Supercritical CO2 Assisted Solvothermal Preparation of CoO/Graphene Nanocomposites for High Performance Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation of CoO/Graphene Composites (CoO/G)
2.3. Characterization
2.4. Electrochemical Measurements
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Goodenough, J.B. Evolution of Strategies for Modern Rechargeable Batteries. Acc. Chem. Res. 2013, 46, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.-M. Li-O2 and Li-S Batteries with High Energy Storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef]
- Wu, Y.; Xie, L.; Ming, H.; Guo, Y.; Hwang, J.-Y.; Wang, W.; He, X.; Wang, L.; Alshareef, H.N.; Sun, Y.-K.; et al. An Empirical Model for the Design of Batteries with High Energy Density. ACS Energy Lett. 2020, 5, 807–816. [Google Scholar] [CrossRef]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.M. Nano-Sized Transition-Metal-Oxides as Negative-Electrode Materials for Lithium-Ion Batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Zhang, P.; Sun, N.; Anasori, B.; Zhu, Q.-Z.; Liu, H.; Gogotsi, Y.; Xu, B. Self-Assembly of Transition Metal Oxide Nanostructures on MXene Nanosheets for Fast and Stable Lithium Storage. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef]
- Deng, M.; Li, S.; Hong, W.; Jiang, Y.; Xu, W.; Shuai, H.; Zou, G.; Hu, Y.; Hou, H.; Wang, W.; et al. Octahedral Sb2O3 as High-Performance Anode for Lithium and Sodium Storage. Mater. Chem. Phys. 2019, 223, 46–52. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, H.; Xiong, T.; Adekoya, D.; Qiu, W.; Wang, Z.; Zhang, S.; Balogun, M.S. Adsorption Energy Engineering of Nickel Oxide Hybrid Nanosheets for High Areal Capacity Flexible Lithium-Ion Batteries. Energy Storage Mater. 2020, 25, 41–51. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C.; Chen, X.; Wang, X.; Li, X.; Yamauchi, Y.; Xu, X.; Wang, J.; Lin, C.; Luo, D.; et al. MoOx Nanoparticles Anchored on N-doped Porous Carbon as Li-Ion Battery Electrode. Chem. Eng. J. 2020, 381. [Google Scholar] [CrossRef]
- Yu, S.-H.; Lee, S.H.; Lee, D.J.; Sung, Y.-E.; Hyeon, T. Conversion Reaction-Based Oxide Nanomaterials for Lithium Ion Battery Anodes. Small 2016, 12, 2146–2172. [Google Scholar] [CrossRef]
- Liang, C.; Gao, M.; Pan, H.; Liu, Y.; Yan, M. Lithium Alloys and Metal Oxides as High-Capacity Anode Materials for Lithium-ion Batteries. J. Alloys Compd. 2013, 575, 246–256. [Google Scholar] [CrossRef]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.-M. Nanomaterials for Rechargeable Lithium Batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. Graphene, Related Two-Dimensional Crystals, and Hybrid Systems for Energy Conversion and Storage. Science 2015, 347. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, J.; Ding, R.; Ji, X.; Hu, Y.; Li, X.; Hu, A.; Wu, F.; Zhu, Z.; Huang, X. Direct Synthesis of CoO Porous Nanowire Arrays on Ti Substrate and Their Application as Lithium-Ion Battery Electrodes. J. Phys. Chem. C 2010, 114, 929–932. [Google Scholar] [CrossRef]
- Wu, F.D.; Wang, Y. Self-Assembled Echinus-Like Nanostructures of Mesoporous CoO Nanorod@CNT for Lithium-Ion Batteries. J. Mater. Chem. 2011, 21, 6636–6641. [Google Scholar] [CrossRef]
- Wu, Z.; Qin, L.; Pan, Q. Fabrication and Electrochemical Behavior of Flower-Like ZnO-CoO-C Nanowall Arrays as Anodes for Lithium-Ion Batteries. J. Alloys Compd. 2011, 509, 9207–9213. [Google Scholar] [CrossRef]
- Yao, W.; Chen, J.; Cheng, H. Platelike CoO/Carbon Nanofiber Composite Electrode with Improved Electrochemical Performance for Lithium Ion Batteries. J. Solid State Electrochem. 2011, 15, 183–188. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Luo, W.; Huang, Y. Ultrathin CoO/Graphene Hybrid Nanosheets: A Highly Stable Anode Material for Lithium-Ion Batteries. J. Phys. Chem. C 2012, 116, 20794–20799. [Google Scholar] [CrossRef]
- Yang, S.; Cui, G.; Pang, S.; Cao, Q.; Kolb, U.; Feng, X.; Maier, J.; Mullen, K. Fabrication of Cobalt and Cobalt Oxide/Graphene Composites: Towards High-Performance Anode Materials for Lithium Ion Batteries. ChemSusChem 2010, 3, 236–239. [Google Scholar] [CrossRef]
- Pan, E.; Jin, Y.; Wang, Y.; Zhao, C.; Bo, X.; Jia, M. Facile Synthesis of Mesoporous 3D CoO/Nitrogen-Doped Graphene Aerogel as High-Performance Anode Materials for Lithium Storage. Microporous Mesoporous Mater. 2018, 267, 93–99. [Google Scholar] [CrossRef]
- Bindumadhavan, K.; Yeh, M.-H.; Chou, T.-C.; Chang, P.-Y.; Doong, R. Ultrafine CoO Embedded Reduced Graphene Oxide Nanocomposites: A High Rate Anode for Li-Ion Battery. ChemistrySelect 2016, 1, 5758–5767. [Google Scholar] [CrossRef]
- Shi, W.; Song, S.; Zhang, H. Hydrothermal Synthetic Strategies of Inorganic Semiconducting Nanostructures. Chem. Soc. Rev. 2013, 42, 5714–5743. [Google Scholar] [CrossRef]
- Gu, W.; Tripp, C.P. Reaction of Silanes in Supercritical CO2 with TiO2 and Al2O3. Langmuir 2006, 22, 5748–5752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Heinonen, S.; Levanen, E. Applications of Supercritical Carbon Dioxide in Materials Processing and Synthesis. RSC Adv. 2014, 4, 61137–61152. [Google Scholar] [CrossRef]
- Li, M.-J.; Zhu, H.-H.; Guo, J.-Q.; Wang, K.; Tao, W.-Q. The Development Technology and Applications of Supercritical CO2 Power Cycle in Nuclear Energy, Solar Energy and Other Energy Industries. Appl. Therm. Eng. 2017, 126, 255–275. [Google Scholar] [CrossRef]
- Meng, M.; Qiu, Z. Experiment Study of Mechanical Properties and Microstructures of Bituminous Coals Influenced by Supercritical Carbon Dioxide. Fuel 2018, 219, 223–238. [Google Scholar] [CrossRef]
- Xu, J.; Sun, E.; Li, M.; Liu, H.; Zhu, B. Key Issues and Solution Strategies for Supercritical Carbon Dioxide Coal Fired Power Plant. Energy 2018, 157, 227–246. [Google Scholar] [CrossRef]
- Zhang, J.; Zhuo, L.; Zhang, L.; Wu, C.; Zhang, X.; Wang, L. Synthesis and Electrochemical Properties of LiFePO4/C Composite Cathode Material Prepared by a New Route Using Supercritical Carbon Dioxide as a Solvent. J. Mater. Chem. 2011, 21, 6975–6980. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, Z.; Han, B.; An, G. Supercritical Carbon Dioxide-Assisted Deposition of Tin Oxide on Carbon Nanotubes. Mater. Lett. 2007, 61, 4565–4568. [Google Scholar] [CrossRef]
- Wang, L.; Zhuo, L.; Zhang, C.; Zhao, F. Carbon Dioxide-Induced Homogeneous Deposition of Nanometer-sized Cobalt Ferrite (CoFe2O4) on Graphene as High-Rate and Cycle-Stable Anode Materials for Lithium-Ion Batteries. J. Power Sources 2015, 275, 650–659. [Google Scholar] [CrossRef]
- An, G.; Na, N.; Zhang, X.; Miao, Z.; Miao, S.; Ding, K.; Liu, Z. SnO2/Carbon Nanotube Nanocomposites Synthesized in Supercritical Fluids: Highly Efficient Materials for Use as a Chemical Sensor and as the Anode of a Lithium-Ion Battery. Nanotechnology 2007, 18, 435707. [Google Scholar] [CrossRef]
- Zhuo, L.; Wu, Y.; Zhou, W.; Wang, L.; Yu, Y.; Zhang, X.; Zhao, F. Trace Amounts of Water-Induced Distinct Growth Behaviors of NiO Nanostructures on Graphene in CO2-Expanded Ethanol and Their Applications in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2013, 5, 7065–7071. [Google Scholar] [CrossRef]
- Hu, X.; Ma, M.; Zeng, M.; Sun, Y.; Chen, L.; Xue, Y.; Zhang, T.; Ai, X.; Mendes, R.G.; Ruemmeli, M.H.; et al. Supercritical Carbon Dioxide Anchored Fe3O4 Nanoparticles on Graphene Foam and Lithium Battery Performance. ACS Appl. Mater. Interfaces 2014, 6, 22527–22533. [Google Scholar] [CrossRef]
- Wang, L.; Zhuo, L.; Zhang, C.; Zhao, F. Embedding NiCo2O4 Nanoparticles into a 3DHPC Assisted by CO2-Expanded Ethanol: A Potential Lithium-Ion Battery Anode with High Performance. ACS Appl. Mater. Interfaces 2014, 6, 10813–10820. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, Z.; Qin, Y.; Hu, W. Fabrication of Co3O4/Graphene Oxide Composites Using Supercritical Fluid and Their Catalytic Application for the Decomposition of Ammonium Perchlorate. CrystEngComm 2014, 16, 2001–2008. [Google Scholar] [CrossRef]
- Ming, J.; Wu, C.; Cheng, H.; Yu, Y.; Zhao, F. Reaction of Hydrous Inorganic Metal Salts in CO2 Expanded Ethanol: Fabrication of Nanostructured Materials via Supercritical Technology. J. Supercrit. Fluids 2011, 57, 137–142. [Google Scholar] [CrossRef]
- Fujita, J.; Martell, A.E.; Nakamoto, K. Infrared spectra of metal chelate compounds. VIII. Infrared spectra of co(III) carbonato complexes. J. Chem. Phys. 1962, 36, 339. [Google Scholar] [CrossRef]
- Addison, C.C.; Gatehouse, B.M. The infrared spectra of anhydrous transition-metal nitrates. J. Chem. Soc. 1960, 613–616. [Google Scholar] [CrossRef]
- Stober, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in micron size range. J. Colloid Interface Sci. 1968, 26, 62. [Google Scholar] [CrossRef]
- Zotov, N.; Petrov, K.; Dimitrovapankova, M. Infrared-spectra of Cu(II)-Co(II) mixed hydroxide nitrates. J. Phys. Chem. Solids 1990, 51, 1199–1205. [Google Scholar] [CrossRef]
- Petrov, K.; Zotov, N.; Mirtcheva, E.; Garcia Martinez, O.; Rojas, R.M. Effect of composition on the lattice-parameters and thermal-behavior of nickel(II)-cobalt(II) hydroxide nitrate solid-solutions. J. Mater. Chem. 1994, 4, 611–614. [Google Scholar] [CrossRef]
- Leng, X.; Ding, X.; Hu, J.; Wei, S.; Jiang, Z.; Lian, J.; Wang, G.; Jiang, Q.; Liu, J. In Situ Prepared Reduced Graphene Oxide/CoO Nanowires Mutually-Supporting Porous Structure with Enhanced Lithium Storage Performance. Electrochim. Acta 2016, 190, 276–284. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, D.-W.; Yin, L.-C.; Li, N.; Li, F.; Cheng, H.-M. Oxygen Bridges between NiO Nanosheets and Graphene for Improvement of Lithium Storage. ACS Nano 2012, 6, 3214–3223. [Google Scholar] [CrossRef]
- Xu, Y.; Wei, J.; Tan, L.; Yu, J.; Chen, Y. A Facile Approach to NiCoO2 Intimately Sstanding on Nitrogen Doped Graphene Sheets by One-Step Hydrothermal Synthesis for Supercapacitors. J. Mater. Chem. A 2015, 3, 7121–7131. [Google Scholar] [CrossRef]
- Ren, M.; Li, F.; Liu, W.; Li, M.; Li, G.; Hei, J.; Su, L.; Wang, L. CoO@N-Doped Carbon Composite Nanotubes as Excellent Anodes for Lithium-Ion Batteries. ChemElectroChem 2017, 4, 2862–2869. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, H.; Du, N.; Yang, D. Highly Loaded CoO/Graphene Nanocomposites as Lithium-Ion Anodes with Superior Reversible Capacity. J. Mater. Chem. A 2013, 1, 2337–2342. [Google Scholar] [CrossRef]
- Reddy, M.V.; Prithvi, G.; Loh, K.P.; Chowdari, B.V.R. Li Storage and Impedance Spectroscopy Studies on Co3O4, CoO, and CoN for Li-Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Wu, P.; Zhou, Y.; Ye, Y.; Wang, H.; Tang, Y.; Zhou, Y.; Lu, T. Nitrogen-Doped Carbon-Wrapped Porous Single-Crystalline CoO Nanocubes for High-Performance Lithium Storage. ACS Appl. Mater. Interfaces 2014, 6, 10602–10607. [Google Scholar] [CrossRef]
- Liu, G.; Shao, J. Pomegranate-Like CoO@ Nitrogen-Doped Carbon Microspheres with Outstanding Rate Behavior and Stability for Lithium Storage. J. Mater. Chem. A 2017, 5, 9801–9806. [Google Scholar] [CrossRef]
- Chen, D.; Ji, G.; Ma, Y.; Lee, J.Y.; Lu, J. Graphene-Encapsulated Hollow Fe3O4 Nanoparticle Aggregates as a High-Performance Anode Material for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2011, 3, 3078–3083. [Google Scholar] [CrossRef]
- Cao, L.; Kang, Q.; Li, J.; Huang, J.; Cheng, Y. Assembly Control of CoO/Reduced Graphene Oxide Composites for Their Enhanced Lithium Storage Behavior. Appl. Surf. Sci. 2018, 455, 96–105. [Google Scholar] [CrossRef]
- Tang, S.B.; Lai, M.O.; Lu, L. Study on Li+-Ion Diffusion in Nano-Crystalline LiMn2O4 Thin Film Cathode Grown by Pulsed Laser Deposition Using CV, EIS and PITT Techniques. Mater. Chem. Phys. 2008, 111, 149–153. [Google Scholar] [CrossRef]
- Wen, H.; Kang, W.; Liu, X.; Li, W.; Zhang, L.; Zhang, C. Two-Phase Interface Hydrothermal Synthesis of Binder-free SnS2/Graphene Flexible Paper Electrodes for High-Performance Li-Ion Batteries. RSC Adv. 2019, 9, 23607–23613. [Google Scholar] [CrossRef]
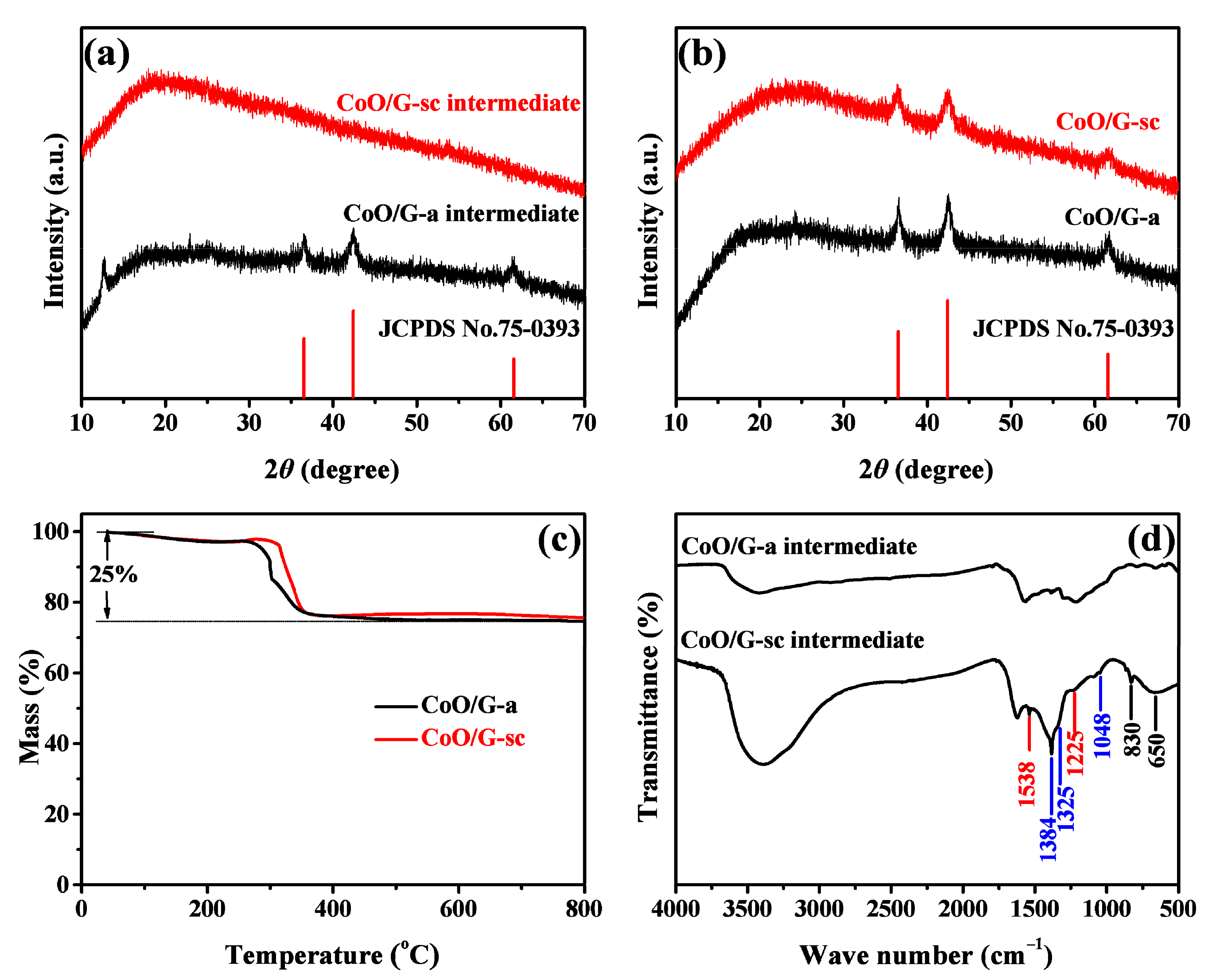




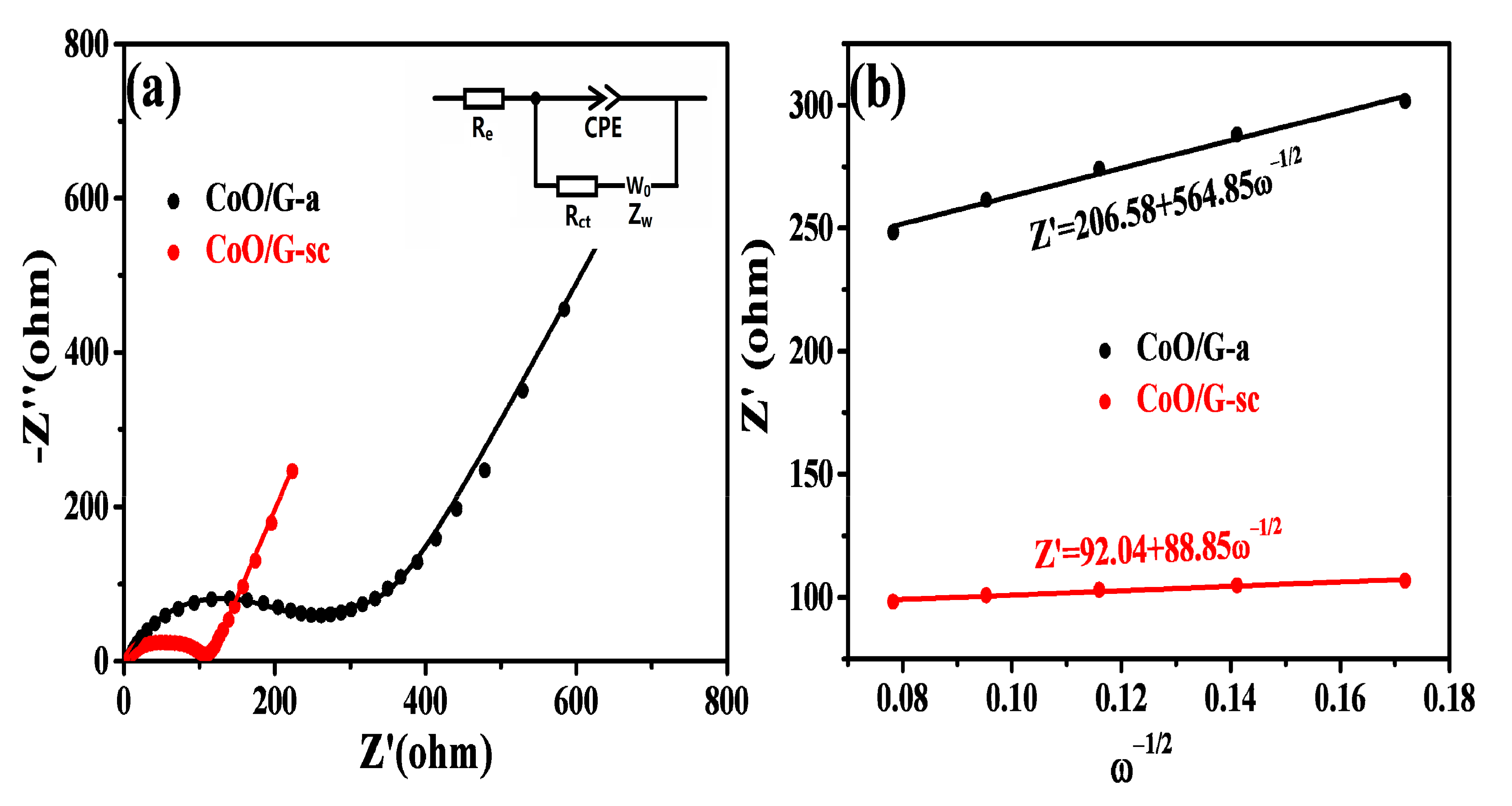
| Sample | Re (Ω) | Rct (Ω) | CPE (μF) | Zw (Ω s−1/2) |
|---|---|---|---|---|
| CoO/G-a | 5.79 | 207.3 | 9.5 | 462.8 |
| CoO/G-sc | 3.70 | 96.1 | 49.90 | 45.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, R.; Wen, H.; Zeng, L.; Li, X.; Liu, X.; Zhang, C. Supercritical CO2 Assisted Solvothermal Preparation of CoO/Graphene Nanocomposites for High Performance Lithium-Ion Batteries. Nanomaterials 2021, 11, 694. https://doi.org/10.3390/nano11030694
Yuan R, Wen H, Zeng L, Li X, Liu X, Zhang C. Supercritical CO2 Assisted Solvothermal Preparation of CoO/Graphene Nanocomposites for High Performance Lithium-Ion Batteries. Nanomaterials. 2021; 11(3):694. https://doi.org/10.3390/nano11030694
Chicago/Turabian StyleYuan, Ruoxin, Hao Wen, Li Zeng, Xi Li, Xingang Liu, and Chuhong Zhang. 2021. "Supercritical CO2 Assisted Solvothermal Preparation of CoO/Graphene Nanocomposites for High Performance Lithium-Ion Batteries" Nanomaterials 11, no. 3: 694. https://doi.org/10.3390/nano11030694
APA StyleYuan, R., Wen, H., Zeng, L., Li, X., Liu, X., & Zhang, C. (2021). Supercritical CO2 Assisted Solvothermal Preparation of CoO/Graphene Nanocomposites for High Performance Lithium-Ion Batteries. Nanomaterials, 11(3), 694. https://doi.org/10.3390/nano11030694







