Incorporation of Manganese Complexes within Hybrid Resol-Silica and Carbon-Silica Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
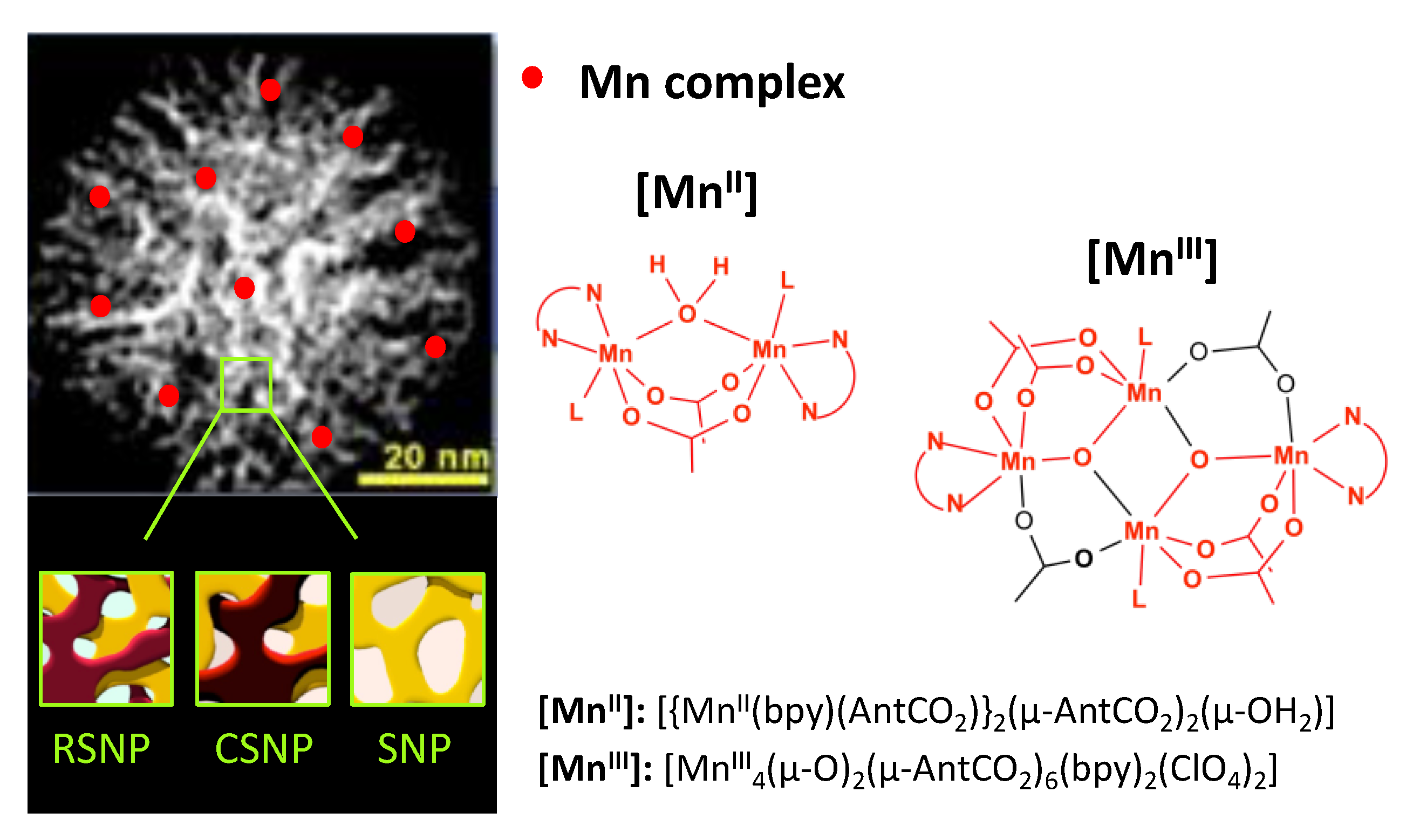
2.1. Synthesis of the Nanoparticles
2.1.1. Mesoporous Resol-Silica Nanoparticles (RSNP)
2.1.2. Mesoporous Carbon-Silica Nanoparticles (CSNP)
2.1.3. Mesoporous Silica Nanoparticles (SNP)
2.2. Synthesis of [Mn]@NP
2.2.1. Synthesis of Mesoporous [MnIII]@Resol-Silica Nanoparticles ([MnIII]@RSNP)
2.2.2. Synthesis of Mesoporous [MnIII]@Carbon-Silica Nanoparticles ([MnIII]@CSNP)
2.2.3. Synthesis of Mesoporous [MnIII]@Silica Nanoparticles ([MnIII]@SNP)
2.3. Synthesis of [MnII]@NP
2.4. Characterization
3. Results and Discussion
3.1. Synthesis of the Materials Using Three Types of Mesoporous Nanoparticles
3.2. Characterization of the Nanoparticles
3.2.1. Infrared Spectroscopy
3.2.2. Raman Spectroscopy
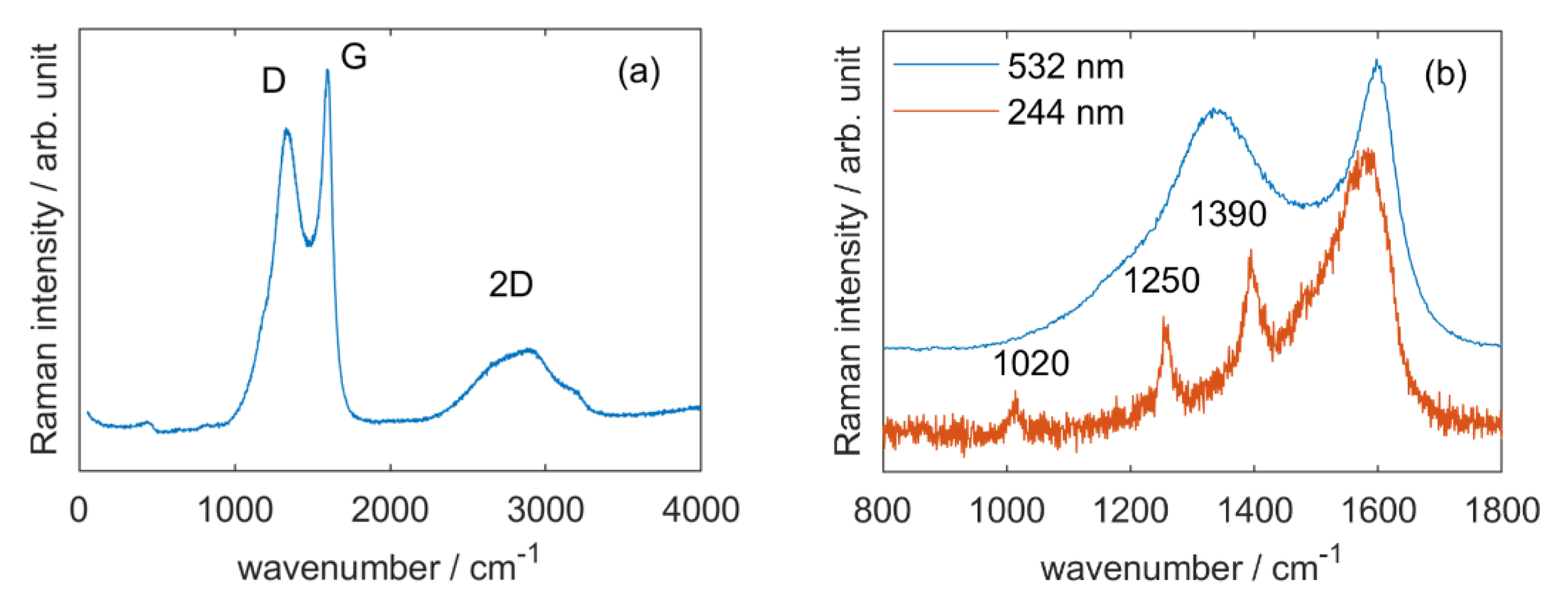
3.2.3. NMR Spectroscopy
3.2.4. Thermogravimetrical Analysis (TGA)
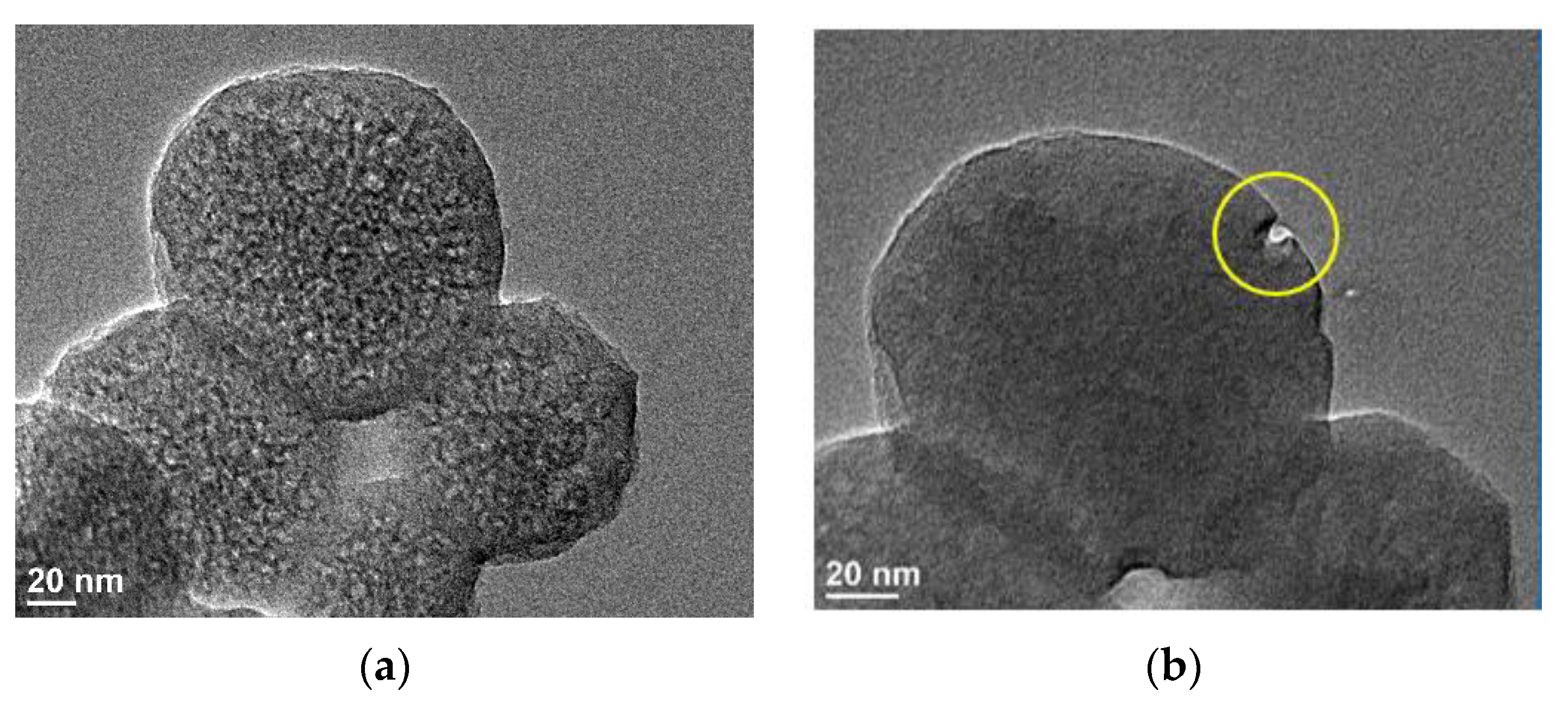
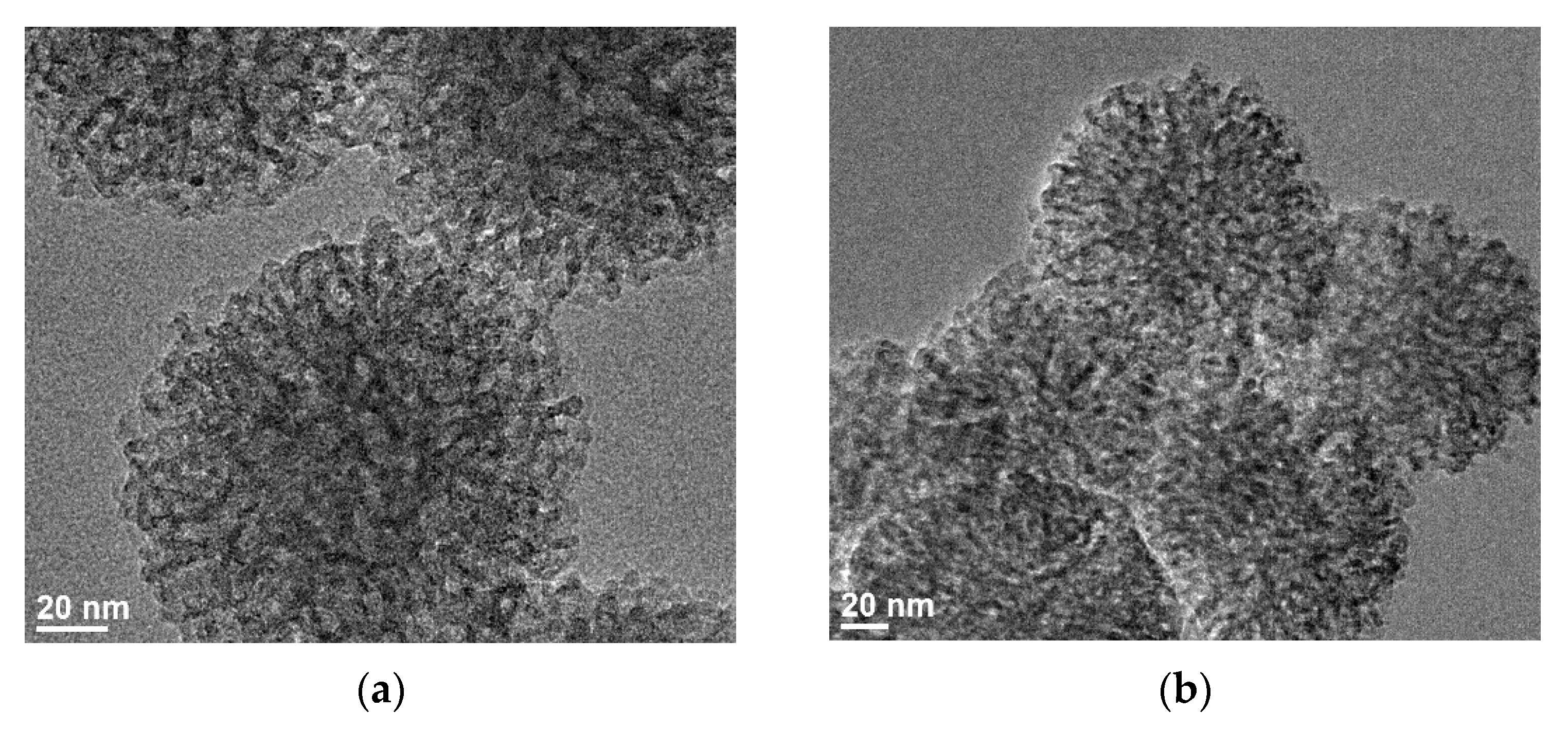
3.2.5. Evaluation of the Size and Porosity
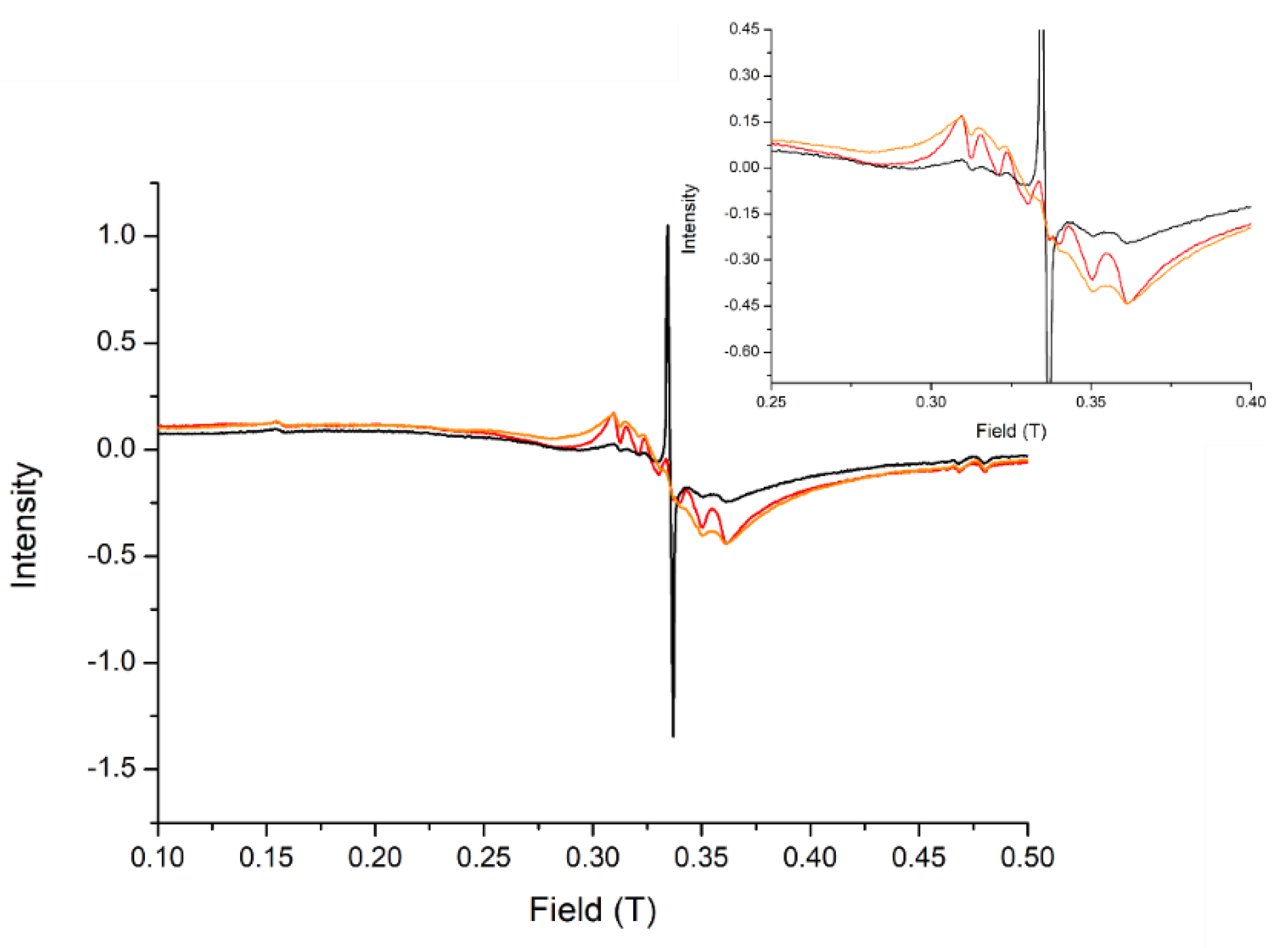
3.2.6. Study of the Integrity of the Complex inside the Nanoparticles and Estimation of the Load of Complex
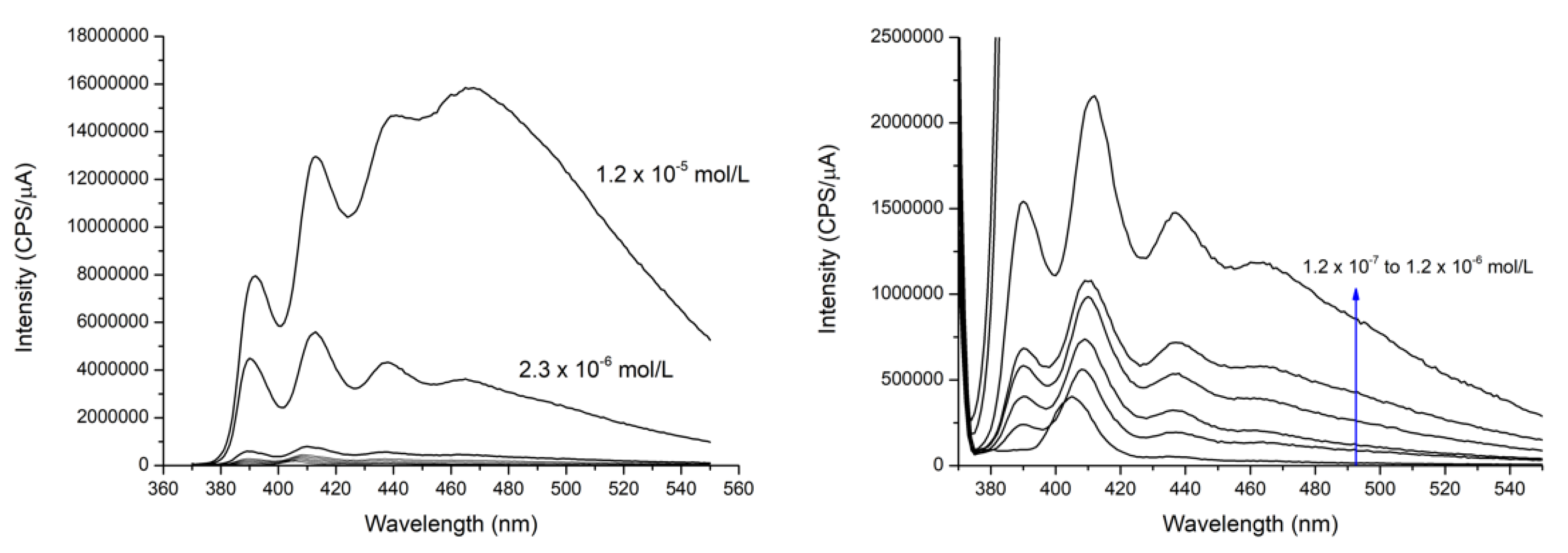
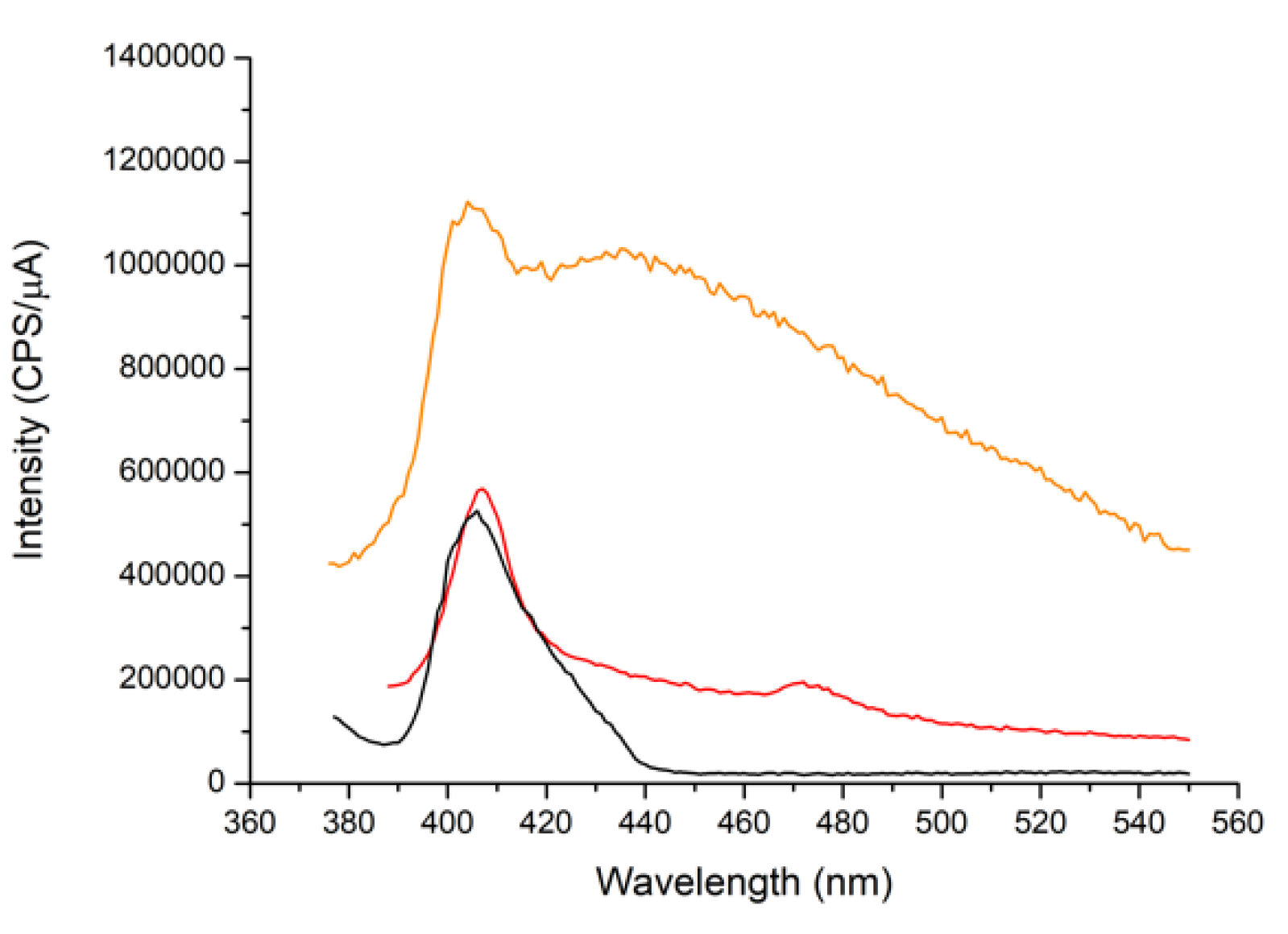
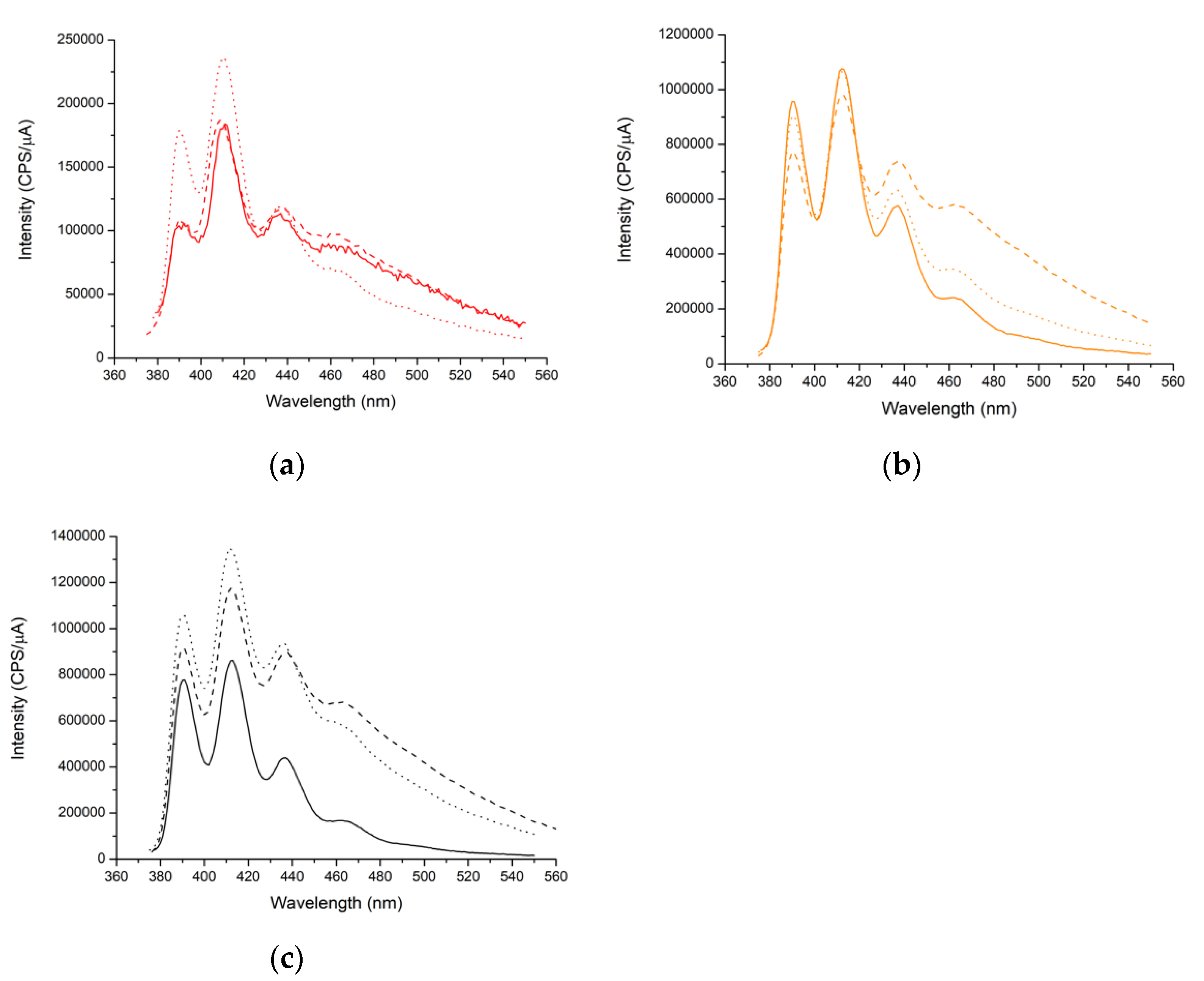
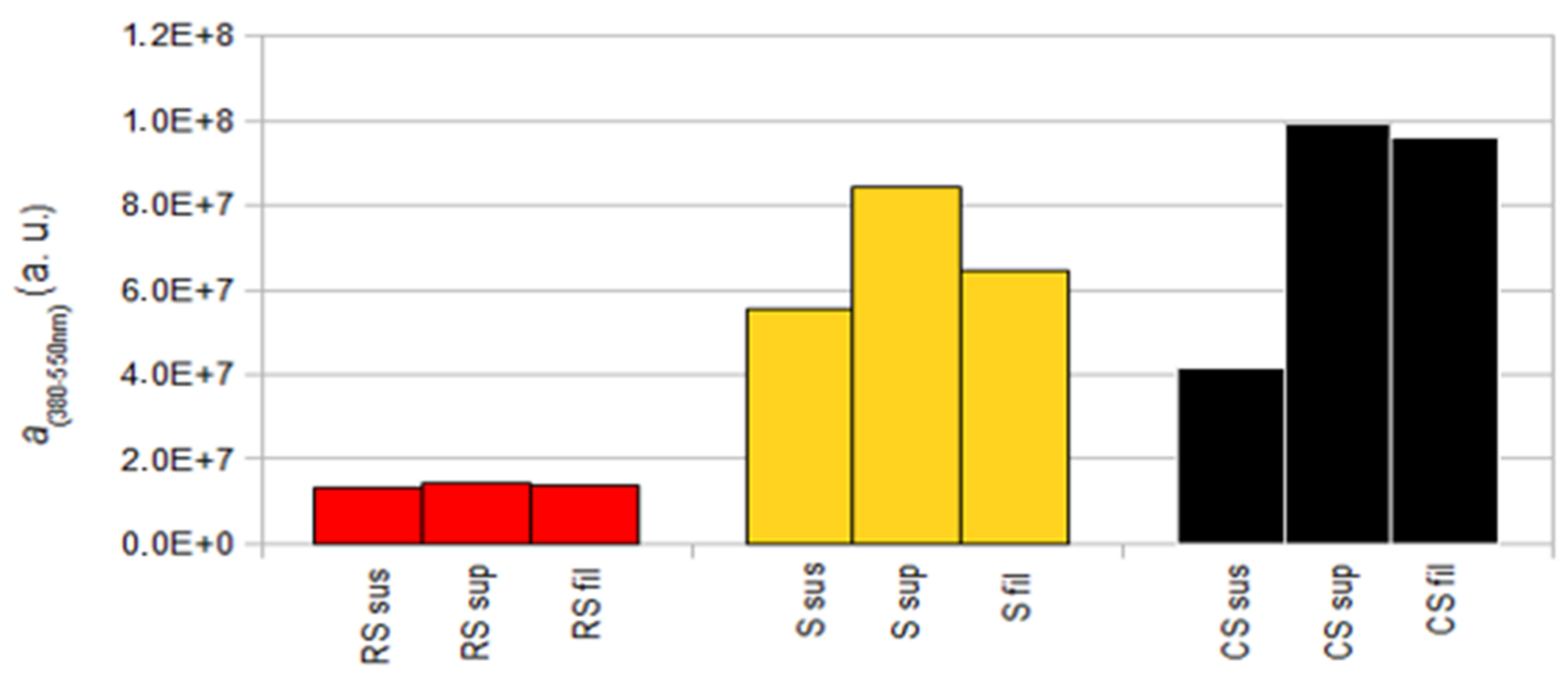
3.3. Study of the Luminescent Properties of the Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suarez, P.L.; Garcia-Cortes, M.; Fernandez-Arguelles, M.T.; Encinar, J.R.; Valledor, M.; Ferrero, F.J.; Campo, J.C.; Costa-Fernandez, J.M. Functionalized phosphorescent nanoparticles in (bio)chemical sensing and imaging—A review. Anal. Chim. Acta 2019, 1046, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Mako, T.L.; Racicot, J.M.; Levine, M. Supramolecular Luminescent Sensors. Chem. Rev. 2019, 119, 322–477. [Google Scholar] [CrossRef] [PubMed]
- Baeza, A.; Colilla, M.; Vallet-Regi, M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery. Expert Opin. Drug. Del. 2015, 12, 319–337. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Qju, H.L.; Prasad, P.N.; Chen, X.Y. Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef] [PubMed]
- Mamaeva, V.; Sahlgren, C.; Linden, M. Mesoporous silica nanoparticles in medicine-Recent advances. Adv. Drug Deliver. Rev. 2013, 65, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.; Chakraborty, I.; Dominguez, A.; Martinez-Gonzalez, J.; Sameera, W.M.C.; Mascharak, P.K. A Luminescent Manganese PhotoCORM for CO Delivery to Cellular Targets under the Control of Visible Light. Inorg. Chem. 2018, 57, 1766–1773. [Google Scholar] [CrossRef] [PubMed]
- Sidman, J.W. Electronic and Vibrational States of Anthracene. J. Chem. Phys. 1956, 25, 115–121. [Google Scholar] [CrossRef]
- Tigoianu, R.-I.; Airinei, A.; Dorohoi, D.-O. Solvent influence on the electronic fluorescence spectra of anthracene. Rev. Chim. 2010, 61, 491–494. [Google Scholar]
- Ware, W.R.; Baldwin, B.A. Absorption intensity + fluorescence lifetimes of molecules. J. Chem. Phys. 1964, 40, 1703–1705. [Google Scholar] [CrossRef]
- Dawson, W.R.; Windsor, M.W. Fluorescence yields of aromatic compounds. J. Phys. Chem. 1968, 72, 3251–3260. [Google Scholar] [CrossRef]
- Lampert, R.A.; Chewter, L.A.; Phillips, D.; Oconnor, D.V.; Roberts, A.J.; Meech, S.R. Standards for nanosecond fluorescence decay time measurements. Anal. Chem. 1983, 55, 68–73. [Google Scholar] [CrossRef]
- Bazilevskaya, N.S.; Cherkasov, A.S. Excited dimers of anthracene derivatives I. Opt. Spectrosc.-Ussr 1965, 18, 30. [Google Scholar]
- Werner, T.C.; Hercules, D.M. Fluorescence of 9-anthroic acid and its esters. environmental effects on excited-state behavior. J. Phys. Chem. 1969, 73, 2005–2011. [Google Scholar] [CrossRef]
- Suzuki, S.; Fujii, T.; Yoshiike, N.; Komatsu, S.; Iida, T. Absorption and fluorescence-spectra of anthracenecarboxylic acids.1. 9-anthroic acid and formation of excimer. Bull. Chem. Soc. Jpn. 1978, 51, 2460–2466. [Google Scholar] [CrossRef]
- Swayambunathan, V.; Lim, E.C. Electronic-structure and spectra of 9-anthroic acid and its esters in supersonic free jets. J. Phys. Chem. 1987, 91, 6359–6364. [Google Scholar] [CrossRef]
- Ghoneim, N.; Scherrer, D.; Suppan, P. Dual luminescence, structure and excimers of 9-anthracene carboxylic-acid. J. Lumin. 1993, 55, 271–275. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, M.S.A.; Galal, H.R.; Dessouky, A.F.M.; El-Naggar, M.; Mekkawi, D.; Ali, S.S.; Attya, G.M. Fluorescence and photostability studies of anthracene-9-carboxylic acid in different media. Int. J. Photoenergy 2000, 2, 47–53. [Google Scholar] [CrossRef]
- Momiji, I.; Yoza, C.; Matsui, K. Fluorescence spectra of 9-anthracenecarboxylic acid in heterogeneous environments. J. Phys. Chem. B 2000, 104, 1552–1555. [Google Scholar] [CrossRef]
- Rodriguez-Cordoba, W.; Noria-Moreno, R.; Navarro, P.; Peon, J. Ultrafast fluorescence study of the effect of carboxylic and carboxylate substituents on the excited state properties of anthracene. J. Lumin. 2014, 145, 697–707. [Google Scholar] [CrossRef]
- Rowe, J.M.; Hay, J.M.; Maza, W.A.; Chapleski, R.C., Jr.; Soderstrom, E.; Troya, D.; Morris, A.J. Systematic investigation of the excited-state properties of anthracene-dicarboxylic acids. J. Photochem. Photobiol. A Chem. 2017, 337, 207–215. [Google Scholar] [CrossRef]
- Abou-Hatab, S.; Spata, V.A.; Matsika, S. Substituent Effects on the Absorption and Fluorescence Properties of Anthracene. J. Phys. Chem. A 2017, 121, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.D.; Arnold, A.E.; Gallant, S.T.; Grinton, C.R.; Locke, J.K.; Mills, N.D.; Snow, C.A.; Uhlig, T.B.; Vessey, C.N. The polarity sensitivity factor of some fluorescent probe molecules used for studying supramolecular systems and other heterogeneous environments. Can. J. Chem. 2018, 96, 629–635. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, T.Q.; Shan, B.Q.; Liu, P.C.; Peng, B.; Xue, Q.S.; Yuan, E.H.; Wu, P.; Albela, B.; Bonneviot, L. Dendritic and Core-Shell-Corona Mesoporous Sister Nanospheres from Polymer-Surfactant-Silica Self-Entanglement. Chem. Eur. J. 2018, 24, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Turquet, F.-X.; Garcia-Cirera, B.; Albela, B.; Bonneviot, L.; Font-Bardia, M.; Corbella, M. Reactivity of 9-anthracenecarboxylate in the presence of Mn(II) and Mn(III) ions: Biomimetic aerobic oxidative decarboxylation catalysed by a manganese(III) 2,2′-bipyridine complex. Inorg. Chim. Acta 2020, 513, 119949. [Google Scholar] [CrossRef]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Escriche-Tur, L.; Albela, B.; Font-Bardia, M.; Corbella, M. Singlet ground states in compounds with a (Mn4O2)-O-III (8+) core due to broken degeneration. New J. Chem. 2017, 41, 2934–2940. [Google Scholar] [CrossRef]
- Liu, J.; Yang, T.; Wang, D.-W.; Lu, G.Q.; Zhao, D.; Qiao, S.Z. A facile soft-template synthesis of mesoporous polymeric and carbonaceous nanospheres. Nat. Commun. 2013, 4, 2798. [Google Scholar] [CrossRef]
- Tan, L.L.; Ong, W.J.; Chai, S.P.; Mohamed, A.R. Reduced graphene oxide-TiO2 nanocomposite as a promising visible-light-active photocatalyst for the conversion of carbon dioxide. Nanoscale Res. Lett. 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- El-Khodary, S.A.; El-Enany, G.M.; El-Okr, M.; Ibrahim, M. Preparation and Characterization of Microwave Reduced Graphite Oxide for High-Performance Supercapacitors. Electrochim. Acta 2014, 150, 269–278. [Google Scholar] [CrossRef]
- Pocsik, I.; Hundhausen, M.; Koos, M.; Ley, L. Origin of the D peak in the Raman spectrum of microcrystalline graphite. J. Non-Cryst. Solids 1998, 227, 1083–1086. [Google Scholar] [CrossRef]
- Garcia, H.C.; Diniz, R.; Yoshida, M.I.; de Oliveira, L.F.C. An intriguing hydrogen bond arrangement of polymeric 1D chains of 4,4′-bipyridine coordinated to Co2+, Ni2+, Cu2+ and Zn2+ ions having barbiturate as counterions in a 3D network. Crystengcomm 2009, 11, 881–888. [Google Scholar] [CrossRef]
- Salzillo, T.; Venuti, E.; Femoni, C.; Della Valle, R.G.; Tarroni, R.; Brillante, A. Crystal Structure of the 9-Anthracene-Carboxylic Acid Photochemical Dimer and Its Solvates by X-ray Diffraction and Raman Microscopy. Cryst. Growth Des. 2017, 17, 3361–3370. [Google Scholar] [CrossRef]
- Michota, A.; Bukowska, J. Surface-enhanced Raman scattering (SERS) of 4-mercaptobenzoic acid on silver and gold substrates. J. Raman Spectrosc. 2003, 34, 21–25. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Montagnac, G.; Hao, J.; Pedreira-Segade, U.; Daniel, I. Detection of nucleotides adsorbed onto clay by UV resonant raman spectroscopy: A step towards the search for biosignatures on Mars. Appl. Clay Sci. 2021, 200, 105824. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas solid systems with special reference to the determination of surface-area and porosity (recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Lijewski, S.; Wencka, M.; Hoffmann, S.K.; Kempinski, M.; Kempinski, W.; Sliwinska-Bartkowiak, M. Electron spin relaxation and quantum localization in carbon nanoparticle: Electron spin echo studies. Phys. Rev. B 2008, 77. [Google Scholar] [CrossRef]
- Swarnalatha, S.; Selvi, P.K.; Kumar, A.G.; Sekaran, G. Nanoemulsion drug delivery by ketene based polyester synthesized using electron rich carbon/silica composite surface. Colloids Surf. B Biointerfaces 2008, 65, 292–299. [Google Scholar] [CrossRef]
- Baikousi, M.; Daikopoulos, C.; Georgiou, Y.; Bourlinos, A.; Zboril, R.; Deligiannakis, Y.; Karakassides, M.A. Novel Ordered Mesoporous Carbon with Innate Functionalities and Superior Heavy Metal Uptake. J. Phys. Chem. C 2013, 117, 16961–16971. [Google Scholar] [CrossRef]
- Escriche-Tur, L.; Corbella, M.; Font-Bardia, M.; Castro, I.; Bonneviot, L.; Albela, B. Biomimetic Mn-Catalases Based on Dimeric Manganese Complexes in Mesoporous Silica for Potential Antioxidant Agent. Inorg. Chem. 2015, 54, 10111–10125. [Google Scholar] [CrossRef]
- Chaignon, J.; Stiriba, S.E.; Lloret, F.; Yuste, C.; Pilet, G.; Bonneviot, L.; Albela, B.; Castro, I. Bioinspired manganese(II) complexes with a clickable ligand for immobilisation on a solid support. Dalton Trans. 2014, 43, 9704–9713. [Google Scholar] [CrossRef]
- Garcia-Cirera, B. Compostos Polinuclears de Manganès Mimètics D’enzims Redox. In Inserció en Suports Mesoestructurats. Estudi de les Propietats Magnètiques i Catalítiques; Universitat de Barcelona: Barcelona, Spain, 2016. [Google Scholar]
- Chaignon, J.; Gourgues, M.; Khrouz, L.; Moliner, N.; Bonneviot, L.; Fache, F.; Castro, I.; Albela, B. A bioinspired heterogeneous catalyst based on the model of the manganese-dependent dioxygenase for selective oxidation using dioxygen. RSC Adv. 2017, 7, 17336–17345. [Google Scholar] [CrossRef]
- Escriche-Tur, L.; Font-Bardia, M.; Albela, B.; Montserrat Corbella, C. New insights into the comprehension of the magnetic properties of dinuclear Mn(III) compounds with general formula [{MnL(NN)}2(μ-O)(μ-n-RC6H4COO)2]X2. Dalton Trans. 2016, 45, 11753–11764. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cirera, B.; Gomez-Coca, S.; Font-Bardia, M.; Ruiz, E.; Corbella, M. Influence of the Disposition of the Anisotropy Axes into the Magnetic Properties of Mn-III Dinuclear Compounds with Benzoato Derivative Bridges. Inorg. Chem. 2017, 56, 8135–8146. [Google Scholar] [CrossRef]
- Escriche-Tur, L.; Font-Bardia, M.; Albela, B.; Corbella, M. Determination of ZFS parameters from the EPR spectra of mono-, di- and trinuclear Mn-II complexes: Impact of magnetic coupling. Dalton Trans. 2017, 46, 2699–2714. [Google Scholar] [CrossRef] [PubMed]
- Jakob, A.M.; Schmedake, T.A. A Novel Approach to Monodisperse, Luminescent Silica Spheres. Chem. Mater. 2006, 18, 3173–3175. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, J. Defect-related luminescent materials: Synthesis, emission properties and applications. Chem. Soc. Rev. 2012, 41, 7938–7961. [Google Scholar] [CrossRef] [PubMed]
- Zhanbotin, A.; Soriano, R.B.; Ikonen, A.K.; Nurakhmetov, T.N.; Schmedake, T.A. Luminescent mesoporous colloidal silica: A nanoporous substrate for photosensitization of lanthanide ions. Mater. Lett. 2011, 65, 10–12. [Google Scholar] [CrossRef]
- Guo, S.-R.; Gong, J.-Y.; Jiang, P.; Wu, M.; Lu, Y.; Yu, S.-H. Biocompatible, Luminescent Silver@Phenol Formaldehyde Resin Core/Shell Nanospheres: Large-Scale Synthesis and Application for In Vivo Bioimaging. Adv. Funct. Mater. 2008, 18, 872–879. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, Y.; Lu, Y.; Xu, Q.-Z.; Xu, X.-W.; Dong, L.; Yu, S.-H. Phenol Formaldehyde Resin Nanoparticles Loaded with CdTe Quantum Dots: A Fluorescence Resonance Energy Transfer Probe for Optical Visual Detection of Copper(II) Ions. ACS Nano 2011, 5, 2147–2154. [Google Scholar] [CrossRef]
- Garcia-Cirera, B.; Corbella, M.; Bonneviot, L.; Albela, B. Bio-inspired manganese mesoporous silica hybrid material as a water compatible antioxidant. Microporous Mesoporous Mater. 2018, 261, 150–157. [Google Scholar] [CrossRef]



| Material | wt% [MnII] | Material | wt% [MnIII] |
|---|---|---|---|
| 1-[MnII]@RSNP | 0.6 | 1-[MnIII]@RSNP | 1.0 |
| 3-[MnII]@RSNP | 2.0 | 3-[MnIII]@RSNP | 2.7 |
| 5-[MnII]@RSNP | 2.9 | 5-[MnIII]@RSNP | 4.6 |
| 1-[MnII]@CSNP | 0.4 | 1-[MnIII]@CSNP | 0.9 |
| 3-[MnII]@CSNP | 2.9 | 3-[MnIII]@CSNP | 1.2 |
| 5-[MnII]@CSNP | 4.6 | 5-[MnIII]@CSNP | 4.2 |
| 1-[MnII]@SNP | 1.2 | 1-[MnIII]@SNP | 0.9 |
| 3-[MnII]@SNP | 2.9 | 3-[MnIII]@SNP | 3.5 |
| 5-[MnII]@SNP | 3.9 | 5-[MnIII]@SNP | 3.8 |
| Material | Capture 1 | Centrifugation Release 2 | Filtration Release 3 |
|---|---|---|---|
| 5-[MnII]@RSNP | 88 | 5.8 | 0.7 |
| 5-[MnII]@SNP | 38 | 51 | 17 |
| 5-[MnII]@RSNP | 46 | 138 | 130 |
| Material | Capture 1 | Centrifugation Release 2 | Filtration Release 3 |
|---|---|---|---|
| 5-[MnIII]@SNP | 47 | 87 | 59 |
| 5-[MnIII]@CSNP | 40 | 139 | 125 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turquet, F.-X.; Corbella, M.; Fellah, C.; Montagnac, G.; Reynard, B.; Bonneviot, L.; Zhang, K.; Albela, B. Incorporation of Manganese Complexes within Hybrid Resol-Silica and Carbon-Silica Nanoparticles. Nanomaterials 2021, 11, 774. https://doi.org/10.3390/nano11030774
Turquet F-X, Corbella M, Fellah C, Montagnac G, Reynard B, Bonneviot L, Zhang K, Albela B. Incorporation of Manganese Complexes within Hybrid Resol-Silica and Carbon-Silica Nanoparticles. Nanomaterials. 2021; 11(3):774. https://doi.org/10.3390/nano11030774
Chicago/Turabian StyleTurquet, François-Xavier, Montserrat Corbella, Clémentine Fellah, Gilles Montagnac, Bruno Reynard, Laurent Bonneviot, Kun Zhang, and Belén Albela. 2021. "Incorporation of Manganese Complexes within Hybrid Resol-Silica and Carbon-Silica Nanoparticles" Nanomaterials 11, no. 3: 774. https://doi.org/10.3390/nano11030774
APA StyleTurquet, F.-X., Corbella, M., Fellah, C., Montagnac, G., Reynard, B., Bonneviot, L., Zhang, K., & Albela, B. (2021). Incorporation of Manganese Complexes within Hybrid Resol-Silica and Carbon-Silica Nanoparticles. Nanomaterials, 11(3), 774. https://doi.org/10.3390/nano11030774







