Media. Nanomaterials. 2021; 11(6):1457. https://doi.org/10.3390/nano11061457
Chicago/Turabian StyleMartínez, Manuel A., Elisa E. Greciano, Jorge Cuéllar, José M. Valpuesta, and Luis Sánchez. 2021. "Globular Aggregates Stemming from the Self-Assembly of an Amphiphilic N-Annulated Perylene Bisimide in Aqueous Media" Nanomaterials 11, no. 6: 1457. https://doi.org/10.3390/nano11061457
APA StyleMartínez, M. A., Greciano, E. E., Cuéllar, J., Valpuesta, J. M., & Sánchez, L. (2021). Globular Aggregates Stemming from the Self-Assembly of an Amphiphilic N-Annulated Perylene Bisimide in Aqueous Media. Nanomaterials, 11(6), 1457. https://doi.org/10.3390/nano11061457







