1. Introduction
In the last decades, carbon nanotubes (CNTs) received increasing attention due to their unique characteristics (chemical, physical and mechanical properties), which make them suitable to be used in different areas, including industry, medicine, energy storage and environmental protection [
1,
2,
3].
CNTs are allotropes of carbon consisting of a hexagonal arrangement of hybridized carbon atoms. They are assembled in cylindrical tubes and can be formed by a single sheet of graphene (single-walled carbon nanotubes, SWCNTs) or by multiple sheets of graphene linked through van der Waals non-covalent forces (multi-walled carbon nanotubes, MWCNTs) [
4,
5,
6]. CNTs can be synthetized by several methods, such as arc-discharge, chemical vapor deposition, laser ablation, hydrothermal method and electrolysis. These methods present several disadvantages, like high costs, low purity or catalysts use. Instead, the chemical method seems to be a good option to obtain MWCNTs more easily, because it uses low temperature, it does not require the use of metal catalyst or pressures and is an inexpensively and environment-friendly technique [
7]. The nanotubes’ structure depends closely on the CNTs synthesis method. Thus, the applications of these nanotubes are linked by their structure (such as: number of walls, length, diameter, structural quality, purity, etc.), which gives specific properties to CNTs [
4]. Both types of CNTs have found applications in several fields, such as medicine (due to their optical properties [
8]), solar thermal applications (favorable stability [
9]), biosensing applications (electron transfer kinetics [
10,
11]), antimicrobial applications (strong antimicrobial activity against a variety of bacterial strains [
12,
13,
14]) and drug delivery applications (due to their large surface/weight ratio [
15,
16]). Even so, MWCNTs present advantages compared with SWCNTs, such as easier mass production, low-cost synthesis and enhanced thermal and chemical stability [
17].
With the rapid development of industries, more advanced materials are required. A solution regarding this problem was found in designing hybrid materials that will combine the properties of individual components. Surface modification of carbon nanotubes imparts new properties to these materials for various applications, where enhanced CNTs functionality, dispersion and compatibility are required. The most common methods to functionalize CNTs includes oxidation using various acids, when oxygen functional groups (–OH, –COOH) result. Oxygen-containing groups created on nanotubes’ surface promote the exfoliation of CNT bundles, enhance the solubility in polar media and offer the possibility of further modification [
17].
MWCNTs decorated with nanoparticles (NPs) are a new family of innovative nanocomposites, effective for multiple biomedical applications [
18]. It has been reported that antimicrobial activity of MWCNTs has significantly increased with the presence of NPs onto their surface [
19]. In another study, it was reported that MWCNTs decorated with silver (Ag) NPs inactivated or killed the bacteria once with the increase of the exposure time [
20]. Dinh and co-workers studied the antibacterial effect of MWCNTs, Ag NPs and MWCNTs_Ag nanocomposites against
Escherichia coli and
Staphylococcus aureus bacteria. It was reported that the nanocomposites (MWCNTs_Ag) show a larger inhibition zone, compared with Ag NPs and MWCNTs. These results suggest that the bacterial growth inhibition of the composite sample was a synergetic effect resulting from both the nanoparticles and MWCNTs. Additionally, the interaction and bactericidal mechanism of MWCNTs decorated with Ag NPs was reported to occur due to the physical interaction of the nanomaterials with cell membrane, the faster destructibility of cell membrane and disruption of membrane function, leading to cell death [
21]. On the other hand, Mohan and co-workers investigated the antimicrobial action of hybrid nanocomposites based on MWCNTs and Ag NPs obtained via an environmentally friendly method. It has been reported that higher inhibitory effects were obtained against
E. coli, compared with MWCNTs or Ag NPs, while in the case of
Pseudomonas aregunosa, the MWCNTs decorated with Ag NPs were the least effective [
22]. Additionally, a lesser inhibition zone of MWCNTs decorated with Ag NPs, compared with pure MWCNTs, was reported in other studies. It can be confirmed that in the case of the Gram-negative bacteria, MWCNTs are slightly more effective than CNTs decorated with Ag NPs, while for the Gram-positive bacteria, the decorated nanocomposites are more effective [
23]. Sui and co-workers reported that MWCNTs decorated with zinc oxide (ZnO) NPs presented a powerful bactericidal effect against
E. coli at a concentration of 0.5 mg/mL after 3 h, confirming that the combination of ZnO NPs and MWCNTs improved the antibacterial property of the nanocomposite [
24]. In another study, the antimicrobial efficiency of raw MWCNTs, purified MWCNTs and MWCNTs decorated with ZnO against
E. coli was investigated. It was reported that no obvious antimicrobial activity was observed against
E. coli after being treated with raw MWCNTs (0.5 mg/mL), while for purified MWCNTs, the growth time of the cells was delayed by approximately 4 h, at the same concentration. In the case of MWCNTs coated with ZnO, there was no obvious cell growth observed within 20 h, at the same concentration [
25]. Akhavan and co-workers reported that the antimicrobial activity of ZnO nanoparticles deposited on MWCNTs against
E. coli increased with the gradual increase of the MWCNTs content [
26]. The antimicrobial activity of MWCNTs decorated with hydroxyapatite (HAp) has been very little investigated, for example, Sivaraj and Vijayalakshmi studied the antimicrobial activity of
E. coli and
S. aureus on Ag-substituted HAp deposited on MWCNTs. It was reported that the higher inhibition zone was obtained at an increased concentration of Ag-HA into the walls of the carbon nanotubes, for both types of bacteria. In addition, it was reported that a higher zone of inhibition of
E. coli could be attributed to less cell wall density compared with Gram-positive bacteria. The enhanced antimicrobial activity is related to the amount of Ag, mainly due to the variation in negative surface charge induced from the composite. The Ag ions released from the nanocomposite attack the cell wall membrane, penetrate into the cell and disrupt the DNA structure [
27].
In this study, MWCNTs obtained by a new, inexpensive and simple technique, chemical synthesis, were used as supporting material for the nanoparticles. The major advantage of the chemical method refers to the fact that it does not require the use of metal catalyst, thus becoming an environment-friendly method. Furthermore, the obtained products were already functionalized with carboxylated groups from the synthesis procedure, so the decoration of the MWCNTs with nanoparticles was performed more easily [
18].
The aim of this study was to design and characterize hybrid materials composed by MWCNTs, obtained by a new and environment-friendly technique, and different types of nanoparticles, in order to obtain efficient nanosystems, with low cost, fast to obtain and improved antimicrobial activity. In this study, synthetized MWCNTs were decorated with NPs, such as hydroxyapatite (MWCNTs_HAp), silver (MWCNTs_Ag) and zinc oxide (MWCNTs_ZnO), in order to obtain efficient hybrid materials based on MWCNTs. The obtained nanomaterials were tested against five microbial species, two Gram-positive (S. aureus, Bacillus subtilis), two Gram-negative (Pseudomonas aeruginosa, E. coli) and one yeast (Candida albicans) to cover the most important model opportunistic pathogens.
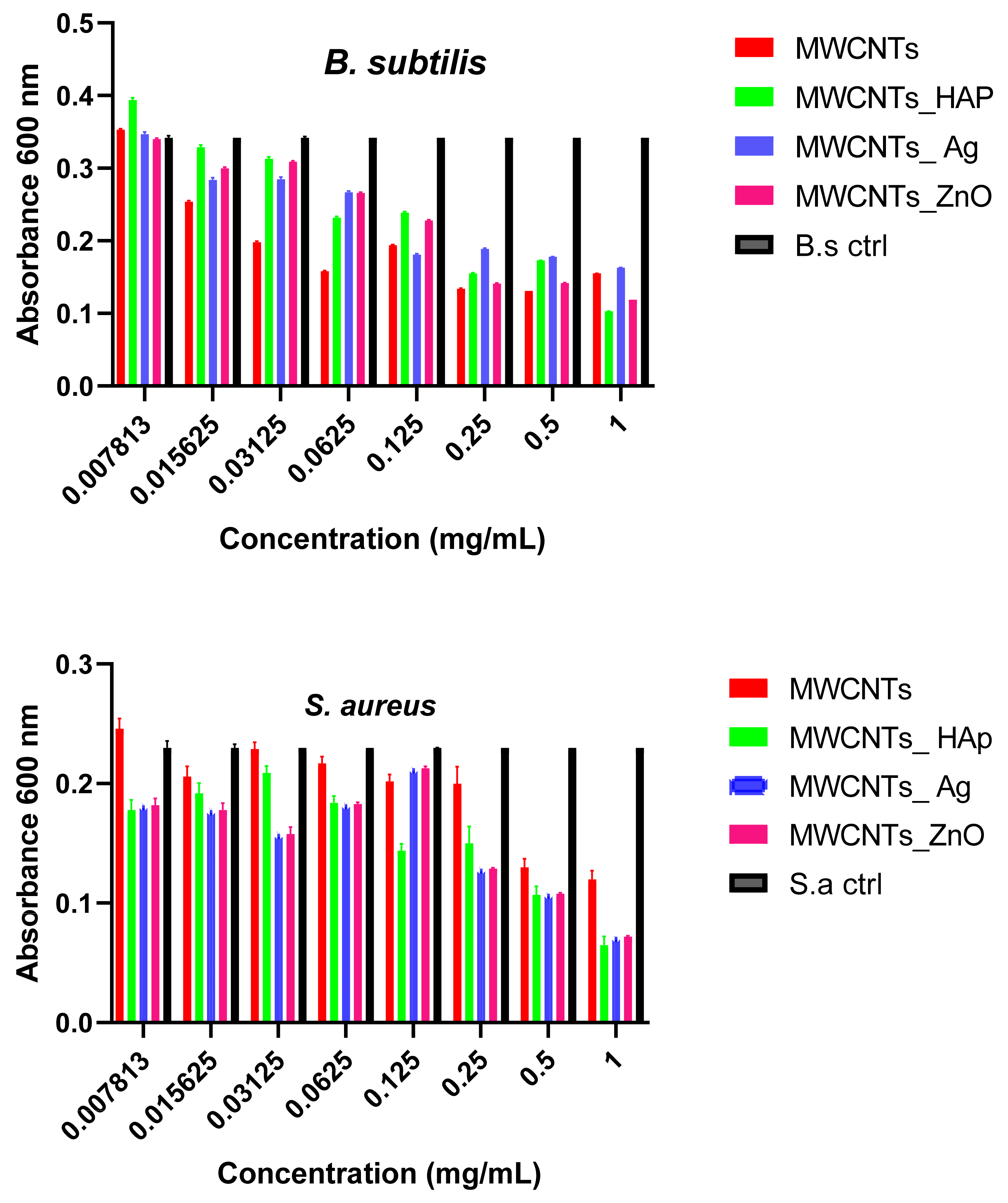
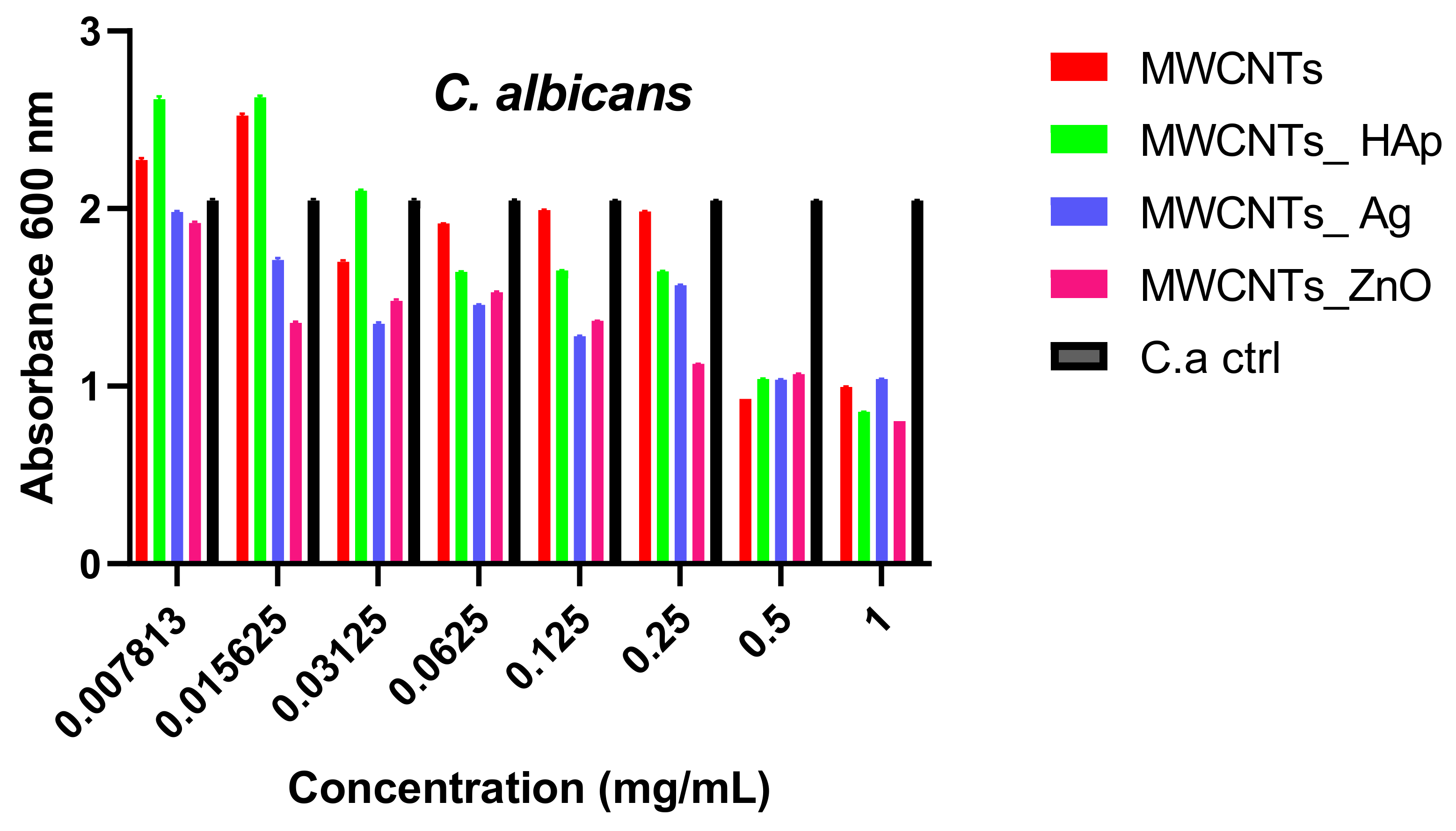
4. Conclusions
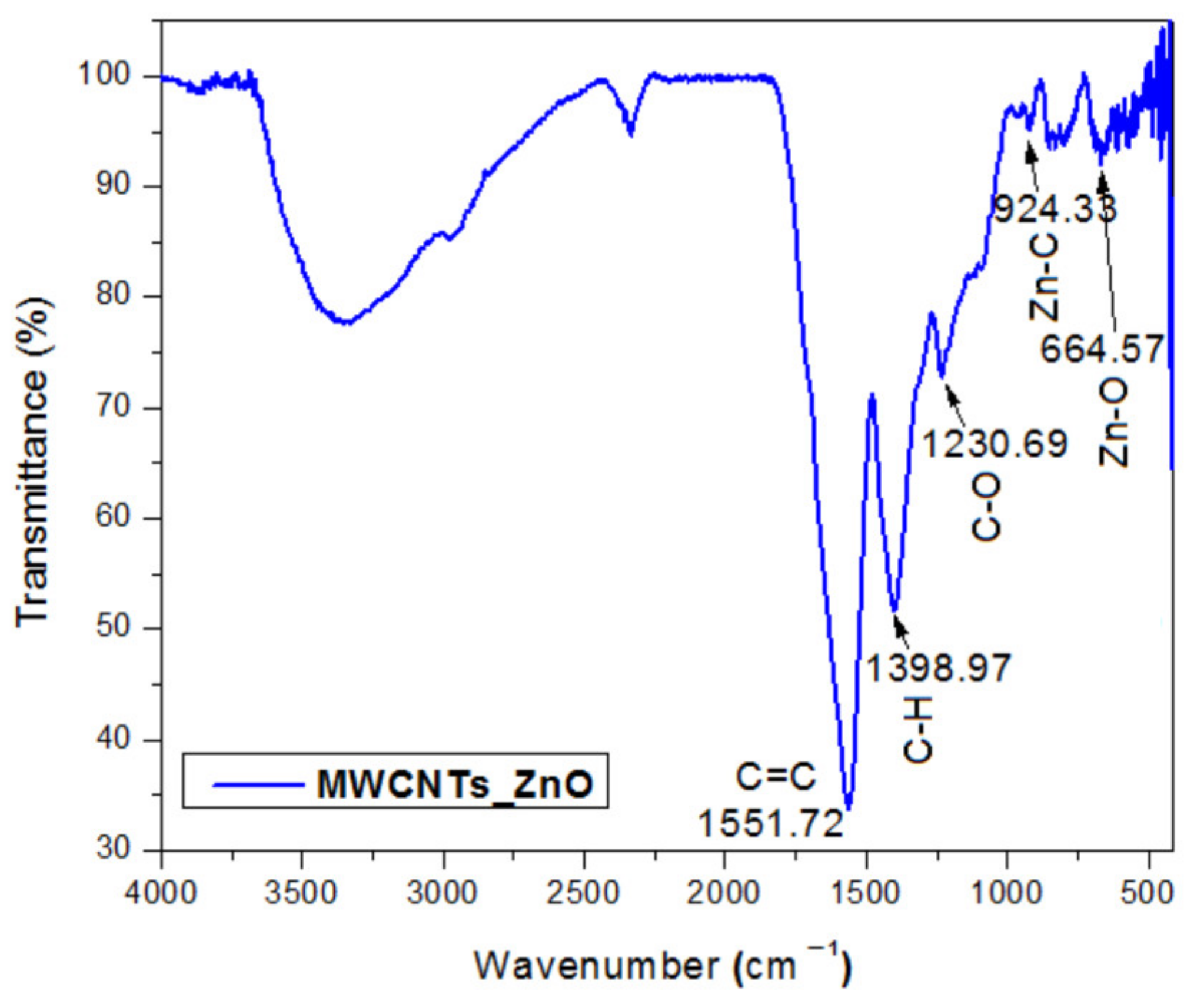
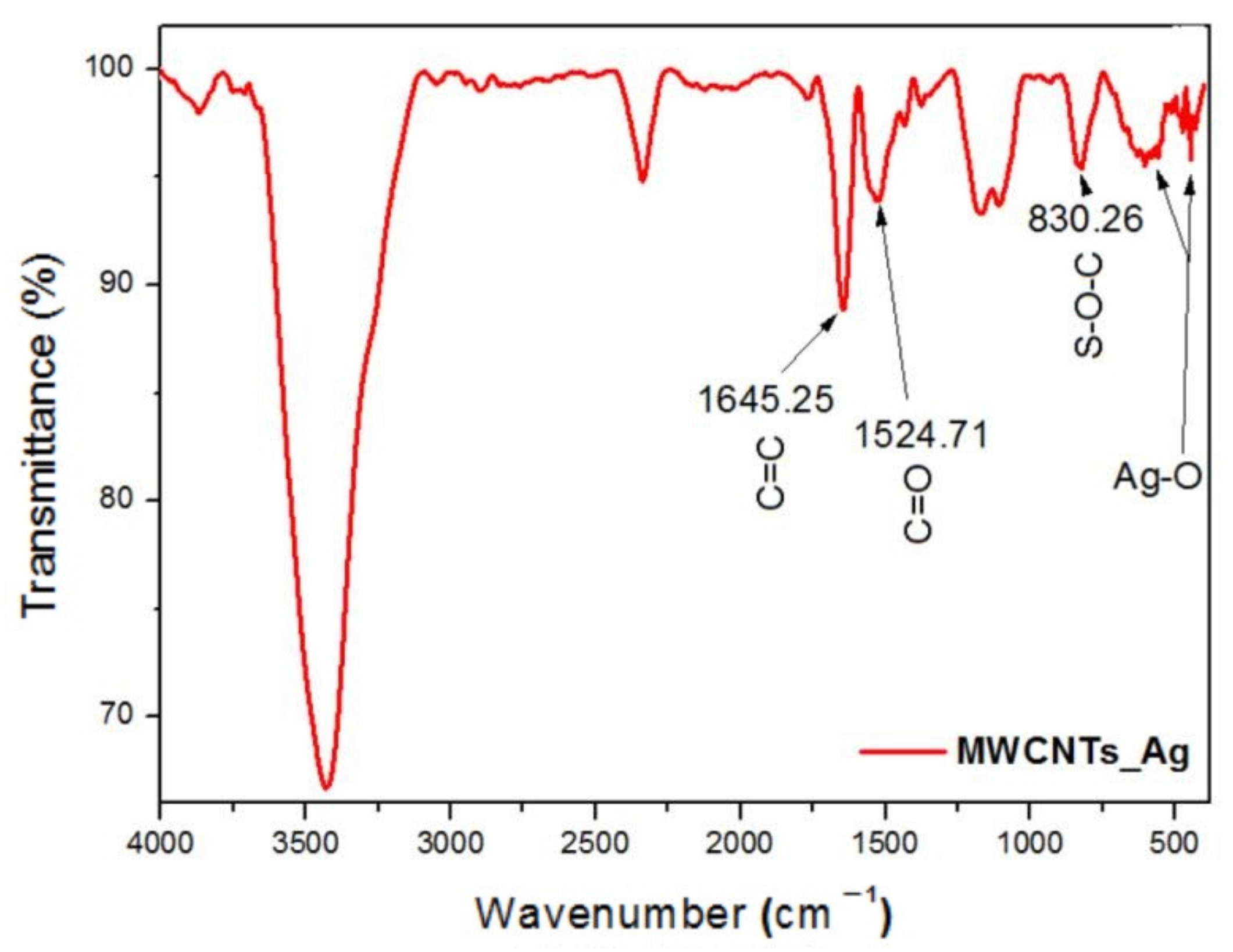
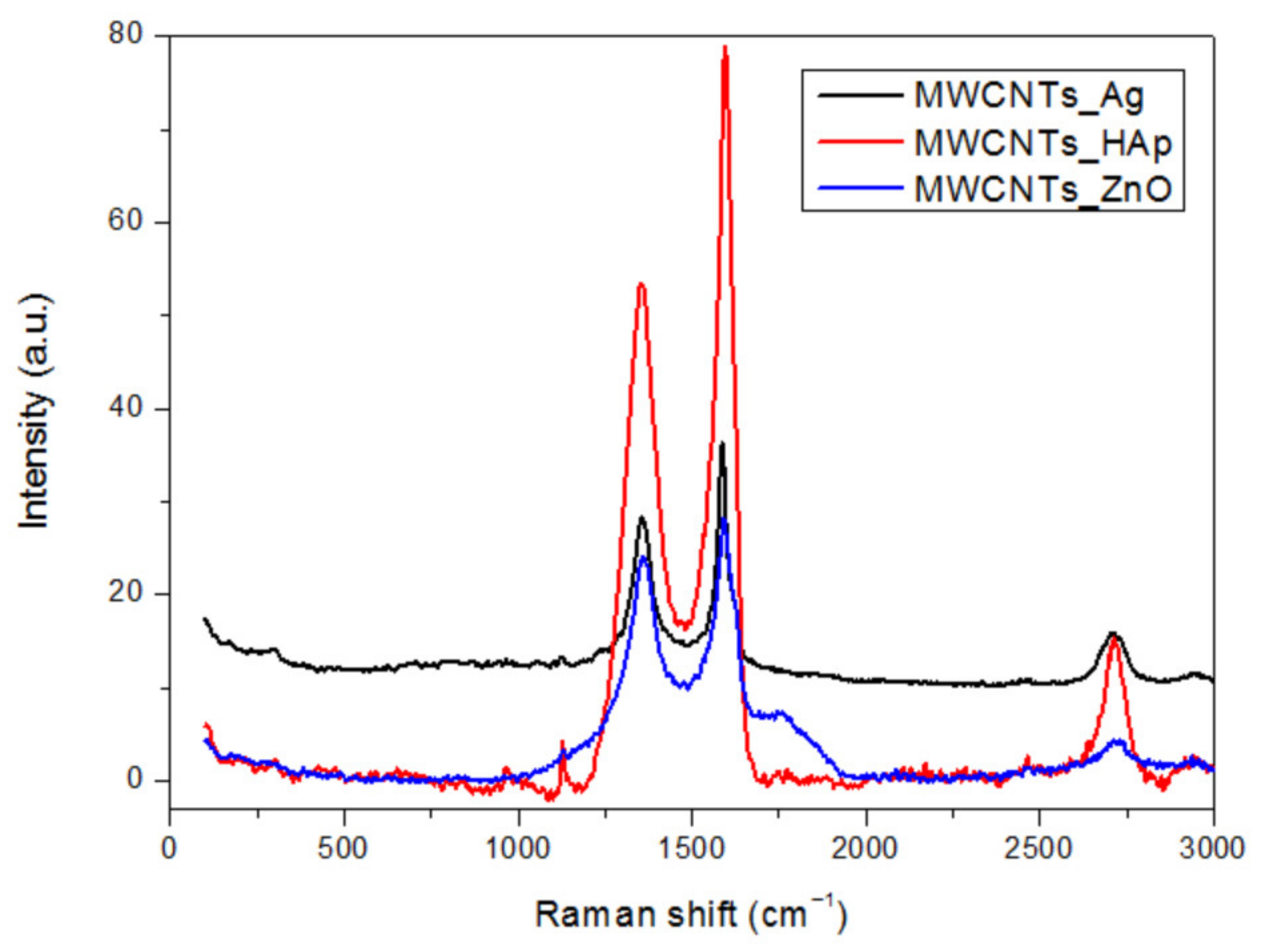
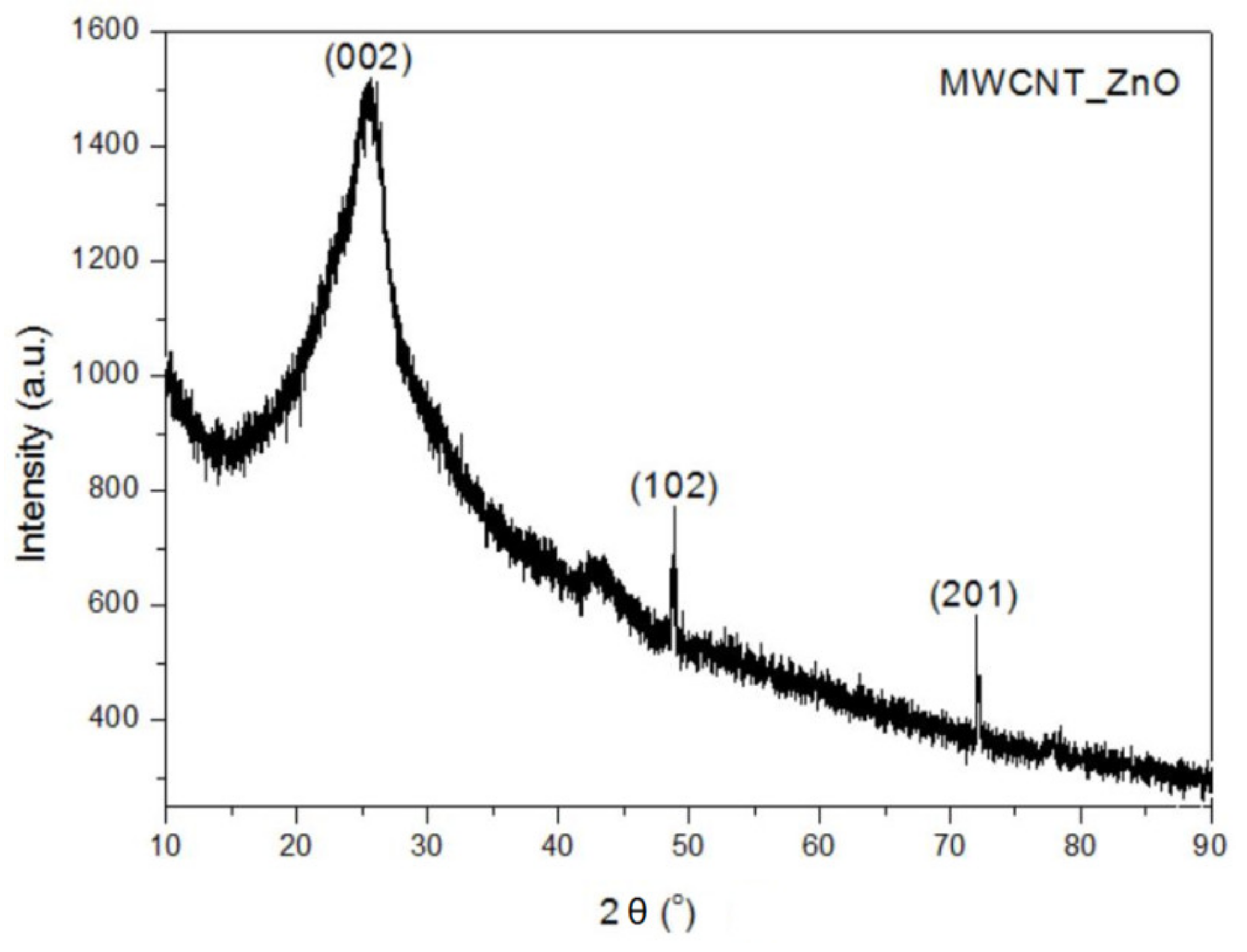
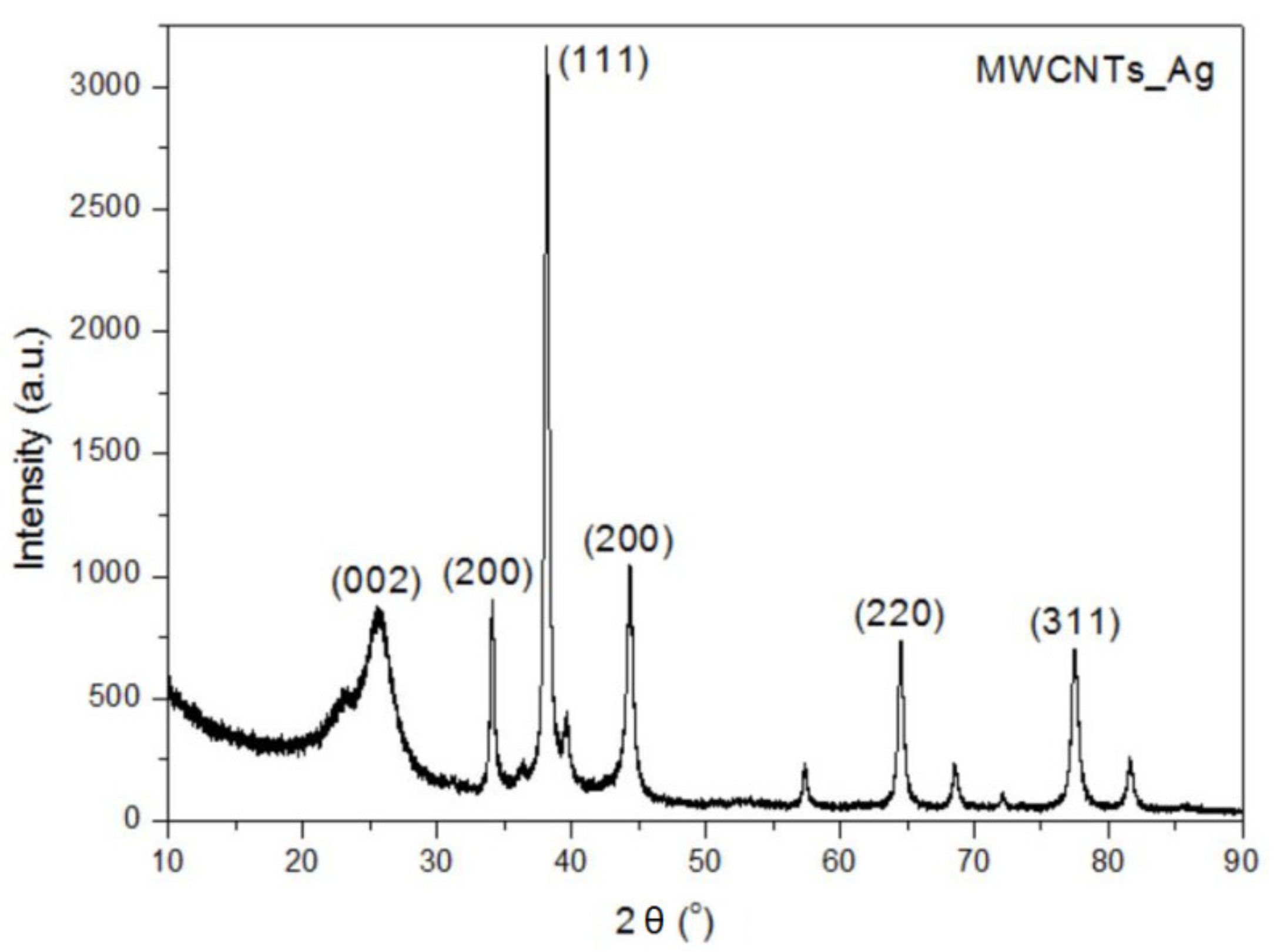
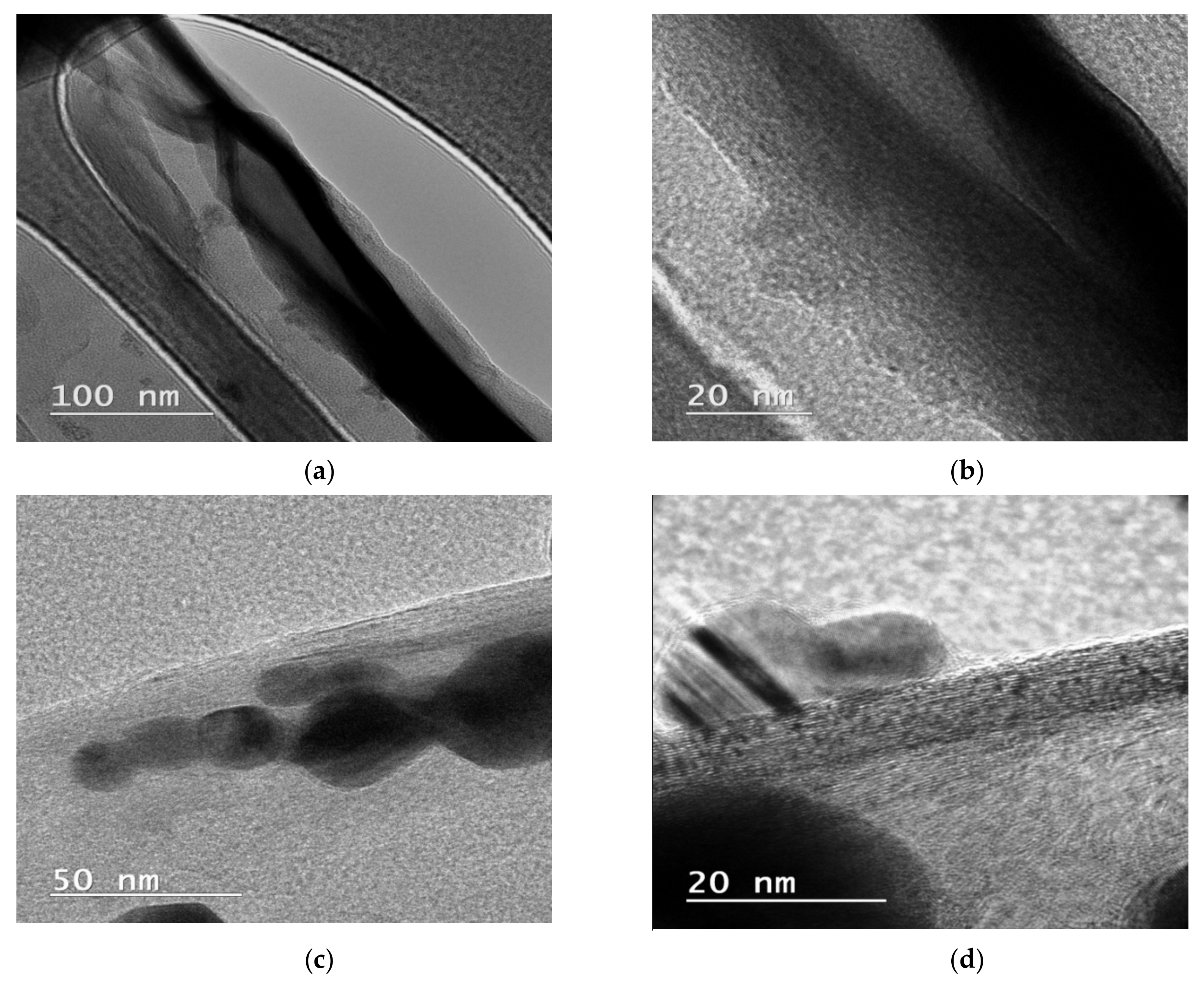
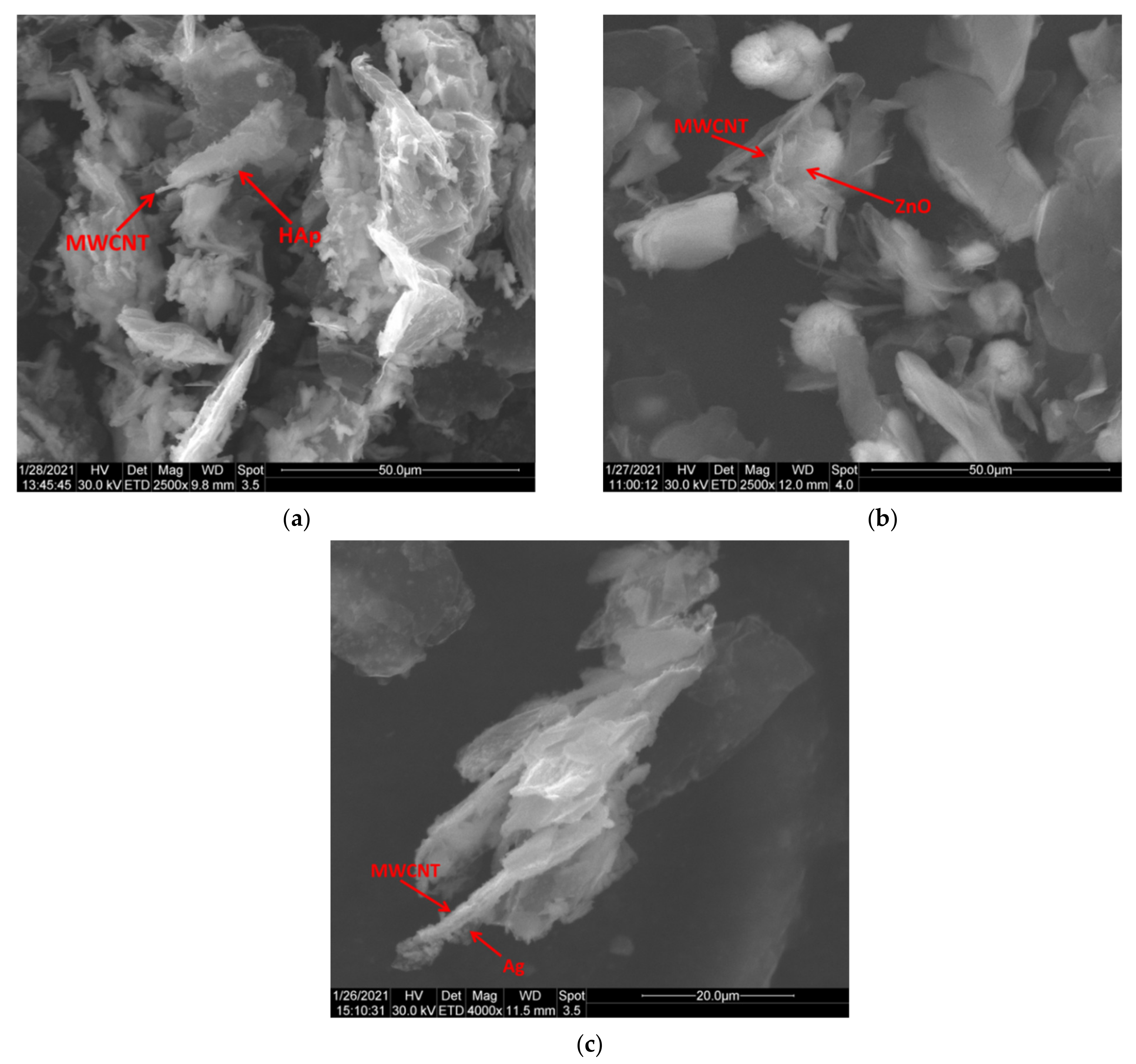
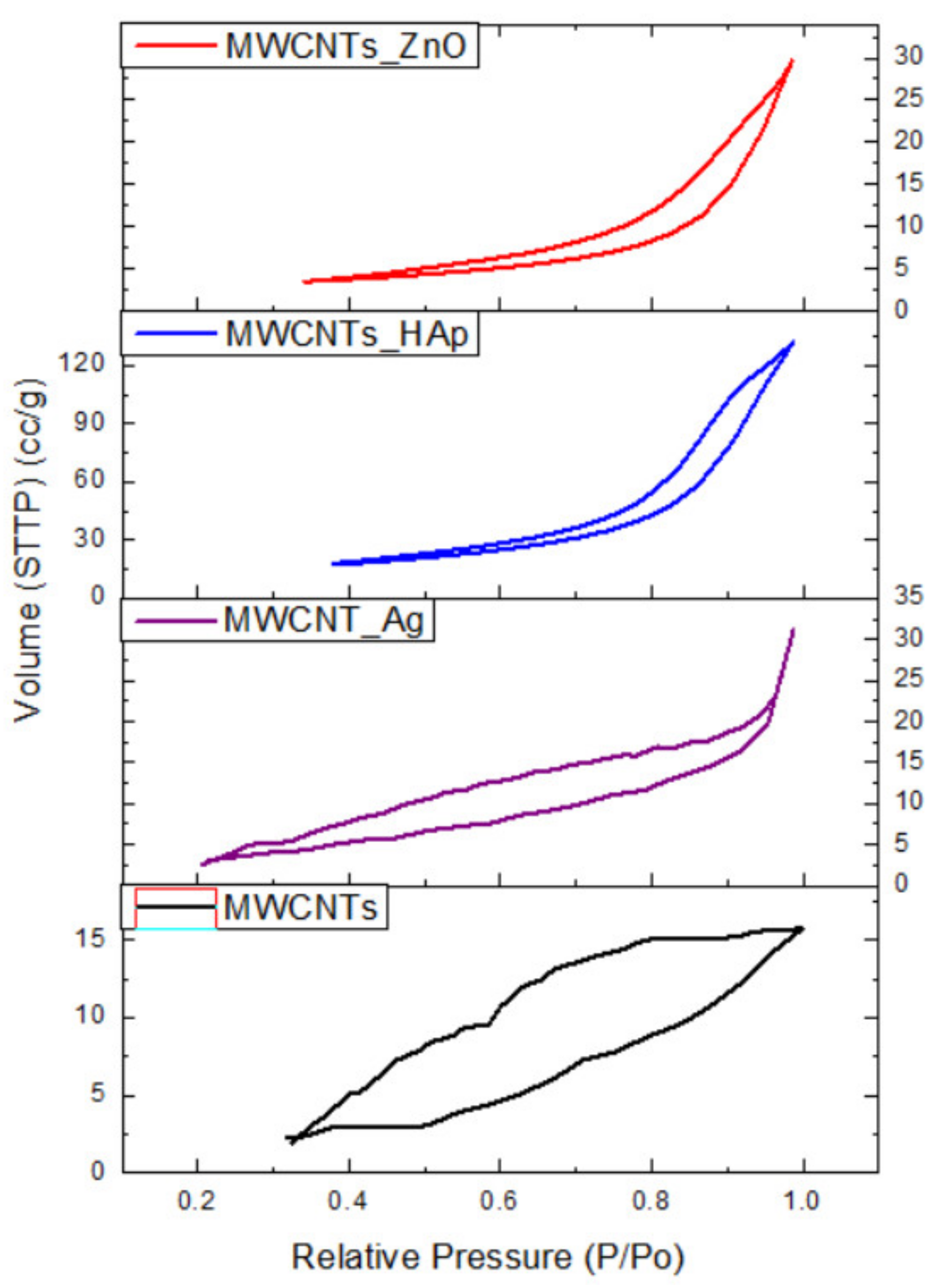
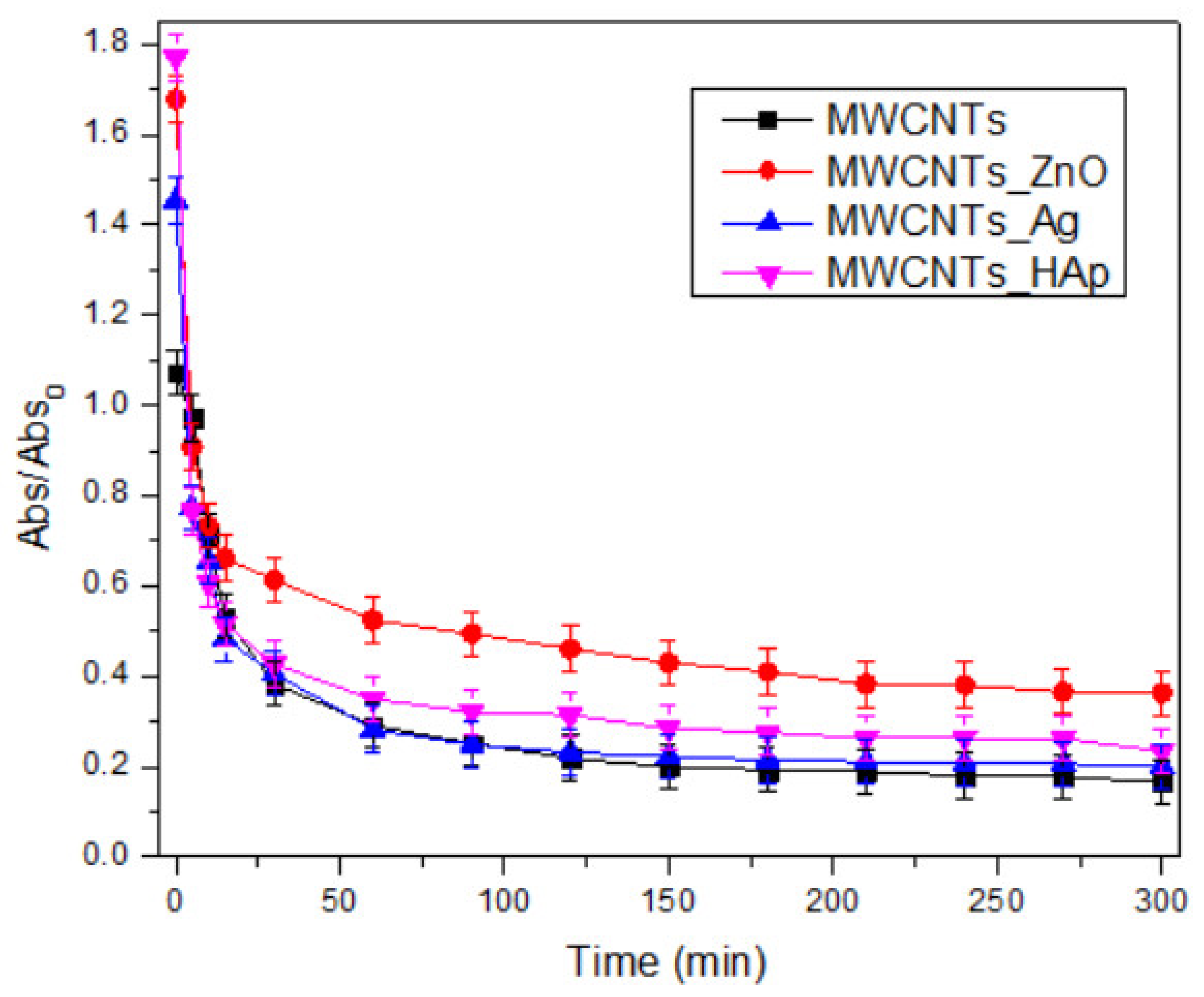
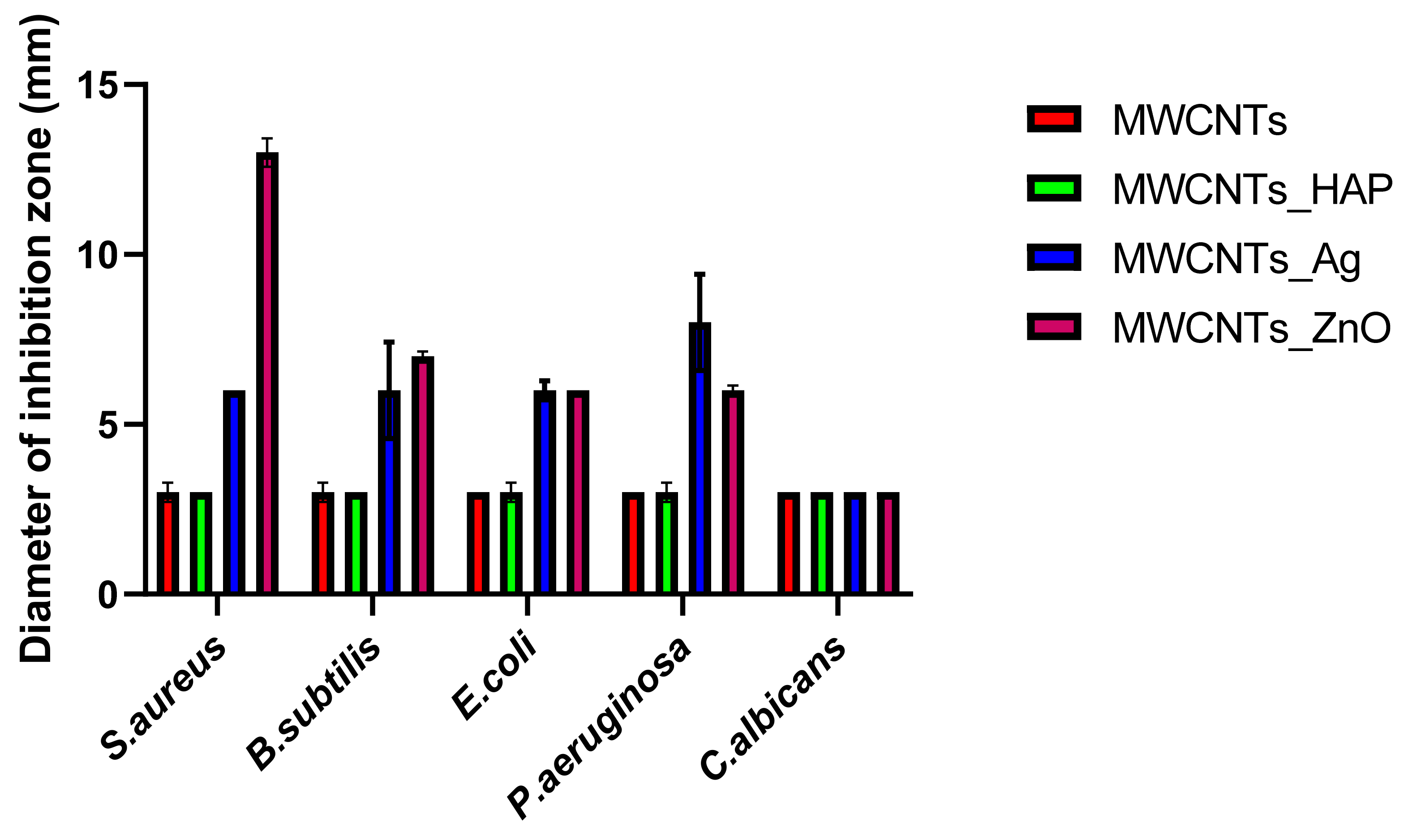
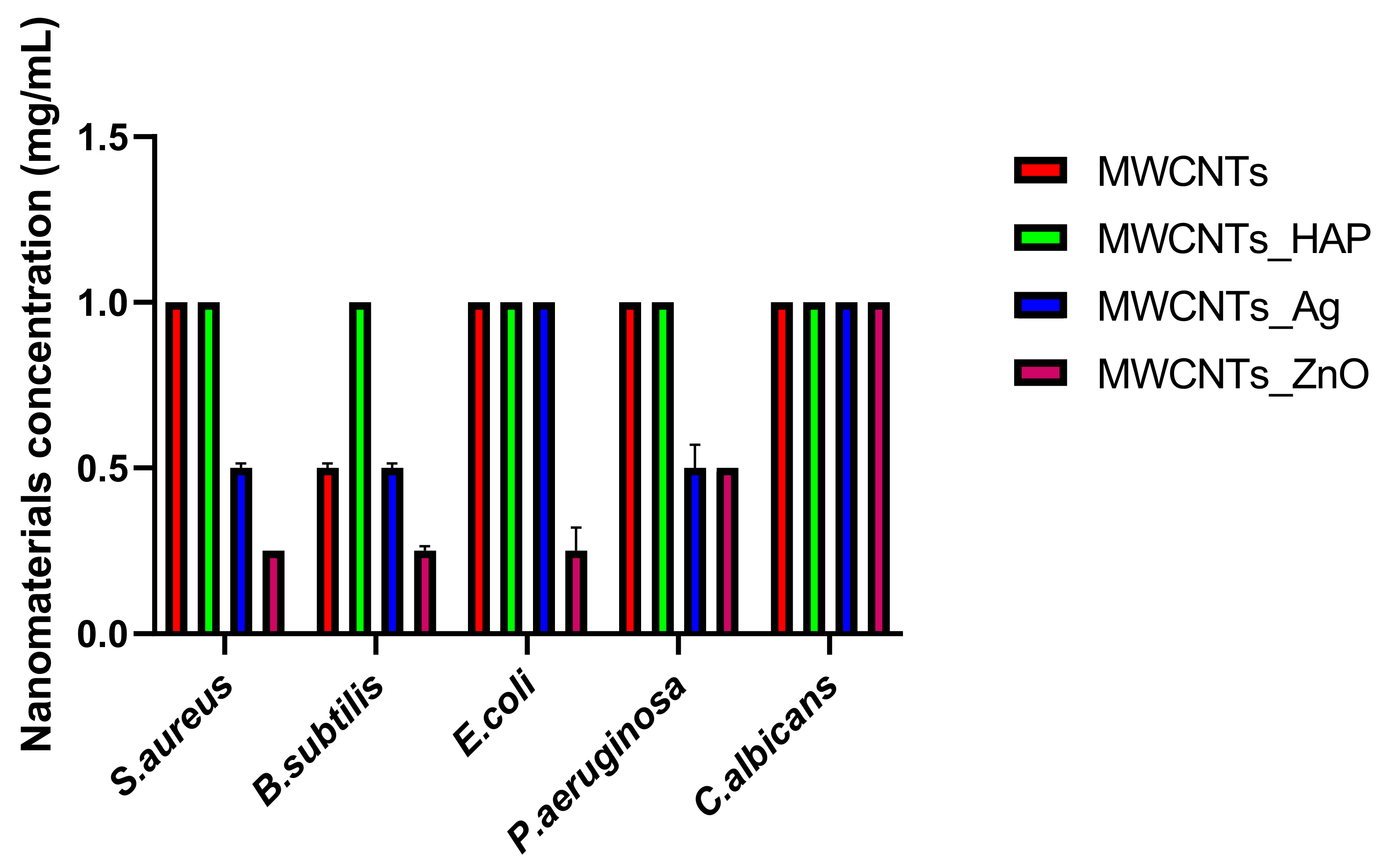
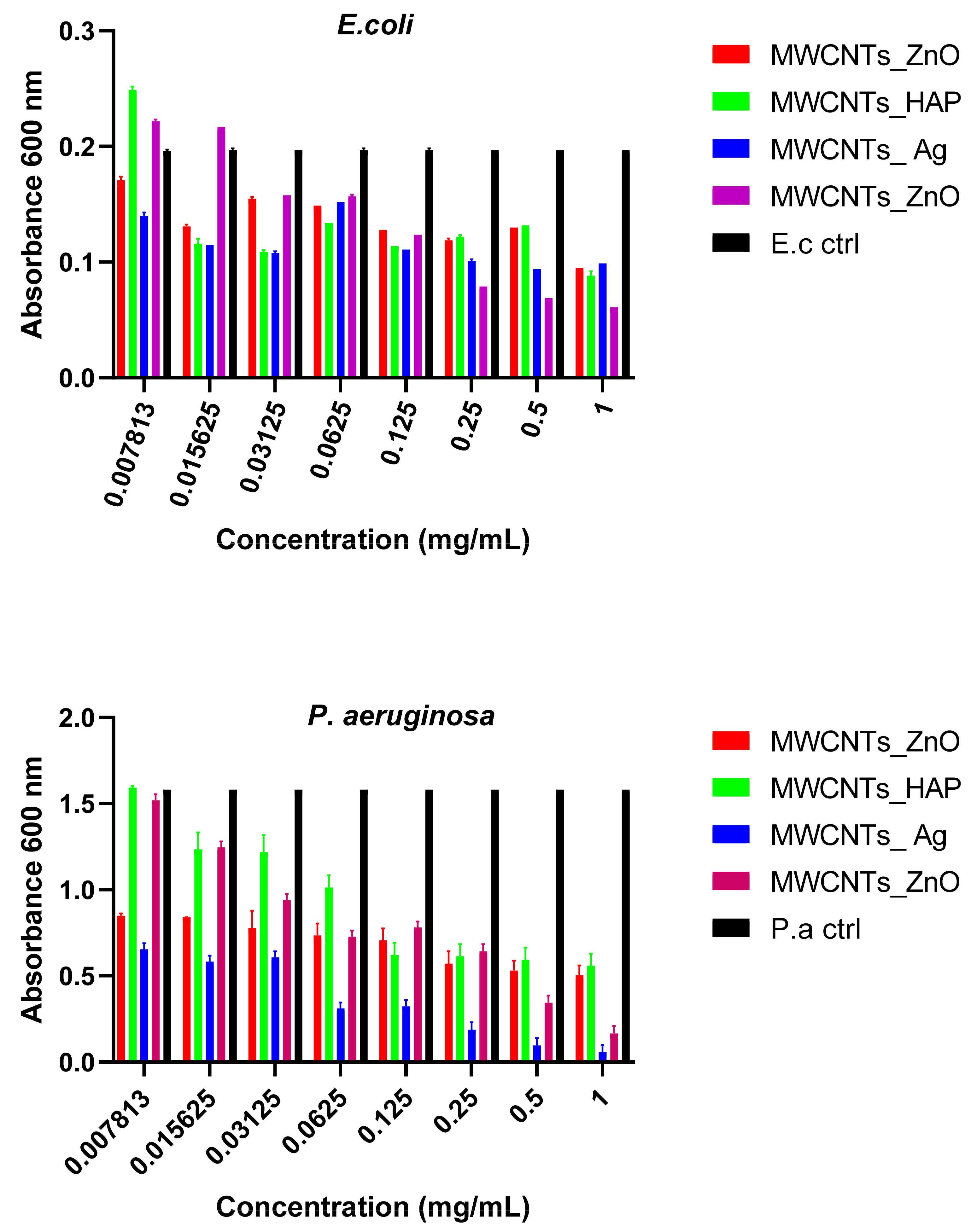
In this study, we reported the decoration of MWCNTs with different types of nanoparticles, such as ZnO, Ag and HAp, in order to obtain hybrid materials with improved antimicrobial activity. The decoration of MWCNTs with NPs was confirmed by structural and morphological analyses, such as Fourier transformed infrared spectroscopy, Raman spectroscopy, X-ray diffraction, transmission electron microscopy, environmental scanning electron microscopy/energy-dispersive X-ray spectroscopy and Brunauer–Emmett–Teller surface area analysis. The nanoparticles attached on the MWCNTs surface presented variable diameters, from 7 to 13 nm for ZnO NPs, 15–33 nm for Ag NPs and about 35 nm for HAp. In all the cases, the NPs were attached and aggregated to the surface of MWCNTs, as demonstrated by SEM analysis. The agglomeration of the NPs indicates the supporting role of the MWCNTs, as a center for the deposition of the clusters. Additionally, the dispersion in aqueous medium of the obtained nanomaterials was investigated and all the decorated MWCNTs presented a better dispersion in water, demonstrating a proper dispersion of the nanomaterials that retain their properties. The in vitro results demonstrated that the obtained nanocomposites have a significant antimicrobial activity and reduce biofilm formation in many relevant microbial strains, such as S. aureus, B. subtilis, P. aeruginosa, E. coli and C. albicans. The higher antimicrobial activity was observed for MWCNTs_ZnO and MWCNTs_Ag, with the largest diameter of inhibition zone being obtained for these nanocomposites (13–6 mm for MWCNTs_ZnO and 6–7 mm for MWCNTs_Ag). The biofilm formation test demonstrated that MWCNTs_ZnO and MWCNTs_Ag presented the highest antimicrobial activity, with the inhibition occurring even at lower concentrations, such as 0.015% and 0.007%. These results were supported by the statistical analysis. The SS was applied in order to investigate the adequacy of the proposed models, with the smallest values being obtained for the MWCNTs_ZnO and MWCNTs_Ag. In addition, based on the F-values of the main factors, the best antimicrobial activity was obtained for MWCNTs_ZnO and MWCNT_Ag. Thus, the obtained materials could be considered as competitive candidates for the development of efficient antimicrobial systems to fight against resistant pathogens and biofilm infections.