Synthesis of Air-Stable Cu Nanoparticles Using Laser Reduction in Liquid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cu NP synthesis
2.3. Instrumentation
2.3.1. Laser Synthesis
2.3.2. UV-Vis Spectroscopy
2.3.3. Transmission Electron Microscopy (TEM)
2.3.4. X-ray Photoelectron Spectroscopy (XPS)
2.3.5. FTIR Spectroscopy
2.3.6. X-ray Diffraction (XRD)
2.3.7. Raman Spectroscopy
2.4. Catalytic Reduction of Para-Nitrophenol (PNP)
3. Results
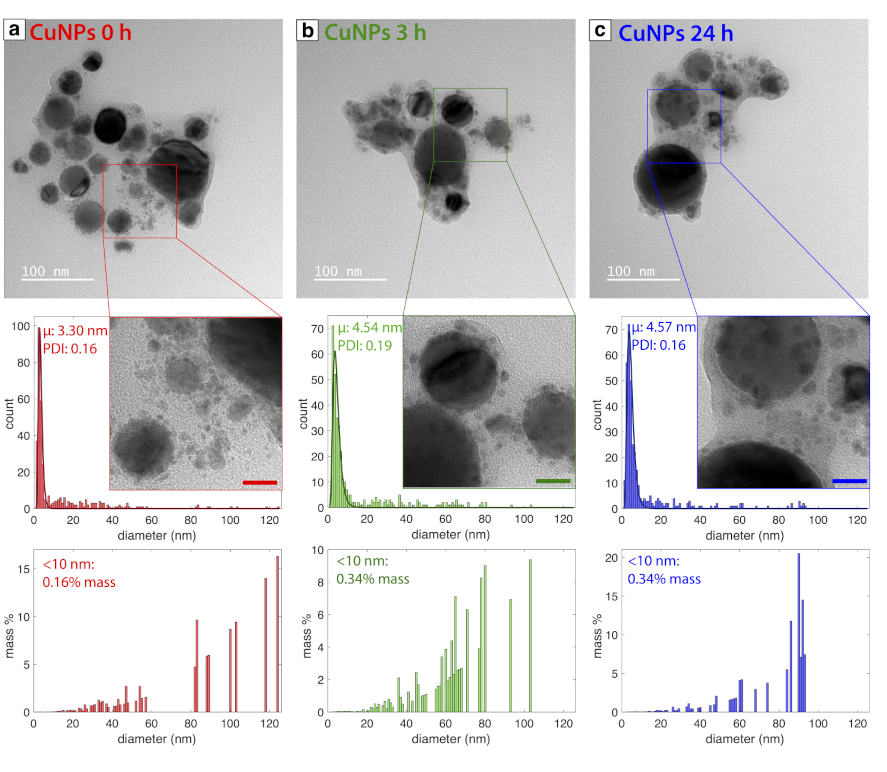
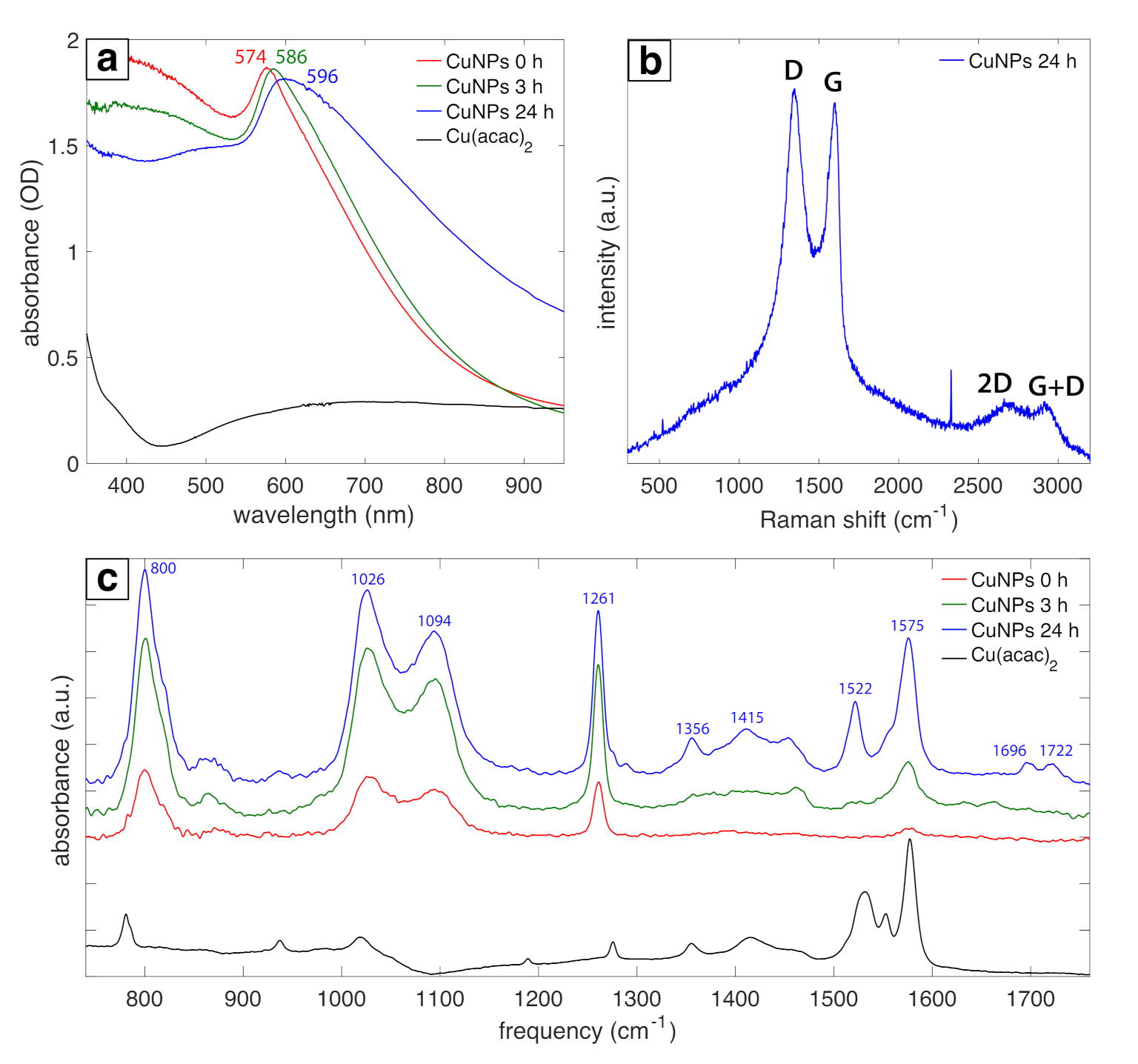
3.1. Physical Characterization
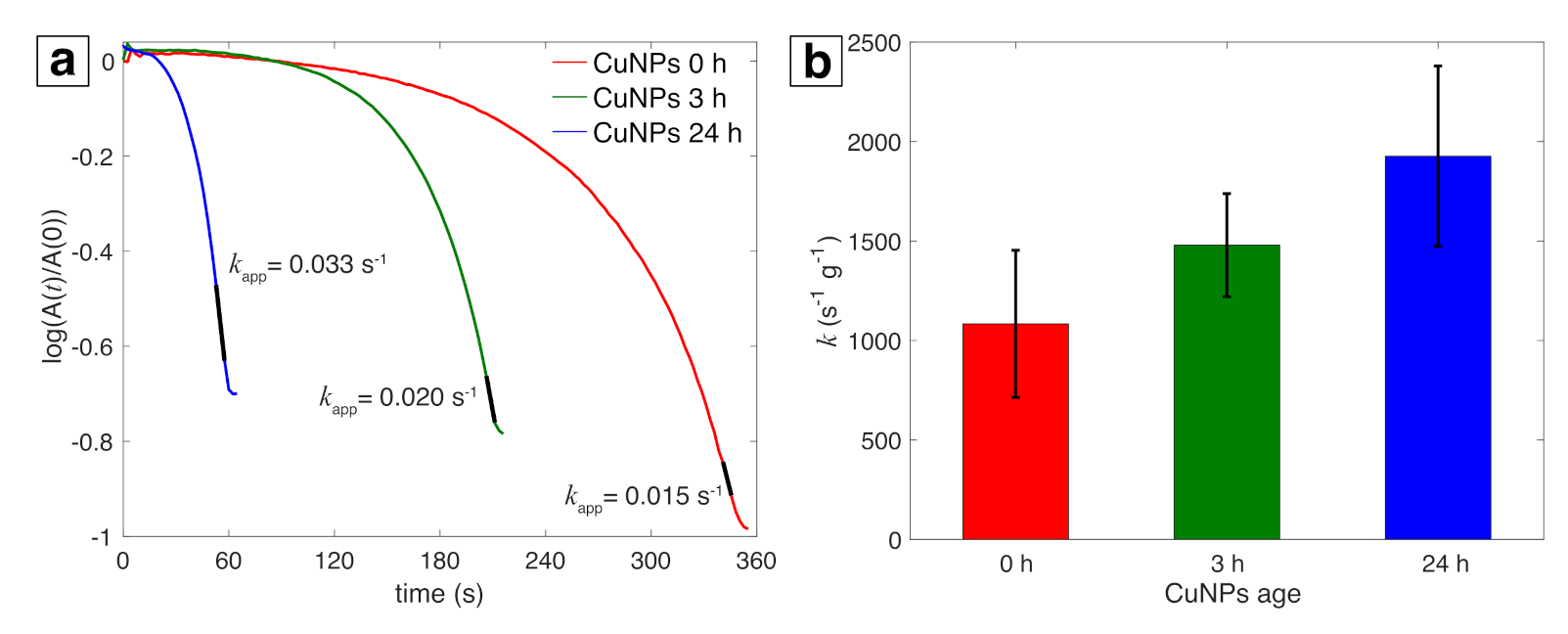
3.2. Catalytic Activity of Cu NPs
3.3. Stability of Cu NPs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, D.; Gökce, B.; Barcikowski, S. Laser Synthesis and Processing of Colloids: Fundamentals and Applications. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef] [PubMed]
- Reichenberger, S.; Marzun, G.; Muhler, M.; Barcikowski, S. Perspective of Surfactant-free Colloidal Nanoparticles in Heterogeneous Catalysis. ChemCatChem 2019, 11, 4489–4518. [Google Scholar] [CrossRef]
- Amans, D.; Cai, W.; Barcikowski, S. Status and demand of research to bring laser generation of nanoparticles in liquids to maturity. Appl. Surf. Sci. 2019, 488, 445–454. [Google Scholar] [CrossRef]
- Swiatkowska-Warkocka, Z.; Pyatenko, A.; Krok, F.; Jany, B.R.; Marszalek, M. Synthesis of new metastable nanoalloys of immiscible metals with a pulse laser technique. Sci. Rep. 2015, 5, 9849. [Google Scholar] [CrossRef]
- Tangeysh, B.; Odhner, J.H.; Wang, Y.; Wayland, B.B.; Levis, R.J. Formation of Copper(I) Oxide- and Copper(I) Cyanide–Polyacetonitrile Nanocomposites through Strong-Field Laser Processing of Acetonitrile Solutions of Copper(II) Acetate Dimer. J. Phys. Chem. A 2019, 123, 6430–6438. [Google Scholar] [CrossRef]
- Streubel, R.; Barcikowski, S.; Gökce, B. Continuous multigram nanoparticle synthesis by high-power, high-repetition-rate ultrafast laser ablation in liquids. Opt. Lett. 2016, 41, 1486–1489. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. What controls the composition and the structure of nanomaterials generated by laser ablation in liquid solution? Phys. Chem. Chem. Phys. 2013, 15, 3027–3046. [Google Scholar] [CrossRef]
- Mastrotto, F.; Caliceti, P.; Amendola, V.; Bersani, S.; Magnusson, J.P.; Meneghetti, M.; Mantovani, G.; Alexander, C.; Salmaso, S. Polymer control of ligand display on gold nanoparticles for multimodal switchable cell targeting. Chem. Commun. 2011, 47, 9846–9848. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; Kibria, M.G.; Mi, Z.; Chaker, M.; Ma, D.; Nechache, R.; Rosei, F. Remarkably enhanced photocatalytic activity of laser ablated Au nanoparticle decorated BiFeO3 nanowires under visible-light. Chem. Commun. 2013, 49, 5856–5858. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Chaker, M.; Rosei, F.; Ma, D. Gold nanoparticle decorated ceria nanotubes with significantly high catalytic activity for the reduction of nitrophenol and mechanism study. Appl. Catal. B-Environ. 2013, 132-133, 107–115. [Google Scholar] [CrossRef]
- Blakemore, J.D.; Gray, H.B.; Winkler, J.R.; Müller, A.M. Co3O4 Nanoparticle Water-Oxidation Catalysts Made by Pulsed-Laser Ablation in Liquids. ACS Catal. 2013, 3, 2497–2500. [Google Scholar] [CrossRef]
- Gu, S.; Kaiser, J.; Marzun, G.; Ott, A.; Lu, Y.; Ballauff, M.; Zaccone, A.; Barcikowski, S.; Wagener, P. Ligand-free Gold Nanoparticles as a Reference Material for Kinetic Modelling of Catalytic Reduction of 4-Nitrophenol. Catal. Lett. 2015, 145, 1105–1112. [Google Scholar] [CrossRef]
- Longano, D.; Ditaranto, N.; Cioffi, N.; Di Niso, F.; Sibillano, T.; Ancona, A.; Conte, A.; Del Nobile, M.A.; Sabbatini, L.; Torsi, L. Analytical characterization of laser-generated copper nanoparticles for antibacterial composite food packaging. Anal. Bioanal. Chem. 2012, 403, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Naddeo, J.; Ratti, M.; O’Malley, S.; Griepenburg, J.; Bubb, D.; Klein, E. Antibacterial Properties of Nanoparticles: A Comparative Review of Chemically Synthesized and Laser-Generated Particles. Adv. Sci. Eng. Med. 2015, 7, 1044–1057. [Google Scholar] [CrossRef]
- Petersen, S.; Barcikowski, S. In Situ Bioconjugation: Single Step Approach to Tailored Nanoparticle-Bioconjugates by Ultrashort Pulsed Laser Ablation. Adv. Funct. Mater. 2009, 19, 1167–1172. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, C.; Liu, J.; Tian, Z.; Shao, G. The formation of onion-like carbon-encapsulated cobalt carbide core/shell nanoparticles by the laser ablation of metallic cobalt in acetone. Carbon 2013, 55, 108–115. [Google Scholar] [CrossRef]
- Yu-jin, K.; Ma, R.; Reddy, D.A.; Kim, T.K. Liquid-phase pulsed laser ablation synthesis of graphitized carbon-encapsulated palladium core–shell nanospheres for catalytic reduction of nitrobenzene to aniline. Appl. Surf. Sci. 2015, 357, 2112–2120. [Google Scholar] [CrossRef]
- Kim, J.H.; Chung, Y.K. Copper nanoparticle-catalyzed cross-coupling of alkyl halides with Grignard reagents. Chem. Commun. 2013, 49, 11101–11103. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Goswami, A.; Felpin, F.X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.T.; Lee, Y.I.; Kim, S.; Lee, K.J.; Choa, Y.H. Full densification of inkjet-printed copper conductive tracks on a flexible substrate utilizing a hydrogen plasma sintering. Appl. Surf. Sci. 2017, 396, 1239–1244. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Edison, T.N.J.I.; Karuppusamy, I.; Kathirvel, B. Inorganic nanoparticles: A potential cancer therapy for human welfare. Int. J. Pharm. 2018, 539, 104–111. [Google Scholar] [CrossRef]
- Park, J.H.; Seo, J.; Kim, C.; Joe, D.J.; Lee, H.E.; Im, T.H.; Seok, J.Y.; Jeong, C.K.; Ma, B.S.; Park, H.K.; et al. Flash-Induced Stretchable Cu Conductor via Multiscale-Interfacial Couplings. Adv. Sci. 2018, 5, 1801146. [Google Scholar] [CrossRef]
- Song, X.; Sun, S.; Zhang, W.; Yin, Z. A method for the synthesis of spherical copper nanoparticles in the organic phase. J. Colloid Interface Sci. 2004, 273, 463–469. [Google Scholar] [CrossRef]
- Dharmadasa, R.; Jha, M.; Amos, D.A.; Druffel, T. Room Temperature Synthesis of a Copper Ink for the Intense Pulsed Light Sintering of Conductive Copper Films. ACS Appl. Mater. Interfaces 2013, 5, 13227–13234. [Google Scholar] [CrossRef]
- Mott, D.; Galkowski, J.; Wang, L.; Luo, J.; Zhong, C.J. Synthesis of Size-Controlled and Shaped Copper Nanoparticles. Langmuir 2007, 23, 5740–5745. [Google Scholar] [CrossRef] [PubMed]
- Swarnkar, R.K.; Singh, S.C.; Gopal, R. Effect of aging on copper nanoparticles synthesized by pulsed laser ablation in water: structural and optical characterizations. Bull. Mater. Sci. 2011, 34, 1363–1369. [Google Scholar] [CrossRef]
- Fernández-Arias, M.; Boutinguiza, M.; Del Val, J.; Covarrubias, C.; Bastias, F.; Gómez, L.; Maureira, M.; Arias-González, F.; Riveiro, A.; Pou, J. Copper nanoparticles obtained by laser ablation in liquids as bactericidal agent for dental applications. Appl. Surf. Sci. 2020, 507, 145032. [Google Scholar] [CrossRef]
- Marzun, G.; Levish, A.; Mackert, V.; Kallio, T.; Barcikowski, S.; Wagener, P. Laser synthesis, structure and chemical properties of colloidal nickel-molybdenum nanoparticles for the substitution of noble metals in heterogeneous catalysis. J. Colloid Interface Sci. 2017, 489, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Goncharova, D.A.; Kharlamova, T.S.; Lapin, I.N.; Svetlichnyi, V.A. Chemical and Morphological Evolution of Copper Nanoparticles Obtained by Pulsed Laser Ablation in Liquid. J. Phys. Chem. C 2019, 123, 21731–21742. [Google Scholar] [CrossRef]
- Goncharova, D.A.; Kharlamova, T.S.; Reutova, O.A.; Svetlichnyi, V.A. Water-ethanol CuOx nanoparticle colloids prepared by laser ablation: Colloid stability and catalytic properties in nitrophenol hydrogenation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126115. [Google Scholar] [CrossRef]
- Rehbock, C.; Merk, V.; Gamrad, L.; Streubel, R.; Barcikowski, S. Size Control of Laser-Fabricated Surfactant-Free Gold Nanoparticles with Highly Diluted Electrolytes and their Subsequent Bioconjugation. Phys. Chem. Chem. Phys. 2013, 15, 3057–3067. [Google Scholar] [CrossRef]
- Sylvestre, J.P.; Kabashin, A.V.; Sacher, E.; Meunier, M. Femtosecond laser ablation of gold in water: influence of the laser-produced plasma on the nanoparticle size distribution. Appl. Phys. A 2005, 80, 753–758. [Google Scholar] [CrossRef]
- Shih, C.Y.; Streubel, R.; Heberle, J.; Letzel, A.; Shugaev, M.V.; Wu, C.; Schmidt, M.; Gökce, B.; Barcikowski, S.; Zhigilei, L.V. Two mechanisms of nanoparticle generation in picosecond laser ablation in liquids: the origin of the bimodal size distribution. Nanoscale 2018, 10, 6900–6910. [Google Scholar] [CrossRef]
- Rodrigues, C.J.; Bobb, J.A.; John, M.G.; Fisenko, S.P.; El-Shall, M.S.; Tibbetts, K.M. Nucleation and growth of gold nanoparticles initiated by nanosecond and femtosecond laser irradiation of aqueous [AuCl4]-. Phys. Chem. Chem. Phys. 2018, 20, 28465–28475. [Google Scholar] [CrossRef]
- Frias Batista, L.M.; Meader, V.K.; Romero, K.; Kunzler, K.; Kabir, F.; Bullock, A.; Tibbetts, K.M. Kinetic Control of [AuCl4]- Photochemical Reduction and Gold Nanoparticle Size with Hydroxyl Radical Scavengers. J. Phys.Chem. B 2019, 123, 7204–7213. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Mochidzuki, Y.; Sato, S. Fabrication of Gold Nanoparticles in Intense Optical Field by Femtosecond Laser Irradiation of Aqueous Solution. J. Mater. Res. 2008, 23, 968–974. [Google Scholar] [CrossRef]
- Tangeysh, B.; Moore Tibbetts, K.; Odhner, J.H.; Wayland, B.B.; Levis, R.J. Gold Nanoparticle Synthesis Using Spatially and Temporally Shaped Femtosecond Laser Pulses: Post-Irradiation Auto-Reduction of Aqueous [AuCl4]-. J. Phys. Chem. C 2013, 117, 18719–18727. [Google Scholar] [CrossRef]
- Meader, V.K.; John, M.G.; Rodrigues, C.J.; Tibbetts, K.M. Roles of Free Electrons and H2O2 in the Optical Breakdown-Induced Photochemical Reduction of Aqueous [AuCl4]-. J. Phys. Chem. A 2017, 121, 6742–6754. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Magara, H.; Herbani, Y.; Sato, S. Fabrication of silver nanoparticles by highly intense laser irradiation of aqueous solution. Appl. Phys. A 2011, 104, 1021. [Google Scholar] [CrossRef]
- Herbani, Y.; Nakamura, T.; Sato, S. Silver nanoparticle formation by femtosecond laser induced reduction of ammonia-containing AgNO 3 solution. J. Phys. Conf. Ser. 2017, 817, 012048. [Google Scholar] [CrossRef]
- Meader, V.K.; John, M.G.; Frias Batista, L.M.; Ahsan, S.; Tibbetts, K.M. Radical Chemistry in a Femtosecond Laser Plasma: Photochemical Reduction of Ag+ in Liquid Ammonia Solution. Molecules 2018, 23, 532. [Google Scholar] [CrossRef]
- Nakamura, T.; Takasaki, K.; Ito, A.; Sato, S. Fabrication of platinum particles by intense, femtosecond laser pulse irradiation of aqueous solution. Appl. Surf. Sci. 2009, 255, 9630–9633. [Google Scholar] [CrossRef]
- Herbani, Y.; Nakamura, T.; Sato, S. Synthesis of platinum-based binary and ternary alloy nanoparticles in an intense laser field. J. Colloid Interface Sci. 2012, 375, 78–87. [Google Scholar] [CrossRef]
- Herbani, Y.; Nakamura, T.; Sato, S. Spectroscopic monitoring on irradiation-induced formation of AuAg alloy nanoparticles by femtosecond laser. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2016; Volume 1711, p. 030005. [Google Scholar] [CrossRef]
- Nguyen, C.M.; Frias Batista, L.M.; John, M.G.; Rodrigues, C.J.; Tibbetts, K.M. Mechanism of Gold–Silver Alloy Nanoparticle Formation by Laser Coreduction of Gold and Silver Ions in Solution. J. Phys. Chem. B 2021, 125, 907–917. [Google Scholar] [CrossRef]
- Okamoto, T.; Nakamura, T.; Kihara, R.; Asahi, T.; Sakota, K.; Yatsuhashi, T. Synthesis of Bare Iron Nanoparticles from Ferrocene Hexane Solution by Femtosecond Laser Pulses. ChemPhysChem 2018, 19, 2480–2485. [Google Scholar] [CrossRef]
- Okamoto, T.; Nakamura, T.; Tahara, Y.O.; Miyata, M.; Sakota, K.; Yatsuhashi, T. Effects of Ligand and Solvent on the Synthesis of Iron Oxide Nanoparticles from Fe(acac)3 Solution by Femtosecond Laser Irradiation. Chem. Lett. 2020, 49, 75–78. [Google Scholar] [CrossRef]
- Dhas, N.A.; Raj, C.P.; Gedanken, A. Synthesis, Characterization, and Properties of Metallic Copper Nanoparticles. Chem. Mater. 1998, 10, 1446–1452. [Google Scholar] [CrossRef]
- John, M.G.; Tibbetts, K.M. One-step femtosecond laser ablation synthesis of sub-3 nm gold nanoparticles stabilized by silica. Appl. Surf. Sci. 2019, 475, 1048–1057. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the ‘Debye-Scherrer equation’. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef] [PubMed]
- Ghodselahi, T.; Vesaghi, M.; Shafiekhani, A.; Baghizadeh, A.; Lameii, M. XPS study of the Cu@Cu2O core-shell nanoparticles. Appl. Surf. Sci. 2008, 255, 2730–2734. [Google Scholar] [CrossRef]
- Wagner, C.; M, R.W.; Davis, L.E.; Moulder, J.F.; Muilenburg, G.E. Handbook of X-ray Photoelectron Spectroscopy; Physical Electronics Division; Perkin-Elmer Corp.: Maharashtra, India, 1979. [Google Scholar]
- Yu, B.; Wang, X.; Qian, X.; Xing, W.; Yang, H.; Ma, L.; Lin, Y.; Jiang, S.; Song, L.; Hu, Y.; et al. Functionalized graphene oxide/phosphoramide oligomer hybrids flame retardant prepared via in situ polymerization for improving the fire safety of polypropylene. RSC Adv. 2014, 4, 31782–31794. [Google Scholar] [CrossRef]
- Rice, K.P.; Walker, E.J.; Stoykovich, M.P.; Saunders, A.E. Solvent-Dependent Surface Plasmon Response and Oxidation of Copper Nanocrystals. J. Phys. Chem. C 2011, 115, 1793–1799. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Hatui, G.; Oraon, R.; De Adhikari, A.; Nayak, G.C. Mixing sequence driven controlled dispersion of graphene oxide in PC/PMMA blend nanocomposite and its effect on thermo-mechanical properties. Curr. Appl. Phys. 2017, 17, 1158–1168. [Google Scholar] [CrossRef]
- Bobb, J.A.; Rodrigues, C.J.; El-Shall, M.S.; Tibbetts, K.M. Laser-assisted synthesis of gold–graphene oxide nanocomposites: effect of pulse duration. Phys. Chem. Chem. Phys. 2020, 22, 18294–18303. [Google Scholar] [CrossRef] [PubMed]
- Baharuddin, A.A.; Ang, B.C.; Wong, Y.H. Self-assembly and electrical characteristics of 4-pentynoic acid functionalized Fe3O4-γ-Fe2O3 nanoparticles on SiO2/n-Si. Appl. Surf. Sci. 2017, 423, 236–244. [Google Scholar] [CrossRef]
- Wunder, S.; Polzer, F.; Lu, Y.; Mei, Y.; Ballauff, M. Kinetic Analysis of Catalytic Reduction of 4-Nitrophenol by Metallic Nanoparticles Immobilized in Spherical Polyelectrolyte Brushes. J. Phys. Chem. C 2010, 114, 8814–8820. [Google Scholar] [CrossRef]
- Aditya, T.; Pal, A.; Pal, T. Nitroarene reduction: a trusted model reaction to test nanoparticle catalysts. Chem. Commun. 2015, 51, 9410–9431. [Google Scholar] [CrossRef] [PubMed]
- Guermoune, A.; Chari, T.; Popescu, F.; Sabri, S.S.; Guillemette, J.; Skulason, H.S.; Szkopek, T.; Siaj, M. Chemical vapor deposition synthesis of graphene on copper with methanol, ethanol, and propanol precursors. Carbon 2011, 49, 4204–4210. [Google Scholar] [CrossRef]
- Galaburda, M.; Kovalska, E.; Hogan, B.T.; Baldycheva, A.; Nikolenko, A.; Dovbeshko, G.I.; Oranska, O.I.; Bogatyrov, V.M. Mechanochemical synthesis of carbon-stabilized Cu/C, Co/C and Ni/C nanocomposites with prolonged resistance to oxidation. Sci. Rep. 2019, 9, 17435. [Google Scholar] [CrossRef]
- Stein, M.; Wieland, J.; Steurer, P.; Tölle, F.; Mülhaupt, R.; Breit, B. Iron Nanoparticles Supported on Chemically-Derived Graphene: Catalytic Hydrogenation with Magnetic Catalyst Separation. Adv. Syn. Catal. 2011, 353, 523–527. [Google Scholar] [CrossRef]
- Tsang, S.C.; Caps, V.; Paraskevas, I.; Chadwick, D.; Thompsett, D. Magnetically Separable, Carbon-Supported Nanocatalysts for the Manufacture of Fine Chemicals. Angew. Chem. Int. Ed. 2004, 43, 5645–5649. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nag, A.; Frias Batista, L.M.; Tibbetts, K.M. Synthesis of Air-Stable Cu Nanoparticles Using Laser Reduction in Liquid. Nanomaterials 2021, 11, 814. https://doi.org/10.3390/nano11030814
Nag A, Frias Batista LM, Tibbetts KM. Synthesis of Air-Stable Cu Nanoparticles Using Laser Reduction in Liquid. Nanomaterials. 2021; 11(3):814. https://doi.org/10.3390/nano11030814
Chicago/Turabian StyleNag, Ashish, Laysa Mariela Frias Batista, and Katharine Moore Tibbetts. 2021. "Synthesis of Air-Stable Cu Nanoparticles Using Laser Reduction in Liquid" Nanomaterials 11, no. 3: 814. https://doi.org/10.3390/nano11030814
APA StyleNag, A., Frias Batista, L. M., & Tibbetts, K. M. (2021). Synthesis of Air-Stable Cu Nanoparticles Using Laser Reduction in Liquid. Nanomaterials, 11(3), 814. https://doi.org/10.3390/nano11030814






