Recent Advances in Nanoporous Anodic Alumina: Principles, Engineering, and Applications
Abstract
1. Introduction
2. Nanoporous Anodic Alumina (NAA): Definition and Formation Mechanism of the Porous Oxide
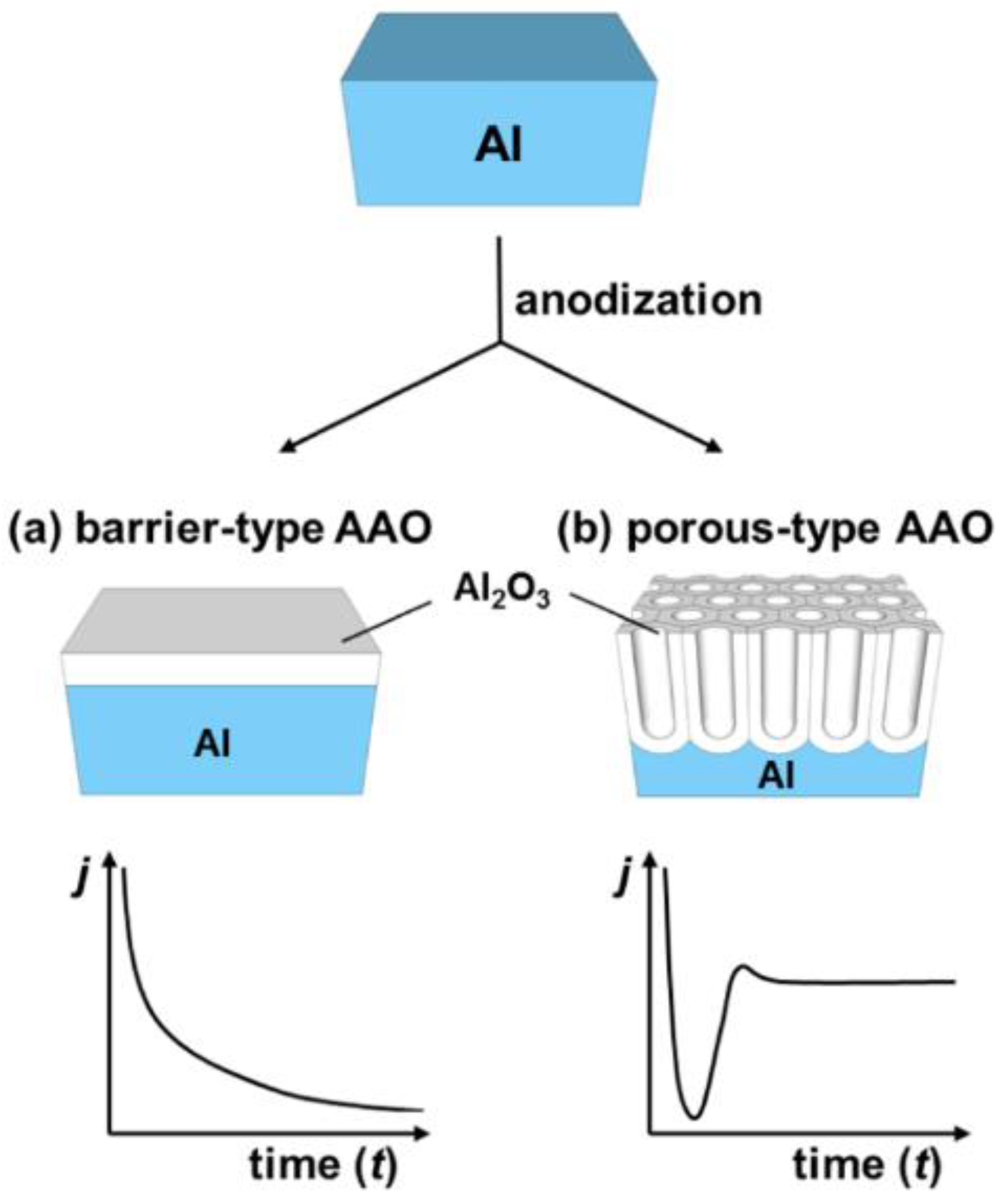
2.1. An Electrolytic Passivation of Aluminum
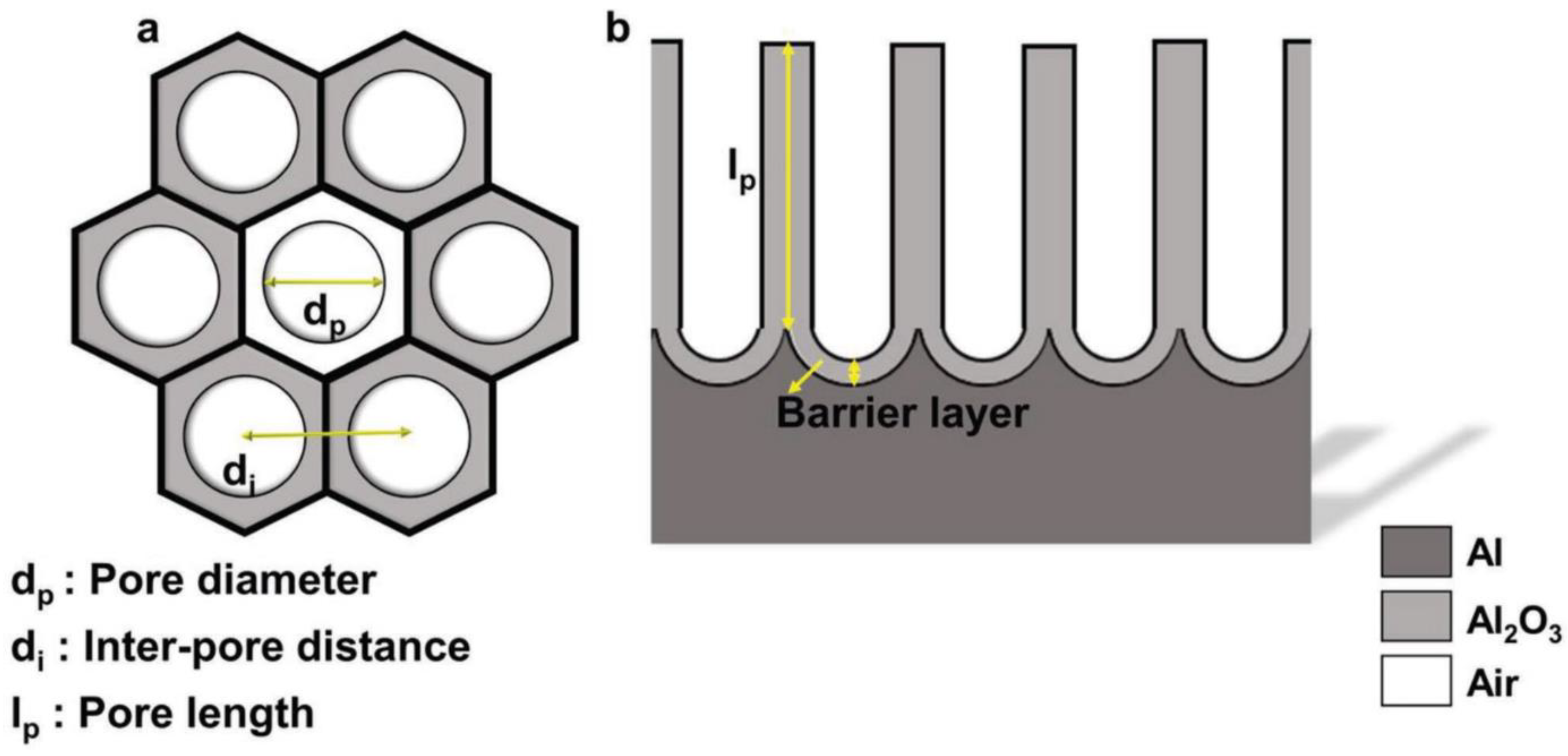
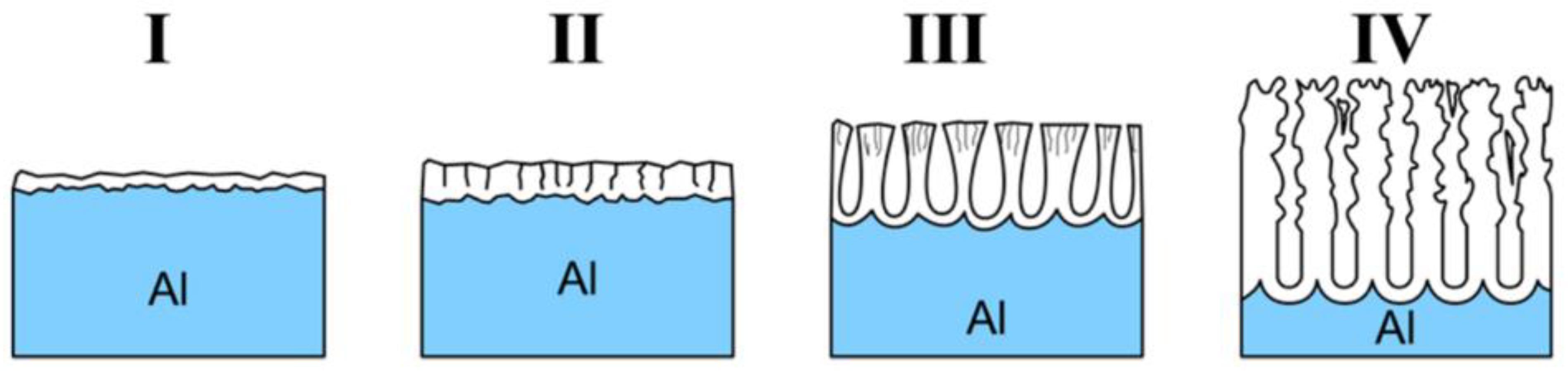
2.2. Pore Growth Mechanism and Spatial Ordering
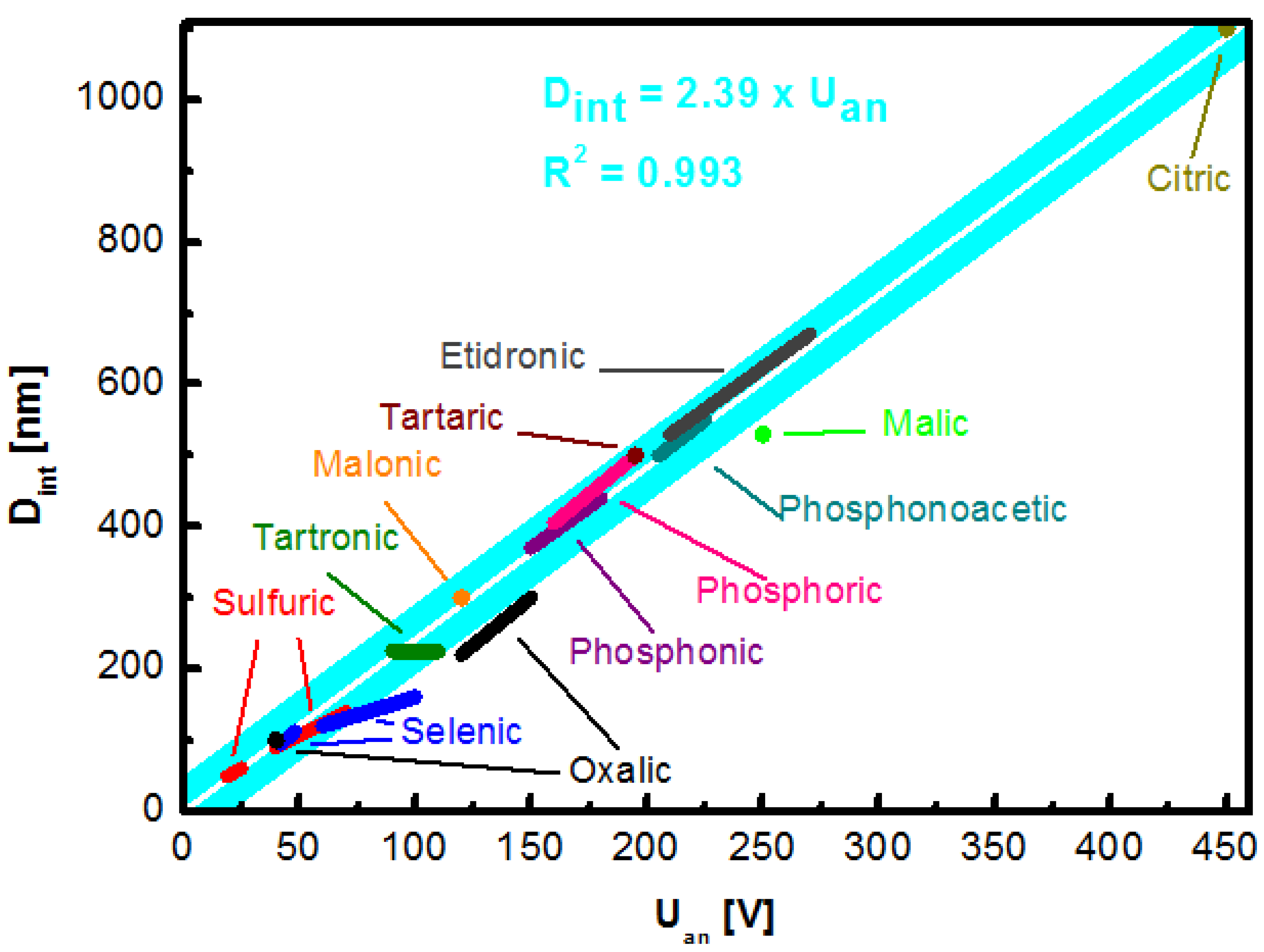
2.3. Electrolyte Specific NAA Geometry
| Interpore Distance [nm] | Electrolyte | Concentration | Applied Potential [V] | Temperature [°C] | Reference |
|---|---|---|---|---|---|
| 50–60 | Sulfuric acid | 0.3 M | 19–25 | 5 | [52] |
| 90–140 | Sulfuric acid | 10 wt % | 40–70 | 0.1 | [84] |
| 95–112 | Selenic acid | 0.3 M | 42–48 | 20 | [23,85] |
| 100 | Oxalic acid | 0.3 M | 40 | 5 | [52] |
| 120–160 | Selenic acid | 0.3 M | 60–100 | 0 | [82] |
| 225 | Tartronic acid | 0.3 M | 90–110 | 0.5 | [86] |
| 220–300 | Oxalic acid | 0.3 M | 120–150 | 1–2 | [24] |
| 300 | Malonic acid | 5.0 M | 120 | 0–1 | [87,88] |
| 370–440 | Phosphonic acid | 0.5–2.0 M | 150–180 | 0–20 | [89] |
| 405–500 | Phosphoric acid | 0.3 M | 160–195 | 5 | [52] |
| 500 | Tartaric acid | 2–4 wt % | 195 | 5 | [90] |
| 530 | Malic acid | 0.5 M | 230 | 5 | [81] |
| 500–550 | Phosphonoacetic acid | 0.1–0.9 M | 205–225 | 10 | [72] |
| 530–670 | Etidronic acid | 0.3 M | 210–270 | 0–40 | [66,91] |
| 1100 | Citric acid | 0.1–1 M | 260–450 | 10–30 | [92] |
Impact of Temperature and Additives
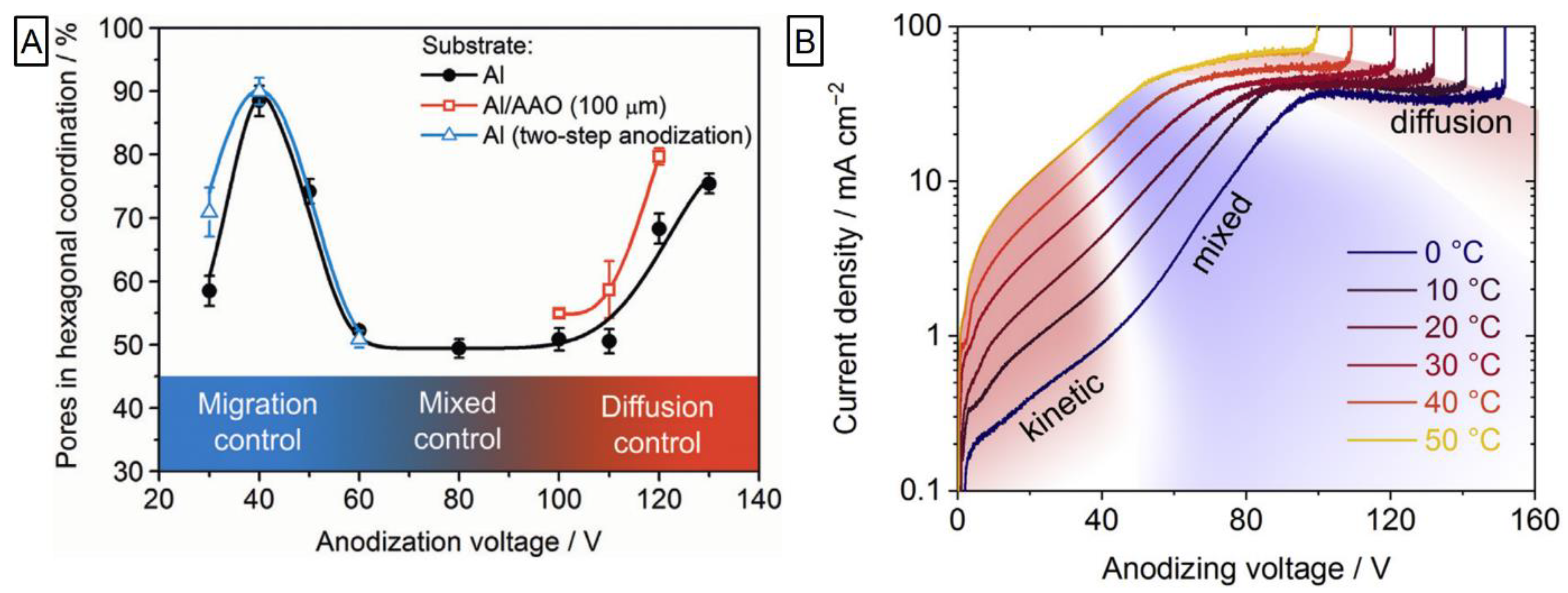
2.4. Mild and Hard Anodization: Two Growth Regimes
2.5. Pore Separation Phenomenon

2.6. Pre- and Post-Anodization Treatments
2.6.1. Pre-Anodization Patterning of the Aluminum Surface
2.6.2. Thermal Annealing
3. Engineered NAA Structures
3.1. Structures Based on the Modulation of the Anodization Current
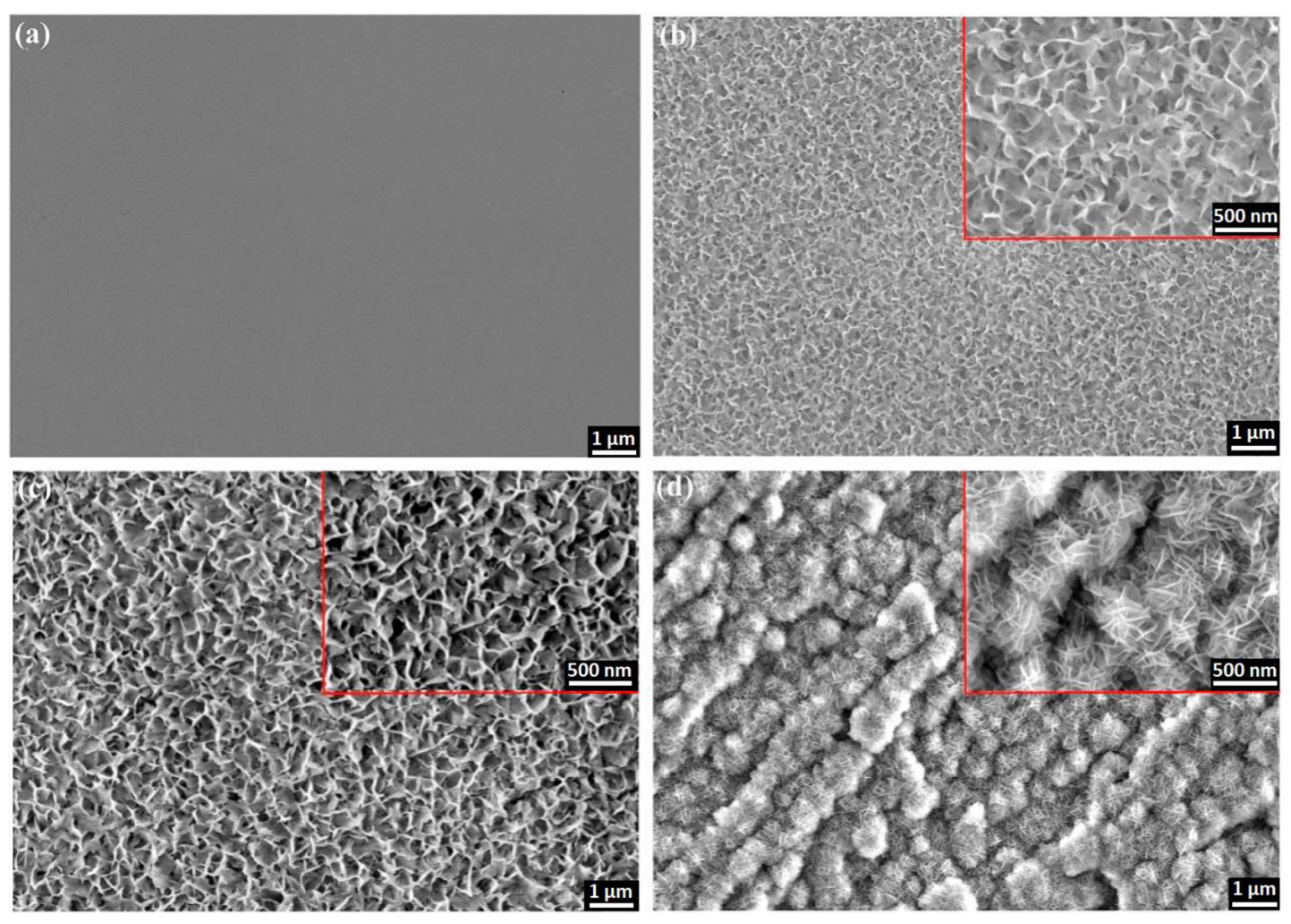
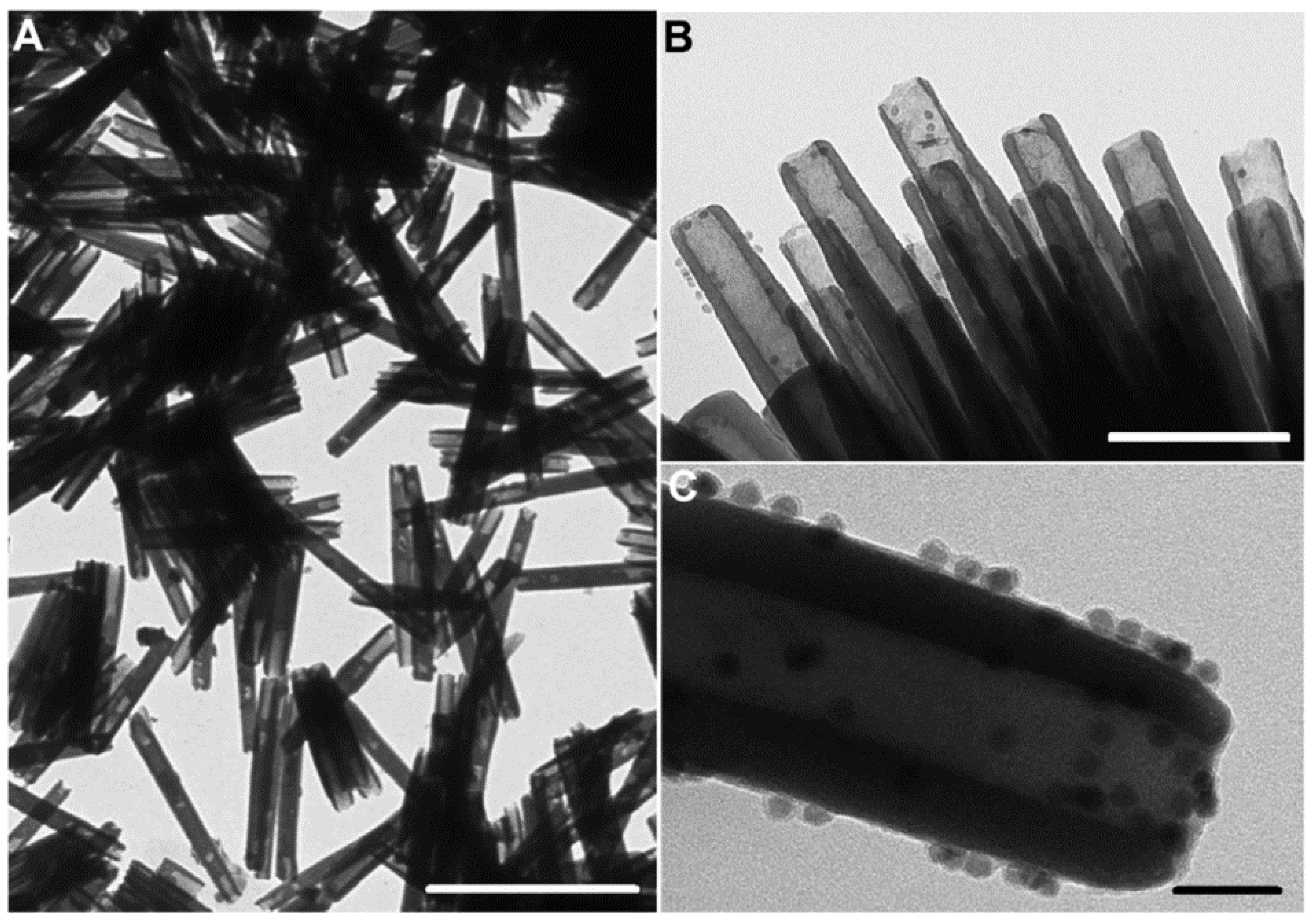
3.2. Nanotubes
3.3. Micro- and Nanoparticles
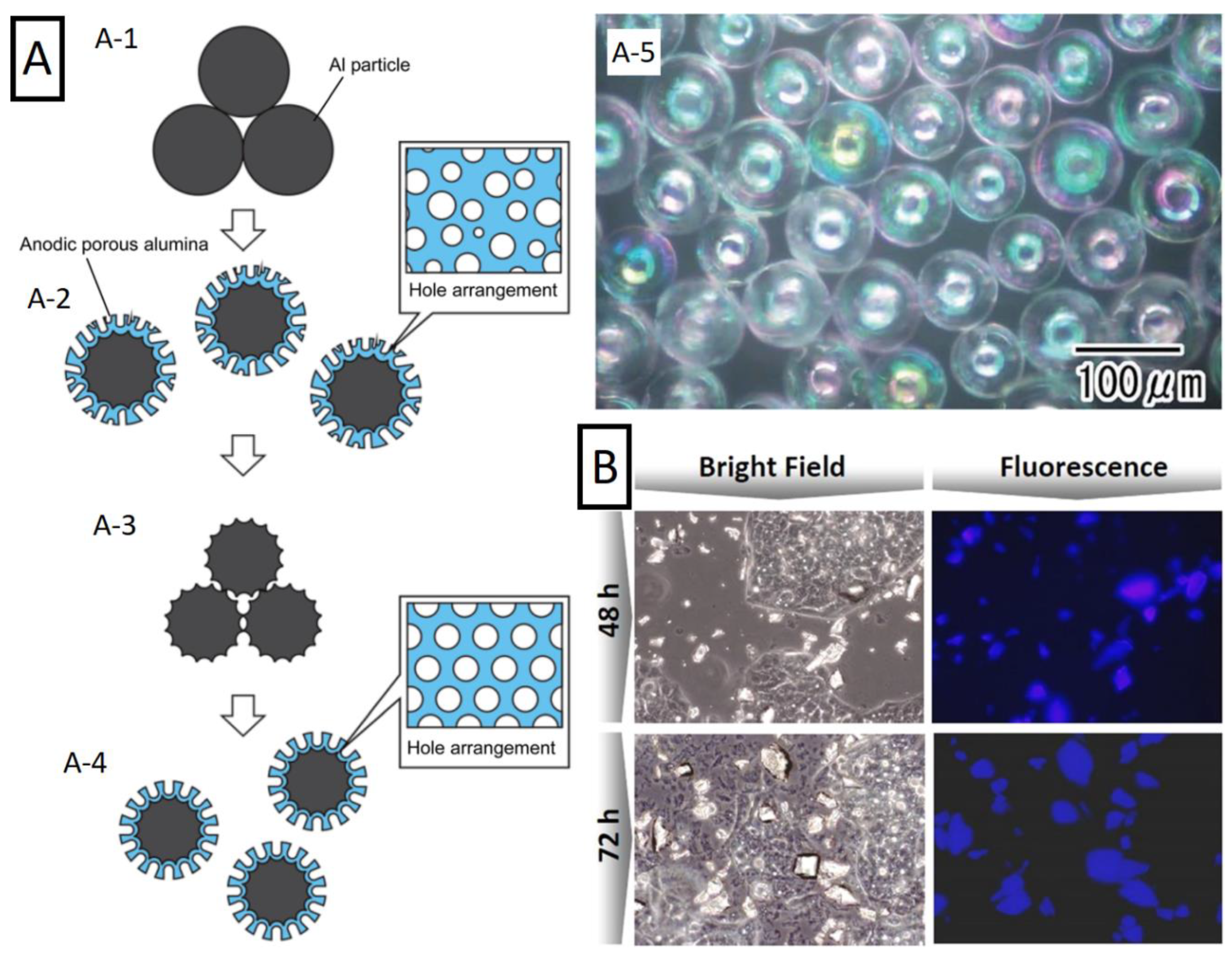
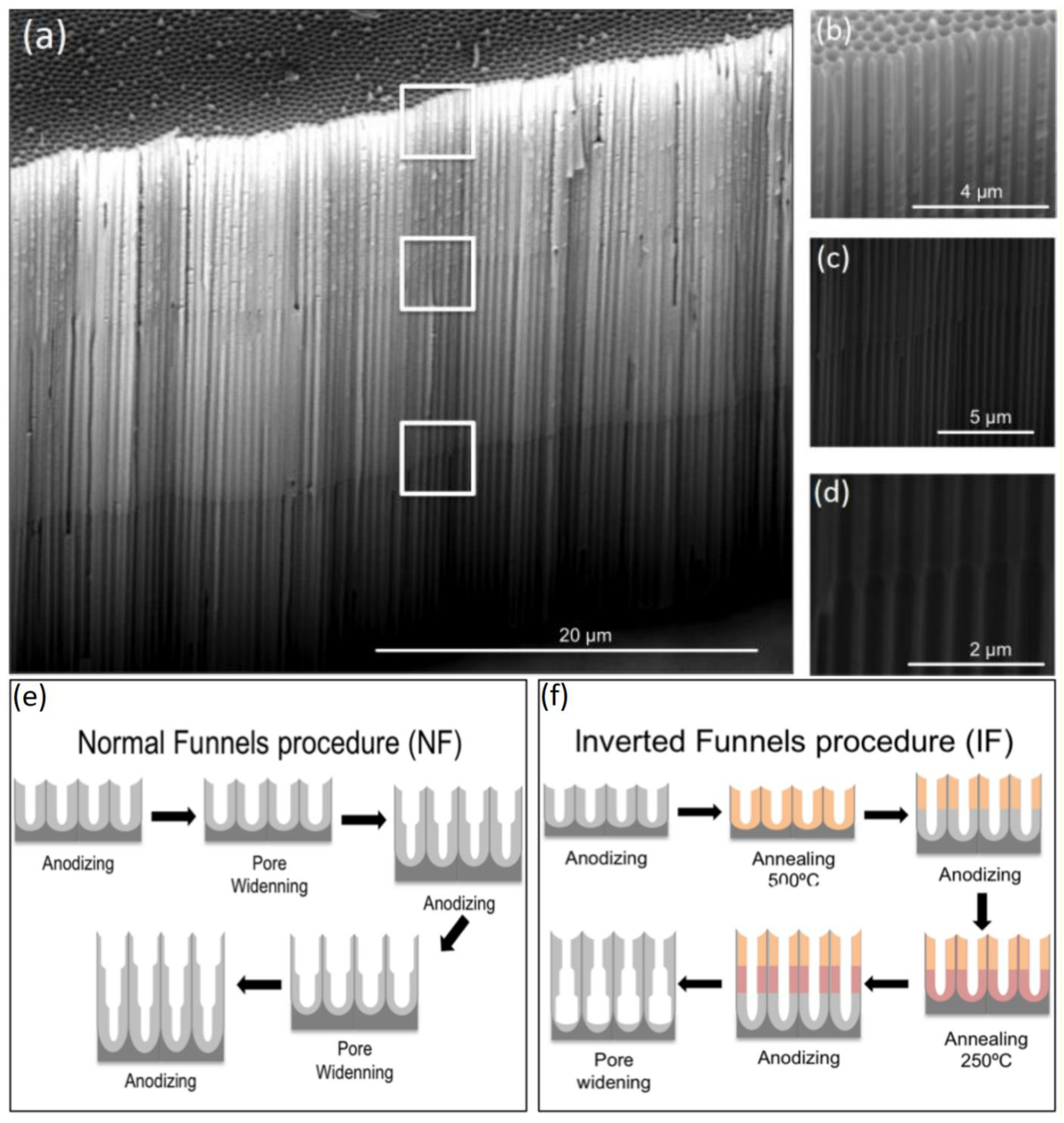
3.4. Funnels and Inverse Funnels
3.5. Hierarchical Pore Structures

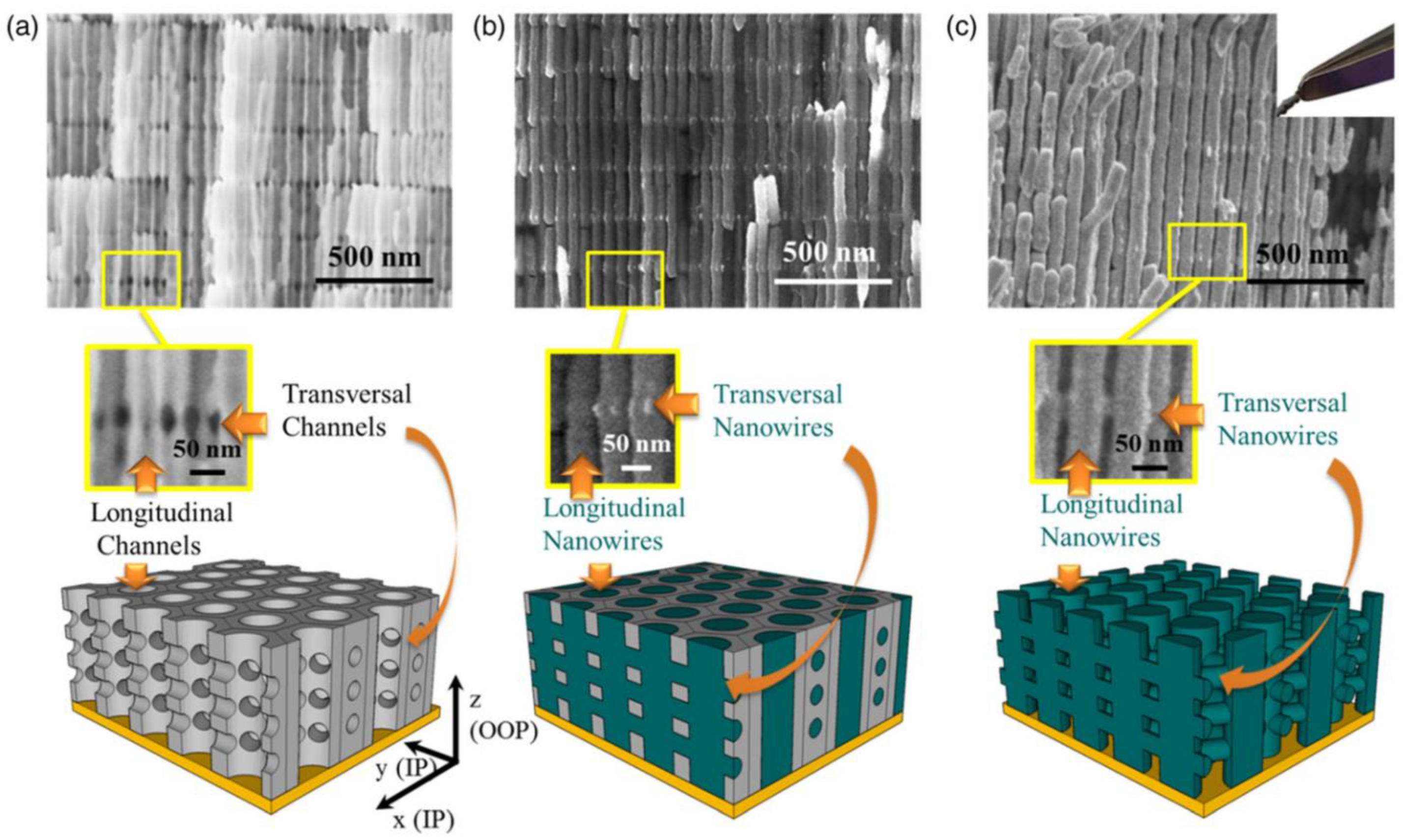
3.6. Three-Dimensional Interconnected Nanoarchitectures
4. Examples of NAA Applications
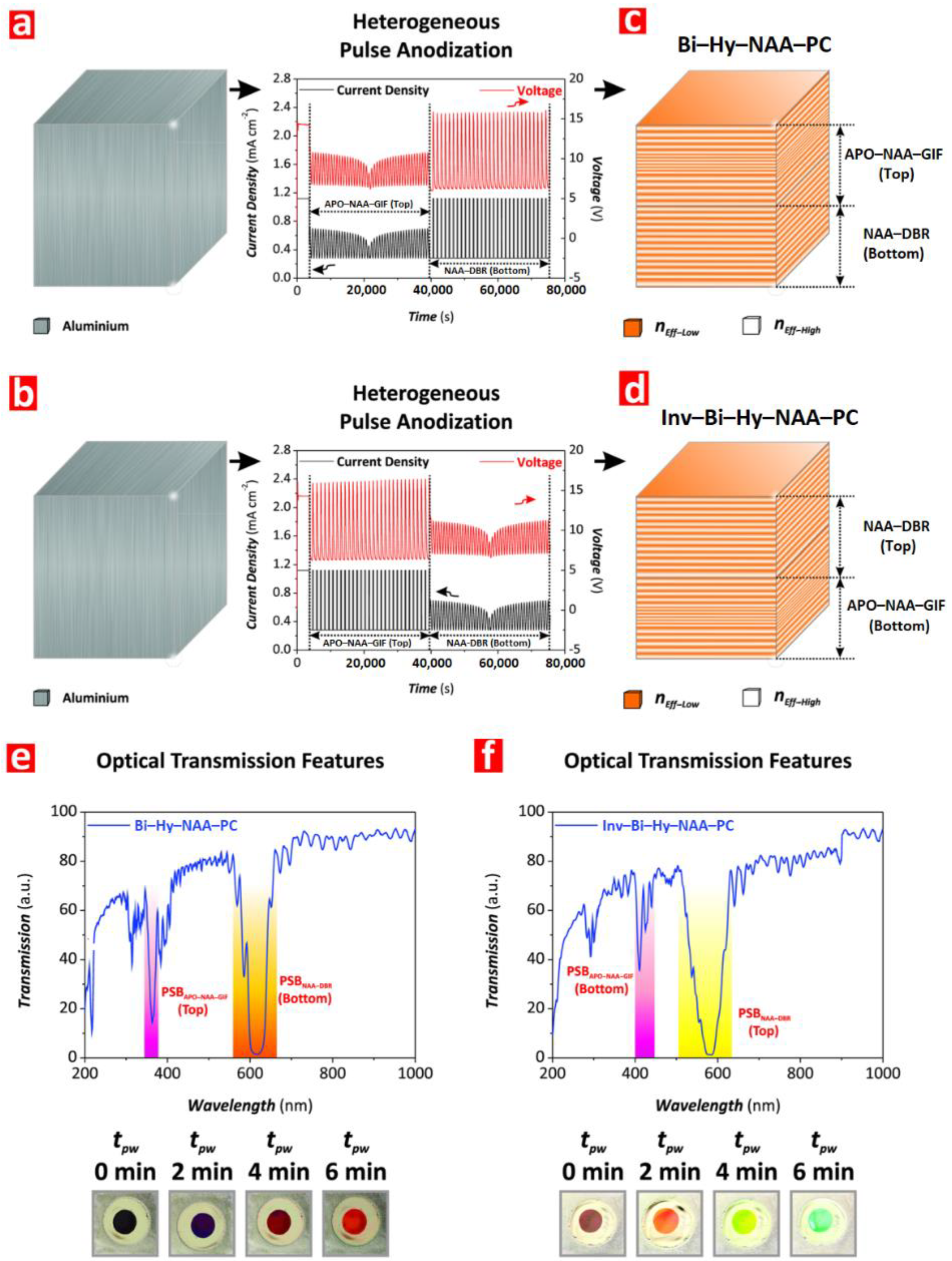
4.1. Photonic Structures
4.2. Sensors
4.3. Templates
4.4. Membranes for Filtering and Separation
4.5. Biological Monitoring and Cell Culture
4.6. Drug Delivery
4.7. Functional Layer for Composites
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Inshakova, E.; Inshakov, O. World market for nanomaterials: Structure and trends. MATEC Web Conf. 2017, 129, 1–5. [Google Scholar] [CrossRef]
- Taylor, A.A.; Freeman, E.L.; van der Ploeg, M.J.C. Regulatory developments and their impacts to the nano-industry: A case study for nano-additives in 3D printing. Ecotoxicol. Environ. Saf. 2020, 207, 111458. [Google Scholar] [CrossRef] [PubMed]
- Rauscher, H.; Rasmussen, K.; Sokull-Klüttgen, B. Regulatory Aspects of Nanomaterials in the EU. Chemie Ingenieur Technik 2017, 89, 224–231. [Google Scholar] [CrossRef]
- Garside, M. Global nanotechnology market value 2010–2020. 5 June 2020. Available online: https://www.statista.com/statistics/1073886/global-market-value-nanotechnology/ (accessed on 22 November 2020).
- Van de Velde, E.; Debergh, P.; Rammer, C.; Schliessler, P.; Gehrke, B.; Wassmann, P.; de Heide, M.; Butter, M.; Wydra, S.; Som, O.; et al. Key Enabling Technologies (KETs) Observatory. Methodology Report. 2015. Available online: https://ec.europa.eu/growth/tools-databases/kets-tools/sites/default/files/documents/data_use_methodology_phase_i_final_report_kets_observatory_en.pdf (accessed on 22 November 2020).
- Michaelis, A. Electrochemical Surface Modification: Thin Films, Functionalization and Characterization; Alkire, R.C., Kolb, D.M., Lipkowski, J., Ross, P.N., Eds.; Willey-VCH: New York, NY, USA, 2008; Chapter 1; pp. 1–106. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Chernozem, R.V.; Pariy, I.O.; Surmeneva, M.A. A review on piezo- and pyroelectric responses of flexible nano- and micropatterned polymer surfaces for biomedical sensing and energy harvesting applications. Nano Energy 2021, 79, 105442. [Google Scholar] [CrossRef]
- Shopska, M.; Paneva, D.; Kolev, H.; Kadinov, G.; Briancin, J.; Fabian, M.; Cherkezova-Zheleva, Z.; Mitov, I. Characterization and catalytic activity in CO oxidation of biogenic lepidocrocite layered on anodic alumina. Catal. Today 2020, 357, 436–441. [Google Scholar] [CrossRef]
- Kasani, S.; Curtin, K.; Wu, N. A review of 2D and 3D plasmonic nanostructure array patterns: Fabrication, light management and sensing applications. Nanophotonics 2019, 8, 2065–2089. [Google Scholar] [CrossRef]
- Wei, Q.; Fu, Y.; Zhang, G.; Yang, D.; Meng, G.; Sun, S. Rational design of novel nanostructured arrays based on porous AAO templates for electrochemical energy storage and conversion. Nano Energy 2019, 55, 234–259. [Google Scholar] [CrossRef]
- Xu, Q.; Meng, G.; Han, F. Porous AAO template-assisted rational synthesis of large-scale 1D hybrid and hierarchically branched nanoarchitectures. Prog. Mater. Sci. 2018, 95, 243–285. [Google Scholar] [CrossRef]
- Rajeev, G.; Simon, B.P.; Marsal, L.F.; Voelcker, N.H. Advances in Nanoporous Anodic Alumina-Based Biosensors to Detect Biomarkers of Clinical Significance: A Review. Adv. Healthc. Mater. 2018, 7, 1–18. [Google Scholar] [CrossRef]
- Mijangos, C.; Hernández, R.; Martín, J. A review on the progress of polymer nanostructures with modulated morphologies and properties, using nanoporous AAO templates. Prog. Polym. Sci. 2016, 54–55, 148–182. [Google Scholar] [CrossRef]
- Zaraska, L.; Gawlak, K.; Gurgul, M.; Chlebda, D.K.; Socha, R.P.; Sulka, G.D. Controlled synthesis of nanoporous tin oxide layers with various pore diameters and their photoelectrochemical properties. Electrochim. Acta 2017, 254, 238–245. [Google Scholar] [CrossRef]
- Fu, Y.; Mo, A. A Review on the Electrochemically Self-organized Titania Nanotube Arrays: Synthesis, Modifications, and Biomedical Applications. Nanoscale Res. Lett. 2018, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Herino, R.; Bomchil, G.; Barla, K.; Bertrand, C. Porosity and Pore Size Distributions of Porous Silicon Layers. J. Electrochem. Soc. 1987, 134, 1994. [Google Scholar] [CrossRef]
- Masuda, H.; Fukuda, K. Ordered Metal Nanohole Arrays Made by a Two-Step Replication of Honeycomb Structures of Anodic Alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- Valiev, R.Z.; Nazarov, A.A. Bulk Nanostructured Materials; Zehetbauer, M.J., Zhu, Y.T., Eds.; Willey-VCH: New York, NY, USA, 2009; Chapter 2; pp. 21–48. [Google Scholar] [CrossRef]
- Lee, W. The anodization of aluminum for nanotechnology applications. JOM 2010, 62, 57–63. [Google Scholar] [CrossRef]
- Gu, P.; Miao, H.; Liu, Z.T.; Wu, X.P.; Zhao, J.H. Investigation of elastic modulus of nanoporous alumina membrane. J. Mater. Sci. 2004, 39, 3369–3373. [Google Scholar] [CrossRef]
- Vojkuvka, L.; Santos, A.; Pallarès, J.; Ferré-Borrull, J.; Marsal, L.F.; Celis, J.P. On the mechanical properties of nanoporous anodized alumina by nanoindentation and sliding tests. Surf. Coat. Technol. 2012, 206, 2115–2124. [Google Scholar] [CrossRef]
- Dai, J.; Singh, J.; Yamamoto, N. Nonbrittle nanopore deformation of anodic aluminum oxide membranes. J. Am. Ceram. Soc. 2018, 101, 2170–2180. [Google Scholar] [CrossRef]
- Nishinaga, O.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Rapid fabrication of self-ordered porous alumina with 10-/sub-10-nm-scale nanostructures by selenic acid anodizing. Sci. Rep. 2013, 3, 1–6. [Google Scholar] [CrossRef]
- Lee, W.; Ji, R.; Gösele, U.; Nielsch, K. Fast fabrication of long-range ordered porous alumina membranes by hard anodization. Nat. Mater. 2006, 5, 741–747. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, W.; Zhang, S.; Zhao, S.; Ai, F.; Zhu, X. Morphology evolution of porous anodic alumina in mixed H3PO4/NH4F electrolytes. Surf. Coat. Technol. 2018, 334, 500–508. [Google Scholar] [CrossRef]
- Ribes, A.; Xifre-Perez, E.; Aznar, E.; Sancenon, F.; Pardo, T.; Marsal, L.F.; Martinez-Manez, R. Molecular gated nanoporous anodic alumina for the detection of cocaine. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.P.; Wood, G.C. Morphology and mechanism of formation of porous anodic films on aluminum. Proc. Roy. Soc. Ser. A Math. Phys. Sci. 1970, 317, 1731. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, Q.; Liu, X.; Gao, Y.; He, J.; Wang, W.; Fan, Z. A Highly Controllable Electrochemical Anodization Process to Fabricate Porous Anodic Aluminum Oxide Membranes. Nanoscale Res. Lett. 2015, 10, 1–8. [Google Scholar] [CrossRef]
- Santos, A.; Ferré-Borrull, J.; Pallarès, J.; Marsal, L.F. Hierarchical nanoporous anodic alumina templates by asymmetric two-step anodization. Phys. Status Solidi Appl. Mater. Sci. 2011, 208, 668–674. [Google Scholar] [CrossRef]
- Vojkuvka, L.; Marsal, L.F.; Ferré-Borrull, J.; Formentin, P.; Pallarés, J. Self-ordered porous alumina membranes with large lattice constant fabricated by hard anodization. Superlattices Microstruct. 2008, 44, 577–582. [Google Scholar] [CrossRef]
- Poznyak, A.; Pligovka, A.; Turavets, U.; Norek, M. On-Aluminum and Barrier Anodic Oxide: Meeting Various Acids and Solutions. Coatings 2020, 10, 875. [Google Scholar] [CrossRef]
- Han, H.; Park, S.J.; Jang, J.S.; Ryu, H.; Kim, K.J.; Baik, S.; Lee, W. In situ determination of the pore opening point during wet-chemical etching of the barrier layer of porous anodic aluminum oxide: Nonuniform Impurity Distribution in Anodic Oxide. ACS Appl. Mater. Interfaces 2013, 5, 3441–3448. [Google Scholar] [CrossRef]
- Garcia-Vergara, S.J.; Habazaki, H.; Skeldon, P.; Thompson, G.E. Tracer studies relating to alloying element behaviour in porous anodic alumina formed in phosphoric acid. Electrochim. Acta 2010, 55, 3175–3184. [Google Scholar] [CrossRef]
- Santos, A. Nanoporous anodic alumina photonic crystals: Fundamentals, developments and perspectives. J. Mater. Chem. C 2017, 5, 5581–5599. [Google Scholar] [CrossRef]
- Vera-Londono, L.; Ruiz-Clavijo, A.; Caballero-Calero, O.; Martín-González, M. Understanding the thermal conductivity variations in nanoporous anodic aluminum oxide. Nanoscale Adv. 2020, 2, 4591–4603. [Google Scholar] [CrossRef]
- Ferre-Borrull, J.; Xifre-Perez, E.; Pallares, J.; Marsal, L.F. Nanoporous Alumina; Losic, D., Santos, A., Eds.; Springer: Cham, Switzerland, 2015; Volume 219, pp. 185–217. [Google Scholar] [CrossRef]
- Santos, A.; Balderrama, V.S.; Alba, M.; Formentin, P.; Ferre-Borrull, J.; Pallares, J.; Marsal, L.F. Tunable fabry-pérot interferometer based on nanoporous anodic alumina for optical biosensing purposes. Nanoscale Res. Lett. 2012, 7, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Kure-Chu, S.-Z.; Osaka, K.; Yashiro, H.; Wada, K.; Segawa, H.; Inoue, S. Facile Fabrication of Ordered Multi-Tiered Hierarchical Porous Alumina Nanostructures with Multiple and Fractional Ratios of Pore Interval toward Multifunctional Nanomaterials. ECS J. Solid State Sci. Technol. 2016, 5, 285–292. [Google Scholar] [CrossRef]
- Li, Y.B.; Zheng, M.J.; Ma, L. High-speed growth and photoluminescence of porous anodic alumina films with controllable interpore distances over a large range. Appl. Phys. Lett. 2007, 91, 8–11. [Google Scholar] [CrossRef]
- Cantelli, L.; Santos, J.S.; Silva, T.F.; Tabacniks, M.H.; Delgado-Silva, A.O.; Trivinho-Strixino, F. Unveiling the origin of photoluminescence in nanoporous anodic alumina (NAA) obtained by constant current regime. J. Lumin. 2019, 207, 63–69. [Google Scholar] [CrossRef]
- Mir, M.A.; Shah, M.A.; Ganai, P.A. Dielectric study of nanoporous alumina fabricated by two-step anodization technique. Chem. Pap. 2020, 0123456789. [Google Scholar] [CrossRef]
- Baranowska, M.; Slota, A.J.; Eravuchira, P.J.; Macias, G.; Xifre-Perez, E.; Pallares, J.; Ferre-Borrull, J.; Marsal, L.F. Protein attachment to nanoporous anodic alumina for biotechnological applications: Influence of pore size, protein size and functionalization path. Coll. Surf. B Biointerfaces 2014, 122, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Porta-i-batalla, M.; Eckstein, C.; Xifré-pérez, E.; Formentín, P.; Marsal, L.F. Sustained, Controlled and Stimuli- Responsive Drug Release Systems Based on Nanoporous Anodic Alumina with Layer-by-Layer Polyelectrolyte. Nanoscale Res. Lett. 2016, 11, 372. [Google Scholar] [CrossRef]
- Eckstein, C.; Acosta, L.K.; Pol, L.; Xifre-Perez, E.; Pallares, J.; Ferre-Borrull, J.; Marsal, L.F. Nanoporous Anodic Alumina Surface Modification by Electrostatic, Covalent, and Immune Complexation Binding Investigated by Capillary Filling. ACS Appl. Mater. Interfaces 2018, 10, 10571–10579. [Google Scholar] [CrossRef]
- Tabrizi, M.A.; Ferre-Borrull, J.; Marsal, L.F. Advances in optical biosensors and sensors using nanoporous anodic alumina. Sensors 2020, 20, 5068. [Google Scholar] [CrossRef]
- Law, C.S.; Lim, S.Y.; Abell, A.D.; Voelcker, N.H.; Santos, A. Nanoporous Anodic Alumina Photonic Crystals for Optical Chemo- and Biosensing: Fundamentals, Advances, and Perspectives. Nanomaterials 2018, 8, 788. [Google Scholar] [CrossRef]
- Zhou, Z.; Nonnenmann, S.S. Progress in nanoporous templates: Beyond anodic aluminum oxide and towards functional complex materials. Materials 2019, 12, 2535. [Google Scholar] [CrossRef]
- Kapruwan, P.; Ferré-borrull, J.; Marsal, L.F. Nanoporous Anodic Alumina Platforms for Drug Delivery Applications: Recent Advances and Perspective. Adv. Mater. Interfaces 2020, 7, 1–17. [Google Scholar] [CrossRef]
- Lee, W.; Park, S.J. Porous anodic aluminum oxide: Anodization and templated synthesis of functional nanostructures. Chem. Rev. 2014, 114, 7487–7556. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Asoh, H.; Ono, S. Effects of electrolyte species and their combination on film structures and dielectric properties of crystalline anodic alumina films formed by two-step anodization. Mater. Trans. 2013, 54, 1993–1999. [Google Scholar] [CrossRef]
- Yazdizadeh, M.; Yelon, A.; Ménard, D. Characterizing and optimizing the electropolishing and pore arrangement in porous anodic aluminum oxide (AAO). J. Porous Mater. 2020, 27, 995–1002. [Google Scholar] [CrossRef]
- Mir, M.A.; Shah, M.A.; Ganai, P.A. Nanoporous anodic alumina (NAA) prepared in different electrolytes with different pore sizes for humidity sensing. J. Solid State Electrochem. 2020, 24, 1679–1686. [Google Scholar] [CrossRef]
- Davies, J.A.; Domeij, B.; Pringle, J.P.S.; Brown, F. The Migration of Metal and Oxygen during Anodic Film Formation. J. Electrochem. Soc. 1965, 112, 675. [Google Scholar] [CrossRef]
- Shimizu, K.; Thompson, G.E.; Wood, G.C.; Xu, Y. Direct observations of ion-implanted xenon marker layers in anodic barrier films on aluminium. Thin Solid Films 1982, 88, 255–262. [Google Scholar] [CrossRef]
- Li, F.; Zhang, L.; Metzger, R.M. On the Growth of Highly Ordered Pores in Anodized Aluminum Oxide. Chem. Mater. 1998, 10, 2470–2480. [Google Scholar] [CrossRef]
- Kumeria, T.; Santos, A. Nanoporous Anodic Alumina for Optical Biosensing. Springer Ser. Mater. Sci. 2015, 219, 293–318. [Google Scholar] [CrossRef]
- Thompson, G.E.; Xu, Y.; Skeldon, P.; Shimizu, K.; Han, S.H.; Wood, G.C. Anodic oxidation of aluminium. Philos. Mag. B Phys. Condens. Matter. 1987, 55, 651–667. [Google Scholar] [CrossRef]
- Thompson, G.E. Porous anodic alumina: Fabrication, characterization and applications. Thin Solid Films 1997, 297, 192–201. [Google Scholar] [CrossRef]
- Jessensky, O.; Müller, F.; Gösele, U. Self-organized formation of hexagonal pore arrays in anodic alumina. Appl. Phys. Lett. 1998, 72, 1173–1175. [Google Scholar] [CrossRef]
- Van Overmeere, Q.; Proost, J. Stress-affected and stress-affecting instabilities during the growth of anodic oxide films. Electrochim. Acta. 2011, 56, 10507–10515. [Google Scholar] [CrossRef]
- Nielsch, K.; Choi, J.; Schwirn, K.; Wehrspohn, R.B. Self-ordering Regimes of Poorus Alumina: The 10% Porosity Rule. Nano Lett. 2002, 2, 1–4. [Google Scholar] [CrossRef]
- Santos, A.; Kumeria, T.; Losic, D. Nanoporous anodic aluminum oxide for chemical sensing and biosensors. TrAC Trends Anal. Chem. 2013, 44, 25–38. [Google Scholar] [CrossRef]
- Sousa, C.T.; Leitao, D.C.; Proenca, M.P.; Ventura, J.; Pereira, A.M.; Araujo, J.P. Nanoporous alumina as templates for multifunctional applications. Appl. Phys. Rev. 2014, 1, 031102. [Google Scholar] [CrossRef]
- Houser, J.E.; Hebert, K.R. The role of viscous flow of oxide in the growth of self-ordered porous anodic alumina films. Nat. Mater. 2009, 8, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Hebert, K.R.; Albu, S.P.; Paramasivam, I.; Schmuki, P. Morphological instability leading to formation of porous anodic oxide films. Nat. Mater. 2012, 11, 162–166. [Google Scholar] [CrossRef]
- Kikuchi, T.; Nishinaga, O.; Natsui, S.; Suzuki, R.O. Fabrication of self-ordered porous alumina via etidronic acid anodizing and structural color generation from submicrometer-scale dimple array. Electrochim. Acta 2015, 156, 235–243. [Google Scholar] [CrossRef]
- Mirzoev, R.A.; Davydov, A.D.; Vystupov, S.I.; Kabanova, T.B. Conditions for self-ordering of porous structure of anodic aluminum oxide in weak and strong acids. Electrochim. Acta 2019, 294, 276–285. [Google Scholar] [CrossRef]
- Masuda, H.; Hasegwa, F. Self-Ordering of Cell Arrangement of Anodic Porous Alumina Formed in Sulfuric Acid Solution. J. Electrochem. Soc. 1997, 144, L127. [Google Scholar] [CrossRef]
- Sulka, G.D.; Parkoła, K.G. Anodising potential influence on well-ordered nanostructures formed by anodisation of aluminium in sulphuric acid. Thin Solid Films 2006, 515, 338–345. [Google Scholar] [CrossRef]
- Raid, A.; Pavan, S.; Fridrici, V.; Poilane, C.; Kapsa, P. Temperature effect on the kinetic alumina layer growth on 5086 aluminum substrate. Mechanika 2017, 23, 923–930. [Google Scholar] [CrossRef][Green Version]
- Arango, F.; Sepúlveda, M.; Aguilar-Sierra, S.; Ricaurte, G.; Echeverría, F. Interferometric colours produced by anodised aluminium diffraction grating. Mater. Sci. Technol. 2020, 36, 1238–1244. [Google Scholar] [CrossRef]
- Takenaga, A.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Self-ordered aluminum anodizing in phosphonoacetic acid and its structural coloration. ECS Solid State Lett. 2015, 4, P55–P58. [Google Scholar] [CrossRef]
- Santos, A.; Balderrama, V.S.; Alba, M.; Formenting, P.; Ferre-Borrull, J.; Pallares, J.; Marsal, L.F. Nanoporous anodic alumina barcodes: Toward smart optical biosensors. Adv. Mater. 2012, 24, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Macias, G.; Ferré-Borrull, J.; Pallarès, J.; Marsal, L.F. 1-D nanoporous anodic alumina rugate filters by means of small current variations for real-time sensing applications. Nanoscale Res. Lett. 2014, 9, 1–6. [Google Scholar] [CrossRef]
- Sun, C.; Hao, S.; Wang, Z.; Xu, Q.; Wang, Y.; Peng, Q.; Lan, T. Rapid fabrication of iridescent alumina films supported on an aluminium substrate by high voltage anodization. Opt. Mater. 2020, 104, 109937. [Google Scholar] [CrossRef]
- Santos, A.; Alba, M.; Rahman, M.M.; Formentin, P.; Ferre-Borrull, J.; Pallares, J.; Marsal, L.F. Structural tuning of photoluminescence in nanoporous anodic alumina by hard anodization in oxalic and malonic acids. Nanoscale Res. Lett. 2012, 7, 1–11. [Google Scholar] [CrossRef]
- Reddy, P.R.; Ajith, K.M.; Udayashankar, N.K. Effect of electrolyte concentration on morphological and photoluminescence properties of free standing porous anodic alumina membranes formed in oxalic acid. Mater. Sci. Semicond. Process. 2020, 106, 104755. [Google Scholar] [CrossRef]
- Akiya, S.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Nanostructural characterization of large-scale porous alumina fabricated via anodizing in arsenic acid solution. Appl. Surf. Sci. 2017, 403, 652–661. [Google Scholar] [CrossRef][Green Version]
- Chernyakova, K.; Karpicz, R.; Zavadski, S.; Poklonskaya, O.; Jagminas, A.; Vrublevsky, I. Structural and fluorescence characterization of anodic alumina/carbon composites formed in tartaric acid solution. J. Lumin. 2017, 182, 233–239. [Google Scholar] [CrossRef]
- González-Rovira, L.; González-Souto, L.; Astola, P.J.; Bravo-Benítez, C.; Botana, F.J. Assessment of the corrosion resistance of self-ordered anodic aluminum oxide (AAO) obtained in tartaric-sulfuric acid (TSA). Surf. Coat. Technol. 2020, 399, 126131. [Google Scholar] [CrossRef]
- Zajączkowska, L.; Siemiaszko, D.; Norek, M. Towards Self-Organized Anodization of Aluminum in Malic Acid Solutions—New Aspects of Anodization in the Organic Acid. Materials 2020, 13, 3899. [Google Scholar] [CrossRef]
- Gordeeva, E.O.; Roslyakov, I.V.; Napolskii, K.S. Aluminium anodizing in selenic acid: Electrochemical behaviour, porous structure, and ordering regimes. Electrochim. Acta 2019, 307, 13–19. [Google Scholar] [CrossRef]
- Sadykov, A.I.; Kushnir, S.E.; Roslyakov, I.V.; Baranchikov, A.E.; Napolskii, K.S. Selenic acid anodizing of aluminium for preparation of 1D photonic crystals. Electrochem. Commun. 2019, 100, 104–107. [Google Scholar] [CrossRef]
- Chu, S.Z.; Wada, K.; Inoue, S.; Isogai, M.; Yasumori, A. Fabrication of ideally ordered nanoporous alumina films and integrated alumina nanotubule arrays by high-field anodization. Adv. Mater. 2005, 17, 2115–2119. [Google Scholar] [CrossRef]
- Kikuchi, T.; Nishinaga, O.; Natsui, S.; Suzuki, R.O. Self-ordering behavior of anodic porous alumina via selenic acid anodizing. Electrochim. Acta 2014, 728–735. [Google Scholar] [CrossRef]
- Pashchanka, M.; Schneider, J.J. Formation of Porous Anodic Alumina under Unstable Electroconvection Flow Regimes: A Case Study of Tartronic Acid Electrolyte. J. Phys. Chem. C 2017, 121, 23683–23692. [Google Scholar] [CrossRef]
- Ono, S.; Saito, M.; Asoh, H. Self-ordering of anodic porous alumina formed in organic acid electrolytes. Electrochim. Acta 2005, 51, 827–833. [Google Scholar] [CrossRef]
- Sun, B.; Li, J.; Jin, X.; Zhou, C.; Hao, Q.; Gao, X. Self-ordered hard anodization in malonic acid and its application in tailoring alumina taper-nanopores with continuously tunable periods in the range of 290–490 nm. Electrochim. Acta 2013, 112, 327–332. [Google Scholar] [CrossRef]
- Akiya, S.; Kikuchi, T.; Natsui, S.; Sakaguchi, N.; Suzuki, R.O. Self-ordered Porous Alumina Fabricated via Phosphonic Acid Anodizing. Electrochim. Acta 2016, 190, 471–479. [Google Scholar] [CrossRef]
- Chu, S.Z.; Wada, K.; Inoue, S.; Isogai, M.; Katsuta, Y.; Yasumori, A. Large-Scale Fabrication of Ordered Nanoporous Alumina Films with Arbitrary Pore Intervals by Critical-Potential Anodization. J. Electrochem. Soc. 2006, 153, B384. [Google Scholar] [CrossRef]
- Huang, H.; Qiu, J.; Sun, M.; Liu, W.; Wei, X.; Sakai, E.; Ito, K. A hard coating with MAO/AAO double layers prepared on aluminum in etidronic acid by DC oxidation. Surf. Coat. Technol. 2019, 360, 307–317. [Google Scholar] [CrossRef]
- Mozalev, A.; Mozaleva, I.; Sakairi, M.; Takahashi, H. Anodic film growth on Al layers and Ta-Al metal bilayers in citric acid electrolytes. Electrochim. Acta 2005, 50, 5065–5075. [Google Scholar] [CrossRef]
- Zaraska, L.; Stȩpniowski, W.J.; Ciepiela, E.; Sulka, G.D. The effect of anodizing temperature on structural features and hexagonal arrangement of nanopores in alumina synthesized by two-step anodizing in oxalic acid. Thin Solid Films 2013, 534, 155–161. [Google Scholar] [CrossRef]
- Stȩpniowski, W.J.; Nowak-Stȩpniowska, A.; Presz, A.; Czujko, T.; Varin, R.A. The effects of time and temperature on the arrangement of anodic aluminum oxide nanopores. Mater. Charact. 2014, 91, 1–9. [Google Scholar] [CrossRef]
- Chernyakova, K.; Tzaneva, B.; Vrublevsky, I.; Videkov, V. Effect of Aluminum Anode Temperature on Growth Rate and Structure of Nanoporous Anodic Alumina. J. Electrochem. Soc. 2020, 167, 103506. [Google Scholar] [CrossRef]
- Resende, P.M.; Martín-González, M. Sub-10 nm porous alumina templates to produce sub-10 nm nanowires. Microporous Mesoporous Mater. 2019, 284, 198–204. [Google Scholar] [CrossRef]
- Pla, L.; Xifre-Perez, E.; Ribes, A.; Aznar, E.; Marcos, D.; Marsal, L.F.; Martinez-Manez, R.; Sancenon, F. A Mycoplasma Genomic DNA Probe using Gated Nanoporous Anodic Alumina. ChemPlusChem 2017, 82, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Asoh, H.; Matsumoto, M.; Hashimoto, H. Effects of ethanol on the efficiency of the formation of anodic alumina in sulfuric acid. Surf. Coat. Technol. 2019, 378, 124947. [Google Scholar] [CrossRef]
- Michalska-Domańska, M.; Stępniowski, W.J.; Jaroszewicz, L.R. Characterization of nanopores arrangement of anodic alumina layers synthesized on low-(AA1050) and high-purity aluminum by two-step anodizing in sulfuric acid with addition of ethylene glycol at low temperature. J. Porous Mater. 2017, 24, 779–786. [Google Scholar] [CrossRef]
- Norek, M.; Zasada, D.; Siemiaszko, D. Systematic study on morphology of anodic alumina produced by hard anodization in the electrolytes modified with ethylene glycol. J. Nano Res. 2017, 46, 165–178. [Google Scholar] [CrossRef]
- Farhan, M.; Anawati, A. Effect of additive ethylene glycol on morphology and mechanical hardness of anodic oxide film formed on AA7075. J. Phys. Conf. Ser. 2019, 1191, 012033. [Google Scholar] [CrossRef]
- Matsumoto, M.; Hashimoto, H.; Asoh, H. Formation Efficiency of Anodic Porous Alumina in Sulfuric Acid Containing Alcohol: Comparison of the Effects of Monohydric and Polyhydric Alcohols as Additives. J. Electrochem. Soc. 2020, 167, 041504. [Google Scholar] [CrossRef]
- Chen, W.; Wu, J.S.; Xia, X.H. Porous anodic alumina with continuously manipulated pore/cell size. ACS Nano 2008, 2, 959–965. [Google Scholar] [CrossRef]
- Abd-Elnaiem, A.M.; Rashad, M. Morphology of anodic aluminum oxide anodized in a mixture of phosphoric acid and lithium phosphate monobasic. Mater. Res. Express 2019, 6, 016412. [Google Scholar] [CrossRef]
- Maekawa, H.; Tanaka, R.; Sato, T.; Fujimaki, Y.; Yamamura, T. Size-dependent ionic conductivity observed for ordered mesoporous alumina-LiI composite. Solid State Ionics 2004, 175, 281–285. [Google Scholar] [CrossRef]
- Christoulaki, A.; Moretti, C.; Chennevière, A.; Dubois, E.; Jouault, N. Improving structural features of nanoporous alumina using deuterated electrolytes. Microporous Mesoporous Mater. 2020, 303, 110201. [Google Scholar] [CrossRef]
- Lämmel, C.; Schneider, M.; Heubner, C.; Beckert, W.; Michaelis, A. Investigations of burning phenomena during the hard anodising of aluminium by local in-operando temperature measurements. Electrochim. Acta 2017, 249, 271–277. [Google Scholar] [CrossRef]
- Noormohammadi, M.; Arani, Z.S.; Ramazani, A.; Kashi, M.A.; Abbasimofrad, S. Super-fast fabrication of self-ordered nanoporous anodic alumina membranes by ultra-hard anodization. Electrochim. Acta 2020, 354, 136766. [Google Scholar] [CrossRef]
- Vega, V.; Garcia, J.; Montero-Moreno, J.M.; Hernando, B.; Bachmann, J.; Prida, V.M.; Nielsch, K. Unveiling the Hard Anodization Regime of Aluminum: Insight into Nanopores Self-Organization and Growth Mechanism. ACS Appl. Mater. Interfaces 2015, 7, 28682–28692. [Google Scholar] [CrossRef]
- Roslyakov, I.V.; Kuratova, N.S.; Koshkodaev, D.S.; Merino, D.H.; Lukashin, A.V.; Napolskii, K.S. Morphology of anodic alumina films obtained by hard anodization: Influence of the rate of anodization voltage increase. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2016, 10, 191–197. [Google Scholar] [CrossRef]
- Roslyakov, I.V.; Gordeeva, E.O.; Napolskii, K.S. Role of Electrode Reaction Kinetics in Self-Ordering of Porous Anodic Alumina. Electrochim. Acta 2017, 241, 362–369. [Google Scholar] [CrossRef]
- Gordeeva, E.O.; Roslyakov, I.V.; Sadykov, A.I.; Suchkova, T.A.; Petukhov, D.I.; Shatalova, T.B.; Napolskii, K.S. Formation Efficiency of Porous Oxide Films in Aluminum Anodizing. Russ. J. Electrochem. 2018, 54, 990–998. [Google Scholar] [CrossRef]
- Leontiev, A.P.; Roslyakov, I.V.; Napolskii, K.S. Complex influence of temperature on oxalic acid anodizing of aluminium. Electrochim. Acta 2019, 319, 88–94. [Google Scholar] [CrossRef]
- Mei, Y.F.; Wu, X.L.; Shao, X.F.; Huang, G.S.; Siu, G.G. Formation mechanism of alumina nanotube array. Phys. Lett. Sect. A 2003, 309, 109–113. [Google Scholar] [CrossRef]
- Yu, M.; Cui, H.; Ai, F.; Jiang, L.; Kong, J.; Zhu, X. Terminated nanotubes: Evidence against the dissolution equilibrium theory. Electrochem. Commun. 2018, 86, 80–84. [Google Scholar] [CrossRef]
- Yu, M.; Chen, Y.; Li, C.; Yan, S.; Cui, H.; Zhu, X.; Kong, J. Studies of oxide growth location on anodization of Al and Ti provide evidence against the field-assisted dissolution and field-assisted ejection theories. Electrochem. Commun. 2018, 87, 76–80. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, X.; Jia, H.; Han, T. Oxygen evolution: The mechanism of formation of porous anodic alumina. Monatshefte fur Chemie 2009, 140, 595–600. [Google Scholar] [CrossRef]
- Zhao, S.; Chan, K.; Yelon, A.; Veres, T. Novel structure of AAO film fabricated by constant current anodization. Adv. Mater. 2007, 19, 3004–3007. [Google Scholar] [CrossRef]
- Pu, L.; Bao, X.; Zou, J.; Feng, D. Individual alumina nanotubes. Angew. Chemie Int. Ed. 2001, 40, 1490–1493. [Google Scholar] [CrossRef]
- Ono, S. Defects in Porous Anodic Films Formed on High Purity Aluminum. J. Electrochem. Soc. 1991, 138, 3705. [Google Scholar] [CrossRef]
- Yi, L.; Zhiyuan, L.; Shuoshuo, C.; Xing, H.; Xinhua, H. Novel AAO films and hollow nanostructures fabricated by ultra-high voltage hard anodization. Chem. Commun. 2010, 46, 309–311. [Google Scholar] [CrossRef]
- Wang, Y.; Santos, A.; Evdokiou, A.; Losic, D. Rational design of ultra-short anodic alumina nanotubes by short-time pulse anodization. Electrochim. Acta 2015, 154, 379–386. [Google Scholar] [CrossRef]
- Norek, M.; Stępniowski, W.J.; Siemiaszko, D. Effect of ethylene glycol on morphology of anodic alumina prepared in hard anodization. J. Electroanal. Chem. 2016, 762, 20–28. [Google Scholar] [CrossRef]
- Lee, W.; Schwirn, K.; Steinhart, M.; Pippel, E.; Scholz, R.; Gösele, U. Structural engineering of nanoporous anodic aluminium oxide by pulse anodization of aluminium. Nat. Nanotechnol. 2008, 3, 234–239. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, L.; Song, Y.; Jia, H.; Yu, H.; Xiao, X.; Yang, X. Oxygen bubble mould effect: Serrated nanopore formation and porous alumina growth. Monatshefte fur Chemie 2008, 139, 999–1003. [Google Scholar] [CrossRef]
- Yu, M.; Li, C.; Yang, Y.; Xu, S.; Zhang, K.; Cui, H.; Zhu, X. Cavities between the double walls of nanotubes: Evidence of oxygen evolution beneath an anion-contaminated layer. Electrochem. Commun. 2018, 90, 34–38. [Google Scholar] [CrossRef]
- Su, Z.; Zhou, W. Formation mechanism of porous anodic aluminium and titanium oxides. Adv. Mater. 2008, 20, 3663–3667. [Google Scholar] [CrossRef]
- Huang, W.; Xu, H.; Ying, Z.; Dan, Y.; Zhou, Q.; Zhang, J.; Zhu, X. Split TiO2 nanotubes − Evidence of oxygen evolution during Ti anodization. Electrochem. Commun. 2019, 106, 106532. [Google Scholar] [CrossRef]
- Zhu, X.; Song, Y.; Yu, D.; Zhang, C.; Yao, W. A novel nanostructure fabricated by an improved two-step anodizing technology. Electrochem. Commun. 2013, 29, 71–74. [Google Scholar] [CrossRef]
- Li, Y.; Peng, N.; Wen, Y.; Liang, L. Effect of hydrothermal treatment on porous anodic alumina generated by one-step anodization. Microporous Mesoporous Mater. 2020, 306, 110412. [Google Scholar] [CrossRef]
- Krupinski, M.; Perzanowski, M.; Maximenko, A.; Zabila, Y.; Marszałek, M. Fabrication of flexible highly ordered porous alumina templates by combined nanosphere lithography and anodization. Nanotechnology 2017, 28, 194003. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.K.; Tu, K.T.; Chang, C.Y.; Peng, Y.C. Fabrication of thin-film spherical anodic alumina oxide templates using a superimposed nano-micro structure. Surf. Coat. Technol. 2019, 361, 170–175. [Google Scholar] [CrossRef]
- Bankova, A.; Videkov, V.; Tzaneva, B.; Mitov, M. Mechanical stability of heat-treated nanoporous anodic alumina subjected to repetitive mechanical deformation. J. Phys. Conf. Ser. 2018, 992, 012055. [Google Scholar] [CrossRef]
- Ozao, R.; Yoshida, H.; Ichimura, Y.; Inada, T.; Ochiai, M. Crystallization of Anodic Alumina Membranes Studied by Simultaneous Tg-Dta / Ftir. J. Therm. Anal. 2001, 64, 915–922. [Google Scholar] [CrossRef]
- Ozao, R.; Ochiai, M.; Yoshida, H.; Ichimura, Y.; Inada, T. Preparation of γ-alumina membranes from sulphuric electrolyte anodic alumina and its transition to α-alumina. J. Therm. Anal. Calorim. 2001, 64, 923–932. [Google Scholar] [CrossRef]
- Marsal, L.F.; Vojkuvka, L.; Formentin, P.; Pallarés, J.; Ferré-Borrull, J. Fabrication and optical characterization of nanoporous alumina films annealed at different temperatures. Opt. Mater. 2009, 31, 860–864. [Google Scholar] [CrossRef]
- Fernández-Romero, L.; Montero-Moreno, J.M.; Pellicer, E.; Peiro, F.; Cornet, A.; Morante, J.R.; Sarret, M.; Muller, C. Assessment of the thermal stability of anodic alumina membranes at high temperatures. Mater. Chem. Phys. 2008, 111, 542–547. [Google Scholar] [CrossRef]
- Roslyakov, I.V.; Kolesnik, I.V.; Levin, E.E.; Katorova, N.S.; Pestrikov, P.P.; Kardash, T.Y.; Solovyov, L.A.; Napolskii, K.S. Annealing induced structural and phase transitions in anodic aluminum oxide prepared in oxalic acid electrolyte. Surf. Coat. Technol. 2020, 381, 125159. [Google Scholar] [CrossRef]
- Aman, J.N.M.; Wied, J.K.; Alhusaini, Q.; Muller, S.; Diehl, K.; Staedler, T.; Schonherr, H.; Jiang, X.; der Gunne, J.S.a. Thermal Hardening and Defects in Anodic Aluminum Oxide Obtained in Oxalic Acid: Implications for the Template Synthesis of Low-Dimensional Nanostructures. ACS Appl. Nano Mater. 2019, 2, 1986–1994. [Google Scholar] [CrossRef]
- Roslyakov, I.V.; Shirin, N.A.; Berekchiian, M.V.; Shatalova, T.B.; Garshev, A.V.; Napolskii, K.S. Coarse-grain alpha-alumina films with highly ordered porous structure. Microporous Mesoporous Mater. 2020, 294, 109840. [Google Scholar] [CrossRef]
- Sulka, G.D.; Parkoła, K.G. Temperature influence on well-ordered nanopore structures grown by anodization of aluminium in sulphuric acid. Electrochim. Acta 2007, 52, 1880–1888. [Google Scholar] [CrossRef]
- Cheng, W.; Steinhart, M.; Gösele, U.; Wehrspohn, R.B. Tree-like alumina nanopores generated in a non-steady-state anodization. J. Mater. Chem. 2007, 17, 3493–3495. [Google Scholar] [CrossRef]
- Montero-Moreno, J.M.; Belenguer, M.; Sarret, M.; Müller, C.M. Production of alumina templates suitable for electrodeposition of nanostructures using stepped techniques. Electrochim. Acta 2009, 54, 2529–2535. [Google Scholar] [CrossRef]
- Lee, W.; Scholz, R.; Gösele, U. A continuous process for structurally well-defined Al2O 3 nanotubes based on pulse anodization of aluminum. Nano Lett. 2008, 8, 2155–2160. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kim, J.C.; Cösele, U. Spontaneous current oscillations during hard anodization of aluminum under potentiostatic conditions. Adv. Funct. Mater. 2010, 20, 21–27. [Google Scholar] [CrossRef]
- Lee, W.; Kim, J.C. Highly ordered porous alumina with tailor-made pore structures fabricated by pulse anodization. Nanotechnology 2010, 21, 485304. [Google Scholar] [CrossRef]
- Peng, Q.; Xie, X.; Xu, Q.; Lan, T.; Sun, C.; Zhang, L.; Dong, M. The effect of the current pulse amplitude on the nanopore structures of 3D-AAO films. Microporous Mesoporous Mater. 2020, 309, 110575. [Google Scholar] [CrossRef]
- Acosta, L.K.; Bertó-Roselló, F.; Xifre-Perez, E.; Santos, A.; Ferré-Borrull, J.; Marsal, L.F. Stacked Nanoporous Anodic Alumina Gradient-Index Filters with Tunable Multispectral Photonic Stopbands as Sensing Platforms. ACS Appl. Mater. Interfaces 2019, 11, 3360–3371. [Google Scholar] [CrossRef]
- Acosta, L.K.; Berto-Rosello, F.; Xifre-Perez, E.; Law, C.S.; Santos, A.; Ferre-Borrull, J.; Marsal, L.F. Tunable Nanoporous Anodic Alumina Photonic Crystals by Gaussian Pulse Anodization. ACS Appl. Mater. Interfaces 2020, 12, 19778–19787. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Law, C.S.; Jiang, L.; Acosta, L.K.; Bachhuka, A.; Marsal, L.F.; Abell, A.D.; Santos, A. Enhancing Forbidden Light Propagation in Nanoporous Anodic Alumina Gradient-Index Filters by Alcohol Additives. ACS Appl. Nano Mater. 2020, 3, 12115–12129. [Google Scholar] [CrossRef]
- Schwirn, K.; Lee, W.; Hillebrand, R.; Steinhart, M.; Nielsch, K.; Gösele, U. Self-ordered anodic aluminum oxide formed by H2SO4 hard anodization. ACS Nano 2008, 2, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.L.; Han, C.Y.; Welp, U.; Wang, H.H.; Kwok, W.K.; Willing, G.A.; Hiller, J.M.; Cook, R.E.; Miller, D.J.; Crabtree, G.W. Fabrication of Alumina Nanotubes and Nanowires by Etching Porous Alumina Membranes. Nano Lett. 2002, 2, 1293–1297. [Google Scholar] [CrossRef]
- Qu, L.; He, C.; Yang, Y.; He, Y.; Liu, Z. Hydrothermal synthesis of alumina nanotubes templated by anionic surfactant. Mater. Lett. 2005, 59, 4034–4037. [Google Scholar] [CrossRef]
- Tang, B.; Ge, J.; Zhuo, L.; Wang, G.; Niu, J.; Shi, Z.; Dong, Y. A facile and controllable synthesis of γ-Al2O3 nanostructures without a surfactant. Eur. J. Inorg. Chem. 2005, 1, 4366–4369. [Google Scholar] [CrossRef]
- Wang, C.C.; Kei, C.C.; Yu, Y.W.; Perng, T.P. Organic nanowire-templated fabrication of alumina nanotubes by atomic layer deposition. Nano Lett. 2007, 7, 1566–1569. [Google Scholar] [CrossRef]
- Lee, K.; Kim, H.; Kim, J.H.; Choi, D. Structural color and near-infrared tunability of ruthenium-coated anodic aluminum oxide by atomic layer deposition. Scr. Mater. 2020, 187, 125–129. [Google Scholar] [CrossRef]
- Peng, T.; Yang, H.; Dai, K.; Nakanishi, K.; Hirao, K. Sol-gel template synthesis of aluminum oxide microtubules. Adv. Eng. Mater. 2004, 6, 241–244. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; He, R.; Zhang, Q.; Zhang, X.; Zhu, J. Synthesis of alumina nanotubes using carbon nanotubes as templates. Chem. Phys. Lett. 2002, 360, 579–584. [Google Scholar] [CrossRef]
- Esmaeily, A.S.; Mills, S.; Coey, J.M.D. Exceptional room-temperature plasticity in amorphous alumina nanotubes fabricated by magnetic hard anodisation. Nanoscale 2017, 9, 5205–5211. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kaur, G.; Zysk, A.; Liapis, V.; Hay, S.; Santos, A.; Losic, D.; Evdokiou, A. Systematic invitro nanotoxicity study on anodic alumina nanotubes with engineered aspect ratio: Understanding nanotoxicity by a nanomaterial model. Biomaterials 2015, 46, 117–130. [Google Scholar] [CrossRef]
- Wang, Y.; Santos, A.; Kaur, G.; Evdokiou, A.; Losic, D. Structurally engineered anodic alumina nanotubes as nano-carriers for delivery of anticancer therapeutics. Biomaterials 2014, 35, 5517–5526. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kaur, G.; Chen, Y.; Santos, A.; Losic, D.; Evdokiou, A. Bioinert Anodic Alumina Nanotubes for Targeting of Endoplasmic Reticulum Stress and Autophagic Signaling: A Combinatorial Nanotube-Based Drug Delivery System for Enhancing Cancer Therapy. ACS Appl. Mater. Interfaces 2015, 7, 27140–27151. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zinonos, I.; Zysk, A.; Panagopoulos, V.; Kaur, G.; Santos, A.; Losic, D.; Evdokiou, A. In vivo toxicological assessment of electrochemically engineered anodic alumina nanotubes: A study of biodistribution, subcutaneous implantation and intravenous injection. J. Mater. Chem. B 2017, 5, 2511–2523. [Google Scholar] [CrossRef]
- Domagalski, J.T.; Xifre-Perez, E.; Santos, A.; Ferre-Borrull, J.; Marsal, L.F. Tailor-engineered structural and physico-chemical properties of anodic alumina nanotubes by pulse anodization: A step forward. Microporous Mesoporous Mater. 2020, 303, 110264. [Google Scholar] [CrossRef]
- Domagalski, J.T.; Xifre-Perez, E.; Tabrizi, M.A.; Ferre-Borrull, J.; Marsal, L.F. Magnetic nanoparticle decorated anodic alumina nanotubes for fluorescent detection of cathepsin B. J. Colloid Interface Sci. 2021, 584, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Xifre-Perez, E.; Guaita-Esteruelas, S.; Baranowska, M.; Pallares, J.; Masana, L.; Marsal, L.F. In Vitro Biocompatibility of Surface-Modified Porous Alumina Particles for HepG2 Tumor Cells: Toward Early Diagnosis and Targeted Treatment. ACS Appl. Mater. Interfaces 2015, 7, 18600–18608. [Google Scholar] [CrossRef]
- Xifre-Perez, E.; Ferré-Borrull, J.; Pallarès, J.; Marsal, L.F. Micro- and nanoparticles of mesoporous anodic alumina: Morphological and physicochemical properties. Microporous Mesoporous Mater. 2017, 239, 363–370. [Google Scholar] [CrossRef]
- Chen, Y.; Santos, A.; Wang, Y.; Kumeria, T.; Li, J.; Wang, C.; Losic, D. Biomimetic Nanoporous Anodic Alumina Distributed Bragg Reflectors in the Form of Films and Microsized Particles for Sensing Applications. ACS Appl. Mater. Interfaces 2015, 7, 19816–19824. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Kitagishi, N.; Fukushima, T.; Yanagishita, T.; Masuda, H. Fabrication of aluminum nanowires by mechanical deformation of Al using anodic porous alumina molds. Mater. Express 2016, 6, 363–366. [Google Scholar] [CrossRef]
- Yanagishita, T.; Imaizumi, M.; Kondo, T.; Masuda, H. Formation of porous Al particles by anisotropic anodic etching. Electrochem. Commun. 2017, 78, 26–28. [Google Scholar] [CrossRef]
- Yanagishita, T.; Imaizumi, M.; Kondo, T.; Masuda, H. Preparation of nanoporous alumina hollow spheres with a highly ordered hole arrangement. RSC Adv. 2018, 8, 2041–4047. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Almalik, A.; Khashab, N.M. Mesoporous Silica and Organosilica Nanoparticles: Physical Chemistry, Biosafety, Delivery Strategies, and Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1–75. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Formentín, P.; Pallarès, J.; Ferré-Borrull, J.; Marsal, L.F. Structural engineering of nanoporous anodic alumina funnels with high aspect ratio. J. Electroanal. Chem. 2011, 655, 73–78. [Google Scholar] [CrossRef]
- Santos, A.; Kumeria, T.; Wang, Y.; Losic, D. In situ monitored engineering of inverted nanoporous anodic alumina funnels: On the precise generation of 3D optical nanostructures. Nanoscale 2014, 6, 9991–9999. [Google Scholar] [CrossRef]
- Porta-i-Batalla, M.; Xifré-Pérez, E.; Eckstein, C.; Ferré-Borrull, J.; Marsal, L. 3D Nanoporous Anodic Alumina Structures for Sustained Drug Release. Nanomaterials 2017, 7, 227. [Google Scholar] [CrossRef] [PubMed]
- Liao, J. Formation of Bottle-Shaped Pores with Petaloid Shoulder within Anodic Alumina. Surf. Eng. Appl. Electrochem. 2018, 54, 555–561. [Google Scholar] [CrossRef]
- Sacco, L.; Florea, I.; Cojocaru, C.S. Fabrication of porous anodic alumina (PAA) templates with straight pores and with hierarchical structures through exponential voltage decrease technique. Surf. Coat. Technol. 2018, 364, 248–255. [Google Scholar] [CrossRef]
- Liu, C.Y.; Biring, S. Nanoplatform based on ideally ordered arrays of short straight and long beer bottle-shaped nanochannels. Microporous Mesoporous Mater. 2019, 287, 71–76. [Google Scholar] [CrossRef]
- Jin, S.; Li, Y.; Li, Z.X.; Hu, X.; Ling, Z.Y.; He, X.H.; Shen, Y.H.; Jin, L. Controllable Fabrication and Microstructure Modulation of Unique AAO Structures Based on Patterned Aluminum Surface. J. Electrochem. Soc. 2016, 163, H1053–H1059. [Google Scholar] [CrossRef]
- Ma, Y.; Wen, Y.; Li, J.; Feng, C.; Zhang, Z.; Gou, T.; Huang, J.; Lu, J.; Cui, Z.; Sun, R. Fabrication of alumina with ordered tapered-nanopore nested in micro-bowl hierarchical structure by a combined anodization. Mater. Chem. Phys. 2020, 239, 122023. [Google Scholar] [CrossRef]
- Martín, J.; Martín-González, M.; Fernández, J.F.; Caballero-Calero, O. Ordered three-dimensional interconnected nanoarchitectures in anodic porous alumina. Nat. Commun. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Abad, B.; Maiz, J.; Ruiz-Clavijo, A.; Caballero-Calero, O.; Martin-Gonzalez, M. Tailoring thermal conductivity via three-dimensional porous alumina. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Ruiz-Clavijo, A.; Ruiz-Gomez, S.; Caballero-Calero, O.; Perez, L.; Martin-Gonzalez, M. Tailoring Magnetic Anisotropy at Will in 3D Interconnected Nanowire Networks. Phys. Status Solidi Rapid Res. Lett. 2019, 13, 1–7. [Google Scholar] [CrossRef]
- Ruiz-Clavijo, A.; Tsurimaki, Y.; Caballero-Calero, O.; Ni, G.; Chen, G.; Boriskina, S.V.; Martin-Gonzalez, M. Engineering a Full Gamut of Structural Colors in All-Dielectric Mesoporous Network Metamaterials. ACS Photonics 2018, 5, 2120–2128. [Google Scholar] [CrossRef]
- Resende, P.M.; Sanz, R.; Caballero-Calero, O.; Martín-González, M. Cost-Effective, Flexible, Hydrophobic, and Tunable Structural Color Polymeric Bragg Reflector Metastructures. Adv. Opt. Mater. 2018, 6, 1–7. [Google Scholar] [CrossRef]
- Santos, A.; Kumeria, T.; Losic, D. Nanoporous anodic alumina: A versatile platform for optical biosensors. Materials 2014, 7, 4297–4320. [Google Scholar] [CrossRef]
- Yablonovitch, E. Inhibited Spontaneous Emission in Solid-State Physics and Electronics. Phys. Rev. Lett. 1987, 58, 2059–2062. [Google Scholar] [CrossRef] [PubMed]
- John, S. Strong localization of photons in certain disordered dielectric superlattices. Phys. Rev. Lett. 1987, 58, 2486–2489. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C. Materials Aspects of Photonic Crystals. Adv. Mater. 2003, 15, 1679–1704. [Google Scholar] [CrossRef]
- Ferro, L.M.M.; Lemos, S.G.; Ferreira, M.; Trivinho-Strixino, F. Use of multivariate analysis on Fabry-Pérot interference spectra of nanoporous anodic alumina (NAA) for optical sensors purposes. Sens. Actuators B Chem. 2017, 248, 718–723. [Google Scholar] [CrossRef]
- Law, C.S.; Lim, S.Y.; Liu, L.; Abell, A.D.; Marsal, L.F.; Santos, A. Realization of high-quality optical nanoporous gradient-index filters by optimal combination of anodization conditions. Nanoscale 2020, 12, 9404–9415. [Google Scholar] [CrossRef]
- Lim, S.Y.; Law, C.S.; Liu, L.; Markovic, M.; Abell, A.D.; Santos, A. Integrating surface plasmon resonance and slow photon effects in nanoporous anodic alumina photonic crystals for photocatalysis. Catal. Sci. Technol. 2019, 9, 3158–3176. [Google Scholar] [CrossRef]
- Santos, A.; Pereira, T.; Law, C.S.; Losic, D. Rational engineering of nanoporous anodic alumina optical bandpass filters. Nanoscale 2016, 8, 14846–14857. [Google Scholar] [CrossRef]
- Law, C.S.; Lim, S.Y.; Abell, A.D.; Santos, A. Real-Time Binding Monitoring between Human Blood Proteins and Heavy Metal Ions in Nanoporous Anodic Alumina Photonic Crystals. Anal. Chem. 2018, 90, 10039–10048. [Google Scholar] [CrossRef]
- Kushnir, S.E.; Komarova, T.Y.; Napolskii, K.S. High-quality-factor anodic alumina optical microcavities prepared by cyclic anodizing with voltage: Versus optical path length modulation. J. Mater. Chem. C 2020, 8, 3991–3995. [Google Scholar] [CrossRef]
- Lim, S.Y.; Law, C.S.; Marsal, L.F.; Santos, A. Engineering of hybrid nanoporous anodic alumina photonic crystals by heterogeneous pulse anodization. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Busch, M.; Kityk, A.V.; Piecek, W.; Hofmann, T.; Wallacher, D.; Calus, S.; Kula, P.; Steinhart, M.; Eich, M.; Huber, P. A ferroelectric liquid crystal confined in cylindrical nanopores: Reversible smectic layer buckling, enhanced light rotation and extremely fast electro-optically active Goldstone excitations. Nanoscale 2017, 9, 19086–19099. [Google Scholar] [CrossRef]
- Yildirim, A.; Szymoniak, P.; Sentker, K.; Butschies, M.; Buhlmeyer, A.; Huber, P.; Laschat, S.; Schonhals, A. Dynamics and ionic conductivity of ionic liquid crystals forming a hexagonal columnar mesophase. Phys. Chem. Chem. Phys. 2018, 20, 5626–5635. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Sentker, K.; Smales, G.J.; Pauw, B.R.; Huber, P.; Schönhals, A. Collective orientational order and phase behavior of a discotic liquid crystal under nanoscale confinement. Nanoscale Adv. 2019, 1, 1104–1116. [Google Scholar] [CrossRef]
- Yildirim, A.; Buhlmeyer, A.; Hayashi, S.; Haenle, J.C.; Sentker, K.; Krause, C.; Huber, P.; Laschat, S.; Schonhals, A. Multiple glassy dynamics in dipole functionalized triphenylene-based discotic liquid crystals revealed by broadband dielectric spectroscopy and advanced calorimetry-assessment of the molecular origin. Phys. Chem. Chem. Phys. 2019, 21, 18265–18277. [Google Scholar] [CrossRef] [PubMed]
- Sentker, K.; Yildririm, A.; Lippomann, M.; Zantop, A.W.; Bertram, F.; Hofmann, T.; Seeck, O.H.; Kityk, A.V.; Mazza, M.G.; Schonhals, A.; et al. Self-Assembly of liquid crystals in nanoporous solids for adaptive photonic metamaterials. Nanoscale 2019, 11, 23304–23317. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.M.; Shaban, M. Nanoporous chromium thin film for active detection of toxic heavy metals traces using surface-enhanced Raman spectroscopy. Mater. Res. Express. 2020, 7, 015084. [Google Scholar] [CrossRef]
- Malinovskis, U.; Poplausks, R.; Erts, D.; Ramser, K.; Tamulevicius, S.; Tamuleviciene, A.; Gu, Y.; Prikulis, J. High-density plasmonic nanoparticle arrays deposited on nanoporous anodic alumina templates for optical sensor applications. Nanomaterials 2019, 9, 531. [Google Scholar] [CrossRef]
- Hao, Q.; Huang, H.; Fan, X.; Yin, Y.; Wang, J.; Li, W.; Qiu, T.; Ma, L.; Chu, P.K.; Schmidt, O.G. Controlled Patterning of Plasmonic Dimers by Using an Ultrathin Nanoporous Alumina Membrane as a Shadow Mask. ACS Appl. Mater. Interfaces 2017, 9, 36199–36205. [Google Scholar] [CrossRef]
- Aguilar-Pujol, M.; Ramirez-Jimenez, R.; Xifre-Perez, E.; Cortijo-Campos, S.; Bartolome, J.; Marsal, L.F.; de Andres, A. Supported ultra-thin alumina membranes with graphene as efficient interference enhanced raman scattering platforms for sensing. Nanomaterials 2020, 10, 830. [Google Scholar] [CrossRef]
- Rajeev, G.; Xifre-Perez, E.; Simon, B.P.; Cowin, A.J.; Marsal, L.F.; Voelcker, N.H. A label-free optical biosensor based on nanoporous anodic alumina for tumour necrosis factor-alpha detection in chronic wounds. Sens. Actuators B Chem. 2018, 257, 116–123. [Google Scholar] [CrossRef]
- Kaur, S.; Law, C.S.; Williamson, N.H.; Kempson, I.; Popat, A.; Kumeria, T.; Santos, A. Environmental Copper Sensor Based on Polyethylenimine-Functionalized Nanoporous Anodic Alumina Interferometers. Anal. Chem. 2019, 91, 5011–5020. [Google Scholar] [CrossRef]
- Pol, L.; Eckstein, C.; Acosta, L.K.; Xifré-Pérez, E.; Ferré-Borrull, J.; Marsal, L.F. Real-time monitoring of biotinylated molecules detection dynamics in nanoporous anodic alumina for bio-sensing. Nanomaterials 2019, 9, 478. [Google Scholar] [CrossRef] [PubMed]
- Pla, L.; Santiago-Felipe, S.; Tormo-Mas, M.A.; Peman, J.; Sancenon, F.; Aznar, E.; Martinez-Manez, R. Aptamer-Capped nanoporous anodic alumina for Staphylococcus aureus detection. Sens. Actuators B Chem. 2020, 320, 128281. [Google Scholar] [CrossRef]
- Tabrizi, M.A.; Ferré-Borrull, J.; Marsal, L.F. Highly sensitive remote biosensor for the determination of lead (II) ions by using nanoporous anodic alumina modified with DNAzyme. Sens. Actuators B Chem. 2020, 321, 128314. [Google Scholar] [CrossRef]
- Tabrizi, M.A.; Ferré-Borrull, J.; Marsal, L.F. Remote biosensor for the determination of trypsin by using nanoporous anodic alumina as a three-dimensional nanostructured material. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Tabrizi, M.A.; Ferré-Borrull, J.; Marsal, L.F. Remote sensing of Salmonella-specific DNA fragment by using nanoporous alumina modified with the single-strand DNA probe. Sens. Actuators B Chem. 2020, 304, 127302. [Google Scholar] [CrossRef]
- Ribes, À.; Aznar, E.; Santiago-Felipe, S.; Xifre-Perez, E.; Tormo-Mas, M.A.; Peman, J.; Marsal, L.F.; Martinez-Manez, R. Selective and Sensitive Probe Based in Oligonucleotide-Capped Nanoporous Alumina for the Rapid Screening of Infection Produced by Candida albicans. ACS Sens. 2019, 4, 1291–1298. [Google Scholar] [CrossRef]
- Jeon, G.J.; Kim, W.Y.; Shim, H.B.; Lee, H.C. Nanoporous Pirani sensor based on anodic aluminum oxide. Appl. Phys. Lett. 2016, 109, 123505. [Google Scholar] [CrossRef]
- Hernández-Vélez, M. Nanowires and 1D arrays fabrication: An overview. Thin Solid Films 2006, 495, 51–63. [Google Scholar] [CrossRef]
- Lim, S.Y.; Law, C.S.; Berto-Rosello, F.; Liu, L.; Markovic, M.; Ferre-Borrull, J.; Abell, A.D.; Voelcker, N.H.; Marsla, L.F.; Santos, A. Tailor-engineered plasmonic single-lattices: Harnessing localized surface plasmon resonances for visible-NIR light-enhanced photocatalysis. Catal. Sci. Technol. 2020, 10, 3195–3211. [Google Scholar] [CrossRef]
- Hu, M.; Xing, Z.; Cao, Y.; Li, Z.; Yan, X.; Xiu, Z.; Zhao, T.; Yang, S.; Zhou, W. Ti3+ self-doped mesoporous black TiO2/SiO2/g-C3N4 sheets heterojunctions as remarkable visible-lightdriven photocatalysts. Appl. Catal. B Environ. 2018, 226, 499–508. [Google Scholar] [CrossRef]
- Goncharova, A.S.; Napolskii, K.S.; Skryabina, O.V.; Stolyarov, V.S.; Levin, E.E.; Egorov, S.V.; Eliseev, A.A.; Kasumov, Y.A.; Ryazanov, V.V.; Tsirilina, G.A. Bismuth nanowires: Electrochemical fabrication, structural features, and transport properties. Phys. Chem. Chem. Phys. 2020, 22, 14953–14964. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Li, T.Y.; Zhang, J.; Chiao, Z.Y.; Wei, P.C.; Fu, H.C.; Hu, L.; Yu, M.J.; Ahmed, G.H.; Guan, X.; et al. Designed growth and patterning of perovskite nanowires for lasing and wide color gamut phosphors with long-term stability. Nano Energy 2020, 73, 104801. [Google Scholar] [CrossRef]
- Ganapathi, A.; Swaminathan, P.; Neelakantan, L. Anodic Aluminum Oxide Template Assisted Synthesis of Copper Nanowires using a Galvanic Displacement Process for Electrochemical Denitrification. ACS Appl. Nano Mater. 2019, 2, 5981–5988. [Google Scholar] [CrossRef]
- Li, H.; Wu, L.; Zhang, H.; Dai, W.; Hao, J.; Wu, H.; Ren, F.; Liu, C. Self-Assembly of Carbon Black/AAO Templates on Nanoporous Si for Broadband Infrared Absorption. ACS Appl. Mater. Interfaces 2020, 12, 4081–4087. [Google Scholar] [CrossRef]
- Bayat, H.; Raoufi, M.; Zamrik, I.; Schönherr, H. Poly(diethylene glycol methylether methacrylate) Brush-Functionalized Anodic Alumina Nanopores: Curvature-Dependent Polymerization Kinetics and Nanopore Filling. Langmuir 2020, 36, 2663–2672. [Google Scholar] [CrossRef]
- Sanguansap, Y.; Karn-orachai, K.; Laocharoensuk, R. Tailor-made porous striped gold-silver nanowires for surface enhanced Raman scattering based trace detection of β-hydroxybutyric acid. Appl. Surf. Sci. 2020, 500, 144049. [Google Scholar] [CrossRef]
- Salaheldeen, M.; Méndez, M.; Vega, V.; Fernández, A.; Prida, V.M. Tuning Nanohole Sizes in Ni Hexagonal Antidot Arrays: Large Perpendicular Magnetic Anisotropy for Spintronic Applications. ACS Appl. Nano Mater. 2019, 2, 1866–1875. [Google Scholar] [CrossRef]
- Garcia, J.; Prida, V.M.; Vega, V.; Rosa, W.O.; Caballero-Flores, R.; Iglesias, I.; Hernando, B. 2D and 3D ordered arrays of Co magnetic nanowires. J. Magn. Magn. Mater. 2015, 383, 88–93. [Google Scholar] [CrossRef]
- Riva, J.S.; Juárez, A.V.; Urreta, S.E.; Yudi, L.M. Catalytic properties of Fe–Pd ferromagnetic nanowires at liquid/liquid interfaces. Electrochim. Acta 2019, 2998, 379–388. [Google Scholar] [CrossRef]
- Galdun, L.; Vega, V.; Vargova, Z.; Barriga-Castro, E.D.; Luna, C.; Varga, R.; Prida, V.M. Intermetallic Co2FeIn Heusler Alloy Nanowires for Spintronics Applications. ACS Appl. Nano Mater. 2018, 1, 7066–7074. [Google Scholar] [CrossRef]
- Rath, A.; Theato, P. Advanced AAO Templating of Nanostructured Stimuli-Responsive Polymers: Hype or Hope? Adv. Funct. Mater. 2020, 30, 1–16. [Google Scholar] [CrossRef]
- Belwalkar, A.; Grasing, E.; van Geertruyden, W.; Huang, Z.; Misiolek, W.Z. Effect of processing parameters on pore structure and thickness of anodic aluminum oxide (AAO) tubular membranes. J. Memb. Sci. 2008, 319, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Xiao, A.; Zhang, C.; Wang, Y. Growing covalent organic frameworks on porous substrates for molecule-sieving membranes with pores tunable from ultra- to nanofiltration. J. Memb. Sci. 2019, 576, 116–122. [Google Scholar] [CrossRef]
- Schneider, J.J.; Engstler, J.; Budna, K.P.; Teichert, C.; Franzka, S. Freestanding, highly flexible, large area, nanoporous alumina membranes with complete through-hole pore morphology. Eur. J. Inorg. Chem. 2005, 12, 2352–2359. [Google Scholar] [CrossRef]
- Yanagishita, T.; Kato, A.; Kondo, T.; Masuda, H. Preparation of freestanding tubular alumina through-hole membranes by two-layer anodization. Jpn. J. Appl. Phys. 2020, 59, 038002. [Google Scholar] [CrossRef]
- Najma, B.; Kasi, A.K.; Kasi, J.K.; Akbar, A.; Bokhari, S.M.A.; Stroe, I.R. ZnO/AAO photocatalytic membranes for efficient water disinfection: Synthesis, characterization and antibacterial assay. Appl. Surf. Sci. 2018, 448, 104–114. [Google Scholar] [CrossRef]
- Aghili, H.; Hashemi, B.; Bahrololoom, M.E.; Jahromi, S.A.J. Fabrication and characterization of nanoporous anodic alumina membrane using commercial pure aluminium to remove Coliform bacteria from wastewater. Process. Appl. Ceram. 2019, 13, 235–243. [Google Scholar] [CrossRef]
- Mohajeri, M.; Akbarpour, H.; Karimkhani, V. Synthesis of highly ordered carbon nanotubes/nanoporous anodic alumina composite membrane and potential application in heavy metal ions removal from industrial wastewater. Mater. Today Proc. 2017, 4, 4906–4911. [Google Scholar] [CrossRef]
- Maghsodi, A.; Adlnasab, L.; Shabanian, M.; Javanbakht, M. Optimization of effective parameters in the synthesis of nanopore anodic aluminum oxide membrane and arsenic removal by prepared magnetic iron oxide nanoparicles in anodic aluminum oxide membrane via ultrasonic-hydrothermal method. Ultrason. Sonochem. 2018, 48, 441–452. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. A smart membrane with antifouling capability and switchable oil wettability for high-efficiency oil/water emulsions separation. J. Memb. Sci. 2018, 555, 69–77. [Google Scholar] [CrossRef]
- Sadilov, I.S.; Petukhov, D.I.; Eliseev, A.A. Enhancing gas separation efficiency by surface functionalization of nanoporous membranes. Sep. Purif. Technol. 2019, 221, 74–82. [Google Scholar] [CrossRef]
- Petukhov, D.I.; Chernova, E.A.; Kapitanova, O.O.; Boytsova, O.V.; Chumakov, R.G.V.A.P.; Konovalov, O.V.; Eliseev, A.A. Thin graphene oxide membranes for gas dehumidification. J. Memb. Sci. 2019, 577, 184–194. [Google Scholar] [CrossRef]
- Han, E.D.; Park, C.W.; Lee, S.H.; Kim, B.H.; Seo, Y.H. Polar molecule filtration using charged cellulose nanofiber membrane on the nanoporous alumina support for high rejection efficiency. Cellulose 2020, 27, 2685–2694. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, Z.; Li, C.; Gao, L.; Zhao, Y.; Yang, L.; Wen, L.; Jiang, L. Engineered Nanochannel Membranes with Diode-like Behavior for Energy Conversion over a Wide pH Range. ACS Appl. Mater. Interfaces 2019, 11, 23815–23821. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, Y.; Zheng, M.; Fang, H.; Meng, X. Nanostructures confined self-assembled in biomimetic nanochannels for enhancing the sensitivity of biological molecules response. J. Mater. Sci. Mater. Electron. 2018, 29, 19757–19767. [Google Scholar] [CrossRef]
- Chen, Z.; Ni, S.; Han, S.; Crawford, R.; Lu, S.; Wei, F.; Chang, J.; Wu, C.; Xiao, Y. Nanoporous microstructures mediate osteogenesis by modulating the osteo-immune response of macrophages. Nanoscale 2017, 9, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Sui, J.; Wu, Y.; Liang, G.; Zhang, Y.; Pu, Y.; Tian, Y.; Liu, S.; Jiang, L. Bioinspired in Vitro Lung Airway Model for Inflammatory Analysis via Hydrophobic Nanochannel Membrane with Joint Three-Phase Interface. Anal. Chem. 2019, 91, 15804–15810. [Google Scholar] [CrossRef]
- de la Escosura-Muñiz, A.; Espinoza-Castañeda, M.; Chamorro-García, A.; Rodríguez-Hernández, C.J.; de Torres, C.; Merkoçi, A. In situ monitoring of PTHLH secretion in neuroblastoma cells cultured onto nanoporous membranes. Biosens. Bioelectron. 2018, 107, 62–86. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, B.; Liu, F.; Duan, J.; Wang, S.; Qiu, J.; Li, D.; Sang, Y.; Liu, C.; Liu, D.; et al. Polylactic Acid Nanopillar Array-Driven Osteogenic Differentiation of Human Adipose-Derived Stem Cells Determined by Pillar Diameter. Nano Lett. 2018, 18, 2243–2253. [Google Scholar] [CrossRef] [PubMed]
- El Merhie, A.; Salerno, M.; Toccafondi, C.; Dante, S. Neuronal-like response of N2a living cells to nanoporous patterns of thin supported anodic alumina. Colloids Surf. B Biointerfaces 2019, 178, 32–37. [Google Scholar] [CrossRef]
- Formentín, P.; Catalán, Ú.; Pol, L.; Fernández-Castillejo, S.; Solà, R.; Marsal, L.F. Collagen and fibronectin surface modification of nanoporous anodic alumina and macroporous silicon for endothelial cell cultures. J. Biol. Eng. 2018, 12, 1–9. [Google Scholar] [CrossRef]
- Rahman, S.; Atkins, G.J.; Findlay, D.M.; Losic, D. Nanoengineered drug releasing aluminium wire implants: A model study for localized bone therapy. J. Mater. Chem. B 2015, 3, 3288–3296. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, M.; Nasiri-Tabrizi, B.; Dabbagh, A.; Basirun, W.J.; Sukiman, N.L. Optimized nanoporous alumina coating on AA3003-H14 aluminum alloy with enhanced tribo-corrosion performance in palm oil. Ceram. Int. 2020, 46, 7306–7323. [Google Scholar] [CrossRef]
- Sundararajan, M.; Subramani, S.; Devarajan, M.; Jaafar, M. Synthesis and analysis of anodic aluminum oxide-nanopore structure on Al substrates for efficient thermal management in electronic packaging. J. Mater. Sci. Mater. Electron. 2020, 31, 9641–9649. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, J.; Su, Y.S.; Du, W.; Ma, Y.G. Daytime passive radiative cooler using porous alumina. Sol. Energy Mater. Sol. Cells 2019, 191, 50–54. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, D.; Ye, Y.; Ma, L.; Minhas, B.; Liu, B.; Terryn, H.A.; Mol, J.M.C.; Li, X. Durable lubricant-infused anodic aluminum oxide surfaces with high-aspect-ratio nanochannels. Chem. Eng. J. 2019, 368, 138–147. [Google Scholar] [CrossRef]
- Tishkevich, D.I.; Vorobjova, A.I.; Shimanovich, D.I.; Vinnik, D.A.; Zubar, T.I.; Kozlovskiy, A.I.; Zdorovets, M.V.; Yakimchuk, D.V.; Trukhanov, S.V.; Trukhanov, A.V. Formation and corrosion properties of Ni-based composite material in the anodic alumina porous matrix. J. Alloys Compd. 2019, 804, 139–146. [Google Scholar] [CrossRef]


| Application | Reported Utility | Refs |
|---|---|---|
| Photonic structures | Reviews on photonic structures | [34,46,186] |
| Principles of photonic crystals | [187,188,189] | |
| Fabry-Perót interferometer | [190] | |
| Gradient-index filters | [191] | |
| Distributed Bragg reflectors | [192] | |
| Optical bandpass filters | [193] | |
| Human protein heavy ions real-time interaction monitoring | [194] | |
| Tailoring of optical properties with pulse anodization | [149] | |
| Design of phase shift defect in effective refractive index | [195] | |
| Hybrid distributed DBRs and apodized GIF photonic structure | [196] | |
| Characterization of thermotropic ferroelectric liquid crystals confined in the NAA | [197,198,199] | |
| Glass transition of discotic liquid crystals in one-dimensional fluid | [200] | |
| Adjustable optical anisotropy by self-assembly of liquid crystals confined in porous structure | [201] | |
| Sensors | NAA template-assisted fabrication of chromium substrate for SERS detection of heavy ions in aqueous solutions | [202] |
| Au NPs array on NAA for SERS detection of hemoglobin | [203] | |
| Controlled fabrication of periodic plasmonic dimer arrays for SERS | [204] | |
| Graphene-NAA composite for SERS sensing | [205] | |
| Label-free optical sensor based on interferometric reflectance spectroscopy for TNF-α detection | [206] | |
| NAA-based interferometer for copper sensing | [207] | |
| [208] | ||
| Rhodamine B release triggered by Staphylococcus aureus detection | [209] | |
| Determination of Pb2+ with DNAzyme | [210] | |
| Reflectance spectroscopy based biosensor for determination of trypsin | [211] | |
| Salmonella sensing through DNA sequence recognition | [212] | |
| Reusable, molecular gated-NAA for detection of Mycoplasma fermentans | [97] | |
| Molecular gated-NAA for detection of Candida albicans | [213] | |
| Molecular gated-NAA for sensitive detection of cocaine | [26] | |
| Adjustable sensor based on the metallic resistor suspended on NAA membrane | [214] | |
| Templates | Reviews of NAA template-assisted fabrication | [47,215] |
| Fabrication of 2D Au nanodot arrays with tailorable geometric features for photocatalysis enhancement | [216] | |
| Nanostructured surface for photocatalysis | [217] | |
| Fabrication of Bi NWs for microelectronics | [218] | |
| Perowskite NWs with a tunable emission wavelength | [219] | |
| Electrodes for the electrochemical denitrification | [220] | |
| Metal-free coating for the broadband infrared absorption | [221] | |
| Polymer-brush structure confined in the NAA | [222] | |
| Segment Au-Ag nanowires for SERS detection | [223] | |
| Fabrication of Ni antidot arrays for spinotronic applications | [224] | |
| Review on NAA-derived electrochemical energy storage devices | [10] | |
| Co magnetic NWs | [225] | |
| Fe-Pd NWs for magnetic/catalytic spinoelectronics | [226] | |
| Ca2FeIn Hensler alloy NWs for spintronics application | [227] | |
| Review on NAA-molded stimuli-responsive polymer structures | [228] | |
| Membranes for filtering and separation | Tailor-engineering of narrow pore NAA membranes | [229] |
| Growing covalent organic frameworks on porous substrates for molecule-sieving membranes with pores tunable from ultra- to nanofiltration | [230] | |
| Mechanical properties of NAA membranes | [231,232] | |
| Photocatalytic membrane for water disinfection | [233] | |
| Steric-based removal of Coliform bacteria | [234] | |
| Removal of Cu2+ and Cd2+ with pH controlled permeability | [235] | |
| Removal of As from aqueous media | [236] | |
| Switchable hydrophobicity of the membrane for selective oil/water emulsion separation | [237] | |
| Efficient separation of hydrocarbons | [238] | |
| Gas dehumidification method with selective water vapor-permeable membrane | [239] | |
| Selective rejection of polar molecules | [240] | |
| The electric eel inspired structure for energy conversion | [241] | |
| Biological monitoring and cell culture | Reviews on NAA-based biosensors | [12,45] |
| Biomimetic nanochannels for enhanced biomolecule response | [242] | |
| Modulation of osteo-immune response of macrophages | [243] | |
| Structure with three-phase interface for gas exposure as a lung airway model | [244] | |
| NAA-based substrate for in situ monitoring of hormonal release from human cell culture | [245] | |
| Osteogenic differentiation induced with NAA morphology | [246] | |
| Pore size-dependent growth of N2a cells | [247] | |
| Control of cell adhesion with functional coating of collagen and fibronectin | [248] | |
| Drug delivery | Impact of NAA pore geometry on DOX sustained-release profile | [175] |
| pH sensitive NAA platform for sustained drug release | [43] | |
| Bone ex vivo evaluation of drug release from NAA surface-modified aluminum wire implants | [249] | |
| Proof-of-concept cancer therapy with nanotube-based drug delivery system targeting autophagic and endoplasmic reticulum stress | [162] | |
| In vivo nanotubes nanotoxicity study on murine model | [163] | |
| Drug delivery perspectives of NAA-derived materials review | [48] | |
| Functional layer for composites | Enhanced wear resistance through multiphase lubrication mechanism | [250] |
| Tartaric-sulfuric acid NAA as “green” alternative for chromic-NAA protective layers | [80] | |
| NAA coating for reduction of thermal resistance and junction temperature | [251] | |
| Daytime passive radiative cooling layer | [252] | |
| Lubricant infused structure with self-healing properties and enhanced corrosion resistance | [253] | |
| NAA casing to reduce Ni corrosion in brine | [254] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domagalski, J.T.; Xifre-Perez, E.; Marsal, L.F. Recent Advances in Nanoporous Anodic Alumina: Principles, Engineering, and Applications. Nanomaterials 2021, 11, 430. https://doi.org/10.3390/nano11020430
Domagalski JT, Xifre-Perez E, Marsal LF. Recent Advances in Nanoporous Anodic Alumina: Principles, Engineering, and Applications. Nanomaterials. 2021; 11(2):430. https://doi.org/10.3390/nano11020430
Chicago/Turabian StyleDomagalski, Jakub T., Elisabet Xifre-Perez, and Lluis F. Marsal. 2021. "Recent Advances in Nanoporous Anodic Alumina: Principles, Engineering, and Applications" Nanomaterials 11, no. 2: 430. https://doi.org/10.3390/nano11020430
APA StyleDomagalski, J. T., Xifre-Perez, E., & Marsal, L. F. (2021). Recent Advances in Nanoporous Anodic Alumina: Principles, Engineering, and Applications. Nanomaterials, 11(2), 430. https://doi.org/10.3390/nano11020430







