Photodeposition of Silver on Zinc/Calcium Ferrite Nanoparticles: A Contribution to Efficient Effluent Remediation and Catalyst Reutilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Mixed Zinc/Calcium Ferrite Nanoparticles
2.2. Silver Coating by Photodeposition Method
2.3. Structural Characterization
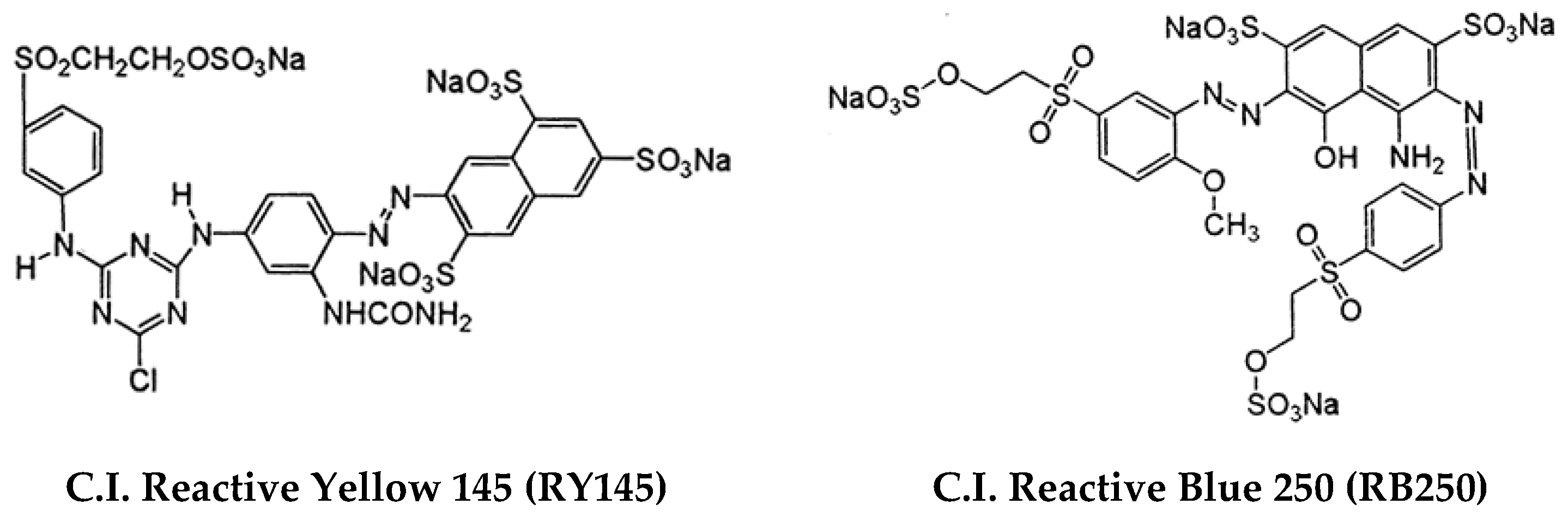
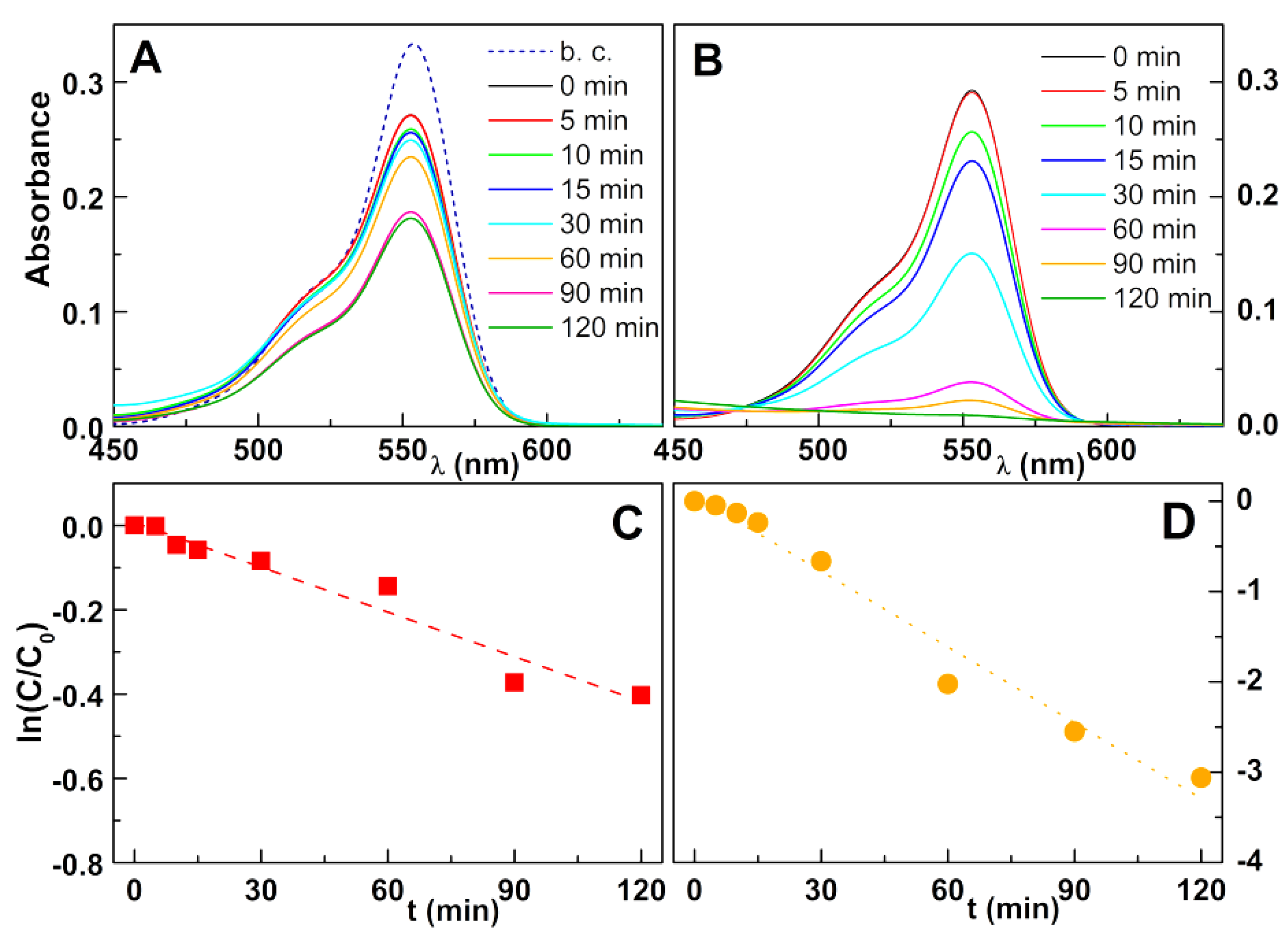
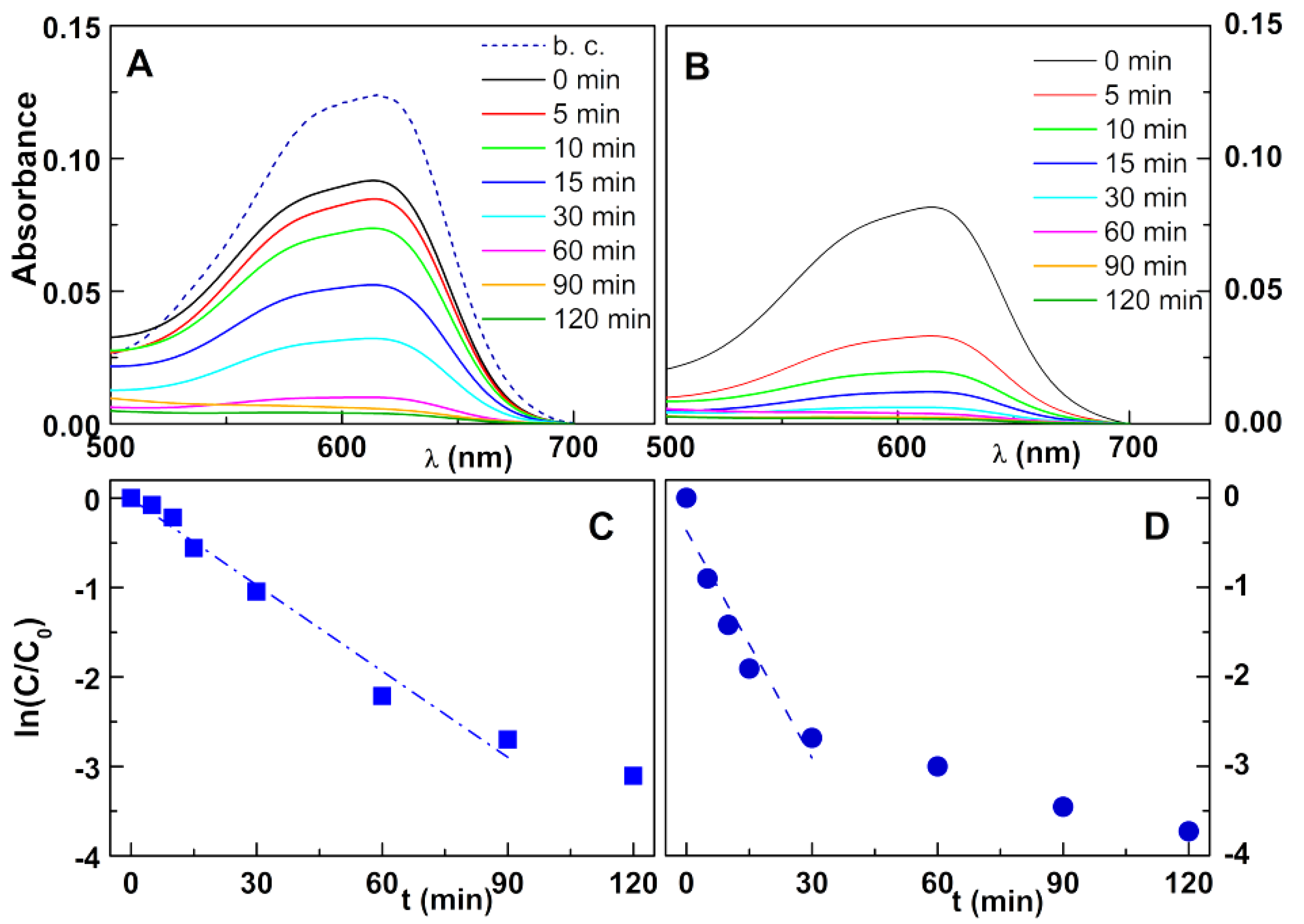
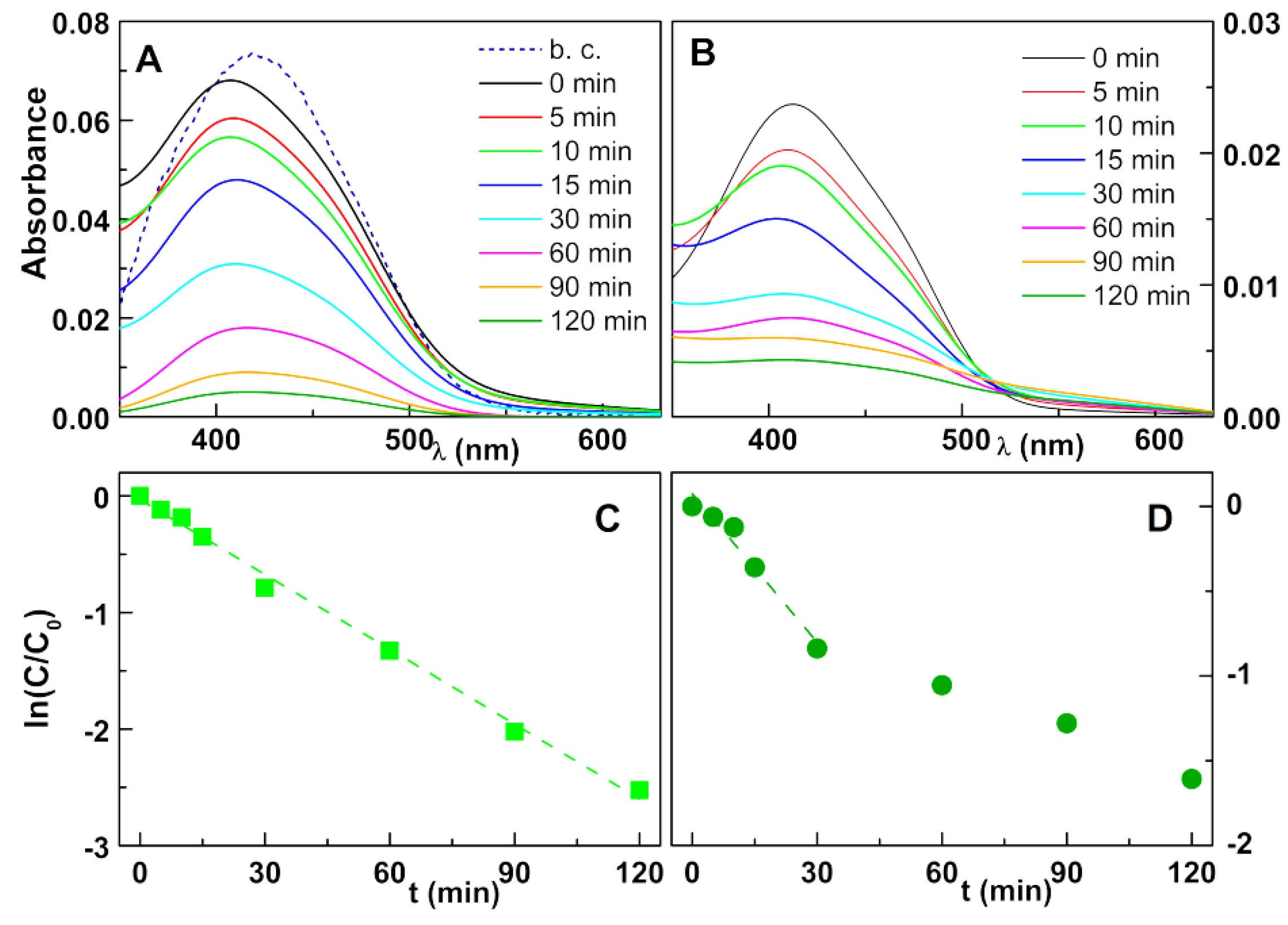
2.4. Photodegradation Assays
3. Results and Discussion
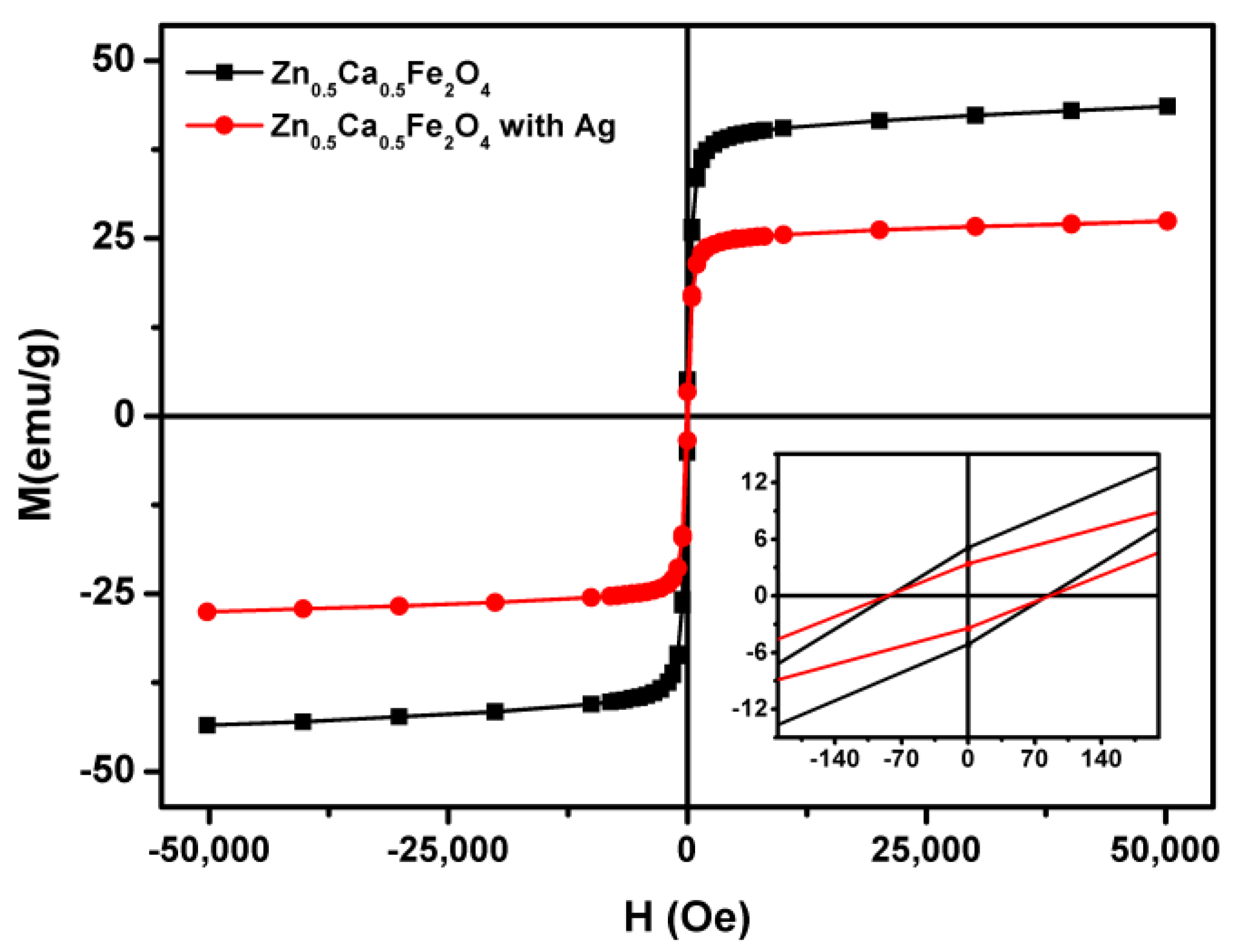
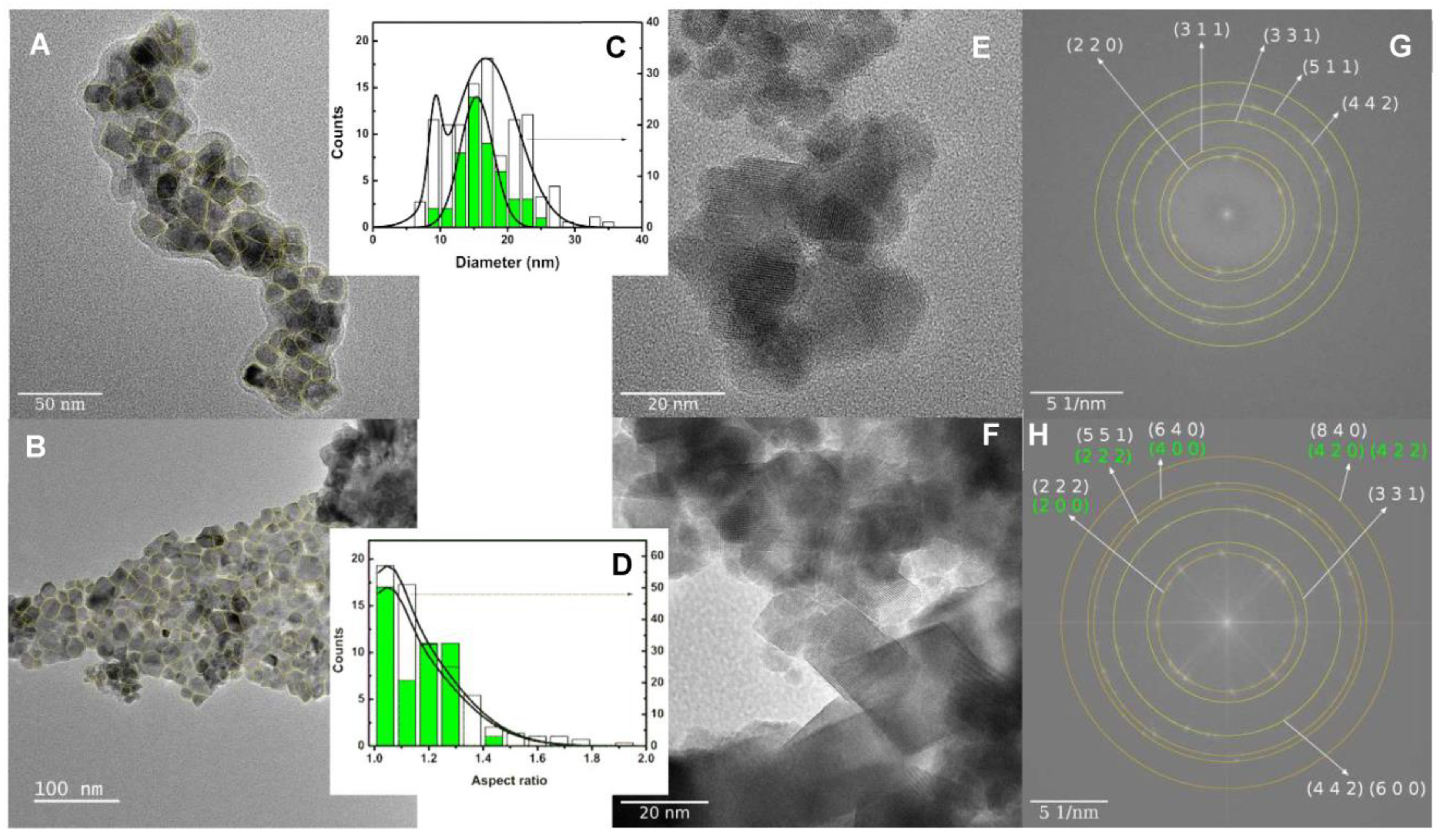
3.1. Nanoparticles Characterization
3.2. Photodegradation Assays
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schweitzer, L.; Noblet, J. Water Contamination and Pollution. In Green Chemistry-An Inclusive Approach; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 3.6; pp. 261–290. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Z. Industrial water pollution, water environment treatment, and health risks in China. Environ. Pollut. 2016, 218, 358–365. [Google Scholar] [CrossRef]
- He, K.; Chen, G.; Zeng, G.; Chen, A.; Huang, Z.; Shi, J.; Huang, T.; Peng, M.; Hu, L. Three-dimensional graphene supported catalysts for organic dyes degradation. Appl. Catal. B Environ. 2018, 228, 19–28. [Google Scholar] [CrossRef]
- Amornpitoksuk, P.; Suwanboon, S.; Randorn, C. Photocatalytic activities of silver compound modified activated carbon@ZnO: Novel ternary composite visible light-driven photocatalysts. Mater. Sci. Semicond. Process. 2017, 84, 50–57. [Google Scholar] [CrossRef]
- Güy, N.; Özacar, M. The influence of noble metals on photocatalytic activity of ZnO for Congo red degradation. Int. J. Hydr. Energy 2016, 41, 20100–20112. [Google Scholar] [CrossRef]
- Kaykhaii, M.; Sasani, M.; Marghzari, S. Removal of Dyes from the Environment by Adsorption Process. Chem. Mater. Eng. 2018, 6, 31–35. [Google Scholar] [CrossRef]
- Li, X.; Xu, P.; Chen, M.; Guangming, Z.; Huang, Z.; Chen, F.; Tang, W.; Chen, C.; Zhang, C.; Tan, X. Application of silver phosphate-based photocatalysts: Barriers and solutions. Chem. Eng. J. 2019, 366, 339–357. [Google Scholar] [CrossRef]
- Nyamukamba, P.; Moloto, M.J.; Mungondori, H. Visible light-active CdS/TiO2 hybrid nanoparticles immobilized on polyacrylonitrile membranes for the photodegradation of dyes in water. J. Nanotechnol. 2019, 2019, 5135618. [Google Scholar] [CrossRef]
- Ambrogi, E.K.; Asenath-Smith, E.; Ballard, W.A.; Moores, L.C.; Brame, J.A. Cross-Comparison of Advanced Oxidation Processes for Remediation of Organic Pollutants in Water Treatment Systems; Technical Report; no. ERDC TR-19-3; Engineer Research and Development Center (USA): Vicksburg, MS, USA, 2019. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Chang, T.-F.M.; Chen, C.-Y.; Sone, M.; Hsu, Y.-J. Mechanistic insights into photodegradation of organic dyes using heterostructure photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef]
- Mahy, J.G.; Wolfs, C.; Mertes, A.; Vreuls, C.; Drot, S.; Smeets, S.; Dircks, S.; Boergers, A.; Tuerk, J.; Lambert, S.D. Advanced photocatalytic oxidation processes for micropollutant elimination from municipal and industrial water. J. Environ. Manag. 2019, 250, 109561. [Google Scholar] [CrossRef]
- Bumajdad, A.; Madkour, M. Understanding the superior photocatalytic activity of noble metals modified titania under UV and visible light irradiation. Phys. Chem. Chem. Phys. 2014, 16, 7146–7158. [Google Scholar] [CrossRef]
- Nanakkal, A.R.; Alexander, L.K. Graphene/BiVO4/TiO2 nanocomposite: Tuning band gap energies for superior photocatalytic activity under visible light. J. Mater. Sci. 2017, 52, 7997–8006. [Google Scholar] [CrossRef]
- Carneiro, J.O.; Teixeira, V.; Portinha, V.A.; Dupák, L.; Magalhães, A.; Coutinho, P. Study of the deposition parameters and Fe-dopant effect in the photocatalytic activity of TiO2 films prepared by dc reactive magnetron sputtering. Vacuum 2005, 78, 37–46. [Google Scholar] [CrossRef]
- Wen, M.Q.; Xiong, T.; Zang, Z.G.; Wei, W.; Tang, W.S.; Dong, F. Synthesis of MoS2/g-C3N4 nanocomposites with enhanced visible-light photocatalytic activity for the removal of nitric oxide (NO). Opt. Express 2016, 24, 10205. [Google Scholar] [CrossRef]
- Fernandes, R.J.C.; Magalhães, C.A.B.; Amorim, C.O.; Amaral, V.S.; Almeida, B.G.; Castanheira, E.M.S.; Coutinho, P.J.G. Magnetic nanoparticles of zinc/calcium ferrite decorated with silver for photodegradation of dyes. Materials 2019, 12, 3582. [Google Scholar] [CrossRef] [PubMed]
- Jasso-Terán, R.A.; Cortés-Hernández, D.A.; Sánchez-Fuentes, H.J.; Reyes-Rodríguez, P.Y.; de-León-Prado, L.E.; Escobedo-Bocardo, J.C.; Almanza-Robles, J.M. Synthesis, characterization and hemolysis studies of Zn(1−x)CaxFe2O4 ferrites synthesized by sol-gel for hyperthermia treatment applications. J. Magn. Magn. Mater. 2017, 427, 241–244. [Google Scholar] [CrossRef]
- Das, A.K.; Govindaraj, R.; Srinivasan, A. Structural and magnetic properties of sol-gel derived CaFe2O4 nanoparticles. J. Magn. Magn. Mater. 2018, 451, 526–531. [Google Scholar] [CrossRef]
- Hufnagel, A.G.; Peters, K.; Müller, A.; Scheu, C.; Fattakhova-Rohlfing, D.; Bein, T. Zinc Ferrite Photoanode Nanomorphologies with Favorable Kinetics for Water-Splitting. Adv. Funct. Mater. 2016, 26, 4435–4443. [Google Scholar] [CrossRef]
- Vaiano, V.; Jaramillo-Paez, C.A.; Matarangolo, M.; Navío, J.A.; Hidalgo, M.C. UV and visible-light driven photocatalytic removal of caffeine using ZnO modified with different noble metals (Pt, Ag and Au). Mater. Res. Bull. 2018, 112, 251–260. [Google Scholar] [CrossRef]
- Cao, X.; Gu, L.; Lan, X.; Zhao, C.; Yao, D.; Sheng, W. Spinel ZnFe2O4 nanoplates embedded with Ag clusters: Preparation, characterization, and photocatalytic application. Mater. Chem. Phys. 2007, 106, 175–180. [Google Scholar] [CrossRef]
- Pica, M.; Nocchetti, M.; Ridolfi, B.; Donnadio, A.; Costantino, F.; Gentili, P.L.; Casciola, M. Nanosized zirconium phosphate/AgCl composite materials: A new synergy for efficient photocatalytic degradation of organic dye pollutants. J. Mater. Chem. A 2015, 3, 5525–5534. [Google Scholar] [CrossRef]
- Nocchetti, M.; Pica, M.; Ridolfi, B.; Donnadio, A.; Boccalon, E.; Zampini, G.; Pietrella, D.; Casciola, M. AgCl-ZnAl layered double hydroxides as catalysts with enhanced photodegradation and antibacterial activities. Inorganics 2019, 7, 120. [Google Scholar] [CrossRef]
- Shirsath, S.E.; Wang, D.; Jadhav, S.S.; Mane, M.L.; Li, S. Ferrites obtained by Sol-Gel Method. In Handbook of Sol-Gel Science and Technology; Klein, L., Aparicio, M., Jitianu, A., Eds.; Springer: Cham, Germany, 2016. [Google Scholar] [CrossRef]
- He, C.; Yu, Y.; Hu, X.; Larbot, A. Influence of silver doping on the photocatalytic activity of titania films. Appl. Surf. Sci. 2002, 200, 239–247. [Google Scholar] [CrossRef]
- Wilhelm, P.; Stephan, D. Photodegradation of rhodamine B in aqueous solution via SiO2@TiO2 nano-spheres. J. Photochem. Photobiol. A Chem. 2007, 185, 19–25. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation of Rhodamine B and methyl orange over boron-doped g-C3N4 under visible light irradiation. Langmuir 2010, 26, 3894–3901. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, R.; Kottam, N.; Girija, C.R.; Nagabhushana, B.M. Photocatalytic degradation of Rhodamine B dye under UV/solar light using ZnO nanopowder synthesized by solution combustion route. Powder Technol. 2012, 215–216, 91–97. [Google Scholar] [CrossRef]
- Samariya, A.; Dolia, S.N.; Prasad, A.S.; Sharma, P.K.; Pareek, S.P.; Dhawan, M.S.; Kumar, S. Size dependent structural and magnetic behaviour of CaFe2O4. Curr. Appl. Phys. 2013, 13, 830–835. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Wu, J. Enhanced Visible-Light Photocatalytic and Antibacterial Activities of Ag-Doped g-C3N4 Nanocomposites. Chem. Sel. 2018, 3, 10630–10636. [Google Scholar] [CrossRef]
- Mogal, S.I.; Gandhi, V.G.; Mishra, M.; Tripathi, S.; Shripathi, T.; Joshi, P.A.; Shah, D.O. Single-Step Synthesis of Silver-Doped Titanium Dioxide: Influence of Silver on Structural, Textural, and Photocatalytic Properties. Ind. Eng. Chem. Res. 2014, 53, 5749–5758. [Google Scholar] [CrossRef]
- Boxia, S.S.; Paria, S. Effect of silver doping on TiO2, CdS, and ZnS nanoparticles for the photocatalytic degradation of metronidazole under visible light. RSC Adv. 2014, 4, 37752–37760. [Google Scholar] [CrossRef]
- Poosinuntakul, N.; Parnklang, T.; Sitiwed, T.; Chaiyo, S.; Kladsomboon, S.; Chailapakul, O.; Apilux, A. Colorimetric assay for determination of Cu(II) ions using L-cysteine functionalized silver nanoplates. Microchem. J. 2020, 158, 105101. [Google Scholar] [CrossRef]
- Dong, C.; Ma, X.; Qiu, N.; Zhang, Y.; Wu, A. An ultra-sensitive colorimetric sensor based on smartphone for pyrophosphate determination. Sens. Act. B 2021, 329, 129066. [Google Scholar] [CrossRef]
- Döbelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystal. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, J.; Friedel, P.; Kleeberg, R. BGMN—A new fundamental parameters based Rietveld program for laboratory X-ray sources, it’s use in quantitative analysis and structure investigations. IUCr Comm. Powder Diffr. Newsl. 1998, 20, 5–8. [Google Scholar]
- Vigneswari, T.; Raji, P. Structural and magnetic properties of calcium doped nickel ferrite nanoparticles by co-precipitation method. J. Molec. Struct. 2017, 1127, 515–521. [Google Scholar] [CrossRef]
- Xu, Z.; Hou, Y.; Sun, S. Magnetic Core/Shell Fe3O4/Au and Fe3O4/Au/Ag Nanoparticles with Tunable Plasmonic Properties. J. Am. Chem. Soc. 2007, 129, 8698–8699. [Google Scholar] [CrossRef]
- Marlina, E.; Goh, S.N.; Wu, T.Y.; Tan, T.; Abd Hamid, S.B.; Juan, J.C. Evaluation on the Photocatalytic Degradation Activity of Reactive Blue 4 using Pure Anatase Nano-TiO2. Sains Malays. 2015, 44, 1011–1019. [Google Scholar] [CrossRef]
- Mohammad, E.J.; Lafta, A.J.; Kahdim, S.K. Photocatalytic removal of reactive yellow 145 dye from simulated textile wastewaters over supported (Co,Ni)3O4/Al2O3 co-catalyst. Pol. J. Chem. Tech. 2016, 18, 1–9. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Z.; Liu, D.; Gao, Z. Preparation of ZnO photocatalyst for the efficient and rapid photocatalytic degradation of azo dyes. Nanoscale Res. Lett. 2017, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, J.; Zeng, M.; Zhao, G.; Xu, J.; Hu, W.; Wang, X. In situ synthesis of water-soluble magnetic graphitic carbon nitride photocatalyst and its synergistic catalytic performance. ACS Appl. Mater. Interfaces 2013, 5, 12735–12743. [Google Scholar] [CrossRef]
- Guo, S.; Yang, Z.; Zhang, H.; Yang, W.; Li, J.; Zhou, K. Enhanced photocatalytic degradation of organic contaminants over CaFe2O4 under visible LED light irradiation mediated by peroxymonosulfate. J. Mater. Sci. Tech. 2021, 62, 34–43. [Google Scholar] [CrossRef]
- Armstrong, D.A.; Huie, R.E.; Lymar, S.; Koppenol, W.H.; Merényi, G.; Neta, P.; Stanbury, D.M.; Steenken, S.; Wardman, P. Standard electrode potentials involving radicals in aqueous solution: Inorganic radicals. Bioinorg. React. Mech. 2013, 9, 59–61. [Google Scholar] [CrossRef]
- Behera, A.; Kandi, D.; Majhi, S.M.; Martha, S.; Parida, K. Facile Synthesis of ZnFe2O4 photocatalysts for decolourization of organic dyes under solar irradiation. Beilstein J. Nanotechnol. 2018, 9, 436–446. [Google Scholar] [CrossRef] [PubMed]

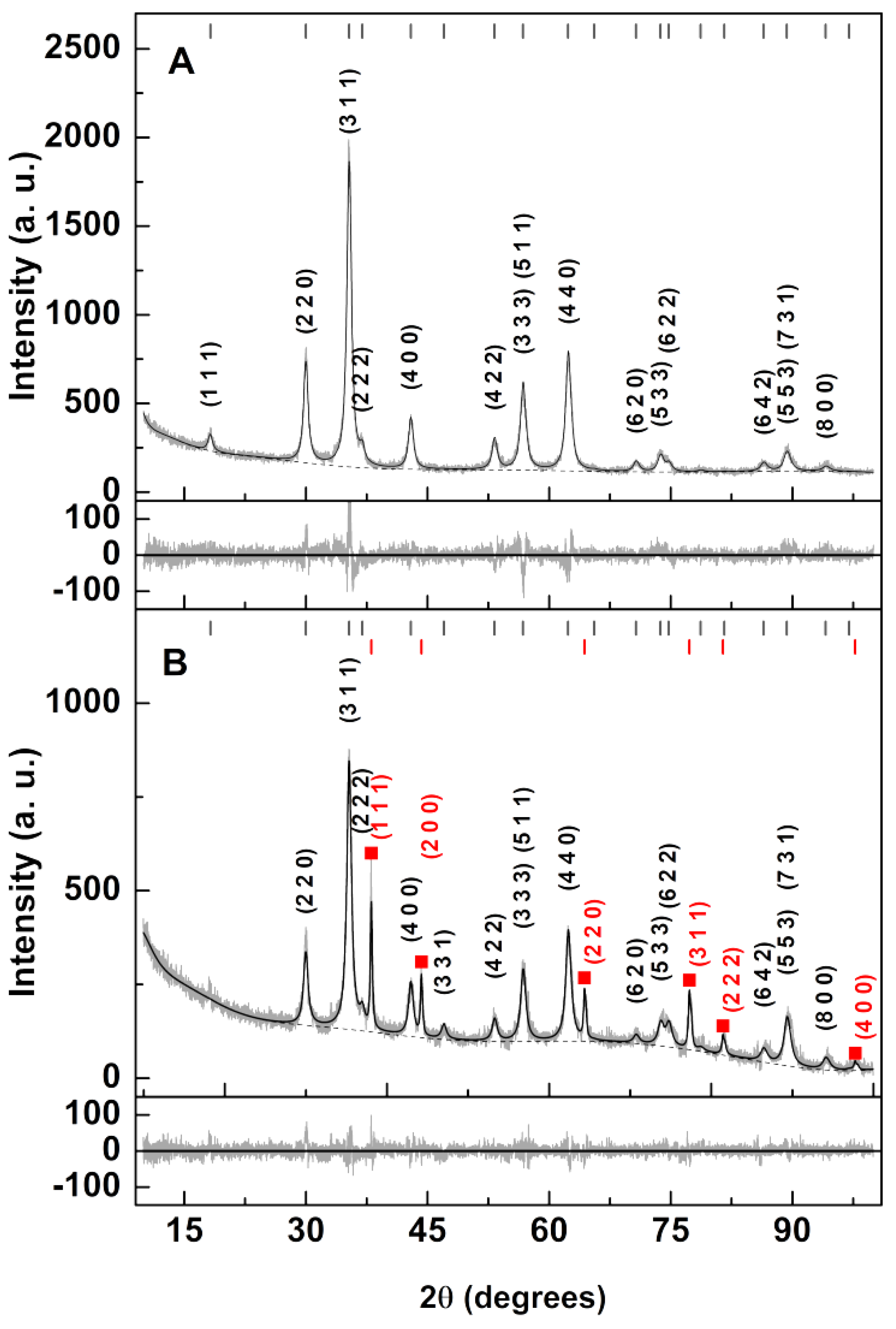

| Sample | Ox,y,z (*) | i (*) | Phase Size (nm) Lattice Constant (nm) Zn/Ca Ferrite|Ag | Ag (wt%) | RP | χ2 |
|---|---|---|---|---|---|---|
| Zn/Ca ferrite | 0.3834 | 1 (+) | 12.3 | ---- 0.8418 | ---- | ----- | 5.89 | 1.16 |
| Zn/Ca ferrite with silver photodeposition | 0.3505 | 1 (+) | 12.4 | 42.4 0.8418(+) | 0.409 | 4.31 | 7.91 | 1.87 |
| Nanoparticles | Ms (emu/g) | Mr (emu/g) | C (Oe) | Mr/Ms |
|---|---|---|---|---|
| Zn0.5Ca0.5Fe2O4 (sol–gel) | 44.22 | 5.03 | 82.66 | 0.11 |
| Zn0.5Ca0.5Fe2O4 (sol–gel)/Ag (photodeposition) | 27.97 | 3.31 | 85.43 | 0.12 |
| k (min−1) | |||
|---|---|---|---|
| Rhodamine B | RB250 | RY145 | |
| Zn0.5Ca0.5Fe2O4 (co-precipitation)/Ag (photodeposition) | 0.0035 | 0.0321 | 0.0214 |
| Zn0.5Ca0.5Fe2O4 (sol–gel)/Ag (photodeposition) | 0.0310 | 0.0847 | 0.0292 |
| Zn0.5Ca0.5Fe2O4 (co-precipitation)/Ag (reflux) [16] | 0.0614 | 0.0104 | 0.0058 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, R.J.C.; Magalhães, C.A.B.; Rodrigues, A.R.O.; Almeida, B.G.; Pires, A.; Pereira, A.M.; Araujo, J.P.; Castanheira, E.M.S.; Coutinho, P.J.G. Photodeposition of Silver on Zinc/Calcium Ferrite Nanoparticles: A Contribution to Efficient Effluent Remediation and Catalyst Reutilization. Nanomaterials 2021, 11, 831. https://doi.org/10.3390/nano11040831
Fernandes RJC, Magalhães CAB, Rodrigues ARO, Almeida BG, Pires A, Pereira AM, Araujo JP, Castanheira EMS, Coutinho PJG. Photodeposition of Silver on Zinc/Calcium Ferrite Nanoparticles: A Contribution to Efficient Effluent Remediation and Catalyst Reutilization. Nanomaterials. 2021; 11(4):831. https://doi.org/10.3390/nano11040831
Chicago/Turabian StyleFernandes, Ricardo J. C., Carlos A. B. Magalhães, Ana Rita O. Rodrigues, Bernardo G. Almeida, Ana Pires, André Miguel Pereira, João Pedro Araujo, Elisabete M. S. Castanheira, and Paulo J. G. Coutinho. 2021. "Photodeposition of Silver on Zinc/Calcium Ferrite Nanoparticles: A Contribution to Efficient Effluent Remediation and Catalyst Reutilization" Nanomaterials 11, no. 4: 831. https://doi.org/10.3390/nano11040831
APA StyleFernandes, R. J. C., Magalhães, C. A. B., Rodrigues, A. R. O., Almeida, B. G., Pires, A., Pereira, A. M., Araujo, J. P., Castanheira, E. M. S., & Coutinho, P. J. G. (2021). Photodeposition of Silver on Zinc/Calcium Ferrite Nanoparticles: A Contribution to Efficient Effluent Remediation and Catalyst Reutilization. Nanomaterials, 11(4), 831. https://doi.org/10.3390/nano11040831







