Towards Control of the Size, Composition and Surface Area of NiO Nanostructures by Sn Doping
Abstract
1. Introduction
2. Experimental Details
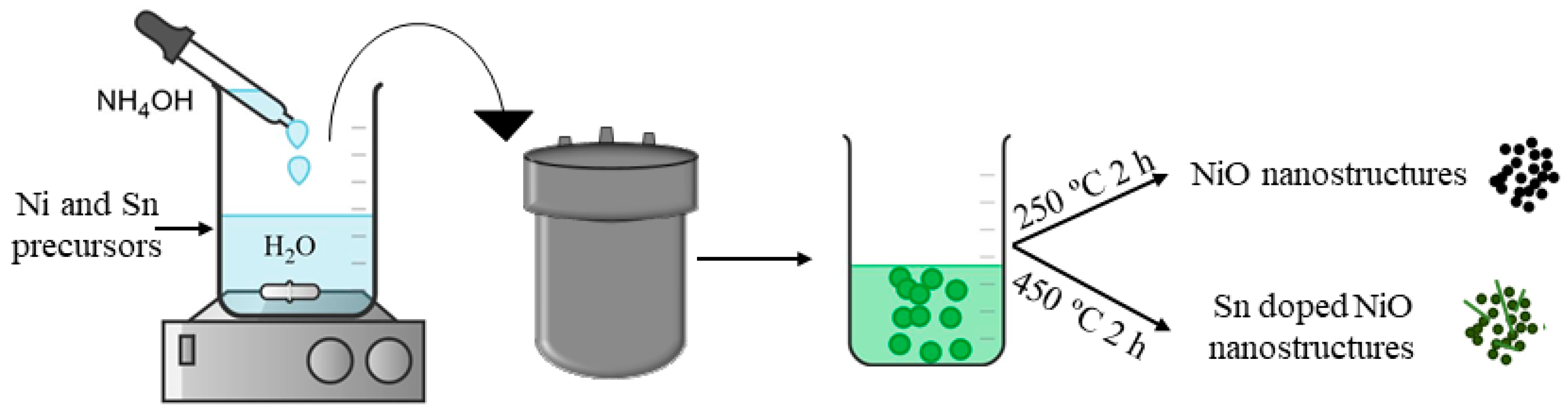
2.1. Synthesis of Undoped and Sn Doped NiO Nanostructures
2.2. Characterization
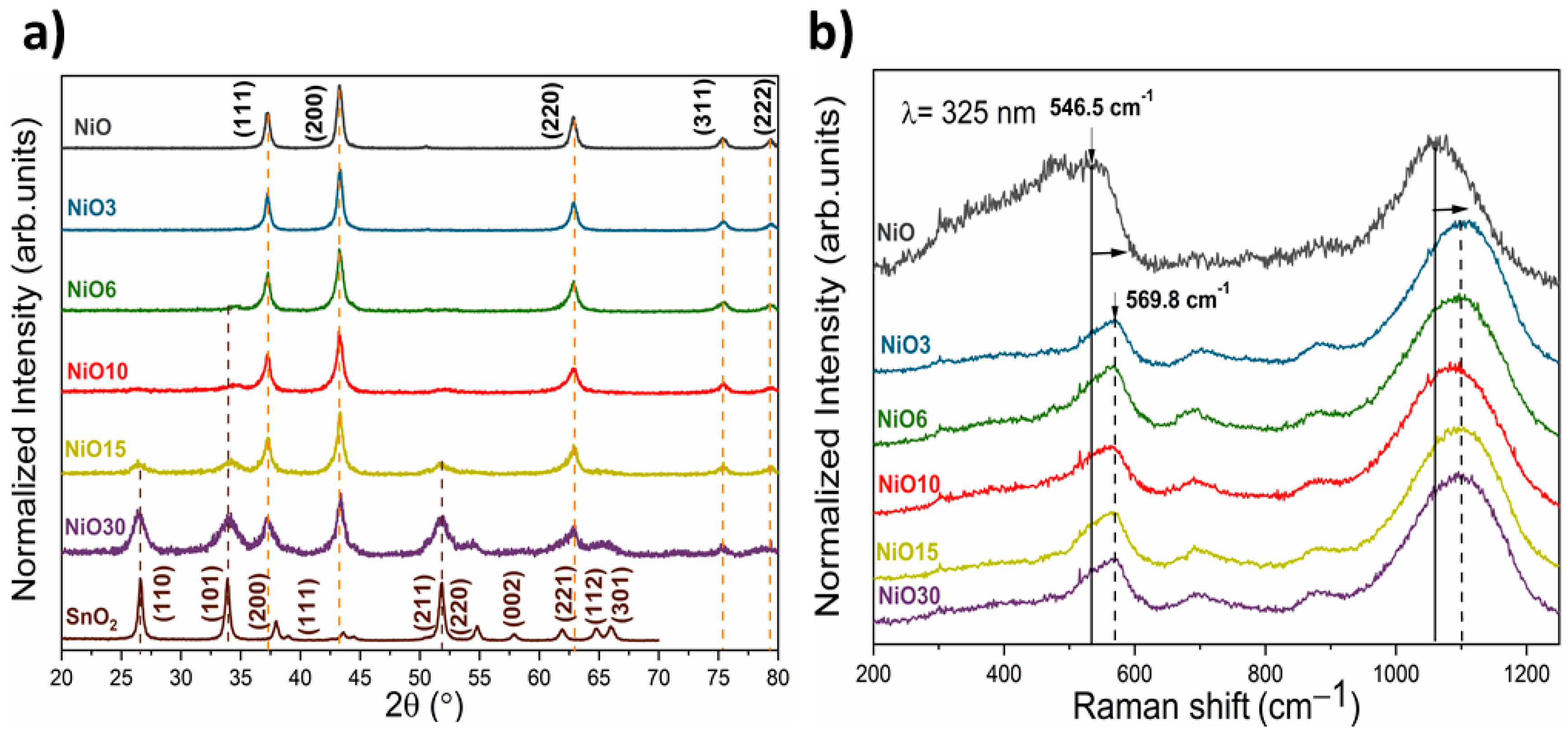
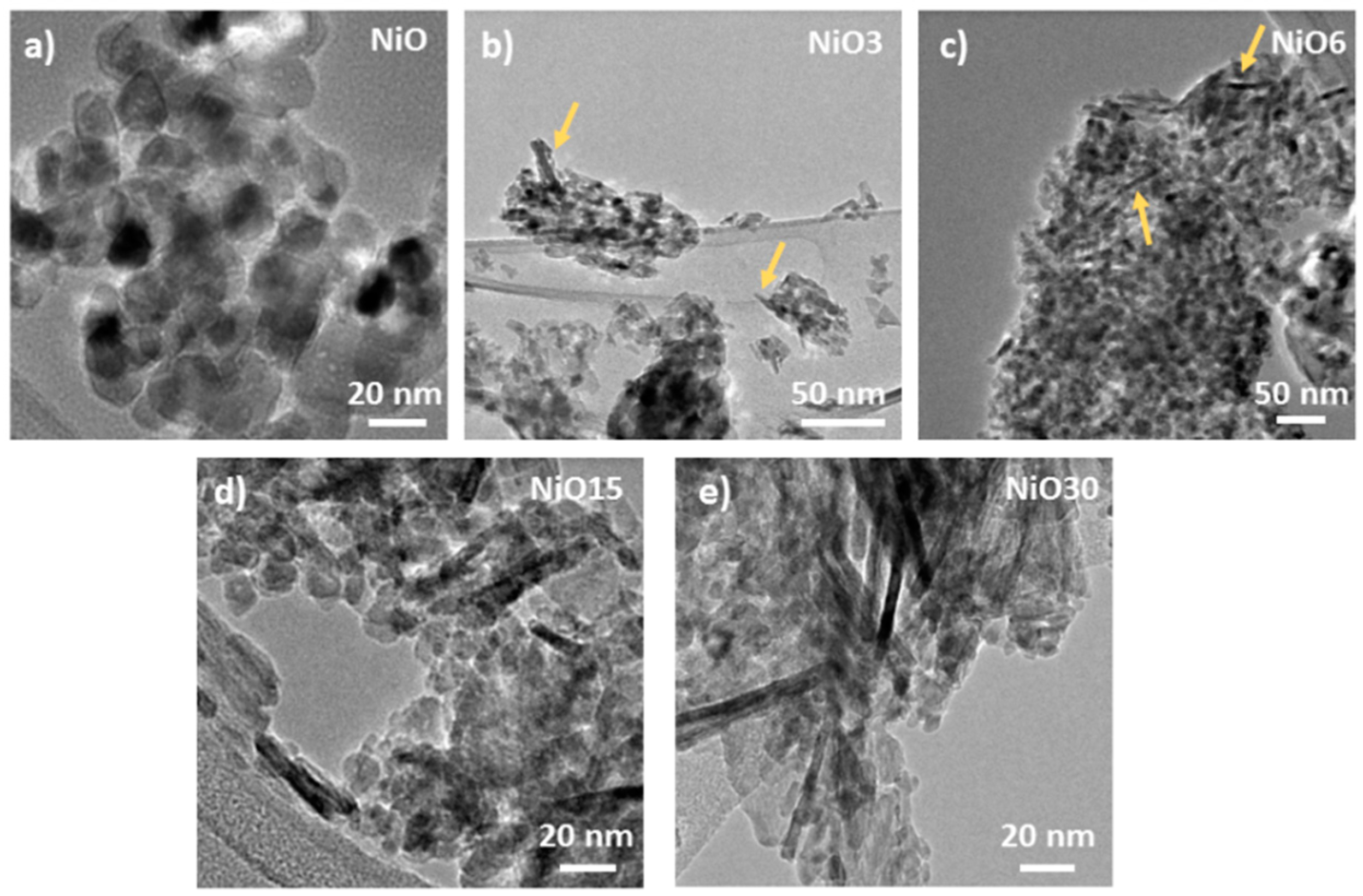
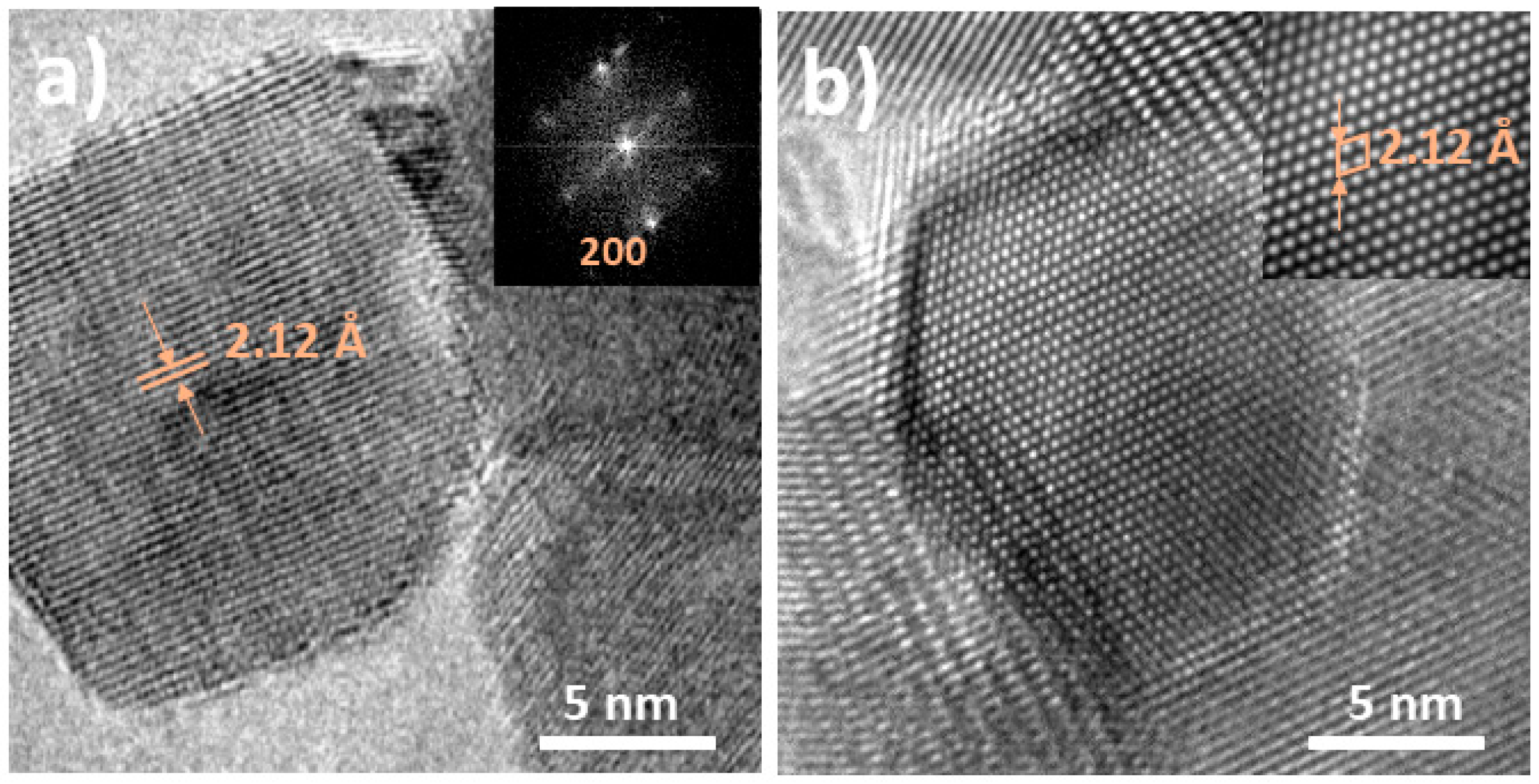
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, L.; Zhang, J.; Lei, J.; Xu, J.; Shang, B.; Liu, L.; Li, N.; Pan, F.-S. O-vacancy Enriched NiO Hexagonal Platelets Fabricated on Ni Foam as Self-supported Electrode for Extraordinary Pseudocapacitance. J. Mater. Chem. A 2018, 6, 7099–7106. [Google Scholar] [CrossRef]
- Lupo, C.; Eberheim, F.; Schlettwein, D. Facile low-temperature synthesis of nickel oxide by an internal combustion reaction for applications in electrochromic devices. J. Mater. Sci. 2020, 55, 14401–14414. [Google Scholar] [CrossRef]
- Cai, G.; Wang, X.; Cui, M.; Darmawan, P.; Wang, J.; Eh, A.L.-S.; Lee, P.S. Electrochromo-supercapacitor based on direct growth of NiO nanoparticles. Nano Energy 2015, 12, 258–267. [Google Scholar] [CrossRef]
- Ramesh, M.; Rao, M.P.C.; Anandan, S.; Nagaraja, H. Adsorption and photocatalytic properties of NiO nanoparticles synthesized via a thermal decomposition process. J. Mater. Res. 2018, 33, 601–610. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, C.; Li, X.; Wang, C.; Zhang, M.; Liu, Y. Electrospun Nanofibers of p-Type NiO/n-Type ZnO Heterojunctions with Enhanced Photocatalytic Activity. ACS Appl. Mater. Interfaces 2010, 2, 2915–2923. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, H.; Han, D.; Gu, F. Electron compensation in p-type 3DOM NiO by Sn doping for enhanced formaldehyde sensing performance. J. Mater. Chem. C 2017, 5, 3254–3263. [Google Scholar] [CrossRef]
- Mokoena, T.P.; Swart, H.C.; Motaung, D.E. A review on recent progress of p-type nickel oxide based gas sensors: Future perspectives. J. Alloy Compd. 2019, 805, 267–294. [Google Scholar] [CrossRef]
- Popescu, I.; Skoufa, Z.; Heracleous, E.; Lemonidou, A.; Marcu, I.-C. Study of M-Doped NiO (M = Li, Mg, Al, Ga, Ti, Nb) Catalysts by in Situ Electrical Conductivity Measurements in Correlation with their Catalytic Performances in Ethane Oxydehydrogenation. In Proceedings of the 2th European Congress on Catalysis—EuropaCat-XII, Kazan, Russia, 30 August–4 September 2015. [Google Scholar]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2, 63. [Google Scholar] [CrossRef]
- Linkov, P.; Artemyev, M.; Efimov, A.E.; Nabiev, I. Comparative advantages and limitations of the basic metrology methods applied to the characterization of nanomaterials. Nanoscale 2013, 5, 8781–8798. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, S.; Huang, J.; Wang, C.; Zhao, R.; Qu, F.; Wang, P.; Yang, M. Highly selective and sensitive xylene sensors based on Nb-doped NiO nanosheets. Sens. Actuators B Chem. 2020, 308, 127520. [Google Scholar] [CrossRef]
- Gao, H.; Wei, D.; Lin, P.; Liu, C.; Sun, P.; Shimanoe, K.; Yamazoe, N.; Lu, G. The design of excellent xylene gas sensor using Sn-doped NiO hierarchical nanostructure. Sens. Actuators B Chem. 2017, 253, 1152–1162. [Google Scholar] [CrossRef]
- Bai, S.; Fu, H.; Shu, X.; Luo, R.; Chen, A.; Li, D.; Liu, C.C. NiO hierarchical hollow microspheres doped Fe to enhance triethylamine sensing properties. Mater. Lett. 2018, 210, 305–308. [Google Scholar] [CrossRef]
- Yun, A.J.; Kim, J.; Hwang, T.; Park, B. Origins of Efficient Perovskite Solar Cells with Low-Temperature Processed SnO2 Electron Transport Layer. ACS Appl. Energy Mater. 2019, 2, 3554–3560. [Google Scholar] [CrossRef]
- Elger, A.-K.; Hess, C. Elucidating the Mechanism of Working SnO2 Gas Sensors Using Combined Operando UV/Vis, Raman, and IR Spectroscopy. Angew. Chem. Int. Ed. 2019, 58, 15057–15061. [Google Scholar] [CrossRef] [PubMed]
- Del Prado, F.; Andersen, H.F.; Taeño, M.; Mæhlen, J.P.; Ramírez-Castellanos, J.; Maestre, D.; Karazhanov, S.; Cremades, A. Comparative study of the implementation of tin and titanium oxide nanoparticles as electrodes materials in Li-ion batteries. Sci. Rep. 2020, 10, 5503. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, V.; Yahia, I.S.; AlFaify, S.; Shkir, M. Sn-doped ZnO nanocrystalline thin films with enhanced linear and nonlinear optical properties for optoelectronic applications. J. Phys. Chem. Solids 2017, 100, 115–125. [Google Scholar] [CrossRef]
- Al-Hadeethi, Y.; Umar, A.; Al-Heniti, S.H.; Kumar, R.; Kim, S.H.; Zhang, X.; Raffah, B.M. 2D Sn-doped ZnO ultrathin nanosheet networks for enhanced acetone gas sensing application. Ceram. Int. 2017, 43, 2418–2423. [Google Scholar] [CrossRef]
- Crockett, B.M.; Jansons, A.W.; Koskela, K.M.; Sharps, M.C.; Johnson, D.W.; Hutchison, J.E. Influence of Nanocrystal Size on the Optoelectronic Properties of Thin, Solution-Cast Sn-Doped In2O3 Films. Chem. Mater. 2019, 31, 3370–3380. [Google Scholar] [CrossRef]
- Guo, D.; Su, Y.; Shi, H.; Li, P.; Zhao, N.; Ye, J.; Wang, S.; Liu, A.; Chen, Z.; Li, C.; et al. Self-Powered Ultraviolet Photodetector with Superhigh Photoresponsivity (3.05 A/W) Based on the GaN/Sn:Ga2O3 pn Junction. ACS Nano 2018, 12, 12827–12835. [Google Scholar] [CrossRef]
- Kim, B.-Y.; Yoon, J.-W.; Kim, J.K.; Kang, Y.C.; Lee, J.-H. Dual Role of Multiroom-Structured Sn-Doped NiO Microspheres for Ultrasensitive and Highly Selective Detection of Xylene. ACS Appl. Mater. Interfaces 2018, 10, 16605–16612. [Google Scholar] [CrossRef]
- del Prado, F.; Taeño, M.; Maestre, D.; Ramírez-Castellanos, J.; González-Calbet, J.M.; Cremades, A. Effect of the synthesis method on the properties of lithium doped graphene oxide composites with tin oxide nanoparticles: Towards white luminescence. J. Phys. Chem. Solids 2019, 129, 133–139. [Google Scholar] [CrossRef]
- Ponnusamy, P.M.; Agilan, S.; Muthukumarasamy, N.; Senthil, T.S.; Rajesh, G.; Venkatraman, M.R.; Velauthapillai, D. Structural, optical and magnetic properties of undoped NiO and Fe-doped NiO nanoparticles synthesized by wet-chemical process. Mater. Charact. 2016, 114, 166–171. [Google Scholar] [CrossRef]
- Mironova-Ulmane, N.; Kuzmin, A.; Steins, I.; Grabis, J.; Sildos, I.; Pärs, M. Raman scattering in nanosized nickel oxide NiO. J. Phys. Conf. Ser. 2007, 93, 012039. [Google Scholar] [CrossRef]
- Lacerda, M.M.; Kargar, F.; Aytan, E.; Samnakay, R.; Debnath, B.; Li, J.X.; Khitun, A.; Lake, R.K.; Shi, J.; Balandin, A.A. Variable-temperature inelastic light scattering spectroscopy of nickel oxide: Disentangling phonons and magnons. Appl. Phys. Lett. 2017, 110, 202406. [Google Scholar] [CrossRef]
- Aytan, E.; Debnath, B.; Kargar, F.; Barlas, Y.; Lacerda, M.M.; Li, J.X.; Lake, R.K.; Shi, J.; Balandin, A.A. Spin-phonon coupling in antiferromagnetic nickel oxide. Appl. Phys. Lett. 2017, 111, 252402. [Google Scholar] [CrossRef]
- Cordoba-Torresi, S.I.; Hugot-Le Goff, A.; Joiret, S. Electrochromic Behavior of Nickel Oxide Electrodes: II. Identification of the Bleached State by Raman Spectroscopy and Nuclear Reactions. J. Electrochem. Soc. 1991, 138, 1554–1559. [Google Scholar] [CrossRef]
- Jang, W.-L.; Lu, Y.-M.; Hwang, W.-S.; Hsiung, T.-L.; Wang, H.P. Point defects in sputtered NiO films. Appl. Phys. Lett. 2009, 94, 062103. [Google Scholar] [CrossRef]
- Kiran, G.K.; Munichandraiah, N.; Vishnu Kamath, P. Effect of non-stoichiometry on the charge storage capacity of NiO conversion anodes in Li-ion batteries. J. Solid State Electrochem. 2018, 22, 3833–3843. [Google Scholar] [CrossRef]
- Dubey, P.; Kaurav, N.; Devan, R.S.; Okram, G.S.; Kuo, Y.K. The effect of stoichiometry on the structural, thermal and electronic properties of thermally decomposed nickel oxide. RSC Adv. 2018, 8, 5882–5890. [Google Scholar] [CrossRef]
- Spanier, J.E.; Robinson, R.D.; Zhang, F.; Chan, S.-W.; Herman, I.P. Size-dependent properties of CeO-y‒2 nanoparticles as studied by Raman scattering. Phys. Rev. B 2001, 64, 245407. [Google Scholar] [CrossRef]
- Duan, W.J.; Lu, S.H.; Wu, Z.L.; Wang, Y.S. Size Effects on Properties of NiO Nanoparticles Grown in Alkalisalts. J. Phys. Chem. C 2012, 116, 26043–26051. [Google Scholar] [CrossRef]
- Varshney, D.; Dwivedi, S. Synthesis, structural, Raman spectroscopic and paramagnetic properties of Sn doped NiO nanoparticles. Superlattices Microstruct. 2015, 86, 430–437. [Google Scholar] [CrossRef]
- Gu, C.; Cui, Y.; Wang, L.; Sheng, E.; Shim, J.-J.; Huang, J. Synthesis of the porous NiO/SnO2 microspheres and microcubes and their enhanced formaldehyde gas sensing performance. Sens. Actuators B Chem. 2017, 241, 298–307. [Google Scholar] [CrossRef]
- Ning, X.; Wang, Z.; Zhang, Z. Fermi Level shifting, Charge Transfer and Induced Magnetic Coupling at La0.7Ca0.3MnO3/LaNiO3 Interface. Sci. Rep. 2015, 5, 8460. [Google Scholar] [CrossRef] [PubMed]
- Naumkin, A.V.; Kraut-Vass, A.; Powell, C.J.; Gaarenstroom, S.W.; National Institute of Standards and Technology U. S. Department of Commerce. NIST X-Ray Photoelectron Spectroscopy Database; NIST: Gaithersburg, MD, USA, 2012.
- Stranick, M.A.; Moskwa, A. SnO2 by XPS. Surf. Sci. Spectra 1993, 2, 50–54. [Google Scholar] [CrossRef]
- Wang, C.; Liu, J.; Yang, Q.; Sun, P.; Gao, Y.; Liu, F.; Zheng, J.; Lu, G. Ultrasensitive and low detection limit of acetone gas sensor based on W-doped NiO hierarchical nanostructure. Sens. Actuators B Chem. 2015, 220, 59–67. [Google Scholar] [CrossRef]
- Ratcliff, E.L.; Meyer, J.; Steirer, K.X.; Garcia, A.; Berry, J.J.; Ginley, D.S.; Olson, D.C.; Kahn, A.; Armstrong, N.R. Evidence for near-Surface NiOOH Species in Solution-Processed NiOx Selective Interlayer Materials: Impact on Energetics and the Performance of Polymer Bulk Heterojunction Photovoltaics. Chem. Mater. 2011, 23, 4988–5000. [Google Scholar] [CrossRef]
- Kuzmin, A.; Mironova-Ulmane, N.; Ronchin, S. Origin of Visible Photoluminescence in NiO and NicMg1-cO Single Crystals; SPIE: Bellingham, DC, USA, 2003; Volume 5122. [Google Scholar]
- Gandhi, A.; Wu, S.Y. Strong Deep-Level-Emission Photoluminescence in NiO Nanoparticles. Nanomaterials 2017, 7, 231. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, A.C.; Huang, C.-Y.; Yang, C.C.; Chan, T.S.; Cheng, C.-L.; Ma, Y.-R.; Wu, S.Y. Growth mechanism and magnon excitation in NiO nanowalls. Nanoscale Res. Lett. 2011, 6, 485. [Google Scholar] [CrossRef]
- Sokolov, V.; Pustovarov, V.A.; Churmanov, V.; Ivanov, V.; Gruzdev, N.; Sokolov, P.S.; Baranov, A.; Moskvin, A. Unusual X-ray excited luminescence spectra of NiO suggestive of a self-trapping of the d-d charge transfer exciton. Phys. Rev. B 2012, 86, 115128. [Google Scholar] [CrossRef]
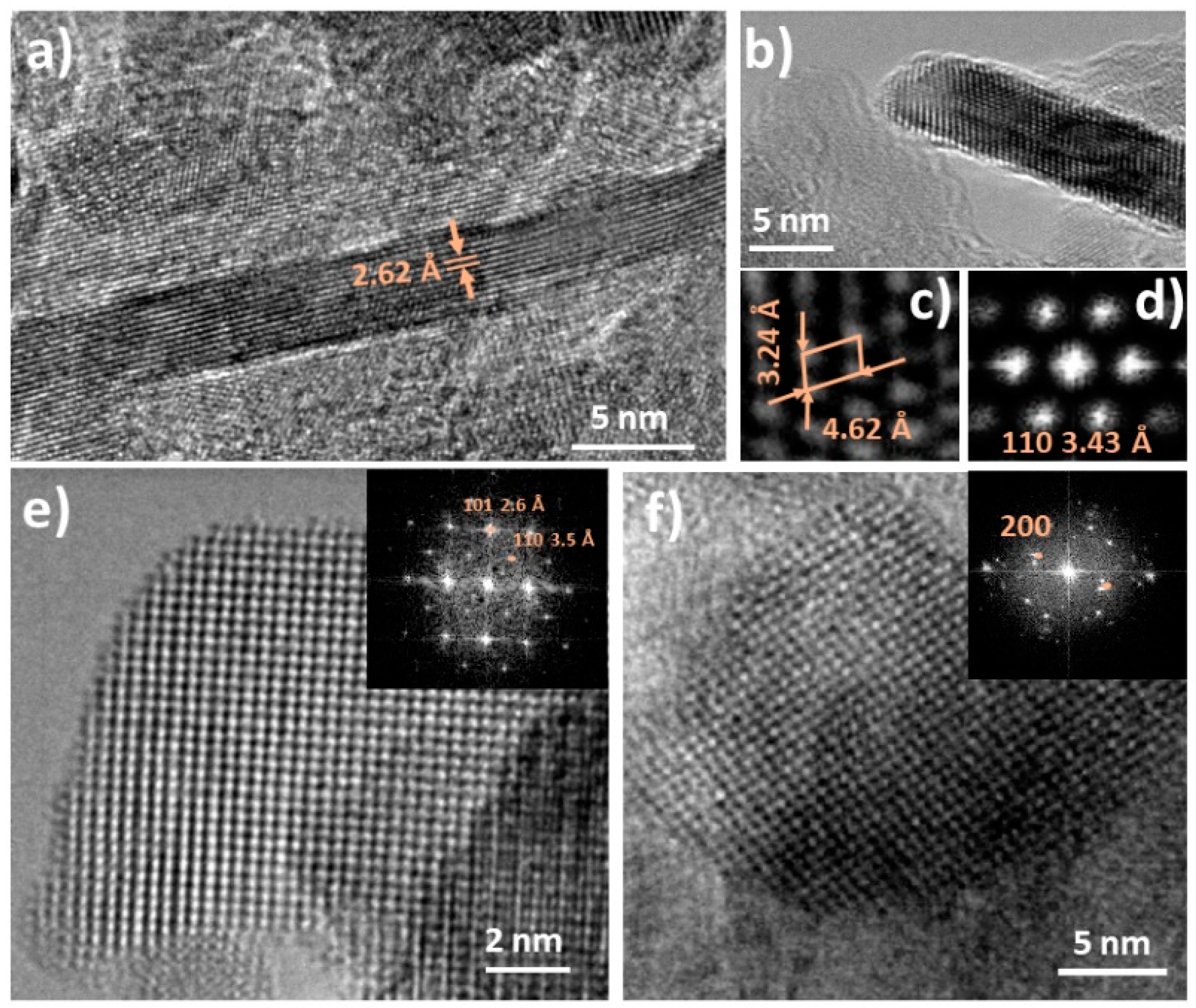
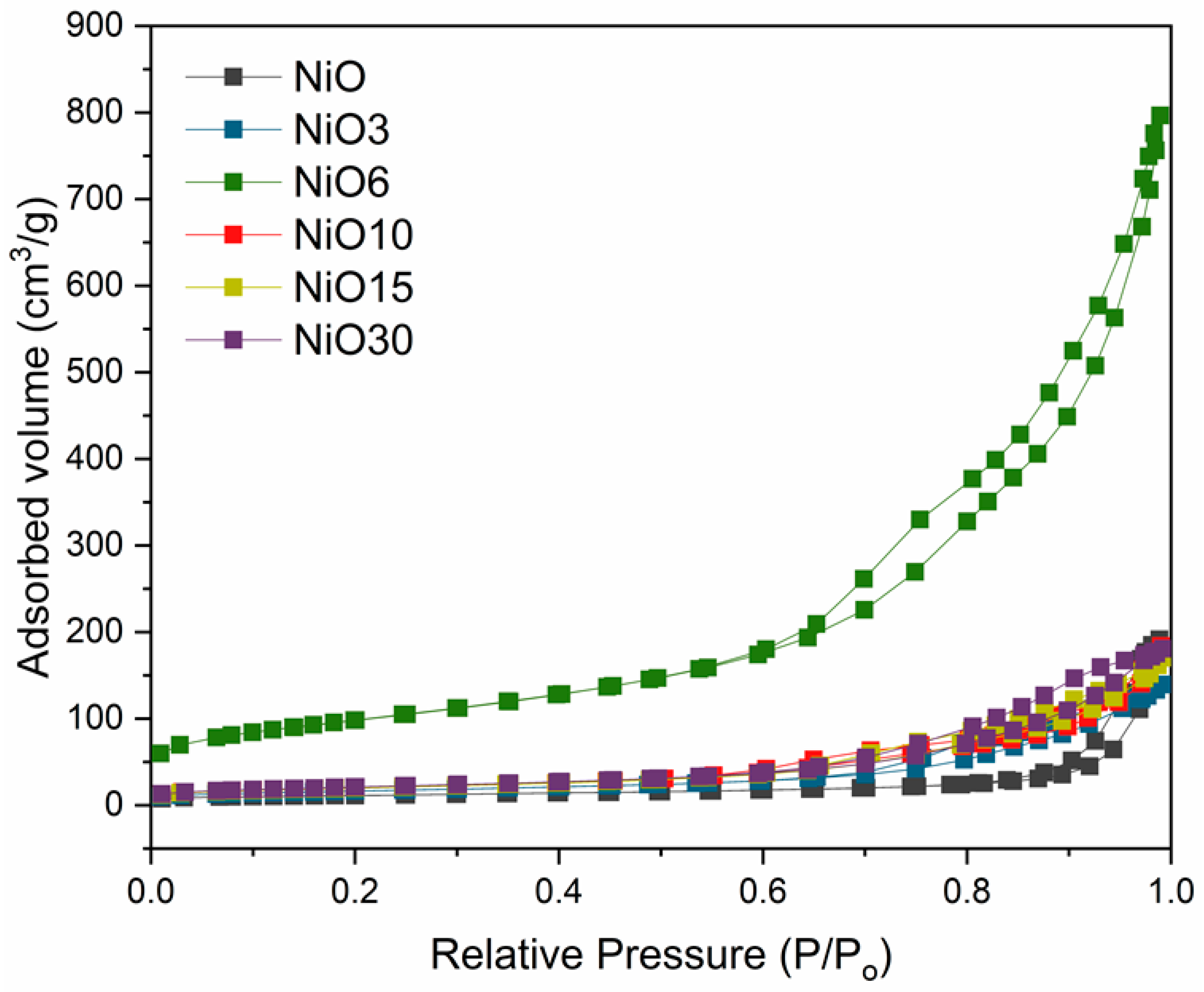
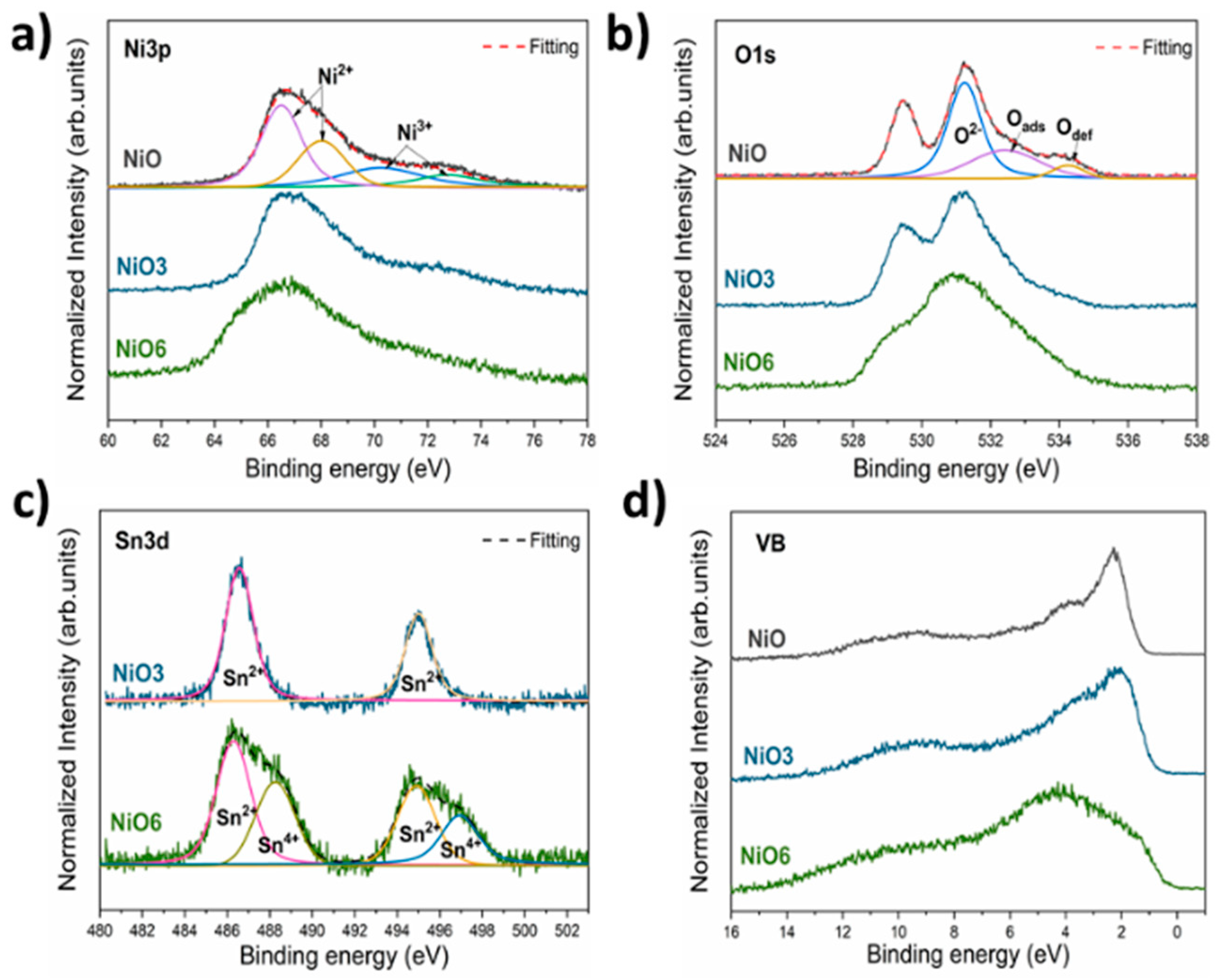

| Sample | Lattice Parameters a a = b = c (Å) | DNiO a (nm) | DNiO b (nm) | Surface Area c (m2/g) |
|---|---|---|---|---|
| NiO | 4.178 | 10.6 | 12 | 39.4 |
| NiO3 | 4.181 | 7.9 | 8.1 | 56.8 |
| NiO6 | 4.180 | 7.3 | 7.6 | 342.4 |
| NiO10 | 4.183 | 6.9 | 6.6 | 71.7 |
| NiO15 | 4.181 | 6.8 | 6.2 | 71.2 |
| NiO30 | 4.167 | 5.7 | 5.4 | 72.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taeño, M.; Maestre, D.; Ramírez-Castellanos, J.; Li, S.; Lee, P.S.; Cremades, A. Towards Control of the Size, Composition and Surface Area of NiO Nanostructures by Sn Doping. Nanomaterials 2021, 11, 444. https://doi.org/10.3390/nano11020444
Taeño M, Maestre D, Ramírez-Castellanos J, Li S, Lee PS, Cremades A. Towards Control of the Size, Composition and Surface Area of NiO Nanostructures by Sn Doping. Nanomaterials. 2021; 11(2):444. https://doi.org/10.3390/nano11020444
Chicago/Turabian StyleTaeño, María, David Maestre, Julio Ramírez-Castellanos, Shaohui Li, Pooi See Lee, and Ana Cremades. 2021. "Towards Control of the Size, Composition and Surface Area of NiO Nanostructures by Sn Doping" Nanomaterials 11, no. 2: 444. https://doi.org/10.3390/nano11020444
APA StyleTaeño, M., Maestre, D., Ramírez-Castellanos, J., Li, S., Lee, P. S., & Cremades, A. (2021). Towards Control of the Size, Composition and Surface Area of NiO Nanostructures by Sn Doping. Nanomaterials, 11(2), 444. https://doi.org/10.3390/nano11020444








