Suitability of Different Titanium Dioxide Nanotube Morphologies for Photocatalytic Water Treatment
Abstract
1. Introduction
2. Methodology
2.1. Material Synthesis
2.2. Morphology and Structural Analysis
2.3. Optical Properties
2.4. Photocatalytic Activity
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Adán, C.; Marugán, J.; Sánchez, E.; Pablos, C.; van Grieken, R. Understanding the effect of morphology on the photocatalytic activity of TiO2 nanotube array electrodes. Electrochim. Acta 2016, 191, 521–529. [Google Scholar] [CrossRef]
- Zhu, X.-D.; Wang, Y.-J.; Sun, R.-J.; Zhou, D.-M. Photocatalytic degradation of tetracycline in aqueous solution by nanosized TiO2. Chemosphere 2013, 92, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, A.; Fragalà, M.E.; Privitera, V.; Impellizzeri, G. ZnO for application in photocatalysis: From thin films to nanostructures. Mater. Sci. Semicond. Process. 2017, 69, 44–51. [Google Scholar] [CrossRef]
- Humayun, M.; Raziq, F.; Khan, A.; Luo, W. Modification strategies of TiO2 for potential applications in photocatalysis: A critical review. Green Chem. Lett. Rev. 2018, 11, 86–102. [Google Scholar] [CrossRef]
- Yan, H.; Wang, X.; Yao, M.; Yao, X. Band structure design of semiconductors for enhanced photocatalytic activity: The case of TiO2. Prog. Nat. Sci. Mater. Int. 2013, 23, 402–407. [Google Scholar] [CrossRef]
- Du, P.; Carneiro, J.T.; Moulijn, J.A.; Mul, G. A novel photocatalytic monolith reactor for multiphase heterogeneous photocatalysis. Appl. Catal. A Gen. 2008, 334, 119–128. [Google Scholar] [CrossRef]
- Baranowska-Wójcik, E.; Szwajgier, D.; Oleszczuk, P.; Winiarska-Mieczan, A. Effects of Titanium Dioxide Nanoparticles Exposure on Human Health—A Review. Biol. Trace Elem. Res. 2020, 193, 118–129. [Google Scholar] [CrossRef]
- Fernández, A.; Lassaletta, G.; Jiménez, V.M.; Justo, A.; González-Elipe, A.R.; Herrmann, J.M.; Tahiri, H.; Ait-Ichou, Y. Preparation and characterization of TiO2 photocatalysts supported on various rigid supports (glass, quartz and stainless steel). Comparative studies of photocatalytic activity in water purification. Appl. Catal. B Environ. 1995, 7, 49–63. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Kawi, S.; Ray, M.B. Photocatalytic degradation of orange II by TiO2 catalysts supported on adsorbents. Catal. Today 2004, 98, 431–439. [Google Scholar] [CrossRef]
- Sanz, R.; Buccheri, M.A.; Zimbone, M.; Scuderi, V.; Amiard, G.; Impellizzeri, G.; Romano, L.; Privitera, V. Photoactive layered nanocomposites obtained by direct transferring of anodic TiO2 nanotubes to commodity thermoplastics. Appl. Surf. Sci. 2017, 399, 451–462. [Google Scholar] [CrossRef]
- Zwilling, V.; Darque-Ceretti, E.; Boutry-Forveille, A.; David, D.; Perrin, M.Y.; Aucouturier, M. Structure and physicochemistry of anodic oxide films on titanium and TA6V alloy. Surf. Interface Anal. 1999, 27, 629–637. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Macak, J.M.; Ghicov, A.; Taveira, L.; Schmuki, P. Self-organized porous TiO2 and ZrO2 produced by anodization. Corros. Sci. 2005, 47, 3324–3335. [Google Scholar] [CrossRef]
- Padiyan, D.P.; Raja, D.H. Synthesis of Various Generations Titania Nanotube Arrays by Electrochemical Anodization for H2 Production. Energy Procedia 2012, 22, 88–100. [Google Scholar] [CrossRef]
- Fu, Y.; Mo, A. A Review on the Electrochemically Self-organized Titania Nanotube Arrays: Synthesis, Modifications, and Biomedical Applications. Nanoscale Res. Lett. 2018, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Panaitescu, E.; Richter, C.; Menon, L. A Study of Titania Nanotube Synthesis in Chloride-Ion-Containing Media. J. Electrochem. Soc. 2008, 155, E7–E13. [Google Scholar] [CrossRef]
- Cheong, Y.L.; Yam, F.K.; Ng, S.; Hassan, Z.; Ng, S.S.; Low, I. Fabrication of titanium dioxide nanotubes in fluoride-free electrolyte via rapid breakdown anodization. J. Porous Mater. 2015, 22, 1437–1444. [Google Scholar] [CrossRef]
- Robertson, J.M.; Robertson, P.K.; Lawton, L.A. A comparison of the effectiveness of TiO2 photocatalysis and UVA photolysis for the destruction of three pathogenic micro-organisms. J. Photochem. Photobiol. A Chem. 2005, 175, 51–56. [Google Scholar] [CrossRef]
- Nagamine, S.; Inohara, K. Photocatalytic microreactor using anodized TiO2 nanotube array. Adv. Powder Technol. 2018, 29, 3100–3106. [Google Scholar] [CrossRef]
- Teodorescu-Soare, C.T.; Catrinescu, C.; Dobromir, M.; Stoian, G.; Arvinte, A.; Luca, D. Growth and characterization of TiO2 nanotube arrays under dynamic anodization. Photocatalytic activity. J. Electroanal. Chem. 2018, 823, 388–396. [Google Scholar] [CrossRef]
- Riboni, F.; Nguyen, N.T.; So, S.; Schmuki, P. Aligned metal oxide nanotube arrays: Key-aspects of anodic TiO2 nanotube formation and properties. Nanoscale Horiz. 2016, 1, 445–466. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Lincho, J.; Domingues, E.; Gmurek, M.; Mazierski, P.; Zaleska-Medynska, A.; Klimczuk, T.; Quinta-Ferreira, R.M.; Martins, R.C. TiO2 nanotube arrays-based reactor for photocatalytic oxidation of parabens mixtures in ultrapure water: Effects of photocatalyst properties, operational parameters and light source. Sci. Total Environ. 2019, 689, 79–89. [Google Scholar] [CrossRef]
- McMichael, S.; Waso, M.; Reyneke, B.; Khan, W.; Byrne, J.A.; Fernandez-Ibanez, P. Electrochemically assisted photocatalysis for the disinfection of rainwater under solar irradiation. Appl. Catal. B Environ. 2021, 281, 119485. [Google Scholar] [CrossRef]
- Kim, H.-I.; Kim, D.; Kim, W.; Ha, Y.-C.; Sim, S.-J.; Kim, S.; Choi, W. Anodic TiO2 nanotube layer directly formed on the inner surface of Ti pipe for a tubular photocatalytic reactor. Appl. Catal. A Gen. 2016, 521, 174–181. [Google Scholar] [CrossRef]
- Bauer, S.; Kleber, S.; Schmuki, P. TiO2 nanotubes: Tailoring the geometry in H3PO4/HF electrolytes. Electrochem. Commun. 2006, 8, 1321–1325. [Google Scholar] [CrossRef]
- Ghicov, A.; Tsuchiya, H.; Macak, J.M.; Schmuki, P. Titanium oxide nanotubes prepared in phosphate electrolytes. Electrochem. Commun. 2005, 7, 505–509. [Google Scholar] [CrossRef]
- Jaroenworaluck, A.; Regonini, D.; Bowen, C.R.; Stevens, R.; Allsopp, D. Macro, micro and nanostructure of TiO2 anodised films prepared in a fluorine-containing electrolyte. J. Mater. Sci. 2007, 42, 6729–6734. [Google Scholar] [CrossRef]
- Regonini, D.; Bowen, C.R.; Stevens, R.; Allsopp, D.; Jaroenworaluck, A. Anodised TiO2 nano-tubes: Voltage ramp influence on the nano-structured oxide and investigation of phase changes promoted by thermal treatments. Phys. Status Solidi (a) 2007, 204, 1814–1819. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.; Oh, C.; Ryu, J.; Kim, H.; Park, E.; No, K.; Hong, S. Effects of NH(4)F and distilled water on structure of pores in TiO(2) nanotube arrays. Sci. Rep. 2018, 8, 12487. [Google Scholar] [CrossRef]
- Fang, D.; Luo, Z.; Huang, K.; Lagoudas, D.C. Effect of heat treatment on morphology, crystalline structure and photocatalysis properties of TiO2 nanotubes on Ti substrate and freestanding membrane. Appl. Surf. Sci. 2011, 257, 6451–6461. [Google Scholar] [CrossRef]
- Barışçı, S.; Turkay, O.; Dimoglo, A. Review on Greywater Treatment and Dye Removal from Aqueous Solution by Ferrate (VI). In Ferrites and Ferrates: Chemistry and Applications in Sustainable Energy and Environmental Remediation; American Chemical Society: Washington, DC, USA, 2016; Volume 1238, pp. 349–409. [Google Scholar]
- Aoudjit, F.; Cherifi, O.; Halliche, D. Simultaneously efficient adsorption and photocatalytic degradation of sodium dodecyl sulfate surfactant by one-pot synthesized TiO2/layered double hydroxide materials. Sep. Sci. Technol. 2019, 54, 1095–1105. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Nabeel, F.; Adeel, M.; Iqbal, H.M.N. Environmentally-related contaminants of high concern: Potential sources and analytical modalities for detection, quantification, and treatment. Environ. Int. 2019, 122, 52–66. [Google Scholar] [CrossRef]
- Turner, R.D.R.; Warne, M.S.J.; Dawes, L.A.; Thompson, K.; Will, G.D. Greywater irrigation as a source of organic micro-pollutants to shallow groundwater and nearby surface water. Sci. Total Environ. 2019, 669, 570–578. [Google Scholar] [CrossRef]
- Van Grieken, R.; Marugán, J.; Pablos, C.; Furones, L.; Lopez, A. Comparison between the photocatalytic inactivation of Gram-positive E. faecalis and Gram-negative E. coli faecal contamination indicator microorganisms. Appl. Catal. B Environ. 2010, 100, 212–220. [Google Scholar] [CrossRef]
- Dunlop, P.S.M.; Sheeran, C.P.; Byrne, J.A.; McMahon, M.A.S.; Boyle, M.A.; McGuigan, K.G. Inactivation of clinically relevant pathogens by photocatalytic coatings. J. Photochem. Photobiol. A Chem. 2010, 216, 303–310. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- ASTM. G173-03, Standard Tables for Reference Solar Spectral Irradiances: Direct Normal and Hemispherical on 37 Tilted Surface; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- I.O.f. Standardisation. Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Determination of Photocatalytic Activity of Surfaces in an Aqueous Medium by Degradation of Methylene Blue; I.O.f. Standardisation: Winterthur, Switzerland, 2010. [Google Scholar]
- Verhoeven, J.W. Glossary of terms used in photochemistry (IUPAC Recommendations 1996). Pure Appl. Chem. 1996, 68, 2223. [Google Scholar] [CrossRef]
- Chen, B.; Hou, J.; Lu, K. Formation Mechanism of TiO2 Nanotubes and Their Applications in Photoelectrochemical Water Splitting and Supercapacitors. Langmuir 2013, 29, 5911–5919. [Google Scholar] [CrossRef]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Mukherjee, N.; Grimes, C.A. Fabrication of tapered, conical-shaped titania nanotubes. J. Mater. Res. 2011, 18, 2588–2593. [Google Scholar] [CrossRef]
- Acevedo-Peña, P.; González, I. TiO2 Nanotubes Formed in Aqueous Media: Relationship between Morphology, Electrochemical Properties and Photoelectrochemical Performance for Water Oxidation. J. Electrochem. Soc. 2013, 160, H452–H458. [Google Scholar] [CrossRef]
- Lockman, Z.; Sreekantan, S.; Ismail, S.; Schmidt-Mende, L.; MacManus-Driscoll, J.L. Influence of anodisation voltage on the dimension of titania nanotubes. J. Alloys Compd. 2010, 503, 359–364. [Google Scholar] [CrossRef]
- Gulati, K.; Santos, A.; Findlay, D.; Losic, D. Optimizing Anodization Conditions for the Growth of Titania Nanotubes on Curved Surfaces. J. Phys. Chem. C 2015, 119, 16033–16045. [Google Scholar] [CrossRef]
- Yang, F.; Feng, X.; Ge, F.; Zhang, T.; Qi, J.; Li, D.; Zhu, X. Rapid growth of titanium oxide nanotubes under the critical breakdown voltage: Evidence against the dissolution reaction of fluoride ions. Electrochem. Commun. 2019, 103, 17–21. [Google Scholar] [CrossRef]
- Marien, C.B.D.; Cottineau, T.; Robert, D.; Drogui, P. TiO2 Nanotube arrays: Influence of tube length on the photocatalytic degradation of Paraquat. Appl. Catal. B Environ. 2016, 194, 1–6. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Q.; Zheng, C.; Yan, W.; Chu, W. Application of ultrasonic wave to clean the surface of the TiO2 nanotubes prepared by the electrochemical anodization. Appl. Surf. Sci. 2011, 257, 8478–8480. [Google Scholar] [CrossRef]
- Zhuang, H.-F.; Lin, C.-J.; Lai, Y.-K.; Sun, L.; Li, J. Some Critical Structure Factors of Titanium Oxide Nanotube Array in Its Photocatalytic Activity. Environ. Sci. Technol. 2007, 41, 4735–4740. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.M.; Aljaber, A.S.; AlQaradawi, S.Y.; Allam, N.K. TiO2 nanotubes with ultrathin walls for enhanced water splitting. Chem. Commun. 2015, 51, 12617–12620. [Google Scholar] [CrossRef] [PubMed]
- Buckeridge, J.; Butler, K.T.; Catlow, C.R.A.; Logsdail, A.J.; Scanlon, D.O.; Shevlin, S.A.; Woodley, S.M.; Sokol, A.A.; Walsh, A. Polymorph Engineering of TiO2: Demonstrating How Absolute Reference Potentials Are Determined by Local Coordination. Chem. Mater. 2015, 27, 3844–3851. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Z.; Li, Y.; Wang, Q.; Wan, L. Photocatalytic degradation of methylene blue in a sparged tube reactor with TiO2 fibers prepared by a properly two-step method. Catal. Commun. 2008, 9, 1178–1183. [Google Scholar] [CrossRef]
- Lu, W.L.; Wang, N.; Gao, P.; Li, C.Y.; Zhao, H.S.; Zhang, Z.T. Effects of anodic titanium dioxide nanotubes of different diameters on macrophage secretion and expression of cytokines and chemokines. Cell Prolif. 2015, 48, 95–104. [Google Scholar] [CrossRef]
- Balaur, E.; Macak, J.M.; Tsuchiya, H.; Schmuki, P. Wetting behaviour of layers of TiO2 nanotubes with different diameters. J. Mater. Chem. 2005, 15, 4488–4491. [Google Scholar] [CrossRef]
- Wang, R.; Sakai, N.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Studies of Surface Wettability Conversion on TiO2 Single-Crystal Surfaces. J. Phys. Chem. B 1999, 103, 2188–2194. [Google Scholar] [CrossRef]
- Sakai, N.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Quantitative Evaluation of the Photoinduced Hydrophilic Conversion Properties of TiO2 Thin Film Surfaces by the Reciprocal of Contact Angle. J. Phys. Chem. B 2003, 107, 1028–1035. [Google Scholar] [CrossRef]
- Sun, R.-D.; Nakajima, A.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Photoinduced Surface Wettability Conversion of ZnO and TiO2 Thin Films. J. Phys. Chem. B 2001, 105, 1984–1990. [Google Scholar] [CrossRef]
- Roy, P.; Kim, D.; Lee, K.; Spiecker, E.; Schmuki, P. TiO2 nanotubes and their application in dye-sensitized solar cells. Nanoscale 2010, 2, 45–59. [Google Scholar] [CrossRef]
- Liang, H.; Han, H.; Wang, F.; Cheng, Z.; Lin, B.; Pan, Y.; Tan, J. Experimental investigation on spectral splitting of photovoltaic/thermal hybrid system with two-axis sun tracking based on SiO2/TiO2 interference thin film. Energy Convers. Manag. 2019, 188, 230–240. [Google Scholar] [CrossRef]
- Serpone, N. Is the Band Gap of Pristine TiO2 Narrowed by Anion- and Cation-Doping of Titanium Dioxide in Second-Generation Photocatalysts? J. Phys. Chem. B 2006, 110, 24287–24293. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-J.; Yu, W.-Y.; Lu, Y.-T.; Chien, S.-H. Fabrication of open-ended high aspect-ratio anodic TiO2 nanotube films for photocatalytic and photoelectrocatalytic applications. Chem. Commun. 2008, 45, 6031–6033. [Google Scholar] [CrossRef]
- Pasikhani, J.V.; Gilani, N.; Pirbazari, A.E. The effect of the anodization voltage on the geometrical characteristics and photocatalytic activity of TiO2 nanotube arrays. Nano-Struct. Nano-Objects 2016, 8, 7–14. [Google Scholar] [CrossRef]
- Mills, A. An overview of the methylene blue ISO test for assessing the activities of photocatalytic films. Appl. Catal. B Environ. 2012, 128, 144–149. [Google Scholar] [CrossRef]
- Valeeva, A.A.; Kozlova, E.A.; Vokhmintsev, A.S.; Kamalov, R.V.; Dorosheva, I.B.; Saraev, A.A.; Weinstein, I.A.; Rempel, A.A. Nonstoichiometric titanium dioxide nanotubes with enhanced catalytical activity under visible light. Sci. Rep. 2018, 8, 9607. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.G.; Devi, L.G. Review on Modified TiO2 Photocatalysis under UV/Visible Light: Selected Results and Related Mechanisms on Interfacial Charge Carrier Transfer Dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, H.C.; Severino, D.; Dias, L.G.; Gugliotti, M.S.; Baptista, M.S. Modulation of methylene blue photochemical properties based on adsorption at aqueous micelle interfaces. Phys. Chem. Chem. Phys. 2002, 4, 2320–2328. [Google Scholar] [CrossRef]
- Pan, L.; Zou, J.-J.; Liu, X.-Y.; Liu, X.-J.; Wang, S.; Zhang, X.; Wang, L. Visible—Light—Induced Photodegradation of Rhodamine B over Hierarchical TiO2: Effects of Storage Period and Water-Mediated Adsorption Switch. Ind. Eng. Chem. Res. 2012, 51, 12782–12786. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, V. TiO2 mediated photocatalytic degradation studies of Reactive Red 198 by UV irradiation. J. Hazard. Mater. 2007, 141, 230–236. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Grätzel, M. Conversion and Storage of Solar Energy using Dye-sensitized Nanocrystalline TiO2 Cells. In Comprehensive Coordination Chemistry II; McCleverty, J.A., Meyer, T.J., Eds.; Pergamon: Oxford, UK, 2003; pp. 719–758. [Google Scholar] [CrossRef]
- Villota, N.; Lomas, J.M.; Camarero, L.M. Study of the paracetamol degradation pathway that generates color and turbidity in oxidized wastewaters by photo-Fenton technology. J. Photochem. Photobiol. A Chem. 2016, 329, 113–119. [Google Scholar] [CrossRef]
- Wang, W.; Huang, G.; Yu, J.C.; Wong, P.K. Advances in photocatalytic disinfection of bacteria: Development of photocatalysts and mechanisms. J. Environ. Sci. 2015, 34, 232–247. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, L.; Zhang, J.; Aslan, H.; Dong, M. Photoactive antimicrobial nanomaterials. J. Mater. Chem. B 2017, 5, 8631–8652. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Li, M.; Yin, J.-J.; Wamer, W.G.; Lo, Y.M. Mechanistic characterization of titanium dioxide nanoparticle-induced toxicity using electron spin resonance. J. Food Drug Anal. 2014, 22, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Anastasescu, C.; Negrila, C.; Angelescu, D.G.; Atkinson, I.; Anastasescu, M.; Spataru, N.; Zaharescu, M.; Balint, I. Particularities of photocatalysis and formation of reactive oxygen species on insulators and semiconductors: Cases of SiO2, TiO2 and their composite SiO2–TiO2. Catal. Sci. Technol. 2018, 8, 5657–5668. [Google Scholar] [CrossRef]
- Lia, F.; Farrugia, C.; Buccheri, M.A.; Rappazzo, G.; Zammit, E.; Rizzo, A.; Grech, M.; Refalo, P.; Abela, S. Effect of the Surface Morphology of TiO2 Nanotubes on Photocatalytic Efficacy Using Electron-Transfer-Based Assays and Antimicrobial Tests. Appl. Sci. 2020, 10, 5243. [Google Scholar] [CrossRef]
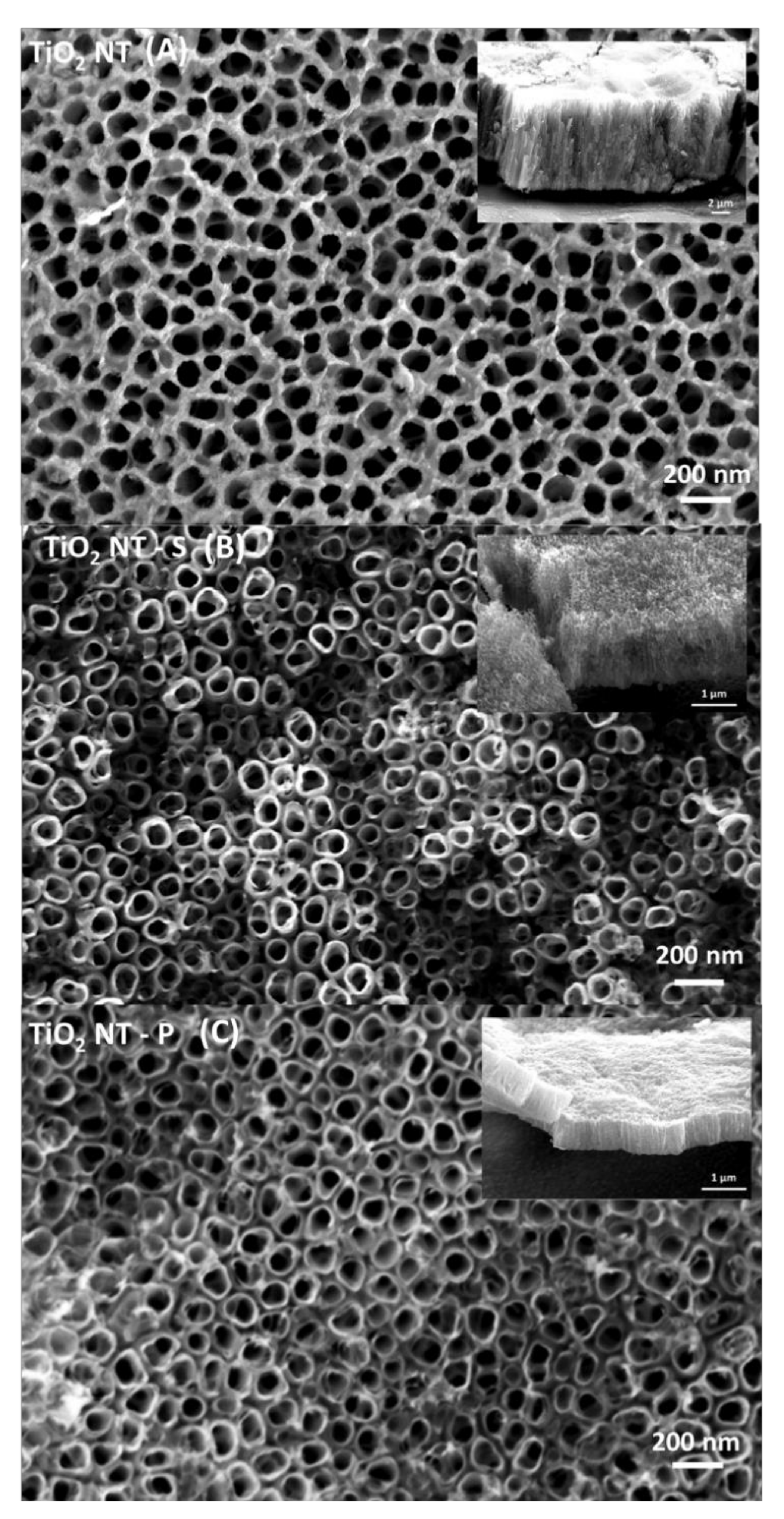
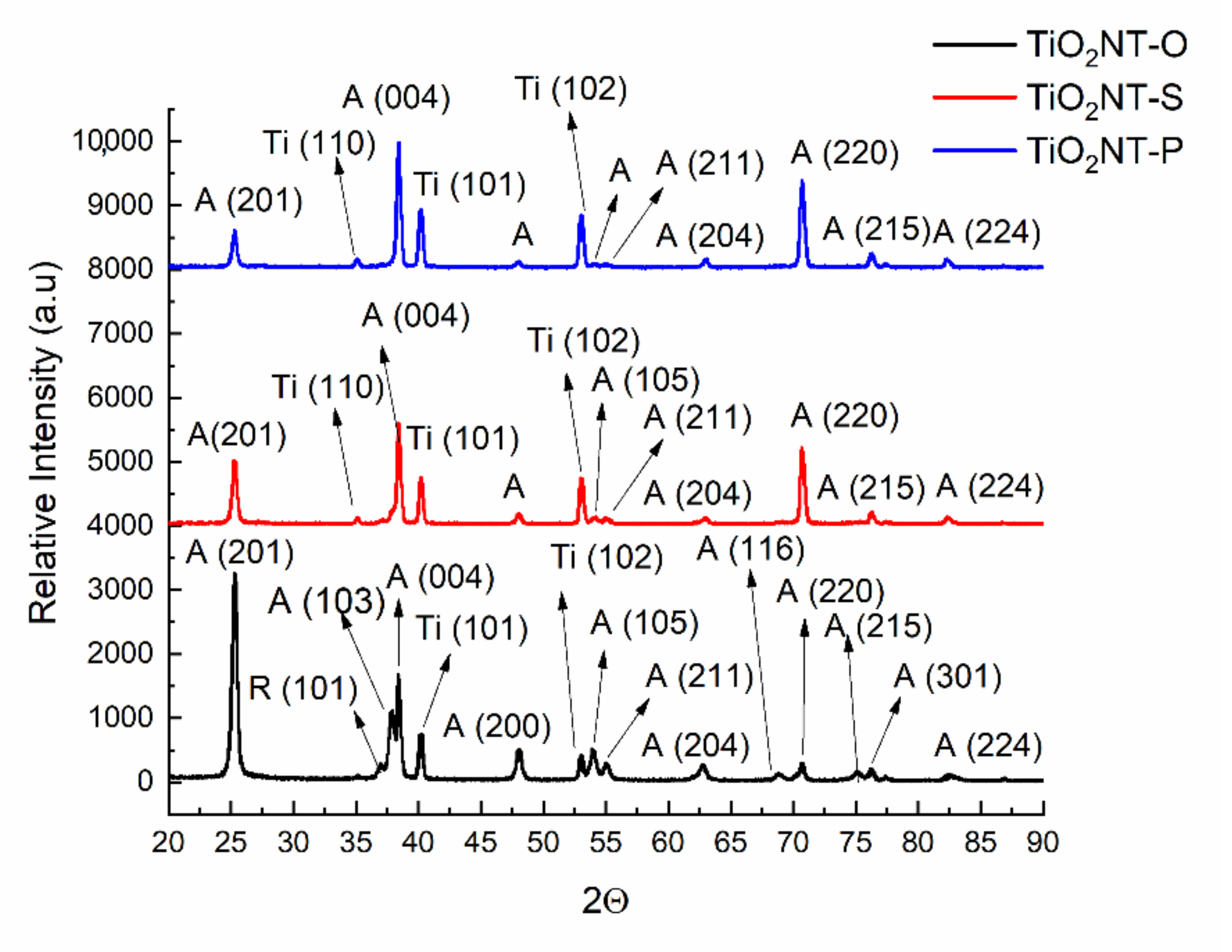
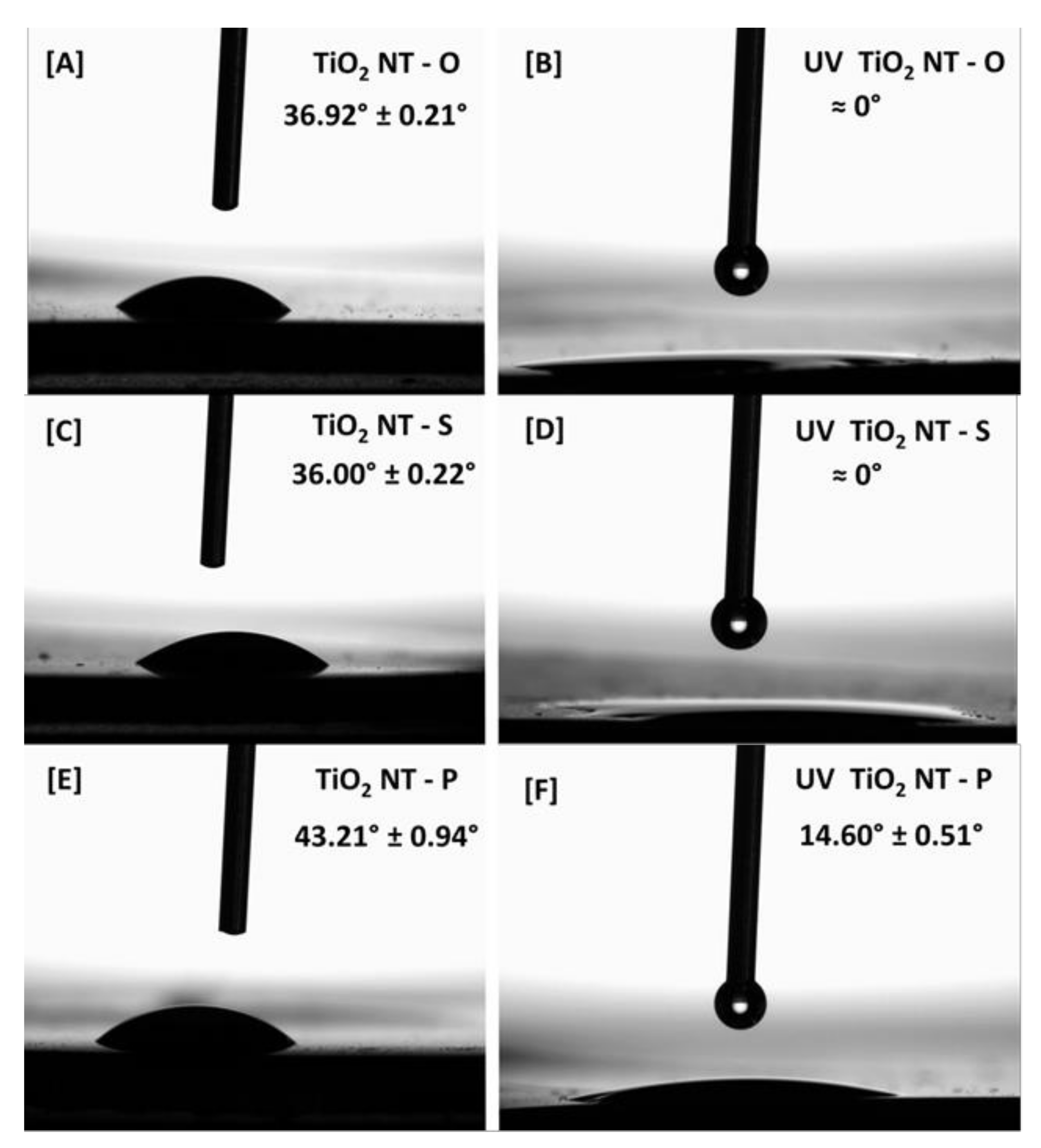
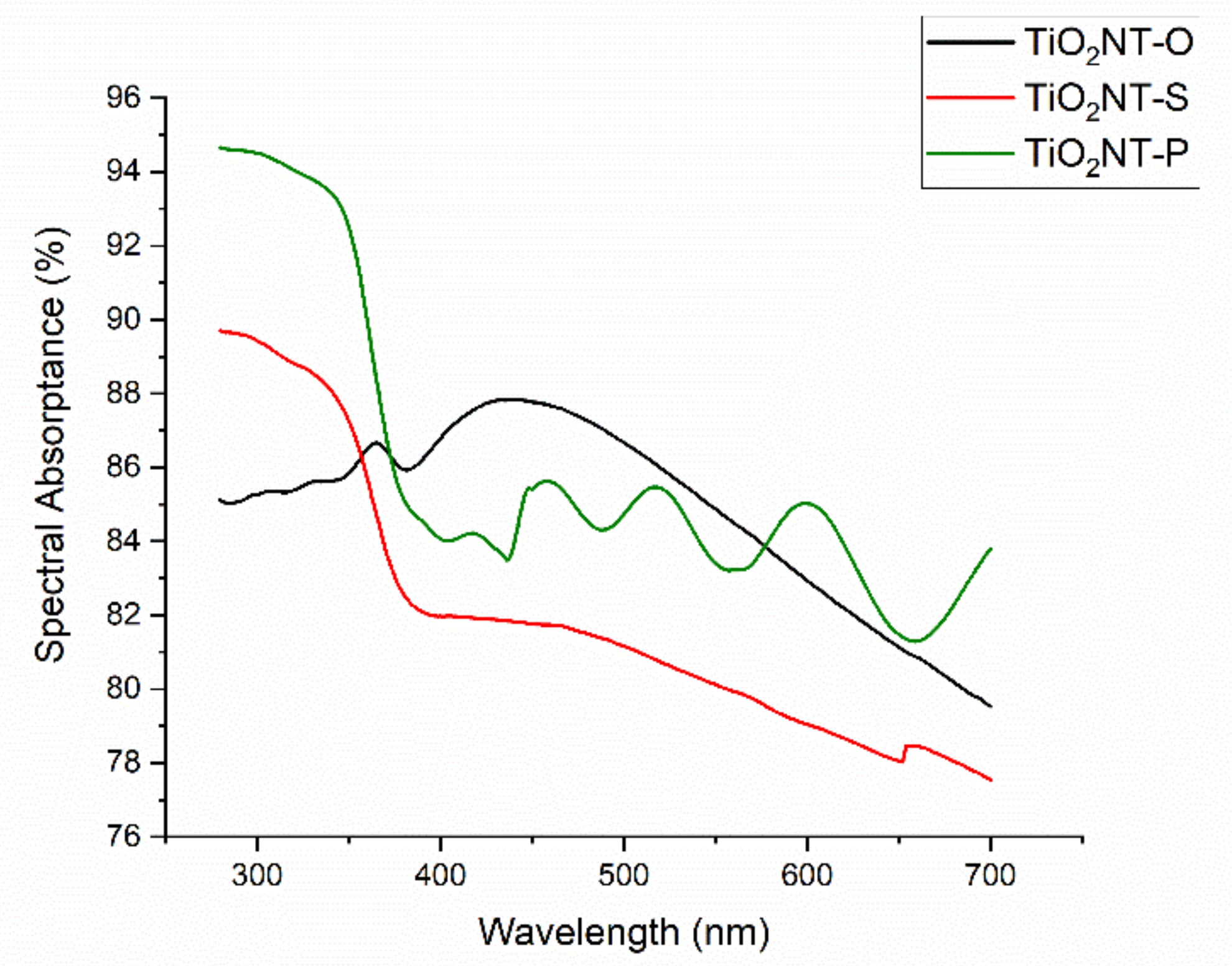
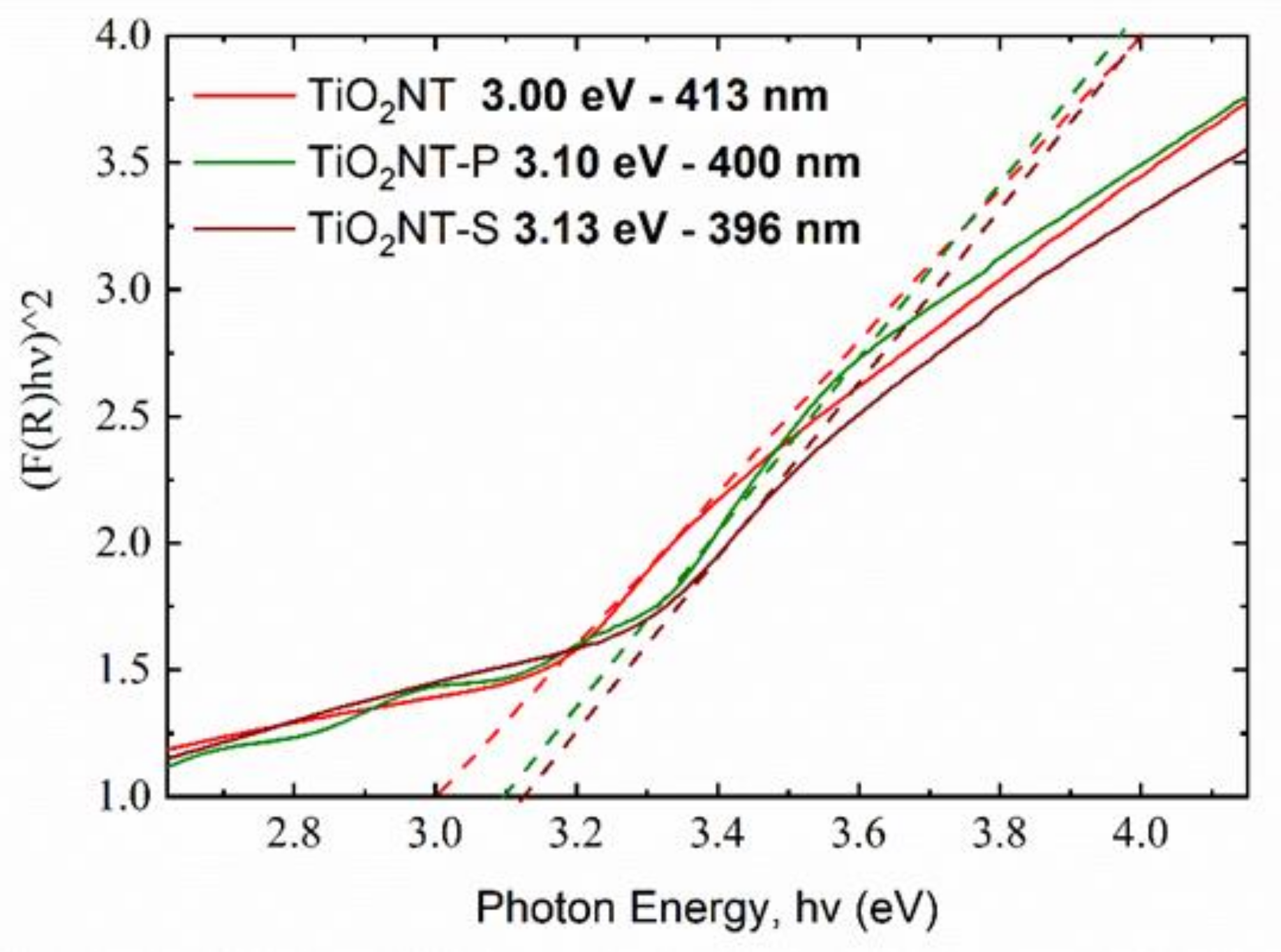
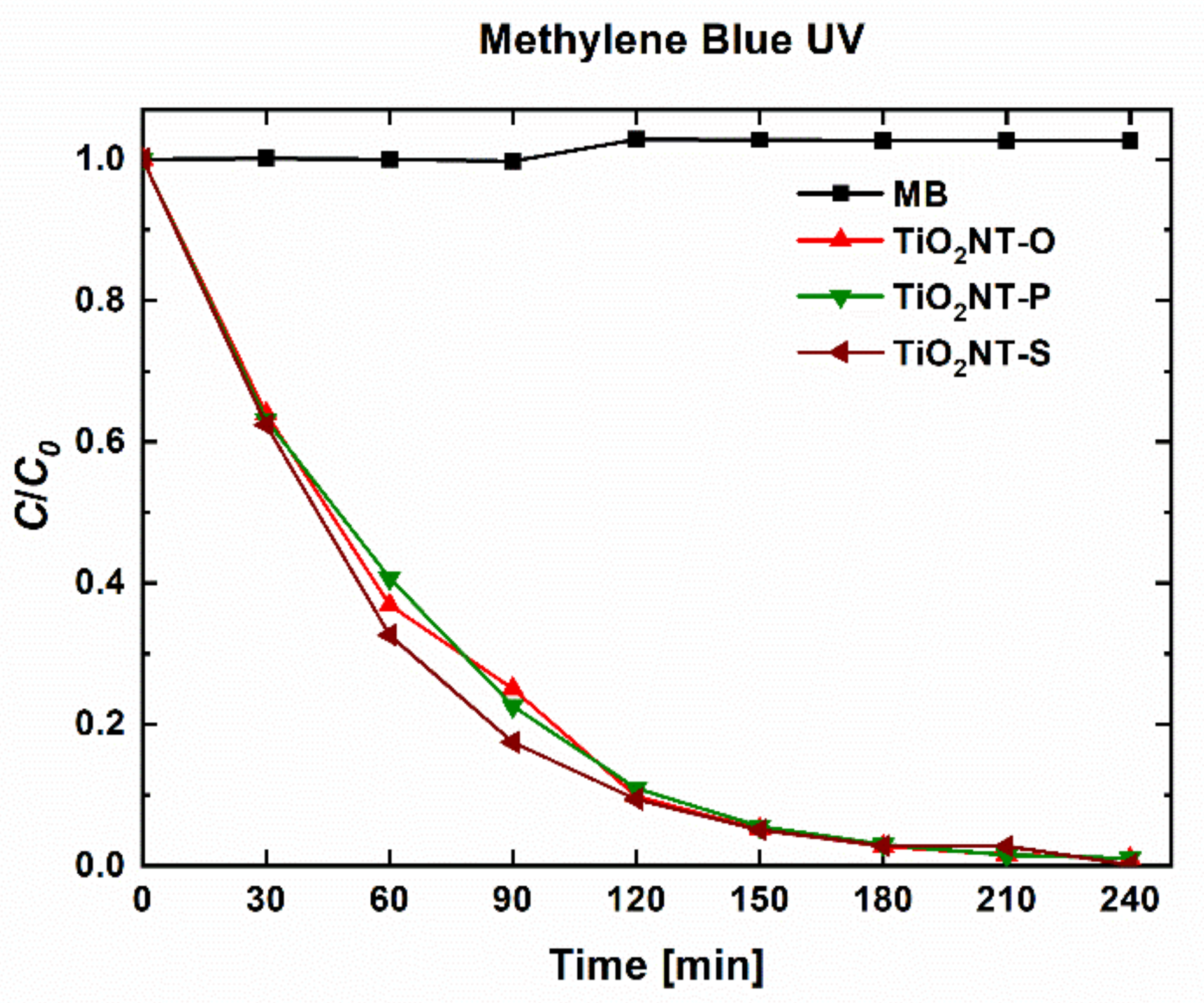
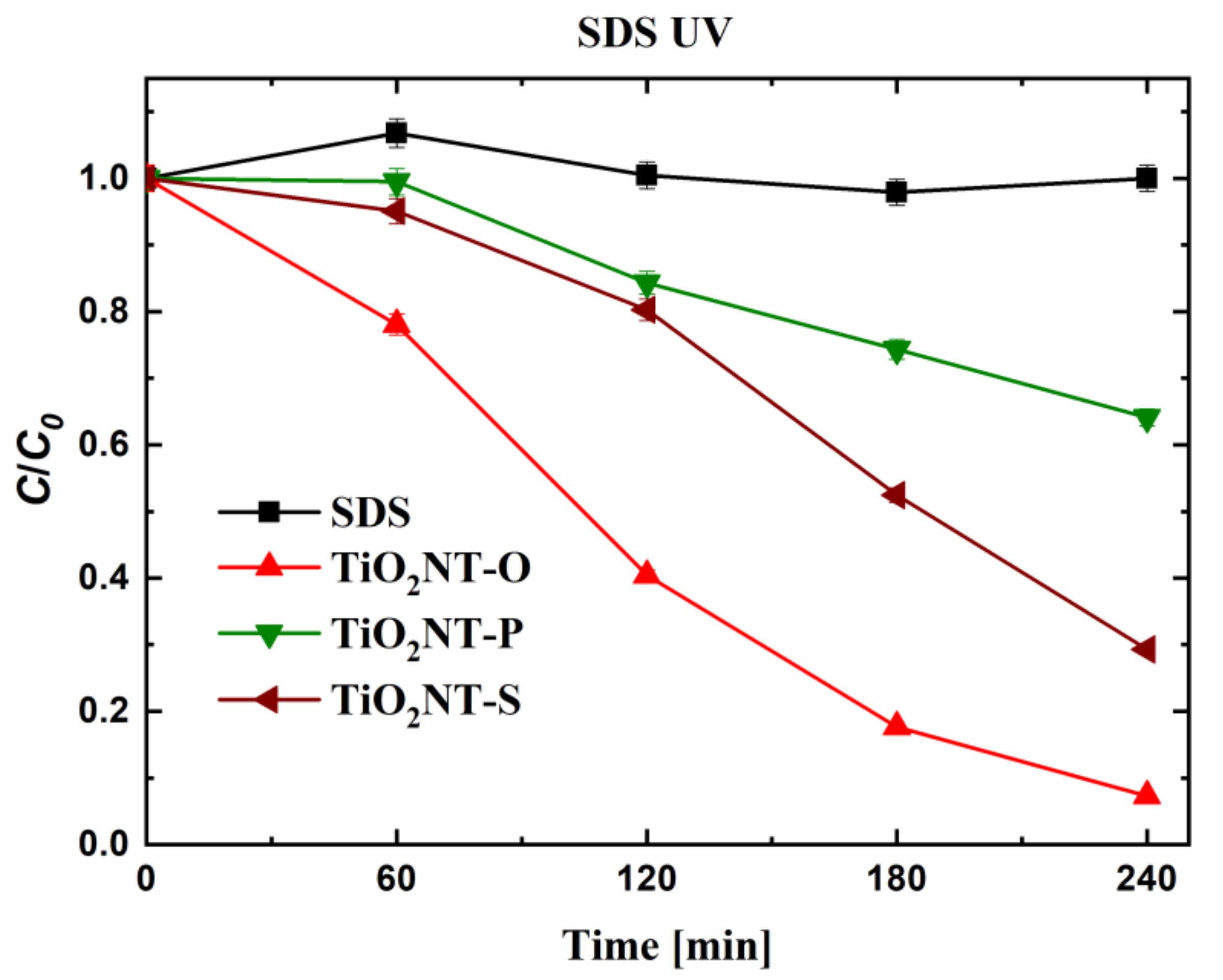
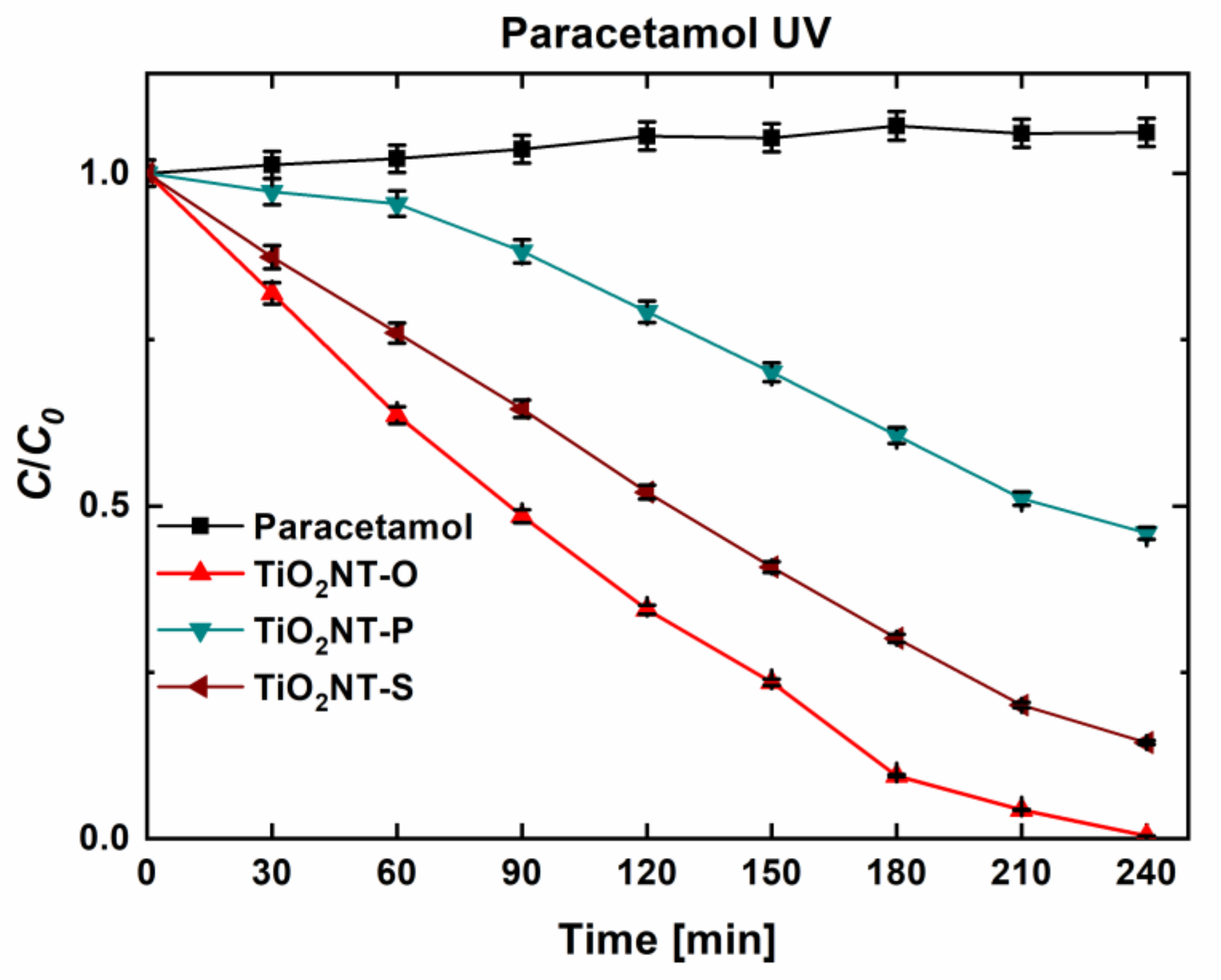
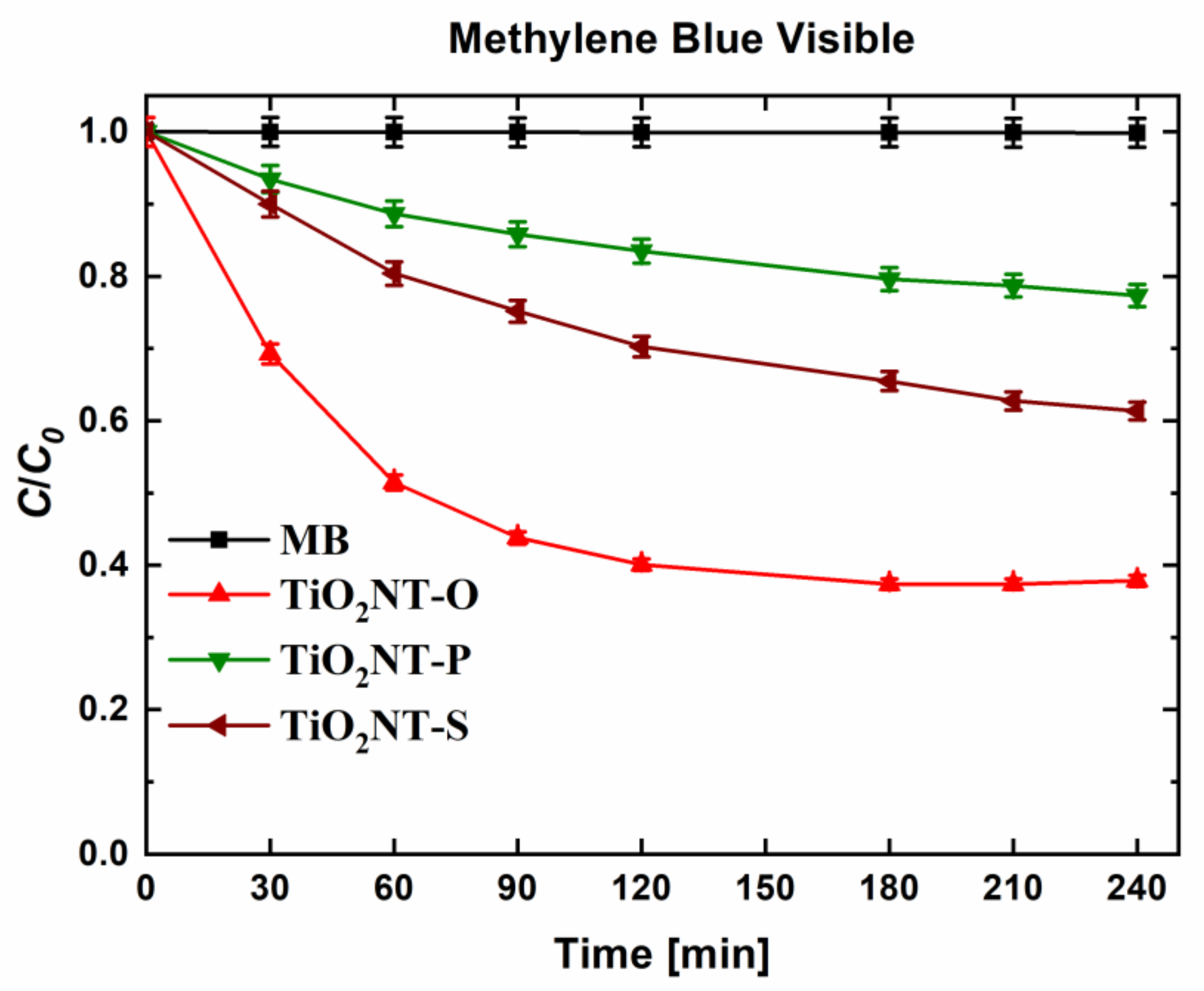

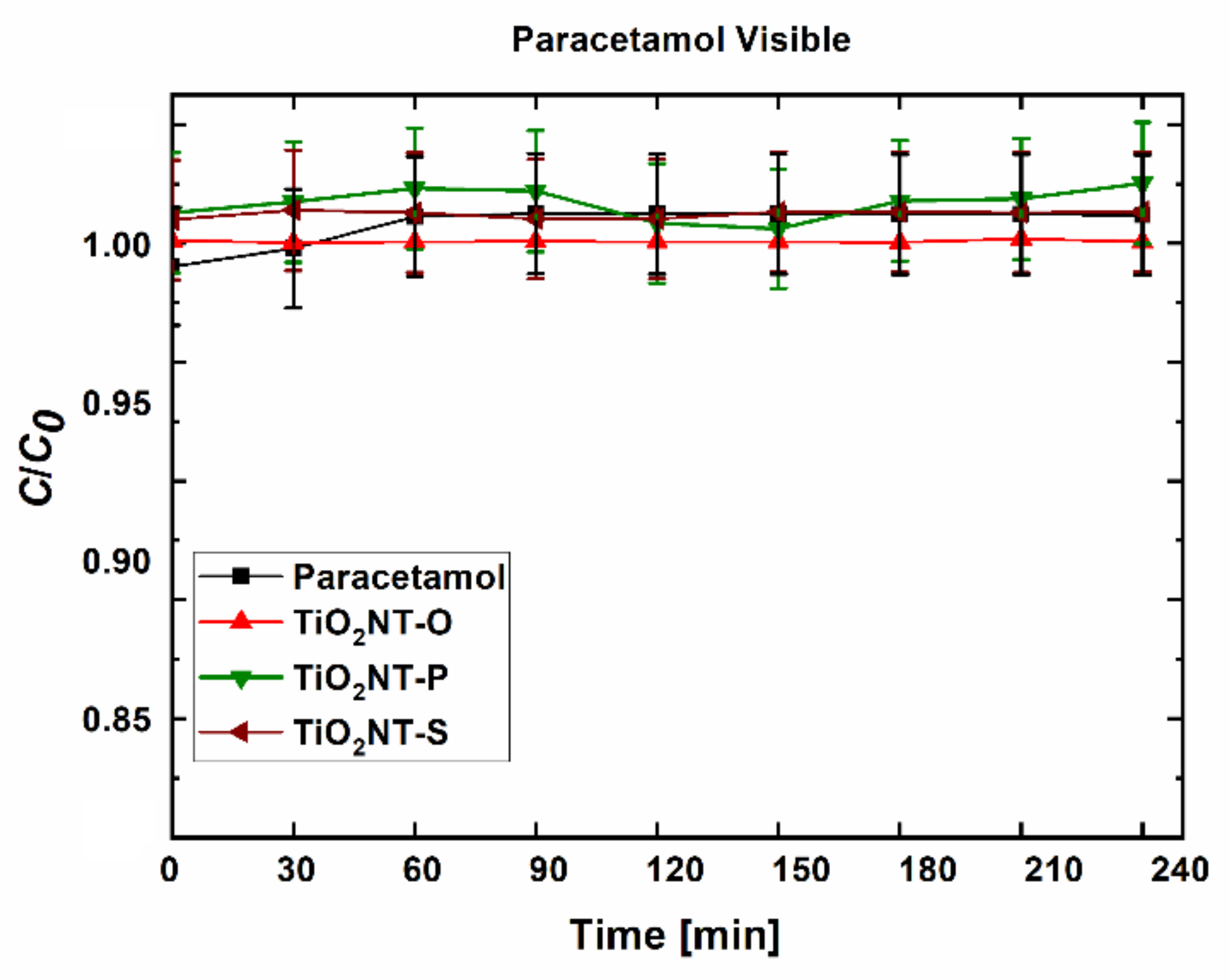

| Material | Parameters | Layer Thickness (µm) | Tube Diameter (nm) | Wall Thickness (nm) | Aspect Ratio |
|---|---|---|---|---|---|
| TiO2NT-O | 70 V, 1 h | 9.99 ± 0.48 | 85–125 | 10.00 ± 2.00 | 79.92–117.52 |
| TiO2NT-S | 20 V, 6 h | 1.45 ± 0.07 | 60–100 | 13.99 ± 2.00 | 14.5–24.17 |
| TiO2NT-P | 20 V, 3 h | 0.68 ± 0.02 | 60–105 | 11.71 ± 2.00 | 6.48–11.33 |
| Material | Solar Absorptance (%) | |||
|---|---|---|---|---|
| UV (280–400 nm) | UV 365 nm | Visible (400–700 nm) | Total (280–700 nm) | |
| TiO2NT-O | 86.1 ± 1.3 | 86.7 ± 0.03 | 84.4 ± 1.2 | 84.6 ± 1.2 |
| TiO2NT-S | 84.9 ± 1.0 | 84.6 ± 0.08 | 79.9 ± 1.4 | 80.4 ± 1.3 |
| TiO2NT-P | 88.7 ± 1.0 | 88.6 ± 0.17 | 83.8 ± 1.1 | 84.3 ± 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farrugia, C.; Di Mauro, A.; Lia, F.; Zammit, E.; Rizzo, A.; Privitera, V.; Impellizzeri, G.; Buccheri, M.A.; Rappazzo, G.; Grech, M.; et al. Suitability of Different Titanium Dioxide Nanotube Morphologies for Photocatalytic Water Treatment. Nanomaterials 2021, 11, 708. https://doi.org/10.3390/nano11030708
Farrugia C, Di Mauro A, Lia F, Zammit E, Rizzo A, Privitera V, Impellizzeri G, Buccheri MA, Rappazzo G, Grech M, et al. Suitability of Different Titanium Dioxide Nanotube Morphologies for Photocatalytic Water Treatment. Nanomaterials. 2021; 11(3):708. https://doi.org/10.3390/nano11030708
Chicago/Turabian StyleFarrugia, Clayton, Alessandro Di Mauro, Frederick Lia, Edwin Zammit, Alex Rizzo, Vittorio Privitera, Giuliana Impellizzeri, Maria Antonietta Buccheri, Giancarlo Rappazzo, Maurice Grech, and et al. 2021. "Suitability of Different Titanium Dioxide Nanotube Morphologies for Photocatalytic Water Treatment" Nanomaterials 11, no. 3: 708. https://doi.org/10.3390/nano11030708
APA StyleFarrugia, C., Di Mauro, A., Lia, F., Zammit, E., Rizzo, A., Privitera, V., Impellizzeri, G., Buccheri, M. A., Rappazzo, G., Grech, M., Refalo, P., & Abela, S. (2021). Suitability of Different Titanium Dioxide Nanotube Morphologies for Photocatalytic Water Treatment. Nanomaterials, 11(3), 708. https://doi.org/10.3390/nano11030708








