Direct Transesterification for Biodiesel Production and Testing the Engine for Performance and Emissions Run on Biodiesel-Diesel-Nano Blends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biodiesel Production by Direct Transesterification (DT)
2.2. The Nano-Additive: Graphene Oxide
2.3. Equipment List and Properties for Analysis
2.4. Engine Testing for Performance and Emissions
Uncertainty Analysis
3. Results and Discussion
3.1. Characterization of NOME and KOME
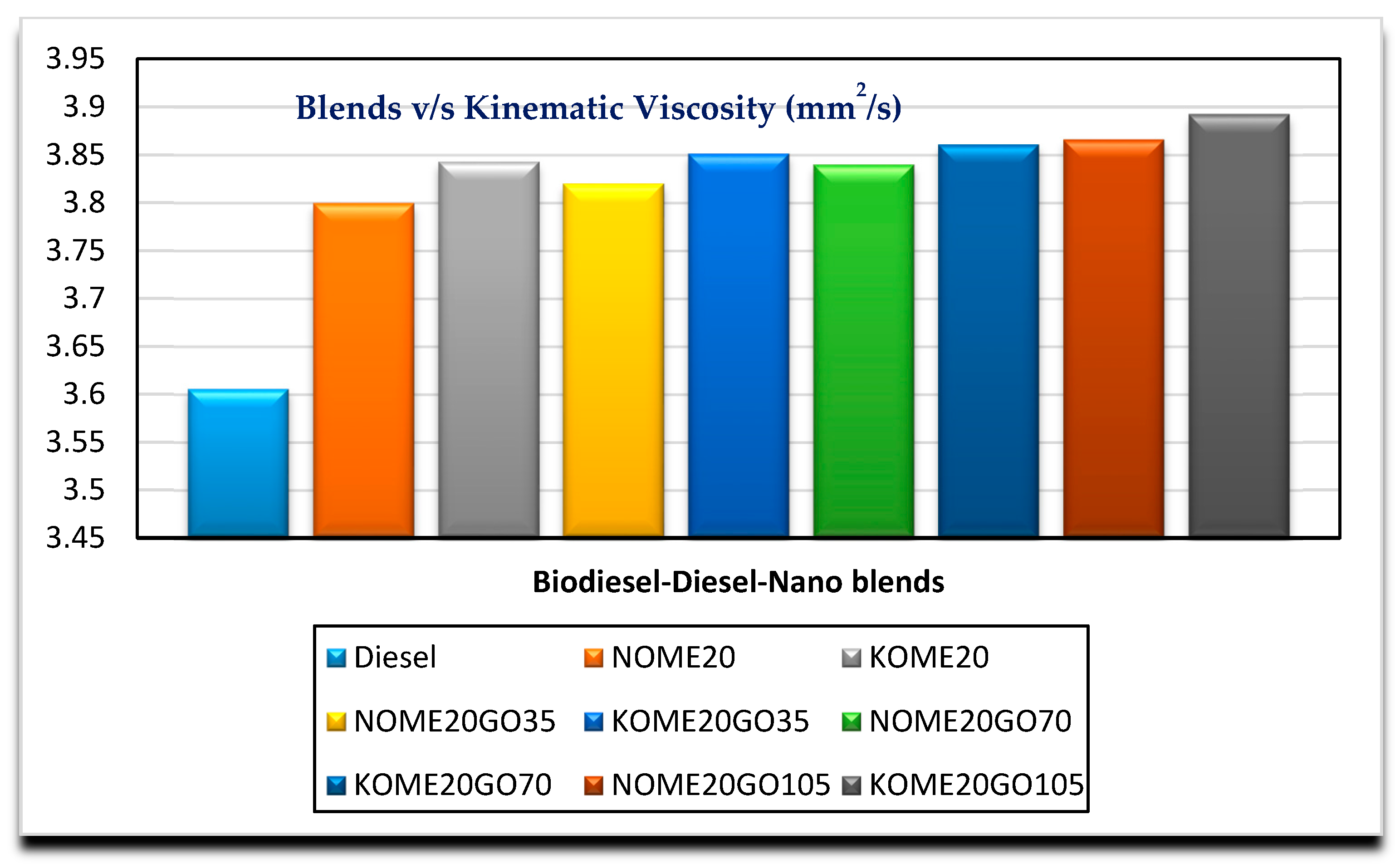
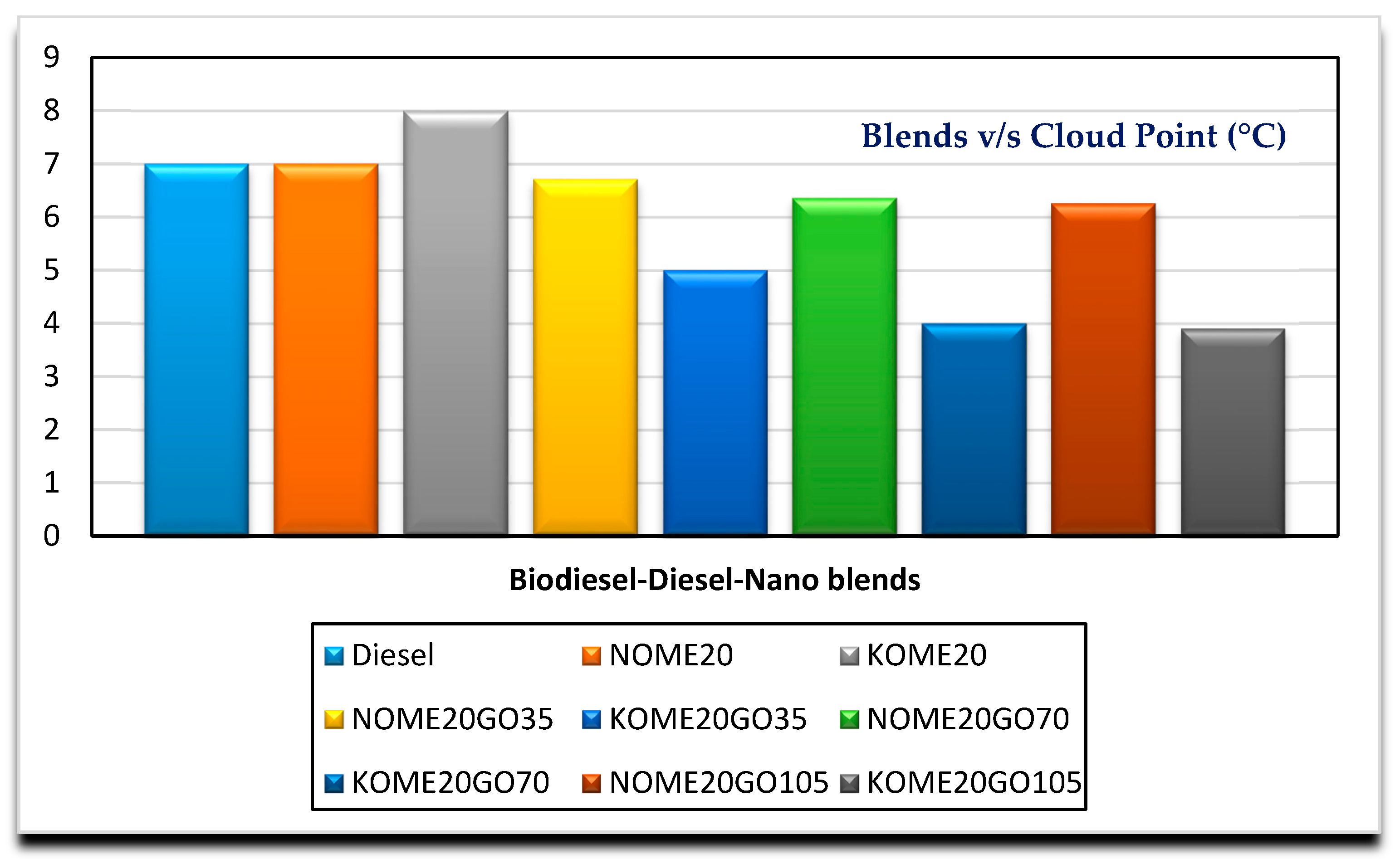
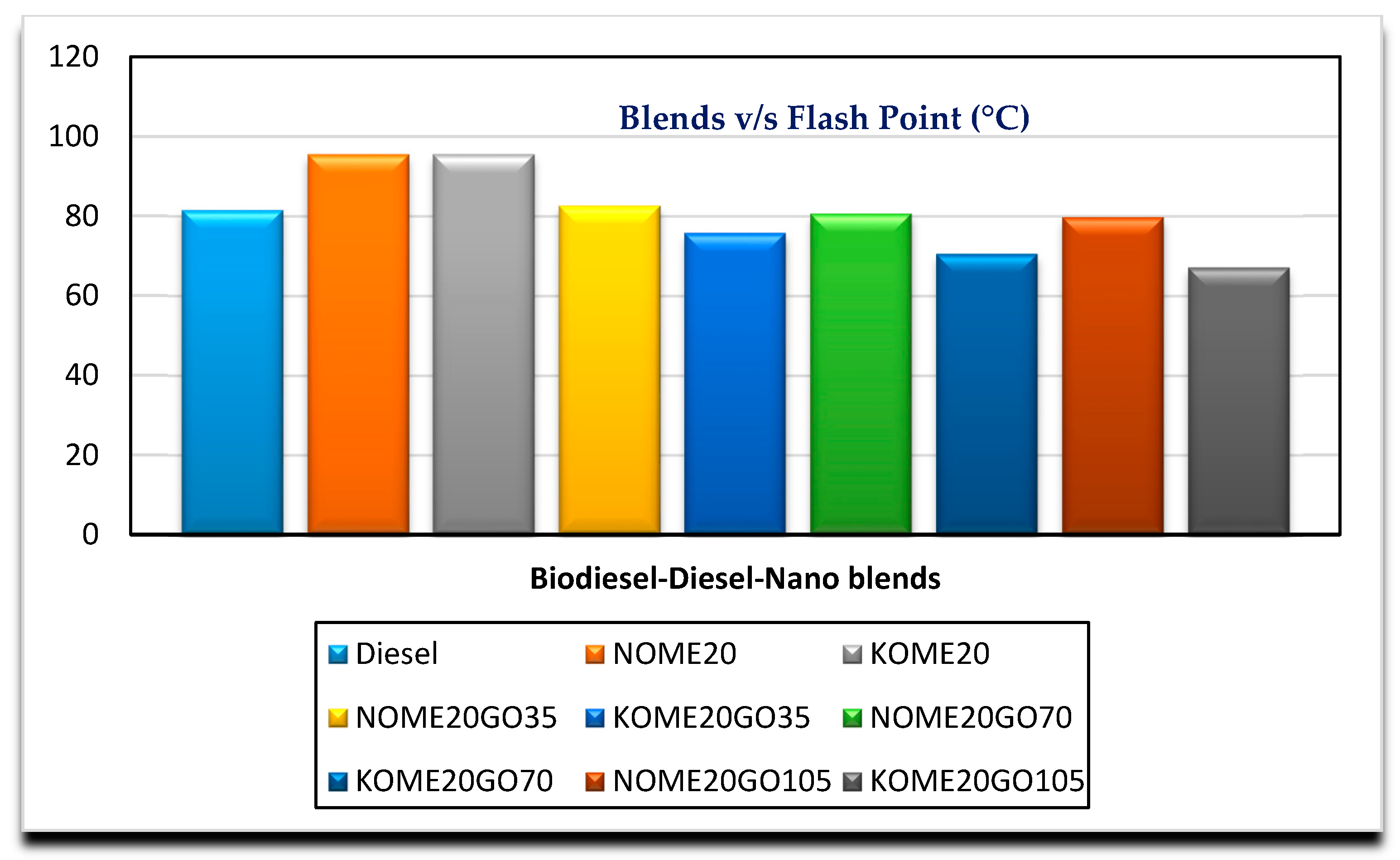
3.2. Effects of GO Nano on Fuel Properties
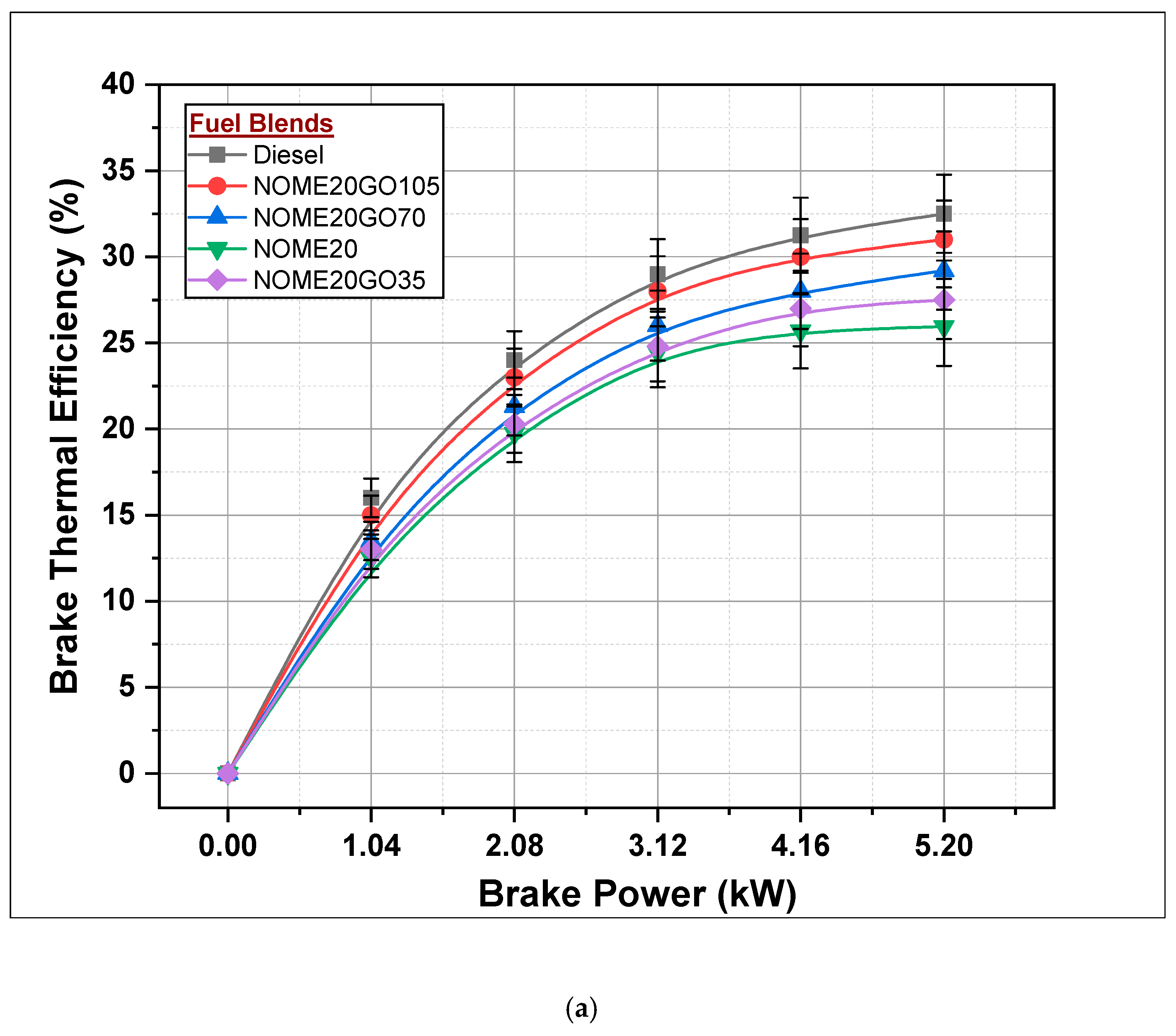
3.2.1. Brake Thermal Efficiency
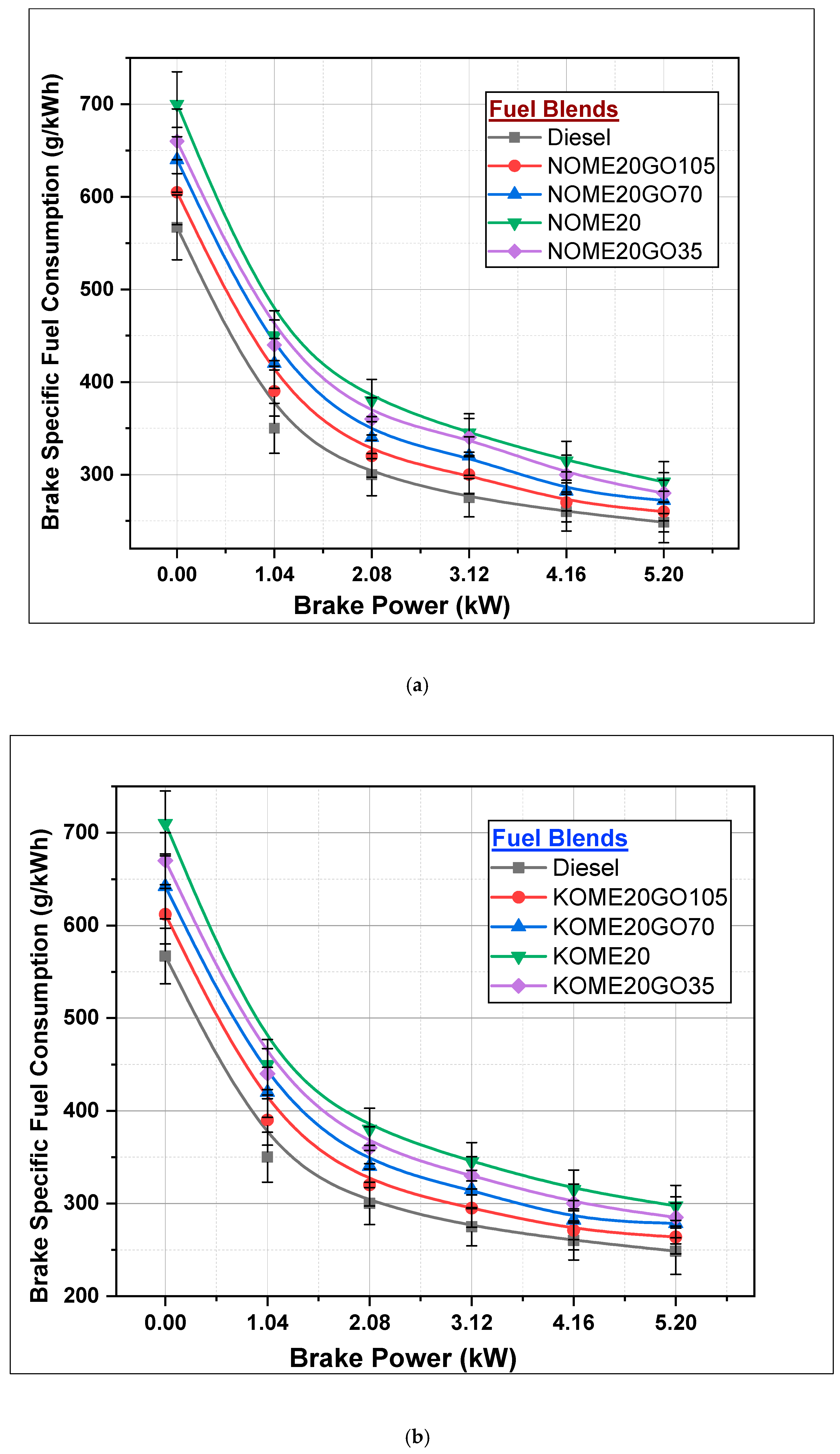
3.2.2. Brake-Specific Fuel Consumption
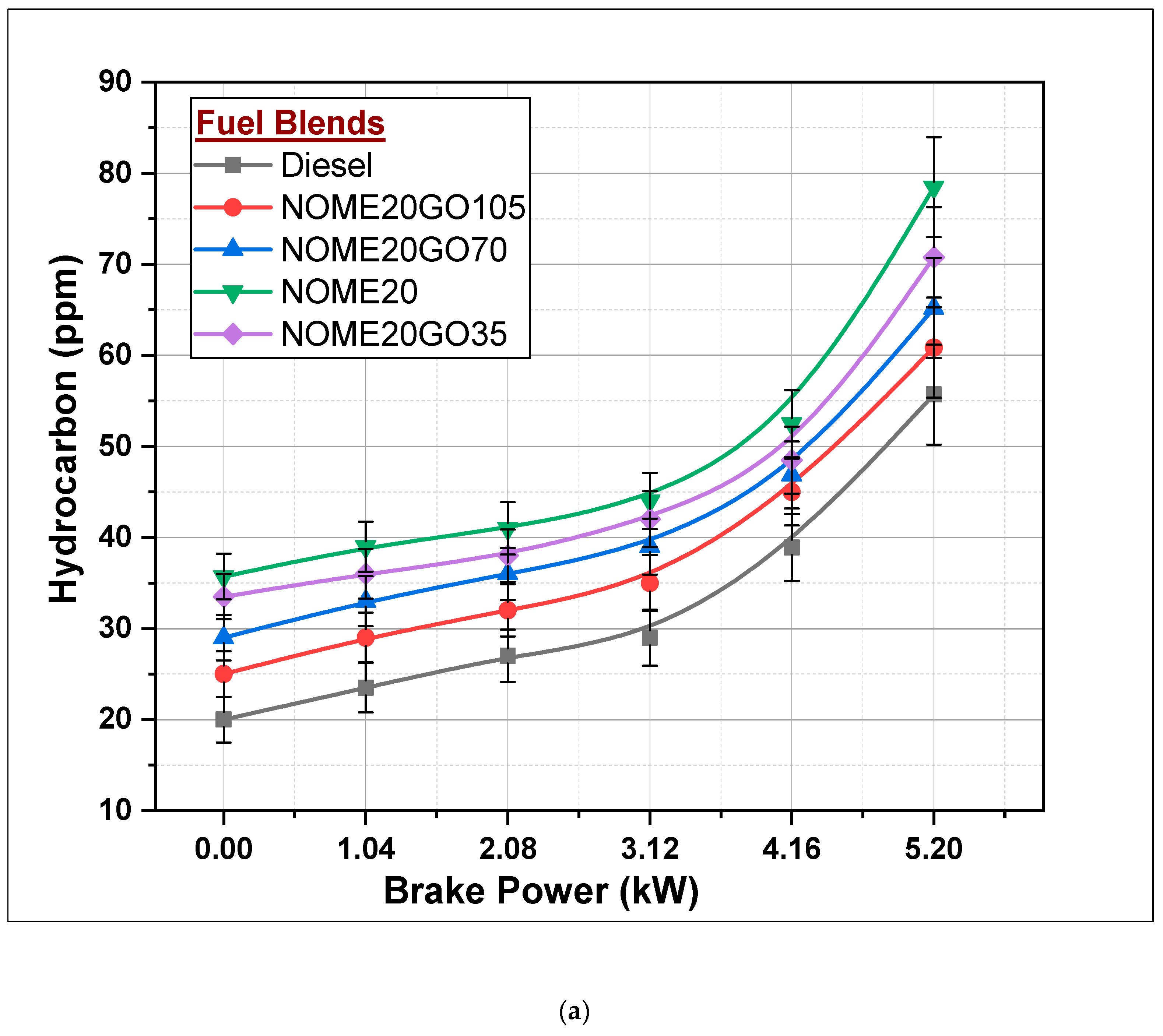
3.2.3. Hydrocarbons (HC)
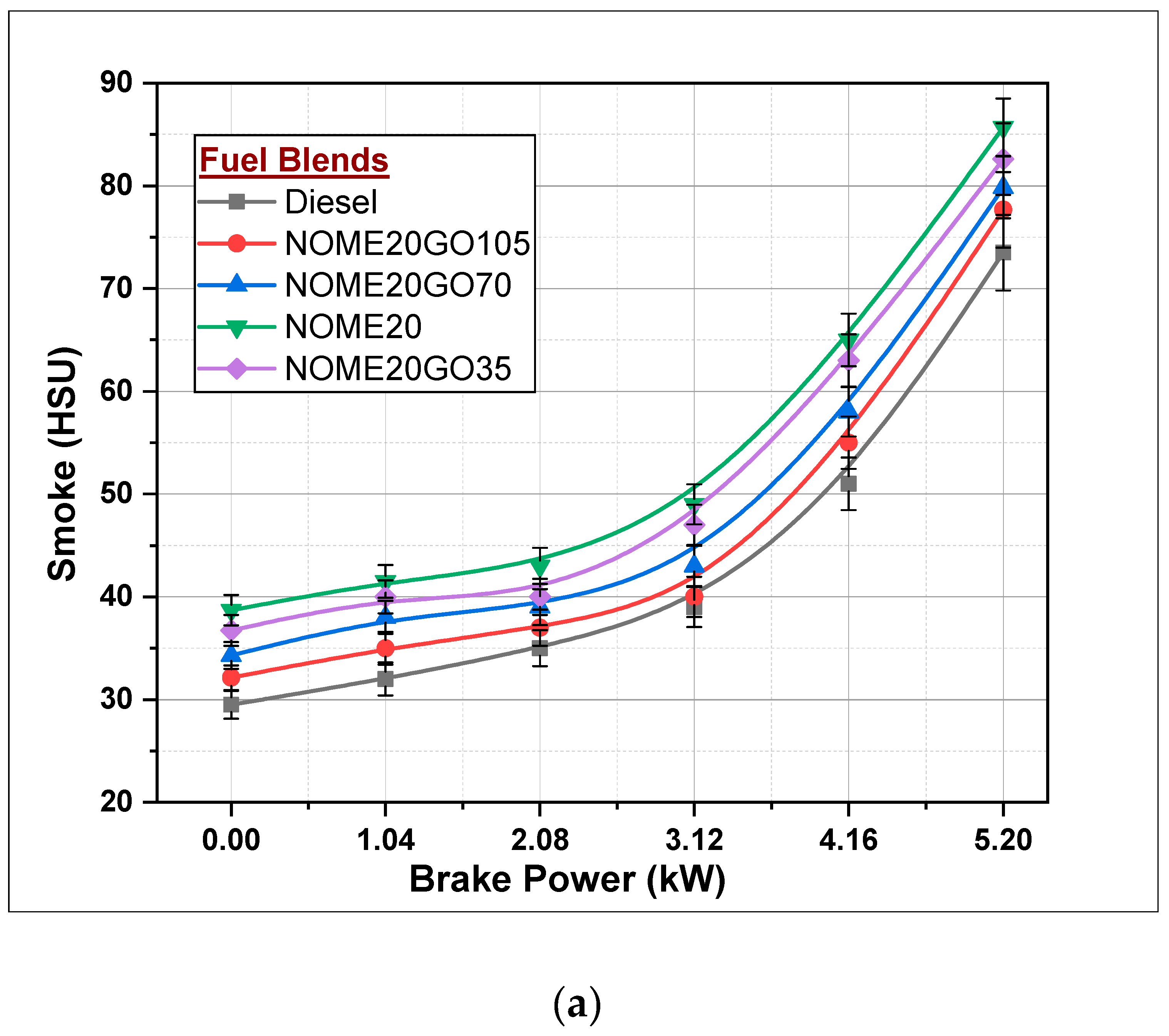
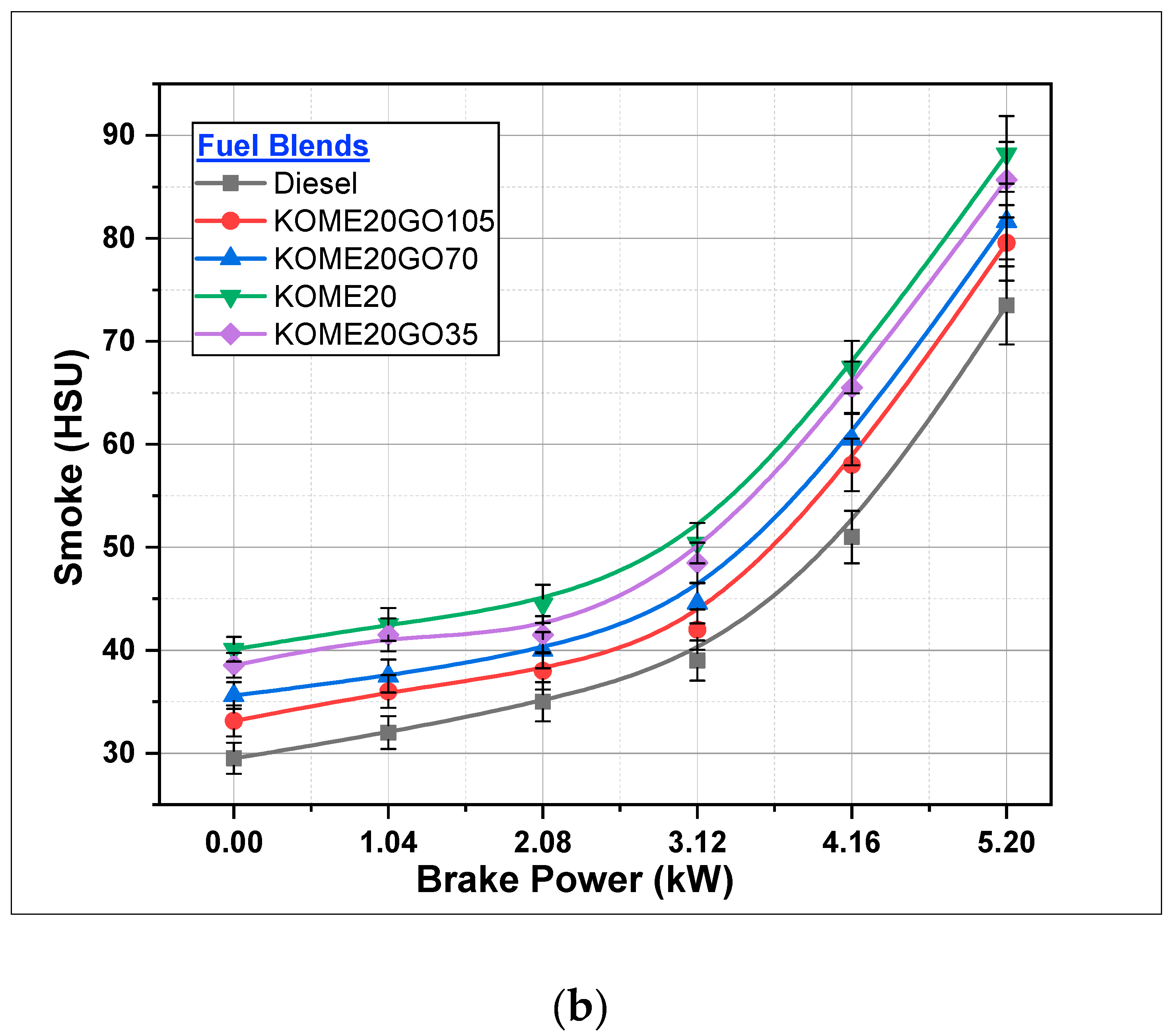
3.2.4. Smoke Formation
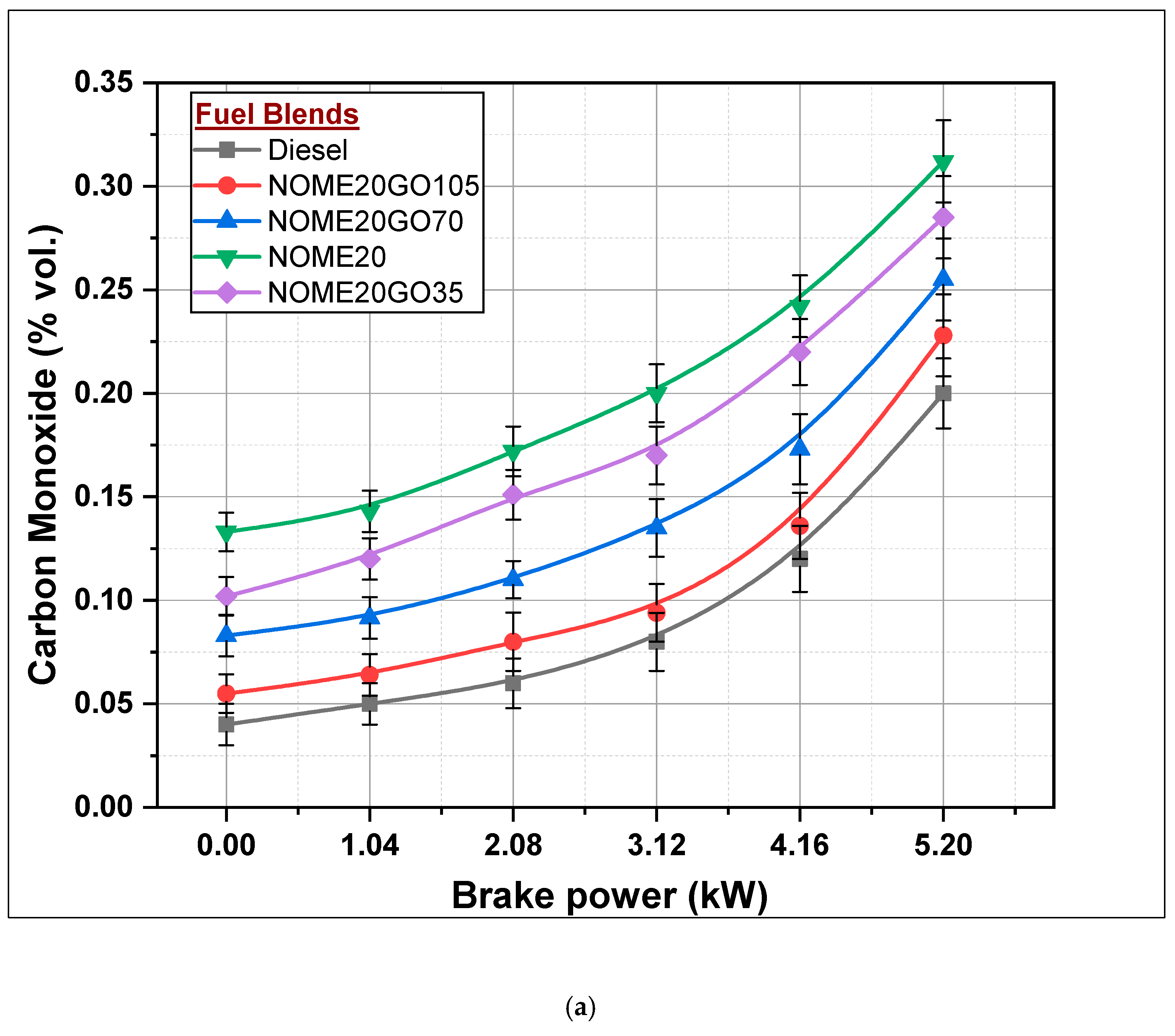
3.2.5. Carbon Monoxide (CO) Formation
4. Conclusions
- Biodiesel prepared by direct transesterification reduced the reaction timing.
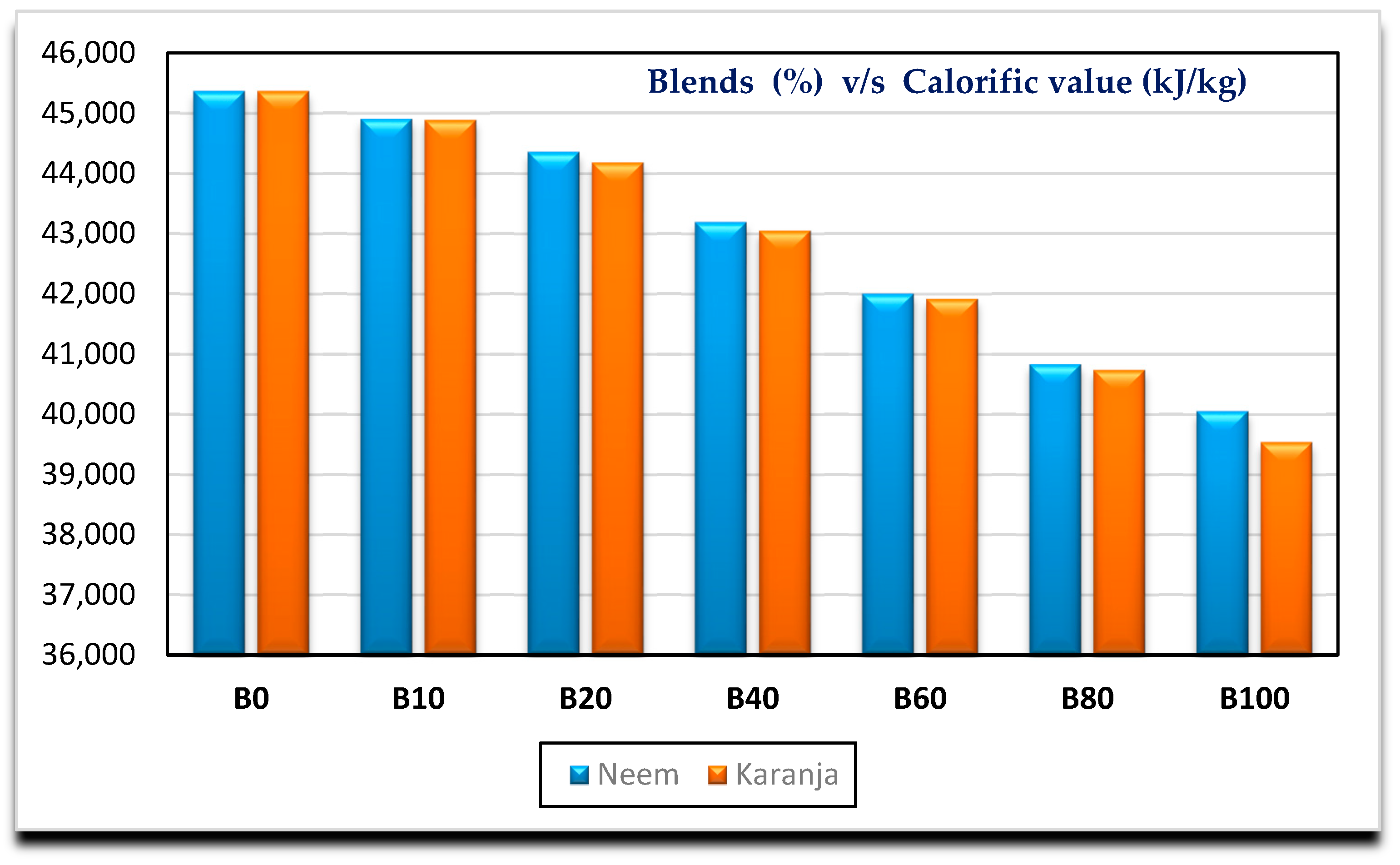
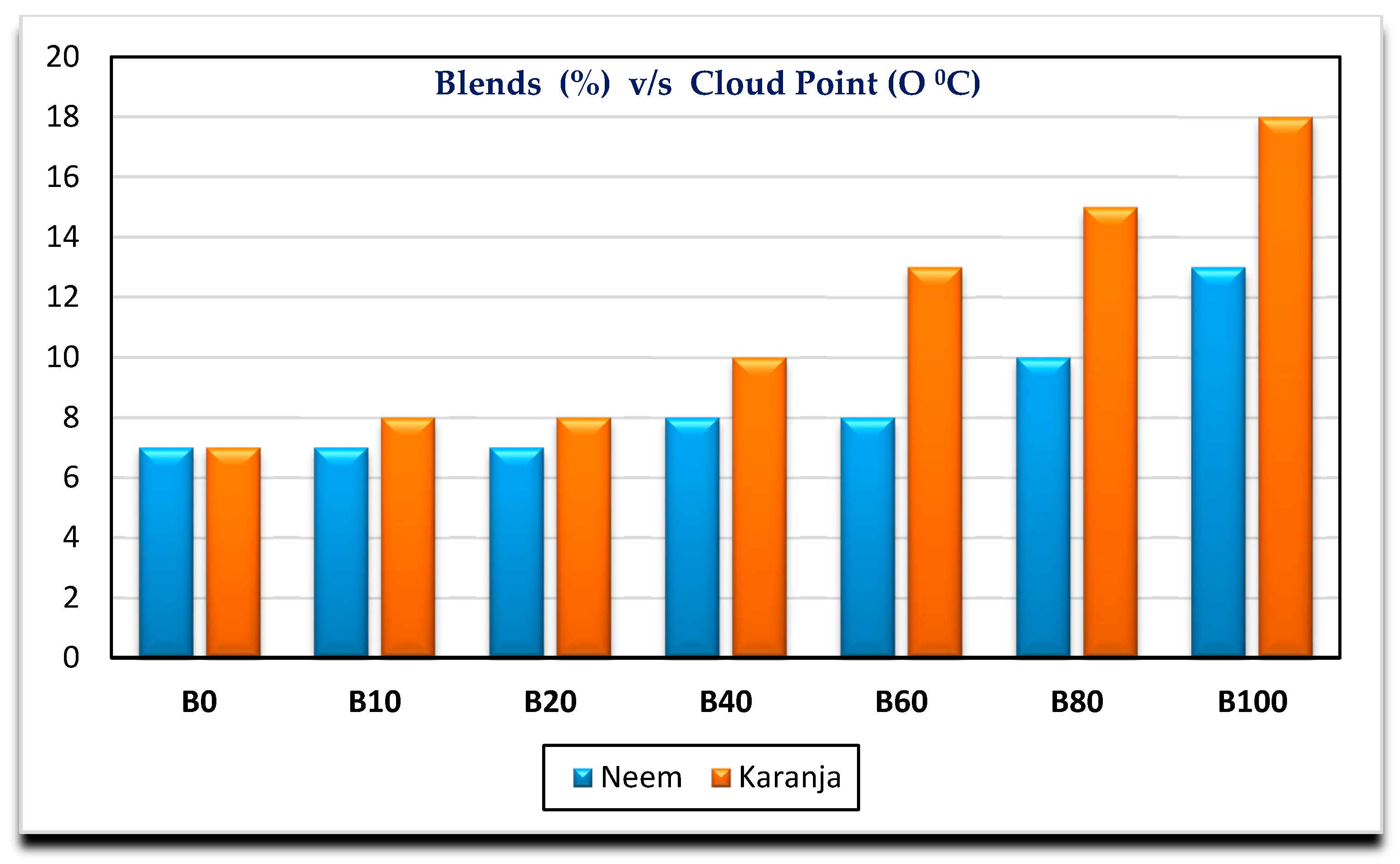
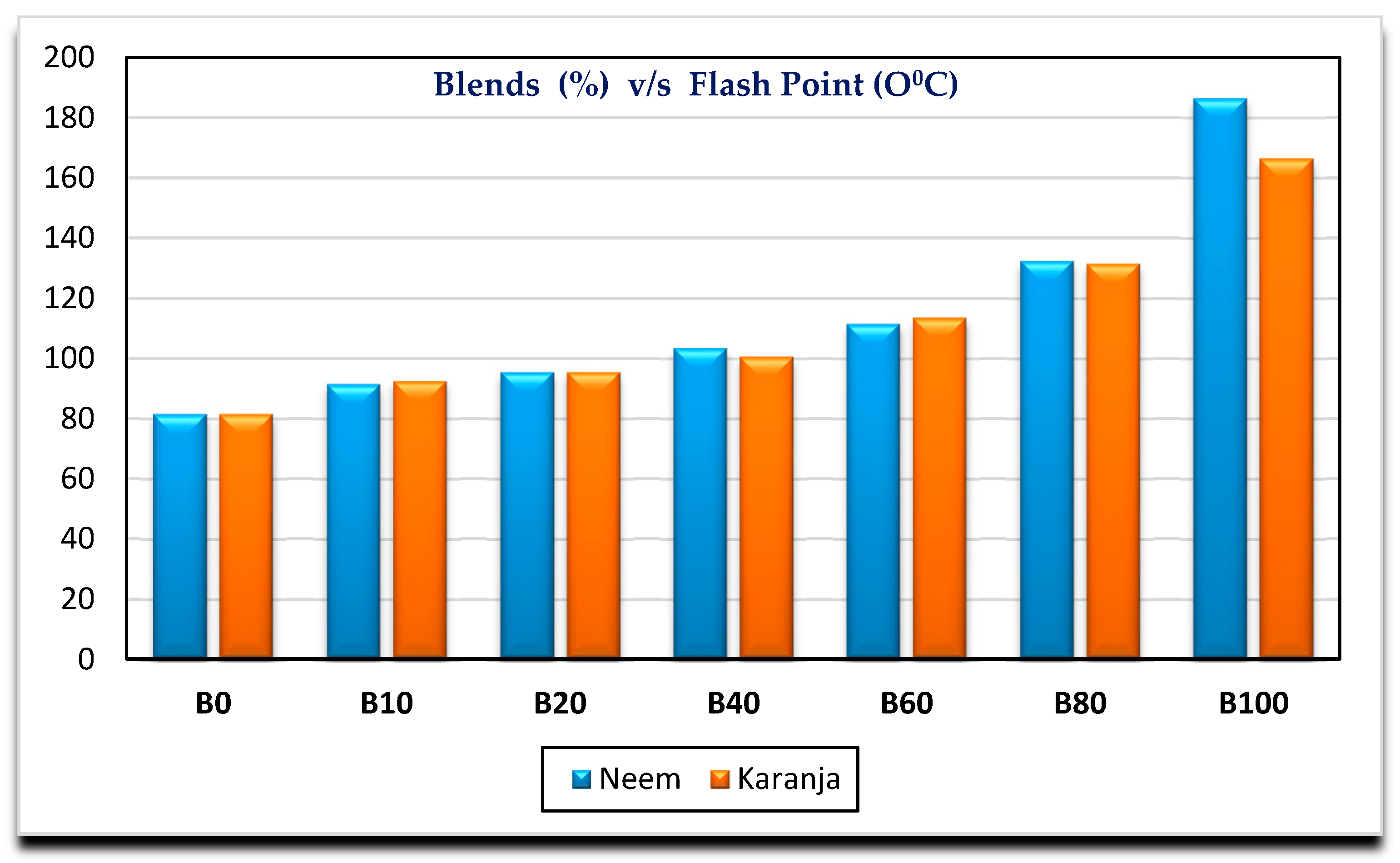
- Neem biodiesel is slightly better compared to Karanja in terms of calorific value, kinematic viscosity. There is a marginal difference in cloud point and flash point.
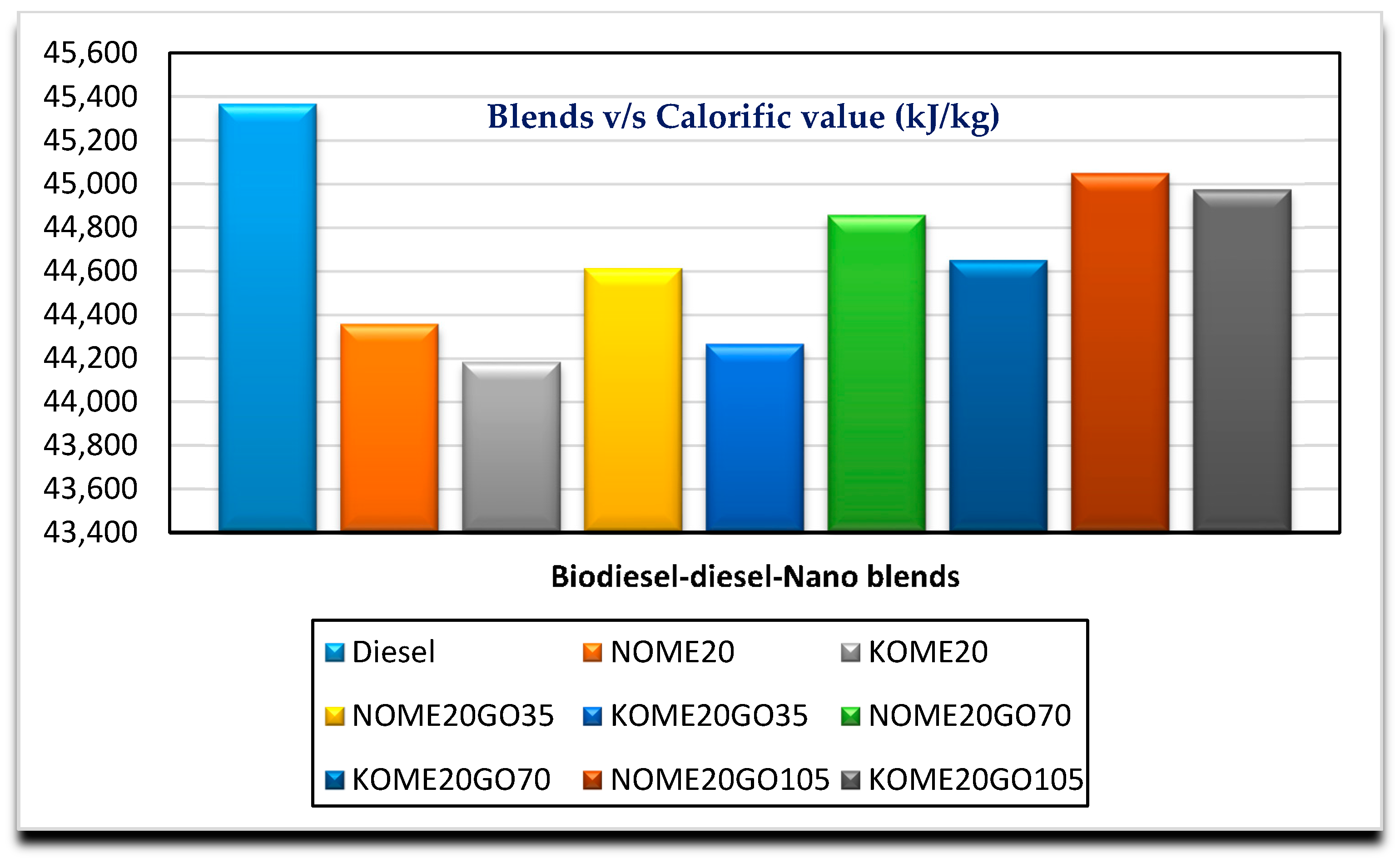
- The fuel properties of both blends improved with addition of higher dosages of graphene oxide nano particles.
- Maximum BTE of diesel, NOME20GO105 and KOMEGO105 is 32.5, 31 and 30.9%, respectively, at full load.
- BSFC for NOME20GO105 and KOME20GO105 was reduced by 10 and 11%, respectively, compared to NOME20 and KOME20.
- CO for NOME20GO105 and KOME20GO105 was reduced by 27 and 29%, respectively, compared to NOME20 and KOME20.
- BTE, BSFC, CO, HC and smoke were slightly better for the diesel-fueled engine compared to NOME20GO105 and KOME20GO10 run engines.
Funding
Acknowledgments
Conflicts of Interest
Future Recommendation
Nomenclature
| NOME | Neem Oil methyl ester |
| KOME | Karanja Oil methyl ester |
| DT | Direct transesterification |
| B0 | Diesel |
| B100 | Pure biodiesel |
| GO | Graphene oxide |
| NPs | Nano particles |
| NOME20 | Neem biodiesel 20% blended with 80% diesel |
| KOME20 | Karanja biodiesel 20% blended with 80% diesel |
| NOME20 GO35 | NOME20 with 35 ppm of GO NPs |
| NOME20GO70 | NOME20 with 70 ppm of GO NPs |
| NOME20GO105 | NOME20 with 105 ppm of GO NPs |
| KOME20GO35 | KOME20 with 35 ppm of GO NPs |
| KOME20GO70 | KOME20 with 70 ppm of GO NPs |
| KOME20GO105 | KOME20 with 105 ppm of GO NPs |
References
- Li, Y.; Du, Q.; Lu, X.; Wu, J.; Han, X. Relationship between the development and CO2 emissions of transport sector in China. Transp. Res. Part D Transp. Environ. 2019, 74, 1–14. [Google Scholar] [CrossRef]
- Thambiran, T.D.; Roseanne, D. Air pollution and climate change co-benefit opportunities in the road transportation sector in Durban, South Africa. Atmos. Environ. 2011, 45, 2683–2689. [Google Scholar] [CrossRef]
- WRI, C. Climate Analysis Indicators Tool: WRI’s Climate Data Explorer; World Resources Institute: Washington, DC, USA, 2014. [Google Scholar]
- Van Fan, Y.P.; Simon Klemeš, J.J.; Lee, C.T. A review on air emissions assessment: Transportation. J. Clean. Prod. 2018, 194, 673–684. [Google Scholar] [CrossRef]
- Available online: https://www.bp.com/en/global/corporate/energy-economics/energy-outlook/demand-by-sector/transport.html (accessed on 16 December 2020).
- Kalghatgi, G. Is it really the end of internal combustion engines and petroleum in transport? Appl. Energy 2018, 225, 965–974. [Google Scholar] [CrossRef]
- Mahlia, T.S.; Mofijur, M.; Abas, A.E.; Bilad, M.R.; Ong, H.C.; Silitonga, A.S. Patent landscape review on biodiesel production: Technology updates. Renew. Sustain. Energy Rev. 2020, 118, 109526. [Google Scholar] [CrossRef]
- D’Adamo, I.; Falcone, P.M.; Gastaldi, M.; Morone, P. RES-T trajectories and an integrated SWOT-AHP analysis for biomethane. Policy implications to support a green revolution in European transport. Energy Policy 2020, 138, 111220. [Google Scholar] [CrossRef]
- Foteinis, S.C.; Litinas, E.A.; Theocharis, T. Used-cooking-oil biodiesel: Life cycle assessment and comparison with first- and third-generation biofuel. Renew. Energy 2020, 153, 588–600. [Google Scholar] [CrossRef]
- Razzaq, L.F.; Mujtaba, M.A.; Sher, F.; Farhan, M.; Hassan, M.T.; Soudagar, M.E.M.; Atabani, A.E.; Kalam, M.A.; Imran, M. Modeling Viscosity and Density of Ethanol-Diesel-Biodiesel Ternary Blends for Sustainable Environment. Sustainability 2020, 12, 5186. [Google Scholar] [CrossRef]
- Ali, S.F.T.; Javed, F.; Hafeez, A.; Akhtar, M.; Haider, B.; Saifur Rehman, M.; Zimmerman, W.B.; Rehman, F. Investigating biodiesel production strategies as a sustainable energy resource for Pakistan. J. Clean. Prod. 2020, 259, 120729. [Google Scholar] [CrossRef]
- Khan, T.M.Y.; Atabani, A.B.; Anjum, I.; Badarudin, A.; Khayoon, M.S.; Triwahyono, S. Recent scenario and technologies to utilize non-edible oils for biodiesel production. Renew. Sustain. Energy Rev. 2014, 37, 840–851. [Google Scholar] [CrossRef]
- Deeba, F.K.; Arora, N.; Singh, S.; Kumar, A.; Han, S.S.; Negi, Y.S. Novel bio-based solid acid catalyst derived from waste yeast residue for biodiesel production. Renew. Energy 2020, 159, 127–139. [Google Scholar] [CrossRef]
- Mirnezami, S.A.Z.; Shayan, A.; Nejad, A. Thermal optimization of a novel solar/hydro/biomass hybrid renewable system for production of low-cost, high-yield, and environmental-friendly biodiesel. Energy 2020, 202, 117562. [Google Scholar] [CrossRef]
- Arumugam, A.; Pooja, S. Biodiesel production and parameter optimization: An approach to utilize residual ash from sugarcane leaf, a novel heterogeneous catalyst, from Calophyllum inophyllum oil. Renew. Energy 2020, 153, 1272–1282. [Google Scholar] [CrossRef]
- Shuit, S.H.; Huat, T.S. Esterification of palm fatty acid distillate with methanol via single-step pervaporation membrane reactor: A novel biodiesel production method. Energy Convers. Manag. 2019, 201, 112110. [Google Scholar] [CrossRef]
- Li, D.; Weifei, W.; Faiza, M.; Yang, B.; Wang, Y. A novel and highly efficient approach for the production of biodiesel from high-acid content waste cooking oil. Catal. Commun. 2017, 102, 76–80. [Google Scholar] [CrossRef]
- Negm, N.A.S.; Galal, H.; Yehia, F.Z.; Habib, O.I.; Mohamed, E.A. Biodiesel production from one-step heterogeneous catalyzed process of Castor oil and Jatropha oil using novel sulphonated phenyl silane montmorillonite catalyst. J. Mol. Liq. 2017, 234, 157–163. [Google Scholar] [CrossRef]
- Griffiths, M.V.H.; Harrison, S.T.L. Selection of direct transesterification as the preferred method for assay of fatty acid content of microalgae. Lipids 2010, 45, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.M.Y.; Badruddin, I.A.A.; Badarudin, R.F.; Hungund, B.S.; Ankalgi, F.R. Biodiesel production by direct transesterification process via sequential use of Acid–Base catalysis. Arab. J. Sci. Eng. 2018, 43, 5929–5936. [Google Scholar] [CrossRef]
- Soudagar, M.E.M. The effects of graphene oxide nanoparticle additive stably dispersed in dairy scum oil biodiesel-diesel fuel blend on CI engine: Performance, emission and combustion characteristics. Fuel 2019, 257, 116015. [Google Scholar] [CrossRef]
- Khond, V.W.K. Effect of nanofluid additives on performances and emissions of emulsified diesel and biodiesel fueled stationary CI engine: A comprehensive review. Renew. Sustain. Energy Rev. 2016, 59, 1338–1348. [Google Scholar] [CrossRef]
- Gardy, J.; Mohammad, R.; Hassanpour, A.; Lai, X.; Abdul-Sattar, N. Advances in nano-catalysts based biodiesel production from non-food feedstocks. J. Environ. Manag. 2019, 249, 109316. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Nik-Nazri, N.-G.; Kalam, A.; Badruddin, I.A.; Banapurmath, N.R.; Akram, N. The effect of nano-additives in diesel-biodiesel fuel blends: A comprehensive review on stability, engine performance and emission characteristics. Energy Convers. Manag. 2018, 178, 146–177. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Banapurmath, N.R.; Afzal, A.; Hossain, N.; Muhammad, M.A.; Hanifa, M.A.C.M.; Naik, B.; Ahmed, W.; Nizamuddin, S.; Mubarak, N.M. Study of diesel engine characteristics by adding nanosized zinc oxide and diethyl ether additives in Mahua biodiesel-diesel fuel blend. Sci. Rep. 2020, 10, 15326. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Afzal, A.; Mohammad, R.S.; Manokar, A.M.; Ahmed, I.; Mujtaba, M.A.; Olusegun, D.S.; Irfan, A.B.; Ahmed, W.; Kiran, S.; et al. Investigation on the efect of cottonseed oil blended with different percentages of octanol and suspended MWCNT nanoparticles on diesel engine characteristics. J. Therm. Anal. Calorim. 2020. [Google Scholar] [CrossRef]
- Ramadhas, A.S.; Simon, J.; Muraleedharan, C. Biodiesel production from high FFA rubber seed oil. Fuel 2005, 84, 335–340. [Google Scholar] [CrossRef]
- Ong, H.M.; Masjuki, T.M.I.; Norhasyima, H.H. Comparison of palm oil, Jatropha curcas and Calophyllum inophyllum for biodiesel: A review. Renew. Sustain. Energy Rev. 2011, 15, 3501–3515. [Google Scholar] [CrossRef]
- Sanjid, A.M.; Kalam, H.H.; Rahman, M.A.; Ashrafur, S.M.; Abedin, M.J.; Palash, S.M. Production of palm and jatropha based biodiesel and investigation of palm-jatropha combined blend properties, performance, exhaust emission and noise in an unmodified diesel engine. J. Clean. Prod. 2014, 65, 295–303. [Google Scholar] [CrossRef]
- Sia, C.B.; Jibrail, K.; Tan, Y.H.; Lee, K.T. Evaluation on biodiesel cold flow properties, oxidative stability and enhancement strategies: A review. Biocatal. Agric. Biotechnol. 2020, 24, 101514. [Google Scholar] [CrossRef]
- Sajith, V.S.; Peterson, G.P. Experimental investigations on the effects of cerium oxide nanoparticle fuel additives on biodiesel. Adv. Mech. Eng. 2010, 2, 581407. [Google Scholar] [CrossRef]
- Venu, H.; Prabhu, A. Al2O3 nano additives blended Polanga biodiesel as a potential alternative fuel for existing unmodified DI diesel engine. Fuel 2020, 279, 118518. [Google Scholar] [CrossRef]
- Kannan, G.; Ramasamy, K.; Anand, R. Effect of metal based additive on performance emission and combustion characteristics of diesel engine fuelled with biodiesel. Appl. Energy 2011, 88, 3694–3703. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Ahmad, T.-A.; Ghobadian, B.; Abbaszadeh-Mayvan, A. Performance and emission characteristics of a CI engine fuelled with carbon nanotubes and diesel-biodiesel blends. Renew. Energy 2017, 111, 201–213. [Google Scholar] [CrossRef]
- Gumus, S.; Hakan, O.; Ozbey, M.; Topaloglu, B. Aluminum oxide and copper oxide nanodiesel fuel properties and usage in a compression ignition engine. Fuel 2016, 163, 80–87. [Google Scholar] [CrossRef]
- Keskin, A.; Metin, G.; Altıparmak, D. Influence of tall oil biodiesel with Mg and Mo based fuel additives on diesel engine performance and emission. Bioresour. Technol. 2008, 99, 6434–6438. [Google Scholar] [CrossRef] [PubMed]
- Guru, M.; Ugur, K.; Altıparmak, D.; Alıcılar, A. Improvement of diesel fuel properties by using additives. Energy Convers. Manag. 2002, 43, 1021–1025. [Google Scholar] [CrossRef]
- El-Seesy, A.I.; Hamdy, H.; Ookawara, S. Effects of graphene nanoplatelet addition to jatropha Biodiesel-diesel mixture on the performance and emission characteristics of a diesel engine. Energy 2018, 147, 1129–1152. [Google Scholar] [CrossRef]
- Mehregan, M.M. Mohammad, Effects of nano-additives on pollutants emission and engine performance in a urea-SCR equipped diesel engine fueled with blended-biodiesel. Fuel 2018, 222, 402–406. [Google Scholar] [CrossRef]
- Gad, M.S.J.S. A comparative study on the effect of nano-additives on the performance and emissions of a diesel engine run on Jatropha biodiesel. Fuel 2020, 267, 117168. [Google Scholar] [CrossRef]
- Perumal, V.I.M. The influence of copper oxide nano particle added pongamia methyl ester biodiesel on the performance, combustion and emission of a diesel engine. Fuel 2018, 232, 791–802. [Google Scholar] [CrossRef]
- Ramesh, D.K.; Dhananjaya, J.L.; Kumar, S.G.H.; Namith, V.; Jambagi, P.B.; Sharath, S. Study on effects of alumina nanoparticles as additive with poultry litter biodiesel on performance, combustion and emission characteristic of diesel engine. Mater. Today Proc. 2018, 5, 1114–1120. [Google Scholar] [CrossRef]
- Selvan, V.A.M.A.; Udayakumar, M. Effects of cerium oxide nanoparticle addition in diesel and diesel-biodiesel-ethanol blends on the performance and emission characteristics of a CI engine. J. Eng. Appl. Sci. 2009, 4, 1819–6608. [Google Scholar]
- Raju, V.D.K.; Nanthagopal, K.; Ashok, B. An experimental study on the effect of nanoparticles with novel tamarind seed methyl ester for diesel engine applications. Energy Convers. Manag. 2018, 164, 655–666. [Google Scholar] [CrossRef]
- Kumar, S.D.P.; Bran, I. Experimental investigation of the effects of nanoparticles as an additive in diesel and biodiesel fuelled engines: A review. Biofuels 2019, 10, 615–622. [Google Scholar] [CrossRef]





| No | Property | Equipment | Manufacturer |
|---|---|---|---|
| 1 | Calorific value | C2000 basic calorimeter—automatic | (IKA, UK) |
| 2 | Kinematic viscosity | SVM 3000 automatic | (Anton Paar, London, UK) |
| 3 | Density | SVM 3000—automatic | (Anton Paar, London, UK) |
| 4 | Cloud and Pour point | Cloud and Pour point tester—automaticNTE 450 | (Normalab, Valliquerville, France) |
| 5 | Cold Filter Plug in Point | Cold filter plugging point—automatic NTL 450 | (Normalab, Valliquerville, France) |
| 6 | Flash Point | Pensky-martens flash point automatic NPM 440 | (Normalab, Valliquerville, France) |
| Type | Single Cylinder, Four Stroke, Variable Compression Ratio Diesel Engine |
|---|---|
| Bore | 87.5 mm |
| Stroke | 110 mm |
| Compression ratio | 17.5:1 |
| Maximum power | 5.2 kW |
| Maximum speed | 1500 rpm |
| Injection system | Direct injection |
| Start of injection | 23° before TDC |
| Injection pressure | 205 bar |
| Cooling system | Water cooled |
| Parameters | Accuracy (±) | Uncertainty (%) |
|---|---|---|
| CO emission (%) | ±0.01% | ±0.15 |
| HC emission (ppm) | ±10 ppm | ±0.12 |
| Smoke meter (HSU) | ±1 | ±0.15 |
| Brake thermal efficiency (%) | - | ±0.20 |
| Brake-specific fuel consumption (g/kW-h) | - | ±0.15 |
| B0 | B10 | B20 | B40 | B60 | B80 | B100 | |
|---|---|---|---|---|---|---|---|
| Calorific value (kJ/kg) | 45,369 | 44,909 | 44,357 | 43,191 | 42,003 | 40,833 | 40,050 |
| Kinematic viscosity (mm2/s) at 40 °C | 3.6056 | 3.7028 | 3.7997 | 4.024 | 4.2807 | 4.556 | 4.8489 |
| Density (kg/m3) at 15 °C | 851.8 | 854.6 | 857.6 | 863.4 | 869.2 | 875.4 | 880.9 |
| Specific gravity at 15 °C | 0.8526 | 0.8554 | 0.8584 | 0.8642 | 0.8700 | 0.8762 | 0.8817 |
| Cloud point (°C) | 7 | 7 | 7 | 8 | 8 | 10 | 13 |
| Pour point (°C) | 2 | 2 | 5 | 8 | 8 | 11 | 14 |
| CFPP (°C) | 0 | 5 | 4 | 4 | 5 | 8 | 12 |
| Flash point (°C) | 81.5 | 91.5 | 95.5 | 103.5 | 111.5 | 132.5 | 186.5 |
| B0 | B10 | B20 | B40 | B60 | B80 | B100 | |
|---|---|---|---|---|---|---|---|
| Calorific value (kJ/kg) | 45,369 | 44,888 | 44,183 | 43,052 | 41,920 | 40,737 | 39,538 |
| Kinematic viscosity (mm2/s) at 40 °C | 3.6056 | 3.7380 | 3.8430 | 4.0615 | 4.3480 | 4.6745 | 5.0901 |
| Density (kg/m3) at 15 °C | 851.8 | 855.6 | 859.6 | 867.4 | 876.0 | 884.10 | 891.7 |
| Specific gravity at 15 °C | 0.8526 | 0.8564 | 0.8604 | 0.8682 | 0.8768 | 0.8849 | 0.8935 |
| Cloud point (°C) | 7 | 8 | 8 | 10 | 13 | 15 | 18 |
| Pour point (°C) | 2 | 5 | 5 | 8 | 8 | 8 | 11 |
| CFPP (°C) | 0 | 6 | 8 | 10 | 12 | 13 | 16 |
| Flash point (°C) | 81.5 | 92.5 | 95.5 | 100.5 | 113.5 | 131.5 | 166.5 |
| ASTM D6751 | NOME20 | NOME20 GO35 | NOME20 GO70 | NOME20 GO105 | ASTM Test Limit | |
|---|---|---|---|---|---|---|
| Calorific value (kJ/kg) | D5865 | 44,357 | 44,614 | 44,858 | 45,050 | Min. 35,000 |
| Kinematic viscosity (mm2/s) at 40 °C | D445 | 3.7997 | 3.82 | 3.84 | 3.866 | 1.9–6 |
| Density (kg/m3) at 15 °C | D4052 | 857.6 | 858.12 | 861.44 | 868.3 | 860–900 |
| Specific gravity at 15 °C | D891 | 0.8584 | 0.867 | 0.88 | 0.894 | 0.87–0.90 |
| Cloud point (°C) | D2500-11 | 7 | 6.71 | 6.636 | 6.26 | −3 to 12 |
| Pour point (°C) | D97-12 | 5 | 4.12 | 3.86 | 3.794 | −15 to 16 |
| CFPP (°C) | D6371 | 4 | 2.67 | 2.15 | 1.92 | -- |
| Flash point (°C) | D93 | 95.5 | 82.62 | 80.66 | 79.74 | Min. 93 |
| ASTM D6751 | KOME20 | KOME20 GO35 | KOME20 GO70 | KOME20 GO105 | ASTM Test Limit | |
|---|---|---|---|---|---|---|
| Calorific value (kJ/kg) | D5865 | 44,183 | 44,266 | 44,650 | 44,975 | Min. 35,000 |
| Kinematic viscosity (mm2/s) at 40 °C | D445 | 3.8430 | 3.8512 | 3.861 | 3.8924 | 1.9–6 |
| Density (kg/m3) at 15 °C | D4052 | 856.6 | 858.11 | 858.38 | 859.0 | 860–900 |
| Specific gravity at 15 °C | D891 | 0.8604 | 0.871 | 0.882 | 0.891 | 0.87–0.90 |
| Cloud point (°C) | D2500-11 | 8 | 5 | 4 | 3.9 | −3 to 12 |
| Pour point (°C) | D97-12 | 5 | 3.89 | 4.15 | 4.61 | −15 to 16 |
| CFPP (°C) | D6371 | 8 | 6.8 | 6.1 | 6 | -- |
| Flash point (°C) | D93 | 95.5 | 75.8 | 70.5 | 67.1 | Min. 93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, T.M.Y. Direct Transesterification for Biodiesel Production and Testing the Engine for Performance and Emissions Run on Biodiesel-Diesel-Nano Blends. Nanomaterials 2021, 11, 417. https://doi.org/10.3390/nano11020417
Khan TMY. Direct Transesterification for Biodiesel Production and Testing the Engine for Performance and Emissions Run on Biodiesel-Diesel-Nano Blends. Nanomaterials. 2021; 11(2):417. https://doi.org/10.3390/nano11020417
Chicago/Turabian StyleKhan, T. M. Yunus. 2021. "Direct Transesterification for Biodiesel Production and Testing the Engine for Performance and Emissions Run on Biodiesel-Diesel-Nano Blends" Nanomaterials 11, no. 2: 417. https://doi.org/10.3390/nano11020417
APA StyleKhan, T. M. Y. (2021). Direct Transesterification for Biodiesel Production and Testing the Engine for Performance and Emissions Run on Biodiesel-Diesel-Nano Blends. Nanomaterials, 11(2), 417. https://doi.org/10.3390/nano11020417






