880 nm NIR-Triggered Organic Small Molecular-Based Nanoparticles for Photothermal Therapy of Tumor
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
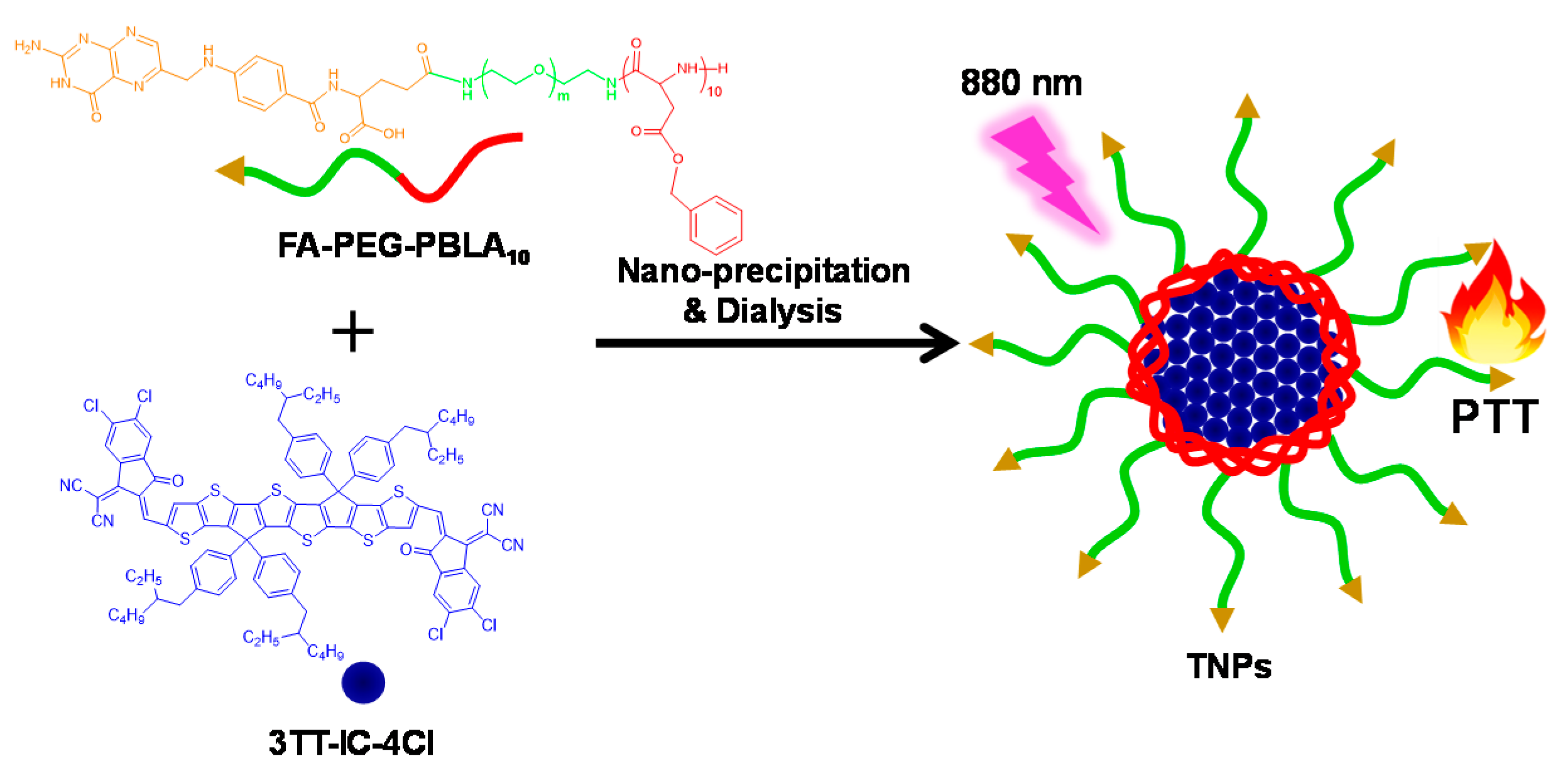
2.3. Preparation of TNPs
2.4. Photothermal Effect
2.5. Stability of TNPs
2.6. In Vitro Phototoxicity and Biocompatibility of TNPs
3. Results and Discussion
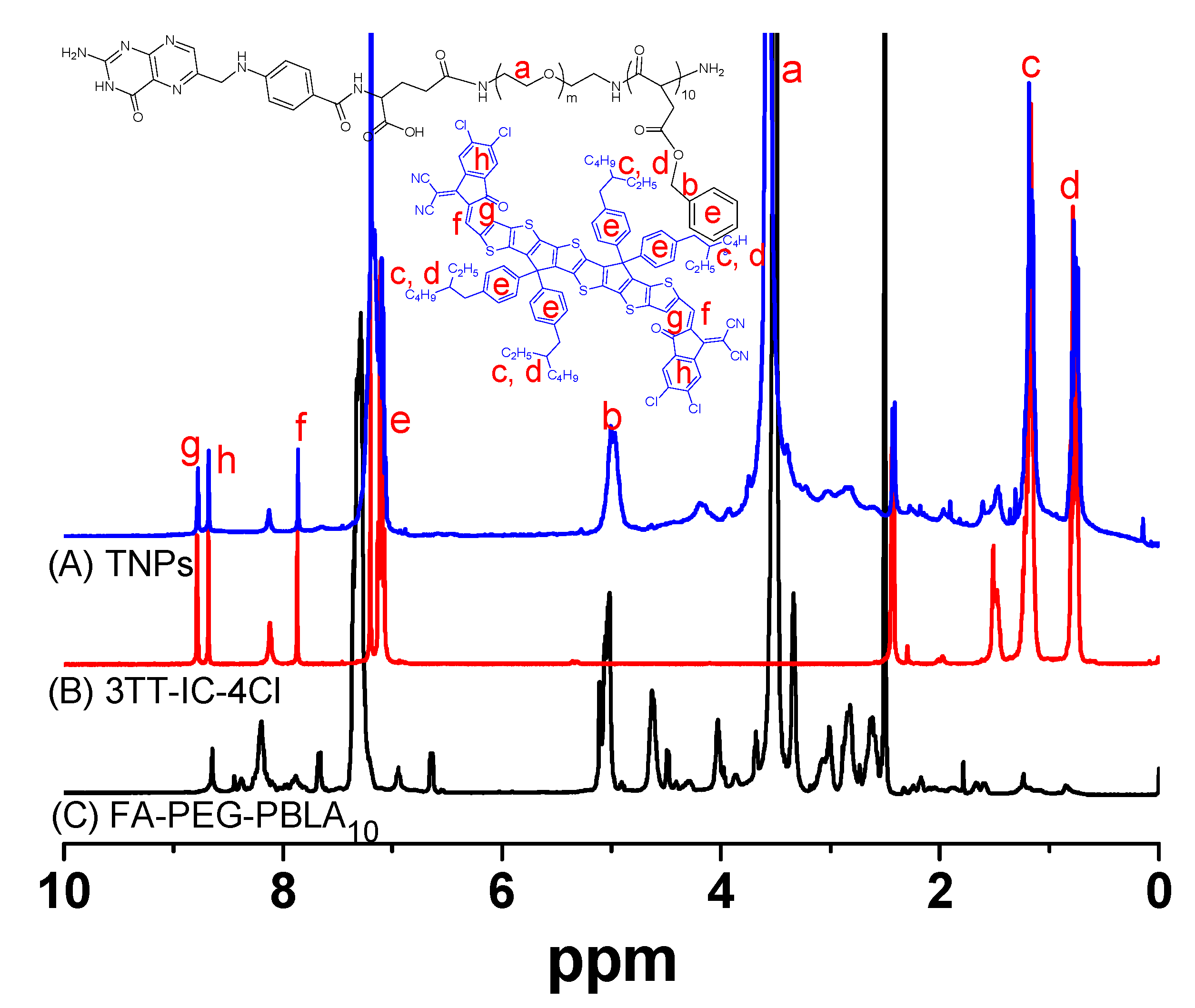
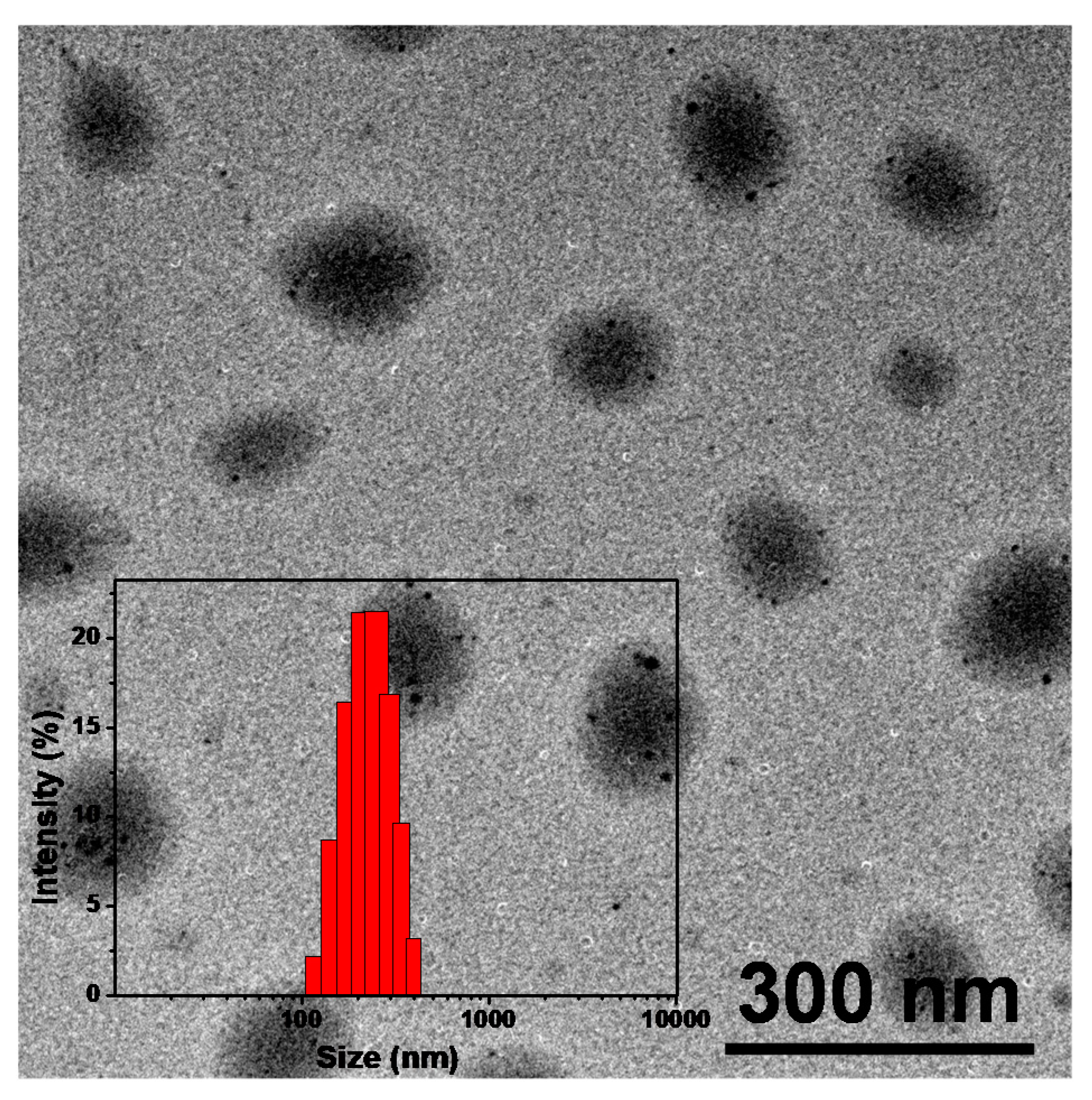
3.1. Synthesis and Characterization of TNPs
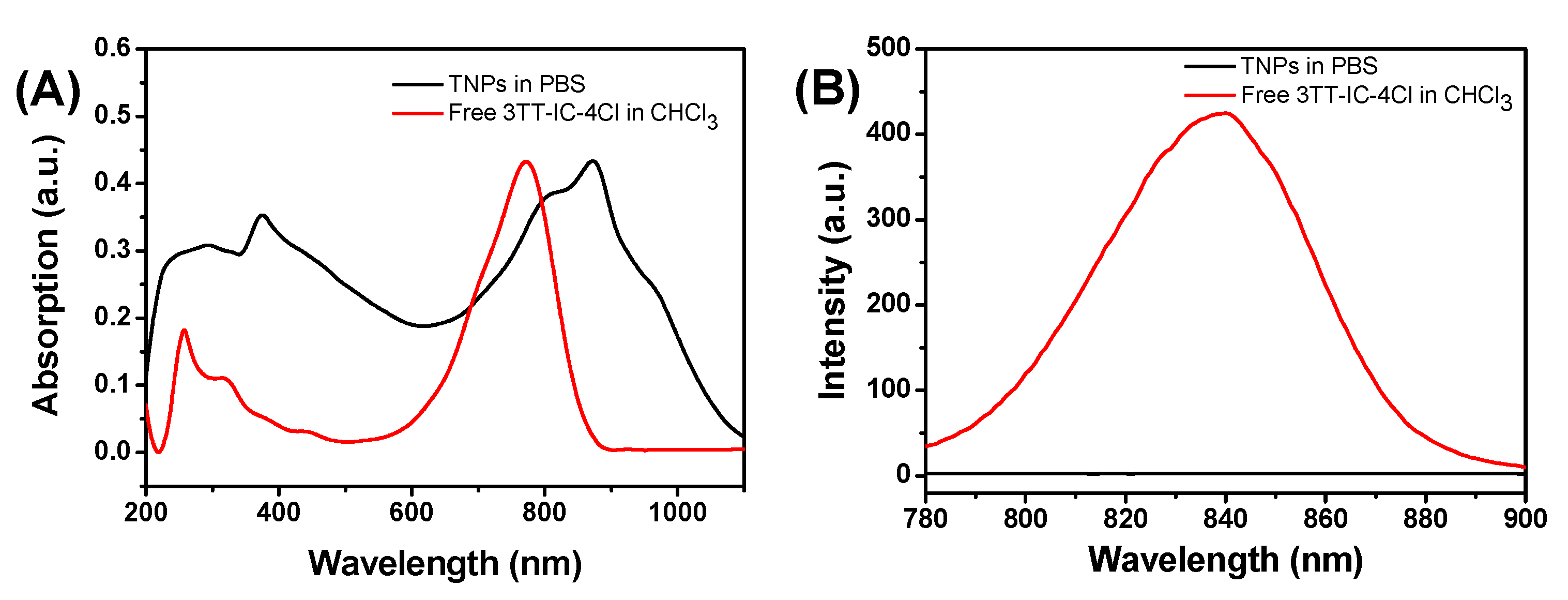
3.2. Optical Properties of TNPs
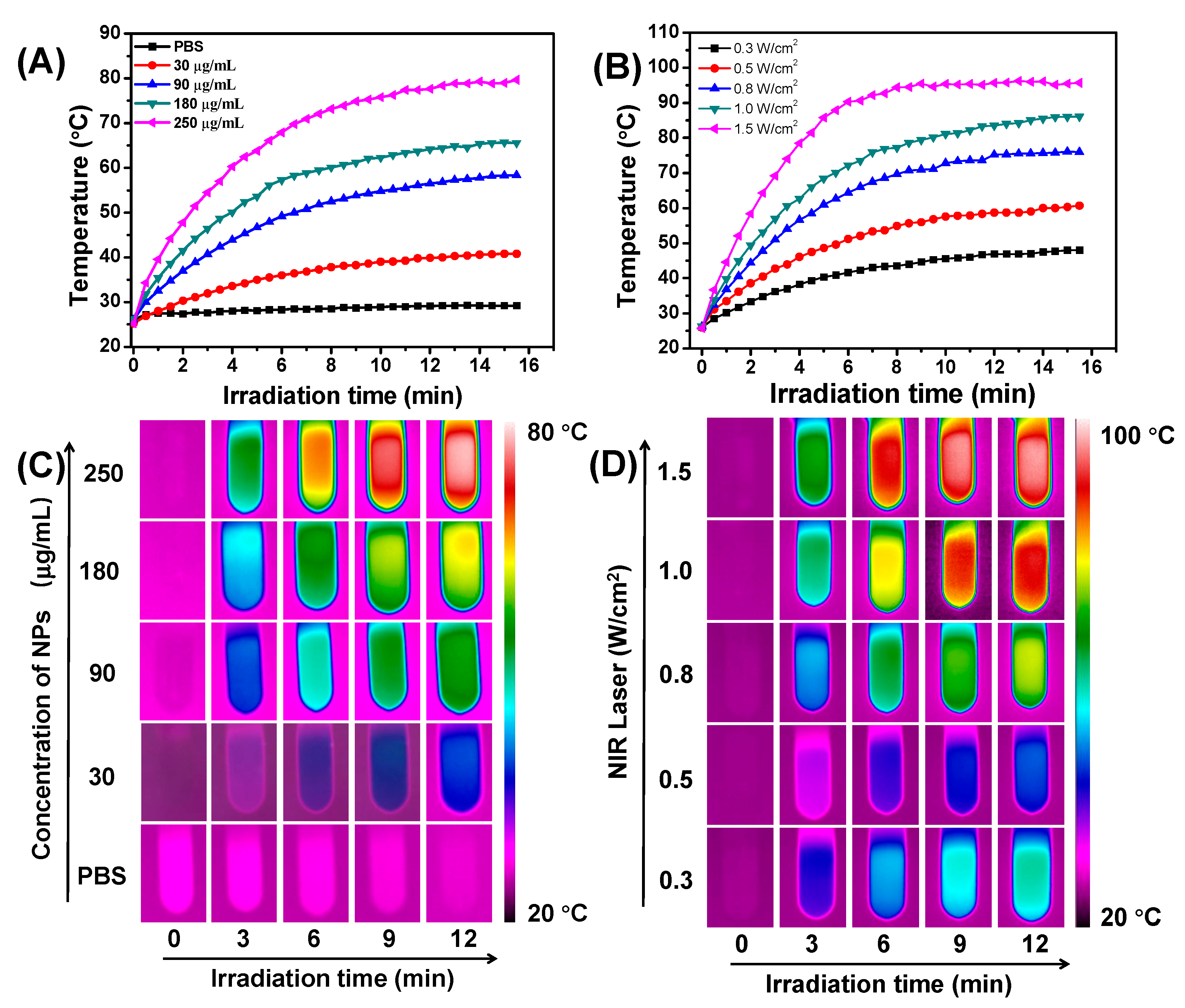
3.3. Photothermal Properties of TNPs In Vitro
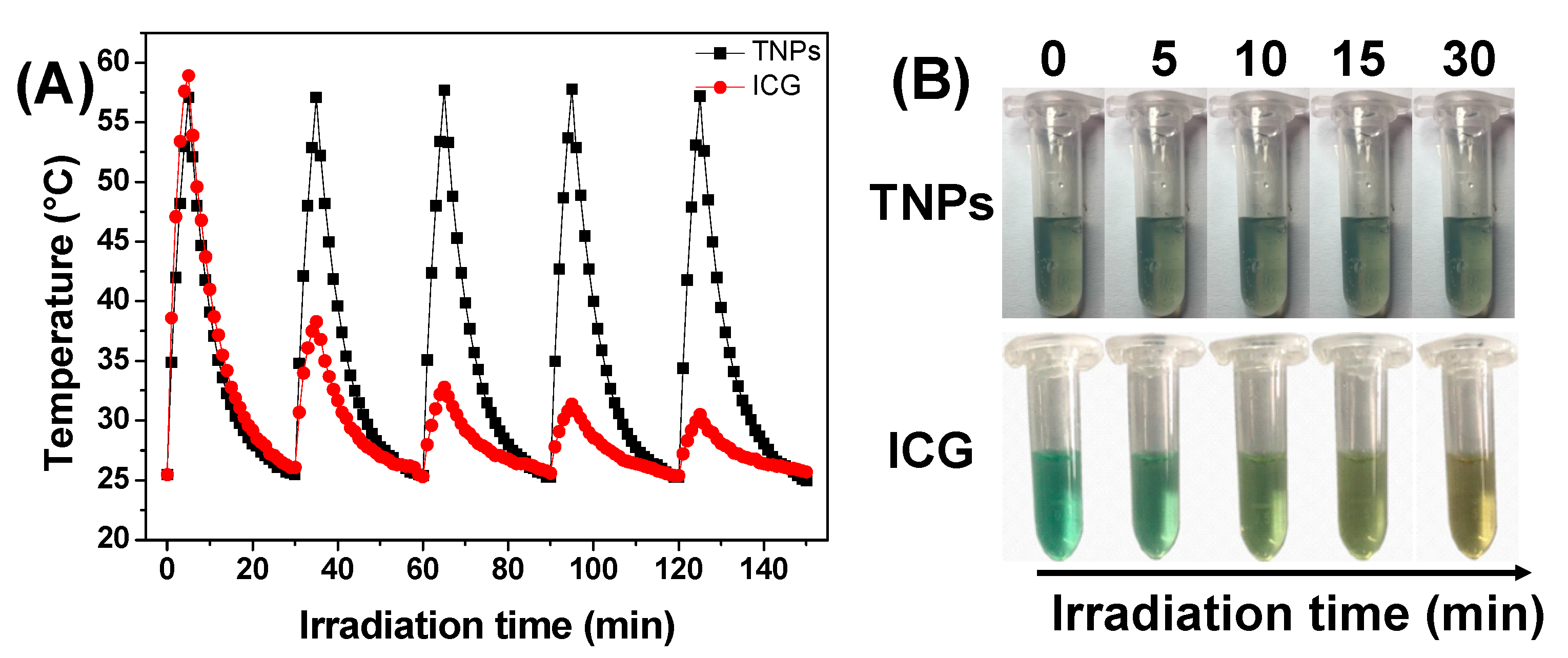
3.4. Photothermal Stability of TNPs
3.5. In Vitro Cell Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, J.; Pu, K. Development of organic semiconducting materials for deep-tissue optical imaging, phototherapy and photoactivation. Chem. Soc. Rev. 2019, 48, 38–71. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, T.Y.; Luo, Y.; Liu, Q.; Xiao, W.; Guo, W.; Lac, D.; Zhang, H.; Feng, C.; Wachs-mann-Hogiu, S.; et al. A smart and versatile theranostic nanomedicine platform based on nanoporphyrin. Nat. Commun. 2014, 5, 4712. [Google Scholar] [CrossRef] [PubMed]
- Antaris, A.L.; Chen, H.; Cheng, K.; Sun, Y.; Hong, G.; Qu, C.; Diao, S.; Deng, Z.; Hu, X.; Zhang, B.; et al. A small-molecule dye for NIR-II imaging. Nat. Mater. 2015, 15, 235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jeon, M.; Rich, L.J.; Hong, H.; Geng, J.; Zhang, Y.; Shi, S.; Barnhart, T.E.; Alexan-dridis, P.; Huizinga, J.D.; et al. Non-invasive multimodal functional imaging of the intestine with frozen micellar naphthalocyanines. Nat. Nanotechnol. 2014, 9, 631. [Google Scholar] [CrossRef] [PubMed]
- Abuteen, A.; Zanganeh, S.; Akhigbe, J.; Samankumara, L.P.; Aguirre, A.; Biswal, N.; Braune, M.; Vollertsen, A.; Röder, B.; Brückner, C.; et al. The evaluation of NIR-absorbing porphyrin derivatives as contrast agents in photoacoustic imaging. Phys. Chem. Chem. Phys. 2013, 15, 18502–18509. [Google Scholar] [CrossRef]
- Song, X.; Chen, Q.; Liu, Z. Recent advances in the development of organic photothermal nano-agents. Nano Res. 2015, 8, 340–354. [Google Scholar] [CrossRef]
- Jung, H.S.; Verwilst, P.; Sharma, A.; Shin, J.; Sessler, J.L.; Kim, J.S. Organic molecule-based photothermal agents: An expanding photothermal therapy universe. Chem. Soc. Rev. 2018, 47, 2280–2297. [Google Scholar] [CrossRef]
- Wang, H.; Chang, J.; Shi, M.; Pan, W.; Li, N.; Tang, B. A Dual-targeted organic photothermal agent for enhanced photothermal therapy. Angew. Chem. Int. Ed. 2019, 58, 1057–1061. [Google Scholar] [CrossRef]
- Asadian-Birjand, M.; Bergueiro, J.; Wedepohl, S.; Calderón, M. Near Infrared dye conjugated nanogels for combined photodynamic and photothermal therapies. Macromol. Biosci. 2016, 16, 1432–1441. [Google Scholar] [CrossRef]
- Luo, S.; Tan, X.; Fang, S.; Wang, Y.; Liu, T.; Wang, X.; Yuan, Y.; Sun, H.; Qi, Q.; Shi, C. Mito-chondria-targeted small-molecule fluorophores for dual modal cancer phototherapy. Adv. Funct. Mater. 2016, 26, 2826–2835. [Google Scholar] [CrossRef]
- Yang, W.; Noh, J.; Park, H.; Gwon, S.; Singh, B.; Song, C.; Lee, D. Near infrared dye-conjugated oxidative stress amplifying polymer micelles for dual imaging and synergistic anticancer phototherapy. Biomaterials 2018, 154, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Heshmati Aghda, N.; Abdulsahib, S.M.; Severson, C.; Lara, E.J.; Torres Hurtado, S.; Yildiz, T. Induction of immunogenic cell death of cancer cells through nanoparticle-mediated dual chemotherapy and photothermal therapy. Int. J. Pharm. 2020, 589, 119787. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; He, Z.; Chen, Y.; Zhou, C.; Wang, C.; Liu, Y. Dual drug delivery system of photothermal-sensitive carboxymethyl chitosan nanosphere for photothermal-chemotherapy. Int. J. Biol. Macromol. 2020, 163, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Liang, P.; Tang, Q.; Yang, X.; Si, W.; Huang, W.; Zhang, Q.; Dong, X. Diketo-pyrrolopyrrole–triphenylamine organic nanoparticles as multifunctional reagents for photoacoustic imaging-guided photodynamic/photothermal synergistic tumor therapy. ACS Nano 2017, 11, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Dai, Y.; Xu, J.; Cai, J.; Niu, X.; Zhang, L.; Chen, R.; Shen, Q.; Huang, W.; Fan, Q. All-in-one phototheranostics: Single laser triggers NIR-II fluorescence/photoacoustic imaging guided photothermal/photodynamic/chemo combination therapy. Adv. Funct. Mater. 2019, 29, 1901480. [Google Scholar] [CrossRef]
- Spence, G.T.; Hartland, G.V.; Smith, B.D. Activated photothermal heating using croconaine dyes. Chem. Sci. 2013, 4, 4240–4244. [Google Scholar] [CrossRef]
- Spence, G.T.; Lo, S.S.; Ke, C.; Destecroix, H.; Davis, A.P.; Hartland, G.V.; Smith, B.D. Near-infrared croconaine rotaxanes and doped nanoparticles for enhanced aqueous photothermal heating. Chem. Eur. J. 2014, 20, 12628–12635. [Google Scholar] [CrossRef]
- Lovell, J.F.; Jin, C.S.; Huynh, E.; Jin, H.; Kim, C.; Rubinstein, J.L.; Chan, W.C.W.; Cao, W.; Wang, L.V.; Zheng, G. Porphysome nanovesicles generated by porphyrin bilayers for use as multi-modal biophotonic contrast agents. Nat. Mater. 2011, 10, 324. [Google Scholar] [CrossRef]
- Peng, J.; Zhao, L.; Zhu, X.; Sun, Y.; Feng, W.; Gao, Y.; Wang, L.; Li, F. Hollow silica nanoparticles loaded with hydrophobic phthalocyanine for near-infrared photodynamic and photothermal combination therapy. Biomaterials 2013, 34, 7905–7912. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, C.; Zhang, R.; Chen, R.; Zhang, Z.; Zhang, W.; Peng, S.H.; Chen, X.; Liu, G.; Hsu, C.S.; et al. Biocompatible D–A semiconducting polymer nanoparticle with light-harvesting unit for highly effective photoacoustic imaging guided photothermal therapy. Adv. Funct. Mater. 2017, 27, 1605094. [Google Scholar] [CrossRef]
- Zou, Q.; Abbas, M.; Zhao, L.; Li, S.; Shen, G.; Yan, X. Biological photothermal nanodots based on self-assembly of peptide–porphyrin conjugates for antitumor therapy. J. Am. Chem. Soc. 2017, 139, 1921–1927. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Ma, Y.; Zheng, Z.; Wu, C.; Wang, Y.; Liang, G. Facile syntheses of conjugated polymers for photothermal tumour therapy. Nat. Commun. 2019, 10, 1192. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Sheng, Z.; Hu, D.; Li, A.; Xu, S.; Manghnani, P.N.; Liu, C.; Guo, L.; Zheng, H.; Liu, B. Molecular engineering of conjugated polymers for biocompatible organic nanoparticles with highly efficient photoacoustic and photothermal performance in cancer theranostics. ACS Nano 2017, 11, 10124–10134. [Google Scholar] [CrossRef]
- Yang, T.; Liu, L.; Deng, Y.; Guo, Z.; Zhang, G.; Ge, Z.; Ke, H.; Chen, H. Ultrastable near-infrared conjugated-polymer nanoparticles for dually photoactive tumor inhibition. Adv. Mater. 2017, 29, 1700487. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Xu, L.; Wang, D.; Long, J.; Zhang, M. Near-Infrared-absorbing conjugated polymer nanoparticles loaded with doxorubicin for combinatorial photothermal-chemotherapy of cancer. ACS Appl. Polym. Mater. 2020, 2, 4180–4187. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Wang, Z.; Feng, L. Photothermal conjugated polymers and their biological applications in imaging and therapy. ACS Appl. Polym. Mater. 2020, 2, 4222–4240. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, H.M.; Song, G.; Li, Z.; Zhang, X.B. Conjugated-polymer-based nanomaterials for photothermal therapy. ACS Appl. Polym. Mater. 2020, 2, 4258–4272. [Google Scholar] [CrossRef]
- Huff, M.E.; Gökmen, F.Ö.; Barrera, J.S.; Lara, E.J.; Tunnell, J.; Irvin, J. Induction of immunogenic cell death in breast cancer by conductive polymer nanoparticle-mediated photothermal therapy. ACS Appl. Polym. Mater. 2020, 2, 5602–5620. [Google Scholar] [CrossRef]
- Lu, K.Y.; Jheng, P.R.; Lu, L.S.; Rethi, L.; Mi, F.L.; Chuang, E.Y. Enhanced anticancer effect of ROS-boosted photothermal therapy by using fucoidan-coated polypyrrole nanoparticles. Int. J. Biol. Macromol. 2021, 166, 98–107. [Google Scholar] [CrossRef]
- Prostota, Y.; Kachkovsky, O.D.; Reis, L.V.; Santos, P.F. New unsymmetrical squaraine dyes derived from imidazo[1,5-a]pyridine. Dye. Pigment. 2013, 96, 554–562. [Google Scholar] [CrossRef]
- Gao, F.P.; Lin, Y.X.; Li, L.L.; Liu, Y.; Mayerhöffer, U.; Spenst, P.; Su, J.G.; Li, J.Y.; Würthner, F.; Wang, H. Supramolecular adducts of squaraine and protein for noninvasive tumor imaging and photothermal therapy in vivo. Biomaterials 2014, 35, 1004–1014. [Google Scholar] [CrossRef]
- Guo, Z.; Zou, Y.; He, H.; Rao, J.; Ji, S.; Cui, X.; Ke, H.; Deng, Y.; Yang, H.; Chen, C.; et al. Bifunctional platinated nanoparticles for photoinduced tumor ablation. Adv. Mater. 2016, 28, 10155–10164. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Xu, B.; Fu, T.; Cai, M.; Li, F.; Zhang, Y.; Wang, Q. Near-infrared photoluminescent Ag2S quantum dots from a single source precursor. J. Am. Chem. Soc. 2010, 132, 1470–1471. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yu, M.; Sun, Y.; Zhang, X.; Zhu, X.; Wu, Z.; Wu, D.; Li, F. Fluorine-18-labeled Gd3+/Yb3+/Er3+ codoped NaYF4 nanophosphors for multimodality PET/MR/UCL imaging. Biomaterials 2011, 32, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Atilgan, S.; Ekmekci, Z.; Dogan, A.L.; Guc, D.; Akkaya, E.U. Water soluble distyryl-boradiazaindacenes as efficient photosensitizers for photodynamic therapy. Chem. Commun. 2006, 4398–4400. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhou, J.; Shen, Z.; Ding, L.; Yu, J.S.; Ju, H. A pH-activatable and aniline-substituted photosensitizer for near-infrared cancer theranostics. Chem. Sci. 2015, 6, 5969–5977. [Google Scholar] [CrossRef]
- Drogat, N.; Gady, C.; Granet, R.; Sol, V. Design and synthesis of water-soluble polyaminated chlorins and bacteriochlorins with near-infrared absorption. Dye. Pigment. 2013, 98, 609–614. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Li, S.; Wan, Y.; Chen, J.X.; Tian, S.; Huang, Z.; Xiao, Y.F.; Cui, X.; Xiang, C.; et al. Biodegradable π-conjugated oligomer nano-particles with high photothermal conversion efficiency for cancer theranostics. ACS Nano 2019, 13, 12901–12911. [Google Scholar] [CrossRef]
- He, Z.; Zhao, L.; Zhang, Q.; Chang, M.; Li, C.; Zhang, H.; Lu, Y.; Chen, Y. An acceptor–donor–acceptor structured small molecule for effective nir triggered dual phototherapy of cancer. Adv. Funct. Mater. 2020, 30, 1910301. [Google Scholar] [CrossRef]
- Cai, Y.; Wei, Z.; Song, C.; Tang, C.; Huang, X.; Hu, Q.; Dong, X.; Han, W. Novel acceptor–donor–acceptor structured small molecule-based nanoparticles for highly efficient photothermal therapy. Chem. Commun. 2019, 55, 8967–8970. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Wang, C.; Zhang, S.; Feng, L.; Liu, Z. Photothermally enhanced photodynamic therapy delivered by nano-graphene oxide. ACS Nano 2011, 5, 7000–7009. [Google Scholar] [CrossRef]
- Yan, H.; Wu, H.; Li, K.; Wang, Y.; Tao, X.; Yang, H.; Li, A.; Cheng, R. Influence of the surface structure of graphene oxide on the adsorption of aromatic organic compounds from water. ACS Appl. Mater. Interfaces 2015, 7, 6690–6697. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Shang, W.; Chi, C.; Zeng, C.; Wang, K.; Fang, C.; Chen, Q.; Liu, H.; Fan, Y.; Tian, J. Dye-conjugated single-walled carbon nanotubes induce photothermal therapy under the guidance of near-infrared imaging. Cancer Lett. 2016, 383, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Fang, Y.; Kwok, R.T.K.; Zhang, X.; Hu, X.; Lam, J.W.Y.; Ding, D.; Tang, B.Z. Highly stable organic small molecular nanoparticles as an advanced and biocompatible phototheranostic agent of tumor in living mice. ACS Nano 2017, 11, 7177–7188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Guo, W.; Wei, J.; Li, C.; Liang, X.J.; Yin, M. Terrylenediimide-based intrinsic theranostic nanomedicines with high photothermal conversion efficiency for photoacoustic imaging-guided cancer therapy. ACS Nano 2017, 11, 3797–3805. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.; Park, J.Y.; Tung, C.H.; Kim, I.H.; Choi, Y. Gold nanorod−photosensitizer complex for near-infrared fluorescence imaging and photodynamic/photothermal therapy in vivo. ACS Nano 2011, 5, 1086–1094. [Google Scholar] [CrossRef]
- Gao, H.H.; Sun, Y.; Wan, X.; Ke, X.; Feng, H.; Kan, B.; Wang, Y.; Zhang, Y.; Li, C.; Chen, Y. A new nonfullerene acceptor with near infrared absorption for high performance ternary-blend organic solar cells with efficiency over 13%. Adv. Sci. 2018, 5, 1800307. [Google Scholar] [CrossRef]
- Zhao, L.; Kim, T.H.; Huh, K.M.; Kim, H.W.; Kim, S.Y. Self-assembled photosensitizer-conjugated nanoparticles for targeted photodynamic therapy. J. Biomater. Appl. 2013, 28, 434–447. [Google Scholar] [CrossRef]
- Byrne, J.D.; Betancourt, T.; Brannon-Peppas, L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv. Drug Deliv. Rev. 2008, 60, 1615–1626. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margali, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Lyu, Y.; Fang, Y.; Miao, Q.; Zhen, X.; Ding, D.; Pu, K. Intraparticle molecular orbital engineering of semiconducting polymer nanoparticles as amplified theranostics for in vivo photoacoustic imaging and photothermal therapy. ACS Nano 2016, 10, 4472–4481. [Google Scholar] [CrossRef] [PubMed]
- Hahn, G.M.; Braun, J.; Har-Kedar, I. Thermo/chemotherapy: Synergism between hyperthermia (42–43 degrees) and adriamycin (of bleomycin) in mammalian cell inactivation. Proc. Natl. Acad. Sci. USA 1975, 72, 937–940. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-melanin colloidal nanospheres: An efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Huang, L.; Yang, H.; Ke, H.; He, H.; Guo, Z.; Yang, T.; Zhu, A.; Wu, H.; Chen, H. Cyanine-anchored silica nanochannels for light-driven synergistic thermo-chemotherapy. Small 2017, 13, 1602747. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Xie, H.; Tang, S.; Yu, X.F.; Guo, Z.; Shao, J.; Zhang, H.; Huang, H.; Wang, H.; Chu, P.K. Ultrasmall black phosphorus quantum dots: Synthesis and use as photothermal agents. Angew. Chem. Int. Ed. 2015, 54, 11526–11530. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; He, Z.; Zhang, Q.; Wang, J.; Jia, W.; Jin, L.; Zhao, L.; Lu, Y. 880 nm NIR-Triggered Organic Small Molecular-Based Nanoparticles for Photothermal Therapy of Tumor. Nanomaterials 2021, 11, 773. https://doi.org/10.3390/nano11030773
Zhao Y, He Z, Zhang Q, Wang J, Jia W, Jin L, Zhao L, Lu Y. 880 nm NIR-Triggered Organic Small Molecular-Based Nanoparticles for Photothermal Therapy of Tumor. Nanomaterials. 2021; 11(3):773. https://doi.org/10.3390/nano11030773
Chicago/Turabian StyleZhao, Yunying, Zheng He, Qiang Zhang, Jing Wang, Wenying Jia, Long Jin, Linlin Zhao, and Yan Lu. 2021. "880 nm NIR-Triggered Organic Small Molecular-Based Nanoparticles for Photothermal Therapy of Tumor" Nanomaterials 11, no. 3: 773. https://doi.org/10.3390/nano11030773
APA StyleZhao, Y., He, Z., Zhang, Q., Wang, J., Jia, W., Jin, L., Zhao, L., & Lu, Y. (2021). 880 nm NIR-Triggered Organic Small Molecular-Based Nanoparticles for Photothermal Therapy of Tumor. Nanomaterials, 11(3), 773. https://doi.org/10.3390/nano11030773







