Functional and Morphological Changes Induced in Mytilus Hemocytes by Selected Nanoparticles
Abstract
1. Introduction
- (a)
- investigating the sensitivity of hemocytes to NPs with different surface characteristics and which characteristic of NPs are most potent to provoke the hemocyte response in physiological medium;
- (b)
- defining optimal experimental conditions for a sensitive, alternative, and affordable biological in vitro model for a rapid prescreening strategy for NPs.
2. Materials and Methods
2.1. Nanoparticle Synthesis and Characterization
2.2. Mussels, Hemolymph Collection, Preparation of Hemolymph Serum and Hemocyte Monolayers and Exposure Conditions
2.3. Hemocyte Morphology Using Scanning Electron Microscopy (SEM)
2.4. Hemocyte Functional Assays
2.5. Statistics
3. Results
3.1. NP Characterization
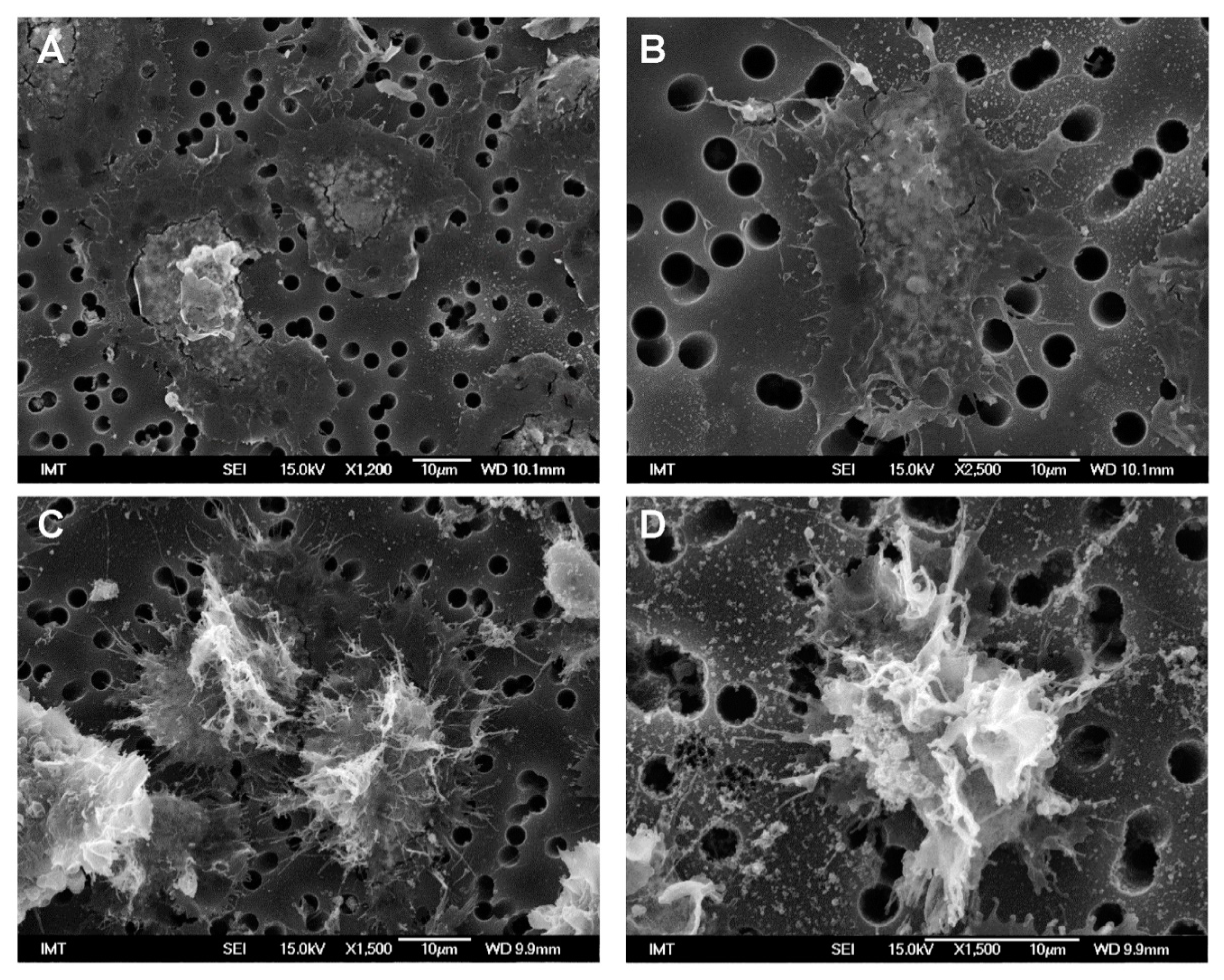
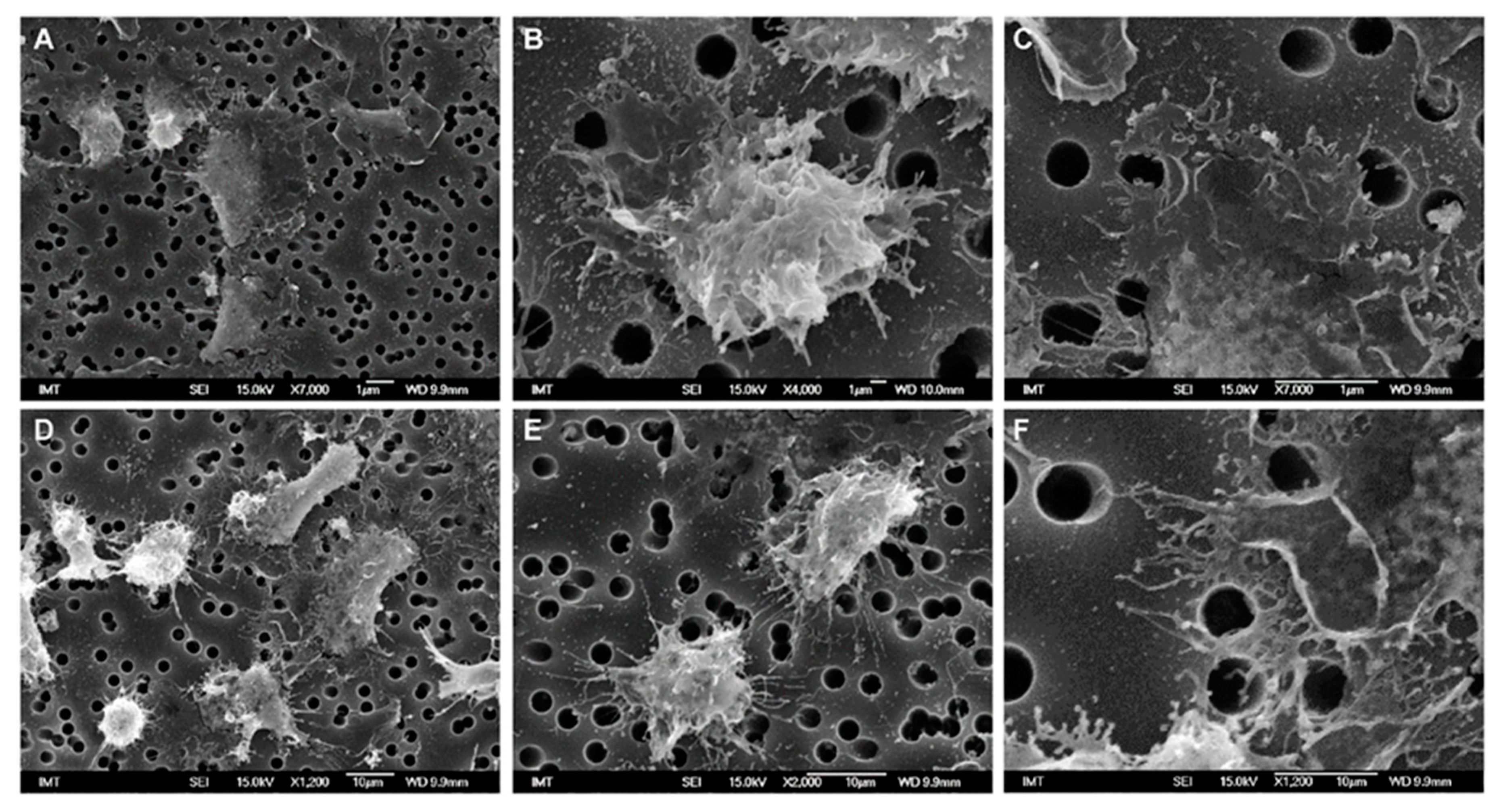
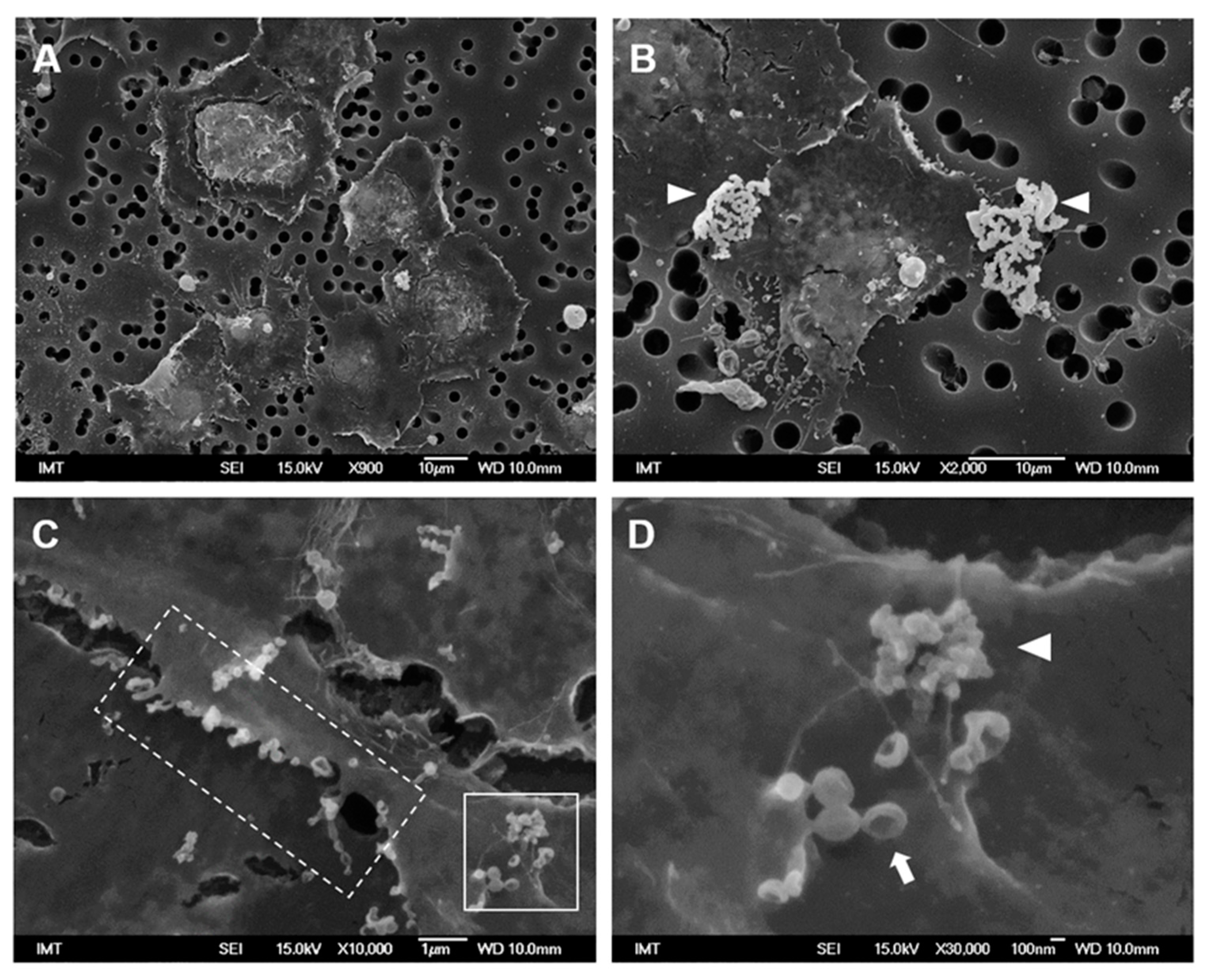
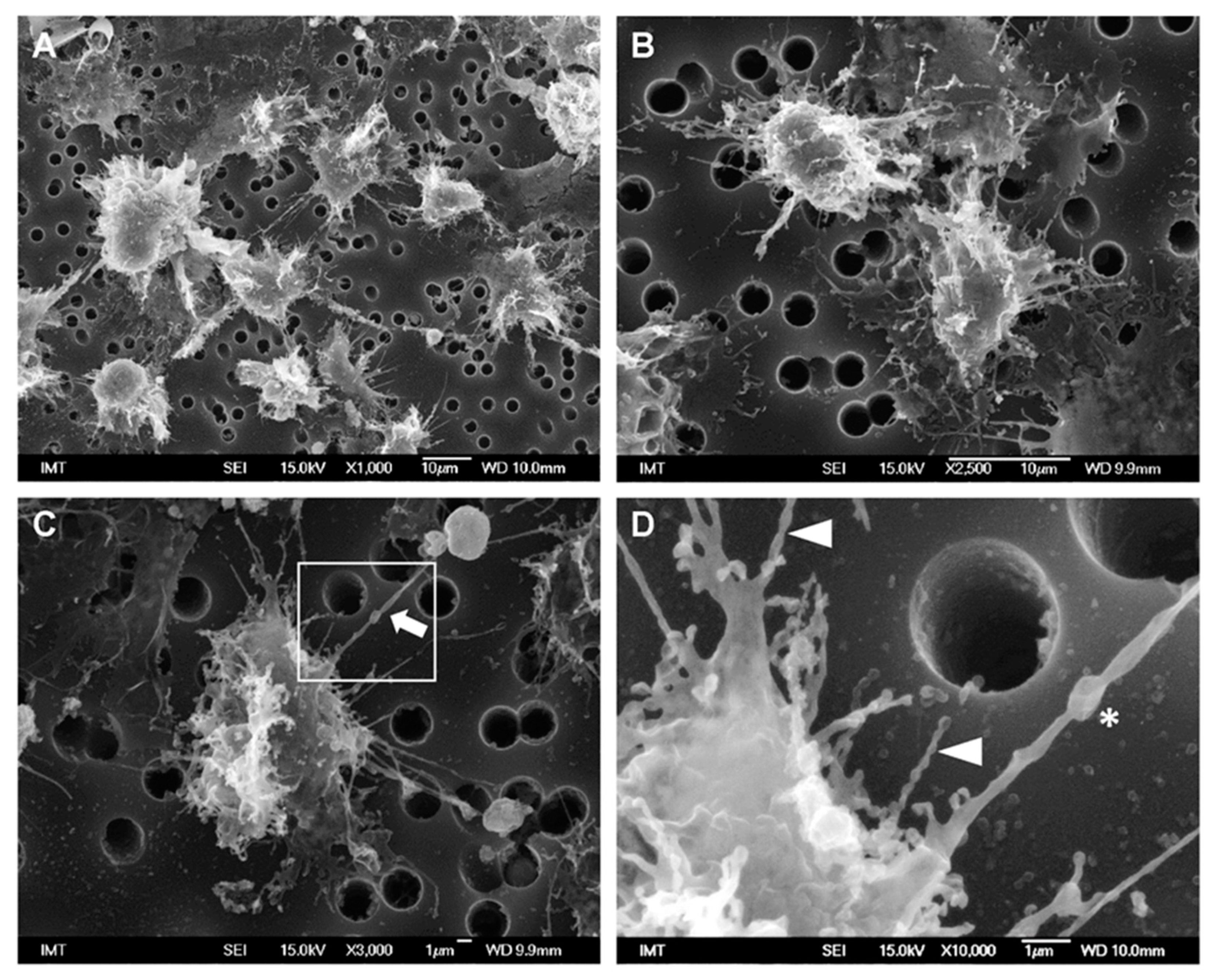
3.2. Effects of NPs on Hemocyte Morphology: SEM
3.3. Hemocyte Functional Parameters
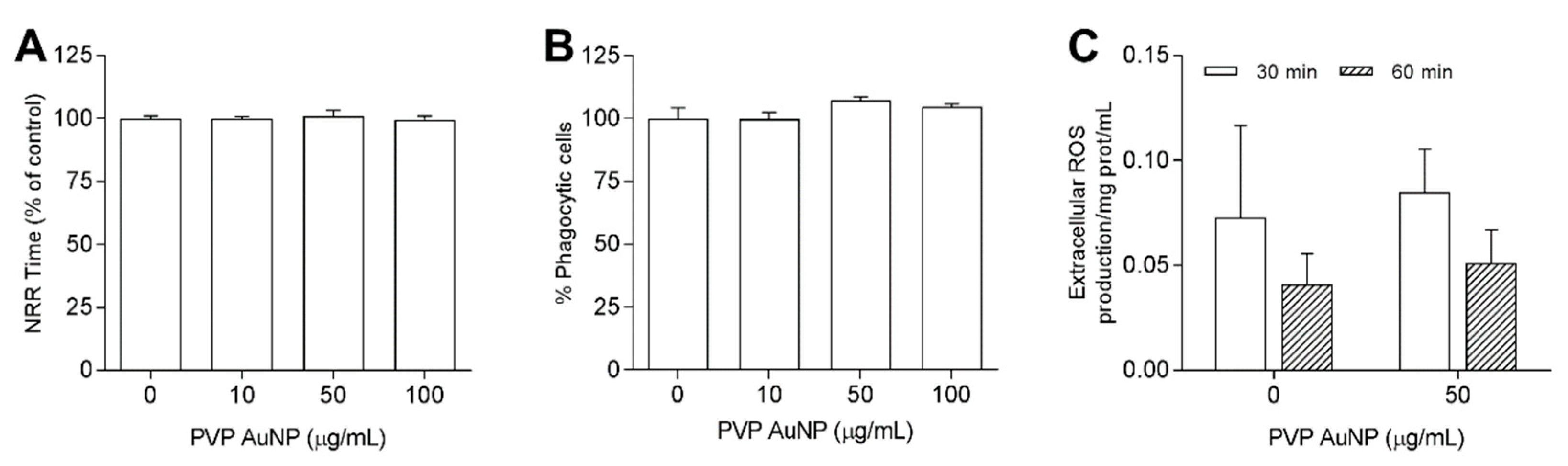
3.3.1. PVP-AuNP
3.3.2. Amino-Modified Nanopolystyrene—PS-NH2
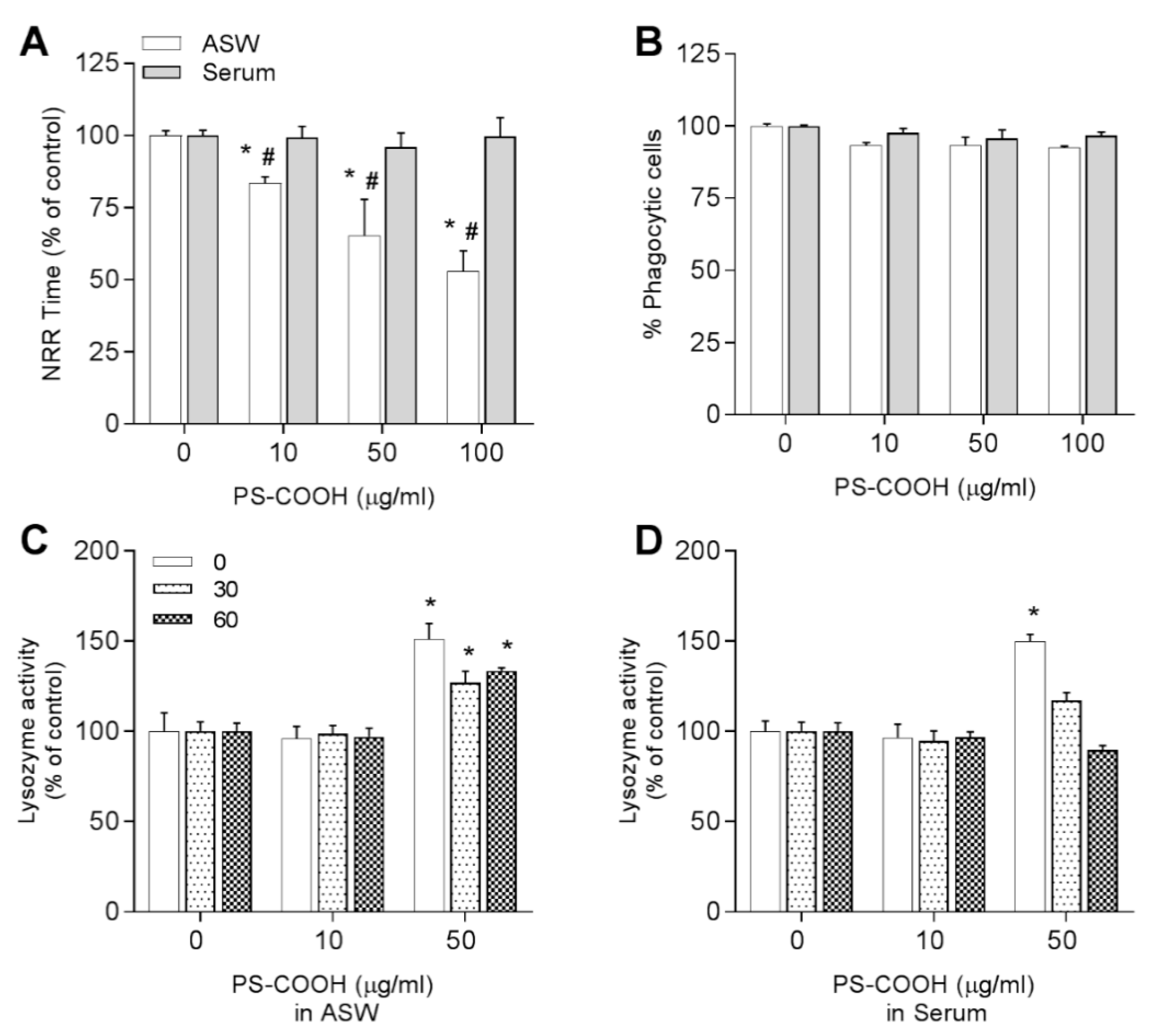
3.3.3. Carboxy-Modified Nanopolystyrene—PS-COOH
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Barrick, A.; Guillet, C.; Mouneyrac, C.; Châtel, A. Investigating the Establishment of Primary Cultures of Hemocytes from Mytilus Edulis. Cytotechnology 2018, 70, 1205–1220. [Google Scholar] [CrossRef] [PubMed]
- Canesi, L.; Ciacci, C.; Fabbri, R.; Marcomini, A.; Pojana, G.; Gallo, G. Bivalve Molluscs as a Unique Target Group for Nanoparticle Toxicity. Mar. Environ. Res. 2012, 76, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Rocha, T.L.; Gomes, T.; Sousa, V.S.; Mestre, N.C.; Bebianno, M.J. Ecotoxicological Impact of Engineered Nanomaterials in Bivalve Molluscs: An Overview. Mar. Environ. Res. 2015, 111, 74–88. [Google Scholar] [CrossRef]
- Canesi, L.; Corsi, I. Effects of Nanomaterials on Marine Invertebrates. Sci. Total Environ. 2016, 565, 933–940. [Google Scholar] [CrossRef]
- Canesi, L.; Auguste, M.; Bebianno, M.J. Sublethal effects of nanoparticles on aquatic invertebrates, from molecular to organism level. In Ecotoxicology of Nanoparticles in Aquatic Systems; CRC Press: Boca Raton, FL, USA, 2019; pp. 38–61. [Google Scholar]
- Moore, M.N. Do Nanoparticles Present Ecotoxicological Risks for the Health of the Aquatic Environment? Environ. Int. 2006, 32, 967–976. [Google Scholar] [CrossRef]
- Allam, B.; Raftos, D. Immune Responses to Infectious Diseases in Bivalves. J. Invertebr. Pathol. 2015, 131, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Gerdol, M.; Gomez-Chiari, M.; Castillo, M.G.; Figueras, A.; Fiorito, G.; Moreira, R.; Novoa, B.; Pallavicini, A.; Ponte, G.; Roumbedakis, K.; et al. Immunity in molluscs: Recognition and effector mechanisms, with a focus on bivalvia. In Advances in Comparative Immunology; Springer: Berlin, Germany, 2018; pp. 225–342. [Google Scholar]
- Ciacci, C.; Canonico, B.; Bilaniĉovă, D.; Fabbri, R.; Cortese, K.; Gallo, G.; Marcomini, A.; Pojana, G.; Canesi, L. Immunomodulation by Different Types of N-Oxides in the Hemocytes of the Marine Bivalve Mytilus Galloprovincialis. PLoS ONE 2012, 7, e36937. [Google Scholar] [CrossRef]
- Canesi, L.; Ciacci, C.; Vallotto, D.; Gallo, G.; Marcomini, A.; Pojana, G. In Vitro Effects of Suspensions of Selected Nanoparticles (C60 Fullerene, TiO2, SiO2) on Mytilus Hemocytes. Aquat. Toxicol. 2010, 96, 151–158. [Google Scholar] [CrossRef]
- Canesi, L.; Ciacci, C.; Bergami, E.; Monopoli, M.P.; Dawson, K.A.; Papa, S.; Canonico, B.; Corsi, I. Evidence for Immunomodulation and Apoptotic Processes Induced by Cationic Polystyrene Nanoparticles in the Hemocytes of the Marine Bivalve Mytilus. Mar. Environ. Res. 2015, 111, 34–40. [Google Scholar] [CrossRef]
- Canesi, L.; Ciacci, C.; Fabbri, R.; Balbi, T.; Salis, A.; Damonte, G.; Cortese, K.; Caratto, V.; Monopoli, M.P.; Dawson, K.; et al. Interactions of Cationic Polystyrene Nanoparticles with Marine Bivalve Hemocytes in a Physiological Environment: Role of Soluble Hemolymph Proteins. Environ. Res. 2016, 150, 73–81. [Google Scholar] [CrossRef]
- Auguste, M.; Ciacci, C.; Balbi, T.; Brunelli, A.; Caratto, V.; Marcomini, A.; Cuppini, R.; Canesi, L. Effects of Nanosilver on Mytilus Galloprovincialis Hemocytes and Early Embryo Development. Aquat. Toxicol. 2018, 203, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Katsumiti, A.; Gilliland, D.; Arostegui, I.; Cajaraville, M.P. Mechanisms of Toxicity of Ag Nanoparticles in Comparison to Bulk and Ionic Ag on Mussel Hemocytes and Gill Cells. PLoS ONE 2015, 10, e0129039. [Google Scholar] [CrossRef]
- Canesi, L.; Ciacci, C.; Balbi, T. Invertebrate Models for Investigating the Impact of Nanomaterials on Innate Immunity: The Example of the Marine Mussel Mytilus Spp. Curr. Bionanotechnol. 2016, 2, 77–83. [Google Scholar] [CrossRef]
- Katsumiti, A.; Arostegui, I.; Oron, M.; Gilliland, D.; Valsami-Jones, E.; Cajaraville, M.P. Cytotoxicity of Au, ZnO and SiO2 NPs Using In Vitro Assays with Mussel Hemocytes and Gill Cells: Relevance of Size, Shape and Additives. Nanotoxicology 2015, 1–9. [Google Scholar] [CrossRef]
- Canesi, L.; Frenzilli, G.; Balbi, T.; Bernardeschi, M.; Ciacci, C.; Corsolini, S.; Della Torre, C.; Fabbri, R.; Faleri, C.; Focardi, S.; et al. Interactive Effects of N-TiO2 and 2,3,7,8-TCDD on the Marine Bivalve Mytilus Galloprovincialis. Aquat. Toxicol. 2014, 153, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Katsumiti, A.; Thorley, A.J.; Arostegui, I.; Reip, P.; Valsami-Jones, E.; Tetley, T.D.; Cajaraville, M.P. Cytotoxicity and Cellular Mechanisms of Toxicity of CuO NPs in Mussel Cells in Vitro and Comparative Sensitivity with Human Cells. Toxicol. Vitr. 2018, 48, 146–158. [Google Scholar] [CrossRef]
- Bruinink, A.; Wang, J.; Wick, P. Effect of Particle Agglomeration in Nanotoxicology. Arch. Toxicol 2015, 89, 659–675. [Google Scholar] [CrossRef] [PubMed]
- Canesi, L.; Balbi, T.; Fabbri, R.; Salis, A.; Damonte, G.; Volland, M.; Blasco, J. Biomolecular Coronas in Invertebrate Species: Implications in the Environmental Impact of Nanoparticles. NanoImpact 2017, 8, 89–98. [Google Scholar] [CrossRef]
- Barbero, F.; Russo, L.; Vitali, M.; Piella, J.; Salvo, I.; Borrajo, M.L.; Busquets-Fité, M.; Grandori, R.; Bastús, N.G.; Casals, E.; et al. Formation of the Protein Corona: The Interface between Nanoparticles and the Immune System. Semin. Immunol. 2017, 34, 52–60. [Google Scholar] [CrossRef]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold Nanoparticles Are Taken Up by Human Cells but Do Not Cause Acute Cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef]
- Sperling, R.A.; Rivera Gil, P.; Zhang, F.; Zanella, M.; Parak, W.J. Biological Applications of Gold Nanoparticles. Chem. Soc. Rev. 2008, 37, 1896. [Google Scholar] [CrossRef]
- Bastús, N.G.; Comenge, J.; Puntes, V. Kinetically Controlled Seeded Growth Synthesis of Citrate-Stabilized Gold Nanoparticles of up to 200 Nm: Size Focusing versus Ostwald Ripening. Langmuir 2011, 27, 11098–11105. [Google Scholar] [CrossRef]
- La Roche, G.; Eisler, R.; Tarzwell, C. Bioassay Procedure for Oil and Oil Dispersant Toxicity Evaluation. J. Water Pollut Control. Fed 1970, 1982–1989. [Google Scholar]
- ASTM D1141-98 Standard Practice for the Preparation of Substitute Ocean Water; American Society for Testing and Materials: West Conshohocken, PA, USA, 2013.
- Grassi, G.; Landi, C.; Della Torre, C.; Bergami, E.; Bini, L.; Corsi, I. Proteomic Profile of the Hard Corona of Charged Polystyrene Nanoparticles Exposed to Sea Urchin Paracentrotus Lividus Coelomic Fluid Highlights Potential Drivers of Toxicity. Environ. Sci. Nano 2019, 6, 2937–2947. [Google Scholar] [CrossRef]
- Hernroth, B. The Influence of Temperature and Dose on Antibacterial Peptide Response against Lipopolysaccharide in the Blue Mussel, Mytilus Edulis. Fish. Shellfish Immunol. 2003, 14, 25–37. [Google Scholar] [CrossRef]
- Li, Y.; Boraschi, D. Endotoxin Contamination: A Key Element in the Interpretation of Nanosafety Studies. Nanomedicine 2016, 11, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, Z.; Radauer-Preiml, I.; Andosch, A.; Casals, E.; Luetz-Meindl, U.; Cobaleda, M.; Lin, Z.; Jaberi-Douraki, M.; Italiani, P.; et al. Bacterial Endotoxin (Lipopolysaccharide) Binds to the Surface of Gold Nanoparticles, Interferes with Biocorona Formation and Induces Human Monocyte Inflammatory Activation. Nanotoxicology 2017, 11, 1157–1175. [Google Scholar] [CrossRef] [PubMed]
- Drobne, D. 3D Imaging of Cells and Tissues by Focused Ion Beam/Scanning Electron Microscopy (FIB/SEM). In Nanoimaging; Sousa, A.A., Kruhlak, M.J., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 275–292. ISBN 978-1-62703-136-3. [Google Scholar]
- Millaku, A.; Drobne, D.; Torkar, M.; Novak, S.; Remškar, M.; Pipan-Tkalec, Ž. Use of Scanning Electron Microscopy to Monitor Nanofibre/Cell Interaction in Digestive Epithelial Cells. J. Hazard. Mater. 2013, 260, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Vig, K.; Dennis, V.; Singh, S. Functionalized Gold Nanoparticles and Their Biomedical Applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Creran, B.; Rotello, V.M. Gold Nanoparticles: Preparation, Properties, and Applications in Bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- Bundschuh, M.; Filser, J.; Lüderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the Environment: Where Do We Come from, Where Do We Go To? Environ. Sci. Eur. 2018, 30, 6. [Google Scholar] [CrossRef] [PubMed]
- García-Negrete, C.A.; Jiménez de Haro, M.C.; Blasco, J.; Soto, M.; Fernández, A. STEM-in-SEM High Resolution Imaging of Gold Nanoparticles and Bivalve Tissues in Bioaccumulation Experiments. Analyst 2015, 140, 3082–3089. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Eken, C.; Sadallah, S.; Martin, P.J.; Treves, S.; Schifferli, J.A. Ectosomes of Polymorphonuclear Neutrophils Activate Multiple Signaling Pathways in Macrophages. Immunobiology 2013, 218, 382–392. [Google Scholar] [CrossRef]
- Oliveri, C.; Peric, L.; Sforzini, S.; Banni, M.; Viarengo, A.; Cavaletto, M.; Marsano, F. Biochemical and Proteomic Characterisation of Haemolymph Serum Reveals the Origin of the Alkali-Labile Phosphate (ALP) in Mussel (Mytilus Galloprovincialis). Comp. Biochem. Physiol. Part. D: Genom. Proteom. 2014, 11, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Pezzati, E.; Canesi, L.; Damonte, G.; Salis, A.; Marsano, F.; Grande, C.; Vezzulli, L.; Pruzzo, C. Susceptibility of V Ibrio Aestuarianu s 01/032 to the Antibacterial Activity of M Ytilus Haemolymph: Identification of a Serum Opsonin Involved in Mannose-Sensitive Interactions: Vibrio Aestuarianus and Bivalve Haemocytes. Environ. Microbiol 2015, 17, 4271–4279. [Google Scholar] [CrossRef] [PubMed]
- Tallec, K.; Blard, O.; González-Fernández, C.; Brotons, G.; Berchel, M.; Soudant, P.; Huvet, A.; Paul-Pont, I. Surface Functionalization Determines Behavior of Nanoplastic Solutions in Model Aquatic Environments. Chemosphere 2019, 225, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, Z.; Wang, S.; Fang, H.; Wang, D. Aquatic Behavior and Toxicity of Polystyrene Nanoplastic Particles with Different Functional Groups: Complex Roles of PH, Dissolved Organic Carbon and Divalent Cations. Chemosphere 2019, 228, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Heckman, C.A.; Plummer, H.K. Filopodia as Sensors. Cell. Signal. 2013, 25, 2298–2311. [Google Scholar] [CrossRef]
- Gauthier, N.C.; Masters, T.A.; Sheetz, M.P. Mechanical Feedback between Membrane Tension and Dynamics. Trends Cell Biol. 2012, 22, 527–535. [Google Scholar] [CrossRef]
- Drab, M.; Stopar, D.; Kralj-Iglič, V.; Iglič, A. Inception Mechanisms of Tunneling Nanotubes. Cells 2019, 8, 626. [Google Scholar] [CrossRef] [PubMed]
- Sisakhtnezhad, S.; Khosravi, L. Emerging Physiological and Pathological Implications of Tunneling Nanotubes Formation between Cells. Eur. J. Cell Biol. 2015, 94, 429–443. [Google Scholar] [CrossRef]
- Korenkova, O.; Pepe, A.; Zurzolo, C. Fine Intercellular Connections in Development: TNTs, Cytonemes, or Intercellular Bridges? CST 2020, 4, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Auguste, M.; Balbi, T.; Ciacci, C.; Canesi, L. Conservation of Cell Communication Systems in Invertebrate Host–Defence Mechanisms: Possible Role in Immunity and Disease. Biology 2020, 9, 234. [Google Scholar] [CrossRef] [PubMed]


| NPs | Medium | Z-Average (nm) | PDI | ζ-Potential (mV) |
|---|---|---|---|---|
| PVP-AuNP | ASW | 58.3 ± 0.16 | 0.12 ± 0.01 | −4.1 ± 0.6 |
| PS-NH2 | MQ 1 | 57 ± 2 | 0.07 ± 0.02 | +42.8 ± 1 |
| ASW 1 | 200 ± 6 | 0.3 ± 0.02 | +14.2 ± 2 | |
| HS 2 | 178 ± 2 | 0.34 ± 0.05 | +14.2 ± 1 | |
| PS-COOH | MQ 3 | 64.5 ± 0.6 | 0.06 ± 0.02 | −59.7 ± 2.6 |
| ASW | 1822 ± 373.6 | 0.228 | −26.9 ± 1.9 | |
| HS | 189.1 ± 48.6 | 0.288 | −11.6 ± 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auguste, M.; Mayall, C.; Barbero, F.; Hočevar, M.; Alberti, S.; Grassi, G.; Puntes, V.F.; Drobne, D.; Canesi, L. Functional and Morphological Changes Induced in Mytilus Hemocytes by Selected Nanoparticles. Nanomaterials 2021, 11, 470. https://doi.org/10.3390/nano11020470
Auguste M, Mayall C, Barbero F, Hočevar M, Alberti S, Grassi G, Puntes VF, Drobne D, Canesi L. Functional and Morphological Changes Induced in Mytilus Hemocytes by Selected Nanoparticles. Nanomaterials. 2021; 11(2):470. https://doi.org/10.3390/nano11020470
Chicago/Turabian StyleAuguste, Manon, Craig Mayall, Francesco Barbero, Matej Hočevar, Stefano Alberti, Giacomo Grassi, Victor F. Puntes, Damjana Drobne, and Laura Canesi. 2021. "Functional and Morphological Changes Induced in Mytilus Hemocytes by Selected Nanoparticles" Nanomaterials 11, no. 2: 470. https://doi.org/10.3390/nano11020470
APA StyleAuguste, M., Mayall, C., Barbero, F., Hočevar, M., Alberti, S., Grassi, G., Puntes, V. F., Drobne, D., & Canesi, L. (2021). Functional and Morphological Changes Induced in Mytilus Hemocytes by Selected Nanoparticles. Nanomaterials, 11(2), 470. https://doi.org/10.3390/nano11020470









