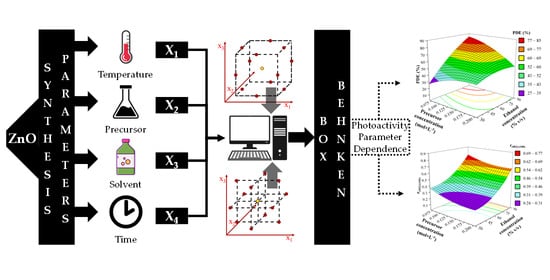
Optimization Method of the Solvothermal Parameters Using Box–Behnken Experimental Design—The Case Study of ZnO Structural and Catalytic Tailoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
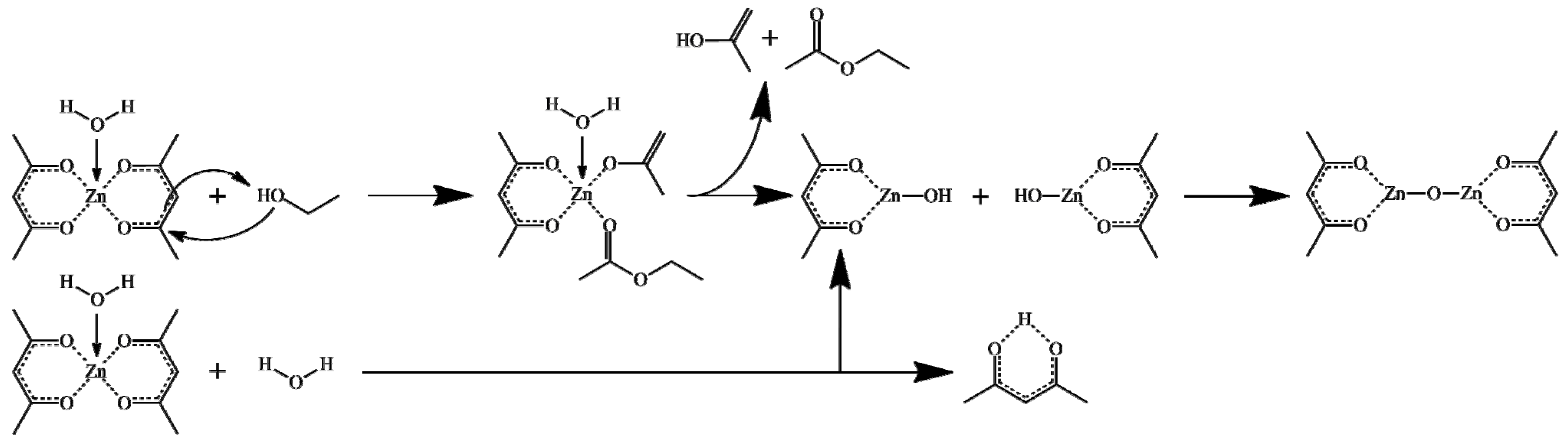
2.2. Solvothermal Synthesis of ZnO
2.3. The Assessment of the Photocatalytic Activity
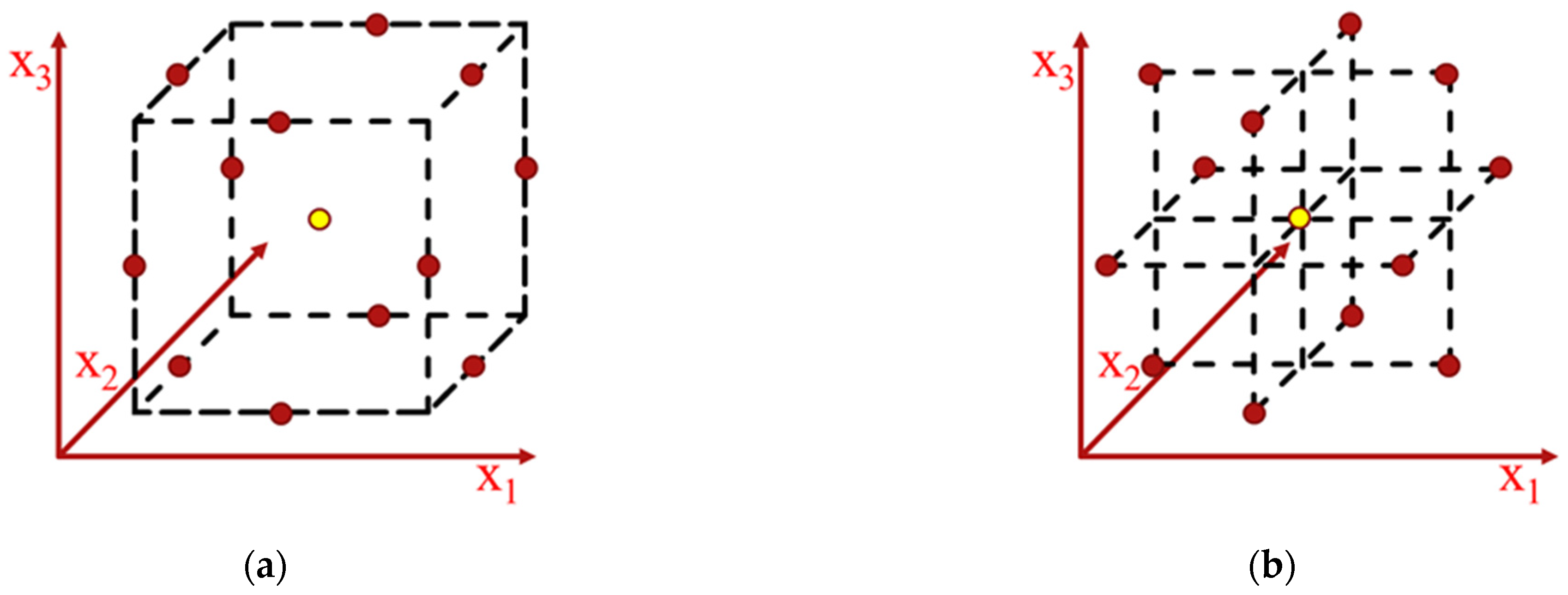
2.4. Experimental Design
3. Results and Discussion
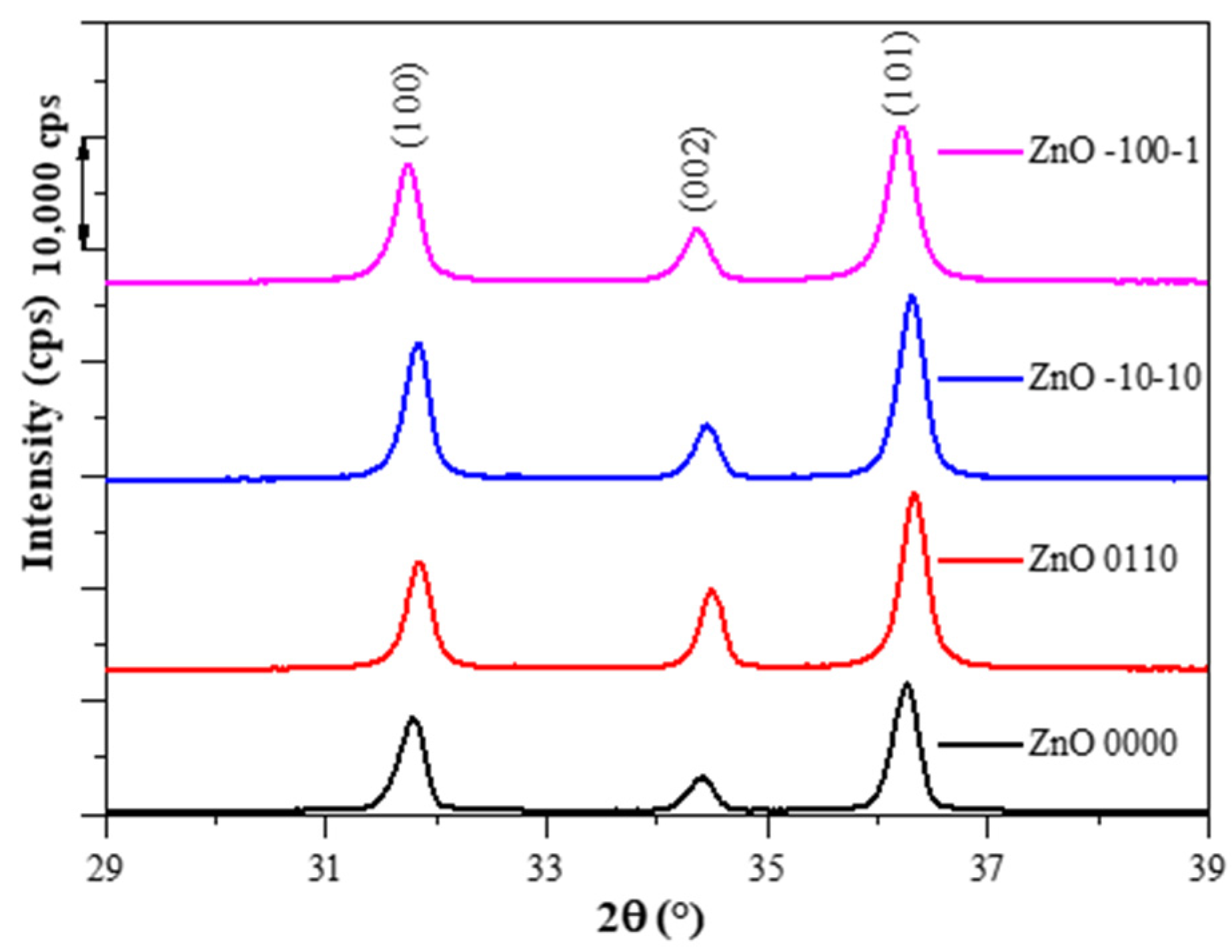
3.1. Characterization
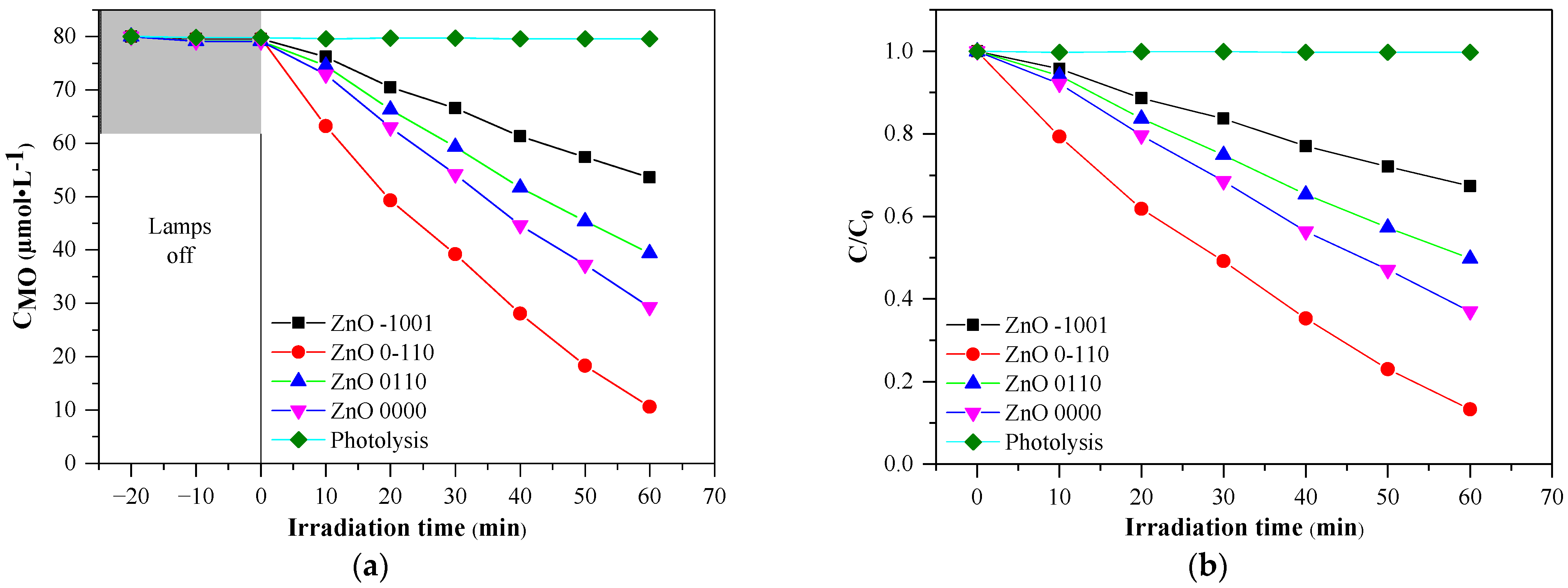
3.2. Photocatalytic Degradation Test
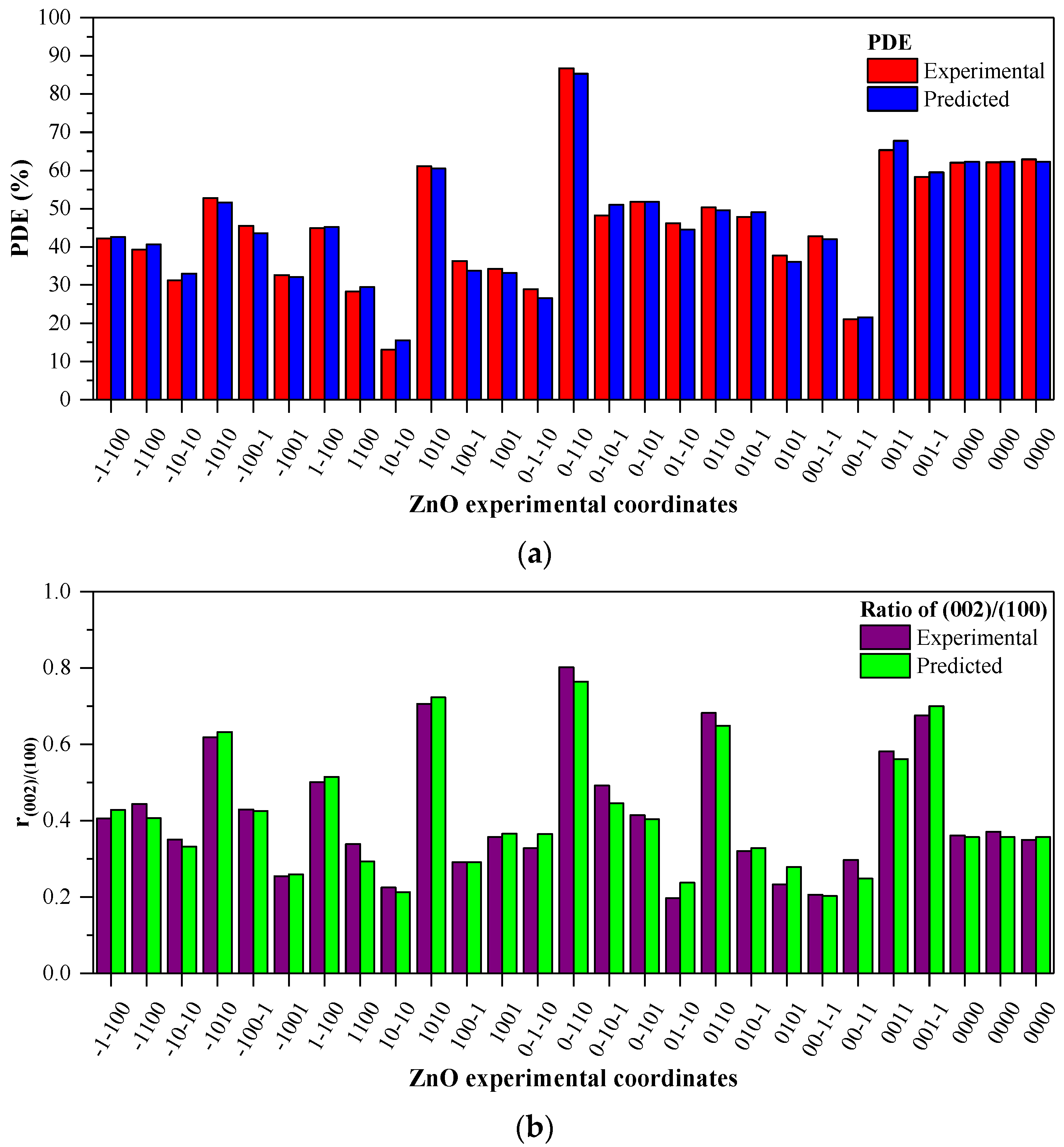
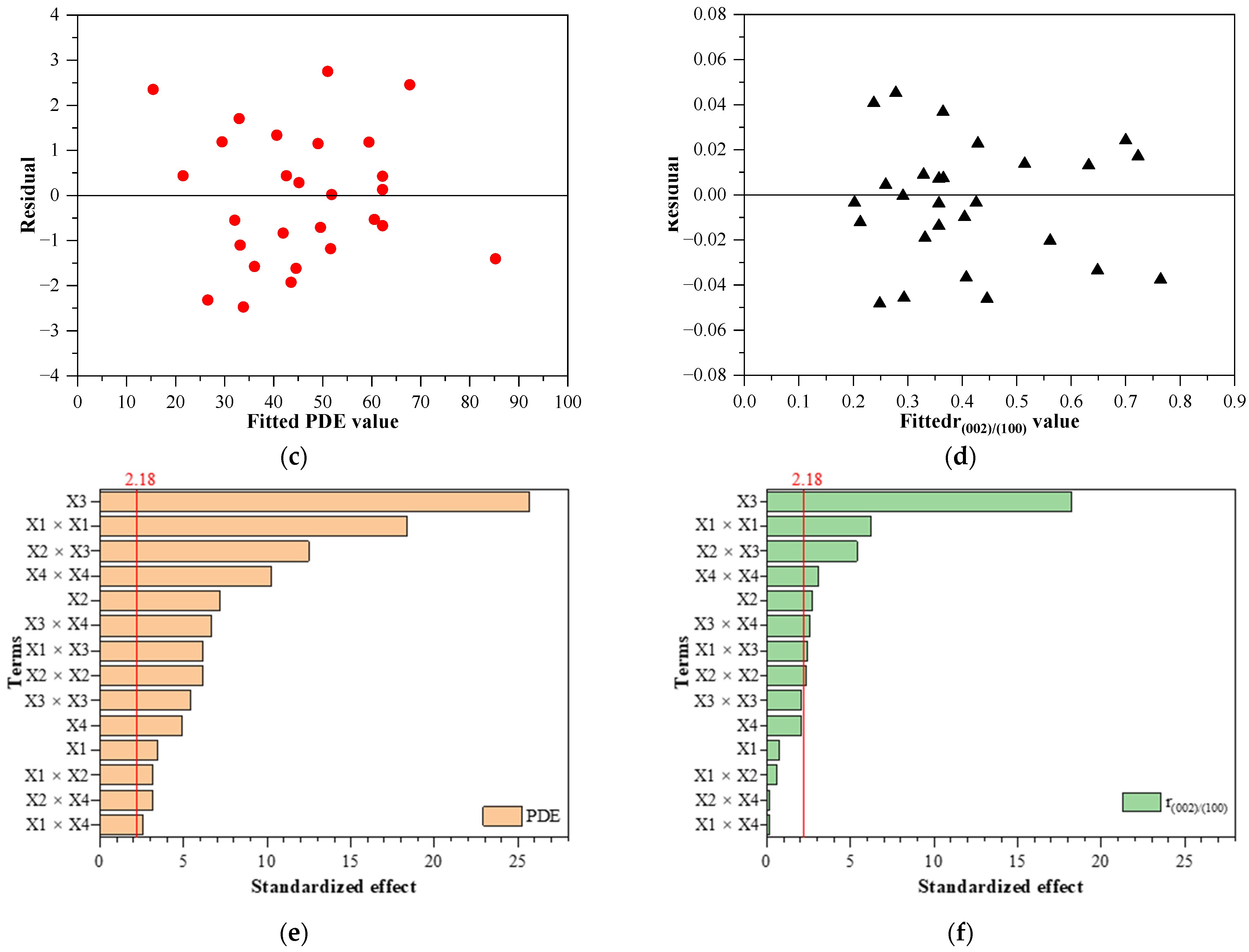
3.3. Model Fitting and ANOVA Analysis
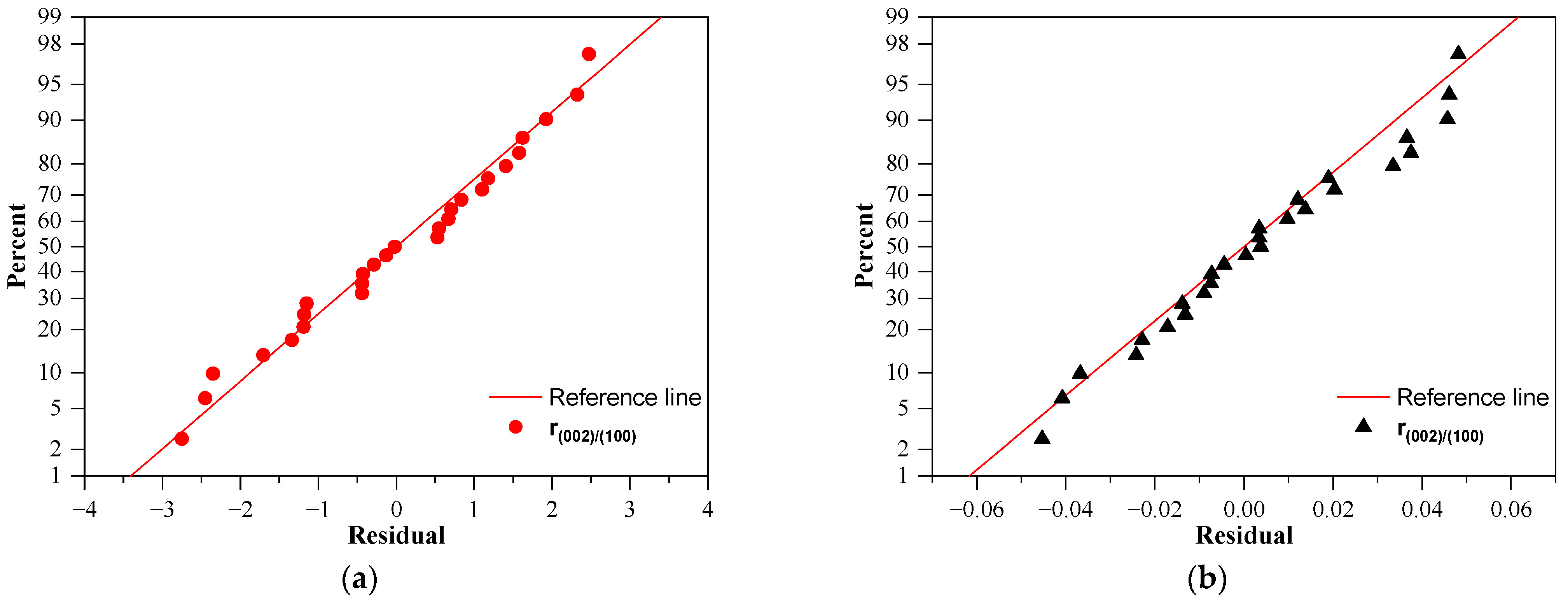
3.4. Adequacy of the Regression Model
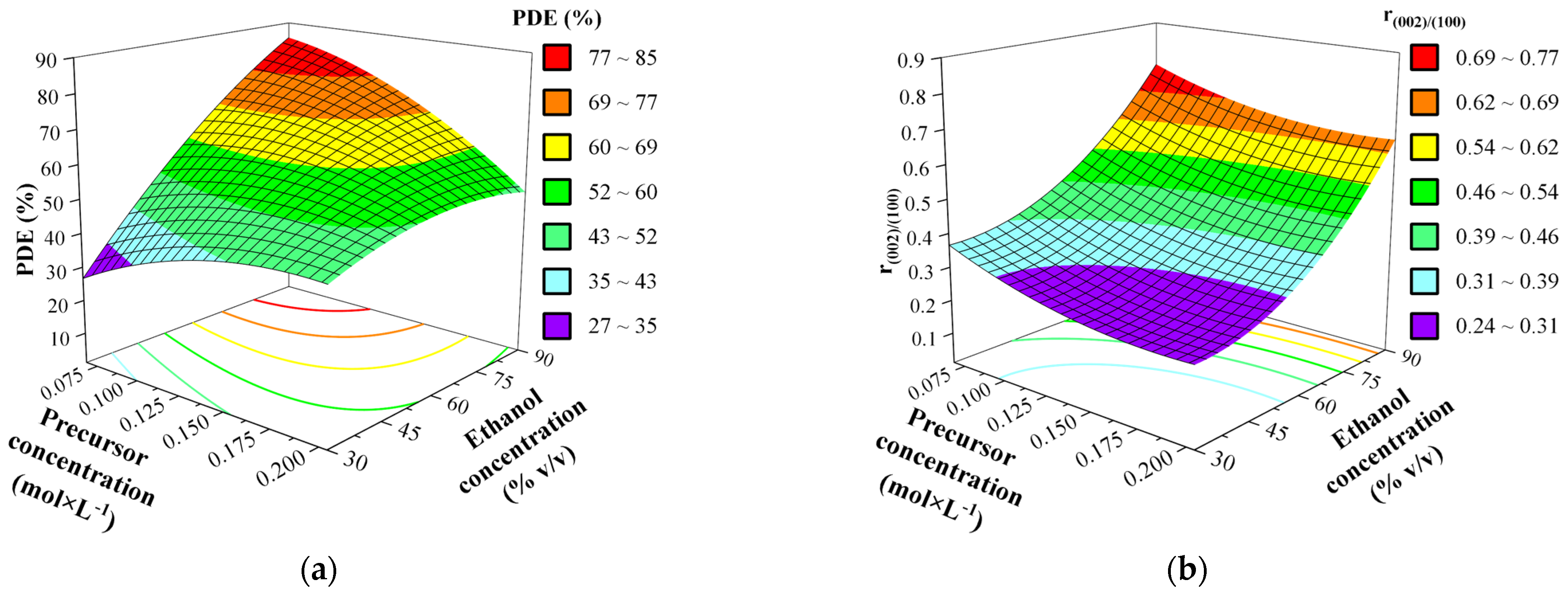
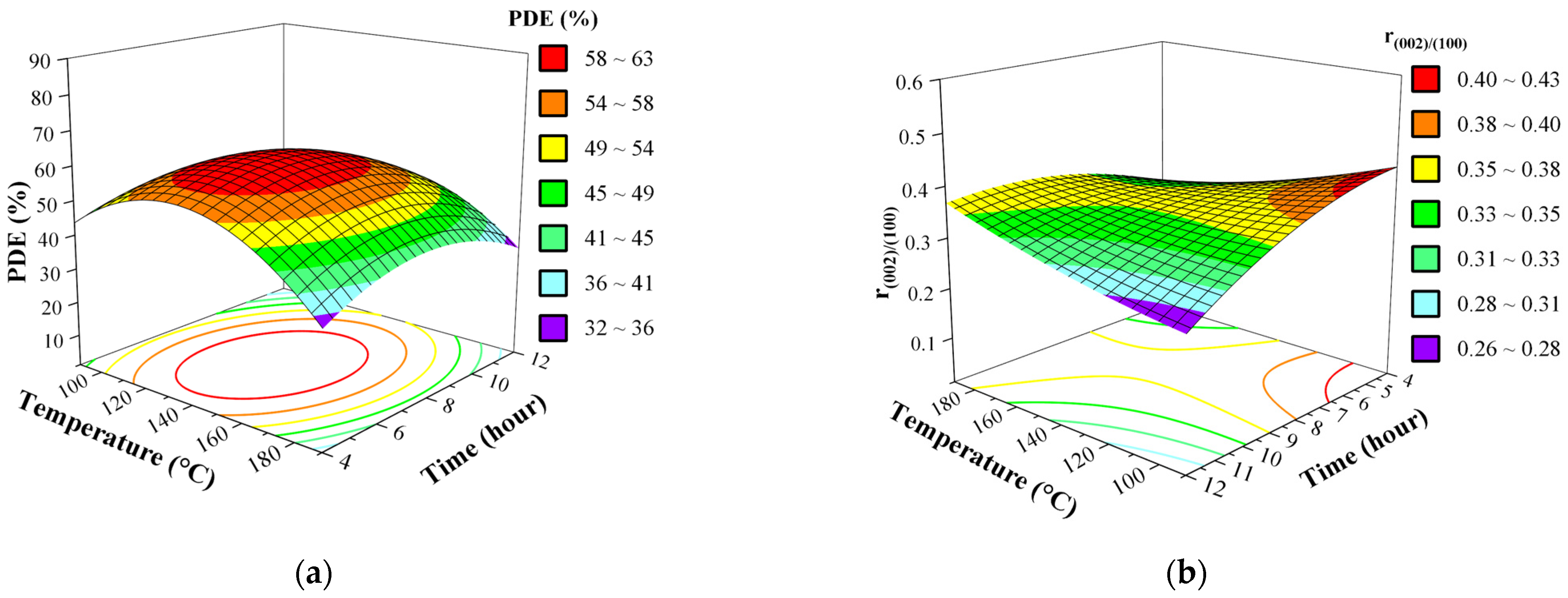
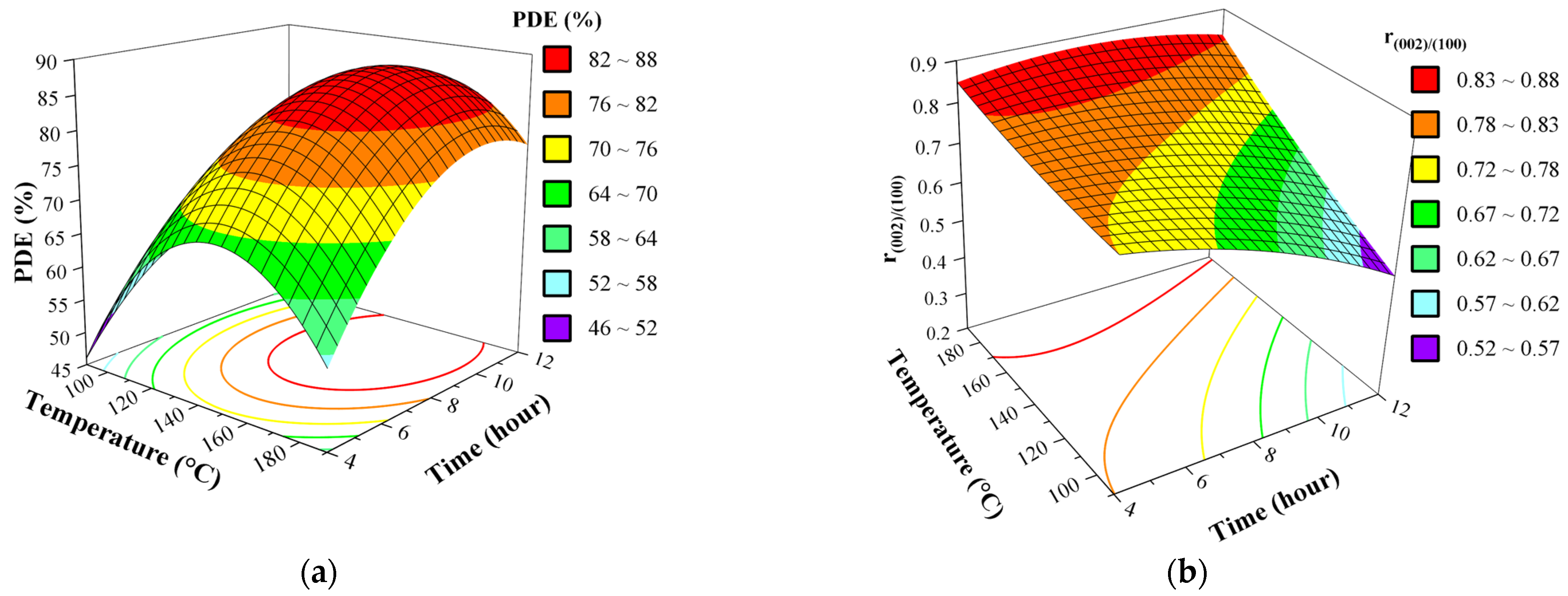
3.5. Effect of Synthesis Factors as Surface and Contour Plots
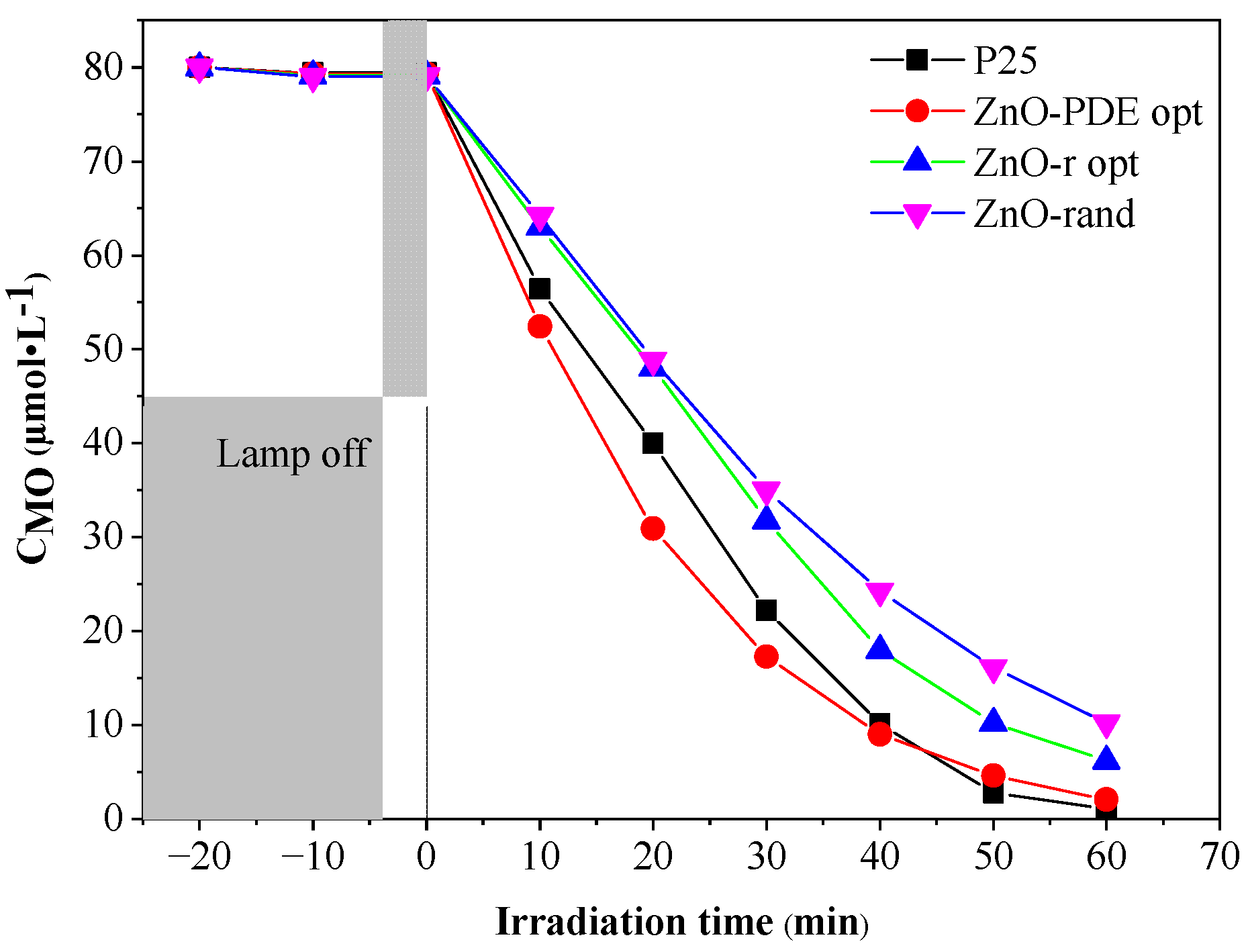
3.6. Optimization and Model Validation
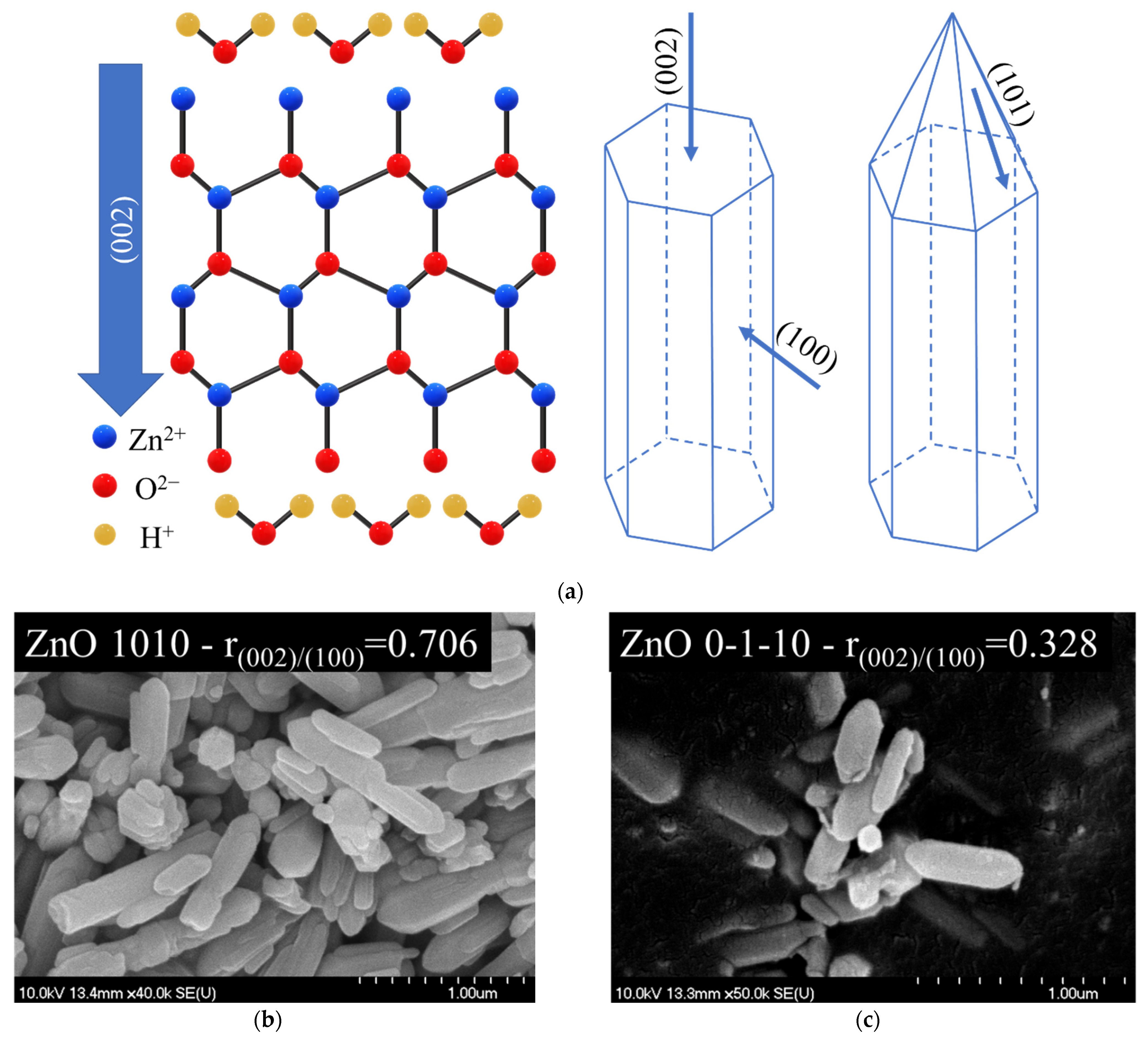
3.7. The Relation of (0 0 2) Peak to the Photocatalytic Activity of ZnO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hern, D. Development of alternative photocatalysts to TiO2: Challenges and opportunities. Energy Environ. Sci. 2009, 1231–1257. [Google Scholar] [CrossRef]
- Lee, C.J.; Lee, T.J.; Lyu, S.C.; Zhang, Y.; Ruh, H.; Lee, H.J. Field emission from well-aligned zinc oxide nanowires grown at low temperature. Appl. Phys. Lett. 2002, 81, 3648–3650. [Google Scholar] [CrossRef]
- Gupta, T.K. Application of Zinc Oxide Varistors. J. Am. Ceram. Soc. 1990, 73, 1817–1840. [Google Scholar] [CrossRef]
- Kumar, R.; Al-Dossary, O.; Kumar, G.; Umar, A. Zinc oxide nanostructures for NO2 gas–sensor applications: A review. Nano Micro Lett. 2015, 7, 97–120. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chem. Eng. J. 2016, 284, 582–598. [Google Scholar] [CrossRef]
- Thi, V.H.T.; Lee, B.K. Effective photocatalytic degradation of paracetamol using La-doped ZnO photocatalyst under visible light irradiation. Mater. Res. Bull. 2017, 96, 171–182. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Z.; Ren, T.; Ding, H.; Yao, W.; Zong, R.; Zhu, Y. Influence of defects on the photocatalytic activity of ZnO. J. Phys. Chem. C 2014, 118, 15300–15307. [Google Scholar] [CrossRef]
- Cardona, M.; Yu, P.Y. Optical Properties of Semiconductors. In Comprehensive Semiconductor Science and Technology, 1st ed.; Bhattacharya, P., Fornari, R., Kamimura, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 1–6, pp. 125–195. ISBN 9780444531537. [Google Scholar]
- Refaat, A.A. Biodiesel production using solid metal oxide catalysts. Int. J. Environ. Sci. Technol. 2011, 8, 203–221. [Google Scholar] [CrossRef]
- Chung, Y.T.; Ba-Abbad, M.M.; Mohammad, A.W.; Hairom, N.H.H.; Benamor, A. Synthesis of minimal-size ZnO nanoparticles through sol-gel method: Taguchi design optimisation. Mater. Des. 2015, 87, 780–787. [Google Scholar] [CrossRef]
- Huang, Y.; Wei, Y.; Wu, J.; Guo, C.; Wang, M.; Yin, S.; Sato, T. Low temperature synthesis and photocatalytic properties of highly oriented ZnO/TiO 2-x N y coupled photocatalysts. Appl. Catal. B Environ. 2012, 123, 9–17. [Google Scholar] [CrossRef]
- Anand, V.; Srivastava, V.C. Zinc oxide nanoparticles synthesis by electrochemical method: Optimization of parameters for maximization of productivity and characterization. J. Alloys Compd. 2015, 636, 288–292. [Google Scholar] [CrossRef]
- Wang, S.L.; Jia, X.; Jiang, P.; Fang, H.; Tang, W.H. Large-scale preparation of chestnut-like ZnO and Zn-ZnO hollow nanostructures by chemical vapor deposition. J. Alloys Compd. 2010, 502, 118–122. [Google Scholar] [CrossRef]
- Batault, F.; Héquet, V.; Raillard, C.; Thévenet, F.; Locoge, N.; Le Coq, L. How chemical and physical mechanisms enable the influence of the operating conditions in a photocatalytic indoor air treatment device to be modeled. Chem. Eng. J. 2017, 307, 766–775. [Google Scholar] [CrossRef]
- Du, Y.P.; Zhang, Y.W.; Sun, L.D.; Yan, C.H. Efficient energy transfer in monodisperse Eu-doped ZnO nanocrystals synthesized from metal acetylacetonates in high-boiling solvents. J. Phys. Chem. C 2008, 112, 12234–12241. [Google Scholar] [CrossRef]
- Djuriić, A.B.; Ng, A.M.C.; Chen, X.Y. ZnO nanostructures for optoelectronics: Material properties and device applications. Prog. Quantum Electron. 2010, 34, 191–259. [Google Scholar] [CrossRef]
- Khataee, A.; Soltani, R.D.C.; Hanifehpour, Y.; Safarpour, M.; Gholipour Ranjbar, H.; Joo, S.W. Synthesis and characterization of dysprosium-doped ZnO nanoparticles for photocatalysis of a textile dye under visible light irradiation. Ind. Eng. Chem. Res. 2014, 53, 1924–1932. [Google Scholar] [CrossRef]
- Famengo, A.; Anantharaman, S.; Ischia, G.; Causin, V.; Natile, M.M.; Maccato, C.; Tondello, E.; Bertagnolli, H.; Gross, S. Facile and reproducible synthesis of nanostructured colloidal ZnO nanoparticles from zinc acetylacetonate: Effect of experimental parameters and mechanistic investigations. Eur. J. Inorg. Chem. 2009, 5017–5028. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Opalinska, A.; Chudoba, T.; Gierlotka, S.; Mukhovskyi, R.; Pietrzykowska, E.; Sobczak, K.; Lojkowski, W. Effect of Water Content in Ethylene Glycol Solvent on the Size of ZnO Nanoparticles Prepared Using Microwave Solvothermal Synthesis. J. Nanomater. 2016, 2016. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Koltsov, I.; Gierlotka, S.; Dworakowska, S.; Lojkowski, W. “Size control mechanism of ZnO nanoparticles obtained in microwave solvothermal synthesis”. Nanotechnology 2018, 29, 065601. [Google Scholar] [CrossRef]
- Kanagamani, K.; Muthukrishnan, P.; Saravanakumar, K.; Gierlotka, S.; Dworakowska, S.; Lojkowski, W. “Photocatalytic degradation of environmental perilous gentian violet dye using leucaena-mediated zinc oxide nanoparticle and its anticancer activity”. Rare Met. 2019, 38, 277–286. [Google Scholar] [CrossRef]
- Schneider, J.J.; Hoffmann, R.C.; Engstler, J.; Dilfer, S.; Klyszcz, A.; Erdem, E.; Jakes, P.; Eichel, R.A. Zinc oxide derived from single source precursor chemistry under chimie douce conditions: Formation pathway, defect chemistry and possible applications in thin film printing. J. Mater. Chem. 2009, 19, 1449–1457. [Google Scholar] [CrossRef]
- Liang, Y.; Wicker, S.; Wang, X.; Erichsen, E.; Fu, F. Organozinc Precursor-Derived Crystalline ZnO Nanoparticles: Synthesis, Characterization and Their Spectroscopic Properties. Nanomaterials 2018, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Ludi, B.; Niederberger, M. Zinc oxide nanoparticles: Chemical mechanisms and classical and non-classical crystallization. Dalt. Trans. 2013, 42, 12554–12568. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Ba-Abbad, M.M.; Chai, P.V.; Takriff, M.S.; Benamor, A.; Mohammad, A.W. Optimization of nickel oxide nanoparticle synthesis through the sol-gel method using Box-Behnken design. Mater. Des. 2015, 86, 948–956. [Google Scholar] [CrossRef]
- Kayani, Z.N.; Iqbal, M.; Riaz, S.; Zia, R.; Naseem, S. Fabrication and properties of zinc oxide thin film prepared by sol-gel dip coating method. Mater. Sci. Pol. 2015, 33, 515–520. [Google Scholar] [CrossRef]
- Poortavasoly, H.; Montazer, M.; Harifi, T. Aminolysis of polyethylene terephthalate surface along with in situ synthesis and stabilizing ZnO nanoparticles using triethanolamine optimized with response surface methodology. Mater. Sci. Eng. C 2016, 58, 495–503. [Google Scholar] [CrossRef]
- Liu, H.; Ding, Y.; Somayazulu, M.; Qian, J.; Shu, J.; Häusermann, D.; Mao, H.K. Rietveld refinement study of the pressure dependence of the internal structural parameter u in the wurtzite phase of ZnO. Phys. Rev. B Condens. Matter Mater. Phys. 2005, 71, 1–4. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, G.; Umar, A. Zinc oxide nanomaterials for photocatalytic degradation of methyl orange: A review. Nanosci. Nanotechnol. Lett. 2014, 6, 631–650. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Dong, L.; Qu, B.; Wang, J. Facile synthesis and enhanced photocatalytic performance of 3D ZnO hierarchical structures. J. Nanosci. Nanotechnol. 2011, 11, 5042–5048. [Google Scholar] [CrossRef]
- Chen, T.; Zheng, Y.; Lin, J.M.; Chen, G. Study on the Photocatalytic Degradation of Methyl Orange in Water Using Ag/ZnO as Catalyst by Liquid Chromatography Electrospray Ionization Ion-Trap Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2008. [Google Scholar] [CrossRef] [PubMed]
- Körbahti, B.K.; Rauf, M.A. Response surface methodology (RSM) analysis of photoinduced decoloration of toludine blue. Chem. Eng. J. 2008, 136, 25–30. [Google Scholar] [CrossRef]
- Huang, M.; Weng, S.; Wang, B.; Hu, J.; Fu, X.; Liu, P. Various facet tunable ZnO crystals by a scalable solvothermal synthesis and their facet-dependent photocatalytic activities. J. Phys. Chem. C 2014, 118, 25434–25440. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Šarić, A.; Štefanić, G.; Dražić, G.; Gotić, M. Solvothermal synthesis of zinc oxide microspheres. J. Alloys Compd. 2015, 652, 91–99. [Google Scholar] [CrossRef]
- Kanari, N.; Mishra, D.; Gaballah, I.; Dupré, B. Thermal decomposition of zinc carbonate hydroxide. Thermochim. Acta 2004, 410, 93–100. [Google Scholar] [CrossRef]
- Inubushi, Y.; Takami, R.; Iwasaki, M.; Tada, H.; Ito, S. Mechanism of formation of nanocrystalline ZnO particles through the reaction of [Zn(acac)2] with NaOH in EtOH. J. Colloid Interface Sci. 1998, 200, 220–227. [Google Scholar] [CrossRef]
- Huang, M.; Yan, Y.; Feng, W.; Weng, S.; Zheng, Z.; Fu, X.; Liu, P. Controllable tuning various ratios of ZnO polar facets by crystal seed-assisted growth and their photocatalytic activity. Cryst. Growth Des. 2014, 14, 2179–2186. [Google Scholar] [CrossRef]
- Boppella, R.; Anjaneyulu, K.; Basak, P.; Manorama, S.V. Facile synthesis of face oriented ZnO crystals: Tunable polar facets and shape induced enhanced photocatalytic performance. J. Phys. Chem. C 2013, 117, 4597–4605. [Google Scholar] [CrossRef]
- Chang, J.; Waclawik, E.R. Facet-controlled self-assembly of ZnO nanocrystals by non-hydrolytic aminolysis and their photodegradation activities. Cryst. Eng. Comm. 2012, 14, 4041–4048. [Google Scholar] [CrossRef]
- Trandafilović, L.V.; Jovanović, D.J.; Zhang, X.; Ptasińska, S.; Dramićanin, M.D. Enhanced photocatalytic degradation of methylene blue and methyl orange by ZnO: Eu nanoparticles. Appl. Catal. B Environ. 2017, 203, 740–752. [Google Scholar] [CrossRef]
- Mclaren, A.; Valdes-Solis, T.; Li, G.; Tsang, S.C. Shape and Size Effect of ZnO nanocrystals on Photocatalytic Activity Supporting Information. J. Am. Chem. Soc. 2009, 131, 12540–12541. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ye, L.; Liu, S.; Li, Y.; Ji, X. Photocatalytic reduction of CO2 by ZnO micro/nanomaterials with different morphologies and ratios of {0001} facets. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Elshypany, R.; Selim, H.; Zakaria, K.; Moustafa, A.H.; Sadeek, S.A.; Sharaa, S.I.; Raynaud, P.; Nada, A.A. Magnetic ZnO Crystal Nanoparticle Growth on Reduced Graphene Oxide for Enhanced Photocatalytic Performance under Visible Light Irradiation. Molecules 2021, 26, 2269. [Google Scholar] [CrossRef] [PubMed]
- Thennarasu, G.; Sivasamy, A. Metal ion doped semiconductor metal oxide nanosphere particles prepared by soft chemical method and its visible light photocatalytic activity in degradation of phenol. Powder Technol. 2013, 250, 1–12. [Google Scholar] [CrossRef]
- Kayaci, F.; Vempati, S.; Ozgit-Akgun, C.; Donmez, I.; Biyikli, N.; Uyar, T. Transformation of polymer-ZnO core-shell nanofibers into ZnO hollow nanofibers: Intrinsic defect reorganization in ZnO and its influence on the photocatalysis. Appl. Catal. B Environ. 2015, 176, 646–653. [Google Scholar] [CrossRef]
- Sun, J.H.; Dong, S.Y.; Wang, Y.K.; Sun, S.P. Preparation and photocatalytic property of a novel dumbbell-shaped ZnO microcrystal photocatalyst. J. Hazard. Mater. 2009, 172, 1520–1526. [Google Scholar] [CrossRef]
| Factors | Symbol | Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Temperature [°C] | X1 | 90 | 140 | 190 |
| Concentration of the precursor [M] | X2 | 0.068 | 0.136 | 0.204 |
| Ethanol content of the solvent [% v/v] | X3 | 30 | 60 | 90 |
| Duration of the solvothermal treatment [h] | X4 | 4 | 8 | 12 |
| Analysis of Variance | |||||
|---|---|---|---|---|---|
| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
| Model | 14 | 6326.51 | 451.89 | 97.32 | <10−3 * |
| Linear | 4 | 3443.08 | 860.77 | 185.37 | <10−3 * |
| X1 | 1 | 55.04 | 55.04 | 11.85 | <10−3 * |
| X2 | 1 | 234.97 | 234.97 | 50.6 | <10−3 * |
| X3 | 1 | 3043.27 | 3043.27 | 655.37 | <10−3 * |
| X4 | 1 | 109.81 | 109.81 | 23.65 | <10−3 * |
| Square | 4 | 1657.47 | 414.37 | 89.23 | <10−3 * |
| X12 | 1 | 1549.66 | 1549.66 | 333.72 | <10−3 * |
| X22 | 1 | 173.03 | 173.03 | 37.26 | <10−3 * |
| X32 | 1 | 134.45 | 134.45 | 28.95 | <10−3 * |
| X42 | 1 | 483.45 | 483.45 | 104.11 | <10−3 * |
| 2-Way Interaction | 6 | 1225.96 | 204.33 | 44 | <10−3 * |
| X1 × X2 | 1 | 46.92 | 46.92 | 10.1 | <10−3 * |
| X1 × X3 | 1 | 175.56 | 175.56 | 37.81 | <10−3 * |
| X1 × X4 | 1 | 29.7 | 29.7 | 6.4 | <10−3 * |
| X2 × X3 | 1 | 720.92 | 720.92 | 155.25 | <10−3 * |
| X2 × X4 | 1 | 46.92 | 46.92 | 10.1 | <10−3 * |
| X3 × X4 | 1 | 205.92 | 205.92 | 44.35 | <10−3 * |
| Error | 12 | 55.72 | 4.64 | <10−3 * | |
| Lack-of-Fit | 10 | 55.08 | 5.51 | 17.03 | 0.057 ** |
| Pure Error | 2 | 0.65 | 0.32 | ||
| Total | 26 | 6382.23 | |||
| Analysis of Variance | |||||
|---|---|---|---|---|---|
| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
| Model | 14 | 0.693 | 0.050 | 32.5 | <10−3 * |
| Linear | 4 | 0.557 | 0.139 | 91.31 | <10−3 * |
| X1 | 1 | 0.001 | 0.001 | 0.38 | 0.551 ** |
| X2 | 1 | 0.044 | 0.044 | 28.98 | <10−3 * |
| X3 | 1 | 0.506 | 0.506 | 331.72 | <10−3 * |
| X4 | 1 | 0.006 | 0.006 | 4.17 | 0.064 ** |
| Square | 4 | 0.092 | 0.023 | 15.16 | <10−3 * |
| X12 | 1 | 0.001 | 0.001 | 0.52 | 0.484 ** |
| X22 | 1 | 0.009 | 0.009 | 5.98 | <10−3 * |
| X32 | 1 | 0.059 | 0.059 | 38.92 | <10−3 * |
| X42 | 1 | 0.006 | 0.006 | 4.09 | 0.066 ** |
| 2-Way Interaction | 6 | 0.044 | 0.007 | 4.85 | <10−3 * |
| X1 × X2 | 1 | 0.010 | 0.010 | 6.56 | <10−3 * |
| X1 × X3 | 1 | 0.011 | 0.011 | 7.44 | <10−3 * |
| X1 × X4 | 1 | 0.014 | 0.014 | 9.45 | <10−3 * |
| X2 × X3 | 1 | 0.000 | 0.000 | 0.02 | 0.89 ** |
| X2 × X4 | 1 | 0.000 | 0.000 | 0.01 | 0.91 ** |
| X3 × X4 | 1 | 0.009 | 0.009 | 5.61 | <10−3 * |
| Error | 12 | 0.018 | 0.002 | <10−3 * | |
| Lack-of-Fit | 10 | 0.018 | 0.002 | 16.37 | 0.059 ** |
| Pure Error | 2 | 0.000 | 0.000 | ||
| Total | 26 | 0.712 | |||
| Parameters | Temp. | Precursor Conc. | EtOH Conc. | Duration | Predicted | Experimental | ||
|---|---|---|---|---|---|---|---|---|
| [°C] | [M] | [% v/v] | [h] | PDE | r(002)/(100) | PDE | r(002)/(100) | |
| [%] | [a.u.] | [%] | [a.u.] | |||||
| Optimized r(002)/(100) (ZnO-r opt) | 190 | 0.068 | 90 | 7.56 | 74.90 | 0.873 | 77.30 | 0.834 |
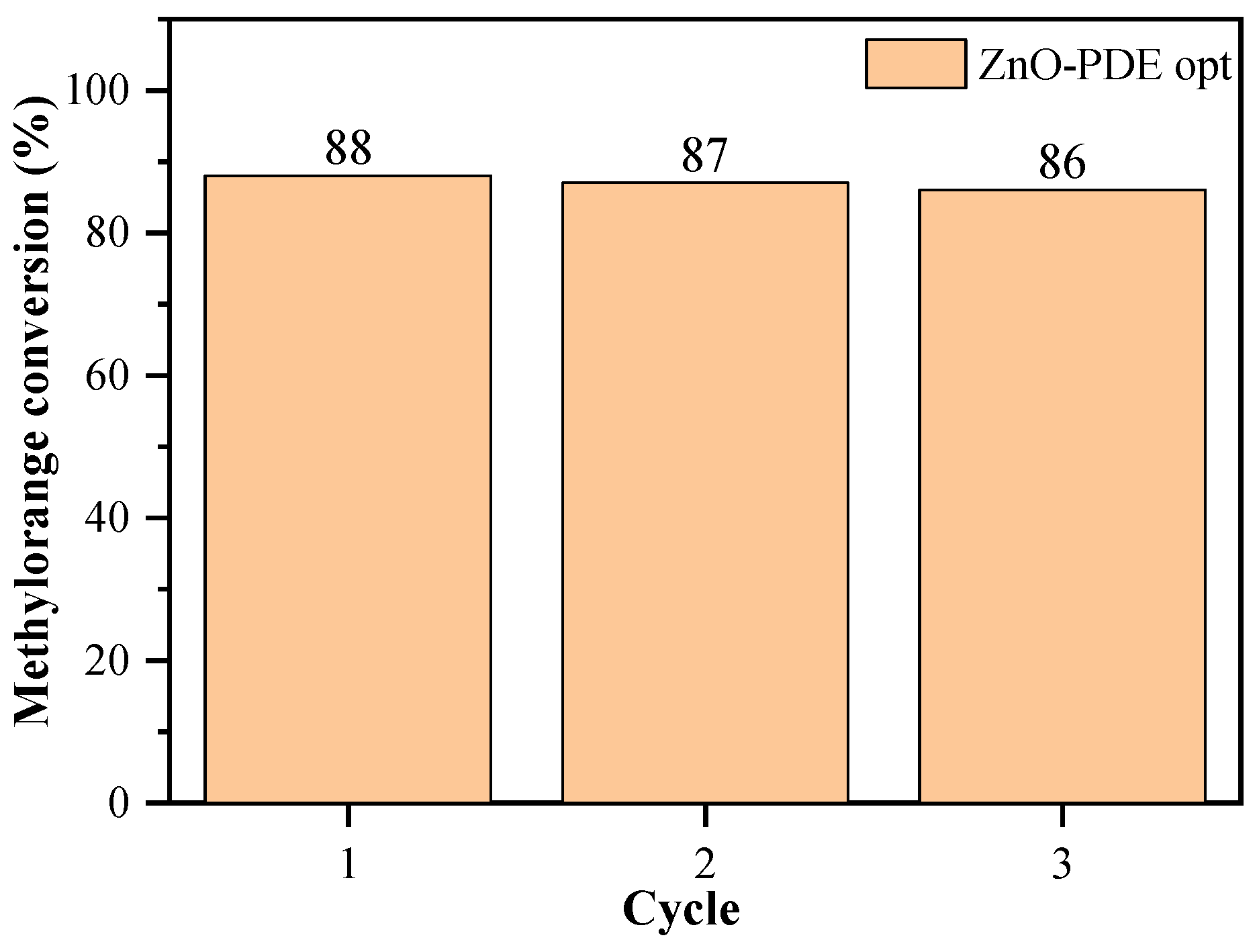
| Optimized PDE (ZnO-PDE opt) | 155 | 0.068 | 90 | 9.75 | 88.03 | 0.766 | 89.10 | 0.799 |
| Randomized (ZnO-rand) | 107 | 0.102 | 69 | 9.00 | 67.18 | 0.516 | 69.30 | 0.534 |
| Synthesis Method | Photocatalytic Activity Experiment | Range of (0 0 2)/(1 0 0) Variation | Observed Trend of Photocatalytic Activity with the Ratio | Reference |
|---|---|---|---|---|
| Solvothermal | Photoreduction CO2 | 0.91–1.27 | Increased | [46] |
| Chemical bath deposition process | Photodegradation of Rhodamine B | 1.28–1.9 | Decreased | [41] |
| High temperature chemical precipitation | 0.63–0.92 | Increased | [43] | |
| Chemical precipitation | Photodegradation of phenol | 0.54–0.93 (I), 0.93–1.13 (II) | I-Increased, II-Decreased | [48] |
| Electrospinning deposition | Photodegradation of Rhodamine B | 0.81–1.06 | Increased | [49] |
| Combustion | Methylene Blue, Crystal Violet | 0.4–0.55 | Increased | [50] |
| Solvothermal | Methylene Blue | 1–5 | Decreased | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, Z.; Molnár, C.; Štangar, U.L.; Cristea, V.-M.; Pap, Z.; Hernadi, K.; Baia, L. Optimization Method of the Solvothermal Parameters Using Box–Behnken Experimental Design—The Case Study of ZnO Structural and Catalytic Tailoring. Nanomaterials 2021, 11, 1334. https://doi.org/10.3390/nano11051334
Kovács Z, Molnár C, Štangar UL, Cristea V-M, Pap Z, Hernadi K, Baia L. Optimization Method of the Solvothermal Parameters Using Box–Behnken Experimental Design—The Case Study of ZnO Structural and Catalytic Tailoring. Nanomaterials. 2021; 11(5):1334. https://doi.org/10.3390/nano11051334
Chicago/Turabian StyleKovács, Zoltán, Csanád Molnár, Urška Lavrenčič Štangar, Vasile-Mircea Cristea, Zsolt Pap, Klara Hernadi, and Lucian Baia. 2021. "Optimization Method of the Solvothermal Parameters Using Box–Behnken Experimental Design—The Case Study of ZnO Structural and Catalytic Tailoring" Nanomaterials 11, no. 5: 1334. https://doi.org/10.3390/nano11051334
APA StyleKovács, Z., Molnár, C., Štangar, U. L., Cristea, V.-M., Pap, Z., Hernadi, K., & Baia, L. (2021). Optimization Method of the Solvothermal Parameters Using Box–Behnken Experimental Design—The Case Study of ZnO Structural and Catalytic Tailoring. Nanomaterials, 11(5), 1334. https://doi.org/10.3390/nano11051334