Characterization of a Novel Nanocomposite Film Based on Functionalized Chitosan–Pt–Fe3O4 Hybrid Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
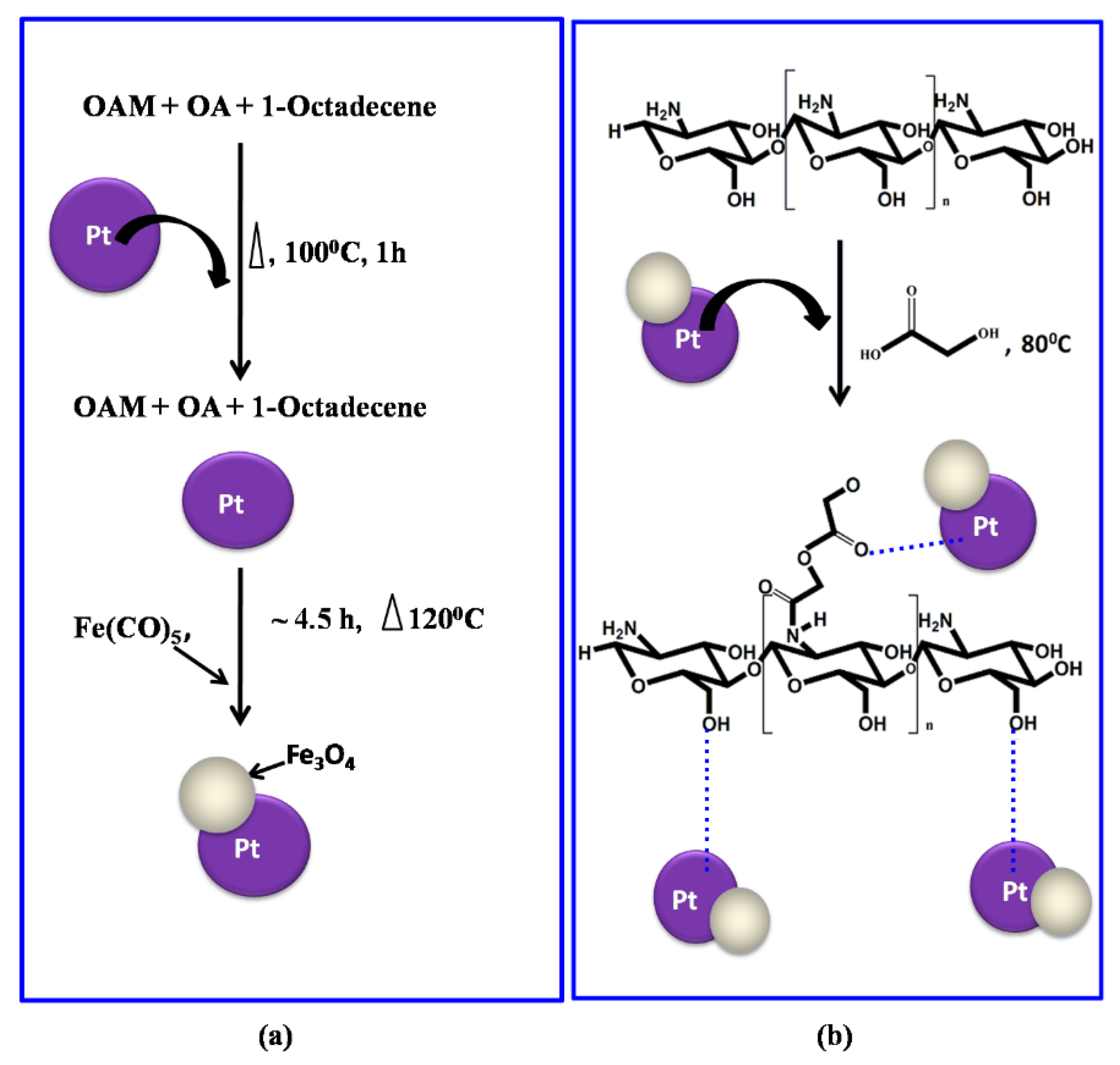
2.1. Pt–Fe3O4 Hybrid Nanoparticle Synthesis
2.2. Preparation of Pt–Fe3O4 Hybrid Nanoparticles-Grafted Chitosan Nanocomposite Film
2.3. Characterization
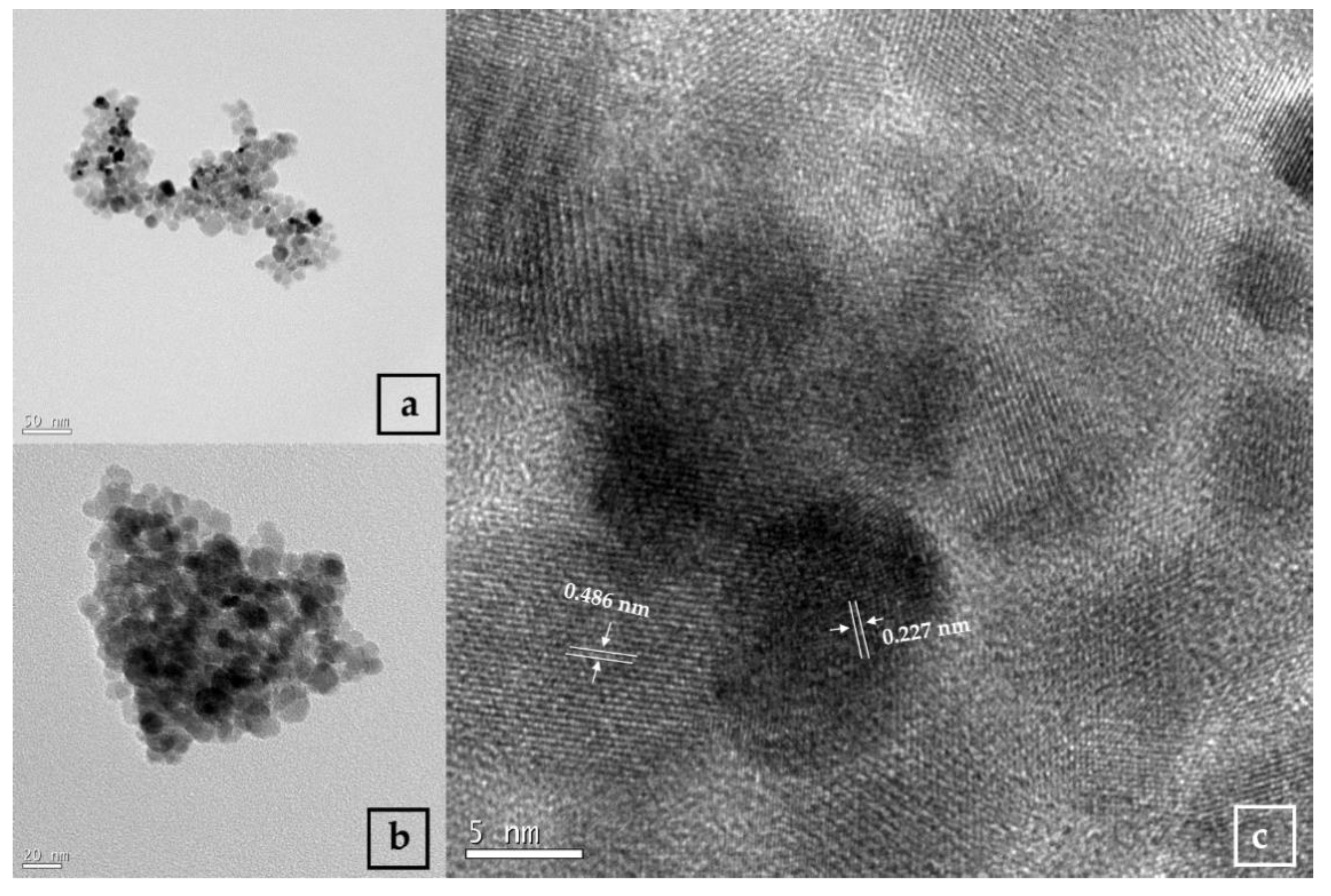
2.3.1. Transmission Electron Microscopy
2.3.2. Physical Property Measuring System
2.3.3. X-ray Diffraction
2.3.4. Atomic Force Microscopy
2.3.5. FTIR Spectra
2.3.6. Thermogravimetric Analysis
2.3.7. Tensile Strength Testing
2.3.8. Water Absorption Study
3. Results and Discussion
3.1. TEM Analysis of Nanoparticles
3.2. Magnetic Property Analysis of the Pt–Fe3O4 Hybrid Nanoparticles
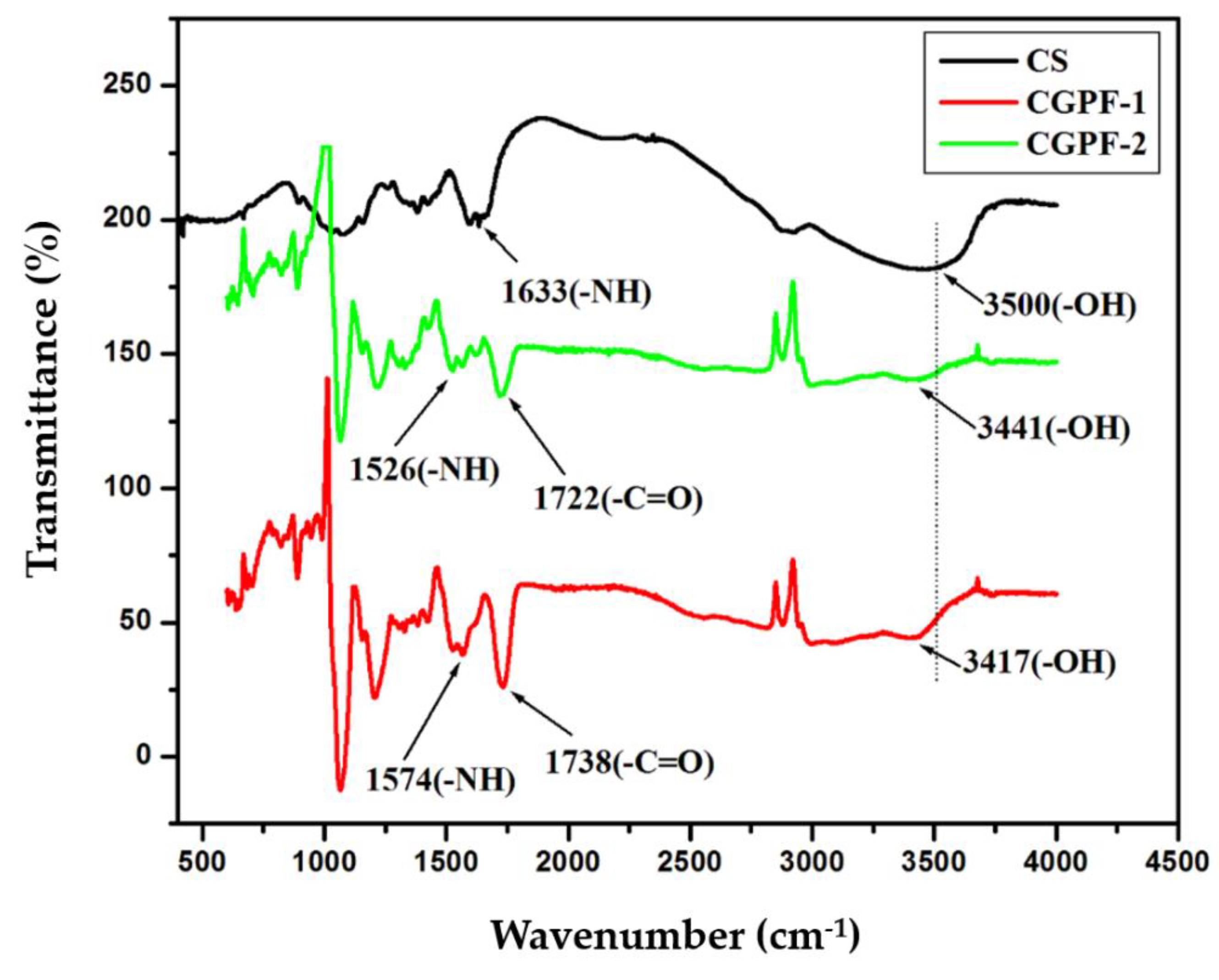
3.3. FTIR Analysis
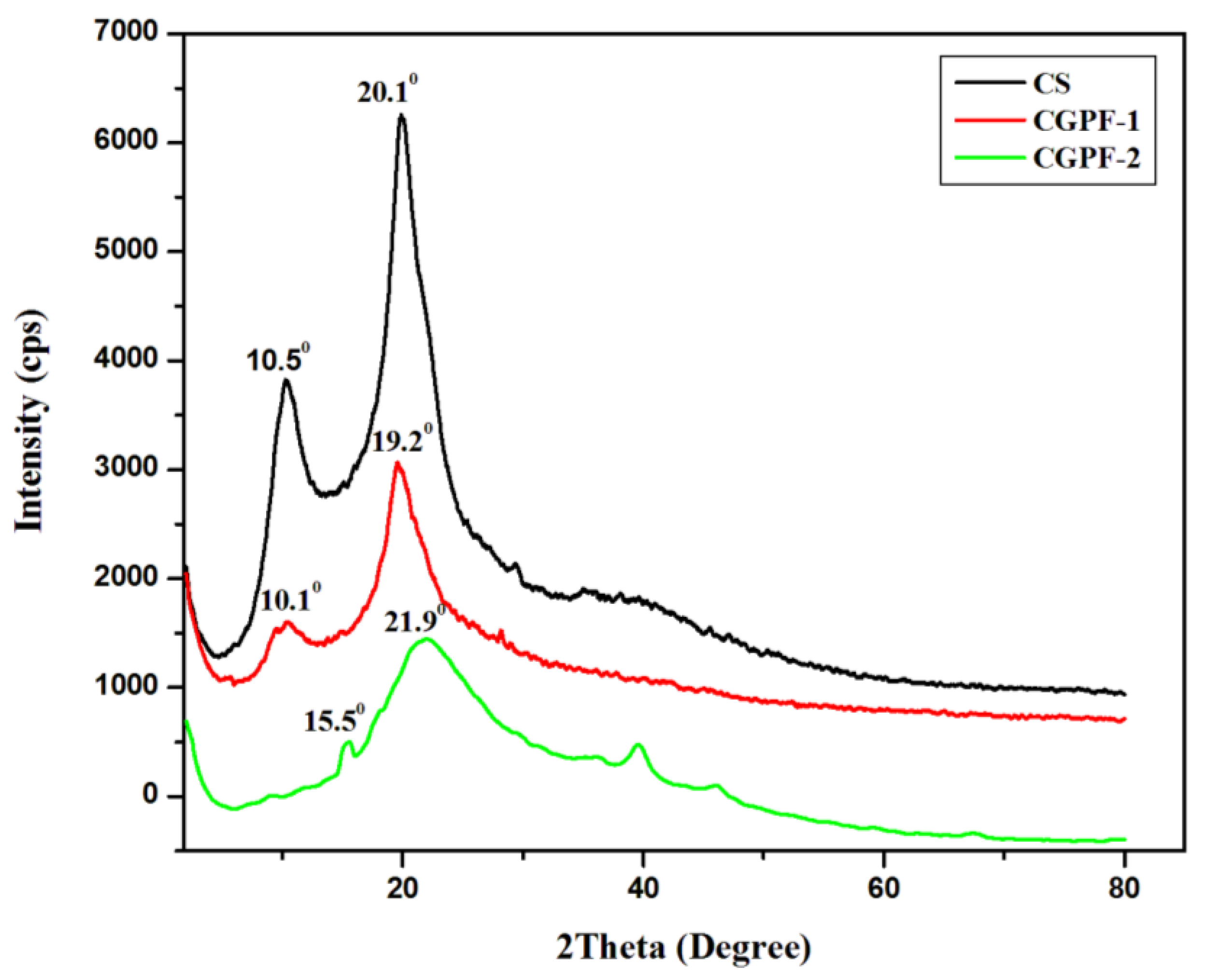
3.4. XRD Analysis
3.5. Morphological Studies
3.6. Water Absorption Studies
3.7. Tensile Strength Testing
3.8. Thermogravimetric Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gómez-Romero, P.; Sanchez, C. Functional Hybrid Materials; Wiley: New York, NY, USA, 2004; pp. 50–85. [Google Scholar]
- Kishnamoorti, R.; Vaia, R.A. Polymer Nanocomposites; ACS: Washington, DC, USA, 2002. [Google Scholar]
- Chapman, R.; Mulvaney, P. Electro-optical shifts in silver nanoparticle films. Chem. Phys. Lett. 2001, 349, 358–362. [Google Scholar] [CrossRef]
- Wilson, O.; Wilson, G.J.; Mulvaney, P. Laser writing in polarized silver nanorod films. Adv. Mater. 2002, 14, 1000–1004. [Google Scholar] [CrossRef]
- Yoon, P.J.; Fornes, T.D.; Paul, D.R. Thermal expansion behavior of nylon 6 nanocomposites. Polymer 2002, 43, 6727–6741. [Google Scholar] [CrossRef]
- Lagaly, G. Introduction: From clay mineral–polymer interactions to clay mineral–polymer nanocomposites. Appl. Clay. Sci. 1999, 15, 1–9. [Google Scholar]
- Luckham, P.F.; Rossi, S. The colloidal and rheological properties of bentonite suspensions. Adv. Coll. Interf. Sci. 1999, 82, 43–92. [Google Scholar] [CrossRef]
- Dobson, J. Magnetic nanoparticle for drug delivery. Drug Dev. Res. 2006, 67, 55–60. [Google Scholar] [CrossRef]
- Francüois, N.J.; Allo, S.; Jacobo, S.E.; Daraio, M.E. Composites of polymeric gels and magnetic nanoparticles: Preparation and drug release behavior. J. Appl. Polym. Sci. 2007, 105, 647–655. [Google Scholar] [CrossRef]
- Zhao, D.L.; Zhang, H.L.; Zeng, X.W.; Xia, Q.S.; Tang, J.T. Inductive heat property of Fe3O4/polymer composite nanoparticles in an ac magnetic field for localized hyperthermia. Biomed. Mater. 2006, 1, 198–201. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S.; Müller, R.; Zeisberger, M. Magnetic particle hyperthermia: Nanoparticle magnetism and materials development for cancer therapy. J. Phys. Condens. Matter. 2006, 18, S2919–S2934. [Google Scholar] [CrossRef]
- Kumari, S.; Singh, R.P. Glycolic acid functionalized chitosan–Au–Fe3O4 hybrid nanoparticle based nanohybrid scaffold for drug delivery. Int. J. Bio. Macromol. 2013, 54, 244–249. [Google Scholar] [CrossRef]
- Kumari, S.; Singh, R.P. Glycolic acid-functionalized chitosan–Co3O4–Fe3O4 hybrid magnetic nanoparticles-based nanohybrid scaffolds for drug-delivery and tissue engineering. J. Mat. Sci. 2013, 48, 1524–1532. [Google Scholar] [CrossRef]
- Gu, H.; Yang, Z.; Gao, J.; Chang, C.K.; Xu, B. Heterodimers of nanoparticle: Formation at a liquid –liquid interface and particle-specific surface modification by functional molecules. J. Am. Chem. Soc. 2005, 127, 34–35. [Google Scholar] [CrossRef]
- Teranishi, T.; Inoue, Y.; Nakaya, M.; Oumi, Y.; Sano, T. Nanocorns: Anisotropically phase-segregated CoPd sulphide nanoparticles. J. Am. Chem. Soc. 2004, 126, 9914–9915. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.W.; Zheng, R.K.; Zhang, X.X.; Xu, B. Facile one-pot synthesis of bimodal heterodimers of nanoparticles: A conjugate of quantum dot and magnetic nanoparticles. J. Am. Chem. Soc. 2004, 126, 5664–5665. [Google Scholar] [CrossRef] [PubMed]
- Hens, Z.; Vanmaekelbergh, D.; Stoffels, E.; van Kempen, H. Effects of crystal shape on the energy levels of zero-dimensional PbS quantum dots. Phys. Rev. Lett. 2002, 88, 236803. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.W.; Shim, M. y-Fe2O3/II-VI Sulfide Nanocrystal Heterojunctions. J. Am. Chem. Soc. 2005, 127, 10269–10275. [Google Scholar] [CrossRef]
- Shi, W.; Zeng, H.; Sahoo, Y.; Ohulchanskyy, T.Y.; Ding, Y.; Wang, Z.L.; Prasad, P.N. General Approach to Binary and Ternary Hybrid Nanocrystals. Nano Lett. 2006, 6, 875–881. [Google Scholar] [CrossRef]
- Yu, H.; Chen, M.; Rice, P.M.; Wang, S.X.; White, R.L.; Sun, S.W.; Sun, S. Dumbell-like bifunctional Au-Fe3O4 nanoparticles. Nano Lett. 2005, 5, 379–382. [Google Scholar] [CrossRef]
- Kudera, S.; Carbone, L.; Casula, M.F.; Cingolani, R.; Falqui, A.; Snoeck, E.; Parak, W.J.; Manna, L. Selective growth of PbSe on one or both tips of colloidal semiconductor nanorods. Nano Lett. 2005, 5, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.M.; El-Zairy, E.M. Chitosan as a biomaterial—Structure, properties, and electrospun nanofibers. Concepts Compd. Altern. Antibact. 2015, 81–101. [Google Scholar] [CrossRef]
- Dos Santos, D.S.; Riul, A., Jr.; Malmegrin, R.R.; Fonseca, F.J.; Oliveira, O.N., Jr.; Mattoso, L.H.C. Color changes in chitosan and poly (allyl amine) films upon metal binding. Macromol. Biosci. 2003, 3, 591–595. [Google Scholar] [CrossRef]
- Xie, M.; Liu, H.H.; Chen, P.; Zhang, Z.L.; Wang, X.H.; Xie, Z.X.; Du, Y.M.; Pand, B.Q.; Pang, D.W. CdSe/ZnS-labeled carboxymethyl chitosan as a bioprobe for live cell imaging. Chem. Commun. 2005, 44, 5518–5520. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, Q.; Lee, J.Y.; Wang, D.I.C. The synthesis of SERS-active gold nanoflower Tags for In Vivo Application. ACS Nano 2008, 2, 2473–2480. [Google Scholar] [CrossRef]
- De Masi, A.; Tonazzini, I.; Masciullo, C.; Mezzena, R.; Chiellini, F.; Puppi, D.; Cecchini, M. Chitosan films for regenerative medicine: Fabrication methods and mechanical characterization of nanostructured chitosan films. Biophys. Rev. 2019, 11, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Sakib, M.N.; Rashid, T.U. Chitosan based bioactive materials in tissue engineering applications-A review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Yin, J.; Khutoryanskaya, O.V.; Khutoryanskiy, V.V. Mucoadhesive and elastic films based on blends of chitosan and hydroxyethylcellulose. Macromol. Biosci. 2008, 8, 184–192. [Google Scholar] [CrossRef]
- Kumari, S.; Singh, R.P. Glycolic acid-g-chitosan–Pt–Fe3O4 nanoparticles nanohybrid scaffold for tissue engineering and drug delivery. Int. J. Bio. Macromol. 2012, 51, 76–82. [Google Scholar] [CrossRef]
- Rodriguez-Fernandez, J.; Perez-Juste, J.; Abajo, F.J.G.; Liz- Marzan, L.M. Seeded growth of sub Au colloids with quadrupole plasmon resonance modes. Langmuir 2006, 22, 7007–7010. [Google Scholar] [CrossRef] [PubMed]
- Schladt, T.D.; Shukoor, M.I.; Schneider, K.; Tahir, M.N.; Natalio, F.; Ament, I.; Becker, J.; Jochum, F.D.; Weber, S.; Köhler, O.; et al. Hybrid nanocomposites for selective dual functionalization and imaging. Angew. Chem. 2010, 49, 3976–3980. [Google Scholar] [CrossRef]
- Lustriane, C.; Dwivany, F.M.; Suendo, V.; Reza, M. Effect of chitosan and chitosan-nanoparticles on post harvest quality of banana fruits. J. Plant. Biotech. 2018, 45, 36–44. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdulwahid, R.T.; Rasheed, M.A.; Abdullah, O.G.; Ahmed, H.M. Polymer blending as a novel approach for tuning the SPR peaks of silver nanoparticles. Polymer 2017, 9, 486. [Google Scholar] [CrossRef]
- Aworska, M.; Sakurai, K.; Gaudon, P.; Guibal, E. Influence of chitosan characteristics on polymer properties. I. Crystallographic properties. Polym. Int. 2003, 2, 198–205. [Google Scholar] [CrossRef]
- Kumar, M.S.; Selvam, V.; Vadivel, M. Synthesis and characterization of silane modified iron (III) oxide nanoparticles reinforced chitosan nanocomposites. Eng. Sci. Adv. Tech. 2012, 2, 1258–1263. [Google Scholar]
- Depan, D.; Kumar, A.P.; Singh, R.P. Preparation and characterization of novel hybrid of chitosan-g-lactic acid and montmorillonite. J. Biomed. Mat. Res. Part. A 2006, 78, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Deng, J.; Yang, F.; Gong, Y.; Zhao, N.; Zhang, X. Study on physical properties and nerve cell affinity of composite films from chitosan and gelatine solutions. Biomat 2003, 24, 2871–2880. [Google Scholar] [CrossRef]
- Chen, R.; Hwa, H. Effect of molecular weight of chitosan with the same degree of deacetylation on the thermal, mechanical, and permeability properties of the prepared membrane. Carbohyd. Polym. 1996, 29, 353–358. [Google Scholar]
- Qurashi, M.; Blair, H.; Allen, S. Studies on modified chitosan membranes.1. Preparation and characterization. J. Appl. Polym. Sci. 1992, 46, 255–261. [Google Scholar] [CrossRef]
- Rodrigues, C.; de Mello, J.M.; Dalcanton, F.; Macuvele, D.L.; Padoin, N.; Fiori, M.A.; Soares, C.; Riella, H.G. Mechanical, thermal and antimicrobial properties of chitosan-based-nanocomposite with potential applications for food packaging. J. Poly. Env. 2020, 28, 1216–1236. [Google Scholar] [CrossRef]
- Wang, S.F.; Chen, L.; Tong, Y.J. Structure-property relationship in chitosan-based biopolymer/montmorillonite nanocomposites. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 686–696. [Google Scholar] [CrossRef]


| Sample Code | Chitosan (g) | Glycolic Acid (g) | Nanoparticle (mg) |
|---|---|---|---|
| CS | 1 | 0 | 0 |
| CGPF-1 | 1 | 1 | 0 |
| CGPF-2 | 1 | 1 | 20 |
| CGPF-3 | 1 | 1 | 40 |
| Sample Code | Water Absorption (%) with SD |
|---|---|
| CS | 54 ± 0.5 |
| CGPF−1 | 77.7 ± 0.5 |
| CGPF-2 | 62 ± 0.5 |
| CGPF-3 | 33.2 ± 0.5 |
| Sample Code | Break Stress (σ) | Break Strain (ε) with SD (%) |
|---|---|---|
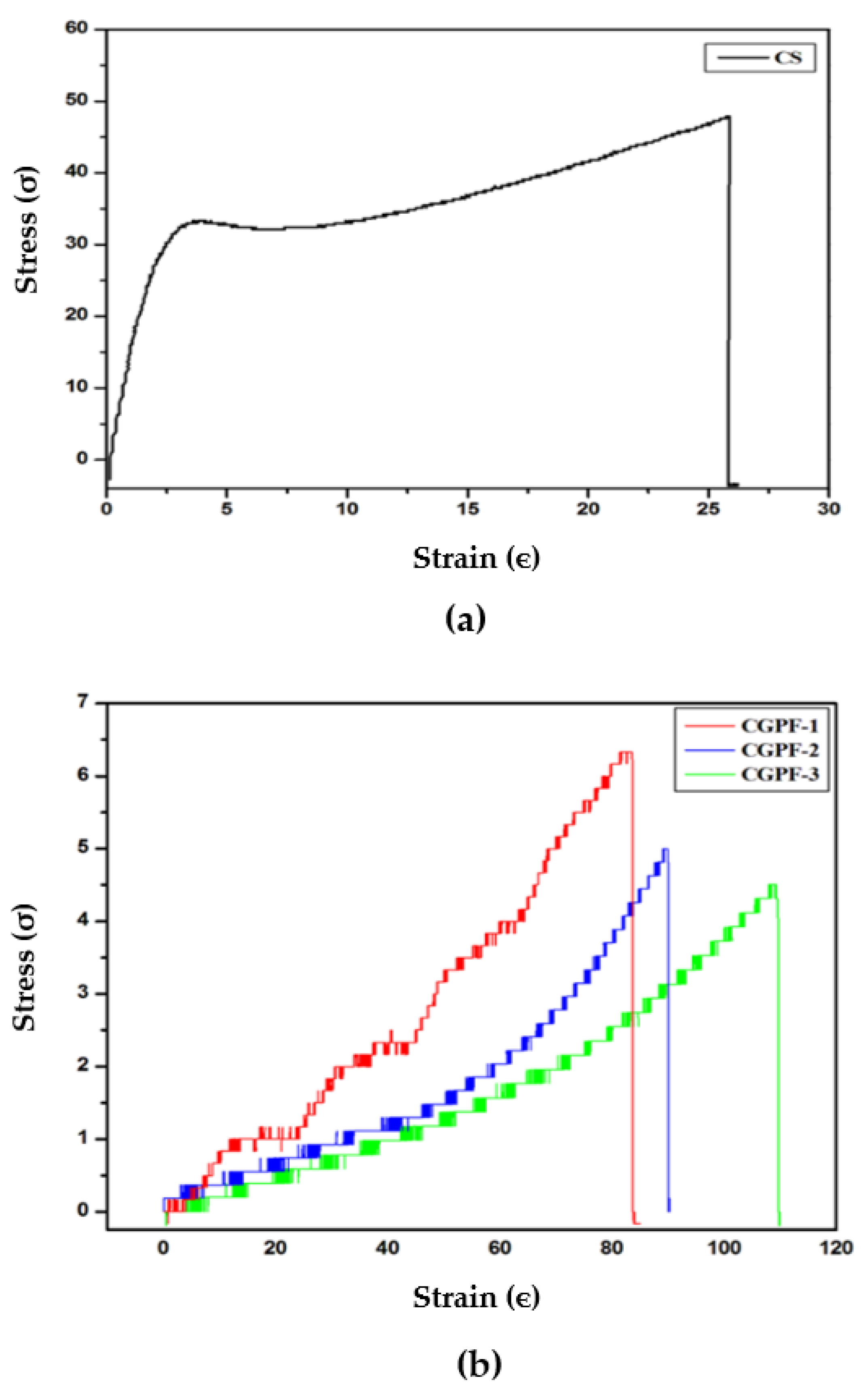
| CS | 47.97 | 25.83 ± 1 |
| CGPF−1 | 6.38 | 83.29 ± 1 |
| CGPF-2 | 4.99 | 89.76 ± 1 |
| CGPF-3 | 4.50 | 109.20 ± 1 |
| Sample Code | Temperature at 20% Loss °C | Temperature at 50% Loss °C | Char 900 °C (wt %) |
|---|---|---|---|
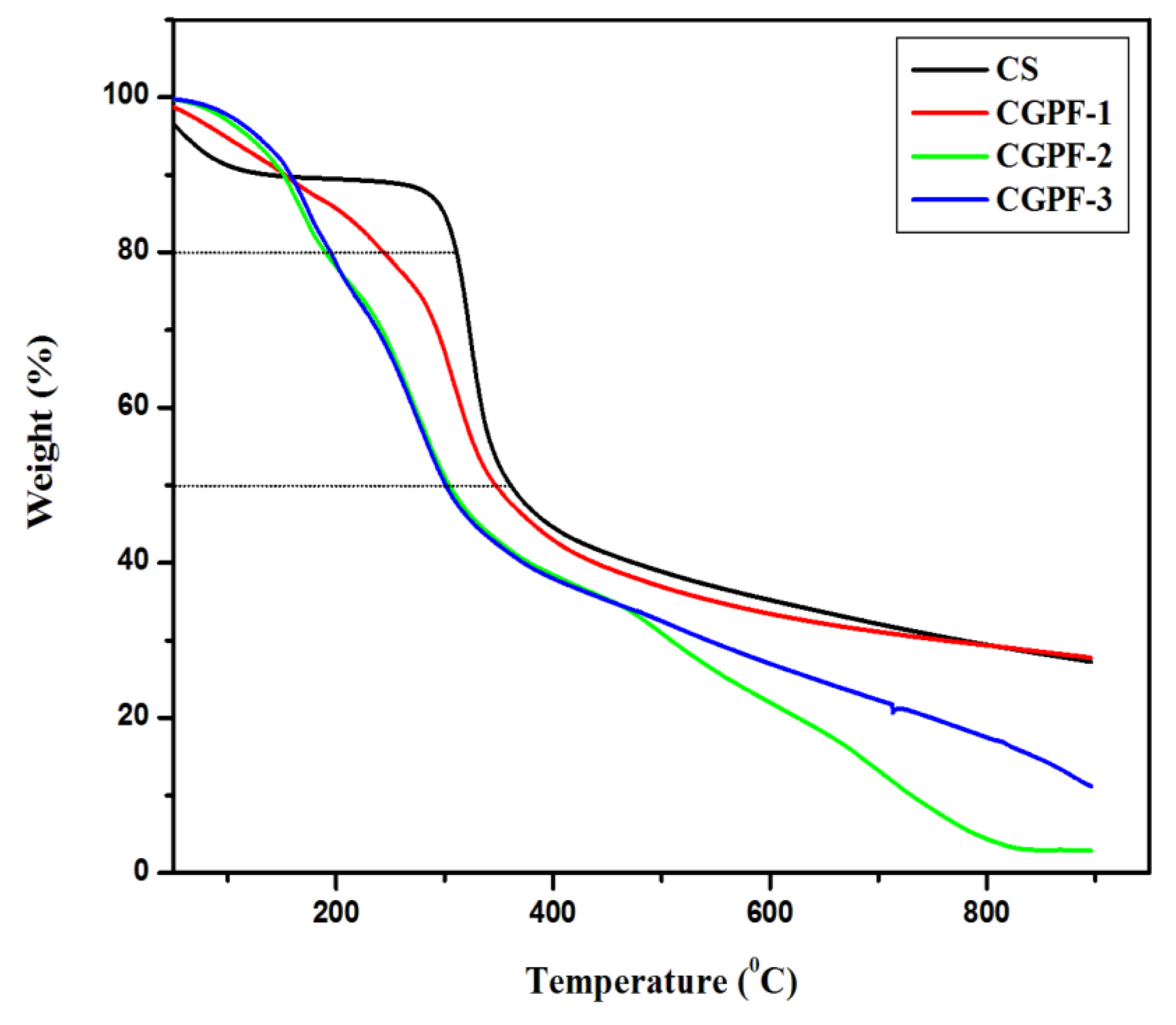
| CS | 310 | 360 | 27.1 |
| CGPF−1 | 246 | 347 | 27.7 |
| CGPF-2 | 188 | 305 | 2.91 |
| CGPF-3 | 197 | 300 | 11.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, S.; Singh, R.P.; Chavan, N.N.; Sahi, S.V.; Sharma, N. Characterization of a Novel Nanocomposite Film Based on Functionalized Chitosan–Pt–Fe3O4 Hybrid Nanoparticles. Nanomaterials 2021, 11, 1275. https://doi.org/10.3390/nano11051275
Kumari S, Singh RP, Chavan NN, Sahi SV, Sharma N. Characterization of a Novel Nanocomposite Film Based on Functionalized Chitosan–Pt–Fe3O4 Hybrid Nanoparticles. Nanomaterials. 2021; 11(5):1275. https://doi.org/10.3390/nano11051275
Chicago/Turabian StyleKumari, Sangeeta, Raj Pal Singh, Nayaku N. Chavan, Shivendra V. Sahi, and Nilesh Sharma. 2021. "Characterization of a Novel Nanocomposite Film Based on Functionalized Chitosan–Pt–Fe3O4 Hybrid Nanoparticles" Nanomaterials 11, no. 5: 1275. https://doi.org/10.3390/nano11051275
APA StyleKumari, S., Singh, R. P., Chavan, N. N., Sahi, S. V., & Sharma, N. (2021). Characterization of a Novel Nanocomposite Film Based on Functionalized Chitosan–Pt–Fe3O4 Hybrid Nanoparticles. Nanomaterials, 11(5), 1275. https://doi.org/10.3390/nano11051275





