Anti-Inflammatory Effect and Cellular Uptake Mechanism of Carbon Nanodots in in Human Microvascular Endothelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. CND Synthesis and Characterization
2.3. CND and TNF-α Treatments
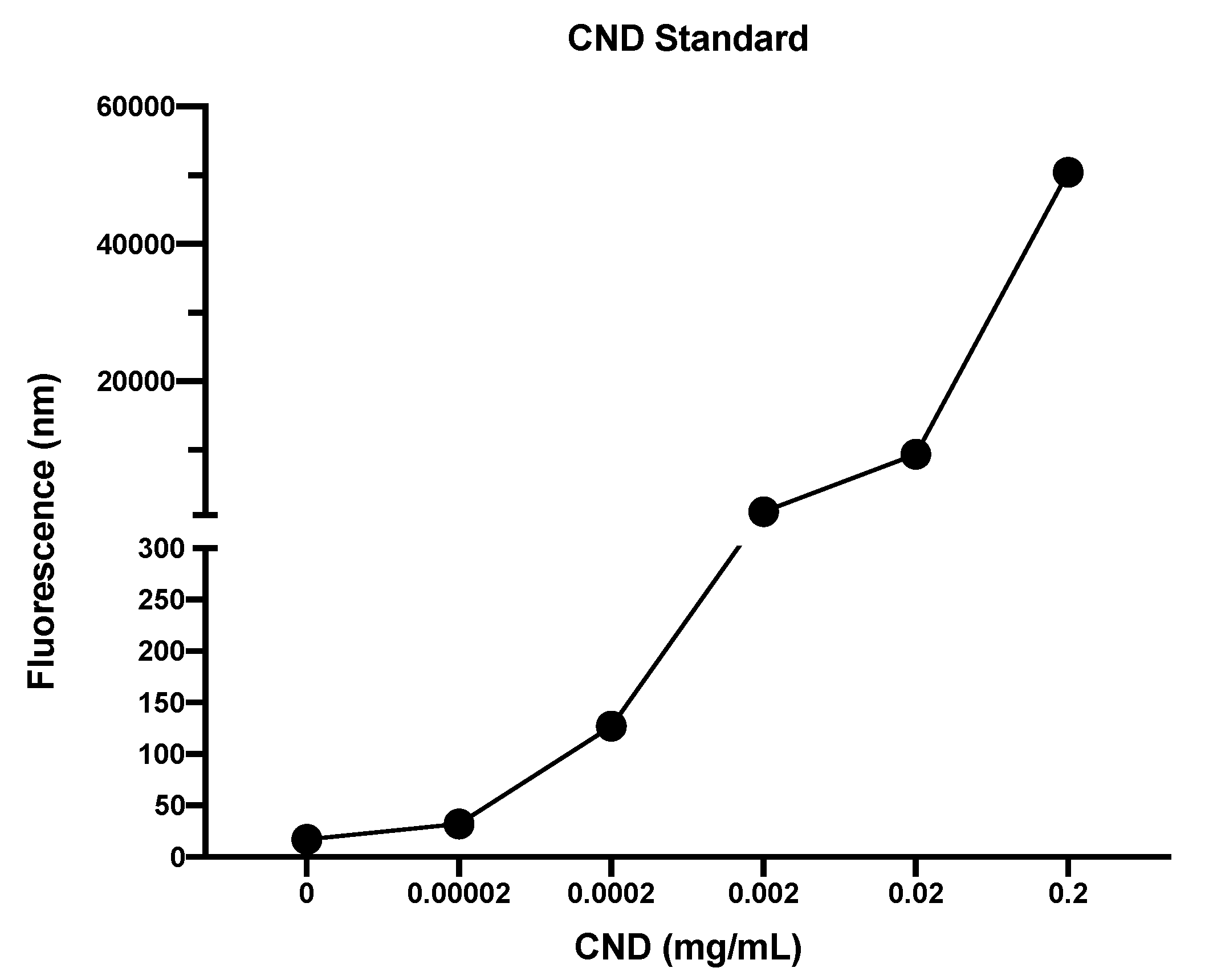
2.4. CND Uptake Assay
2.5. Cell Viability with MTT Assay
2.6. Measurements of IL-8 and sICAM-1 Protein Molecules
2.7. RNA Extraction
2.8. cDNA Synthesis
2.9. Quantitative Real Time-Polymerase Chain Reaction (qRT-PCR)
2.10. IDT® Human Primer Sequences
2.11. Statistical Analysis
3. Results
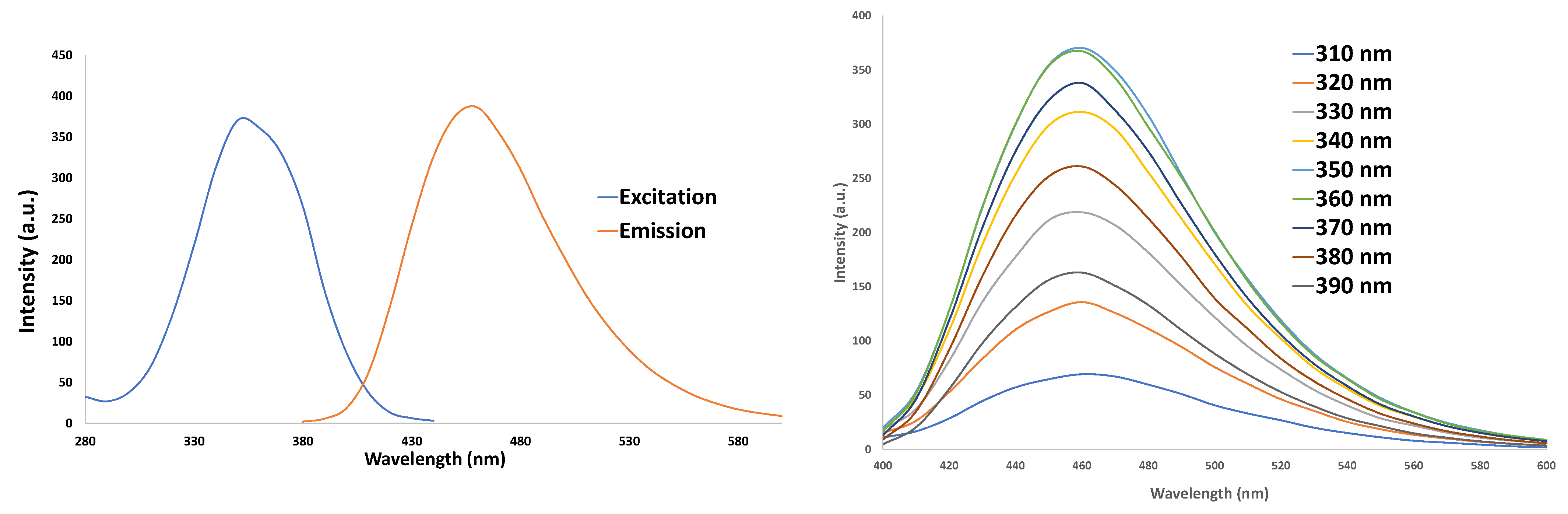
3.1. Characterization of CNDs: UV–VIS
3.2. Cell Viability with MTT
3.3. Quantitative Real Time-Polymerase Chain Reaction (qRT-PCR) for Proinflammatory Genes
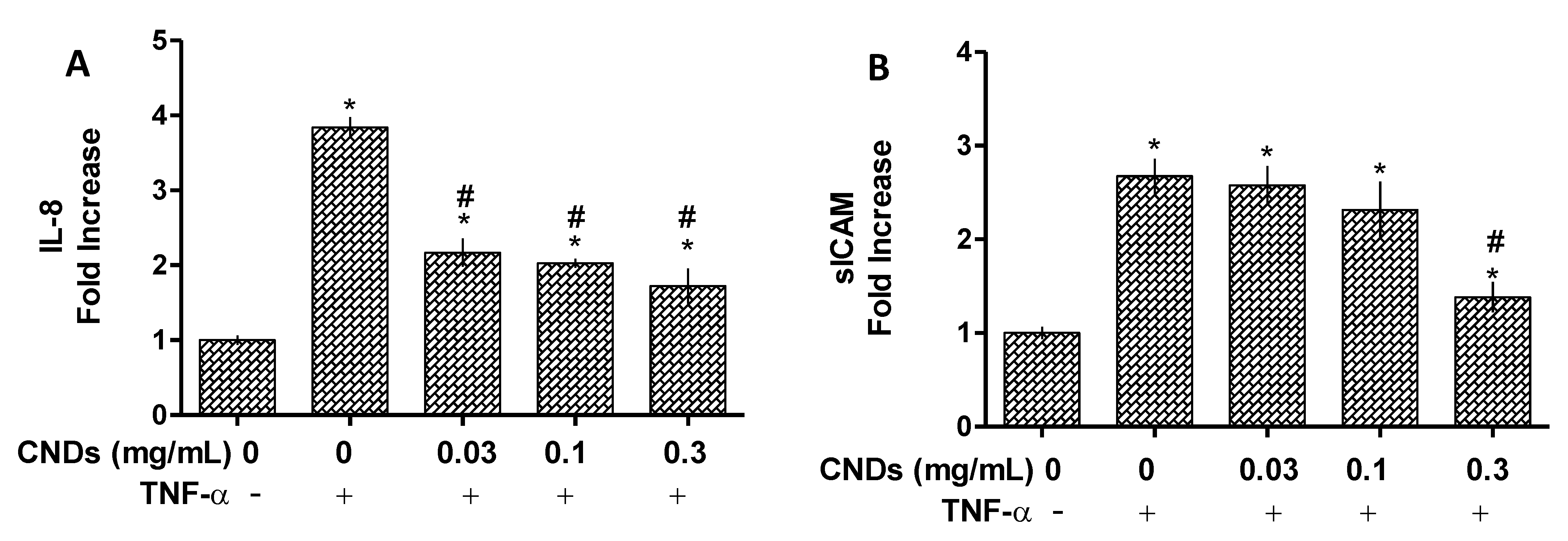
3.4. ELISA Assay for IL-8 and ICAM Protein Quantification
3.5. Quantitative Real Time-Polymerase Chain Reaction (qRT-PCR) for ROS Detoxification Gene Expression
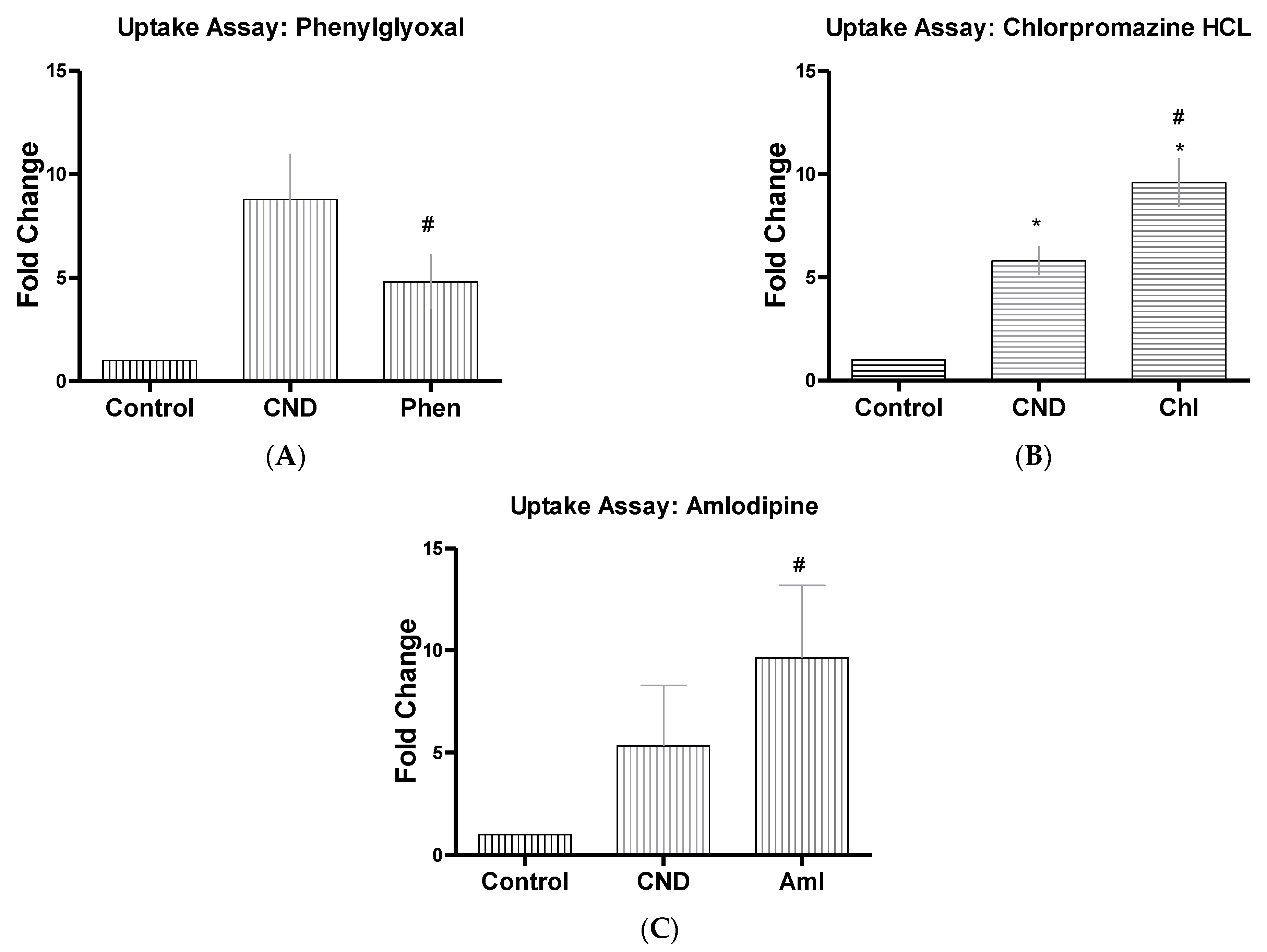
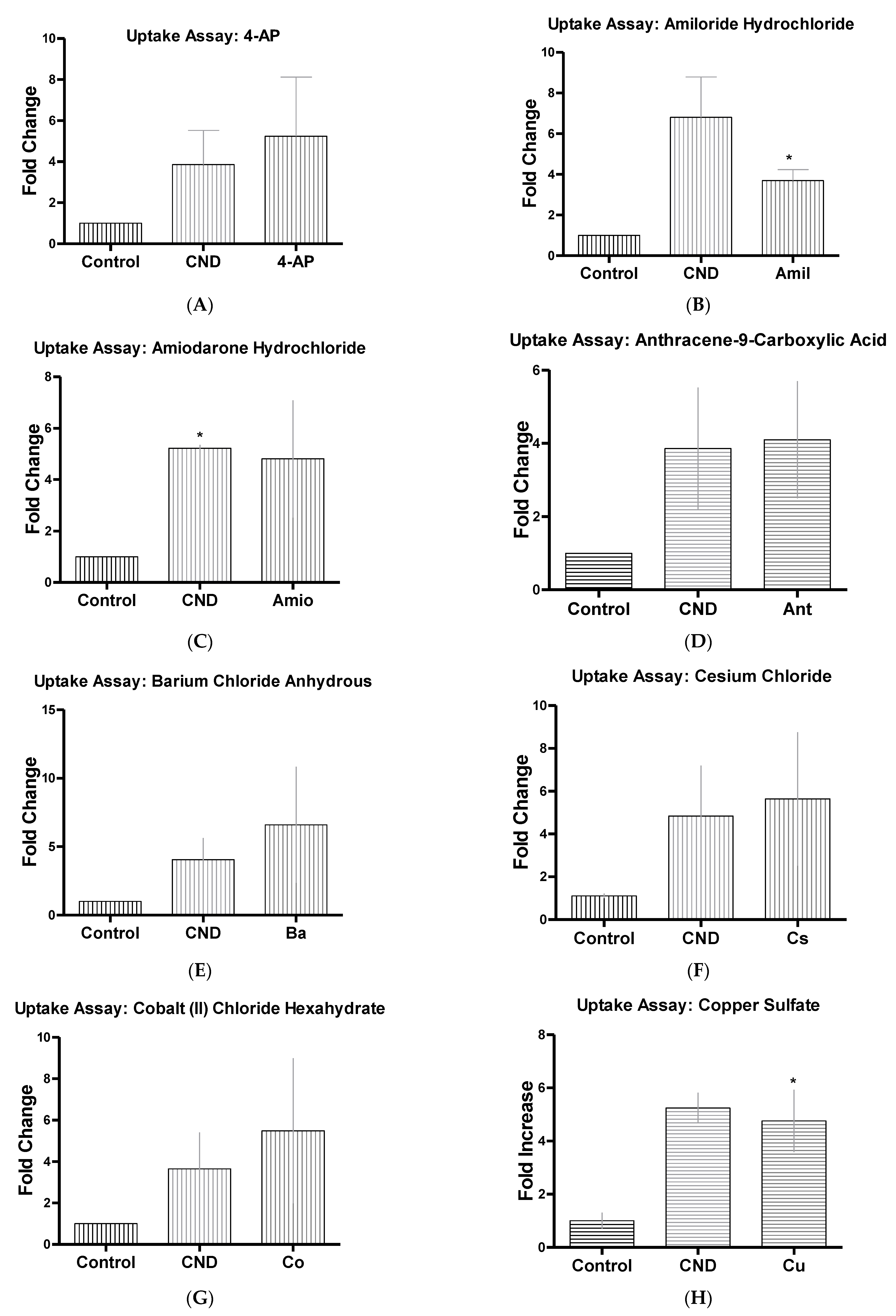
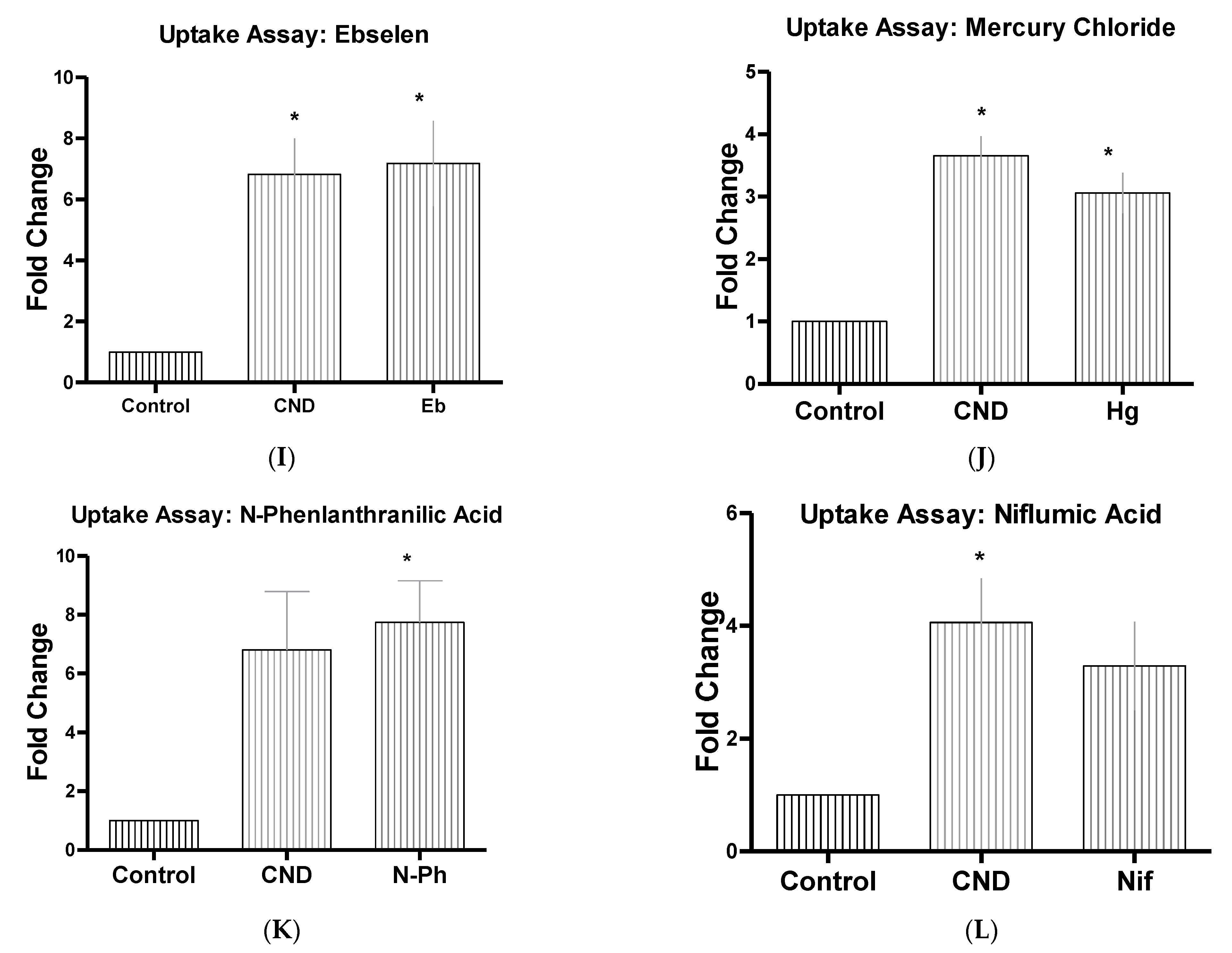
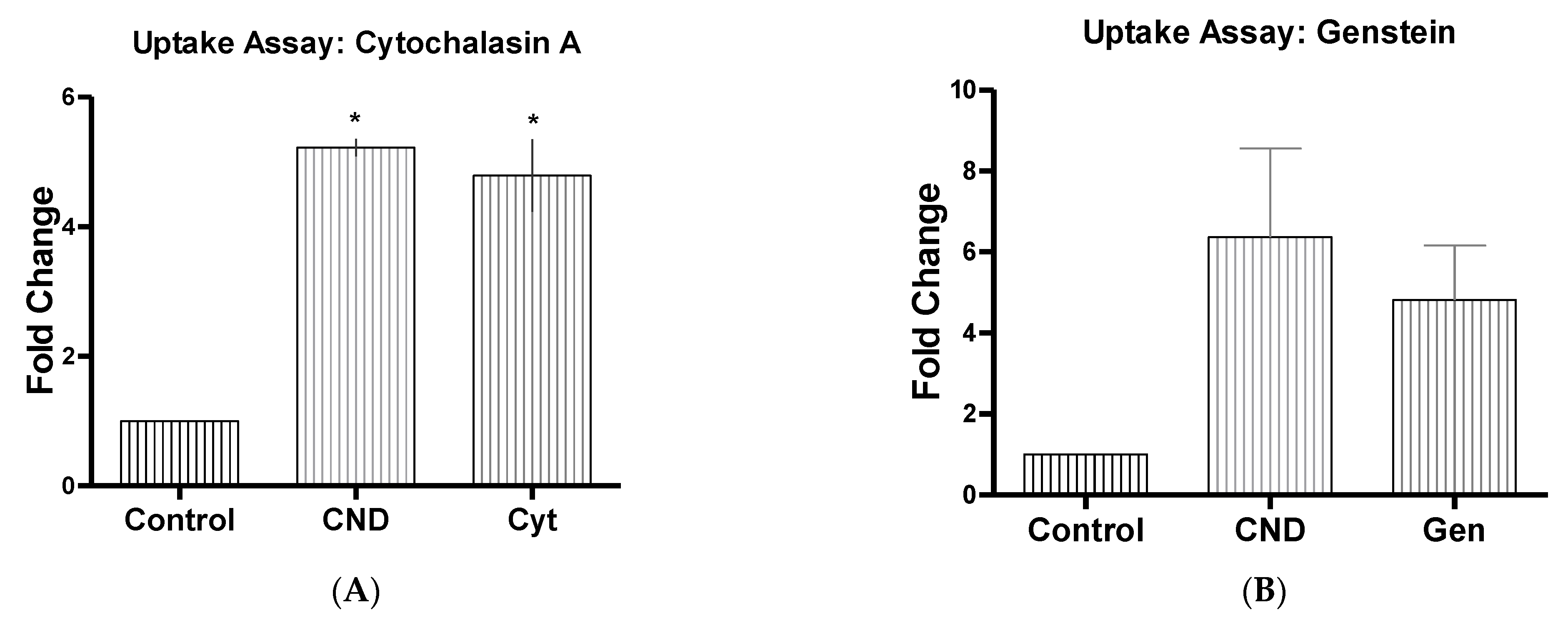
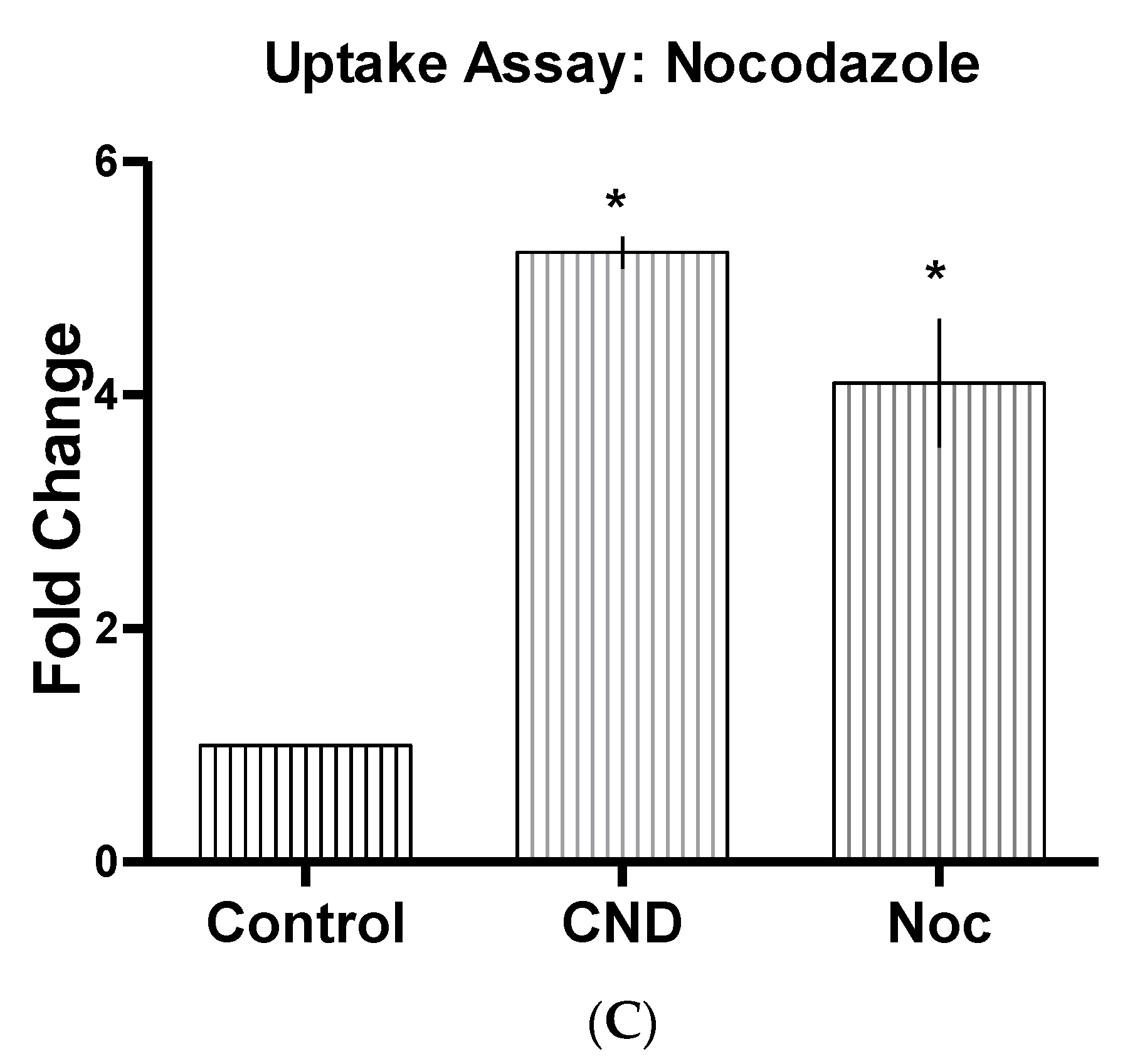
3.6. CND Uptake Assay
| Inhibitor Name | Abbrev | Concentrate | Function |
|---|---|---|---|
| 4-Aminopyridine ~98% | 4-AP | 5 mM | Ion channel blocker (K+) [35] |
| Amiloride Hydrochloride Dihydrous | Amil | 50 µM | Inhibits micropinocytosis: blocks Na+/H+ exchanger pump [36,37,38] |
| Amiodarone Hydrochloride | Amio | 10 µM | Non-selective ion channel blocker [39] |
| Amlodipine | Aml | 10 µM | Ion channel blocker (Ca+) [40] |
| Anthracene-9-Carboxilic Acid | Ant | 100 µM | Ion channel blocker (Cl−) [41] |
| Barium Chloride Anhydrous | Ba | 350 µM | Ion channel blocker (K+) [35] |
| Cesium Chloride, 99% | Cs | 1 mM | Ion channel blocker (K+) [42] |
| Chlorpromazine HCL | Chl | 10 µM | Suppresses clathrin disassembly [32,36] |
| Cobalt (II) Chloride | Co | 2 mM | Ion channel blocker (Ca+) [43] |
| Copper Sulfate | Cu | 100 µM | hAQP3 Aquaporins [44] |
| Cytochalasin A | Cyt | 5 µg/mL | Actin disruptor [32] |
| Ebselen | Eb | 15 µM | Inhibits mammalian H+, K+-ATPase [45] |
| Genstein | Gen | 200 µM | Inhibits tyrosine kinase receptors [32] |
| Mercury Chloride | Hg | 50 µM | hAQPI Aquaporins [44] |
| N-Phenlanthranilic Acid | N-Ph | 0.1 mM | Ion channel blocker (Cl−) [46] |
| Niflumic Acid | Nif | 10 µM | Ion channel blocker (Cl−) |
| Nocodazole | Noc | 20 µM | Actin and microtubule disruptor [32] |
| Phenylglyoxal | Phen | 100 µg | Selective inhibitor of phagocytosis [47] |
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics-2020 update: A report from the american heart association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Inflammation and atherosclerosis: The end of a controversy. Circulation 2017, 136, 1875–1877. [Google Scholar] [CrossRef]
- Davignon, J.; Ganz, P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004, 109, III27–III32. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef]
- Moss, J.W.; Ramji, D.P. Cytokines: Roles in atherosclerosis disease progression and potential therapeutic targets. Future Med. Chem. 2016, 8, 1317–1330. [Google Scholar] [CrossRef]
- Tedgui, A.; Mallat, Z. Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiol. Rev. 2006, 86, 515–581. [Google Scholar] [CrossRef] [PubMed]
- Mehra, V.C.; Ramgolam, V.S.; Bender, J.R. Cytokines and cardiovascular disease. J. Leukoc. Biol. 2005, 78, 805–818. [Google Scholar] [CrossRef] [PubMed]
- Nallasamy, P.; Si, H.; Babu, P.V.; Pan, D.; Fu, Y.; Brooke, E.A.; Shah, H.; Zhen, W.; Zhu, H.; Liu, D.; et al. Sulforaphane reduces vascular inflammation in mice and prevents TNF-α-induced monocyte adhesion to primary endothelial cells through interfering with the NF-κB pathway. J. Nutr. Biochem. 2014, 25, 824–833. [Google Scholar] [CrossRef]
- Hill, S.; Galan, M.C. Fluorescent carbon dots from mono- and polysaccharides: Synthesis, properties and applications. Beilstein J. Org. Chem. 2017, 13, 675–693. [Google Scholar] [CrossRef]
- Hoshino, A.; Manabe, N.; Fujioka, K.; Suzuki, K.; Yasuhara, M.; Yamamoto, K. Use of fluorescent quantum dot bioconjugates for cellular imaging of immune cells, cell organelle labeling, and nanomedicine: Surface modification regulates biological function, including cytotoxicity. J. Artif. Organs 2007, 10, 149–157. [Google Scholar] [CrossRef]
- Lin, L.; Song, X.; Chen, Y.; Rong, M.; Zhao, T.; Wang, Y.; Jiang, Y.; Chen, X. Intrinsic peroxidase-like catalytic activity of nitrogen-doped graphene quantum dots and their application in the colorimetric detection of H2O2 and glucose. Anal. Chim. Acta 2015, 869, 89–95. [Google Scholar] [CrossRef]
- Miao, P.; Han, K.; Tang, Y.; Wang, B.; Lin, T.; Cheng, W. Recent advances in carbon nanodots: Synthesis, properties and biomedical applications. Nanoscale 2015, 7, 1586–1595. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Rajendran, R.; Jeong, M.S.; Ko, H.Y.; Joo, J.Y.; Cho, S.; Chang, Y.W.; Kim, S. Bioimaging of targeting cancers using aptamer-conjugated carbon nanodots. Chem. Commun. 2013, 49, 6543–6545. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, K.; Zhao, L.; Li, C.; Bu, W.; Shen, Y.; Gu, Z.; Chang, B.; Zheng, C.; Lin, C.; et al. Aspirin-based carbon dots, a good biocompatibility of material applied for bioimaging and anti-inflammation. ACS Appl. Mater. Interfaces 2016, 8, 32706–32716. [Google Scholar] [CrossRef]
- Zong, J.; Yang, X.; Trinchi, A.; Hardin, S.; Cole, I.; Zhu, Y.; Li, C.; Muster, T.; Wei, G. Carbon dots as fluorescent probes for “off-on” detection of Cu2+ and L-cysteine in aqueous solution. Biosens. Bioelectron. 2014, 51, 330–335. [Google Scholar] [CrossRef]
- Ma, J.-L.; Yin, B.-C.; Wu, X.; Ye, B.-C. Simple and cost-effective glucose detection based on carbon nanodots supported on silver nanoparticles. Anal. Chem. 2017, 89, 1323. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.K.; Yap, S.H.K.; Yong, K.T. Biogreen synthesis of carbon dots for biotechnology and nanomedicine applications. Nanomicro Lett. 2018, 10, 72. [Google Scholar] [CrossRef]
- Sun, H.; Gao, N.; Dong, K.; Ren, J.; Qu, X. Graphene quantum dots-band-aids used for wound disinfection. ACS Nano 2014, 8, 6202–6210. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, S.; Tang, Q.; Zhang, K.; Yu, W.; Sun, H.; Yang, B. One-step hydrothermal synthesis of photoluminescent carbon nanodots with selective antibacterial activity against Porphyromonas gingivalis. Nanoscale 2017, 9, 7135–7142. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Dadhich, P.; Pal, P.; Srivas, P.K.; Bankoti, K.; Dhara, S. Carbon nanodots from date molasses: New nanolights for the in vitro scavenging of reactive oxygen species. J. Mater. Chem. B 2014, 2, 6839–6847. [Google Scholar] [CrossRef]
- Picchi, A.; Gao, X.; Belmadani, S.; Potter, B.J.; Focardi, M.; Chilian, W.M.; Zhang, C. Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ. Res. 2006, 99, 69–77. [Google Scholar] [CrossRef]
- Dunphy, A.; Patel, K.; Belperain, S.; Pennington, A.; Chiu, N.H.L.; Yin, Z.; Zhu, X.; Priebe, B.; Tian, S.; Wei, J.; et al. Modulation of macrophage polarization by carbon nanodots and elucidation of carbon nanodot uptake routes in macrophages. Nanomaterials 2021, 11, 1116. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Yin, Z.; Jia, Z.; Wei, J. Carbon nanodots derived from urea and citric acid in living cells: Cellular uptake and antioxidation effect. Langmuir 2020, 36, 8632–8640. [Google Scholar] [CrossRef]
- Zhang, W.; Chavez, J.; Zeng, Z.; Bloom, B.; Sheardy, A.; Ji, Z.; Yin, Z.; Waldeck, D.H.; Jia, Z.; Wei, J. Antioxidant Capacity of Nitrogen and Sulfur Codoped Carbon Nanodots. ACS Appl. Nano Mater. 2018, 1, 2699–2708. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Sittampalam, G.S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Austin, C.P., Baell, J., Bejcek, B., Caaveiro, J.M.M., Chung, T.D.Y., et al., Eds.; Bethesda: Rockville, MD, USA, 2004. [Google Scholar]
- Maus, U.; Henning, S.; Wenschuh, H.; Mayer, K.; Seeger, W.; Lohmeyer, J. Role of endothelial MCP-1 in monocyte adhesion to inflamed human endothelium under physiological flow. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H2584–H2591. [Google Scholar] [CrossRef]
- Soares, M.P.; Seldon, M.P.; Gregoire, I.P.; Vassilevskaia, T.; Berberat, P.O.; Yu, J.; Tsui, T.Y.; Bach, F.H. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J. Immunol. 2004, 172, 3553–3563. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.; Siegel, D. Functions of NQO1 in cellular protection and CoQ10 metabolism and its potential role as a redox sensitive molecular switch. Front. Physiol. 2017, 8, 595. [Google Scholar] [CrossRef] [PubMed]
- Satta, S.; Mahmoud, A.M.; Wilkinson, F.L.; Yvonne Alexander, M.; White, S.J. The Role of Nrf2 in Cardiovascular Function and Disease. Oxidative Med. Cell. Longev. 2017, 2017, 9237263. [Google Scholar] [CrossRef] [PubMed]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- Goodwin, J.E.; Feng, Y.; Velazquez, H.; Sessa, W.C. Endothelial glucocorticoid receptor is required for protection against sepsis. Proc. Natl. Acad. Sci. USA 2013, 110, 306–311. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, T.; Varela, J.; Lynch, I.; Salvati, A.; Dawson, K.A.; Schnur, J.M.E. Effects of transport inhibitors on the cellular uptake of carboxylated polystyrene nanoparticles in different cell lines. PLoS ONE 2011, 6, 1–10. [Google Scholar] [CrossRef]
- Strauss, V.; Margraf, J.T.; Dolle, C.; Butz, B.; Nacken, T.J.; Walter, J.; Bauer, W.; Peukert, W.; Spiecker, E.; Clark, T.; et al. Carbon nanodots: Toward a comprehensive understanding of their photoluminescence. J. Am. Chem. Soc. 2014, 136, 17308–17316. [Google Scholar] [CrossRef]
- Essner, J.B.; McCay, R.N.; Smith Ii, C.J.; Cobb, S.M.; Laber, C.H.; Baker, G.A. A switchable peroxidase mimic derived from the reversible co-assembly of cytochrome c and carbon dots. J. Mater. Chem. B 2016, 4, 2163–2170. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.; Palacios, J.; Jofre, I.; Paz, C.; Nwokocha, C.R.; Paredes, A.; Cifuentes, F. Aristoteline, an indole-alkaloid, induces relaxation by activating potassium channels and blocking calcium channels in isolated rat aorta. Molecules 2019, 24, 2748. [Google Scholar] [CrossRef]
- Park, S.; Lee, S.J.; Chung, H.; Her, S.; Choi, Y.; Kim, K.; Choi, K.; Kwon, I.C. Cellular uptake pathway and drug release characteristics of drug-encapsulated glycol chitosan nanoparticles in live cells. Microsc. Res. Tech. 2010, 73, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Francia, V.; Reker-Smit, C.; Boel, G.; Salvati, A. Limits and challenges in using transport inhibitors to characterize how nano-sized drug carriers enter cells. Nanomedicine 2019, 14, 1533–1549. [Google Scholar] [CrossRef]
- Koivusalo, M.; Welch, C.; Hayashi, H.; Scott, C.C.; Kim, M.; Alexander, T.; Touret, N.; Hahn, K.M.; Grinstein, S. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J. Cell Biol. 2010, 188, 547–563. [Google Scholar] [CrossRef]
- Roden, D.M. Pharmacogenetics of potassium channel blockers. Card. Electrophysiol. Clin. 2016, 8, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Alawdi, S.H.; Eidi, H.; Safar, M.M.; Abdel-Wahhab, M.A. Loading amlodipine on diamond nanoparticles: A novel drug delivery system. Nanotechnol. Sci. Appl. 2019, 12, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Cherian, O.L.; Menini, A.; Boccaccio, A. Multiple effects of anthracene-9-carboxylic acid on the TMEM16B/anoctamin2 calcium-activated chloride channel. Biochim. Biophys. Acta 2015, 1848, 1005–1013. [Google Scholar] [CrossRef]
- Rouzaire-Dubois, B.; Dubois, J.M. K+ channel block-induced mammalian neuroblastoma cell swelling: A possible mechanism to influence proliferation. J. Physiol. 1998, 510, 93–102. [Google Scholar] [CrossRef]
- Wu, D.; Yotnda, P. Induction and testing of hypoxia in cell culture. J. Vis. Exp. 2011, 12, 2899. [Google Scholar] [CrossRef]
- Alejandra, R.; Natalia, S.; Alicia, E.D. The blocking of aquaporin-3 (AQP3) impairs extravillous trophoblast cell migration. Biochem. Biophys. Res. Commun. 2018, 499, 227–232. [Google Scholar] [CrossRef]
- Kjellerup, L.; Gordon, S.; Cohrt, K.O.; Brown, W.D.; Fuglsang, A.T.; Winther, A.L. Identification of antifungal H(+)-ATPase inhibitors with effect on plasma membrane potential. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.K.; Boneham, G.C.; Pirie, B.L.; Collin, H.B.; Campbell, T.J. Chloride ion channels are associated with adherence of lymphatic endothelial cells. Microvasc. Res. 1996, 52, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Wieth, J.O.; Bjerrum, P.J.; Borders, C.L., Jr. Irreversible inactivation of red cell chloride exchange with phenylglyoxal, and arginine-specific reagent. J. Gen. Physiol. 1982, 79, 283–312. [Google Scholar] [CrossRef]
- Aboyans, V.; Lacroix, P.; Criqui, M.H. Large and small vessels atherosclerosis: Similarities and differences. Prog. Cardiovasc. Dis. 2007, 50, 112–125. [Google Scholar] [CrossRef]
- Ades, E.W.; Candal, F.J.; Swerlick, R.A.; George, V.G.; Summers, S.; Bosse, D.C.; Lawley, T.J. HMEC-1: Establishment of an immortalized human microvascular endothelial cell line. J. Investig. Dermatol. 1992, 99, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Oude Nijhuis, C.S.; Vellenga, E.; Daenen, S.M.; Kamps, W.A.; De Bont, E.S. Endothelial cells are main producers of interleukin 8 through Toll-like receptor 2 and 4 signaling during bacterial infection in leukopenic cancer patients. Clin. Diagn. Lab. Immunol. 2003, 10, 558–563. [Google Scholar] [CrossRef]
- Bickel, M. The role of interleukin-8 in inflammation and mechanisms of regulation. J. Periodontol. 1993, 64, 456–460. [Google Scholar]
- Yeh, M.; Leitinger, N.; de Martin, R.; Onai, N.; Matsushima, K.; Vora, D.K.; Berliner, J.A.; Reddy, S.T. Increased transcription of IL-8 in endothelial cells is differentially regulated by TNF-alpha and oxidized phospholipids. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1585–1591. [Google Scholar] [CrossRef]
- Clark, P.R.; Manes, T.D.; Pober, J.S.; Kluger, M.S. Increased ICAM-1 expression causes endothelial cell leakiness, cytoskeletal reorganization and junctional alterations. J. Investig. Dermatol. 2007, 127, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Lawson, C.; Wolf, S. ICAM-1 signaling in endothelial cells. Pharmacol. Rep. 2009, 61, 22–32. [Google Scholar] [CrossRef]
- Frank, P.G.; Lisanti, M.P. ICAM-1: Role in inflammation and in the regulation of vascular permeability. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H926–H927. [Google Scholar] [CrossRef]
- Puhlmann, M.; Weinreich, D.M.; Farma, J.M.; Carroll, N.M.; Turner, E.M.; Alexander, H.R., Jr. Interleukin-1beta induced vascular permeability is dependent on induction of endothelial tissue factor (TF) activity. J. Transl. Med. 2005, 3, 37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zeng, Z.; Wei, J. Electrochemical study of DPPH radical scavenging for evaluating the antioxidant capacity of carbon nanodots. J. Phys. Chem. C 2017, 121, 18635–18642. [Google Scholar] [CrossRef]
- Radeke, H.H.; Meier, B.; Topley, N.; Floge, J.; Habermehl, G.G.; Resch, K. Interleukin 1-alpha and tumor necrosis factor-alpha induce oxygen radical production in mesangial cells. Kidney Int. 1990, 37, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jia, Z.; Strobl, J.S.; Ehrich, M.; Misra, H.P.; Li, Y. Potent induction of total cellular and mitochondrial antioxidants and phase 2 enzymes by cruciferous sulforaphane in rat aortic smooth muscle cells: Cytoprotection against oxidative and electrophilic stress. Cardiovasc. Toxicol. 2008, 8, 115–125. [Google Scholar] [CrossRef]
- Corda, S.; Laplace, C.; Vicaut, E.; Duranteau, J. Rapid reactive oxygen species production by mitochondria in endothelial cells exposed to tumor necrosis factor-alpha is mediated by ceramide. Am. J. Respir. Cell Mol. Biol. 2001, 24, 762–768. [Google Scholar] [CrossRef]
- Kim, Y.S.; Morgan, M.J.; Choksi, S.; Liu, Z.G. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol. Cell 2007, 26, 675–687. [Google Scholar] [CrossRef]
- Panday, A.; Sahoo, M.K.; Osorio, D.; Batra, S. NADPH oxidases: An overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 2015, 12, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Li, C.; Fan, J.; He, D.; Li, L. Androgen receptor (AR) signaling promotes RCC progression via increased endothelial cell proliferation and recruitment by modulating AKT → NF-κB → CXCL5 signaling. Sci. Rep. 2016, 6, 37085. [Google Scholar] [CrossRef]
- Araujo, J.A.; Zhang, M.; Yin, F. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front. Pharmacol. 2012, 3, 119. [Google Scholar] [CrossRef]
- Choi, A.M.; Alam, J. Heme oxygenase-1: Function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am. J. Respir. Cell Mol. Biol. 1996, 15, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Taha, H.; Skrzypek, K.; Guevara, I.; Nigisch, A.; Mustafa, S.; Grochot-Przeczek, A.; Ferdek, P.; Was, H.; Kotlinowski, J.; Kozakowska, M.; et al. Role of heme oxygenase-1 in human endothelial cells: Lesson from the promoter allelic variants. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1634–1641. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-kappaB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Zupancic, G.; Ogden, D.; Magnus, C.J.; Wheeler-Jones, C.; Carter, T.D. Differential exocytosis from human endothelial cells evoked by high intracellular Ca(2+) concentration. J. Physiol. 2002, 544, 741–755. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belperain, S.; Kang, Z.Y.; Dunphy, A.; Priebe, B.; Chiu, N.H.L.; Jia, Z. Anti-Inflammatory Effect and Cellular Uptake Mechanism of Carbon Nanodots in in Human Microvascular Endothelial Cells. Nanomaterials 2021, 11, 1247. https://doi.org/10.3390/nano11051247
Belperain S, Kang ZY, Dunphy A, Priebe B, Chiu NHL, Jia Z. Anti-Inflammatory Effect and Cellular Uptake Mechanism of Carbon Nanodots in in Human Microvascular Endothelial Cells. Nanomaterials. 2021; 11(5):1247. https://doi.org/10.3390/nano11051247
Chicago/Turabian StyleBelperain, Sarah, Zi Yae Kang, Andrew Dunphy, Brandon Priebe, Norman H. L. Chiu, and Zhenquan Jia. 2021. "Anti-Inflammatory Effect and Cellular Uptake Mechanism of Carbon Nanodots in in Human Microvascular Endothelial Cells" Nanomaterials 11, no. 5: 1247. https://doi.org/10.3390/nano11051247
APA StyleBelperain, S., Kang, Z. Y., Dunphy, A., Priebe, B., Chiu, N. H. L., & Jia, Z. (2021). Anti-Inflammatory Effect and Cellular Uptake Mechanism of Carbon Nanodots in in Human Microvascular Endothelial Cells. Nanomaterials, 11(5), 1247. https://doi.org/10.3390/nano11051247







