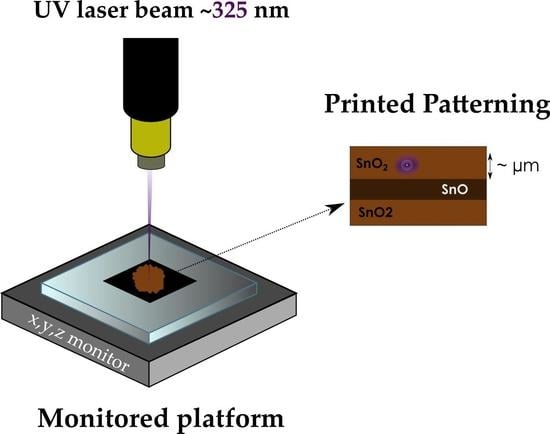
In Situ Local Oxidation of SnO Induced by Laser Irradiation: A Stability Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of SnO Nanoparticles and Nanostructures
2.2. Characterization Techniques
3. Results
3.1. TEM and XRD
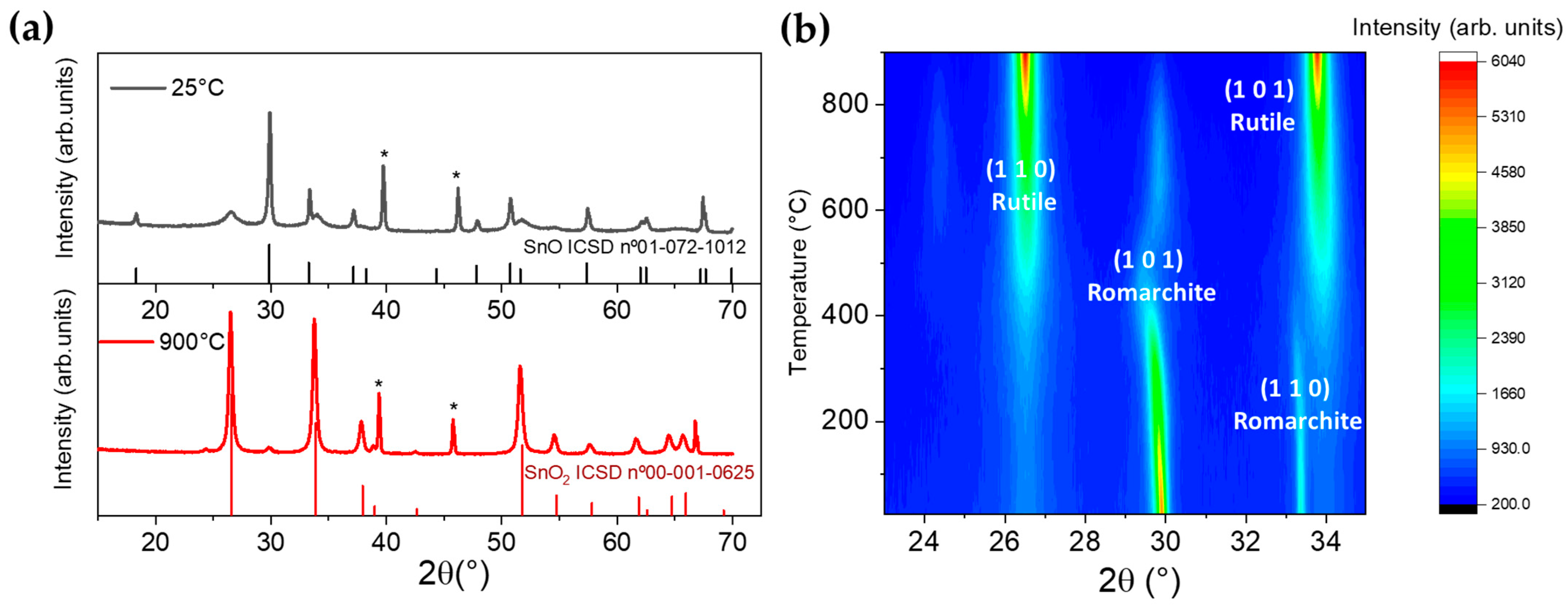
3.2. Thermo XRD
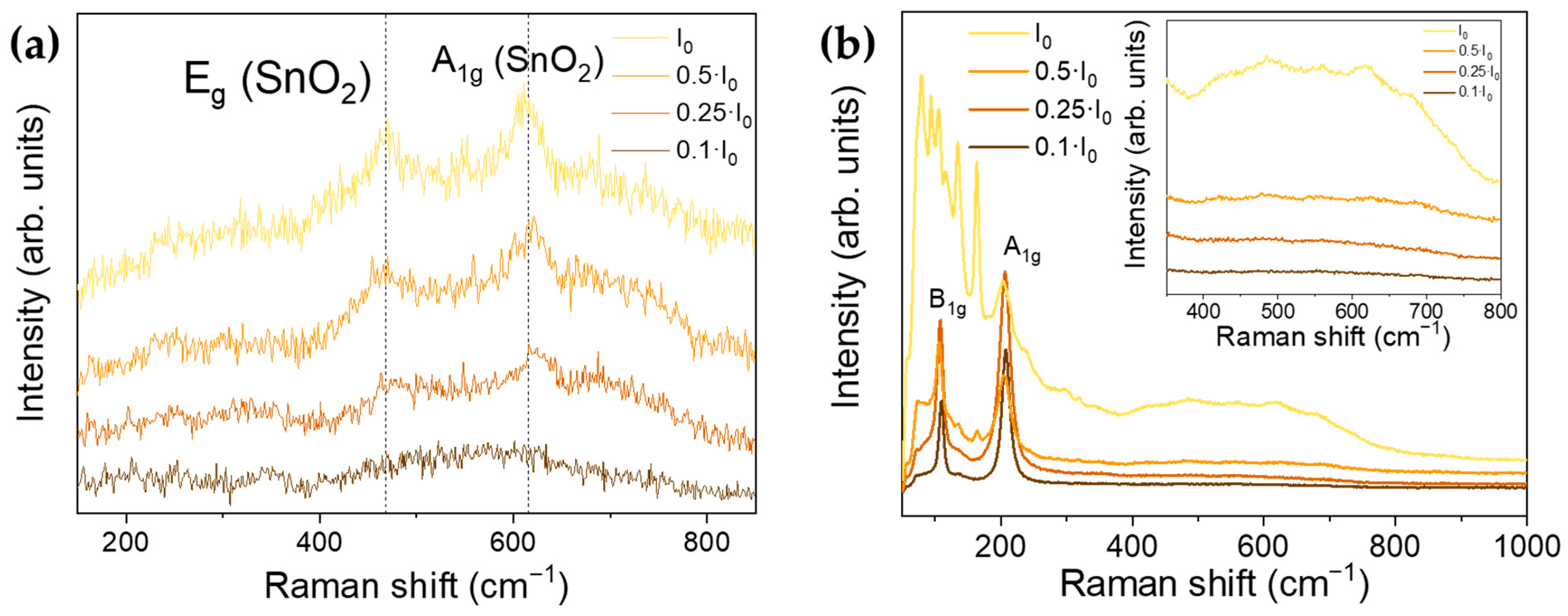
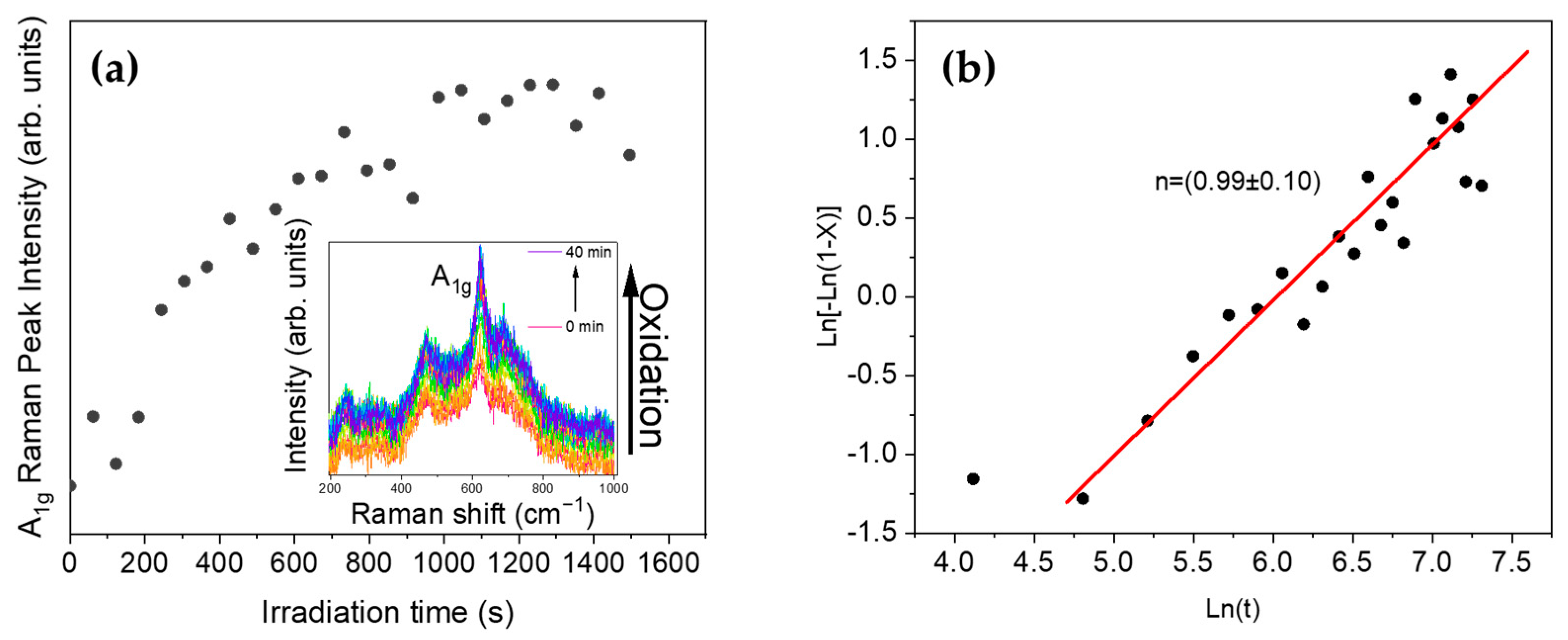
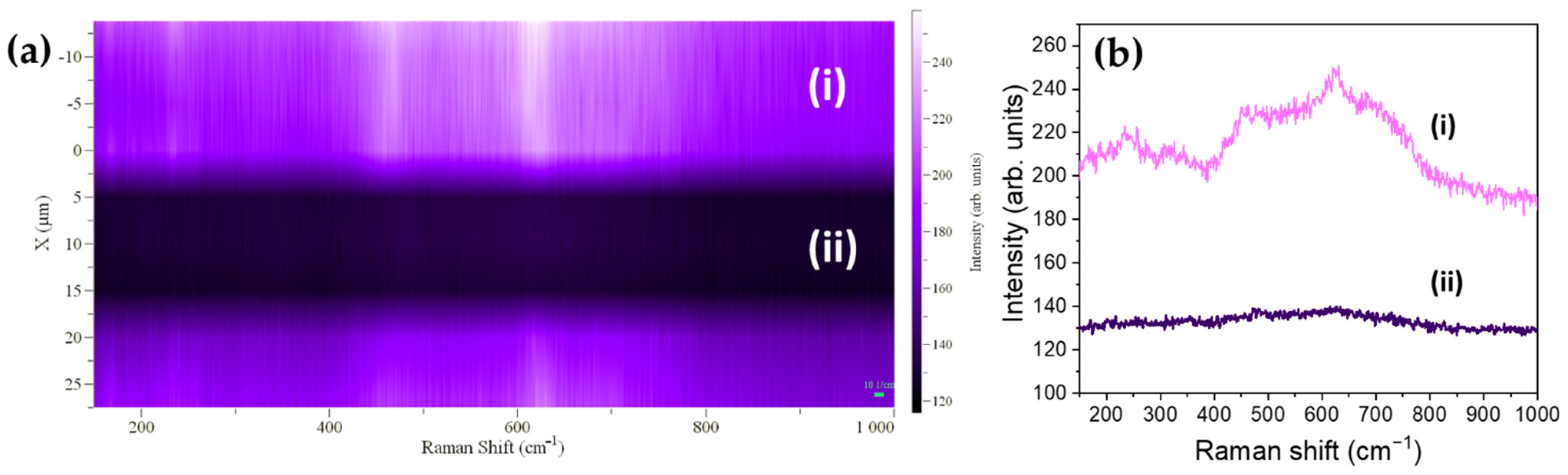
3.3. Raman Spectroscopy
3.4. Laser-Induced Phase Transition
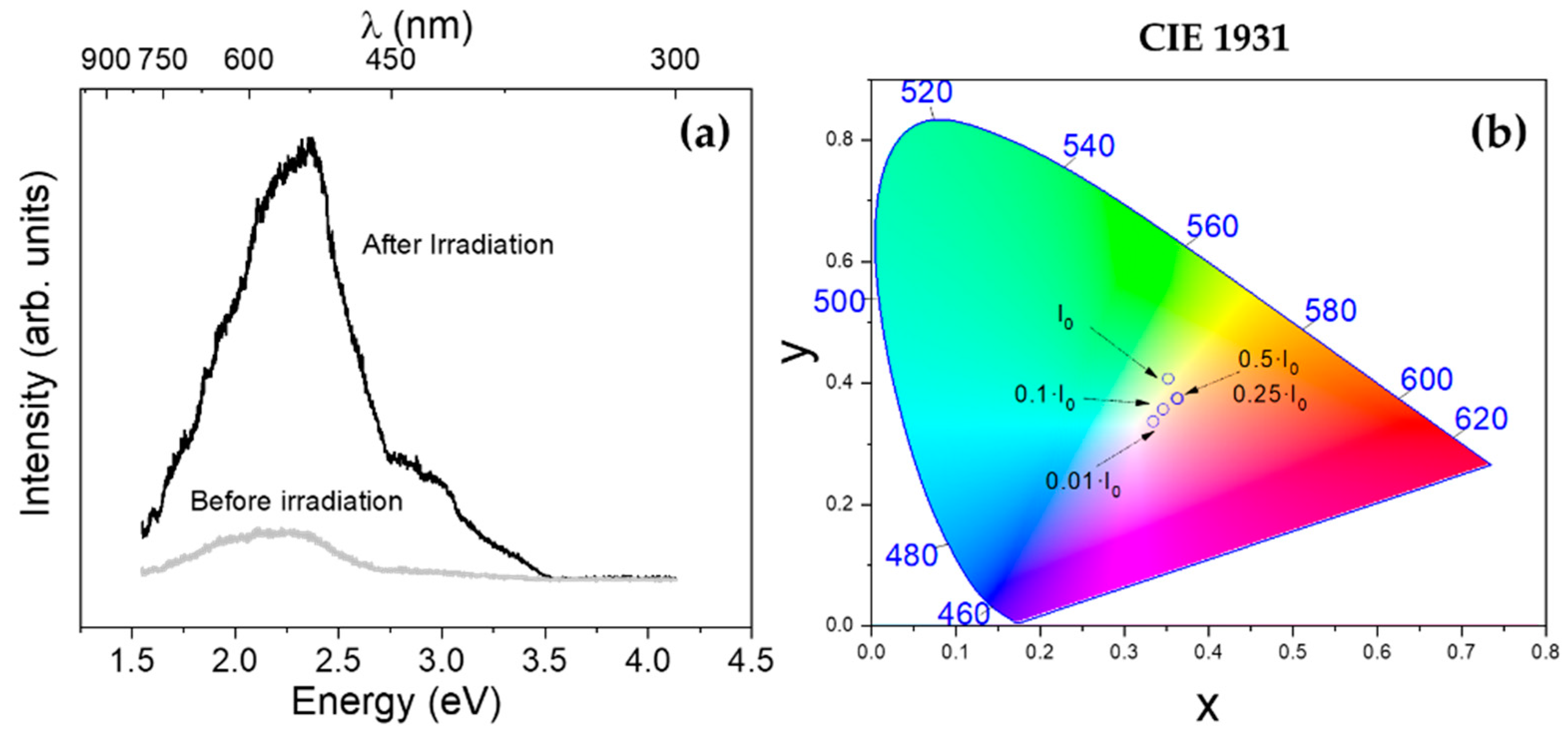
3.5. Photoluminescence
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Batzill, M.; Diebold, U. The surface and materials science of tin oxide. Prog. Surf. Sci. 2005, 79, 47–154. [Google Scholar] [CrossRef]
- Vázquez-López, A.; Maestre, D.; Ramírez-Castellanos, J.; González-Calbet, J.M.; Pis, I.; Nappini, S.; Yuca, N.; Cremades, A. Influence of Doping and Controlled Sn Charge State on the Properties and Performance of SnO2 Nanoparticles as Anodes in Li-Ion Batteries. J. Phys. Chem. C 2020, 124, 18490–18501. [Google Scholar] [CrossRef]
- Dias, J.S.; Batista, F.R.M.; Bacani, R.; Triboni, E.R. Structural characterization of SnO nanoparticles synthesized by the hydrothermal and microwave routes. Sci. Rep. 2020, 10, 9446. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, J.; Taeño, M.; Vázquez-lópez, A.; Prado, F.; García-Tecedor, M.; Cristian, G.; Ramírez-Castellanos, J.; Cremades, A. Oxide-Based Materials and Structures; Savkina, R., Khomenkova, L., Eds.; Taylor and Francis: Abingdon, UK, 2020. [Google Scholar]
- Chen, J.S.; Lou, X.W.D. SnO2 -Based Nanomaterials: Synthesis and Application in Lithium-Ion Batteries. Small 2013, 9, 1877–1893. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Jayaraman, V. SnO2: A comprehensive review on structures and gas sensors. Prog. Mater. Sci. 2014, 66, 112–255. [Google Scholar] [CrossRef]
- Prasad, P.D.; Reddy, S.P.; Deepthi, A. Synthesis, Characterization of Tin Oxide (SnO) Nanoparticles via Autoclave synthesis protocol for H2 sensing. Int. J. Nanotechnol. Appl. 2017, 11, 265–276. [Google Scholar]
- Vázquez-López, A.; Yaseen, A.; Maestre, D.; Ramírez-Castellanos, J.; Marstein, E.S.; Karazhanov, S.Z.; Cremades, A. Synergetic Improvement of Stability and Conductivity of Hybrid Composites formed by PEDOT: PSS and SnO Nanoparticles. Molecules 2020, 25, 695. [Google Scholar] [CrossRef]
- Liu, W.; Yin, L.; Zhang, R.; Yang, H.; Ma, J.; Cao, W. One-step synthesis of SnO hierarchical architectures under room temperature and their photocatalytic properties. Nanotechnology 2018, 29, 284002. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Q.; Zhang, J.; Yin, L.; Li, Y.; Yin, S.; Cao, W. Morphology modulation of SnO photocatalyst: From microplate to hierarchical architectures self-assembled with thickness controllable nanosheets. CrystEngComm 2018, 20, 4651–4665. [Google Scholar] [CrossRef]
- Solola, G.T.; Klopov, M.; Akinami, J.O.; Afolabi, T.A.; Karazhanov, S.Z.; Adebayo, G. First principle calculations of structural, electronic, optical and thermoelectric properties of tin (II) oxide. Mater. Res. Express 2019, 6, 125915. [Google Scholar] [CrossRef]
- Miller, S.A.; Gorai, P.; Aydemir, U.; Mason, T.O.; Stevanović, V.; Toberer, E.S.; Snyder, G.J. SnO as a potential oxide thermoelectric candidate. J. Mater. Chem. C 2017, 5, 8854–8861. [Google Scholar] [CrossRef]
- Huda, A.; Handoko, C.T.; Bustan, M.D.; Yudono, B.; Gulo, F. New route in the synthesis of Tin (II) oxide micro-sheets and its thermal transformation. Mater. Lett. 2018, 211, 293–295. [Google Scholar] [CrossRef]
- Pires, F.I.; Joanni, E.; Savu, R.; Zaghete, M.A.; Longo, E.; Varela, J.A. Microwave-assisted hydrothermal synthesis of nanocrystalline SnO powders. Mater. Lett. 2008, 62, 239–242. [Google Scholar] [CrossRef]
- Gervillié, C.; Boisard, A.; Labbé, J.; Berthon-Fabry, S.; Guérin, K. Influence upon cycling of oxygen amount in tin-based compound used as negative electrode in lithium-ion battery. Synth. Met. 2020, 267, 116477. [Google Scholar] [CrossRef]
- Jaśkaniec, S.; Kavanagh, S.R.; Coelho, J.; Ryan, S.; Hobbsb, C.; Walsh, A.; Scanlon, D.O.; Valeria, N. Solvent Engineered Synthesis of Layered SnO Nanoparticles for High-Performance Anodes. npj 2D Mater. Appl. 2020, 5, 27. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, J.; Zhang, D.; Schwingenschlögl, U.; Alshareef, H.N. Two-Dimensional SnO Anodes with a Tunable Number of Atomic Layers for Sodium Ion Batteries. Nano Lett. 2017, 17, 1302–1311. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeon, K.M.; Park, J.S.; Kang, Y.C. Excellent Li-ion storage performances of hierarchical SnO-SnO2 composite powders and SnO nanoplates prepared by one-pot spray pyrolysis. J. Power Sources 2017, 359, 363–370. [Google Scholar] [CrossRef]
- Li, L.; Zhang, C.; Chen, W. Fabrication of SnO2-SnO nanocomposites with p–n heterojunctions for the low-temperature sensing of NO2 gas. Nanoscale 2015, 7, 12133–12142. [Google Scholar] [CrossRef]
- Shanmugasundaram, A.; Basak, P.; Satyanarayana, L.; Manorama, S.V. Hierarchical SnO/SnO2 nanocomposites: Formation of in situ p–n junctions and enhanced H2 sensing. Sens. Actuators B Chem. 2013, 185, 265–273. [Google Scholar] [CrossRef]
- Yin, G.; Sun, J.; Zhang, F.; Yu, W.; Peng, F.; Sun, Y.; Chen, X.; Xu, L.; Lu, J.; Luo, C.; et al. Enhanced gas selectivity induced by surface active oxygen in SnO/SnO2 heterojunction structures at different temperatures. RSC Adv. 2019, 9, 1903–1908. [Google Scholar] [CrossRef]
- Li, N.; Fan, Y.; Shi, Y.; Xiang, Q.; Wang, X.; Xu, J. A low temperature formaldehyde gas sensor based on hierarchical SnO/SnO2 nano-flowers assembled from ultrathin nanosheets: Synthesis, sensing performance and mechanism. Sens. Actuators B Chem. 2019, 294, 106–115. [Google Scholar] [CrossRef]
- Palneedi, H.; Park, J.H.; Maurya, D.; Peddigari, M.; Hwang, G.-T.; Annapureddy, V.; Kim, J.-W.; Choi, J.-J.; Hahn, B.-D.; Priya, S.; et al. Laser Processing of Metal Oxides: Laser Irradiation of Metal Oxide Films and Nanostructures: Applications and Advances. Adv. Mater. 2018, 30, 1870094. [Google Scholar] [CrossRef]
- Vila, M.; Díaz-Guerra, C.; Piqueras, J. Laser irradiation-induced α to δ phase transformation in Bi2O3 ceramics and nanowires. Appl. Phys. Lett. 2012, 101, 71905. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Wang, F.; Zhao, H.; Rafique, M.Y.; Wang, J.; Li, Q. Structural and electrochemical properties of SnO nanoflowers as an anode material for lithium ion batteries. Scr. Mater. 2012, 67, 665–668. [Google Scholar] [CrossRef]
- Sun, Y.H.; Dong, P.P.; Lang, X.; Nan, J.M. A novel rose flower-like SnO hierarchical structure synthesized by a hydrothermal method in an ethanol/water system. Chin. Chem. Lett. 2014, 25, 915–918. [Google Scholar] [CrossRef]
- Pan, X.Q.; Fu, L. Oxidation and phase transitions of epitaxial tin oxide thin films on (1012) sapphire. J. Appl. Phys. 2001, 89, 6048–6055. [Google Scholar] [CrossRef]
- Campo, C.M.; Rodríguez, J.E.; Ramírez, A.E. Thermal behaviour of romarchite phase SnO in different atmospheres: A hypothesis about the phase transformation. Heliyon 2016, 2, e00112. [Google Scholar] [CrossRef]
- Leitner, J.; Sedmidubský, D. Thermodynamic Modeling of Oxidation of Tin Nanoparticles. J. Phase Equilibria Diffus. 2019, 40, 10–20. [Google Scholar] [CrossRef]
- Cahen, S.; David, N.; Fiorani, J.M.; Maître, A.; Vilasi, M. Thermodynamic modelling of the O-Sn system. Thermochim. Acta 2003, 403, 275–285. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, X.; Zhou, J.; Sham, T.K.; Ding, Z. Observation of single tin dioxide nanoribbons by confocal raman microspectroscopy. J. Phys. Chem. C 2007, 111, 18839–18843. [Google Scholar] [CrossRef]
- Diéguez, A.; Romano-Rodríguez, A.; Vilà, A.; Morante, J.R. The complete Raman spectrum of nanometric SnO2 particles. J. Appl. Phys. 2001, 90, 1550–1557. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, F.X.; Loa, I.; Syassen, K.; Hanfland, M.; Mathis, Y.-L. Structural properties, infrared reflectivity, and Raman modes of SnO at high pressure. Phys. Status Solidi 2004, 241, 3168–3178. [Google Scholar] [CrossRef]
- Chen, X.; Grandbois, M. In situ Raman spectroscopic observation of sequential hydrolysis of stannous chloride to abhurite, hydroromarchite, and romarchite. J. Raman Spectrosc. 2013, 44, 501–506. [Google Scholar] [CrossRef]
- Guillén, C.; Herrero, J. P-type SnO thin films prepared by reactive sputtering at high deposition rates. J. Mater. Sci. Technol. 2019, 35, 1706–1711. [Google Scholar] [CrossRef]
- Eifert, B.; Becker, M.; Reindl, C.T.; Giar, M.; Zheng, L.; Polity, A.; He, Y.; Heiliger, C.; Klar, P.J. Raman studies of the intermediate tin-oxide phase. Phys. Rev. Mater. 2017, 1, 14602. [Google Scholar] [CrossRef]
- Shebanova, O.N.; Lazor, P. Raman study of magnetite (Fe3O4): Laser-induced thermal effects and oxidation. J. Raman Spectrosc. 2003, 34, 845–852. [Google Scholar] [CrossRef]
- Maestre, D.; Cremades, A.; Piqueras, J. Cathodoluminescence of defects in sintered tin oxide. J. Appl. Phys. 2004, 95, 3027–3030. [Google Scholar] [CrossRef]
- Zhao, Q.; Tong, Y.; Liu, Y.; Mingzhe, Z. Effect of Gd3+ doping on structural, optical and magnetic properties of SnO crystals. Ceram. Int. 2019, 45, 17529–17535. [Google Scholar] [CrossRef]
- Del Prado, F.; Taeño, M.; Maestre, D.; Ramírez-Castellanos, J.; González-Calbet, J.M.; Cremades, A. Effect of the synthesis method on the properties of lithium doped graphene oxide composites with tin oxide nanoparticles: Towards white luminescence. J. Phys. Chem. Solids 2019, 129, 133–139. [Google Scholar] [CrossRef]
- Schanda, J. Colorimetry: Understanding the CIE System; John Wiley and Sons: Hoboken, NJ, USA, 2007; ISBN 978-0-470-04904-4. [Google Scholar]
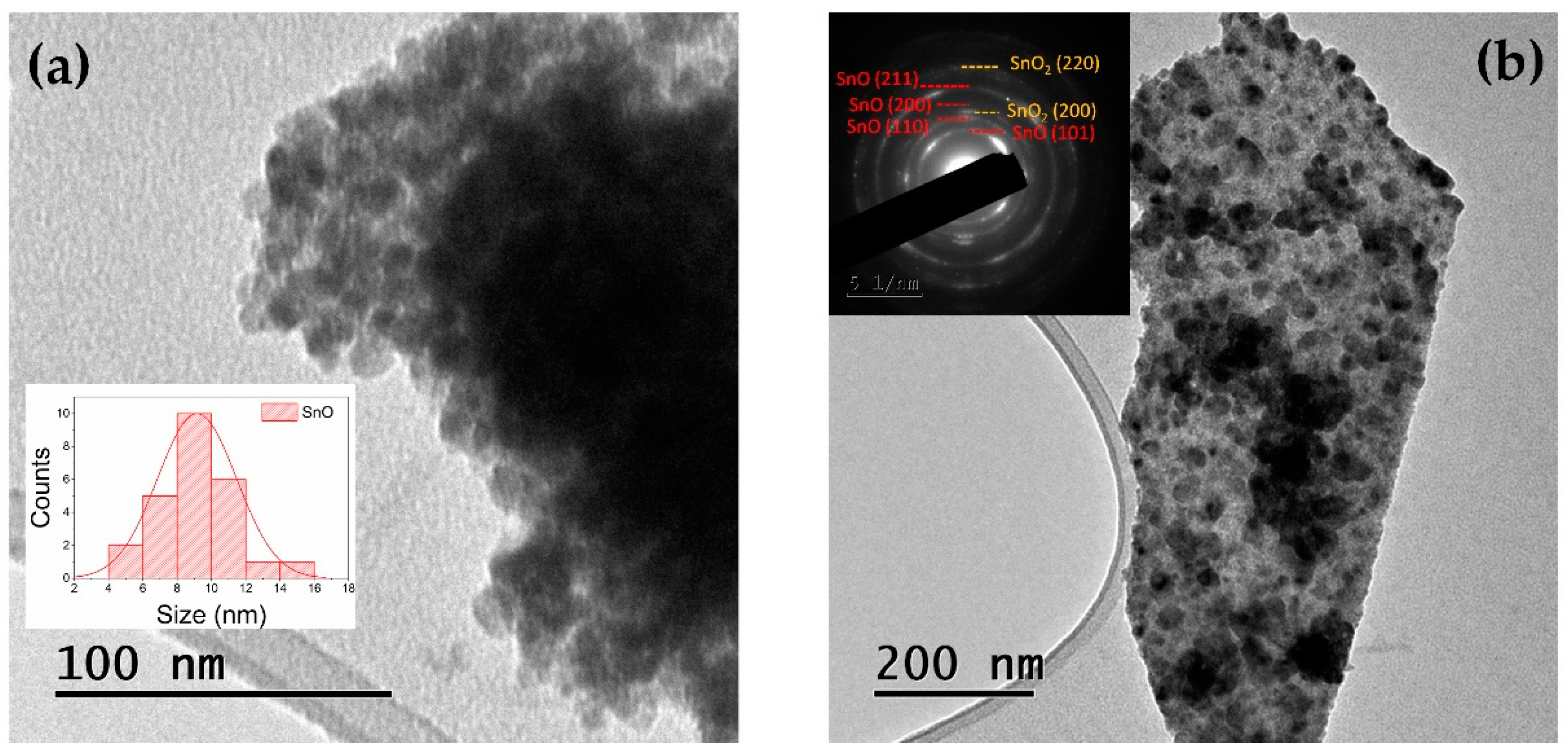
| D(nm) (By TEM) | a(Å) (By XRD) | c(Å) (By XRD) |
|---|---|---|
| 9.14 ± 2.58 | 3.80(1) | 4.83(7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-López, A.; Maestre, D.; Ramírez-Castellanos, J.; Cremades, A. In Situ Local Oxidation of SnO Induced by Laser Irradiation: A Stability Study. Nanomaterials 2021, 11, 976. https://doi.org/10.3390/nano11040976
Vázquez-López A, Maestre D, Ramírez-Castellanos J, Cremades A. In Situ Local Oxidation of SnO Induced by Laser Irradiation: A Stability Study. Nanomaterials. 2021; 11(4):976. https://doi.org/10.3390/nano11040976
Chicago/Turabian StyleVázquez-López, Antonio, David Maestre, Julio Ramírez-Castellanos, and Ana Cremades. 2021. "In Situ Local Oxidation of SnO Induced by Laser Irradiation: A Stability Study" Nanomaterials 11, no. 4: 976. https://doi.org/10.3390/nano11040976
APA StyleVázquez-López, A., Maestre, D., Ramírez-Castellanos, J., & Cremades, A. (2021). In Situ Local Oxidation of SnO Induced by Laser Irradiation: A Stability Study. Nanomaterials, 11(4), 976. https://doi.org/10.3390/nano11040976