The Efficient Photocatalytic Degradation of Organic Pollutants on the MnFe2O4/BGA Composite under Visible Light
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
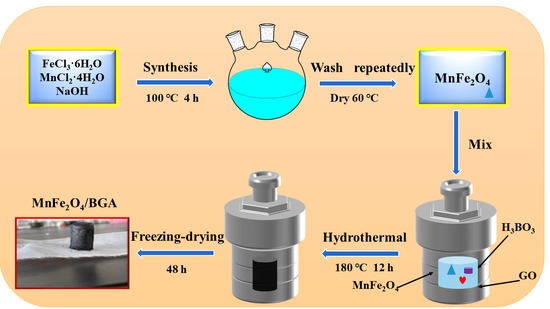
2.2. Preparation of the MnFe2O4/BGA Composite
2.3. Photo-Fenton Degradation of Organic Pollutants on the MnFe2O4/BGA Composite
2.4. Characterization Methods
3. Results
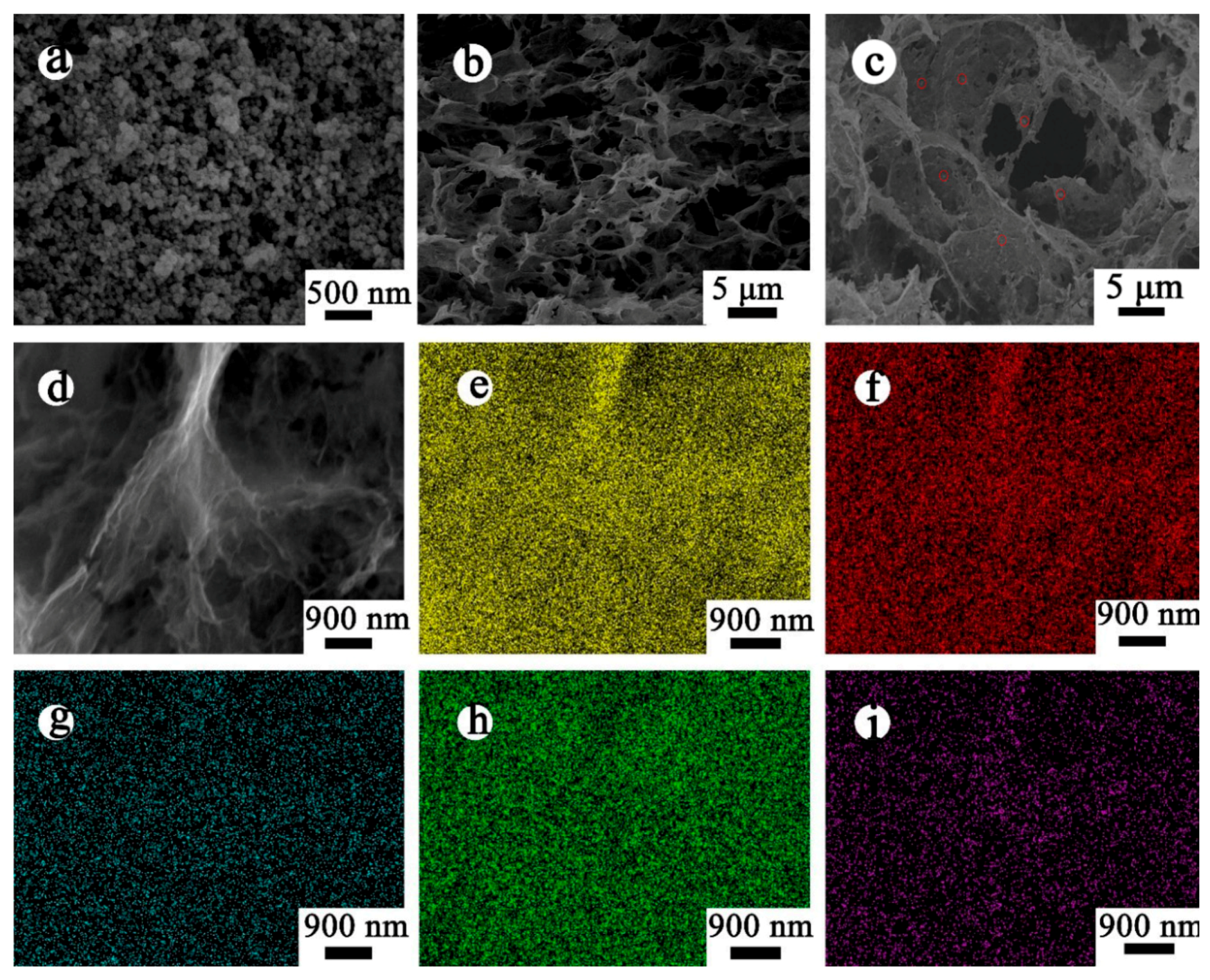
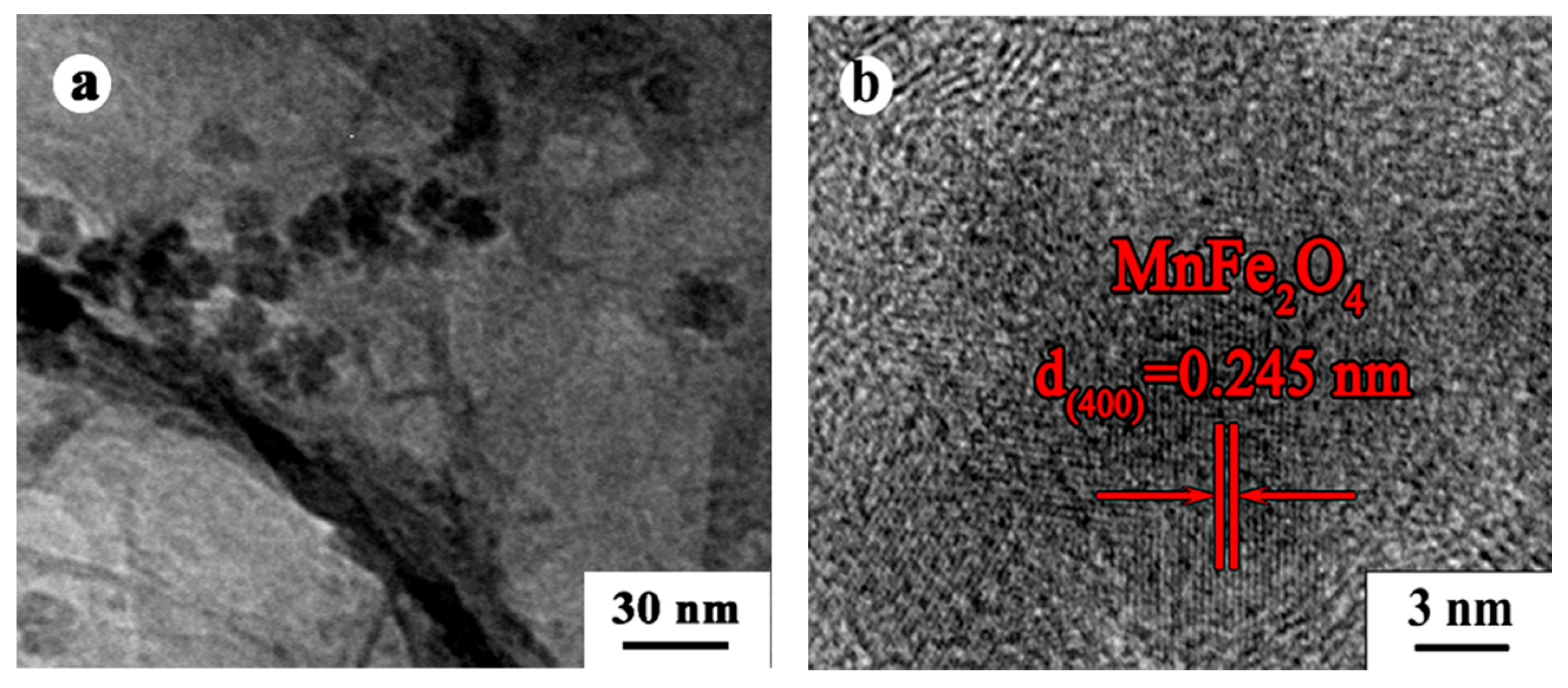
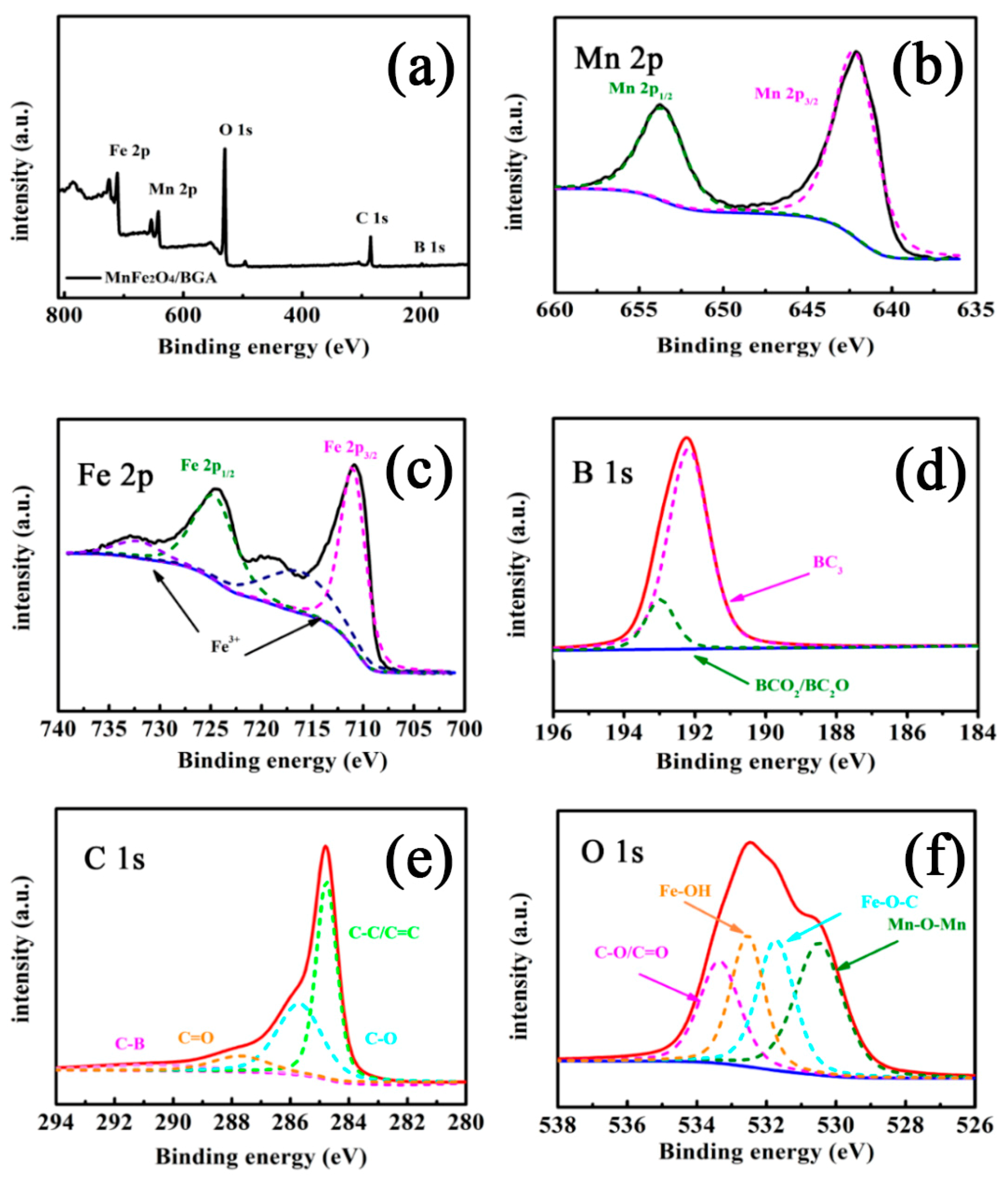
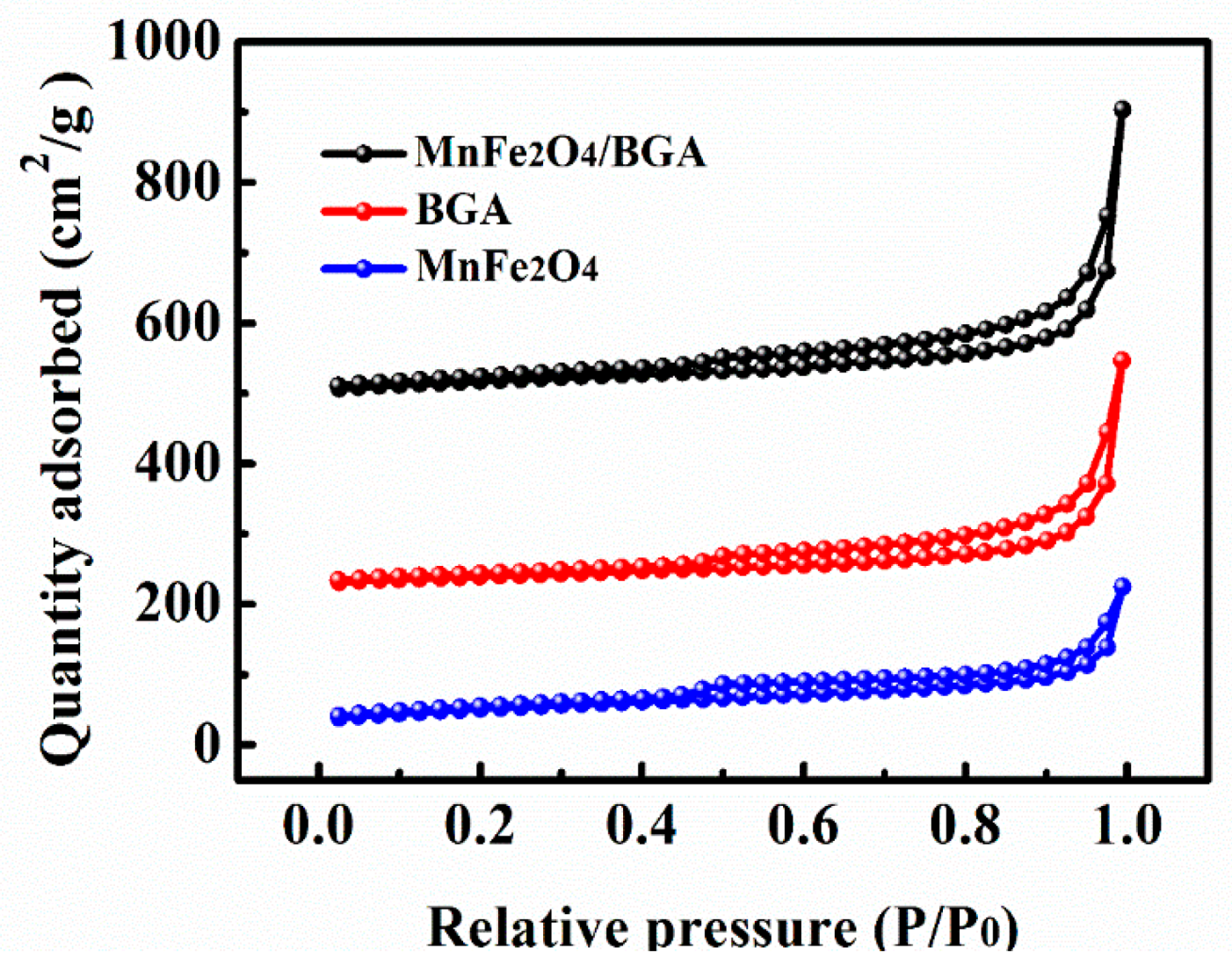
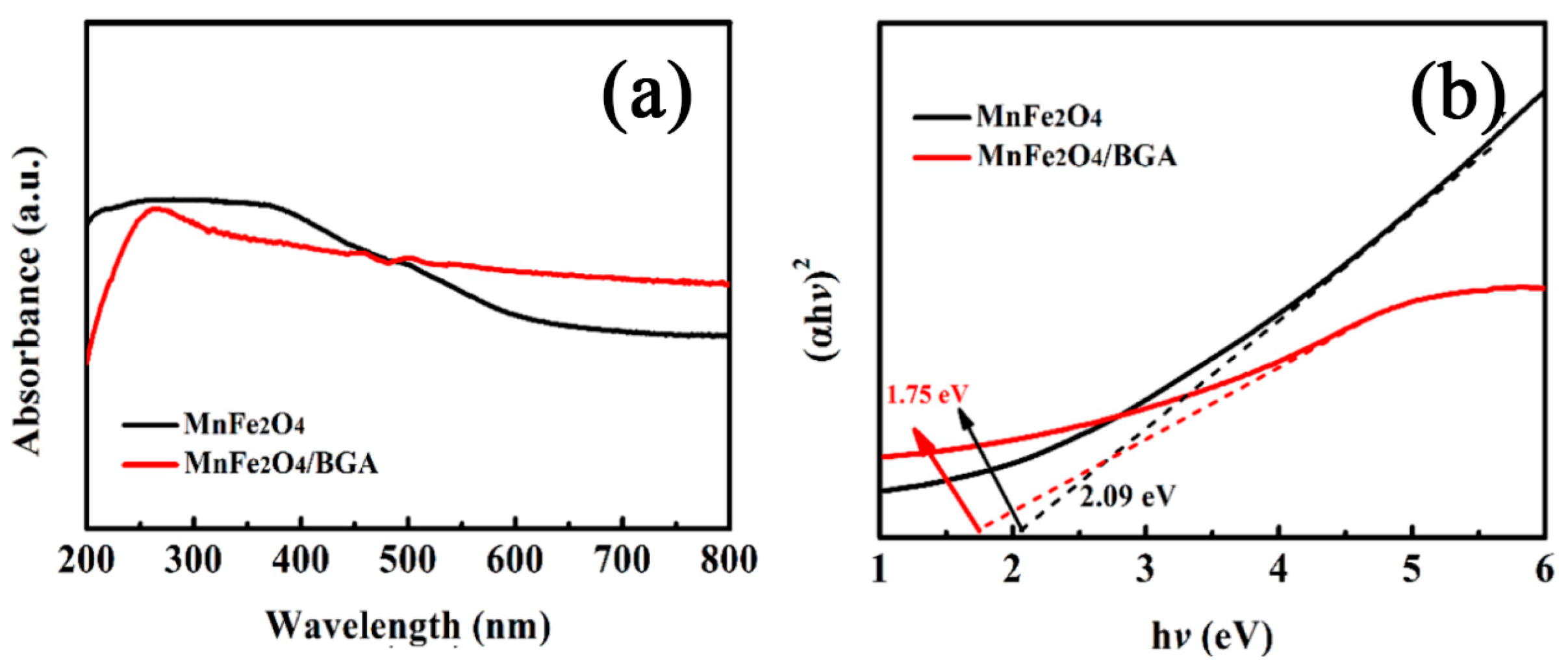
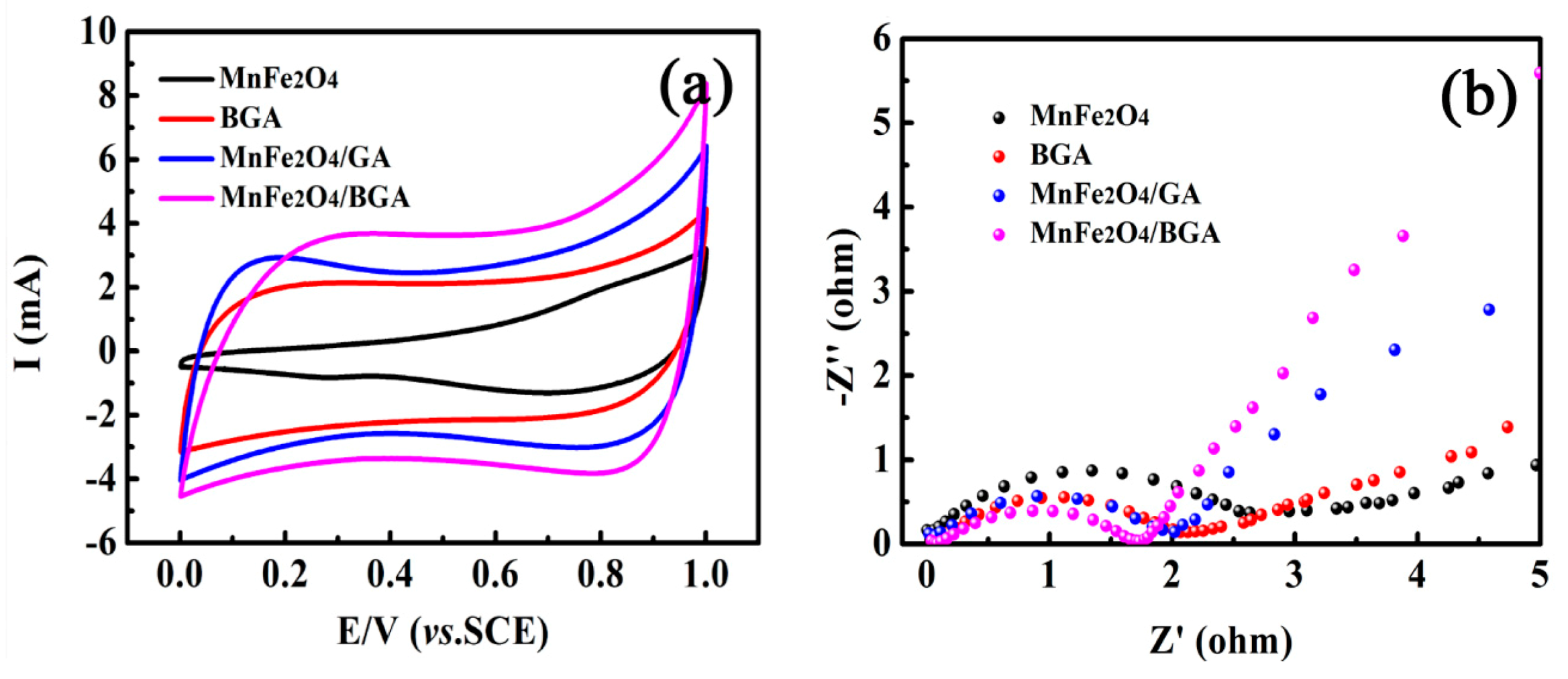
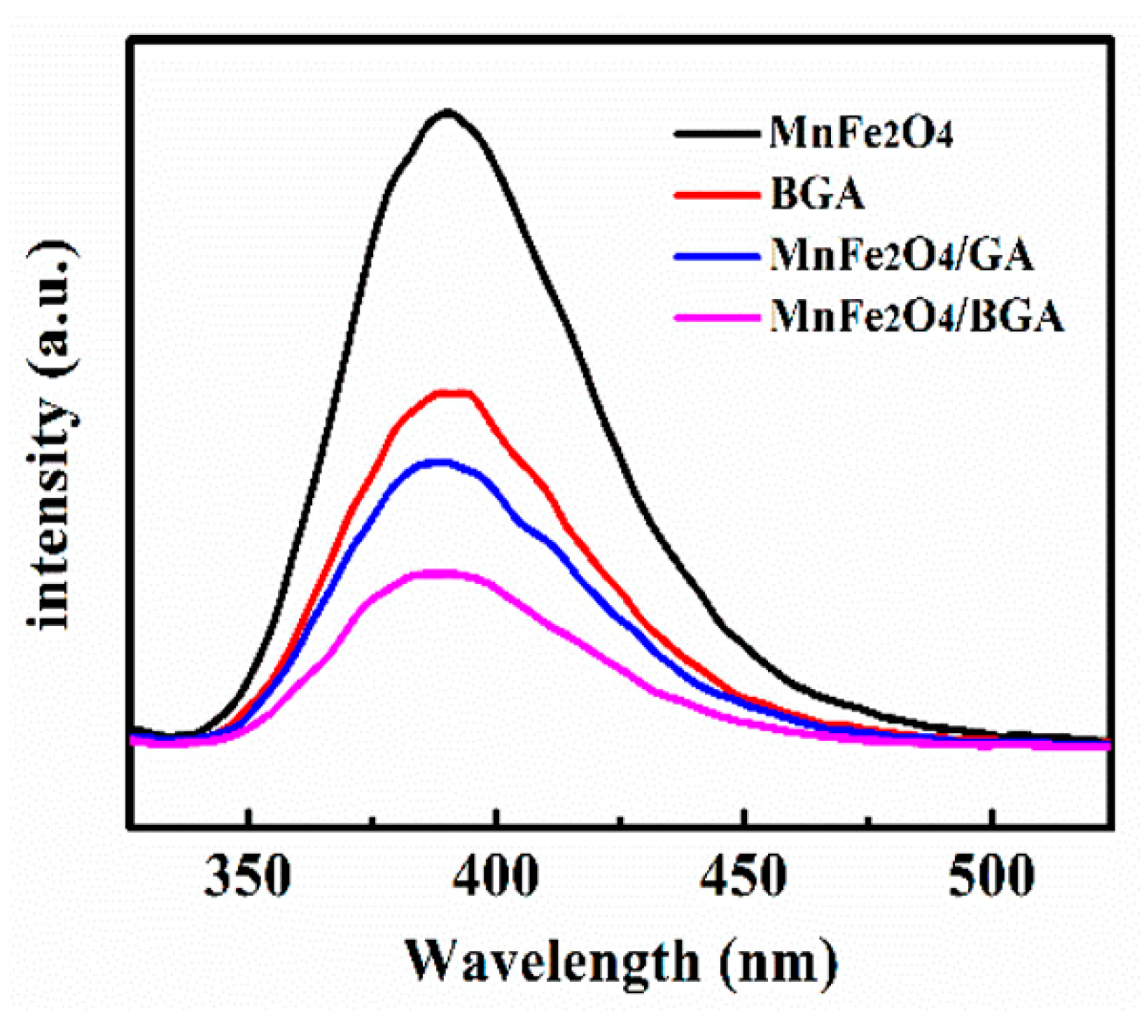
3.1. Materials Characterizations
3.2. Degradation of Organic Pollutants on the MnFe2O4/BGA Composite
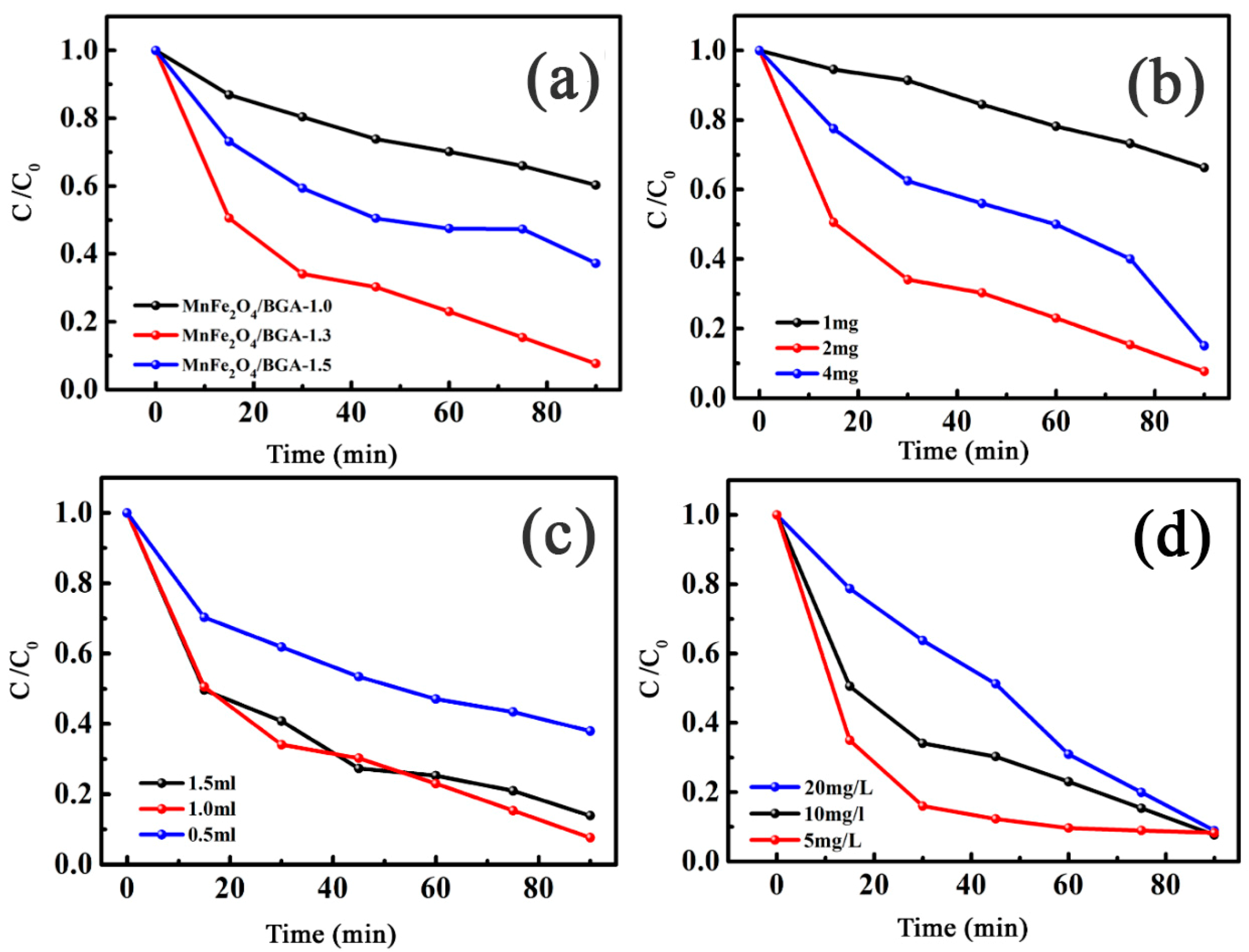
3.2.1. The Optimized Experimental Conditions of Photo-Fenton Catalytic Degradation
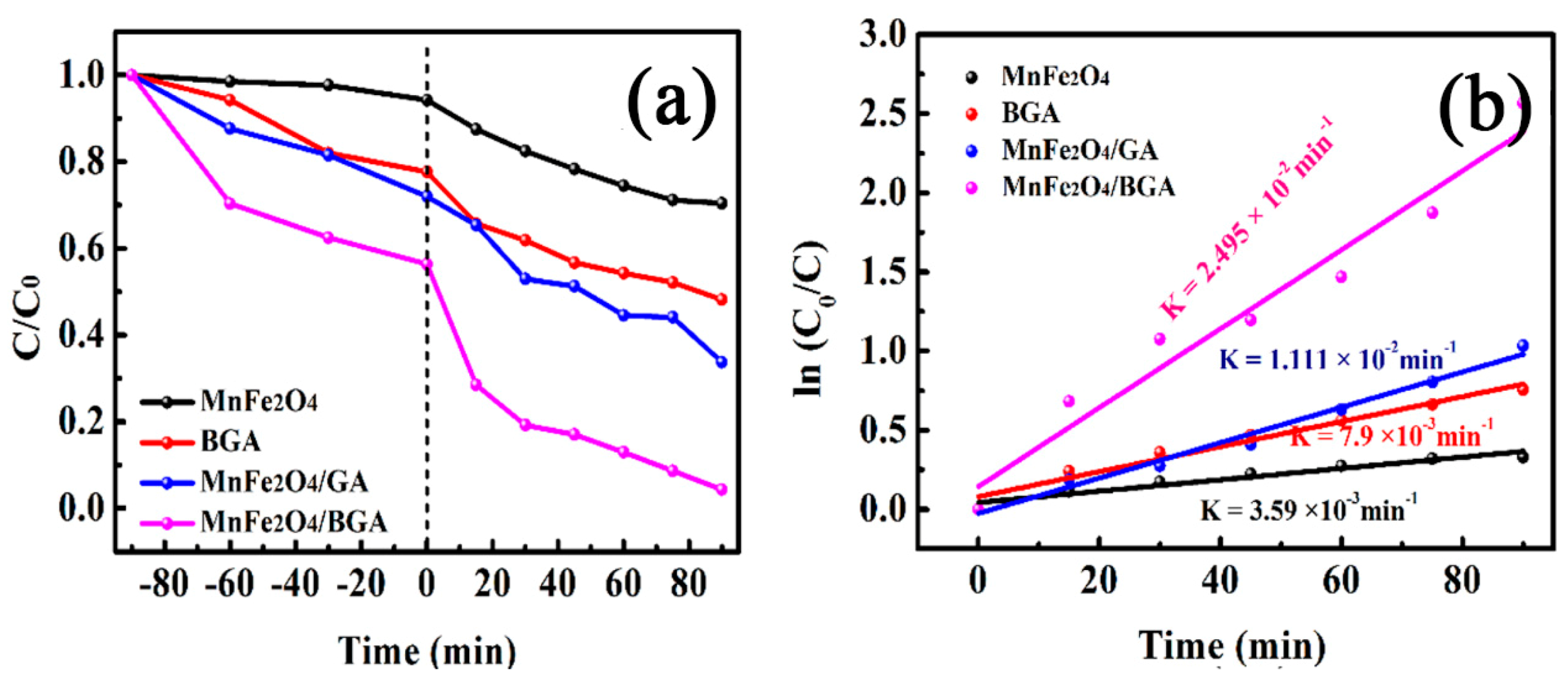
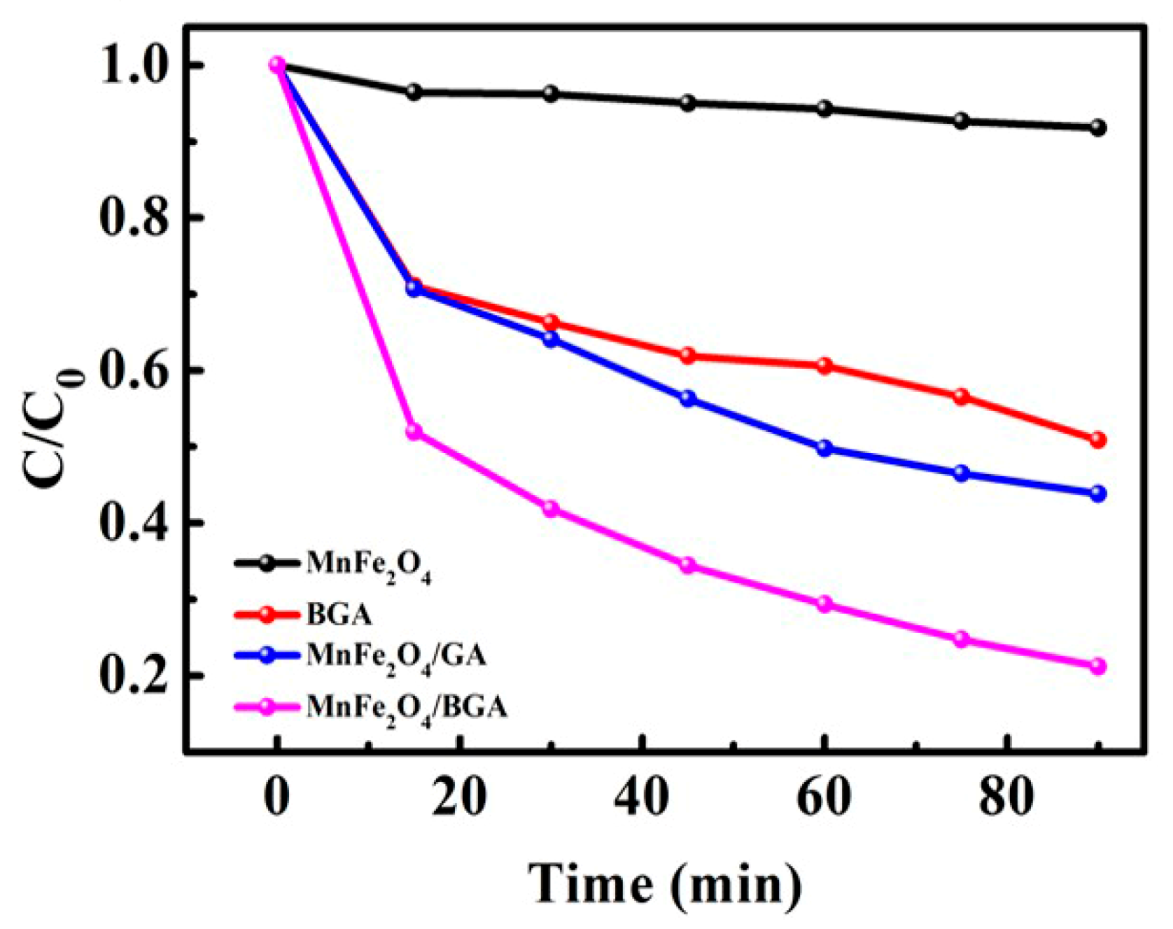
3.2.2. Degradation of Rhodamine B on Relevant Photo-Fenton Catalysts
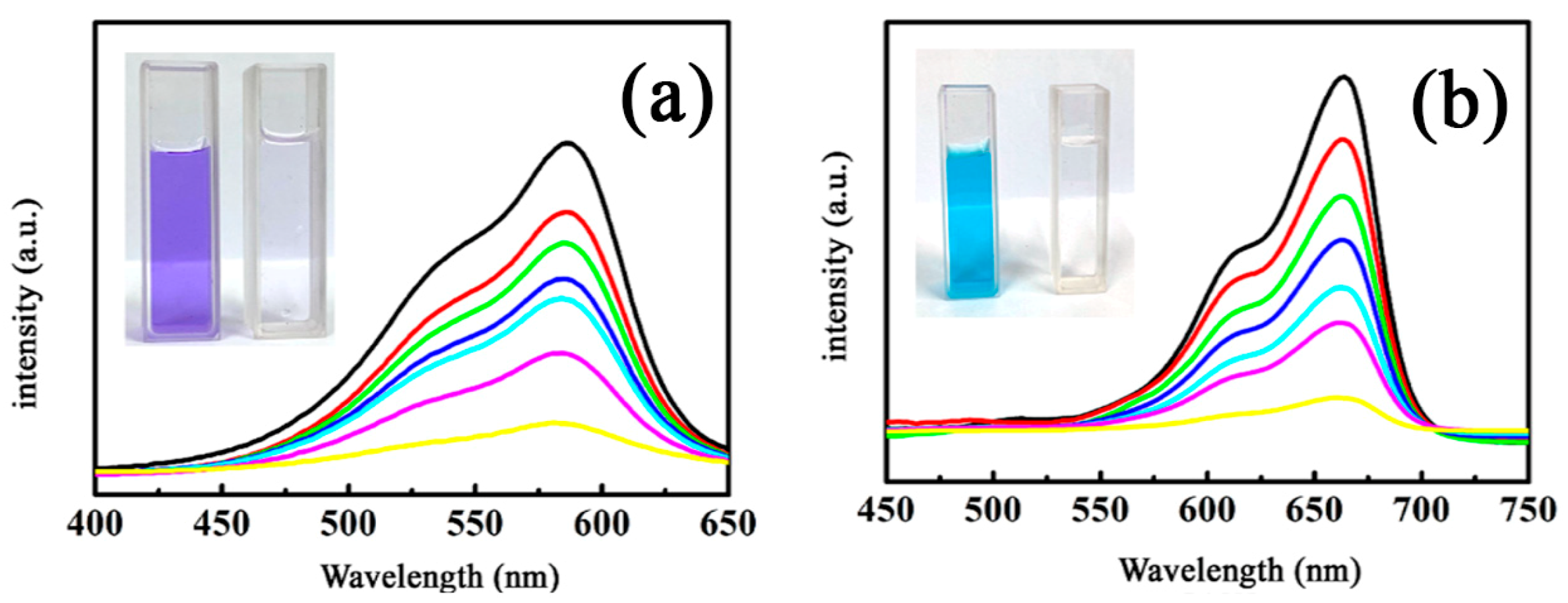
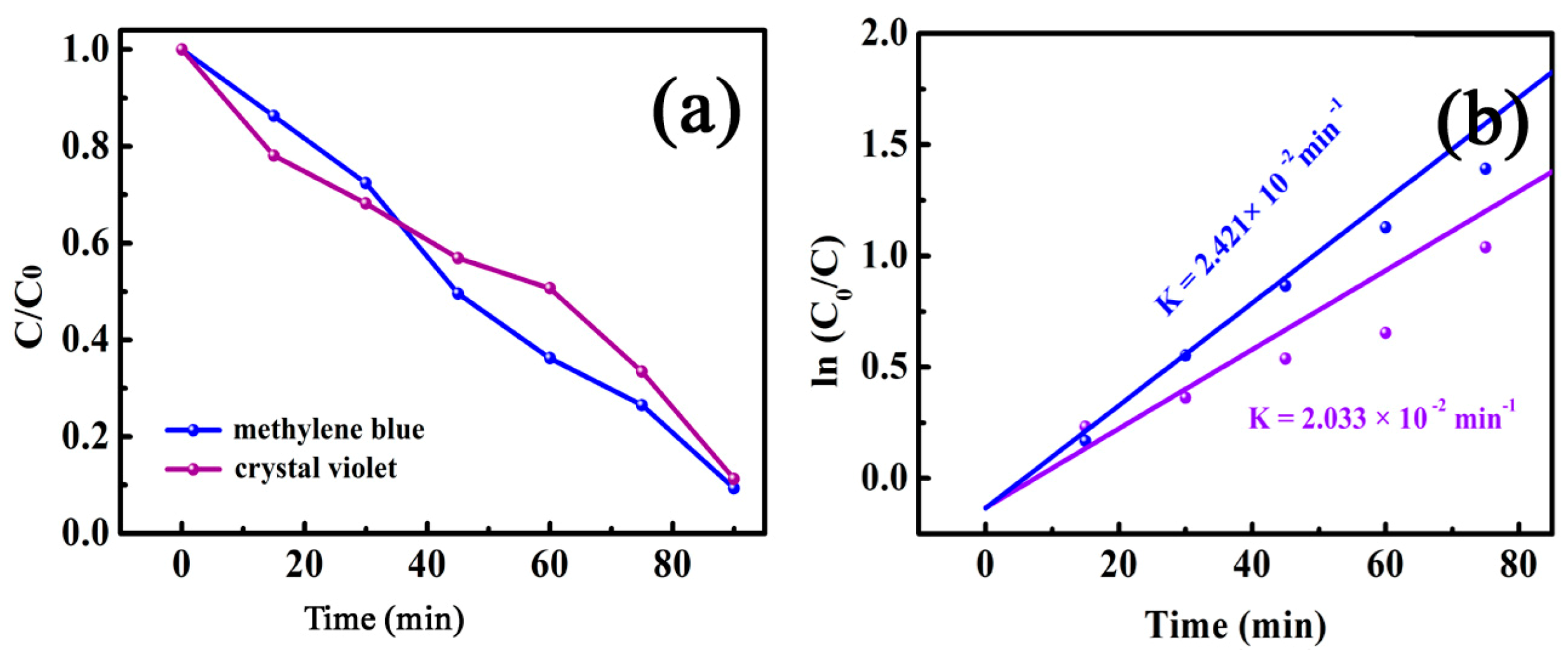
3.2.3. Degradation of Crystal Violet and Methylene Blue on the MnFe2O4/BGA Composite
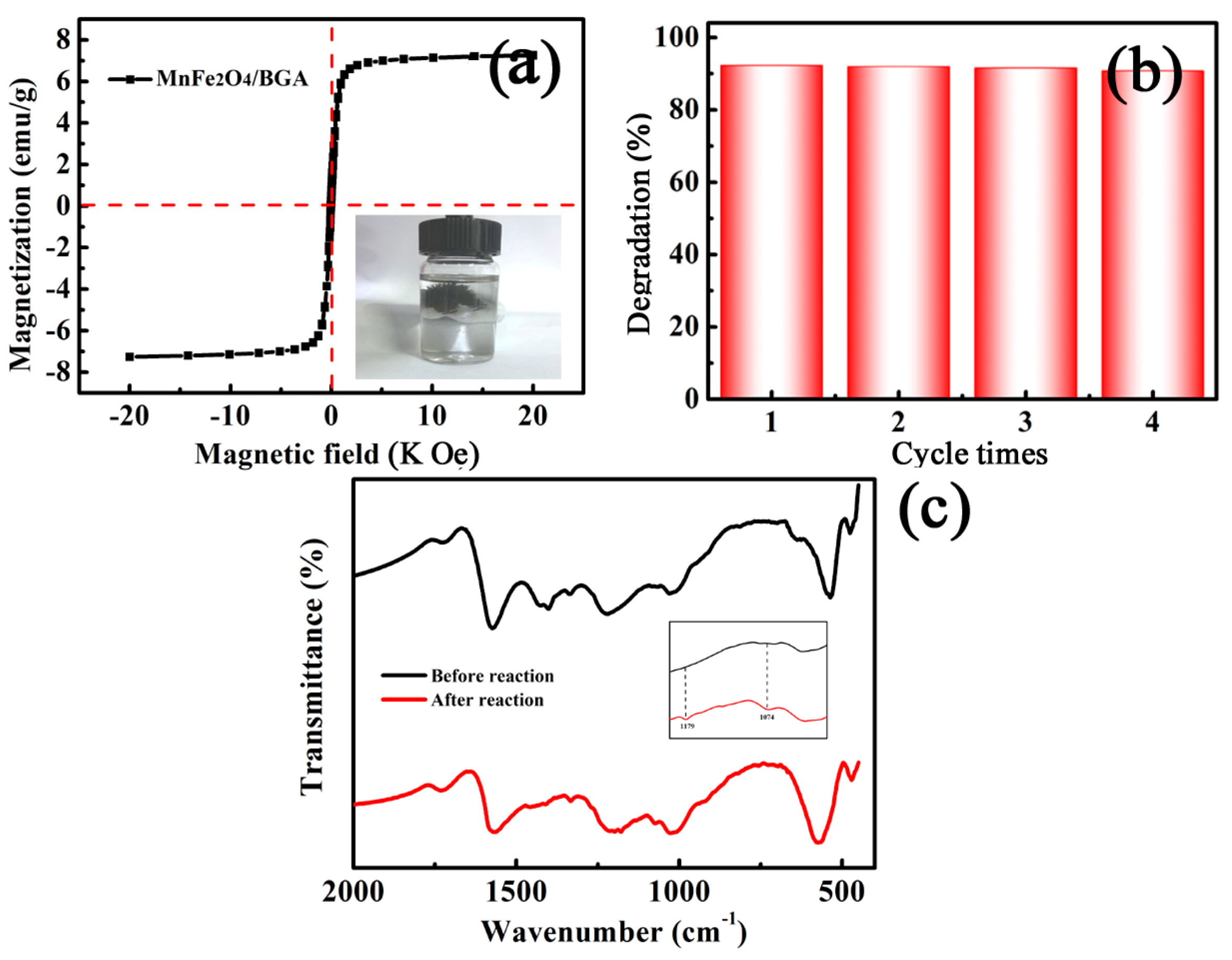
3.2.4. Stability and Reusability of the MnFe2O4/BGA Composite
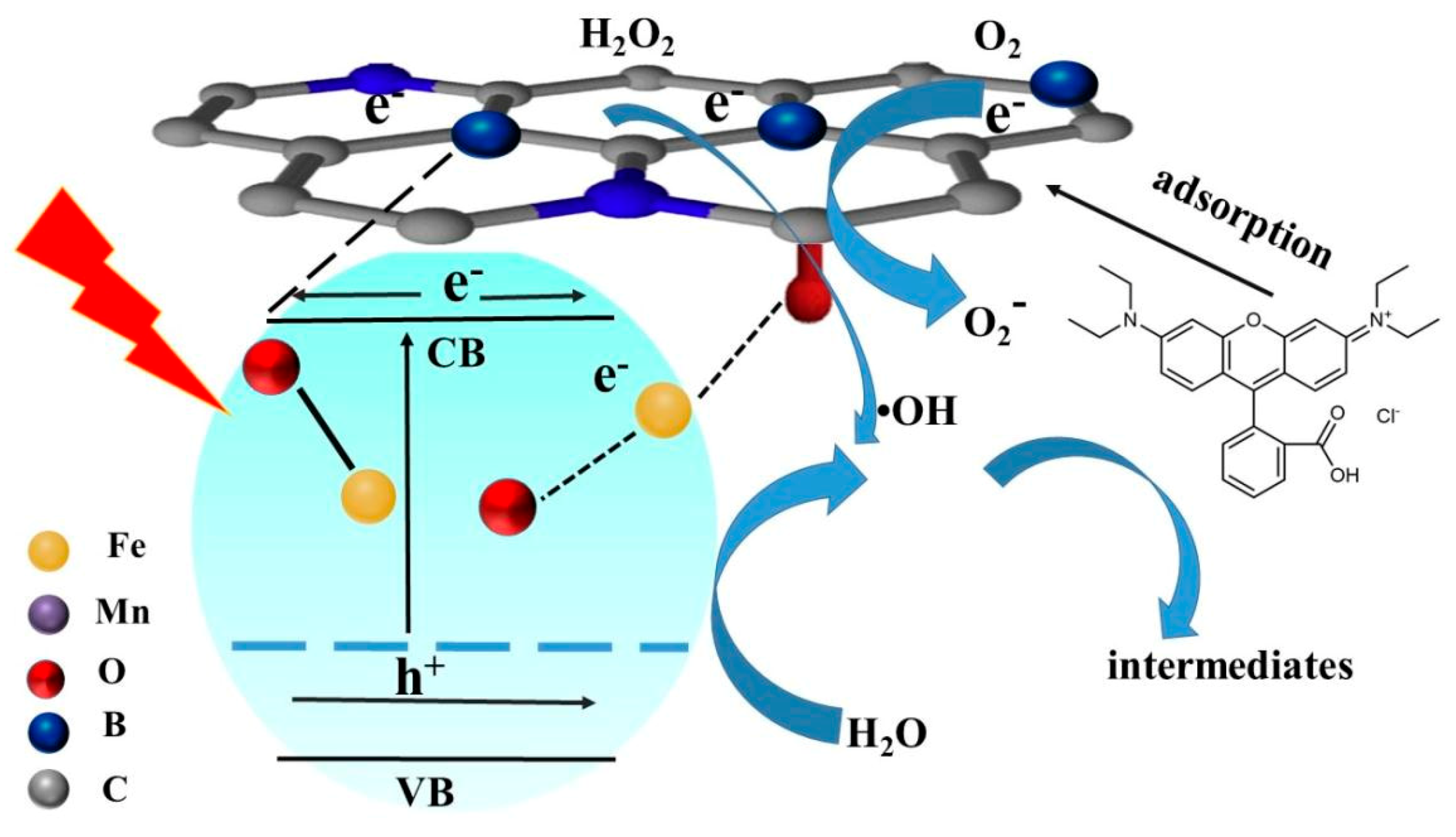
3.2.5. Possible Degradation Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reddy, D.H.K.; Yun, Y.S. Spinel ferrite magnetic adsorbents: Alternative future materials for water purification? Coord. Chem. Rev. 2016, 315, 90–111. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, X.; Gao, Y.; Zhu, G.; Cheng, Q.; Cheng, X. Novel magnetic MnO2/MnFe2O4 nanocomposite as a heterogeneous catalyst for activation of peroxymonosulfate (PMS) toward oxidation of organic pollutants. Sep. Purif. Technol. 2019, 213, 456–464. [Google Scholar] [CrossRef]
- Abroshan, E.; Farhadi, S.; Zabardasti, A. Novel magnetically separable Ag3PO4/MnFe2O4 nanocomposite and its high photocatalytic degradation performance for organic dyes under solar-light irradiation. Sol. Energy Mater. Sol. Cells 2018, 178, 154–163. [Google Scholar] [CrossRef]
- Sakho, E.H.M.; Jose, J.; Thomas, S.; Kalarikkal, N.; Oluwafemi, O.S. Antimicrobial properties of MFe2O4 (M = Mn, Mg)/reduced graphene oxide composites synthesized via solvothermal method. Mater. Sci. Eng. C 2019, 95, 43–48. [Google Scholar] [CrossRef]
- Qin, L.; Wang, Z.H.; Fu, Y.K.; Lai, C.; Liu, X.; Li, B.; Liu, S.; Yi, H.; Li, L.; Zhang, M.; et al. Gold nanoparticles-modified MnFe2O4 with synergistic catalysis for photo-Fenton degradation of tetracycline under neutral pH. J. Hazard. Mater. 2021, 414, 125448–125462. [Google Scholar] [CrossRef]
- Zhao, W.; Wei, Z.; Zhang, X.; Ding, M.; Huang, S. PH-controlled MnFe2O4@ SnS2 nanocomposites for the visible-light photo-Fenton degradation. Mater. Res. Bull. 2020, 124, 110749. [Google Scholar] [CrossRef]
- Zhao, W.H.; Wei, Z.Q.; Zhang, X.D.; Ding, M.; Huang, S.; Yang, S. Magnetic recyclable MnFe2O4/CeO2/SnS2 ternary nano-photocatalyst for photo-Fenton degradation. Appl. Catal. A-Gen. 2020, 593, 117443–117452. [Google Scholar] [CrossRef]
- Vignesha, K.; Suganthi, A.; Min, B.K.; Kang, M. Photocatalytic activity of magnetically recoverable MnFe2O4/g-C3N4/TiO2 nanocomposite under simulated solar light irradiation. J. Mol. Catal. A-Chem. 2014, 395, 373–383. [Google Scholar] [CrossRef]
- Fu, Y.; Xiong, P.; Chen, H.Q.; Sun, X.; Wang, X. High Photocatalytic Activity of Magnetically Separable Manganese Ferrite–Graphene Heteroarchitectures. Ind. Eng. Chem. Res. 2011, 51, 725–731. [Google Scholar] [CrossRef]
- Bai, S.; Shen, X.; Zhong, X.; Liu, Y.; Zhu, G.; Xu, X.; Chen, K. One-pot solvothermal preparation of magnetic reduced graphene oxide-ferrite hybrids for organic dye removal. Carbon 2012, 50, 2337–2346. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiao, B.; Liu, S.-Q.; Meng, Z.; Chen, Z.-G.; Zou, C.-Y.; Liu, C.-B.; Chen, F.; Zhou, X. Photo-Fenton degradation of ammonia via a manganese–iron double-active component catalyst of graphene–manganese ferrite under visible light. Chem. Eng. J. 2016, 283, 266–275. [Google Scholar] [CrossRef]
- Huang, X.; Liu, L.; Xi, Z.; Zheng, H.; Dong, W.; Wang, G. One-pot solvothermal synthesis of magnetically separable rGO/MnFe2O4 hybrids as efficient photocatalysts for degradation of MB under visible light. Mater. Chem. Phys. 2019, 231, 68–74. [Google Scholar] [CrossRef]
- Tabasum, A.; Bhatti, I.A.; Nadeem, N.; Zahid, M.; Rehan, Z.A.; Hussain, T.; Jilani, A. Degradation of acetamiprid using graphene-oxide-based metal (Mn and Ni) ferrites as Fenton-like photocatalysts. Water Sci. Technol. 2020, 81, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Luciano, A.J.R.; Soletti, L.D.S.; Ferreira, M.E.C.; Cusioli, L.F.; de Andrade, M.B.; Bergamasco, R.; Yamaguchi, N.U. Manganese ferrite dispersed over graphene sand composite for methylene blue photocatalytic degradation. J. Environ. Chem. Eng. 2020, 8, 104191. [Google Scholar] [CrossRef]
- Feng, Y.; Yao, T.J.; Yang, Y.; Zheng, F.; Chen, P.; Wu, J.; Xin, B. One-step preparation of Fe2O3/reduced graphene oxide aerogel as heterogeneous Fenton-like catalyst for enhanced photo-degradation of organic dyes. ChemistrySelect 2018, 3, 9062–9070. [Google Scholar] [CrossRef]
- Wang, L.P.; Zhang, M.Y.; Xie, J.W. Self-assembled nano-Fe3C embedded in reduced graphene oxide aerogel with efficient Fenton-like catalysis. Nanomaterials 2020, 10, 2348. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.C.; Deng, Y.X.; Du, M.M.; Xing, M.; Zhang, J. Ultradispersed cobalt ferrite nanoparticles assembled in graphene aerogel for continuous photo-Fenton reaction and enhanced Lithium storage performance. Sci. Rep. 2016, 6, 29099–29109. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Liu, X.M.; Zhao, Y.P.; Dionysiou, D.D. Aligned α-FeOOH nanorods anchored on a graphene oxide-carbon nanotubes aerogel can serve as an effective Fenton-like oxidation catalyst. Appl. Catal. B Environ. 2017, 213, 74–86. [Google Scholar] [CrossRef]
- Shi, H.; He, Y.; Li, Y.; Wang, S.; Luo, P. Mixed-dimensional assembled superhydrophilic graphene-based aerogel with enhanced mass/charge transportation for efficient photoredox catalysis. Sep. Purif. Technol. 2020, 252, 117454. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Liu, Z.; Lyu, C.; Niu, S.; Dong, Z.; Lyu, C. Enhanced catalytic oxidation of benzotriazole via peroxymonosulfate activated by CoFe2O4 supported onto nitrogen-doped three-dimensional graphene aerogels. Chem. Eng. J. 2020, 400, 125897. [Google Scholar] [CrossRef]
- Chowdhury, S.; Jiang, Y.; Muthukaruppan, S.; Balasubramanian, R. Effect of boron doping level on the photocatalytic activity of graphene aerogels. Carbon 2018, 128, 237–248. [Google Scholar] [CrossRef]
- Bejigo, K.S.; Park, B.J.; Kim, J.H.; Yoon, H.H. Synthesis and evaluation of graphene aerogel-supported MnxFe3xO4 for oxygen reduction in Urea/O2 fuel cells. ChemistryOpen 2019, 8, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhang, Y.; Chen, Z.; Duan, Y.; Lai, Y.; Fang, Q.; Wang, F.; Li, S. Performance of boron-doped graphene aerogel modified gas diffusion electrode for in-situ metal-free electrochemical advanced oxidation of Bisphenol A. Appl. Catal. B Environ. 2019, 255, 117784. [Google Scholar] [CrossRef]
- Samakchi, S.; Chaibakhs, N.; Moradi-Shoeili, Z. Synthesis of MoS2/MnFe2O4 nanocomposite with highly efficient catalytic performance in visible light photo-Fenton-like process. J. Photochem. Photobiol. A 2018, 367, 420–428. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Zhang, G.; Shu, R.; Xie, Y.; Xia, H.; Gan, Y.; Shi, J.; He, J. Cubic MnFe2O4 particles decorated reduced graphene oxide with excellent microwave absorption properties. Mater. Lett. 2018, 231, 209–212. [Google Scholar] [CrossRef]
- Agnoli, S.; Favaro, M. Doping graphene with boron: A review of synthesis methods, physicochemical characterization, and emerging applications. J. Mater. Chem. A 2016, 4, 45002–45025. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Li, S.W.; Lian, Y.F. The electrochemical performance of the N-doped graphene aerogels and nickel foam composite electrode prepared by one-pot hydrothermal method. Fuller. Nanotub. Carbon Nanostruct. 2019, 27, 582–590. [Google Scholar] [CrossRef]
- Yao, T.J.; Jia, W.J.; Feng, Y.; Lian, Y.; Wu, J.; Zhang, X. Preparation of reduced graphene oxide nanosheet/FexOy/nitrogen-doped carbon layer aerogel as photo-Fenton catalyst with enhanced degradation activity and reusability. J. Hazard. Mater. 2019, 362, 62–71. [Google Scholar] [CrossRef]
- Madhumita, S.; Sreena, K.P.; Vinayan, B.P. Green synthesis of boron doped graphene and its application as high performance anode material in Li ion battery. Mater. Res. Bull. 2015, 61, 383–390. [Google Scholar]
- Lu, J.; Zhou, Y.; Lei, J.; Ao, Z.; Zhou, Y. Fe3O4/graphene aerogels: A stable and efficient persulfate activator for the rapid degradation of malachite green. Chemosphere 2020, 251, 126402–126414. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, J.; Yang, Q.F.; Zhao, L.; Yuan, Q.; Jin, P.; Feng, L. Phosphate doped ultrathin FeP nanosheets as efficient electrocatalyst for hydrogen evolution reaction in acid media. ChemCatChem 2019, 10, 2484–2489. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, G.K.; Jimmy, C.Y. Enhanced photo-Fenton degradation of rhodamine B using graphene oxide-amorphous FePO4 as effective and stable heterogeneous catalyst. J. Colloid Interface Sci. 2015, 448, 460–466. [Google Scholar] [CrossRef]
- Du, J.; Bao, J.; Fu, X.; Lu, C.; Kim, S.H. Mesoporous sulfur-modified iron oxide as an effective Fenton-like catalyst for degradation of bisphenol A. Appl. Catal. B Environ. 2016, 184, 132–141. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, R.; Xi, Y.; Zhu, J.; Zhu, G.; He, H. Strategies for enhancing the heterogeneous Fenton catalytic reactivity: A review. Appl. Catal. B Environ. 2019, 255, 117739. [Google Scholar] [CrossRef]
- Xu, T.; Yu, D.Y.; Du, Z.L.; Huang, W.; Lu, X. Two-dimensional mesoporous carbon materials derived from fullerene microsheets for energy applications. Chem. Eur. J. 2020, 47, 10811–10816. [Google Scholar] [CrossRef]
- Shoueir, K.; El-Sheshtawy, H.; Misbah, M.; El-Hosainy, H.; El-Mehasseb, I.; El-Kemary, M. Fenton-like nanocatalyst for photodegradation of methylene blue under visible light activated by hybrid green DNSA@Chitosan@MnFe2O4. Carbohyd. Polym. 2018, 197, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Yan, M.; Huang, D.L. Synergistic effect of artificial enzyme and 2D nano-structured Bi2WO6 for eco-friendly and efficient biomimetic photocatalysis. Appl. Catal. B Environ. 2019, 250, 52–62. [Google Scholar] [CrossRef]
- Wei, Z.; Huang, S.; Zhang, X.; Lu, C.; He, Y. Hydrothermal synthesis and photo-Fenton degradation of magnetic MnFe2O4/rGO nanocomposites. J. Mater. Sci. Mater. Electron. 2020, 31, 5176–5186. [Google Scholar] [CrossRef]
- Gomathi Devi, L.; Shyamala, R. Photocatalytic activity of SnO2-α-Fe2O3 composite mixtures: Exploration of number of active sites, turnover number and turnover frequency. Mater. Chem. Front. 2018, 2, 796–806. [Google Scholar] [CrossRef]
- Xu, H.D.; Quan, X.C.; Chen, L. A novel combination of bioelectrochemical system with peroxymonosulfate oxidation for enhanced azo dye degradation and MnFe2O4 catalyst regeneration. Chemosphere 2019, 217, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, L.; Li, K.; Wang, J.; Fang, D.; Zhang, Y.; Tian, D.; Zhang, Z.; Dionysiou, D.D. Fabrication of novel Z-scheme SrTiO3/MnFe2O4 system with double-response activity for simultaneous microwave-induced and photocatalytic degradation of tetracycline and mechanism insight. Chem. Eng. J. 2020, 400, 125981. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Jiang, X.; Lian, Y. The Efficient Photocatalytic Degradation of Organic Pollutants on the MnFe2O4/BGA Composite under Visible Light. Nanomaterials 2021, 11, 1276. https://doi.org/10.3390/nano11051276
Li Q, Jiang X, Lian Y. The Efficient Photocatalytic Degradation of Organic Pollutants on the MnFe2O4/BGA Composite under Visible Light. Nanomaterials. 2021; 11(5):1276. https://doi.org/10.3390/nano11051276
Chicago/Turabian StyleLi, Qian, Xiaoyu Jiang, and Yongfu Lian. 2021. "The Efficient Photocatalytic Degradation of Organic Pollutants on the MnFe2O4/BGA Composite under Visible Light" Nanomaterials 11, no. 5: 1276. https://doi.org/10.3390/nano11051276
APA StyleLi, Q., Jiang, X., & Lian, Y. (2021). The Efficient Photocatalytic Degradation of Organic Pollutants on the MnFe2O4/BGA Composite under Visible Light. Nanomaterials, 11(5), 1276. https://doi.org/10.3390/nano11051276