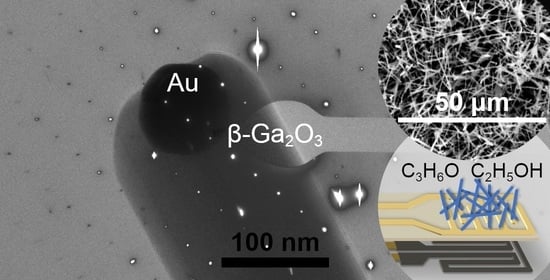
Morphology of Ga2O3 Nanowires and Their Sensitivity to Volatile Organic Compounds
Abstract
1. Introduction
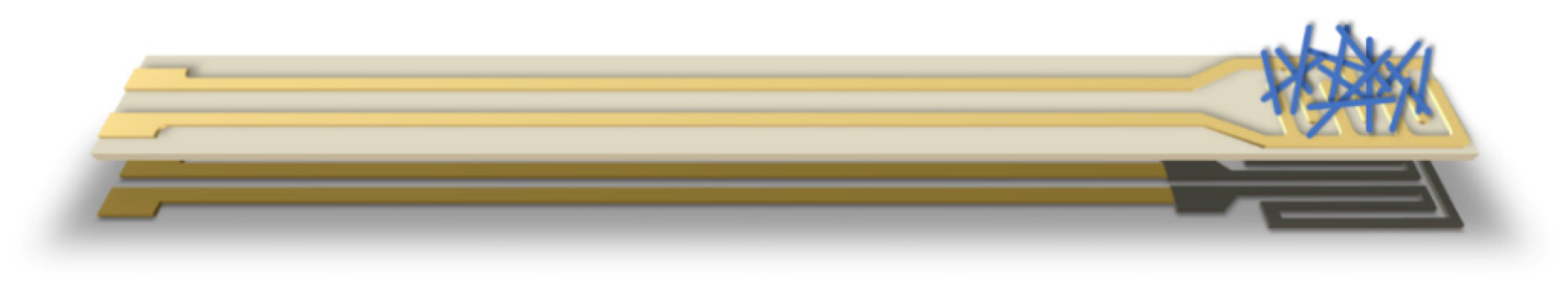
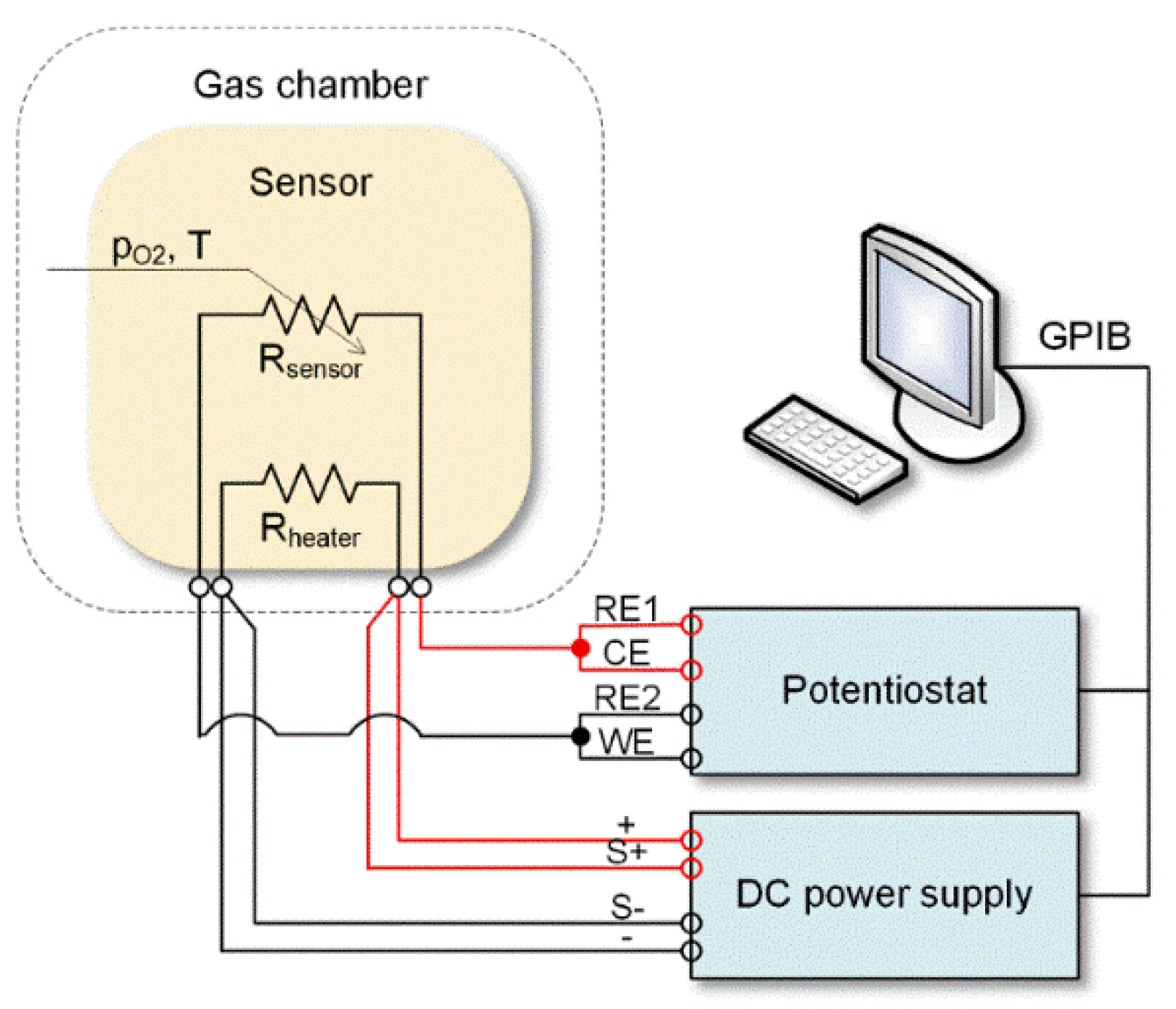
2. Materials and Methods
3. Results
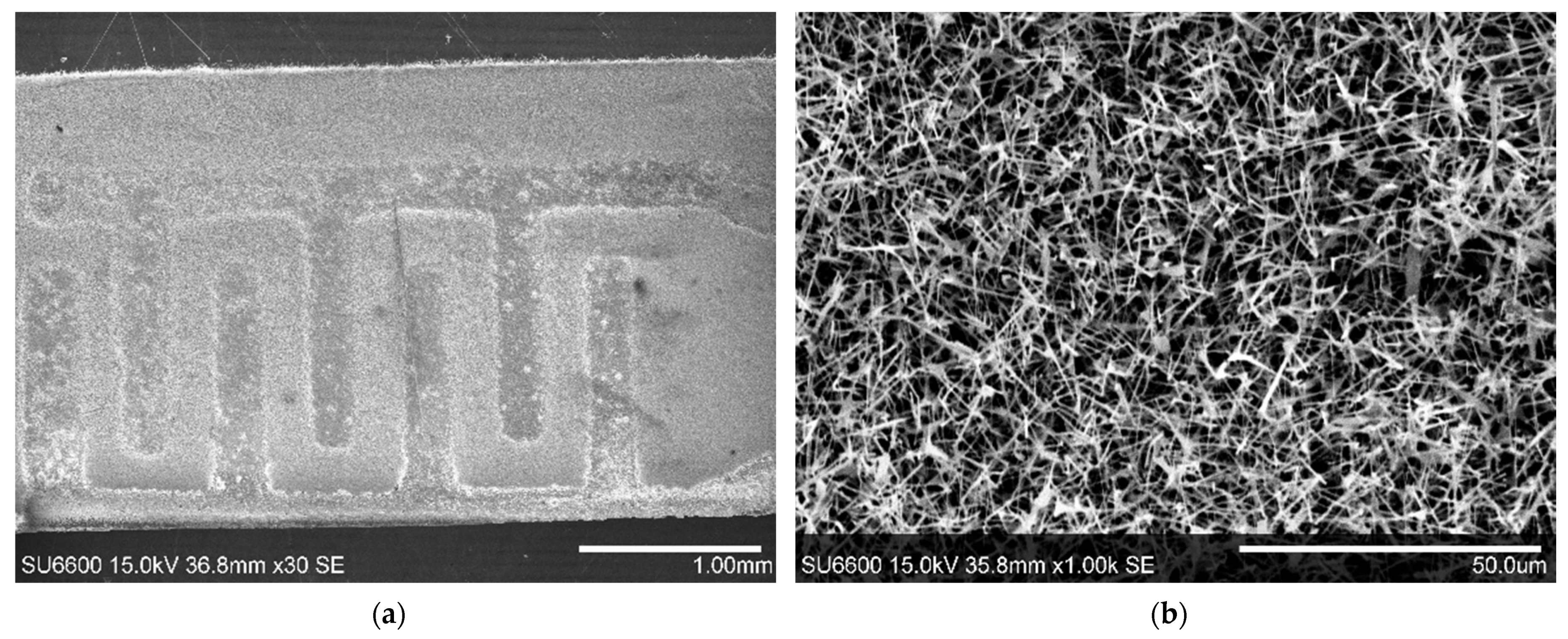
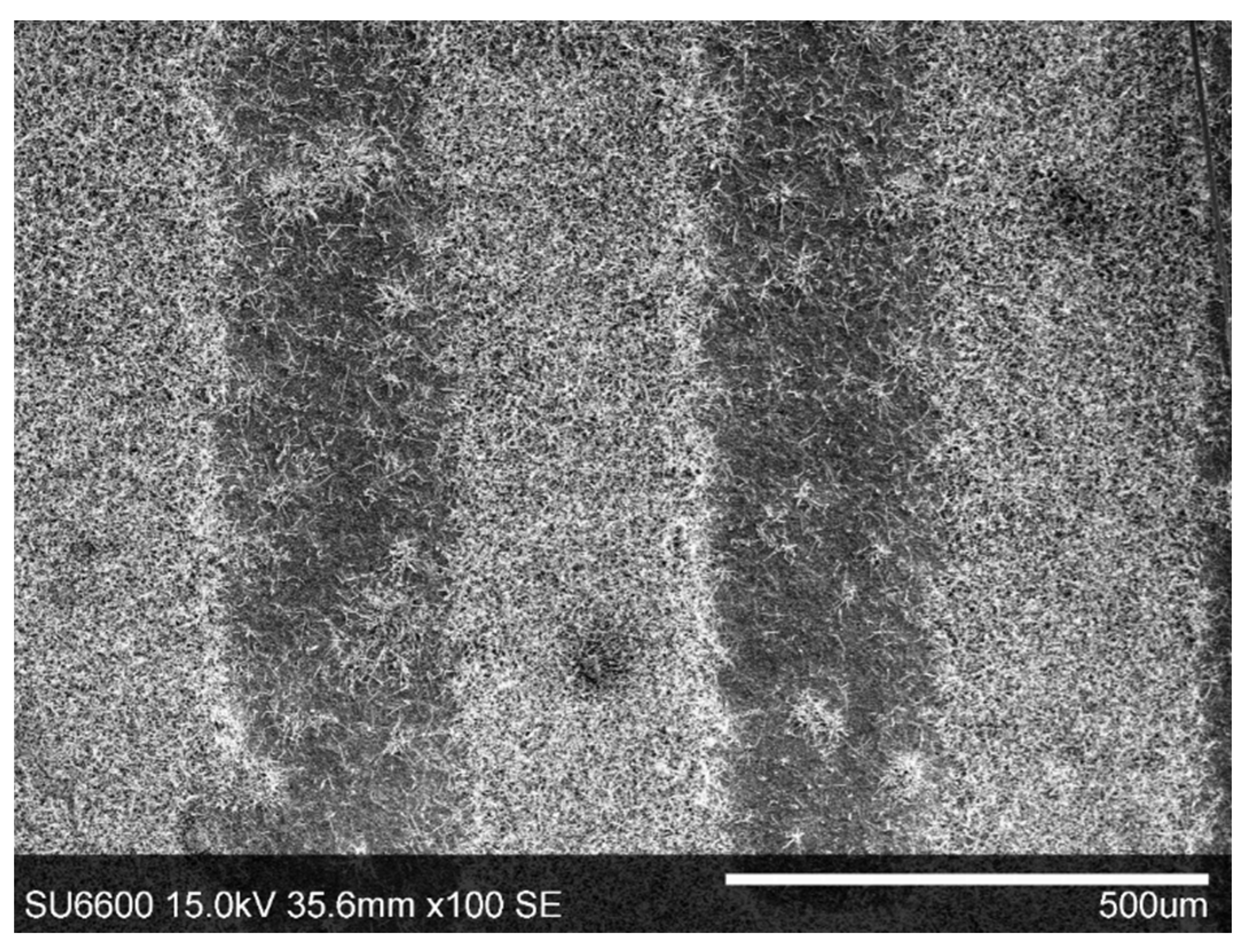
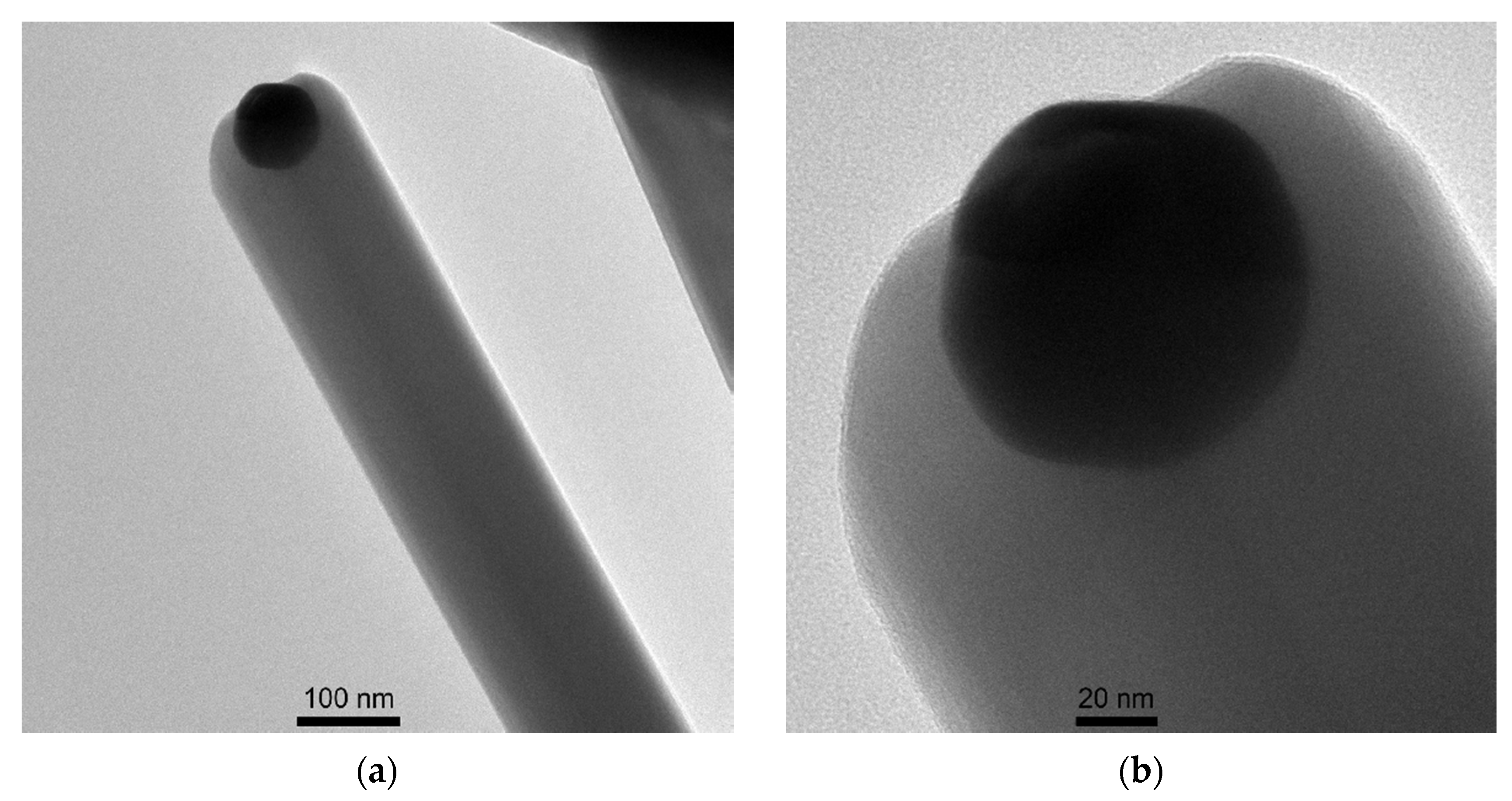
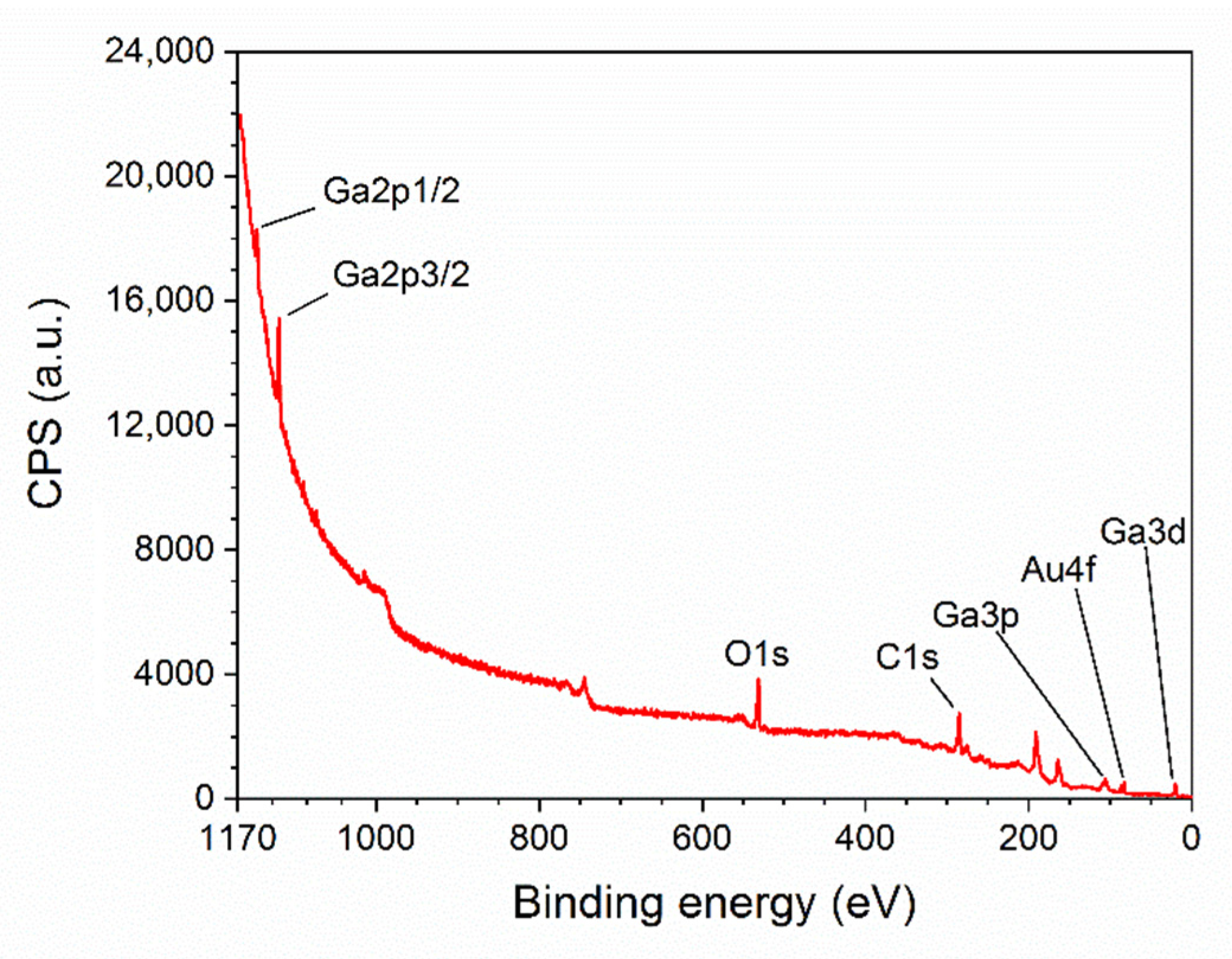
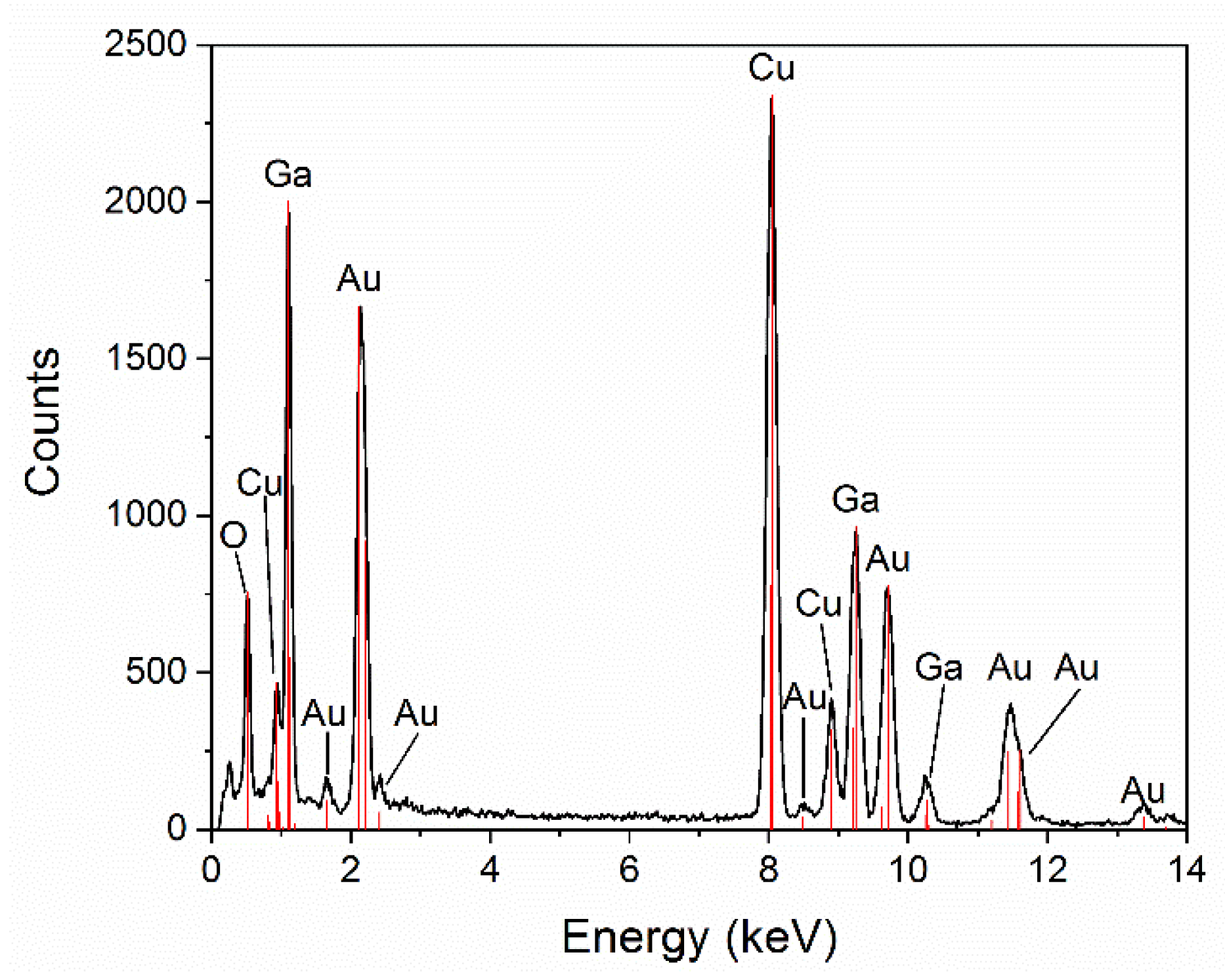
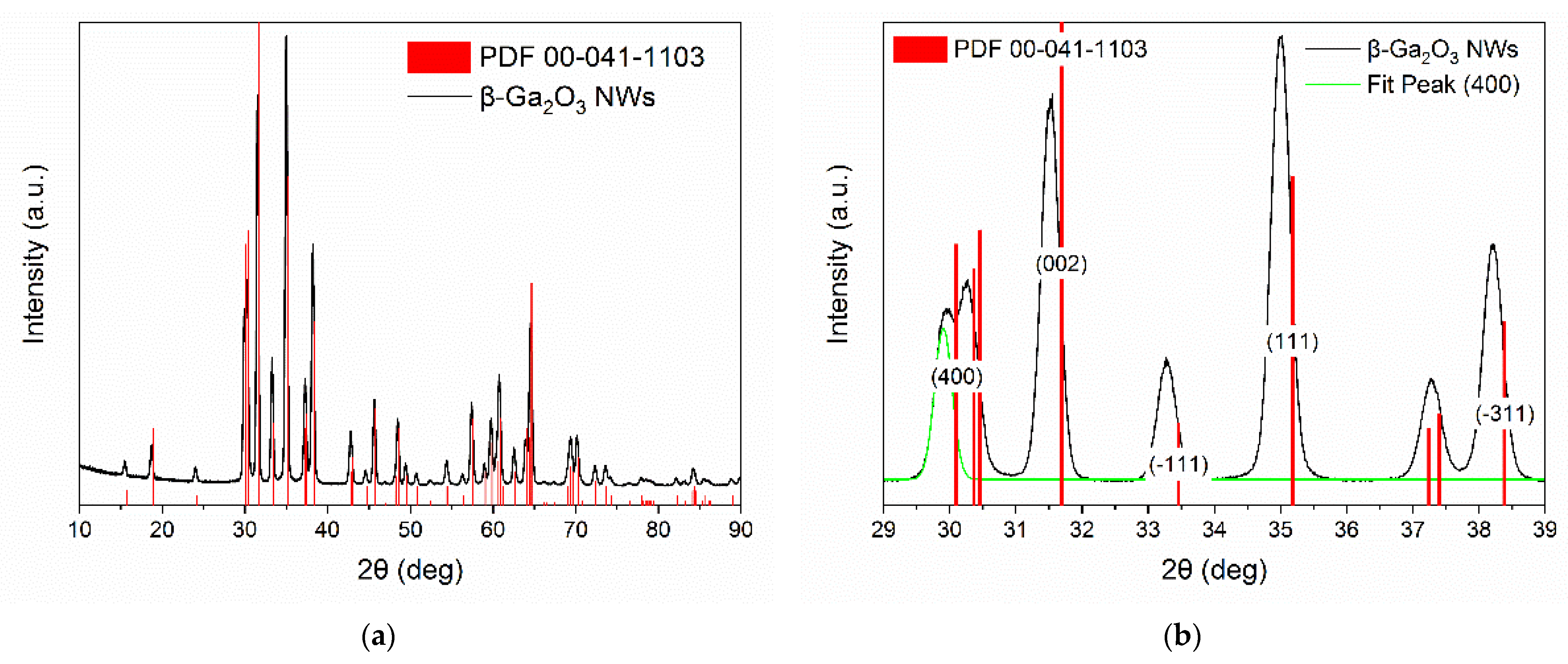
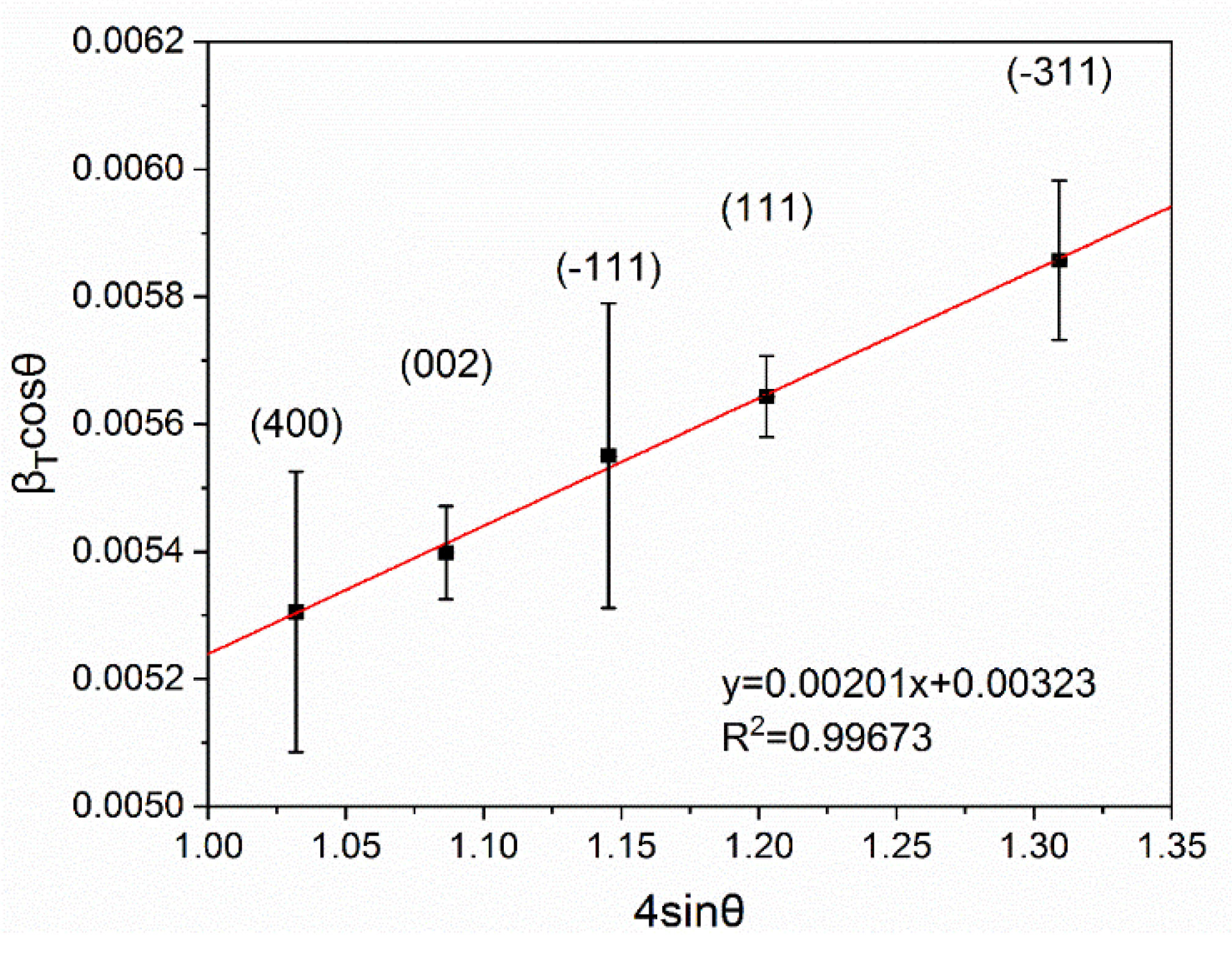
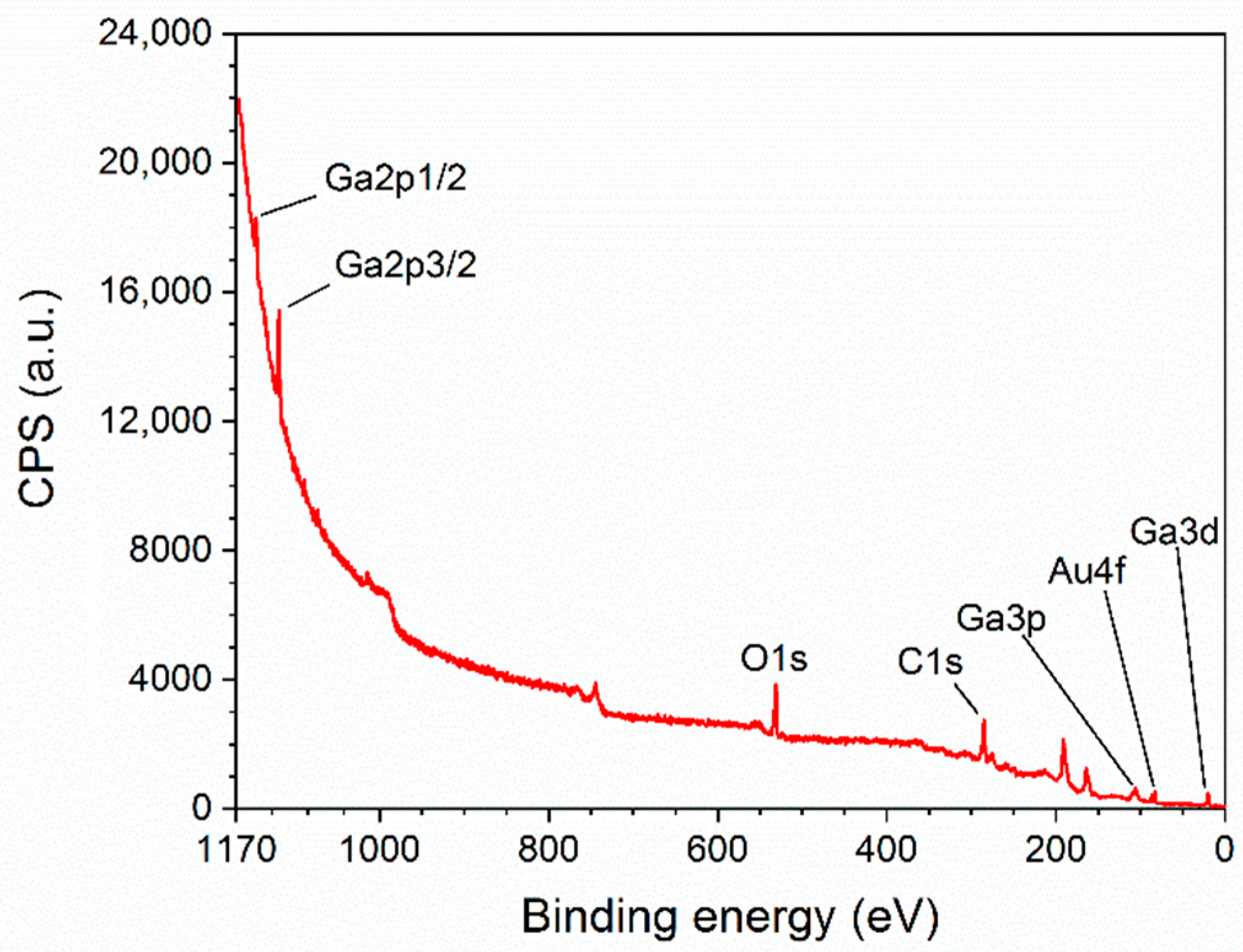
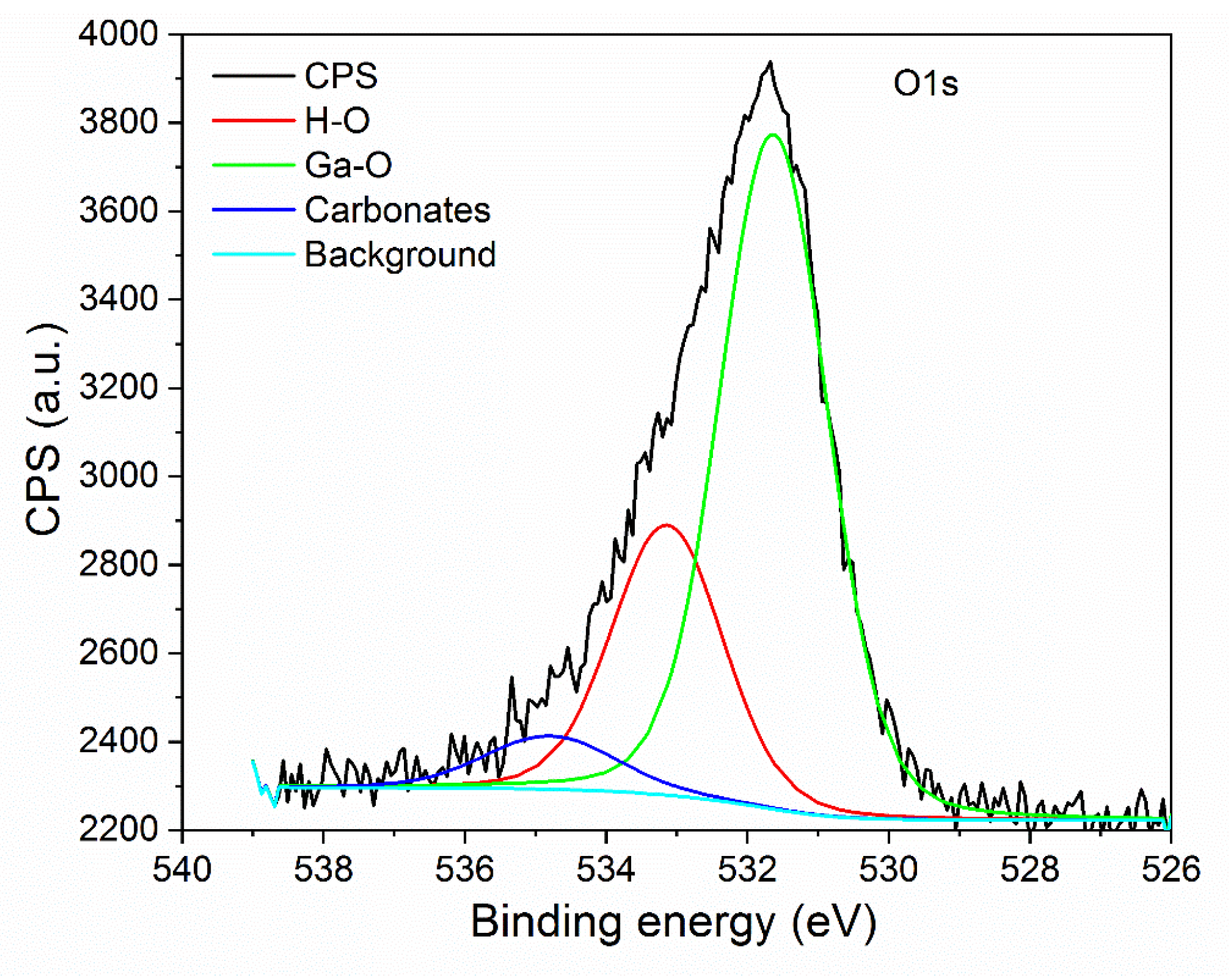
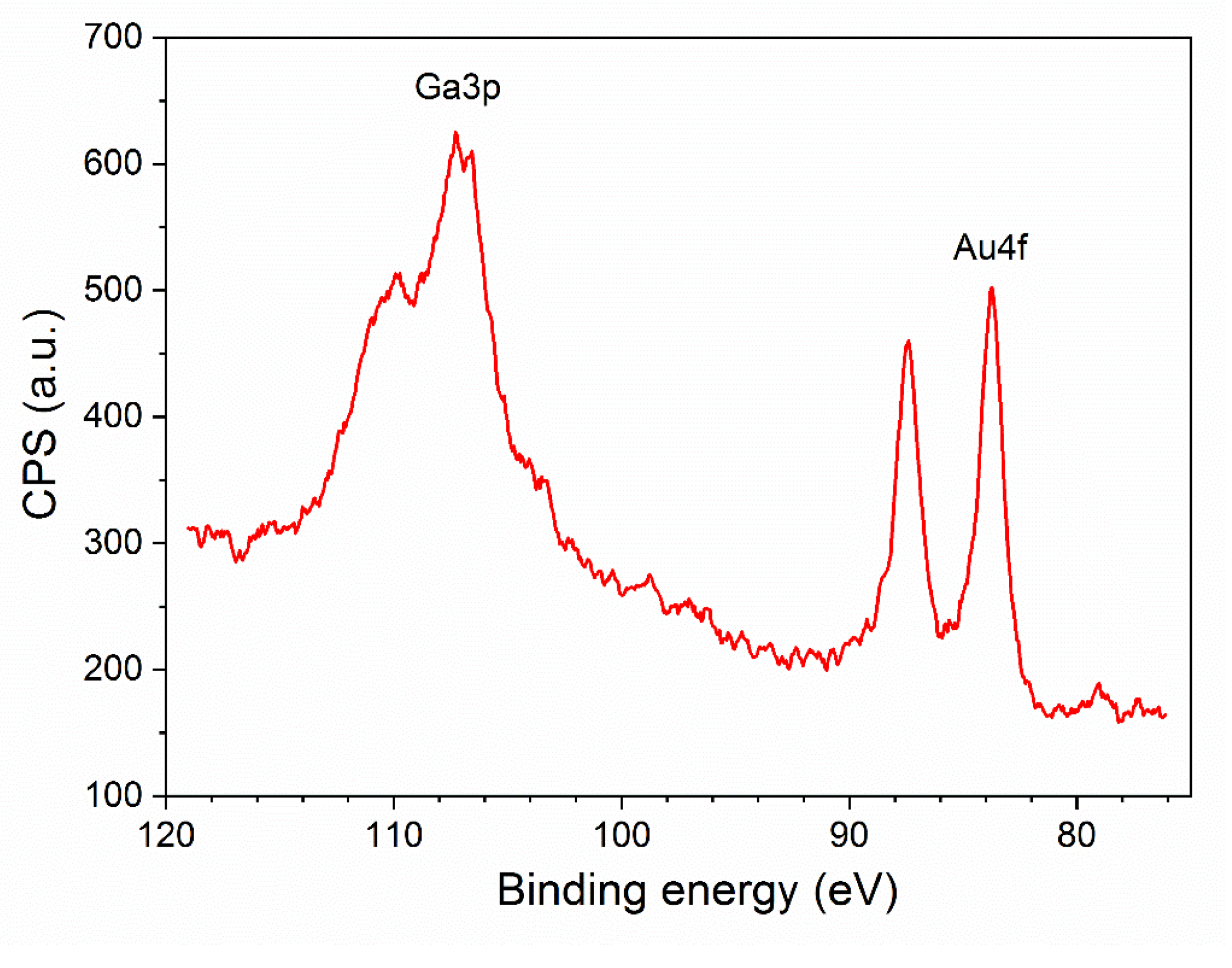
3.1. Structural Characterization of β-Ga2O3 Nanowires
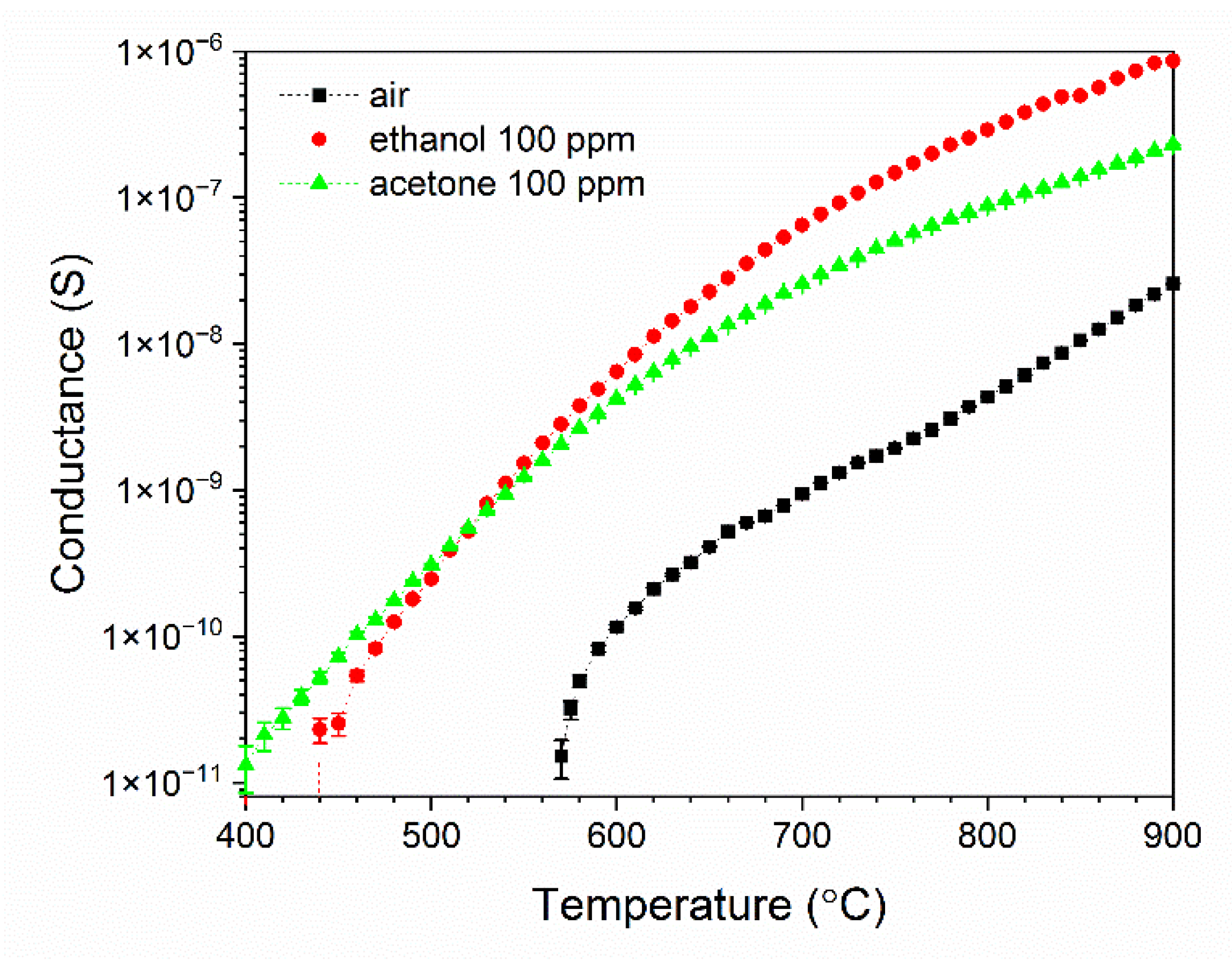
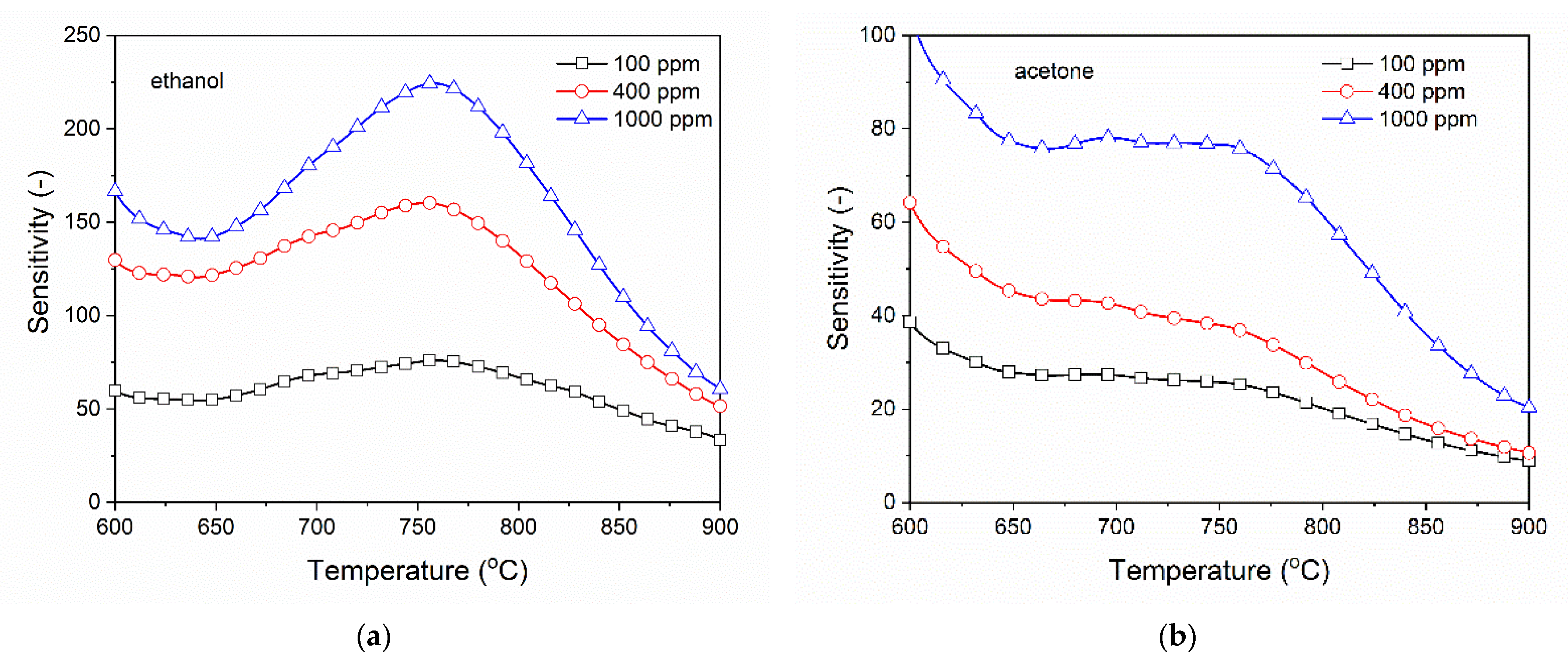
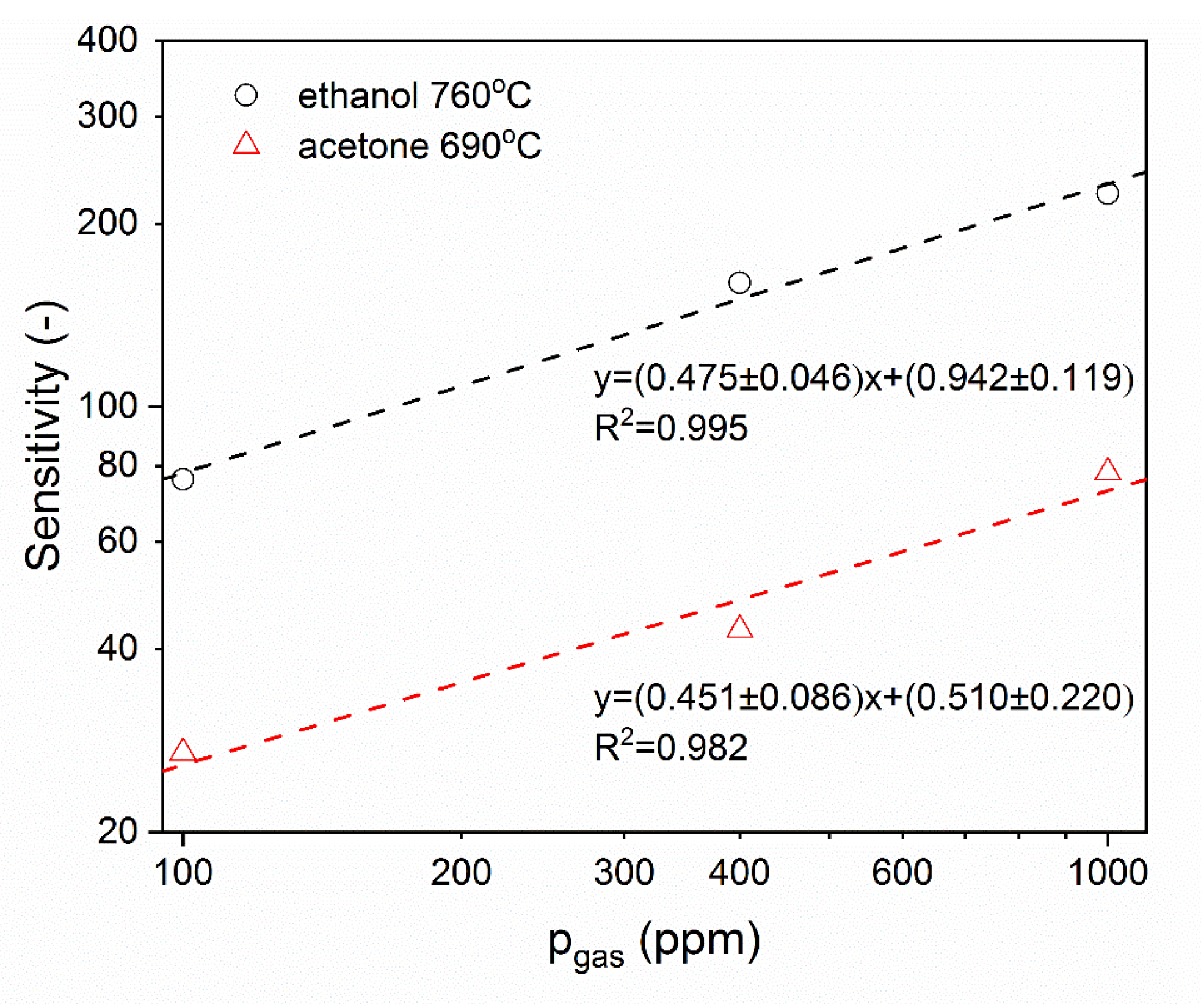
3.2. Analysis of Gas Sensing Properties of β-Ga2O3 Nanowires
- Surface conduction, when ;
- At the grain boundary, when ;
- In the volume of grains, when ;
- At the Schottky contact;
- Surface conduction, when ;
- In the volume of the nanowire, when ;
- At the Schottky contact;
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, T.; Lv, X.; Hu, Z.; Xu, A.; Feng, C. Semiconductor Metal Oxides as Chemoresistive Sensors for Detecting Volatile Organic Compounds. Sensors 2019, 19, 233. [Google Scholar] [CrossRef]
- Teterycz, H.; Halek, P.; Wiśniewski, K.; Halek, G.; Koźlecki, T.; Polowczyk, I. Oxidation of Hydrocarbons on the Surface of Tin Dioxide Chemical Sensors. Sensors 2011, 11, 4425–4437. [Google Scholar] [CrossRef]
- Yamazoe, N. Toward innovations of gas sensor technology. Sens. Actuators B Chem. 2005, 108, 2–14. [Google Scholar] [CrossRef]
- Fiedot-Toboła, M.; Suchorska-Woźniak, P.; Startek, K.; Rac-Rumijowska, O.; Szukiewicz, R.; Kwoka, M.; Teterycz, H. Correlation between Microstructure and Chemical Composition of Zinc Oxide Gas Sensor Layers and Their Gas-Sensitive Properties in Chlorine Atmosphere. Sensors 2020, 20, 6951. [Google Scholar] [CrossRef] [PubMed]
- Suchorska-Woźniak, P.; Rac, O.; Fiedot, M.; Teterycz, H. The Impact of Sepiolite on Sensor Parameters during the Detection of Low Concentrations of Alcohols. Sensors 2016, 16, 1881. [Google Scholar] [CrossRef]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N.; Lin, L. A review on chemiresistive room temperature gas sensors based on metal oxide nanostructures, graphene and 2D transition metal dichalcogenides. Microchim. Acta 2018, 185, 213. [Google Scholar] [CrossRef]
- Galstyan, V.; Poli, N.; Comini, E. Highly Sensitive and Selective H2S Chemical Sensor Based on ZnO Nanomaterial. Appl. Sci. 2019, 9, 1167. [Google Scholar] [CrossRef]
- Nasiri, N.; Clarke, C. Nanostructured Gas Sensors for Medical and Health Applications: Low to High Dimensional Materials. Biosensors 2019, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Shin, G. Soft, Wirelessly Powered Humidity Sensor Based on SnO2 Nanowires for Wireless/Wearable Sensor Application. Materials 2020, 13, 2176. [Google Scholar] [CrossRef]
- Jha, R.K.; D’Costa, J.V.; Sakhuja, N.; Bhat, N. MoSe2 nanoflakes based chemiresistive sensors for ppb-level hydrogen sulfide gas detection. Sens. Actuators B Chem. 2019, 297, 126687. [Google Scholar] [CrossRef]
- Kuchibhatla, S.V.N.T.; Karakoti, A.S.; Bera, D.; Seal, S. One dimensional nanostructured materials. Prog. Mater. Sci. 2007, 52, 699–913. [Google Scholar] [CrossRef]
- Franke, M.E.; Koplin, T.J.; Simon, U. Metal and Metal Oxide Nanoparticles in Chemiresistors: Does the Nanoscale Matter? Small 2006, 2, 36–50. [Google Scholar] [CrossRef]
- Tsao, J.Y.; Chowdhury, S.; Hollis, M.A.; Jena, D.; Johnson, N.M.; Jones, K.A.; Kaplar, R.J.; Rajan, S.; Van de Walle, C.G.; Bellotti, E.; et al. Ultrawide-Bandgap Semiconductors: Research Opportunities and Challenges. Adv. Electron. Mater. 2018, 4, 1600501. [Google Scholar] [CrossRef]
- Baldini, M.; Galazka, Z.; Wagner, G. Recent progress in the growth of β-Ga2O3 for power electronics applications. Mater. Sci. Semicond. Process. 2018, 78, 132–146. [Google Scholar] [CrossRef]
- Galazka, Z. β-Ga2O3 for wide-bandgap electronics and optoelectronics. Semicond. Sci. Technol. 2018, 33, 113001. [Google Scholar] [CrossRef]
- Stepanov, S.I.; Nikolaev, V.I.; Bougrov, V.E.; Romanov, A.E. Gallium oxide: Properties and applications—A review. Rev. Adv. Mater. Sci. 2016, 44, 63–86. [Google Scholar]
- Higashiwaki, M.; Sasaki, K.; Murakami, H.; Kumagai, Y.; Koukitu, A.; Kuramata, A.; Masui, T.; Yamakoshi, S. Recent progress in Ga2O3 power devices. Semicond. Sci. Technol. 2016, 31, 034001. [Google Scholar] [CrossRef]
- Jiang, Z.X.; Wu, Z.Y.; Ma, C.C.; Deng, J.N.; Zhang, H.; Xu, Y.; Ye, J.D.; Fang, Z.L.; Zhang, G.Q.; Kang, J.Y.; et al. P-type β-Ga2O3 metal-semiconductor-metal solar-blind photodetectors with extremely high responsivity and gain-bandwidth product. Mater. Today Phys. 2020, 14, 100226. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, J.; Yang, Y.; Pan, D.; Xing, Y.; Shi, X.; Xia, X.; Liang, H. Catalytic growth and characterization of single crystalline Zn doped p-type β-Ga2O3 nanowires. J. Alloy. Compd. 2016, 687, 964–968. [Google Scholar] [CrossRef]
- Afzal, A. β-Ga2O3 nanowires and thin films for metal oxide semiconductor gas sensors: Sensing mechanisms and performance enhancement strategies. J. Mater. 2019, 5, 542–557. [Google Scholar] [CrossRef]
- Varley, J.B.; Weber, J.R.; Janotti, A.; Van de Walle, C.G. Oxygen vacancies and donor impurities in β-Ga2O3. Appl. Phys. Lett. 2010, 97, 142106. [Google Scholar] [CrossRef]
- King, P.D.C.; Veal, T.D. Conductivity in transparent oxide semiconductors. J. Phys. Condens. Matter 2011, 23, 334214. [Google Scholar] [CrossRef] [PubMed]
- Higashiwaki, M.; Sasaki, K.; Kuramata, A.; Masui, T.; Yamakoshi, S. Gallium oxide (Ga2O3) metal-semiconductor field-effect transistors on single-crystal β-Ga2O3 (010) substrates. Appl. Phys. Lett. 2012, 100, 013504. [Google Scholar] [CrossRef]
- Fleischer, M.; Meixner, H. Gallium oxide thin films: A new material for high-temperature oxygen sensors. Sens. Actuators B Chem. 1991, 4, 437–441. [Google Scholar] [CrossRef]
- Hoefer, U.; Frank, J.; Fleischer, M. High temperature Ga2O3-gas sensors and SnO2-gas sensors: A comparison. Sens. Actuators B Chem. 2001, 78, 6–11. [Google Scholar] [CrossRef]
- Réti, F.; Fleischer, M.; Perczel, I.V.; Meixner, H.; Giber, J. Detection of reducing gases in air by β-Ga2O3 thin films using self-heated and externally (oven-) heated operation modes. Sens. Actuators B Chem. 1996, 34, 378–382. [Google Scholar] [CrossRef]
- Pohle, R.; Fleischer, M.; Meixner, H. In situ infrared emission spectroscopic study of the adsorption of H2O and hydrogen-containing gases on Ga2O3 gas sensors. Sens. Actuators B Chem. 2000, 68, 151–156. [Google Scholar] [CrossRef]
- Yu, M.-F.; Atashbar, M.Z.; Chen, X. Mechanical and electrical characterization of β-Ga2O3 nanostructures for sensing applications. IEEE Sens. J. 2005, 5, 20–25. [Google Scholar] [CrossRef]
- Korotcenkov, G. Current Trends in Nanomaterials for Metal Oxide-Based Conductometric Gas Sensors: Advantages and Limitations. Part 1: 1D and 2D Nanostructures. Nanomaterials 2020, 10, 1392. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.G.; Kim, W.S.; Kim, D.H.; Hong, S.H. Fabrication of Ga2O3/SnO2 core-shell nanowires and their ethanol gas sensing properties. J. Mater. Res. 2011, 26, 2322–2327. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Sun, G.-J.; Lee, C. Synthesis, structure and ethanol sensing properties of Ga2O3-core/WO3-shell nanostructures. Thin Solid Film. 2015, 591, 341–345. [Google Scholar] [CrossRef]
- Korbutowicz, R.; Stafiniak, A.; Serafińczuk, J. Ga2O3 nanowires preparation at atmospheric pressure. Mater. Sci. 2017, 35, 412–420. [Google Scholar] [CrossRef][Green Version]
- Jubu, P.R.; Yam, F.K.; Chahrour, K.M. Structural and morphological properties of β-Ga2O3 nanostructures synthesized at various deposition temperatures. Phys. E Low Dimens. Syst. Nanostructures 2020, 123, 114153. [Google Scholar] [CrossRef]
- Jia, C.; Jeon, D.-W.; Xu, J.; Yi, X.; Park, J.-H.; Zhang, Y. Catalyst-Assisted Large-Area Growth of Single-Crystal β-Ga2O3 Nanowires on Sapphire Substrates by Metal–Organic Chemical Vapor Deposition. Nanomaterials 2020, 10, 1031. [Google Scholar] [CrossRef]
- McIntyre, P.C.; Fontcuberta i Morral, A. Semiconductor nanowires: To grow or not to grow? Mater. Today Nano 2020, 9, 100058. [Google Scholar] [CrossRef]
- Sutter, E.; Idrobo, J.C.; Sutter, P. Synthesis and optoelectronic properties of ultrathin Ga2O3 nanowires. J. Mater. Chem. C 2020, 8, 11555–11562. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, V.; Singh, R. Diameter Tuning of β-Ga2O3 Nanowires Using Chemical Vapor Deposition Technique. Nanoscale Res. Lett. 2017, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Suchorska-Woźniak, P.; Rac, O.; Klimkiewicz, R.; Fiedot, M.; Teterycz, H. Dehydrogenation properties of ZnO and the impact of gold nanoparticles on the process. Appl. Catal. A Gen. 2016, 514, 135–145. [Google Scholar] [CrossRef]
- Williamson, G.; Hall, W. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Mote, V.; Purushotham, Y.; Dole, B. Williamson-Hall analysis in estimation of lattice strain in nanometer-sized ZnO particles. J. Theor. Appl. Phys. 2012, 6, 6. [Google Scholar] [CrossRef]
- Li, W.; Zamani, R.; Rivera Gil, P.; Pelaz, B.; Ibáñez, M.; Cadavid, D.; Shavel, A.; Alvarez-Puebla, R.A.; Parak, W.J.; Arbiol, J.; et al. CuTe Nanocrystals: Shape and Size Control, Plasmonic Properties, and Use as SERS Probes and Photothermal Agents. J. Am. Chem. Soc. 2013, 135, 7098–7101. [Google Scholar] [CrossRef]
- McCluskey, M.D. Point defects in Ga2O3. J. Appl. Phys. 2020, 127, 101101. [Google Scholar] [CrossRef]
- Qi, W.H.; Wang, M.P.; Liu, Q.H. Shape factor of nonspherical nanoparticles. J. Mater. Sci. 2005, 40, 2737–2739. [Google Scholar] [CrossRef]
- Schmeißer, D.; Henkel, K. Intrinsic electronic defects and multiple-atom processes in the oxidic semiconductor Ga2O3. J. Appl. Phys. 2018, 123, 161596. [Google Scholar] [CrossRef]
- Mahmoodinezhad, A.; Janowitz, C.; Naumann, F.; Plate, P.; Gargouri, H.; Henkel, K.; Schmeißer, D.; Flege, J.I. Low-temperature growth of gallium oxide thin films by plasma-enhanced atomic layer deposition. J. Vac. Sci. Technol. A 2020, 38, 022404. [Google Scholar] [CrossRef]
- Radnik, J.; Mohr, C.; Claus, P. On the origin of binding energy shifts of core levels of supported gold nanoparticles and dependence of pretreatment and material synthesis. Phys. Chem. Chem. Phys. 2003, 5, 172–177. [Google Scholar] [CrossRef]
- Sankar, M.; He, Q.; Morad, M.; Pritchard, J.; Freakley, S.J.; Edwards, J.K.; Taylor, S.H.; Morgan, D.J.; Carley, A.F.; Knight, D.W.; et al. Synthesis of Stable Ligand-free Gold–Palladium Nanoparticles Using a Simple Excess Anion Method. ACS Nano 2012, 6, 6600–6613. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Park, S. Enhancement mechanisms of ethanol-sensing properties based on Cr2O3 nanoparticle-anchored SnO2 nanowires. J. Mater. Res. Technol. 2020, 9, 271–281. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, L.; Deng, Z.; Liao, J. Electrically Transduced Gas Sensors Based on Semiconducting Metal Oxide Nanowires. Sensors 2020, 20, 6781. [Google Scholar] [CrossRef]
- Fleischer, M.; Meixner, H. Selectivity in high-temperature operated semiconductor gas-sensors. Sens. Actuators B Chem. 1998, 52, 179–187. [Google Scholar] [CrossRef]
- Xu, C.; Tamaki, J.; Miura, N.; Yamazoe, N. Grain size effects on gas sensitivity of porous SnO2-based elements. Sens. Actuators B Chem. 1991, 3, 147–155. [Google Scholar] [CrossRef]
- Bârsan, N.; Hübner, M.; Weimar, U. Conduction mechanisms in SnO2 based polycrystalline thick film gas sensors exposed to CO and H2 in different oxygen backgrounds. Sens. Actuators B Chem. 2011, 157, 510–517. [Google Scholar] [CrossRef]
- Tonezzer, M.; Hieu, N.V. Size-dependent response of single-nanowire gas sensors. Sens. Actuators B Chem. 2012, 163, 146–152. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Rao, M.V. Gallium Nitride (GaN) Nanostructures and Their Gas Sensing Properties: A Review. Sensors 2020, 20, 3889. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, M.; Meixner, H. Sensitive, selective and stable CH4 detection using semiconducting Ga2O3 thin films. Sens. Actuators B Chem. 1995, 26, 81–84. [Google Scholar] [CrossRef]
- Schwebel, T.; Fleischer, M.; Meixner, H.; Kohl, C.-D. CO-Sensor for domestic use based on high temperature stable Ga2O3 thin films. Sens. Actuators B Chem. 1998, 49, 46–51. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krawczyk, M.; Suchorska-Woźniak, P.; Szukiewicz, R.; Kuchowicz, M.; Korbutowicz, R.; Teterycz, H. Morphology of Ga2O3 Nanowires and Their Sensitivity to Volatile Organic Compounds. Nanomaterials 2021, 11, 456. https://doi.org/10.3390/nano11020456
Krawczyk M, Suchorska-Woźniak P, Szukiewicz R, Kuchowicz M, Korbutowicz R, Teterycz H. Morphology of Ga2O3 Nanowires and Their Sensitivity to Volatile Organic Compounds. Nanomaterials. 2021; 11(2):456. https://doi.org/10.3390/nano11020456
Chicago/Turabian StyleKrawczyk, Maciej, Patrycja Suchorska-Woźniak, Rafał Szukiewicz, Maciej Kuchowicz, Ryszard Korbutowicz, and Helena Teterycz. 2021. "Morphology of Ga2O3 Nanowires and Their Sensitivity to Volatile Organic Compounds" Nanomaterials 11, no. 2: 456. https://doi.org/10.3390/nano11020456
APA StyleKrawczyk, M., Suchorska-Woźniak, P., Szukiewicz, R., Kuchowicz, M., Korbutowicz, R., & Teterycz, H. (2021). Morphology of Ga2O3 Nanowires and Their Sensitivity to Volatile Organic Compounds. Nanomaterials, 11(2), 456. https://doi.org/10.3390/nano11020456