Peculiarities of Thermodynamic Behaviors of Xenon Adsorption on the Activated Carbon Prepared from Silicon Carbide
Abstract
1. Introduction
- Increasing utilization of electric propulsion systems in satellite and rocket launches;
- Growing demand for anesthetic in the medical industry;
- Widening applicability in electronics and semiconducting industry;
- Massive employment in the R&D projects, including dark matter investigations.
2. Materials and Methods
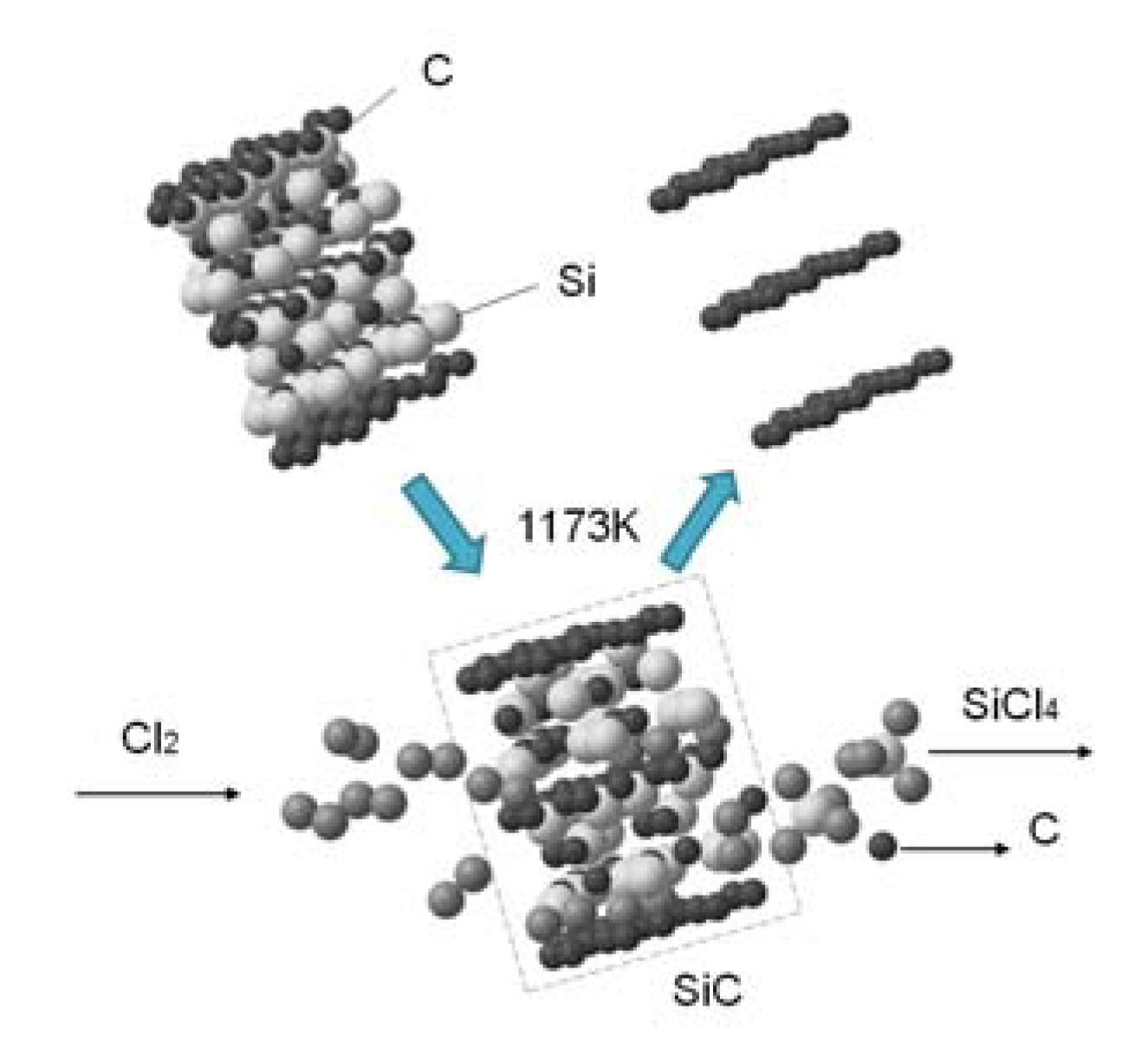
2.1. Adsorbent
2.2. Adsorptive
2.3. Methods
- A system calibration algorithm, which takes into account a deformation effect of the setup itself induced by xenon adsorption or temperature under experimental conditions, was employed. The dilatometer was calibrated using a fused quartz mockup, the shape and size of which were identical to that of the tested SiC-AC sample—a rod, which was 54.0 mm long and 11.4 mm in diameter. Quartz was selected due to its low compressibility. Calibration curves were recorded with a gradual increase in pressure up to 6 MPa (or temperature from 213 to 573K) and used for evaluating the systematic amendments;
- An adsorption stabilization procedure of the SiC-AC sample consisting of a gradual increase in xenon (or nitrogen for thermal deformation measurements) pressure up to 6 MPa, holding for 30 min, followed by xenon export using a vacuum pump. A sequence of ten adsorption/desorption cycles made it possible to verify the reversibility of adsorption (or temperature)-induced deformations of SiC-AC. Before each measurement, the adsorbent was regenerated at 623 K for 2 h to the pressure of 0.01 Pa;
- The signal stability was ensured by storing the measuring units for 3 h at each experimental temperature. The dilatometer unit containing the SiC-AC sample was thermostatted at each experimental temperature with an accuracy of 0.2 K.
- Adsorption-induced deformation was measured in the temperature range of 216 to 393 K and at pressures varying from 0.1 to 6 MPa. Thermal deformation of SiC-AC was measured within the range of temperatures of 260 to 575 K.
3. Results
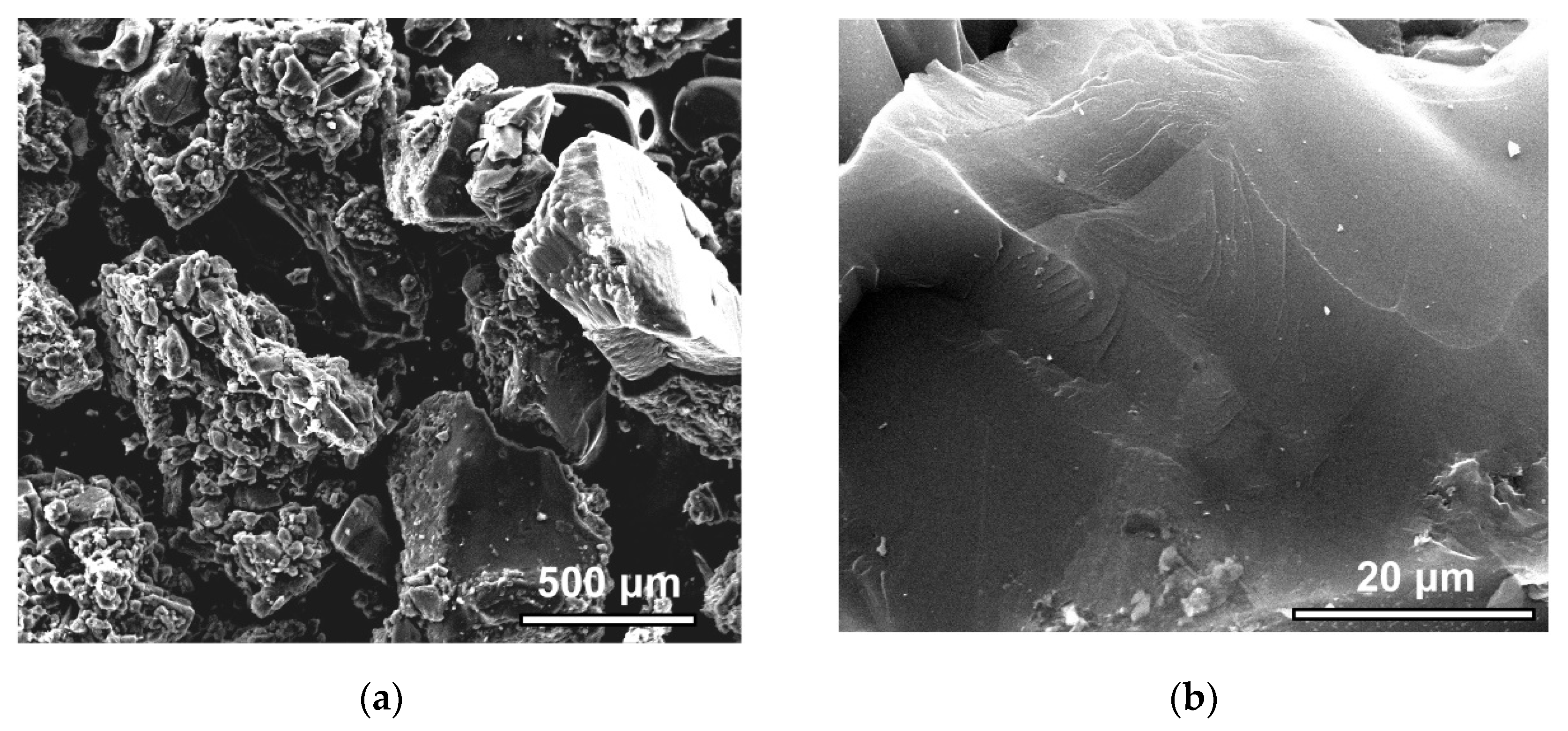
3.1. Structure and Morphology Characterization of the SiC-AC Adsorbent
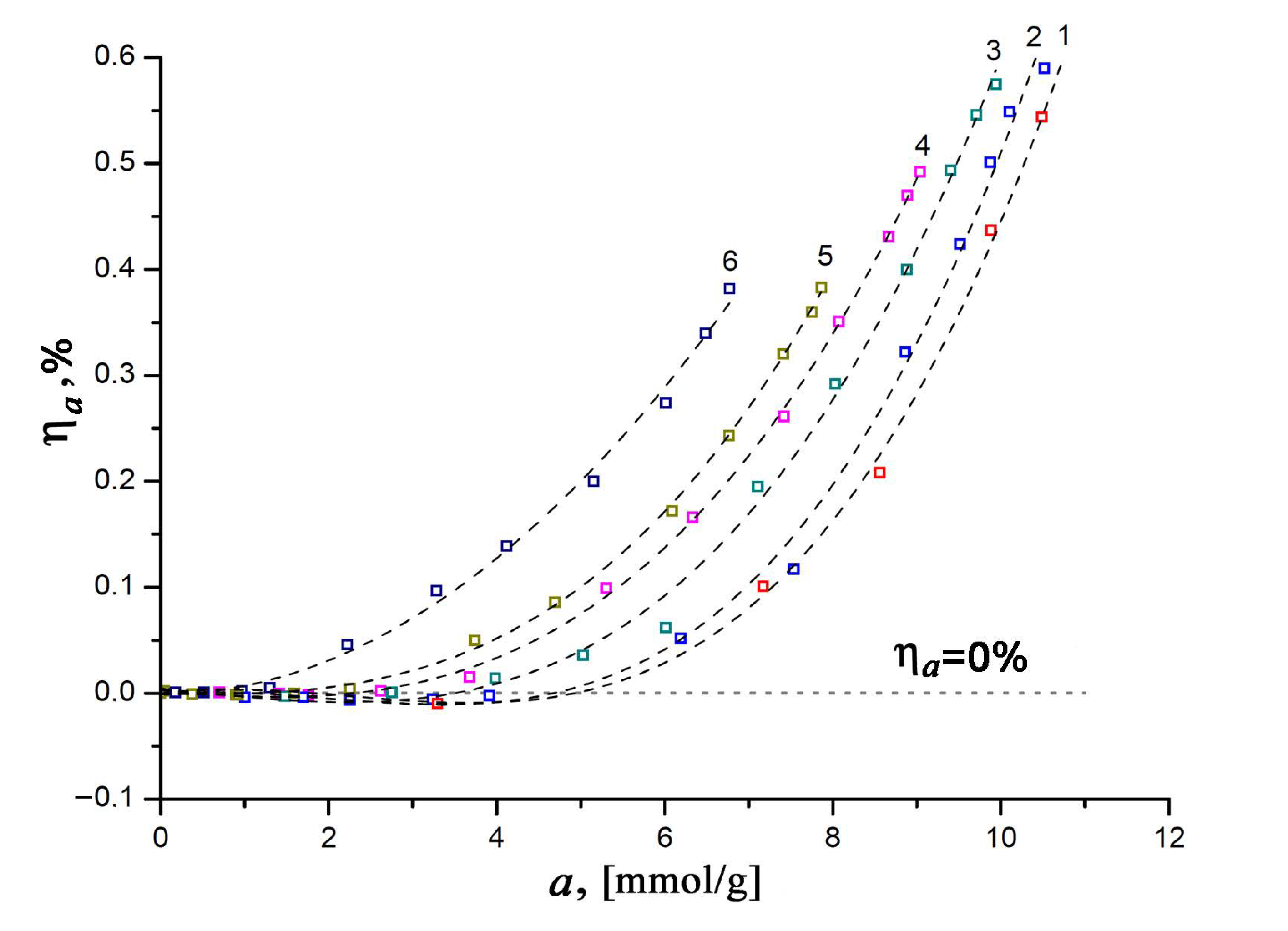
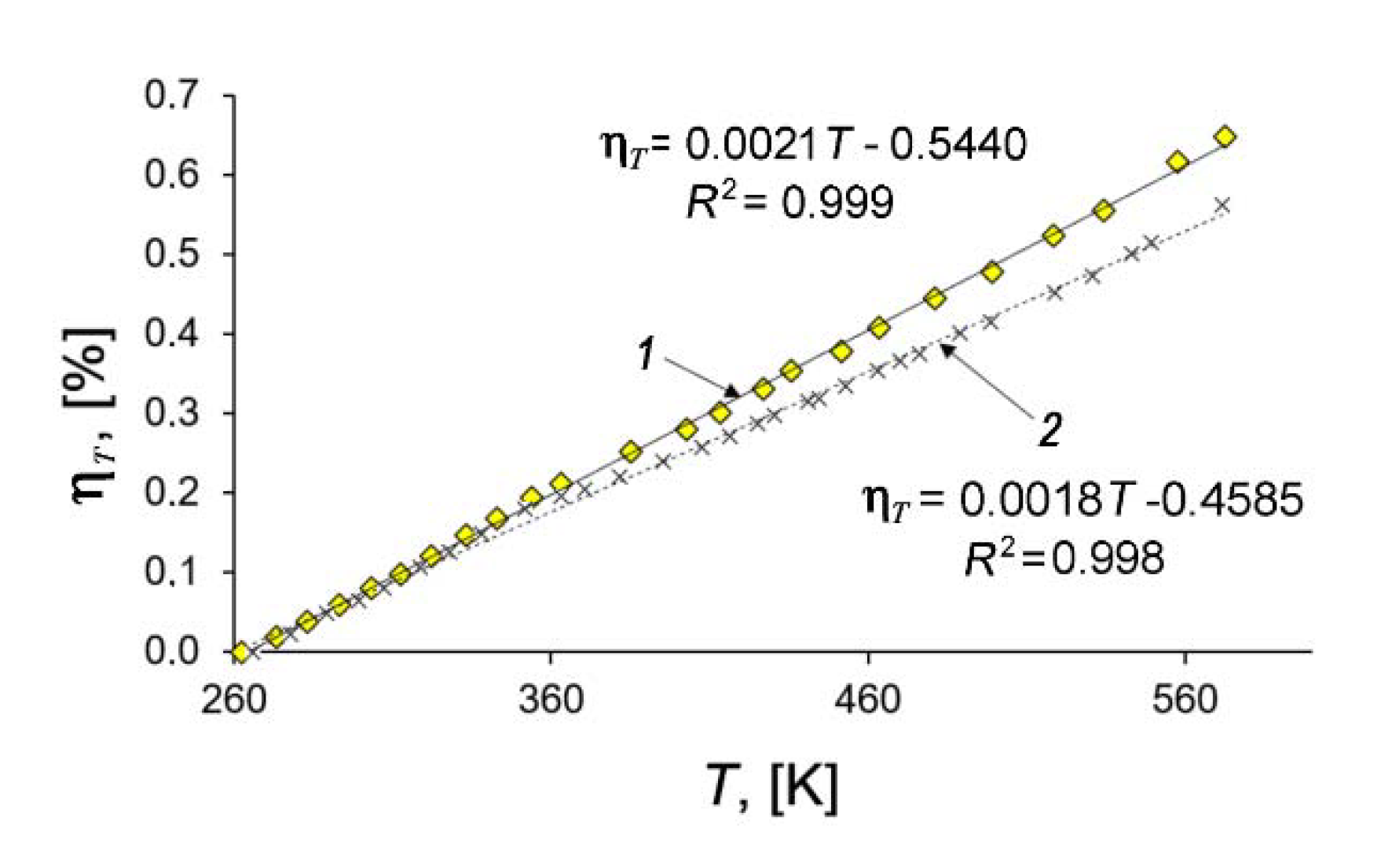
3.2. Adsorption- and Temperature-Induced Deformation of the SiC-AC Adsorbent
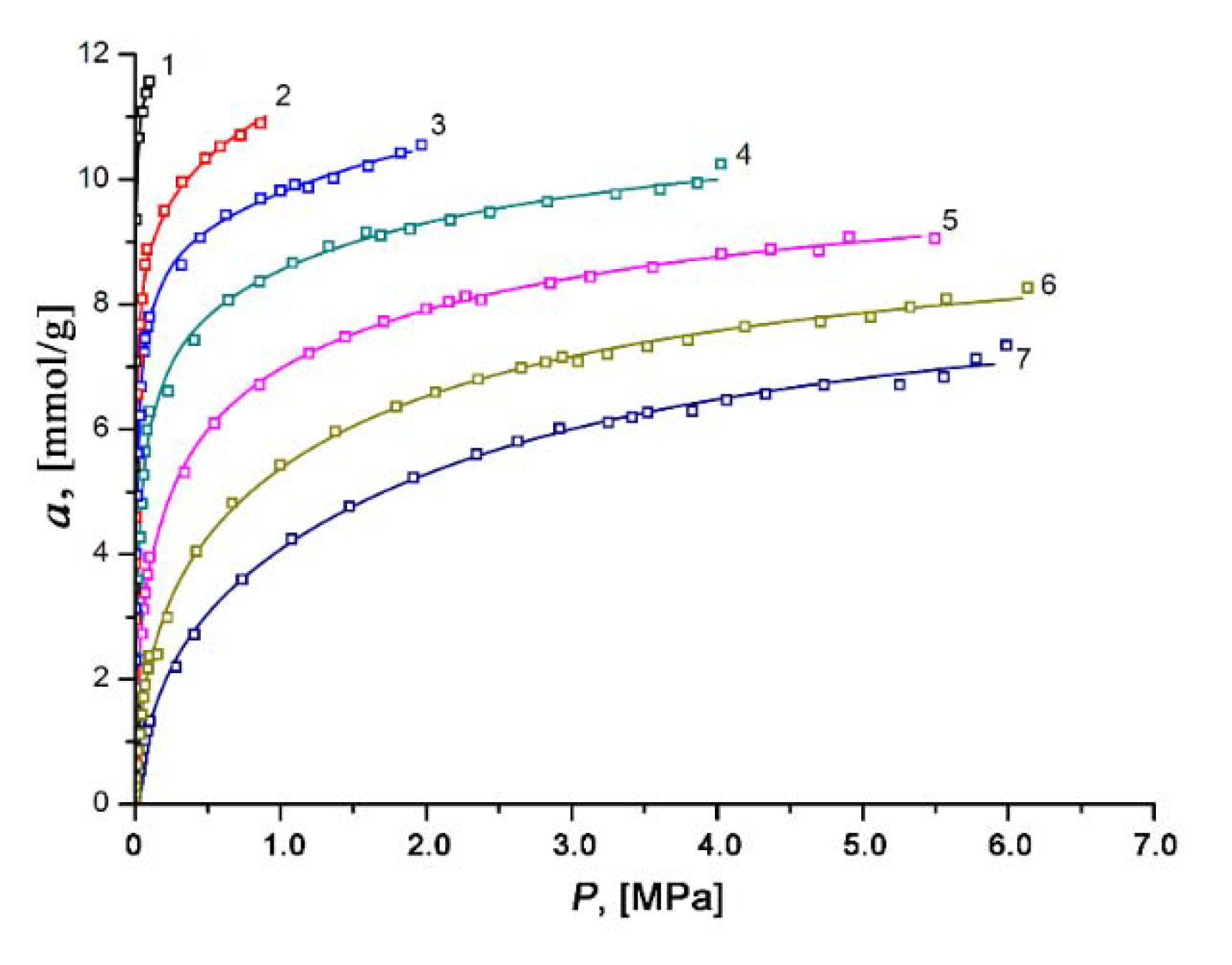
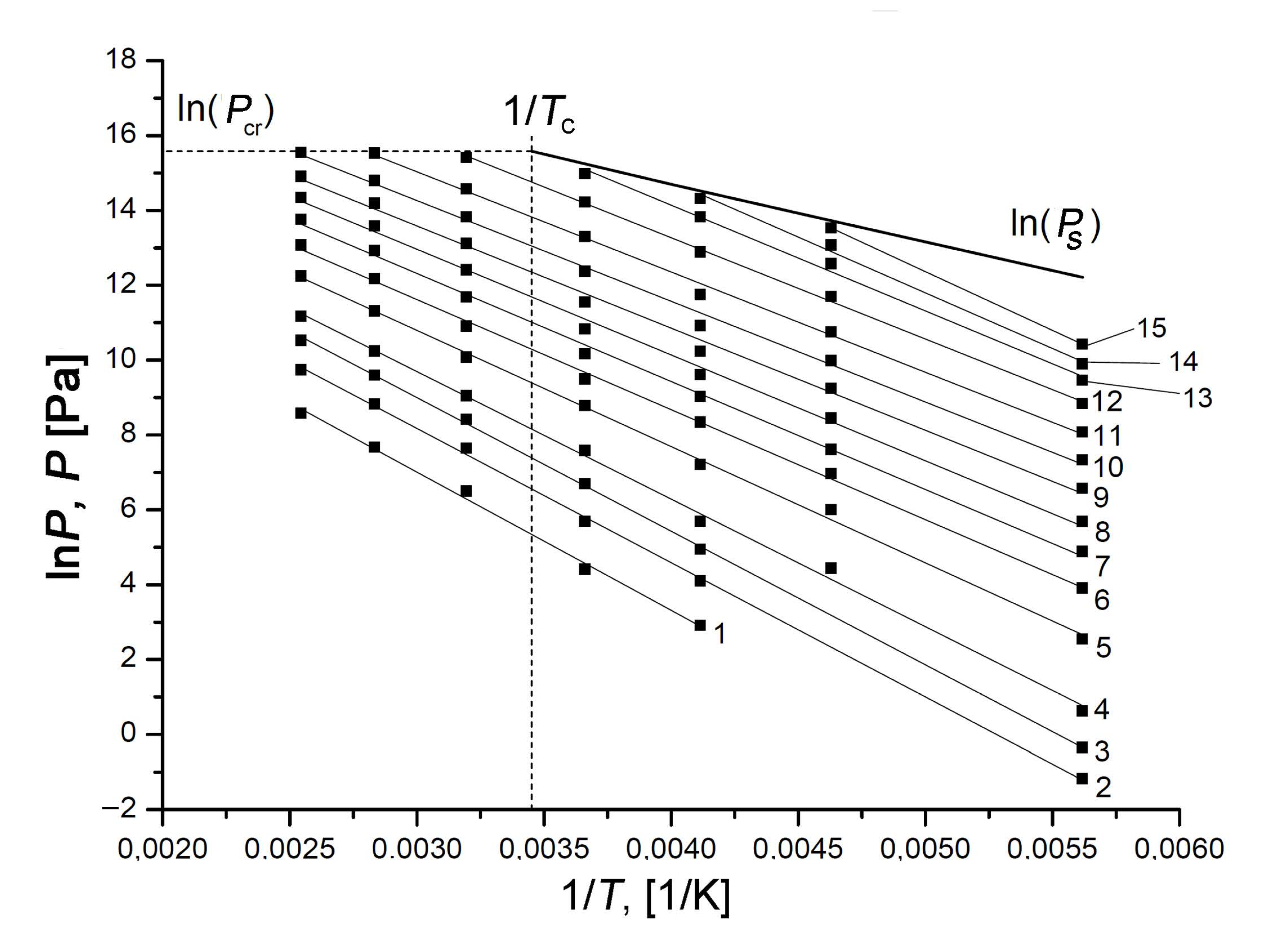
3.3. Xenon Adsorption on the SiC-AC Adsorbent
3.4. Thermodynamic Behaviors of the Xe/ SiC-AC Adsorption System
3.4.1. Differential Molar Heat of Absolute Xe Adsorption on the SiC-AC Carbon Adsorbent
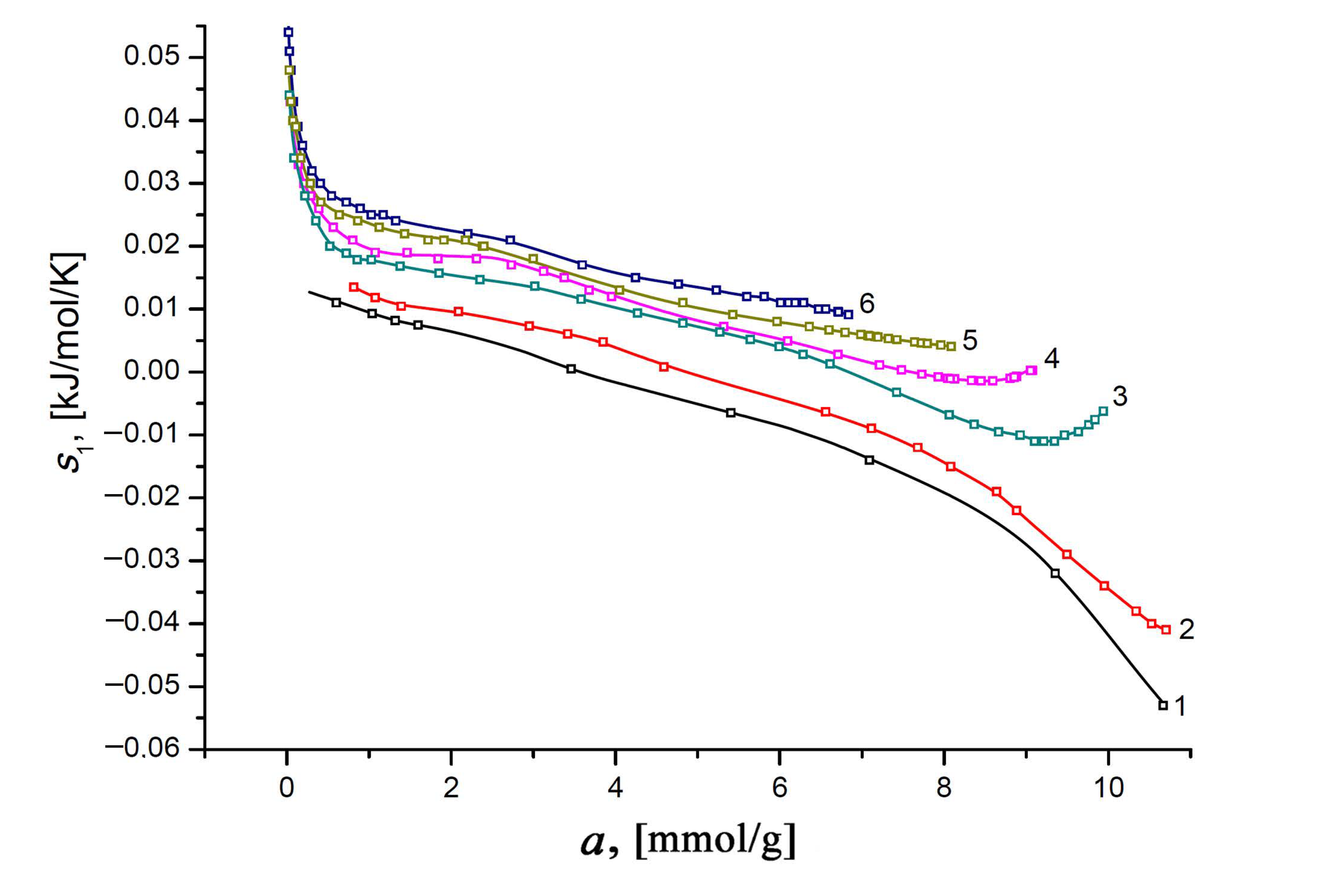
3.4.2. Differential Molar Entropy of Xe Adsorption on the SiC-AC Adsorbent
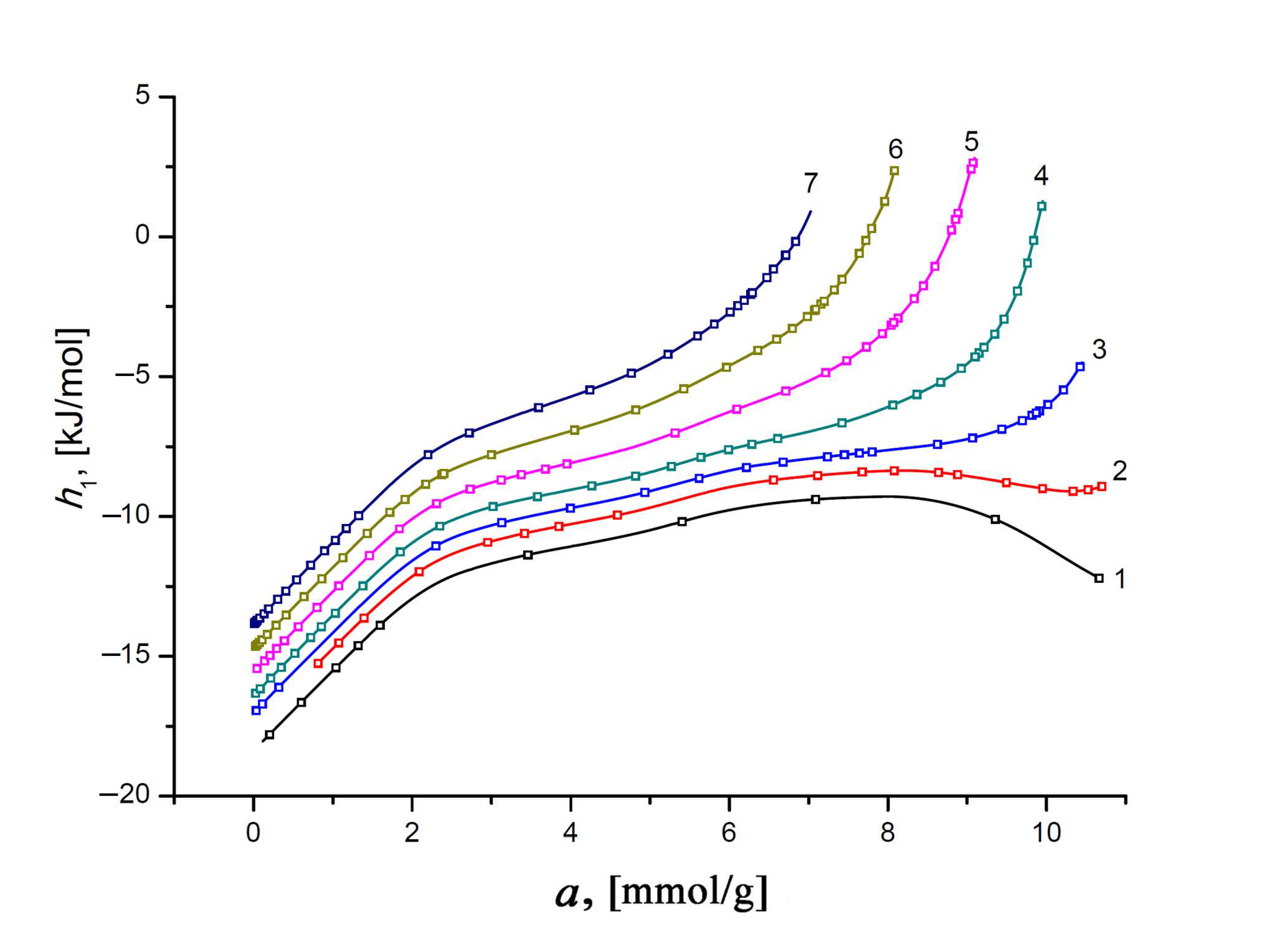
3.4.3. Differential Molar Enthalpy of Xe Adsorption on the SiC-AC Adsorbent
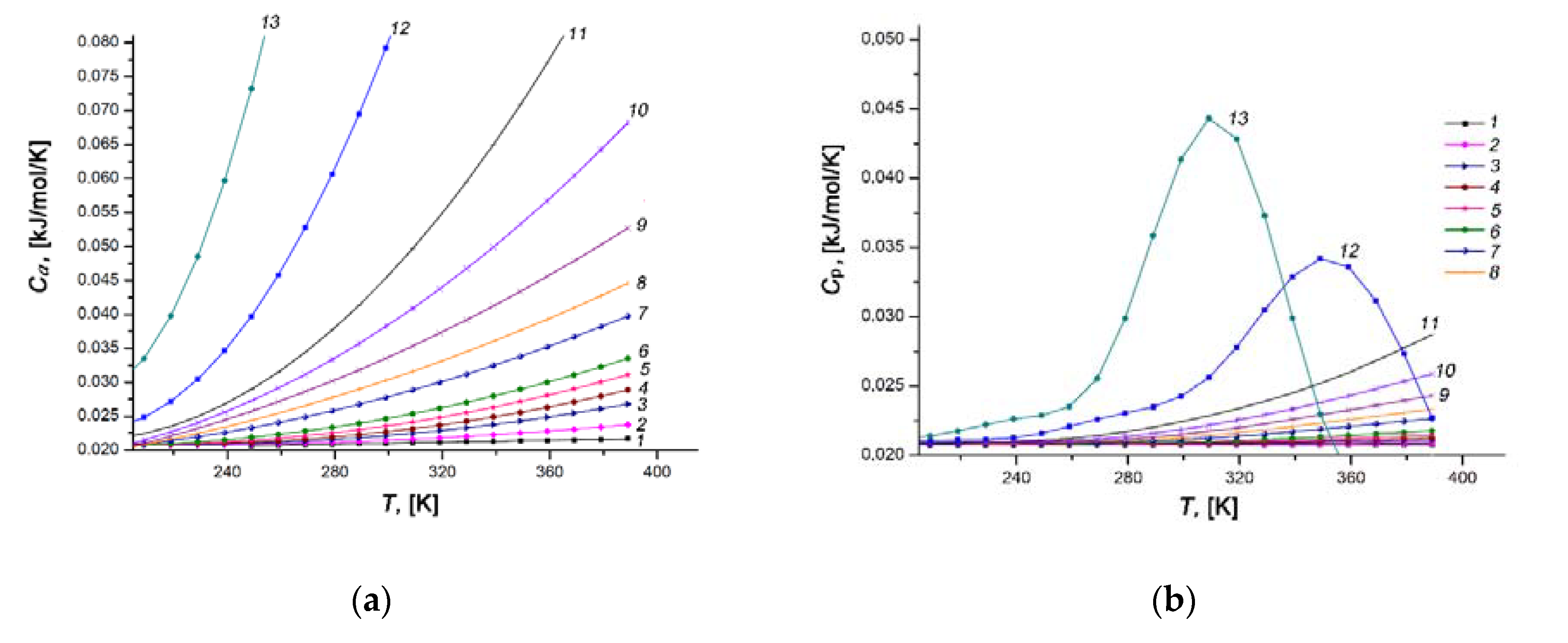
3.4.4. Differential Molar Isosteric Heat Capacity of the Xe/SiC-AC Adsorption System
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rabinovich, V.A.; Vasserman, A.A.; Nedostup, V.I.; Veksler, L.S. Thermal-Physical Properties of Neon, Argon, Krypton, and Xenon, 1st ed.; Izd-vo standartov: Moscow, Russia, 1976; p. 636. [Google Scholar]
- Esencan, E.; Yuksel, S.; Tosun, Y.B.; Robinot, A.; Solaroglu, I.; Zhang, J.H. Xenon in medical area: Emphasis on neuroprotection in hypoxia and anesthesia. MGR 2013, 3. Available online: http://www.medicalgasresearch.com/content/3/1/4 (accessed on 9 February 2021). [CrossRef]
- Arkharov, A.M.; Savinov, M.Y.; Bondarenko, V.L.; Kolpakov, M.Y.; Vorotyntsev, V.B. Industrial plant for Xenon extraction from tail streams of air fractionating plants. Chem. Petrol. Eng. 2004, 40, 595–599. [Google Scholar] [CrossRef]
- Gordienko, V.A.; Brykin, S.N.; Kuzins, R.E.; Serebryakov, I.S.; Starkova, M.V.; Tairov, T.N. Nuclear power: For or against? Comparative analysis of radioactive contamination from NPP and coal-fired TPP. Moscow Univ. Bull. Ser. 3 Physics Astronomy 2012, 67, 123–130. [Google Scholar]
- Kitani, S.; Takada, J. Adsorption of Krypton and Xenon on Various Adsorbents. J. Nucl. Sci. Technol. 1961, 2, 51–56. [Google Scholar] [CrossRef]
- Chepel, V.; Lopes, M.I.; Solovov, V.; Ferreira Marques, R.; Policarpo, A.J.P.L. Development of liquid xenon detectors for medical imaging. In Technique and Application of Xenon Detectors, 1st ed.; Suzuki, Y., Nakahata, M., Koshio, Y., Moriyama, S., Eds.; World Scientific Publishing Co. Pte. Ltd.: Singapore, Singapore, 2003; pp. 28–40. [Google Scholar]
- Chhandak, A.K.; Israni, R.; Trivedi, A.V. Real Life Application of Xenon: A Critical Review. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2063–2068. [Google Scholar] [CrossRef][Green Version]
- Xenon-Xe. Available online: https://www.niikm.ru/articles/element_articles/xenon/ (accessed on 9 February 2021).
- Rare Gases. Available online: http://chrom-rg.ru/rare-gases/uncategorised/redkie-gazy (accessed on 9 February 2021).
- Xenon Market Size, Share&Covid-19 Impact Analysis, By Application (Imaging and Lighting, Medical, satellite, Electronics&Semiconductors, and others (including R&D), and Regional Forecast, 2020–2027. Available online: https://www.fortunebusinessinsights.com/xenon-market-101965 (accessed on 9 February 2021).
- Price of Xenon Gas. Available online: https://www.alibaba.com/showroom/xenon-gas-price.html (accessed on 10 March 2021).
- Goto, T.; Nakata, Y.; Morita, S. Will Xenon Be a Stranger or a Friend? The Cost, Benefit, and Future of Xenon Anesthesia. Anesthesiology 2003, 98, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Potapov, V.N.; Potapov, S.V.; Shkolin, A.V.; Potapov, A.V. Xenon Concentration Block and Method of its Operation. RU Patent 2 670 635 C9, 24 October 2018. [Google Scholar]
- Dubinin, M.M. Physical Adsorption of Gases and Vapors in Micropores. Progress Surface Membrane Sci. 1975, 9, 1–70. [Google Scholar]
- Dubinin, M.M. Fundamentals of the theory of adsorption in micropores of carbon adsorbents: Characteristics of their adsorption properties and microporous structures. Carbon 1989, 27, 457–467. [Google Scholar] [CrossRef]
- Underhill, D.W.; DiCello, D.C.; Scaglia, L.A.; Watson, J.A. Factors Affecting the Adsorption of Xenon on Activated Carbon. Nucl. Sci. Eng. 1986, 93, 411–414. [Google Scholar] [CrossRef]
- Bazan, R.E.; Bastos-Neto, M.; Moeller, A.; Dreisbach, F.; Staudt, R. Adsorption equilibria of O2, Ar, Kr and Xe on activated carbon and zeolites: Single component and mixture data. Adsorption 2011, 17, 371–383. [Google Scholar] [CrossRef]
- Shkolin, A.V.; Fomkin, A.A.; Potapov, S.V. Adsorption concentrating of xenon. Khim. Techn. 2013, 14, 528–535. [Google Scholar]
- Thallapally, P.K.; Grate, J.W.; Motkuri, R.K. Facile xenon capture and release at room temperature using a metal-organic framework: A comparison with activated charcoal. Chem. Commun. (Camb). 2012, 48, 347–349. [Google Scholar] [CrossRef]
- Fomkin, A.A.; Pulin, A.L. Adsorption deformation of zeolite at xenon high pressure. Russ. Chem Bull. 1996, 45, 321–323. [Google Scholar] [CrossRef]
- Wang, Q.; Wen, S.; Hao, F.; Huang, Z.; Peng, S. Rare Gas Adsorption to Silver-Exchanged Zeolites. Adv. Condens. Matter Phys. 2015. [Google Scholar] [CrossRef]
- Machin, W.D.; Golding, P.D. Adsorption of xenon on silica gel. Part 2.—Hysteresis and capillary phase transitions. Chem. Soc. Faraday Trans. 1990, 86, 175–179. [Google Scholar] [CrossRef]
- Banerjee, D.; Simon, C.M.; Elsaidi, S.K.; Haranczyk, M.; Thallapally, P.K. Xenon Gas Separation and Storage Using Metal-Organic Frameworks. Chem 2018, 4, 466–494. [Google Scholar] [CrossRef]
- Banerjee, D.; Simon, C.M.; Plonka, A.M.; Motkuri, R.K.; Liu, J.; Chen, X.; Smit, B.; Parise, J.B.; Haranczyk, M.; Thallapally, P.K. Metal-organic framework with optimally selective xenon adsorption and separation. Nat. Comm. 2016, 7, 11831. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Reiss, P.S.; Chong, S.Y.; Holden, D.; Jelfs, K.E.; Hasell, T.; Little, M.A.; Kewley, A.; Briggs, M.E.; Stephenson, A.; et al. Separation of rare gases and chiral molecules by selective binding in porous organic cages. Nat. Mater. 2014, 13, 954–960. [Google Scholar] [CrossRef]
- Simon, C.M.; Mercado, R.; Schnell, S.K.; Smit, B.; Haranczyk, M. What Are the Best Materials To Separate a Xenon/Krypton Mixture? Chem. Mater. 2015, 27, 4459–4475. [Google Scholar] [CrossRef]
- Hulvey, Z.; Lawler, K.; Qiao, Z.; Zhou, J. Noble gas adsorption in Copper Trimesate, HKUST-1: An experimental and computational study. J. Phys. Chem. C. 2013, 117, 20116–20126. [Google Scholar] [CrossRef]
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New synthetic routes towards MOF production at scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef]
- DeSantis, D.; Mason, J.A.; James, B.D.; Houchins, C.; Long, J.R.; Veenstra, M. Techno-economic Analysis of Metal−Organic Frameworks for Hydrogen and Natural Gas Storage. Energy Fuels 2017, 31, 2024–2032. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F. Activated Carbon and Adsorption. In Encyclopedia of Materials: Science and Technology, 2nd ed.; Buschow, J.K.H., Cahn, R.W., Flemings, M., Ilschner, B., Kramer, E., Mahajan, S., Veyssierre, P., Eds.; Elsevier: Amsterdam, The Netherland, 2001; pp. 22–34. [Google Scholar]
- Mukhin, V.M.; Tarasov, A.V.; Klushin, V.N. Aktivnie ugli Rossii (Active Carbons of Russia), 1st ed.; Metallurgiya: Moscow, Russia, 2000; p. 352. (In Russian) [Google Scholar]
- Bakaev, V.A. Molecular Theory of Physical Adsorption. Ph.D. Thesis, Moscow State University, Moscow, Russia, May 1990. (In Russian). [Google Scholar]
- Guggenheim, E.A. Modern Thermodynamics by the Methods of Willard Gibbs; Methuen & Co. Ltd.: London, UK, 1933; p. 206. [Google Scholar]
- Guggenheim, E.A. Thermodynamics: An Advanced Treatment for Chemist and Physicist, 5th ed.; North-Holland Publ. Comp.: Amsterdam, The Netherland, 1967; pp. 166–169. [Google Scholar]
- Hill, T.L. Theory of Physical Adsorption in Advances in Catalysis and Related Subjects; Frankerburg, Y.I., Ed.; Acad. Press: New York, NY, USA, 1952; Volume 4, pp. 211–258. [Google Scholar]
- Fedorov, N.F.; Ivakhnyuk, G.K.; Gavrilov, D.N.; Tetenov, V.V.; Smetanin, G.N.; Samonin, V.V.; Babkin, O.E.; Zaitsev, Y.A. Carbon adsorbents and their industrial applications, 1st ed.; Nauka: Moscow, Russia, 1983; p. 20. [Google Scholar]
- Xenon. Available online: https://webbook.nist.gov/cgi/inchi?ID=C7440633&Mask=4 (accessed on 12 January 2021).
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Ravikovitch, P.I.; Vishnyakov, A.; Russo, R.; Neimark, A.V. Unified approach to pore size characterization of microporous carbonaceous materials from N2, Ar, and CO2 adsorption isotherms. Langmuir. 2000, 16, 2311–2320. [Google Scholar] [CrossRef]
- Pribylov, A.A.; Serpinskii, V.V.; Kalashnikov, S.M. Adsorption of Gases by Microporous Adsorbents under Pressures up to Hundreds of Megapascals. Zeolites. 1991, 11, 846–849. [Google Scholar] [CrossRef]
- Shkolin, A.V.; Fomkin, A.A.; Pulin, A.L.; Yakovlev, V.Y. A technique for measuring an adsorption-induced deformation. Instr. Exper. Techn. 2008, 51, 150–155. [Google Scholar] [CrossRef]
- GOST 34100.3-2017/ISO/IEC Guide 98-3:2008. Part 3. Uncertainty of Measurement. Part 3. Guide to the Expression of Uncertainty in Measurement. Available online: https://files.stroyinf.ru/Data/651/65118.pdf (accessed on 18 June 2020).
- Fomkin, A.A.; Seliverstova, I.I.; Serpinskii, V.V. Determination of the parameters of the microprobe structure of solid adsorbents. Communication 1. Method of determination of the specific volume of totally microporous adsorbents. Russ. Chem. Bull. 1986, 35, 256–259. [Google Scholar] [CrossRef]
- Men’shchikov, I.E.; Shkolin, A.V.; Fomkin, A.A. Measurements of adsorption and thermal deformations of microporous carbon adsorbents. Meas. Tech. 2018, 60, 1051–1058. [Google Scholar] [CrossRef]
- ISO 11095:1996. Statistical Methods. Linear Calibration Using Reference Materials. Available online: https://www.iso.org/standard/1060.html (accessed on 6 September 2019).
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Oliver, J.P.; Rodrigues-Reinoso, F.; Rouquerol, J.; Sing, K. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer Science & Business Media: New York, NY, USA; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; p. 101. [Google Scholar]
- Pauling, L. The Nature of the Chemical Bond and the Structure of Molecules and Crystals: An Introduction to Modern Structural Chemistry; Cornell University Press: Ithaca, NY, USA, 1939; p. 429. [Google Scholar]
- Men’shchikov, I.E.; Shiryaev, A.A.; Shkolin, A.V.; Vysotskii, V.V.; Khozina, E.V.; Fomkin, A.A. Carbon adsorbents for methane storage: Genesis, synthesis, porosity, adsorption. Korean, J. Chem. Eng. 2021, 38, 276–291. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F.; Kaneko, K. Nanoporous Materials for Gas Storage; Rodríguez-Reinoso, F., Kaneko, K., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2019; p. 403. [Google Scholar]
- Rutman, A.M.; Skakov, Y.A. Radial distribution functions of atoms and interference functions of partly ordered carbon materials. I. Influence of edge atoms of disperse layers. Sov. Phys. Cryst. 1989, 34, 338–341. [Google Scholar]
- Fujimoto, H. Theoretical X-ray scattering intensity of carbons with turbostratic stacking and AB stacking structures. Carbon 2003, 41, 1585–1592. [Google Scholar] [CrossRef]
- Ishikawa, S.; Saito, T.; Kuwahara, K. Carbon Materials with Nano-sized Pores Derived from Carbides. SEI Tech. Rev. 2016, 82, 152–157. [Google Scholar]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-based supercapacitors produced by activation of graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef]
- Men’shchikov, I.E.; Shkolin, A.V.; Strizhenov, E.M.; Khozina, E.V.; Chugaev, S.S.; Shiryaev, A.A.; Fomkin, A.A.; Zherdev, A.A. Thermodynamic Behaviors of Adsorbed Methane Storage Systems Based on Nanoporous Carbon Adsorbents Prepared from Coconut Shells. Nanomaterials 2020, 10, 2243. [Google Scholar] [CrossRef]
- Men’shchikov, I.E.; Shkolin, A.V.; Khozina, E.V.; Fomkin, A.A. Thermodynamics of Adsorbed Methane Storage Systems Based on Peat-Derived Activated Carbons. Nanomaterials 2020, 10, 1379. [Google Scholar] [CrossRef] [PubMed]
- Fomkin, A.A. Adsorption of gases, vapors, and liquids by microporous adsorbents. Adsorption 2005, 11, 425–436. [Google Scholar] [CrossRef]
- Haines, R.S.; McIntosh, R. Length changes of activated carbon rods caused by adsorption of vapors. J. Chem. Phys. 1947, 15, 28–38. [Google Scholar] [CrossRef]
- Lakhanpal, M.L.; Flood, E.A. Stresses and strains in Adsorbate-adsorbent systems. IV Contraction of activated carbon on adsorption of gases and vapors at low initial pressures. Can. J. Chem. 1957, 35, 887–899. [Google Scholar] [CrossRef]
- Fomkin, A.A.; Regent, N.I.; Sinitsyn, V.A. Adsorption deformation in the microporous carbon adsorbent-benzene system and porous structure of adsorbents. Russ. Chem Bull. 2000, 49, 1012–1016. [Google Scholar] [CrossRef]
- Yakovlev, V.Y.; Fomkin, A.A.; Tvardovski, A.V.; Sinitsyn, V.A.; Pulin, A.L. Adsorption-stimulated deformation of microporous carbon adsorbent. Russ. Chem Bull. 2003, 52, 354–358. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Ciach, A.; Neimark, A. Adsorption-Induced Deformation of Microporous Carbons: Pore Size Distribution Effect. Langmuir 2008, 24, 6603–6608. [Google Scholar] [CrossRef]
- Shkolin, A.V.; Fomkin, A.A. Deformation of AUK microporous carbon adsorbent induced by methane adsorption. Colloid J. 2009, 71, 119–124. [Google Scholar] [CrossRef]
- Potapov, S.V.; Shkolin, A.V.; Fomkin, A.A. Deformation of AUK microporous carbon adsorbent induced by krypton adsorption. Colloid J. 2014, 76, 351–357. [Google Scholar] [CrossRef]
- Shkolin, A.V.; Fomkin, A.A.; Men’shchikov, I.E.; Pulin, A.L.; Yakovlev, V.Y. Adsorption-Induced and Thermal Deformation of Microporous Carbon Adsorbent upon n-Octane adsorption. Colloid J. 2019, 81, 797–803. [Google Scholar] [CrossRef]
- Shkolin, A.V.; Fomkin, A.A.; Men’shchikov, I.E.; Pulin, A.L.; Yakovlev, V.Y. Deformation of AUK Adsorbent and Adsorbate Structure upon n-Octane adsorption. Colloid J. 2019, 81, 613–620. [Google Scholar] [CrossRef]
- Balzer, C.; Brameier, S.; Neimark, A.V.; Reichenauer, G. Deformation of Microporous Carbon during Adsorption of Nitrogen, Argon, Carbon Dioxide, and Water Studied by in Situ Dilatometry. Langmuir 2015, 31, 12512–12519. [Google Scholar] [CrossRef] [PubMed]
- Bering, B.; Krasil’nikova, O.; Sarakhov, A.; Serpinskii, V.; Dubinin, M. Alteration of zeolite granule dimensions under krypton adsorption. Russ. Chem. Bull. 1977, 26, 2258–2261. [Google Scholar] [CrossRef]
- Coudert, F.-X.; Boutin, A.; Fuchs, A.H.; Neimark, A.V. Adsorption Deformation and Structural Transitions in Metal-Organic Frameworks: From the Unit Cell to the Crystal. J. Phys.Chem. Lett. 2013, 4, 3198–3205. [Google Scholar] [CrossRef]
- Neimark, A.V.; Coudert, F.-X.; Boutin, A.; Fuchs, A.H. Stress-Based Model for the Breathing of Metal-Organic Frameworks. J. Phys.Chem. Lett. 2010, 1, 445–449. [Google Scholar] [CrossRef]
- Neimark, A.V.; Grenev, I. Adsorption-Induced Deformation of Microporous Solids: A New Insight from a Century-Old Theory. J. Phys. Chem. C. 2020, 124, 749–755. [Google Scholar] [CrossRef]
- Polanyi, M. Adsorption from the point of view of the of the Third Law of Thermodynamics. Verh. Deut. Phys. Ges. 1914, 16, 1012–1016. [Google Scholar]
- Harris, G.L. Miscellaneous Properties of SiC. In Properties of Silicon Carbide; IET: London, UK, 1995; pp. 9–12. [Google Scholar]
- Stockmeier, M.; Müller, R.; Sakwe, S.A.; Wellmann, P.J.; Mager, A. On the lattice parameters of silicon carbide. J. Appl. Phys. 2009, 105, id033511(1–4). [Google Scholar] [CrossRef]
- Bakaev, V.A. The statistical thermodynamics of adsorption equilibriums in the case of zeolites. Dokl. Acad. Nauk SSSR [Dokl.Chem. (Engl. Transl.)] 1966, 167, 369–372. [Google Scholar]
- Pribylov, A.A.; Murdmaa, K.A. Adsorption of gases onto Polymer Adsorbent MN-270 in the region of supercritical temperatures and pressures. Prot. Met. Phys. Chem. Surf. 2020, 56, 115–121. [Google Scholar] [CrossRef]
- Pribylov, A.A.; Kalinnikova, I.A.; Murdmaa, K.O. Determination of the average heat and characteristic energy from the adsorption isotherm. Russ. Chem. Bull. Int. Ed. 2016, 65, 972–977. [Google Scholar] [CrossRef]
- Liu, B.-Y.; Gong, Y.-J.; Wu, X.-N.; Liu, Q.; Li, W.; Xiong, S.-S.; Hu, S.; Wang, X.-L. Enhanced xenon adsorption and separation with an anionic indium–organic framework by ion exchange with Co 2+. RSC Adv. 2017, 7, 55012–55019. [Google Scholar] [CrossRef]
- Elsaidi, S.K.; Ongari, D.; Xu, W.; Mohamed, M.H.; Haranczyk, M.; Thallapally, P.K. Xenon Recovery at Room Temperature using Metal Organic Frameworks. Chem.-Eur. J. 2017, 23, 10758–10762. [Google Scholar] [CrossRef]
- Fomkin, A.A.; Serpinsky, V.V.; Bering, B.P. Investigation of the adsorption of xenon on NaX zeolite within a broad range of pressures and temperatures. Russ. Chem. Bull. 1975, 24, 1147–1150. [Google Scholar] [CrossRef]
- Deliere, L.; Topin, S.; Coasne, B.; Fontaine, J.-P.; De Vito, S.; Auwer, C.D.; Solari, P.L.; Daniel, C.; Schuurman, Y.; Farrusseng, D. Role of Silver Nanoparticles in Enhanced Xenon Adsorption Using Silver-Loaded Zeolites. J. Phys. Chem. C. 2014, 118, 25032–25040. [Google Scholar] [CrossRef]
- Zhong, S.; Qian Wang, Q.; Dapeng Cao, D. ZIF-Derived Nitrogen-Doped Porous Carbons for Xe Adsorption and Separation. Sci. Rep. 2016, 6, 21295. [Google Scholar] [CrossRef]
- Pickett, S.D.; Nowak, A.K.; Thomas, J.M.; Peterson, B.K.; Swift, J.F.P.; Cheetham, A.K.; den Ouden, C.J.J.; Smit, B.; Post, M.F.M. Mobility of Adsorbed Species in Zeolites: A Molecular Dynamics. Simulation of enon in Silicalite. J. Phys. Chem. 1990, 94, 1233–1236. [Google Scholar]
- Bering, B.P.; Zhukovskaya, E.G.; Rakhmukov, B.K.; Serpinskii, V.V. Adsorption in micropores. Communication 1. Theoretical aspects. Izv. Akademii Nauk SSSR. Ser. Khim. 1967, 8, 1656–1659. (In Russian) [Google Scholar]
- Bering, B.P.; Zhukovskaya, E.G.; Rakhmukov, B.K.; Serpinskii, V.V. Adsorption in micropores. Communication 2. Experimental isosteres of adsorption. Izv. Akademii Nauk SSSR. Ser. Khim. 1967, 16, 1662–1669. [Google Scholar]
- Bering, B.P.; Zhukovskaya, E.G.; Rakhmukov, B.K.; Serpinskii, V.V. Adsorption in micropores. Communication 3. Methods of calculation of parameters of adsorption equilibrium. Izv. Akademii Nauk SSSR. Ser. Khim. 1968, 17, 30–36. [Google Scholar]
- Bülow, M.; Shen, D.; Jale, S. Measurement of sorption equilibria under isosteric conditions: The principles, advantages and limitations. Appl. Surf. Sci. 2002, 196, 157–172. [Google Scholar] [CrossRef]
- Barrer, R.M.; Papadopoulos, R. The sorption of krypton and xenon in zeolites at high pressures and temperatures I. Chabazite. Proc. R. Soc. Lond. Math. Phys. Sci. 1972, 326, 315–330. [Google Scholar]
- Fomkin, A.A. Adsorbzija Ar na Zeolite NaX. In Adsorbzija v Mikroporach; Dubinin, M.M., Serpinsky, V.V., Eds.; Nauka: Moscow, Russia, 1983; pp. 98–99. [Google Scholar]
- Shkolin, A.V.; Fomkin, A.A.; Yakovlev, V.Y. Analysis of adsorption isosteres of gas and vapor on microporous adsorbents. Russ. Chem. Bull. 2007, 56, 393–396. [Google Scholar] [CrossRef]
- Potapov, S.V.; Fomkin, A.A.; Sinitsyn, V.A.; Shkolin, A.V. Nitrogen adsorption by microporous adsorbents in the range of high pressures and supercritical temperatures. Prot. Met. Phys. Chem. Surf. 2010, 46, 519–523. [Google Scholar] [CrossRef]
- Potapov, S.V.; Fomkin, A.A.; Sinitsyn, V.A.; Shkolin, A.V. Krypton adsorption on microporous adsorbents at higher pressures. Prot. Met. Phys. Chem. Surf. 2010, 46, 639–643. [Google Scholar] [CrossRef]
- Shkolin, A.V.; Fomkin, A.A.; Potapov, S.V. Thermodynamics of krypton adsorption on microporous carbon adsorbent at high pressures. Russ. Chem. Bull. 2017, 66, 607–613. [Google Scholar] [CrossRef]
- Men’shchikov, I.E.; Fomkin, A.A.; Strizhenov, E.M.; Zaitsev, D.S.; Tvardovskii, A.V. The energy of adsorption of methane on microporous carbon adsorbents. Prot. Met. Phys. Chem. Surf. 2017, 53, 780–785. [Google Scholar] [CrossRef]
- Shkolin, A.V.; Fomkin, A.A.; Tsivadze, A.Y.; Anuchin, K.M.; Men’shchikov, I.E.; Pulin, A.L. Experimental study and numerical modeling: Methane adsorption in microporous carbon adsorbent over the subcritical and supercritical temperature regions. Prot. Met. Phys. Chem. Surf. 2016, 52, 955–963. [Google Scholar] [CrossRef]
- Tolmachev, A.M. Adsorption of Gases, Vapors, and Solutions: I. Thermodynamics of Adsorption. Prot. Met. Phys. Chem. Phys. Surf. 2010, 46, 955–963. [Google Scholar] [CrossRef]
- Gusev, V.; Fomkin, A.A. High-Pressure Adsorption of Xe on NaX Zeolite by Microcalorimetry and Isosteric Analisis. J. Colloid Interface Sci. 1994, 162, 279–283. [Google Scholar] [CrossRef]
- Handy, B.E.; Sharma, S.B.; Spiewak, B.E.; Dumesic, J.A. A Tian-Calvet heat-flux microcalorimeter for measurement of differential heats of adsorption. Meas. Sci. Technol. 1993, 4, 1350–1356. [Google Scholar]
- Bakaev, V.A. One possible formulation of the thermodynamics of sorption equilibrium. Bull. Acad. Sci. USSR Div. Chem. Sci. 1971, 20, 2516–2520. [Google Scholar] [CrossRef]
- Guggenheim, E.A. The thermodynamics of interfaces in systems of several components. J. Trans. Faraday Soc. 1940, 36, 397–412. [Google Scholar] [CrossRef]
- Lopatkin, A.A. Theoretical Fundamentals of Physical Adsorption; Moscow University Publication: Moscow, Russia, 1983; pp. 112–120. [Google Scholar]
- Tian, Y.; Wu, J. Differential Heat of Adsorption and Isosteres. Langmuir 2017, 33, 996–1003. [Google Scholar] [CrossRef]
- Shekhovtsova, L.G.; Fomkin, A.A. Two methods of describing adsorption equilibrium. Russ. Chem. Bull. 1992, 41, 10–13. [Google Scholar] [CrossRef]
- Shen, D.; Bülow, M.; Siperstein, F.; Engelhard, M.; Myers, A.L. Comparison of Experimental Techniques for Measuring Isosteric Heat of Adsorption. Adsorption. 2000, 6, 275–286. [Google Scholar] [CrossRef]
- Whittaker, P.B.; Wang, X.; Zimmermann, W.; Regenauer-Lieb, K.; Chua, H.T. Predicting the Integral Heat of Adsorption for Gas Physisorption on Microporous and Mesoporous Adsorbents. J. Phys. Chem. C. 2014, 118, 8350–8358. [Google Scholar] [CrossRef]
- Giraldo, L.; Rodriguez-Estupiñán, P.; Carlos Moreno-Piraján, J. Isosteric Heat: Comparative Study between Clausius–Clapeyron, CSK and Adsorption Calorimetry Methods. Processes 2019, 7, 203. [Google Scholar] [CrossRef]
- Šifner, O.; Klomar, J. Thermodynamic properties of xenon from the triple point to 800 K with Pressures up to 350 MPa. J. Phys. Chem. Ref. Data. 1994, 23, 63–152. [Google Scholar] [CrossRef]
- Tykodi, R.J.; Aston, J.G.; Schreiner, G.D.L. Thermodynamic Properties of Neon Adsorbed on Titanium Dioxide. J. Am. Chem. Soc. 1955, 77, 2168–2171. [Google Scholar] [CrossRef]
- Pires, J. Chapter 12. Textural and surface chemistry characterization of zeolites via adsorption phenomena. In Handbook of Surfaces and Interfaces of Materials; Elsevier: Amsterdam, The Netherland, 2001; Volume 2, pp. 481–507. [Google Scholar]
- Chkhaidze, E.V.; Fomkin, A.A.; Serpinskii, V.V.; Tsitsishvili, G.V.; Dubinin, M.M. Methane adsorption on a microporous carbon adsorbent in the precritical and hypercritical regions. Russ. Chem. Bull. 1986, 35, 847–849. [Google Scholar] [CrossRef]
- Shkolin, A.V.; Fomkin, A.A. Thermodynamics of methane adsorption on the microporous carbon adsorbent ACC. Russ. Chem. Bull. 2008, 57, 1799–1805. [Google Scholar] [CrossRef]
- Anuchin, K.M.; Fomkin, A.A.; Korotych, A.P.; Tolmachev, A.M. Adsorption concentration of methane. Dependence of adsorbate density on the width of slit-shaped micropores in activated carbons. Prot. Met. Phys. Chem. Surf. 2014, 50, 173–177. [Google Scholar] [CrossRef]
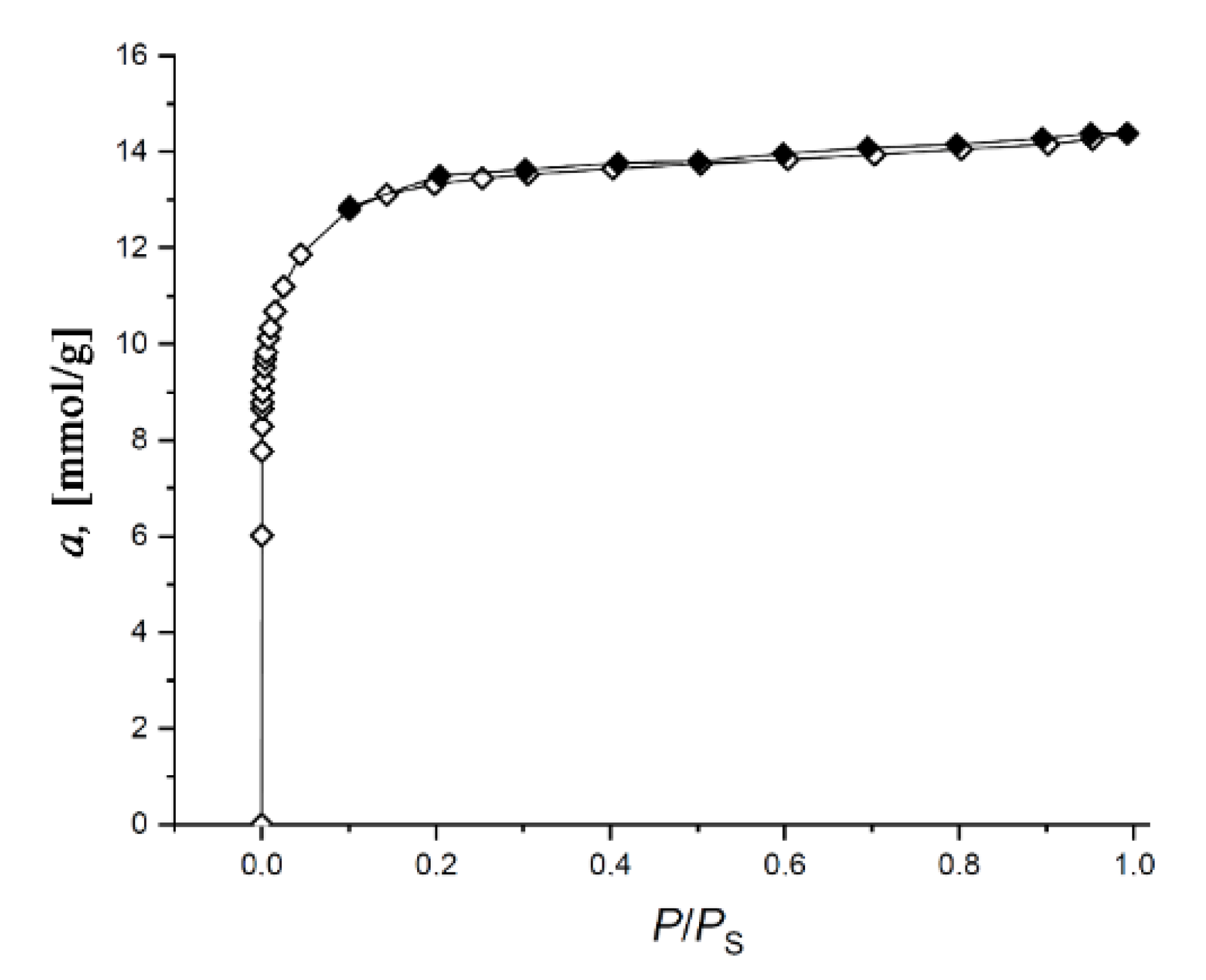
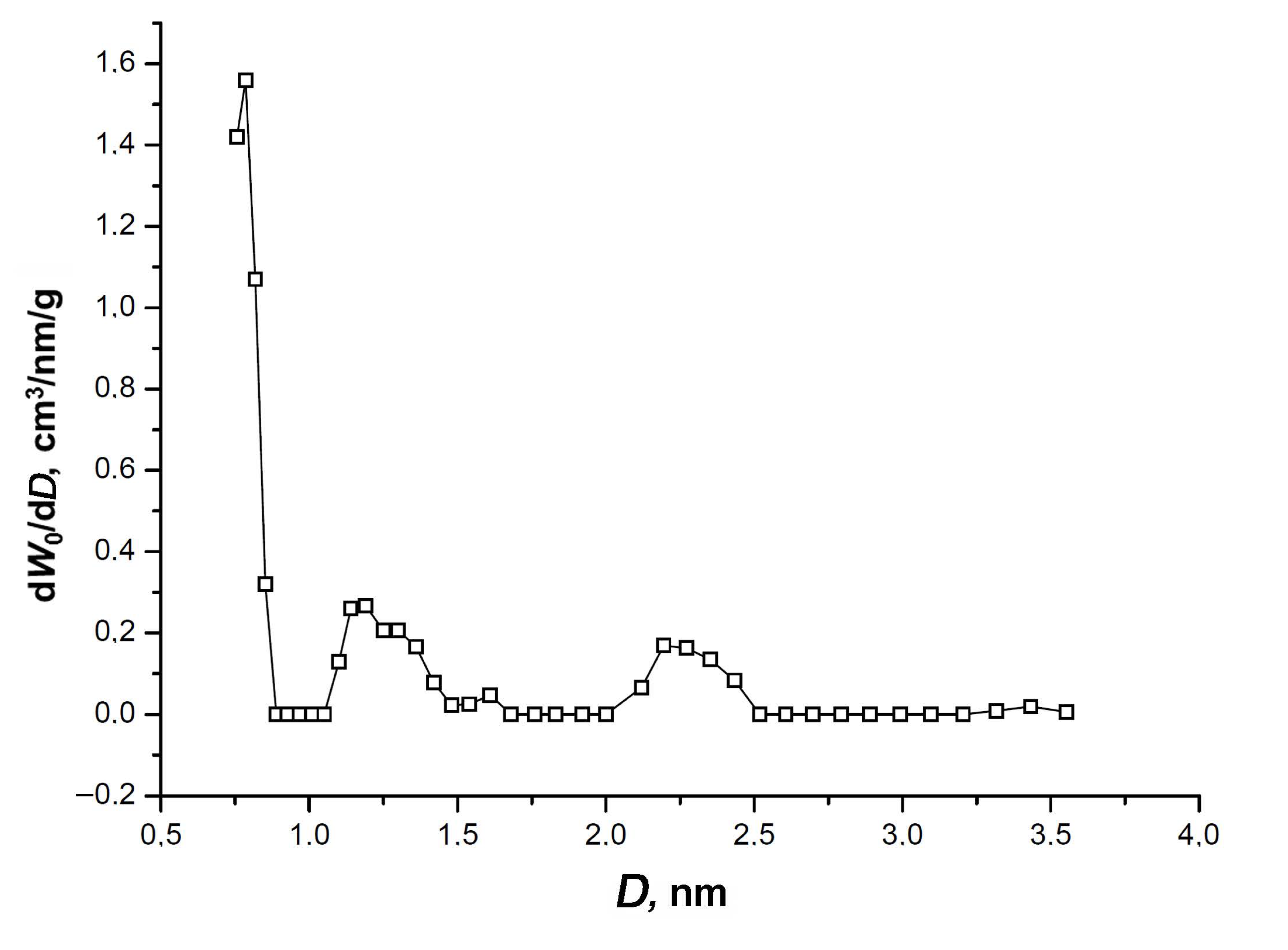
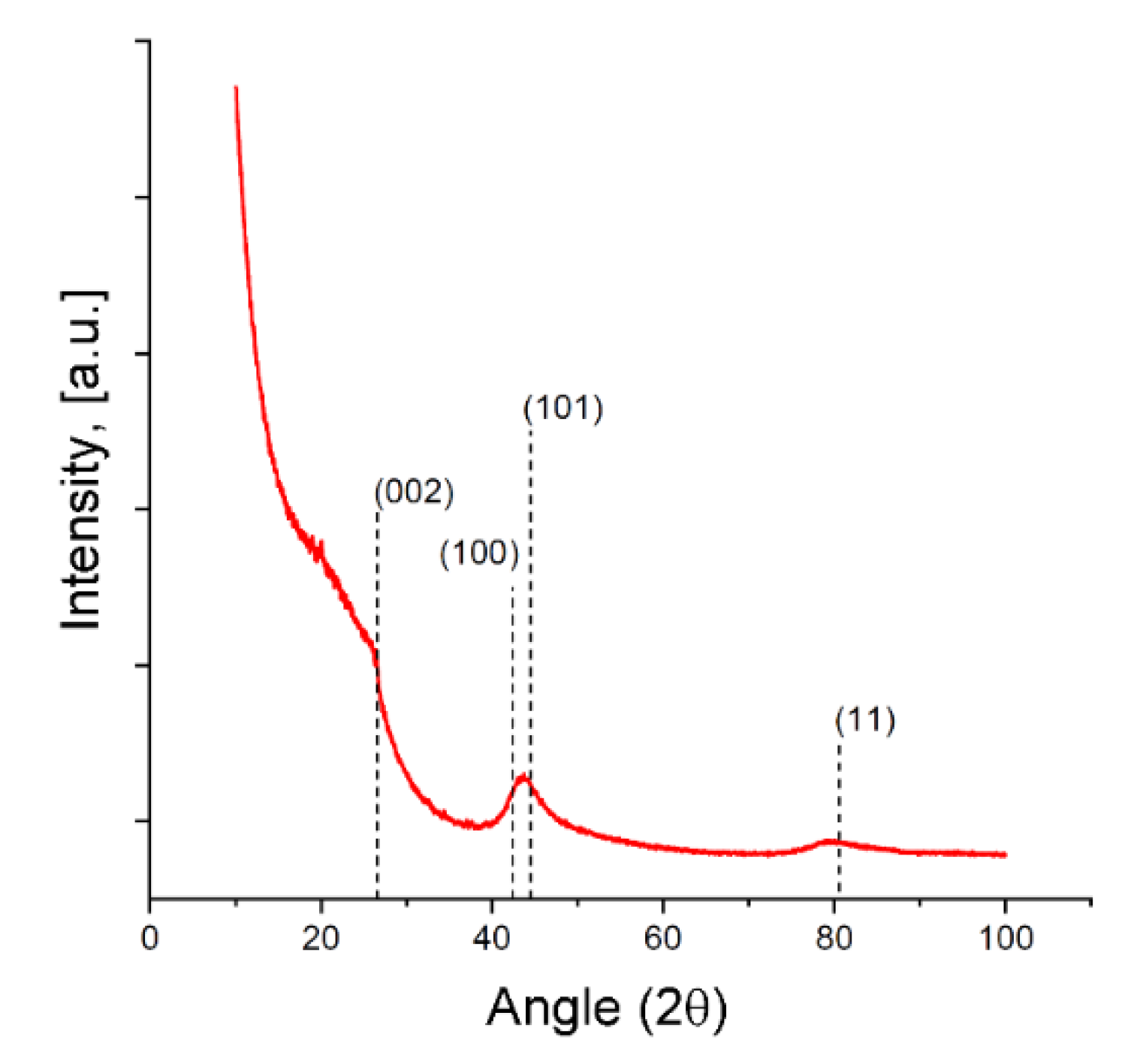


| W0, cm3/g | E0(N2), kJ/mol | E0(C6H6) 1, kJ/mol | x0, nm | SBET, m2/g | Wtotal, cm3/g | Wmeso, cm3/g |
|---|---|---|---|---|---|---|
| 0.48 | 9.6 | 29.2 | 0.41 | 1110 | 0.50 | 0.02 |
| Units | Elements | |||
|---|---|---|---|---|
| C | O | Si | Cl | |
| wt. % | 93.89 | 4.20 | 1.04 | 0.86 |
| at. % | 96.02 | 3.23 | 0.46 | 0.30 |
| No | Adsorbent | Pore Size, D, nm | Xe Adsorption, mmol/g; (T,P-Conditions) | Ref. |
|---|---|---|---|---|
| 1 | Co+2-CPM-6 | 0.54 ≤ D ≤ 0.86 | 3.2 (298K, 1 bar) data | [78] |
| 2 | PCN-12 | 0.78; 0.77; 1.45 | 5.4 (298 K, 1 bar) data | [79] |
| 3 | NiDOBDC | 1.1 | 4.3 (298 K, 1 bar) | [79] |
| 4 | CC3 | 0.36 | 2.2 (298, 1 bar) | [25] |
| 5 | Zeolite Koestrolith 13X-K2 | 0.9 | 4.8 (303 K, 0.9 bar) | [17] |
| 6 | Zeolite NaX | 0.62 | 2.7 (295 K, 1 bar) | [80] |
| 7 | Ag-doped ZSM-5 | 0.5 | 1.8 (298 K, 1 bar) | [81] |
| 8 | SorboNorit B3 | 0.8 | 3.17 (303 K, 0.9 bar) | [17] |
| 9 | Carbon-Zx | 0.2 ≤ D ≤ 2 | 4.42 (298 K, 1 bar) | [82] |
| 10 | SiC-AC | 0.82 | 3.9 (313 K, 1 bar) | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Men’shchikov, I.; Shkolin, A.; Khozina, E.; Fomkin, A. Peculiarities of Thermodynamic Behaviors of Xenon Adsorption on the Activated Carbon Prepared from Silicon Carbide. Nanomaterials 2021, 11, 971. https://doi.org/10.3390/nano11040971
Men’shchikov I, Shkolin A, Khozina E, Fomkin A. Peculiarities of Thermodynamic Behaviors of Xenon Adsorption on the Activated Carbon Prepared from Silicon Carbide. Nanomaterials. 2021; 11(4):971. https://doi.org/10.3390/nano11040971
Chicago/Turabian StyleMen’shchikov, Ilya, Andrey Shkolin, Elena Khozina, and Anatoly Fomkin. 2021. "Peculiarities of Thermodynamic Behaviors of Xenon Adsorption on the Activated Carbon Prepared from Silicon Carbide" Nanomaterials 11, no. 4: 971. https://doi.org/10.3390/nano11040971
APA StyleMen’shchikov, I., Shkolin, A., Khozina, E., & Fomkin, A. (2021). Peculiarities of Thermodynamic Behaviors of Xenon Adsorption on the Activated Carbon Prepared from Silicon Carbide. Nanomaterials, 11(4), 971. https://doi.org/10.3390/nano11040971






