Graphene-Based Scaffolds for Regenerative Medicine
Abstract
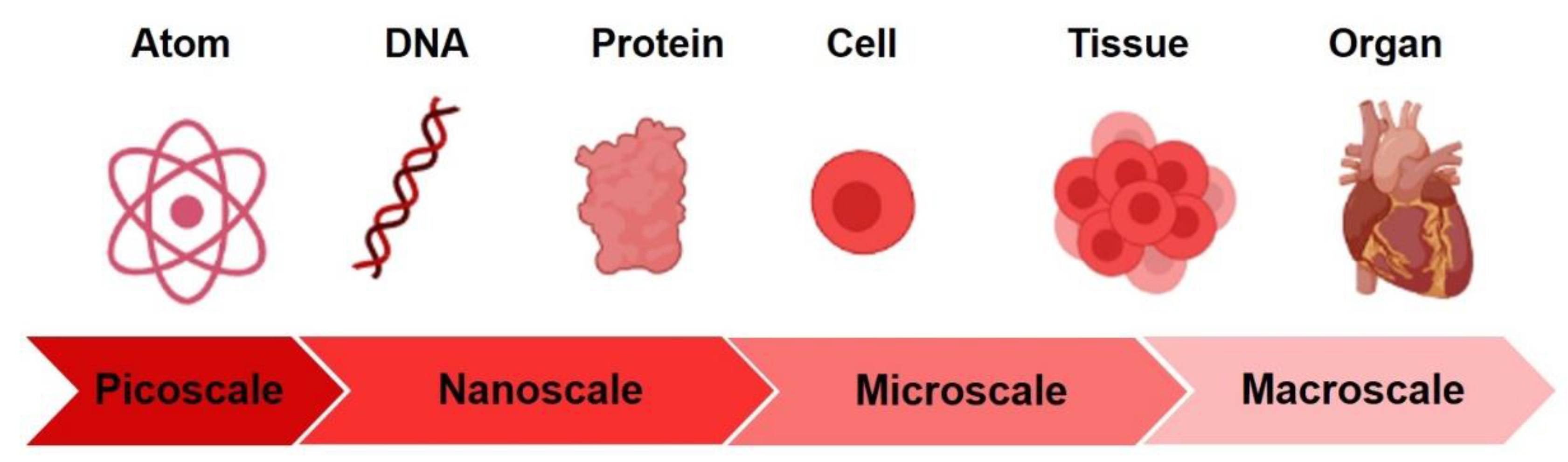
1. Introduction
2. Graphene-Based Scaffolds
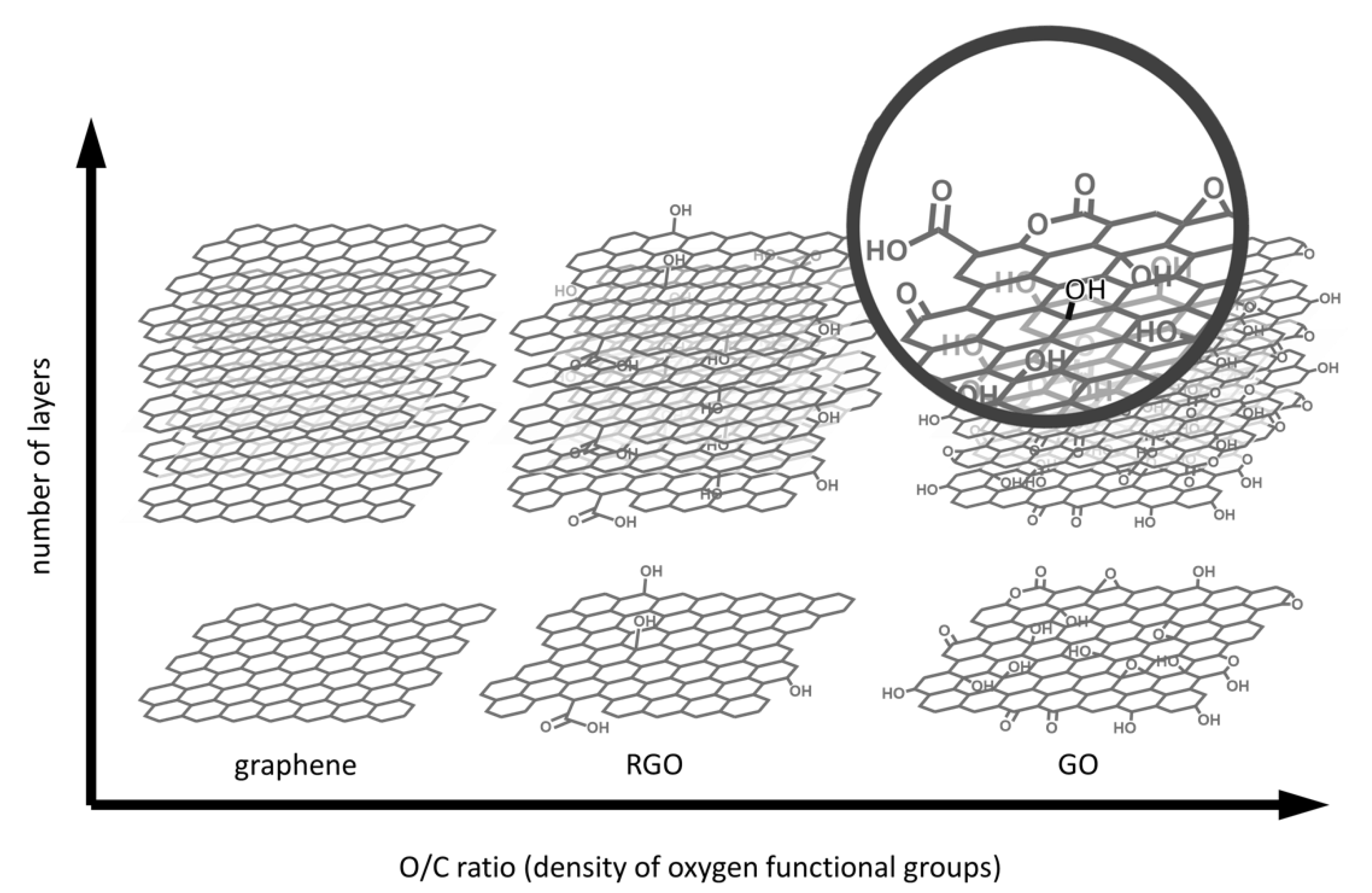
2.1. Methods for GBM Synthesis
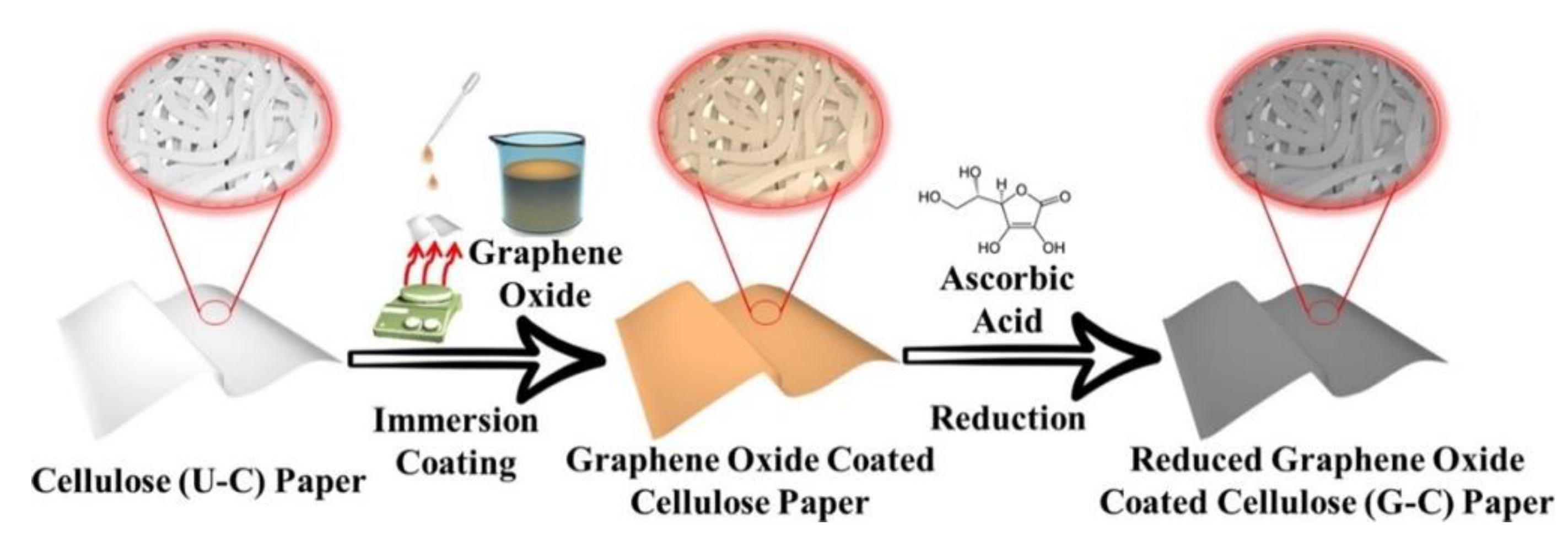
2.2. Two-Dimensional Scaffolds
2.3. Three-Dimensional Scaffolds
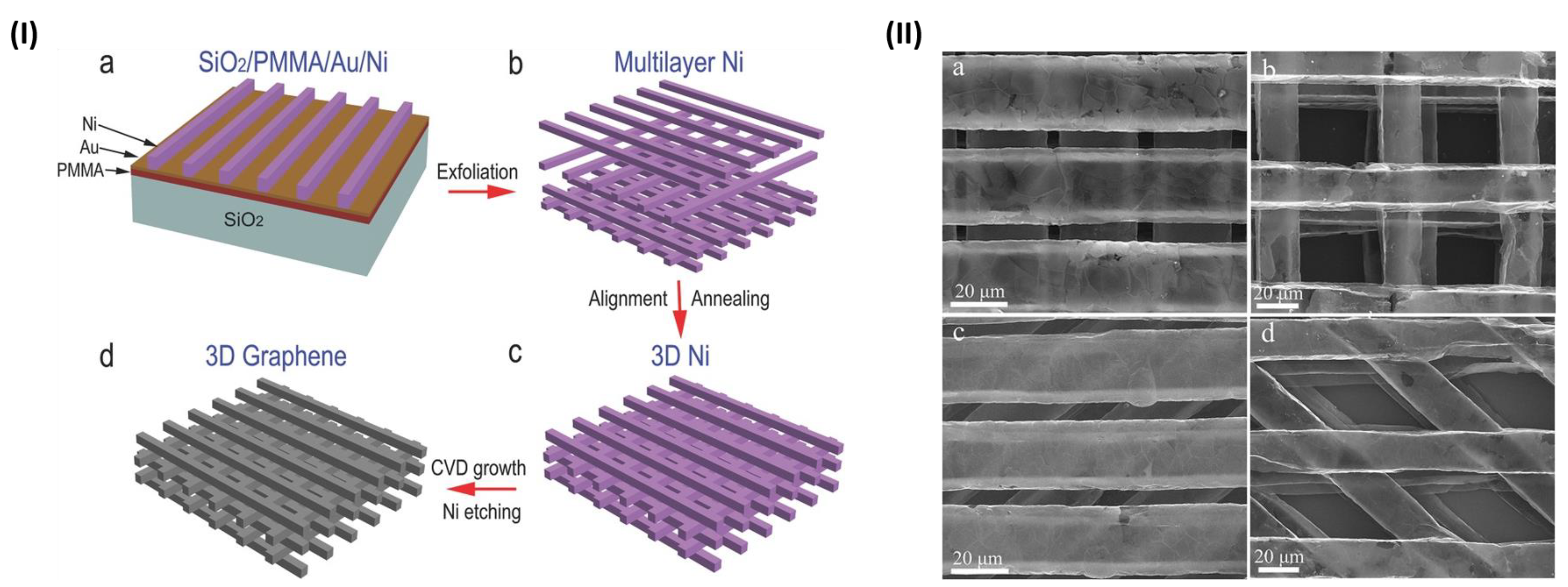
2.3.1. Foams
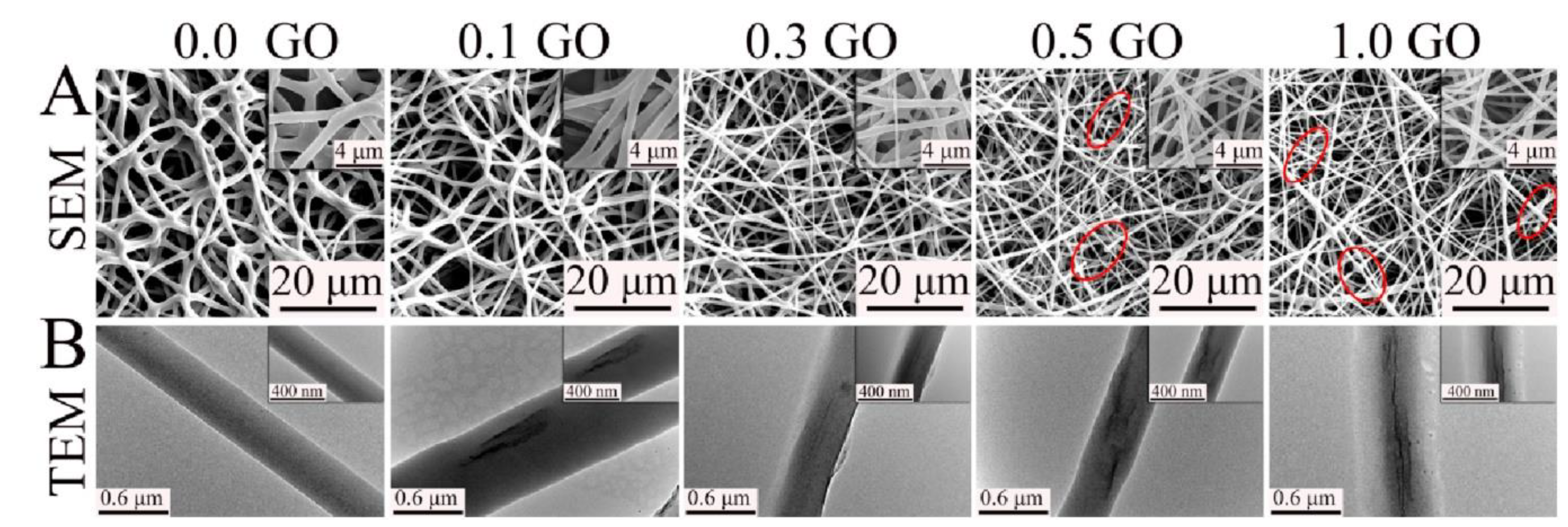
2.3.2. Electrospun Fibers
2.3.3. Hydrogels
3. Stem Cell Differentiation and Mechano-Transduction
3.1. Tissue Engineering and Stem Cells
3.2. Cues Controlling Stem Cell Behavior
3.3. The Importance of the “Nanoscale”
3.4. Role of the Biomolecular Corona
4. Nanotoxicology and Functionalization
4.1. In Vitro Cytotoxicity
4.2. Hemocompatibility and Interaction with Immune System Cells
4.3. In Vivo Biocompatibility
4.4. Minimizing GBM Toxicity
5. Examples of Tissue Regeneration
5.1. Bone Regeneration
5.2. Muscle Regeneration
5.3. Nerve Regeneration
5.4. Wound Healing
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Patel, K.D.; Singh, R.K.; Kim, H.-W. Carbon-based nanomaterials as an emerging platform for theranostics. Mater. Horizons 2019, 6, 434–469. [Google Scholar] [CrossRef]
- Sharma, H.; Mondal, S. Functionalized Graphene Oxide for Chemotherapeutic Drug Delivery and Cancer Treatment: A Promising Material in Nanomedicine. Int. J. Mol. Sci. 2020, 21, 6280. [Google Scholar] [CrossRef]
- Gazzi, A.; Fusco, L.; Khan, A.; Bedognetti, D.; Zavan, B.; Vitale, F.; Yilmazer, A.; Delogu, L.G. Photodynamic Therapy Based on Graphene and MXene in Cancer Theranostics. Front. Bioeng. Biotechnol. 2019, 7, 295. [Google Scholar] [CrossRef]
- Kim, N.Y.; Blake, S.; De, D.; Ouyang, J.; Shi, J.; Kong, N. Two-Dimensional Nanosheet-Based Photonic Nanomedicine for Combined Gene and Photothermal Therapy. Front. Pharmacol. 2020, 10, 1573. [Google Scholar] [CrossRef]
- Priya Swetha, P.D.; Manisha, H.; Sudhakaraprasad, K. Graphene and Graphene-Based Materials in Biomedical Science. Part. Part. Syst. Charact. 2018, 35, 1800105. [Google Scholar] [CrossRef]
- Weng, Y.; Jiang, B.; Yang, K.; Sui, Z.; Zhang, L.; Zhang, Y. Polyethyleneimine-modified graphene oxide nanocomposites for effective protein functionalization. Nanoscale 2015, 7, 14284–14291. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Skafidas, E. Detection of Protein Conformational Changes with Multilayer Graphene Nanopore Sensors. ACS Appl. Mater. Interfaces 2014, 6, 16777–16781. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, X.; Zhou, X.; Zhang, Y. Review on the graphene based optical fiber chemical and biological sensors. Sensors Actuators B Chem. 2016, 231, 324–340. [Google Scholar] [CrossRef]
- Omar, N.A.; Fen, Y.W.; Saleviter, S.; Daniyal, W.M.; Anas, N.A.; Ramdzan, N.S.; Roshidi, M.D. Development of a Graphene-Based Surface Plasmon Resonance Optical Sensor Chip for Potential Biomedical Application. Materials 2019, 12, 1928. [Google Scholar] [CrossRef]
- Younis, M.R.; He, G.; Lin, J.; Huang, P. Recent Advances on Graphene Quantum Dots for Bioimaging Applications. Front. Chem. 2020, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhao, X.; Wei, G.; Su, Z. Recent Advances in the Cancer Bioimaging with Graphene Quantum Dots. Curr. Med. Chem. 2018, 25, 2876–2893. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, Y.; Bidram, E.; Zarrabi, A.; Amini, A.; Cheng, C. Graphene oxide and its derivatives as promising In-vitro bio-imaging platforms. Sci. Rep. 2020, 10, 18052. [Google Scholar] [CrossRef]
- Jagiełło, J.; Chlanda, A.; Baran, M.; Gwiazda, M.; Lipińska, L. Synthesis and Characterization of Graphene Oxide and Reduced Graphene Oxide Composites with Inorganic Nanoparticles for Biomedical Applications. Nanomaterials 2020, 10, 1846. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Dvir, T.; Timko, B.P.; Kohane, D.S.; Langer, R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 2011, 6, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.-I.; Xiang, S.; Chen, C.-S.; Chin, W.-C.; Nelson, A.J.; Wang, C.; Lu, J. Carbon nanotubes promote neuron differentiation from human embryonic stem cells. Biochem. Biophys. Res. Commun. 2009, 384, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-J.; Park, S.; Bhang, S.H.; Yoon, J.-K.; Jo, I.; Jeong, G.-J.; Hong, B.H.; Kim, B.-S. Graphene enhances the cardiomyogenic differentiation of human embryonic stem cells. Biochem. Biophys. Res. Commun. 2014, 452, 174–180. [Google Scholar] [CrossRef]
- Barnes, J.M.; Przybyla, L.; Weaver, V.M. Tissue mechanics regulate brain development, homeostasis and disease. J. Cell Sci. 2017, 130, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Ege, D.; Kamali, A.R.; Boccaccini, A.R. Graphene Oxide/Polymer-Based Biomaterials. Adv. Eng. Mater. 2017, 19, 1700627. [Google Scholar] [CrossRef]
- He, Z.; Zhang, S.; Song, Q.; Li, W.; Liu, D.; Li, H.; Tang, M.; Chai, R. The structural development of primary cultured hippocampal neurons on a graphene substrate. Colloids Surf. B. Biointerfaces 2016, 146, 442–451. [Google Scholar] [CrossRef]
- Da Silva, L.P.; Kundu, S.C.; Reis, R.L.; Correlo, V.M. Electric Phenomenon: A Disregarded Tool in Tissue Engineering and Regenerative Medicine. Trends Biotechnol. 2020, 38, 24–49. [Google Scholar] [CrossRef]
- Burnstine-Townley, A.; Eshel, Y.; Amdursky, N. Conductive Scaffolds for Cardiac and Neuronal Tissue Engineering: Governing Factors and Mechanisms. Adv. Funct. Mater. 2020, 30, 1901369. [Google Scholar] [CrossRef]
- Plachá, D.; Jampilek, J. Graphenic Materials for Biomedical Applications. Nanomaterials 2019, 9, 1758. [Google Scholar] [CrossRef]
- Seabra, A.B.; Paula, A.J.; De Lima, R.; Alves, O.L.; Durán, N. Nanotoxicity of Graphene and Graphene Oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.; Komvopoulos, K. A review of graphene synthesis by indirect and direct deposition methods. J. Mater. Res. 2020, 35, 76–89. [Google Scholar] [CrossRef]
- Yu, Q.; Lian, J.; Siriponglert, S.; Li, H.; Chen, Y.P.; Pei, S.-S. Graphene segregated on Ni surfaces and transferred to insulators. Appl. Phys. Lett. 2008, 93, 113103. [Google Scholar] [CrossRef]
- Reina, A.; Thiele, S.; Jia, X.; Bhaviripudi, S.; Dresselhaus, M.S.; Schaefer, J.A.; Kong, J. Growth of large-area single- and Bi-layer graphene by controlled carbon precipitation on polycrystalline Ni surfaces. Nano Res. 2009, 2, 509–516. [Google Scholar] [CrossRef]
- Liu, W.; Li, H.; Xu, C.; Khatami, Y.; Banerjee, K. Synthesis of high-quality monolayer and bilayer graphene on copper using chemical vapor deposition. Carbon N. Y. 2011, 49, 4122–4130. [Google Scholar] [CrossRef]
- Ciesielski, A.; Samorì, P. Graphene via sonication assisted liquid-phase exfoliation. Chem. Soc. Rev. 2014, 43, 381–398. [Google Scholar] [CrossRef]
- Yi, M.; Shen, Z. A review on mechanical exfoliation for the scalable production of graphene. J. Mater. Chem. A 2015, 3, 11700–11715. [Google Scholar] [CrossRef]
- Narayan, R.; Kim, S.O. Surfactant mediated liquid phase exfoliation of graphene. Nano Converg. 2015, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cao, H.; Xue, Y.; Li, B.; Cai, W. Liquid-Phase Exfoliation of Graphene: An Overview on Exfoliation Media, Techniques, and Challenges. Nanomaterials 2018, 8, 942. [Google Scholar] [CrossRef]
- Le Ba, T.; Mahian, O.; Wongwises, S.; Szilágyi, I.M. Review on the recent progress in the preparation and stability of graphene-based nanofluids. J. Therm. Anal. Calorim. 2020, 142, 1145–1172. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef]
- Polyakova, E.Y.; Rim, K.T.; Eom, D.; Douglass, K.; Opila, R.L.; Heinz, T.F.; Teplyakov, A.V.; Flynn, G.W. Scanning Tunneling Microscopy and X-ray Photoelectron Spectroscopy Studies of Graphene Films Prepared by Sonication-Assisted Dispersion. ACS Nano 2011, 5, 6102–6108. [Google Scholar] [CrossRef]
- Skaltsas, T.; Ke, X.; Bittencourt, C.; Tagmatarchis, N. Ultrasonication Induces Oxygenated Species and Defects onto Exfoliated Graphene. J. Phys. Chem. C 2013, 117, 23272–23278. [Google Scholar] [CrossRef]
- Seiler, S.; Halbig, C.E.; Grote, F.; Rietsch, P.; Börrnert, F.; Kaiser, U.; Meyer, B.; Eigler, S. Effect of friction on oxidative graphite intercalation and high-quality graphene formation. Nat. Commun. 2018, 9, 836. [Google Scholar] [CrossRef]
- Eigler, S.; Enzelberger-Heim, M.; Grimm, S.; Hofmann, P.; Kroener, W.; Geworski, A.; Dotzer, C.; Röckert, M.; Xiao, J.; Papp, C.; et al. Wet Chemical Synthesis of Graphene. Adv. Mater. 2013, 25, 3583–3587. [Google Scholar] [CrossRef]
- Eigler, S.; Grimm, S.; Hirsch, A. Investigation of the Thermal Stability of the Carbon Framework of Graphene Oxide. Chem. A Eur. J. 2014, 20, 984–989. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef]
- Eigler, S.; Grimm, S.; Enzelberger-Heim, M.; Müller, P.; Hirsch, A. Graphene oxide: Efficiency of reducing agents. Chem. Commun. 2013, 49, 7391–7393. [Google Scholar] [CrossRef]
- Eigler, S.; Grimm, S.; Hof, F.; Hirsch, A. Graphene oxide: A stable carbon framework for functionalization. J. Mater. Chem. A 2013, 1, 11559–11562. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Kim, Y.J.; Chang Su, J.; Lim, K.T.; Seonwoo, H.; Cho, S.P.; Chung, T.D.; Choung, P.H.; Choung, Y.H.; et al. Monolayer graphene-directed growth and neuronal differentiation of mesenchymal stem cells. J. Biomed. Nanotechnol. 2015, 11, 2024–2033. [Google Scholar] [CrossRef] [PubMed]
- Nayak, T.R.; Andersen, H.; Makam, V.S.; Khaw, C.; Bae, S.; Xu, X.; Ee, P.L.R.; Ahn, J.H.; Hong, B.H.; Pastorin, G.; et al. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano 2011, 5, 4670–4678. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bae, W.G.; Park, S.; Kim, Y.J.; Jo, I.; Park, S.; Jeon, N.L.; Kwak, W.; Cho, S.; Park, J.; et al. Engineering structures and functions of mesenchymal stem cells by suspended large-area graphene nanopatterns. 2D Mater. 2016, 3, 35013. [Google Scholar] [CrossRef]
- Tonellato, M.; Piccione, M.; Gasparotto, M.; Bellet, P.; Tibaudo, L.; Vicentini, N.; Bergantino, E.; Menna, E.; Vitiello, L.; Di Liddo, R.; et al. Commitment of autologous human multipotent stem cells on biomimetic poly-l-lactic acid-based scaffolds is strongly influenced by structure and concentration of carbon nanomaterial. Nanomaterials 2020, 10, 415. [Google Scholar] [CrossRef]
- Jasim, D.A.; Lozano, N.; Bussy, C.; Barbolina, I.; Rodrigues, A.F.; Novoselov, K.S.; Kostarelos, K. Graphene-based papers as substrates for cell growth: Characterisation and impact on mammalian cells. FlatChem 2018, 12, 17–25. [Google Scholar] [CrossRef]
- Rasch, F.; Schütt, F.; Saure, L.M.; Kaps, S.; Strobel, J.; Polonskyi, O.; Nia, A.S.; Lohe, M.R.; Mishra, Y.K.; Faupel, F.; et al. Wet-Chemical Assembly of 2D Nanomaterials into Lightweight, Microtube-Shaped, and Macroscopic 3D Networks. ACS Appl. Mater. Interfaces 2019, 11, 44652–44663. [Google Scholar] [CrossRef]
- Vicentini, N.; Gatti, T.; Salice, P.; Scapin, G.; Marega, C.; Filippini, F.; Menna, E. Covalent functionalization enables good dispersion and anisotropic orientation of multi-walled carbon nanotubes in a poly(l-lactic acid) electrospun nanofibrous matrix boosting neuronal differentiation. Carbon N. Y. 2015, 95, 725–730. [Google Scholar] [CrossRef]
- Ligorio, C.; Zhou, M.; Wychowaniec, J.K.; Zhu, X.; Bartlam, C.; Miller, A.F.; Vijayaraghavan, A.; Hoyland, J.A.; Saiani, A. Graphene oxide containing self-assembling peptide hybrid hydrogels as a potential 3D injectable cell delivery platform for intervertebral disc repair applications. Acta Biomater. 2019, 92, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, S.R.; Kim, Y.K.; Kim, M.H.; Min, D.H. Behaviors of NIH-3T3 fibroblasts on graphene/carbon nanotubes: Proliferation, focal adhesion, and gene transfection studies. ACS Nano 2010, 4, 6587–6598. [Google Scholar] [CrossRef]
- Pandele, A.M.; Dinescu, S.; Costache, M.; Vasile, E.; Obreja, C.; Iovu, H.; Ionita, M. Preparation and in vitro, bulk, and surface investigation of chitosan/graphene oxide composite films. Polym. Compos. 2013, 34, 2116–2124. [Google Scholar] [CrossRef]
- Jin, L.; Zeng, Z.; Kuddannaya, S.; Wu, D.; Zhang, Y.; Wang, Z. Biocompatible, Free-Standing Film Composed of Bacterial Cellulose Nanofibers-Graphene Composite. ACS Appl. Mater. Interfaces 2016, 8, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, X.; Tomaskovic-Crook, E.; Crook, J.M.; Wallace, G.G. Smart graphene-cellulose paper for 2D or 3D “origami-inspired” human stem cell support and differentiation. Colloids Surfaces B Biointerfaces 2019, 176, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Kim, M.S.; Shin, Y.C.; Eom, S.U.; Lee, J.H.; Shin, D.M.; Hong, S.W.; Kim, B.; Park, J.C.; Shin, B.S.; et al. Cell migration according to shape of graphene oxide micropatterns. Micromachines 2016, 7, 186. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.S.T.; Yoo, H.; Yi, H.; Ahn, E.H.; Lee, J.H.; Shao, G.; Nagornyak, E.; Laflamme, M.A.; Murry, C.E.; Kim, D.H. Micro-and nano-patterned conductive graphene-PEG hybrid scaffolds for cardiac tissue engineering. Chem. Commun. 2017, 53, 7412–7415. [Google Scholar] [CrossRef]
- Qi, Z.; Chen, X.; Guo, W.; Fu, C.; Pan, S. Theanine-Modified Graphene Oxide Composite Films for Neural Stem Cells Proliferation and Differentiation. J. Nanomater. 2020, 2020, 3068173. [Google Scholar] [CrossRef]
- Vicentini, N.; Gatti, T.; Salerno, M.; Hernandez Gomez, Y.S.; Bellon, M.; Gallio, S.; Marega, C.; Filippini, F.; Menna, E. Effect of different functionalized carbon nanostructures as fillers on the physical properties of biocompatible poly(L-lactic acid) composites. Mater. Chem. Phys. 2018, 214, 265–276. [Google Scholar] [CrossRef]
- Lutzweiler, G.; Halili, A.N.; Vrana, N.E. The overview of porous, bioactive scaffolds as instructive biomaterials for tissue regeneration and their clinical translation. Pharmaceutics 2020, 12, 602. [Google Scholar] [CrossRef]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes—different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef]
- Reilly, G.C.; Engler, A.J. Intrinsic extracellular matrix properties regulate stem cell differentiation. J. Biomech. 2010, 43, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Bajo, A.; González-Mayorga, A.; Guerrero, C.R.; Palomares, F.J.; García, R.; López-Dolado, E.; Serrano, M.C. Myelinated axons and functional blood vessels populate mechanically compliant rGO foams in chronic cervical hemisected rats. Biomaterials 2019, 192, 461–474. [Google Scholar] [CrossRef]
- Serrano, M.C.; Patiño, J.; García-Rama, C.; Ferrer, M.L.; Fierro, J.L.G.; Tamayo, A.; Collazos-Castro, J.E.; Del Monte, F.; Gutiérrez, M.C. 3D free-standing porous scaffolds made of graphene oxide as substrates for neural cell growth. J. Mater. Chem. B 2014, 2, 5698–5706. [Google Scholar] [CrossRef]
- Liao, J.F.; Qu, Y.; Chu, B.; Zhang, X.; Qian, Z. Biodegradable CSMA/PECA/graphene porous hybrid scaffold for cartilage tissue engineering. Sci. Rep. 2015, 5, 9879. [Google Scholar] [CrossRef]
- Hermenean, A.; Codreanu, A.; Herman, H.; Balta, C.; Rosu, M.; Mihali, C.V.; Ivan, A.; Dinescu, S.; Ionita, M.; Costache, M. Chitosan-Graphene Oxide 3D scaffolds as Promising Tools for Bone Regeneration in Critical-Size Mouse Calvarial Defects. Sci. Rep. 2017, 7, 16641. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.J.; Güneş, F.; Kim, K.K.; Kim, E.S.; Han, G.H.; Kim, S.M.; Shin, H.-J.; Yoon, S.-M.; Choi, J.-Y.; Park, M.H.; et al. Synthesis of Large-Area Graphene Layers on Poly-Nickel Substrate by Chemical Vapor Deposition: Wrinkle Formation. Adv. Mater. 2009, 21, 2328–2333. [Google Scholar] [CrossRef]
- Ma, Q.; Yang, L.; Jiang, Z.; Song, Q.; Xiao, M.; Zhang, D.; Ma, X.; Wen, T.; Cheng, G. Three-Dimensional Stiff Graphene Scaffold on Neural Stem Cells Behavior. ACS Appl. Mater. Interfaces 2016, 8, 34227–34233. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Q.; Gao, S.; Song, Q.; Huang, R.; Wang, L.; Liu, L.; Dai, J.; Tang, M.; Cheng, G. Three-dimensional graphene foam as a biocompatible and conductive scaffold for neural stem cells. Sci. Rep. 2013, 3, 1604. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Kong, T.; Wang, W.; Song, Q.; Zhang, D.; Ma, Q.; Cheng, G. Interconnected Graphene Networks with Uniform Geometry for Flexible Conductors. Adv. Funct. Mater. 2015, 25, 6165–6172. [Google Scholar] [CrossRef]
- Schmitt, C.; Rasch, F.; Cossais, F.; Held-Feindt, J.; Lucius, R.; Vazquez, A.R.; Nia, A.S.; Lohe, M.R.; Feng, X.; Mishra, Y.K.; et al. Glial cell responses on tetrapod-shaped graphene oxide and reduced graphene oxide 3D scaffolds in brain in vitro and ex vivo models of indirect contact. Biomed. Mater. 2020. [Google Scholar] [CrossRef]
- Evlashin, S.; Dyakonov, P.; Tarkhov, M.; Dagesyan, S.; Rodionov, S.; Shpichka, A.; Kostenko, M.; Konev, S.; Sergeichev, I.; Timashev, P.; et al. Flexible polycaprolactone and polycaprolactone/graphene scaffolds for tissue engineering. Materials 2019, 12, 2991. [Google Scholar] [CrossRef] [PubMed]
- Nieto, A.; Dua, R.; Zhang, C.; Boesl, B.; Ramaswamy, S.; Agarwal, A. Three Dimensional Graphene Foam/Polymer Hybrid as a High Strength Biocompatible Scaffold. Adv. Funct. Mater. 2015, 25, 3916–3924. [Google Scholar] [CrossRef]
- Song, F.; Jie, W.; Zhang, T.; Li, W.; Jiang, Y.; Wan, L.; Liu, W.; Li, X.; Liu, B. Room-temperature fabrication of a three-dimensional reduced-graphene oxide/polypyrrole/hydroxyapatite composite scaffold for bone tissue engineering. RSC Adv. 2016, 6, 92804–92812. [Google Scholar] [CrossRef]
- Deliormanlı, A.M.; Türk, M.; Atmaca, H. Preparation and characterization of PCL-coated porous hydroxyapatite scaffolds in the presence of MWCNTs and graphene for orthopedic applications. J. Porous Mater. 2019, 26, 247–259. [Google Scholar] [CrossRef]
- Turk, M.; Deliormanll, A.M. Electrically conductive borate-based bioactive glass scaffolds for bone tissue engineering applications. J. Biomater. Appl. 2017, 32, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Deliormanlı, A.M.; Türk, M. Investigation the in vitro biological performance of graphene/bioactive glass scaffolds using MC3T3-E1 and ATDC5 cells. Mater. Technol. 2018, 33, 854–864. [Google Scholar] [CrossRef]
- Jakus, A.E.; Secor, E.B.; Rutz, A.L.; Jordan, S.W.; Hersam, M.C.; Shah, R.N. Three-dimensional printing of high-content graphene scaffolds for electronic and biomedical applications. ACS Nano 2015, 9, 4636–4648. [Google Scholar] [CrossRef]
- Cabral, C.S.D.; Miguel, S.P.; de Melo-Diogo, D.; Louro, R.O.; Correia, I.J. In situ green reduced graphene oxide functionalized 3D printed scaffolds for bone tissue regeneration. Carbon N. Y. 2019, 146, 513–523. [Google Scholar] [CrossRef]
- Jun, I.; Han, H.-S.; Edwards, J.R.; Jeon, H. Electrospun Fibrous Scaffolds for Tissue Engineering: Viewpoints on Architecture and Fabrication. Int. J. Mol. Sci. 2018, 19, 749. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Progress in the field of electrospinning for tissue engineering applications. Adv. Mater. 2009, 21, 3343–3351. [Google Scholar] [CrossRef] [PubMed]
- Gnavi, S.; Fornasari, B.E.; Tonda-Turo, C.; Laurano, R.; Zanetti, M.; Ciardelli, G.; Geuna, S. The Effect of Electrospun Gelatin Fibers Alignment on Schwann Cell and Axon Behavior and Organization in the Perspective of Artificial Nerve Design. Int. J. Mol. Sci. 2015, 16, 12925–12942. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Shafiei, S.S.; Asadi-Eydivand, M.; Ardeshir, M.; Solati-Hashjin, M. Graphene oxide-enriched poly(ϵ-caprolactone) electrospun nanocomposite scaffold for bone tissue engineering applications. J. Bioact. Compat. Polym. 2017, 32, 325–342. [Google Scholar] [CrossRef]
- Song, J.; Gao, H.; Zhu, G.; Cao, X.; Shi, X.; Wang, Y. The preparation and characterization of polycaprolactone/graphene oxide biocomposite nanofiber scaffolds and their application for directing cell behaviors. Carbon N. Y. 2015, 95, 1039–1050. [Google Scholar] [CrossRef]
- Luo, Y.; Shen, H.; Fang, Y.; Cao, Y.; Huang, J.; Zhang, M.; Dai, J.; Shi, X.; Zhang, Z. Enhanced proliferation and osteogenic differentiation of mesenchymal stem cells on graphene oxide-incorporated electrospun poly(lactic-co-glycolic acid) nanofibrous mats. ACS Appl. Mater. Interfaces 2015, 7, 6331–6339. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Maio, A.; Botta, L.; Rigogliuso, S.; Ghersi, G. Electrospun PCL/GO-g-PEG structures: Processing-morphology-properties relationships. Compos. Part A Appl. Sci. Manuf. 2017, 92, 97–107. [Google Scholar] [CrossRef]
- Basar, A.O.; Sadhu, V.; Turkoglu Sasmazel, H. Preparation of electrospun PCL-based scaffolds by mono/multi-functionalized GO. Biomed. Mater. 2019, 14, 45012. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wong, H.M.; Yeung, K.W.K.; Tjong, S.C. Novel electrospun polylactic acid nanocomposite fiber mats with hybrid graphene oxide and nanohydroxyapatite reinforcements having enhanced biocompatibility. Polymers 2016, 8, 287. [Google Scholar] [CrossRef]
- Jeznach, O.; Kolbuk, D.; Sajkiewicz, P. Aminolysis of Various Aliphatic Polyesters in a Form of Nanofibers and Films. Polymers 2019, 11, 1669. [Google Scholar] [CrossRef] [PubMed]
- Jalili, S.; Mohamadzadeh, M.; Ghanian, M.; Ashtiani, M.; Alimadadi, H.; Baharvand, H.; Martin, I.; Scherberich, A. Polycaprolactone-templated reduced-graphene oxide liquid crystal nanofibers towards biomedical applications. RSC Adv. 2017, 7, 39628–39634. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, Y.; Chen, L.; Zhu, T.; Ye, K.; Jia, C.; Wang, H.; Zhu, M.; Fan, C.; Mo, X. In vitro and in vivo studies of electroactive reduced graphene oxide-modified nanofiber scaffolds for peripheral nerve regeneration. Acta Biomater. 2019, 84, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Wu, D.; Kuddannaya, S.; Zhang, Y.; Wang, Z. Fabrication, Characterization, and Biocompatibility of Polymer Cored Reduced Graphene Oxide Nanofibers. ACS Appl. Mater. Interfaces 2016, 8, 5170–5177. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Zhang, X.; Yang, W.; Liu, X.; Jiao, Y.; Zhou, C. Influence of layer-by-layer assembled electrospun poly (l-lactic acid) nanofiber mats on the bioactivity of endothelial cells. Appl. Surf. Sci. 2016, 390, 838–846. [Google Scholar] [CrossRef]
- Shao, W.; He, J.; Wang, Q.; Cui, S.; Ding, B. Biomineralized Poly(l -lactic-co-glycolic acid)/Graphene Oxide/Tussah Silk Fibroin Nanofiber Scaffolds with Multiple Orthogonal Layers Enhance Osteoblastic Differentiation of Mesenchymal Stem Cells. ACS Biomater. Sci. Eng. 2017, 3, 1370–1380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zheng, H.; Liang, S.; Gao, C. Aligned PLLA nanofibrous scaffolds coated with graphene oxide for promoting neural cell growth. Acta Biomater. 2016, 37, 131–142. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Schäfer, M.K.E.; Altevogt, P. L1CAM malfunction in the nervous system and human carcinomas. Cell. Mol. Life Sci. 2010, 67, 2425–2437. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, K. Stimuli-responsive nanocomposite gels. Colloid Polym. Sci. 2011, 289, 455–473. [Google Scholar] [CrossRef]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-Assembled Graphene Hydrogel via a One-Step Hydrothermal Process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef]
- Yang, X.; Qiu, L.; Cheng, C.; Wu, Y.; Ma, Z.-F.; Li, D. Ordered Gelation of Chemically Converted Graphene for Next-Generation Electroconductive Hydrogel Films. Angew. Chemie Int. Ed. 2011, 50, 7325–7328. [Google Scholar] [CrossRef]
- Liao, G.; Hu, J.; Chen, Z.; Zhang, R.; Wang, G.; Kuang, T. Preparation, Properties, and Applications of Graphene-Based Hydrogels. Front. Chem. 2018, 6, 450. [Google Scholar] [CrossRef]
- Prasadh, S.; Suresh, S.; Wong, R. Osteogenic Potential of Graphene in Bone Tissue Engineering Scaffolds. Materials 2018, 11, 143. [Google Scholar] [CrossRef]
- Spicer, C.D. Hydrogel scaffolds for tissue engineering: The importance of polymer choice. Polym. Chem. 2020, 11, 184–219. [Google Scholar] [CrossRef]
- Moradali, M.F.; Ghods, S.; Rehm, B.H.A. Alginate Biosynthesis and Biotechnological Production BT—Alginates and Their Biomedical Applications; Rehm, B.H.A., Moradali, M.F., Eds.; Springer: Singapore, 2018; pp. 1–25. ISBN 978-981-10-6910-9. [Google Scholar]
- Mansouri, N.; Al-Sarawi, S.F.; Mazumdar, J.; Losic, D. Advancing fabrication and properties of three-dimensional graphene–alginate scaffolds for application in neural tissue engineering. RSC Adv. 2019, 9, 36838–36848. [Google Scholar] [CrossRef]
- Jiao, C.; Xiong, J.; Tao, J.; Xu, S.; Zhang, D.; Lin, H.; Chen, Y. Sodium alginate/graphene oxide aerogel with enhanced strength-toughness and its heavy metal adsorption study. Int. J. Biol. Macromol. 2016, 83, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Sivashankari, P.R.; Prabaharan, M. Three-dimensional porous scaffolds based on agarose/chitosan/graphene oxide composite for tissue engineering. Int. J. Biol. Macromol. 2020, 146, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Balagangadharan, K.; Dhivya, S.; Selvamurugan, N. Chitosan based nanofibers in bone tissue engineering. Int. J. Biol. Macromol. 2017, 104, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludağ, H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, N.; Liu, B.; Song, L.; Wen, B.; Yang, D. Aligned porous chitosan/graphene oxide scaffold for bone tissue engineering. Mater. Lett. 2018, 233, 78–81. [Google Scholar] [CrossRef]
- Ulijn, R.V.; Smith, A.M. Designing peptide based nanomaterials. Chem. Soc. Rev. 2008, 37, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, S. Designer Self-Assembling Peptide Materials. Macromol. Biosci. 2007, 7, 13–22. [Google Scholar] [CrossRef]
- Wang, L.; Song, D.; Zhang, X.; Ding, Z.; Kong, X.; Lu, Q.; Kaplan, D.L. Silk–Graphene Hybrid Hydrogels with Multiple Cues to Induce Nerve Cell Behavior. ACS Biomater. Sci. Eng. 2019, 5, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, G.; Lu, Q.; Kaplan, D.L. Controlling Cell Behavior on Silk Nanofiber Hydrogels with Tunable Anisotropic Structures. ACS Biomater. Sci. Eng. 2018, 4, 933–941. [Google Scholar] [CrossRef]
- Piantanida, E.; Alonci, G.; Bertucci, A.; De Cola, L. Design of Nanocomposite Injectable Hydrogels for Minimally Invasive Surgery. Acc. Chem. Res. 2019, 52, 2101–2112. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Nah, H.; Heo, D.N.; Kim, K.-H.; Seok, J.M.; Heo, M.; Moon, H.-J.; Lee, D.; Lee, J.S.; An, S.Y.; et al. Induction of osteogenic differentiation in a rat calvarial bone defect model using an In situ forming graphene oxide incorporated glycol chitosan/oxidized hyaluronic acid injectable hydrogel. Carbon N. Y. 2020, 168, 264–277. [Google Scholar] [CrossRef]
- Sayyar, S.; Gambhir, S.; Chung, J.; Officer, D.L.; Wallace, G.G. 3D printable conducting hydrogels containing chemically converted graphene. Nanoscale 2017, 9, 2038–2050. [Google Scholar] [CrossRef]
- Fan, L.; Yi, J.; Tong, J.; Zhou, X.; Ge, H.; Zou, S.; Wen, H.; Nie, M. Preparation and characterization of oxidized konjac glucomannan/carboxymethyl chitosan/graphene oxide hydrogel. Int. J. Biol. Macromol. 2016, 91, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Vimalraj, S.; Anuradha, D. Chitosan based thermoresponsive hydrogel containing graphene oxide for bone tissue repair. Biomed. Pharmacother. 2018, 107, 908–917. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Poly(propylene fumarate)/Polyethylene Glycol-Modified Graphene Oxide Nanocomposites for Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 17902–17914. [Google Scholar] [CrossRef]
- Noh, M.; Kim, S.H.; Kim, J.; Lee, J.R.; Jeong, G.J.; Yoon, J.K.; Kang, S.; Bhang, S.H.; Yoon, H.H.; Lee, J.C.; et al. Graphene oxide reinforced hydrogels for osteogenic differentiation of human adipose-derived stem cells. RSC Adv. 2017, 7, 20779–20788. [Google Scholar] [CrossRef]
- Wu, D.; Bäckström, E.; Hakkarainen, M. Starch Derived Nanosized Graphene Oxide Functionalized Bioactive Porous Starch Scaffolds. Macromol. Biosci. 2017, 17, 1600397. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Wang, X.; Yu, Z.; Wang, Z.; Xie, Q.; Zhang, D.; Huang, Y.; Zhou, H.; Bi, X.; Xiao, C.; et al. Enhanced Physiochemical and Mechanical Performance of Chitosan-Grafted Graphene Oxide for Superior Osteoinductivity. Adv. Funct. Mater. 2016, 26, 1085–1097. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, Y.; Chen, S.; Xing, T. Preparation and characterization of cross-linked carboxymethyl chitin porous membrane scaffold for biomedical applications. Carbohydr. Polym. 2015, 126, 150–155. [Google Scholar] [CrossRef]
- Kim, J.A.; Jang, E.Y.; Kang, T.J.; Yoon, S.; Ovalle-Robles, R.; Rhee, W.J.; Kim, T.; Baughman, R.H.; Kim, Y.H.; Park, T.H. Regulation of morphogenesis and neural differentiation of human mesenchymal stem cells using carbon nanotube sheets. Integr. Biol. (Camb). 2012, 4, 587–594. [Google Scholar] [CrossRef]
- Nishikawa, S.-I.; Jakt, L.M.; Era, T. Embryonic stem-cell culture as a tool for developmental cell biology. Nat. Rev. Mol. Cell Biol. 2007, 8, 502–507. [Google Scholar] [CrossRef]
- Spangrude, G.J.; Heimfeld, S.; Weissman, I.L. Purification and characterization of mouse hematopoietic stem cells. Science 1988, 241, 58–62. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.; Lee, K.-H.; Nam, D.-H.; Joo, K.M. Adult human neural stem cell therapeutics: Current developmental status and prospect. World J. Stem Cells 2015, 7, 126–136. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.T.; Alexander, G.J.M. Induced pluripotent stem cells: From Nobel Prizes to clinical applications. J. Hepatol. 2013, 58, 625–629. [Google Scholar] [CrossRef][Green Version]
- Lanza, R.; Russell, D.W.; Nagy, A. Engineering universal cells that evade immune detection. Nat. Rev. Immunol. 2019, 19, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.N.; Boughan, K.; Dahi, P.B.; Devlin, S.M.; Maloy, M.A.; Naputo, K.; Mazis, C.M.; Davis, E.; Nhaissi, M.; Wells, D.; et al. Racial disparities in access to HLA-matched unrelated donor transplants: A prospective 1312-patient analysis. Blood Adv. 2019, 3, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Gornalusse, G.G.; Hirata, R.K.; Funk, S.E.; Riolobos, L.; Lopes, V.S.; Manske, G.; Prunkard, D.; Colunga, A.G.; Hanafi, L.-A.; Clegg, D.O.; et al. HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat. Biotechnol. 2017, 35, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Deuse, T.; Hu, X.; Gravina, A.; Wang, D.; Tediashvili, G.; De, C.; Thayer, W.O.; Wahl, A.; Garcia, J.V.; Reichenspurner, H.; et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 2019, 37, 252–258. [Google Scholar] [CrossRef]
- Xu, H.; Wang, B.; Ono, M.; Kagita, A.; Fujii, K.; Sasakawa, N.; Ueda, T.; Gee, P.; Nishikawa, M.; Nomura, M.; et al. Targeted Disruption of HLA Genes via CRISPR-Cas9 Generates iPSCs with Enhanced Immune Compatibility. Cell Stem Cell 2019, 24, 566–578.e7. [Google Scholar] [CrossRef]
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular matrix assembly: A multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Alberts, P.; Rudge, R.; Hinners, I.; Muzerelle, A.; Martinez-Arca, S.; Irinopoulou, T.; Marthiens, V.; Tooze, S.; Rathjen, F.; Gaspar, P.; et al. Cross talk between tetanus neurotoxin-insensitive vesicle-associated membrane protein-mediated transport and L1-mediated adhesion. Mol. Biol. Cell 2003, 14, 4207–4220. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Sato, C.; Naka, Y.; Kitazawa, A.; Whitby, R.L.D.; Shimizu, N. Neurite outgrowths of neurons with neurotrophin-coated carbon nanotubes. J. Biosci. Bioeng. 2007, 103, 216–220. [Google Scholar] [CrossRef]
- Von der Mark, K.; Park, J.; Bauer, S.; Schmuki, P. Nanoscale engineering of biomimetic surfaces: Cues from the extracellular matrix. Cell Tissue Res. 2010, 339, 131–153. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, L.; Song, W.; Wu, Z.; Li, D. Biocompatible polymer materials: Role of protein–surface interactions. Prog. Polym. Sci. 2008, 33, 1059–1087. [Google Scholar] [CrossRef]
- Shekaran, A.; Garcia, A.J. Nanoscale engineering of extracellular matrix-mimetic bioadhesive surfaces and implants for tissue engineering. Biochim. Biophys. Acta 2011, 1810, 350–360. [Google Scholar] [CrossRef]
- Berezin, V.; Bock, E. NCAM mimetic peptides: Pharmacological and therapeutic potential. J. Mol. Neurosci. 2004, 22, 33–39. [Google Scholar] [CrossRef]
- Scapin, G.; Salice, P.; Tescari, S.; Menna, E.; De Filippis, V.; Filippini, F. Enhanced neuronal cell differentiation combining biomimetic peptides and a carbon nanotube-polymer scaffold. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 621–632. [Google Scholar] [CrossRef]
- Nikkhah, M.; Edalat, F.; Manoucheri, S.; Khademhosseini, A. Engineering microscale topographies to control the cell-substrate interface. Biomaterials 2012, 33, 5230–5246. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Pettikiriarachchi, J.T.S.; Parish, C.L.; Shoichet, M.S.; Forsythe, J.S.; Nisbet, D.R. Biomaterials for Brain Tissue Engineering. Aust. J. Chem. 2010, 63, 1143–1154. [Google Scholar] [CrossRef]
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules 2020, 21, 1968–1994. [Google Scholar] [CrossRef]
- Landers, J.; Turner, J.T.; Heden, G.; Carlson, A.L.; Bennett, N.K.; Moghe, P.V.; Neimark, A. V Carbon nanotube composites as multifunctional substrates for in situ actuation of differentiation of human neural stem cells. Adv. Healthc. Mater. 2014, 3, 1745–1752. [Google Scholar] [CrossRef]
- Yao, L.; Pandit, A.; Yao, S.; McCaig, C.D. Electric field-guided neuron migration: A novel approach in neurogenesis. Tissue Eng. Part B. Rev. 2011, 17, 143–153. [Google Scholar] [CrossRef]
- Ning, C.; Zhou, Z.; Tan, G.; Zhu, Y.; Mao, C. Electroactive polymers for tissue regeneration: Developments and perspectives. Prog. Polym. Sci. 2018, 81, 144–162. [Google Scholar] [CrossRef] [PubMed]
- Fukada, E. History and recent progress in piezoelectric polymers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2000, 47, 1277–1290. [Google Scholar] [CrossRef]
- Yao, L.; McCaig, C.D.; Zhao, M. Electrical signals polarize neuronal organelles, direct neuron migration, and orient cell division. Hippocampus 2009, 19, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.E.; Shastri, V.R.; Vacanti, J.P.; Langer, R. Stimulation of neurite outgrowth using an electrically conducting polymer. Proc. Natl. Acad. Sci. USA 1997, 94, 8948–8953. [Google Scholar] [CrossRef]
- Lovat, V.; Pantarotto, D.; Lagostena, L.; Cacciari, B.; Grandolfo, M.; Righi, M.; Spalluto, G.; Prato, M.; Ballerini, L. Carbon Nanotube Substrates Boost Neuronal Electrical Signaling. Nano Lett. 2005, 5, 1107–1110. [Google Scholar] [CrossRef]
- Isaacson, B.M.; Bloebaum, R.D. Bone bioelectricity: What have we learned in the past 160 years? J. Biomed. Mater. Res. A 2010, 95, 1270–1279. [Google Scholar] [CrossRef]
- Defteralı, Ç.; Verdejo, R.; Majeed, S.; Boschetti-de-Fierro, A.; Méndez-Gómez, H.R.; Díaz-Guerra, E.; Fierro, D.; Buhr, K.; Abetz, C.; Martínez-Murillo, R.; et al. In Vitro Evaluation of Biocompatibility of Uncoated Thermally Reduced Graphene and Carbon Nanotube-Loaded PVDF Membranes with Adult Neural Stem Cell-Derived Neurons and Glia. Front. Bioeng. Biotechnol. 2016, 4, 94. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Fan, L.; Yi, X.; Zhou, Z.; Liu, C.; Fu, R.; Dai, C.; Wang, Z.; Chen, X.; Yu, P.; et al. Soft Conducting Polymer Hydrogels Cross-Linked and Doped by Tannic Acid for Spinal Cord Injury Repair. ACS Nano 2018, 12, 10957–10967. [Google Scholar] [CrossRef]
- Scapin, G.; Bertalot, T.; Vicentini, N.; Gatti, T.; Tescari, S.; De Filippis, V.; Marega, C.; Menna, E.; Gasparella, M.; Parnigotto, P.P.; et al. Neuronal commitment of human circulating multipotent cells by carbon nanotube-polymer scaffolds and biomimetic peptides. Nanomedicine 2016, 11, 1929–1946. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhang, X.; Yu, X.; Wang, S.; Qiu, J.; Tang, W.; Li, L.; Liu, H.; Wang, Z.L. Self-Powered Electrical Stimulation for Enhancing Neural Differentiation of Mesenchymal Stem Cells on Graphene-Poly(3,4-ethylenedioxythiophene) Hybrid Microfibers. ACS Nano 2016, 10, 5086–5095. [Google Scholar] [CrossRef] [PubMed]
- Navaei, A.; Saini, H.; Christenson, W.; Sullivan, R.T.; Ros, R.; Nikkhah, M. Gold nanorod-incorporated gelatin-based conductive hydrogels for engineering cardiac tissue constructs. Acta Biomater. 2016, 41, 133–146. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, J.; Xu, G.; Wei, J.; Zhang, Z.; Li, X. Tuning the conductivity and inner structure of electrospun fibers to promote cardiomyocyte elongation and synchronous beating. Mater. Sci. Eng. C. Mater. Biol. Appl. 2016, 69, 865–874. [Google Scholar] [CrossRef]
- Chen, J.; Yu, M.; Guo, B.; Ma, P.X.; Yin, Z. Conductive nanofibrous composite scaffolds based on in-situ formed polyaniline nanoparticle and polylactide for bone regeneration. J. Colloid Interface Sci. 2018, 514, 517–527. [Google Scholar] [CrossRef]
- Comisar, W.A.; Hsiong, S.X.; Kong, H.-J.; Mooney, D.J.; Linderman, J.J. Multi-scale modeling to predict ligand presentation within RGD nanopatterned hydrogels. Biomaterials 2006, 27, 2322–2329. [Google Scholar] [CrossRef] [PubMed]
- Abrams, G.A.; Goodman, S.L.; Nealey, P.F.; Franco, M.; Murphy, C.J. Nanoscale topography of the basement membrane underlying the corneal epithelium of the rhesus macaque. Cell Tissue Res. 2000, 299, 39–46. [Google Scholar] [CrossRef]
- Curtis, A.S.; Casey, B.; Gallagher, J.O.; Pasqui, D.; Wood, M.A.; Wilkinson, C.D. Substratum nanotopography and the adhesion of biological cells. Are symmetry or regularity of nanotopography important? Biophys. Chem. 2001, 94, 275–283. [Google Scholar] [CrossRef]
- Rodríguez Hernández, J.C.; Salmerón Sánchez, M.; Soria, J.M.; Gómez Ribelles, J.L.; Monleón Pradas, M. Substrate chemistry-dependent conformations of single laminin molecules on polymer surfaces are revealed by the phase signal of atomic force microscopy. Biophys. J. 2007, 93, 202–207. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, N.J.; Lee, S.J.; Atala, A. 1—Biomedical nanomaterials in tissue engineering. In Woodhead Publishing Series in Biomaterials; Gaharwar, A.K., Sant, S., Hancock, M.J., Hacking, S.A., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 1–25. ISBN 978-0-85709-596-1. [Google Scholar]
- Arnold, M.; Cavalcanti-Adam, E.A.; Glass, R.; Blümmel, J.; Eck, W.; Kantlehner, M.; Kessler, H.; Spatz, J.P. Activation of integrin function by nanopatterned adhesive interfaces. Chemphyschem 2004, 5, 383–388. [Google Scholar] [CrossRef]
- Yang, K.; Jung, K.; Ko, E.; Kim, J.; Park, K.I.; Kim, J.; Cho, S.-W. Nanotopographical manipulation of focal adhesion formation for enhanced differentiation of human neural stem cells. ACS Appl. Mater. Interfaces 2013, 5, 10529–10540. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, V.; Maiorano, G.; Rizzello, L.; Sorce, B.; Sabella, S.; Cingolani, R.; Pompa, P.P. Neurons sense nanoscale roughness with nanometer sensitivity. Proc. Natl. Acad. Sci. USA 2010, 107, 6264–6269. [Google Scholar] [CrossRef]
- Staii, C.; Viesselmann, C.; Ballweg, J.; Williams, J.C.; Dent, E.W.; Coppersmith, S.N.; Eriksson, M.A. Distance dependence of neuronal growth on nanopatterned gold surfaces. Langmuir 2011, 27, 233–239. [Google Scholar] [CrossRef]
- Silva, G.A. Nanotechnology approaches for drug and small molecule delivery across the blood brain barrier. Surg. Neurol. 2007, 67, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Kotov, N.A.; Winter, J.O.; Clements, I.P.; Jan, E.; Timko, B.P.; Campidelli, S.; Pathak, S.; Mazzatenta, A.; Lieber, C.M.; Prato, M.; et al. Nanomaterials for Neural Interfaces. Adv. Mater. 2009, 21, 3970–4004. [Google Scholar] [CrossRef]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Umadevi, D.; Sastry, G.N. Impact of the chirality and curvature of carbon nanostructures on their interaction with aromatics and amino acids. ChemPhysChem 2013, 14, 2570–2578. [Google Scholar] [CrossRef]
- Kumar, S.; Parekh, S.H. Linking graphene-based material physicochemical properties with molecular adsorption, structure and cell fate. Commun. Chem. 2020, 3, 8. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of graphene: Covalent and non-covalent approaches, derivatives and applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef] [PubMed]
- Lalwani, G.; D’Agati, M.; Khan, A.M.; Sitharaman, B. Toxicology of graphene-based nanomaterials. Adv. Drug Deliv. Rev. 2016, 105, 109–144. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-based antibacterial paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Kuo, W.-S.; Chen, H.-H.; Chen, S.-Y.; Chang, C.-Y.; Chen, P.-C.; Hou, Y.-I.; Shao, Y.-T.; Kao, H.-F.; Lilian Hsu, C.-L.; Chen, Y.-C.; et al. Graphene quantum dots with nitrogen-doped content dependence for highly efficient dual-modality photodynamic antimicrobial therapy and bioimaging. Biomaterials 2017, 120, 185–194. [Google Scholar] [CrossRef]
- Prasad, K.; Lekshmi, G.S.; Ostrikov, K.; Lussini, V.; Blinco, J.; Mohandas, M.; Vasilev, K.; Bottle, S.; Bazaka, K.; Ostrikov, K. Synergic bactericidal effects of reduced graphene oxide and silver nanoparticles against Gram-positive and Gram-negative bacteria. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Vallabani, N.V.S.; Mittal, S.; Shukla, R.K.; Pandey, A.K.; Dhakate, S.R.; Pasricha, R.; Dhawan, A. Toxicity of graphene in normal human lung cells (BEAS-2B). J. Biomed. Nanotechnol. 2011, 7, 106–107. [Google Scholar] [CrossRef]
- Nasirzadeh, N.; Azari, M.R.; Rasoulzadeh, Y.; Mohammadian, Y. An assessment of the cytotoxic effects of graphene nanoparticles on the epithelial cells of the human lung. Toxicol. Ind. Health 2019, 35, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Take, S.; Tani, R.; Maru, J.; Obara, S.; Endoh, S. Assessment of cytotoxicity and mutagenicity of exfoliated graphene. Toxicol. Vitr. 2018, 52. [Google Scholar] [CrossRef]
- Yang, Z.; Pan, Y.; Chen, T.; Li, L.; Zou, W.; Liu, D.; Xue, D.; Wang, X.; Lin, G. Cytotoxicity and Immune Dysfunction of Dendritic Cells Caused by Graphene Oxide. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Dallavalle, M.; Calvaresi, M.; Bottoni, A.; Melle-Franco, M.; Zerbetto, F. Graphene can wreak havoc with cell membranes. ACS Appl. Mater. Interfaces 2015, 7, 4406–4414. [Google Scholar] [CrossRef]
- Chatterjee, N.; Eom, H.J.; Choi, J. A systems toxicology approach to the surface functionality control of graphene-cell interactions. Biomaterials 2014, 35, 1109–1127. [Google Scholar] [CrossRef]
- Askari, E.; Naghib, S.M.; Seyfoori, A.; Maleki, A.; Rahmanian, M. Ultrasonic-assisted synthesis and in vitro biological assessments of a novel herceptin-stabilized graphene using three dimensional cell spheroid. Ultrason. Sonochem. 2019, 58, 104615. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Kim, H.D.; Han, S.S. Biocompatibility and hemocompatibility of hydrothermally derived reduced graphene oxide using soluble starch as a reducing agent. Colloids Surfaces B Biointerfaces 2020, 185. [Google Scholar] [CrossRef]
- Palmieri, V.; Perini, G.; De Spirito, M.; Papi, M. Graphene oxide touches blood: In vivo interactions of bio-coronated 2D materials. Nanoscale Horizons 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Yu, Y.; Shen, C.; Jiao, Y.; Zhou, C. Impact of graphene oxide on the structure and function of important multiple blood components by a dose-dependent pattern. J. Biomed. Mater. Res. Part A 2015, 103. [Google Scholar] [CrossRef] [PubMed]
- Singh Bakshi, M. Nanotoxicity in Systemic Circulation and Wound Healing. Chem. Res. Toxicol. 2017, 30, 1253–1274. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, S.; Hinzmann, M.; Sawosz, E.; Grodzik, M.; Kutwin, M.; Wierzbicki, M.; Strojny, B.; Vadalasetty, K.P.; Lipińska, L.; Chwalibog, A. Interaction of different forms of graphene with chicken embryo red blood cells. Environ. Sci. Pollut. Res. 2017, 24. [Google Scholar] [CrossRef]
- Duan, G.; Kang, S.G.; Tian, X.; Garate, J.A.; Zhao, L.; Ge, C.; Zhou, R. Protein corona mitigates the cytotoxicity of graphene oxide by reducing its physical interaction with cell membrane. Nanoscale 2015, 7, 15214–15224. [Google Scholar] [CrossRef]
- Sasidharan, A.; Panchakarla, L.S.; Sadanandan, A.R.; Ashokan, A.; Chandran, P.; Girish, C.M.; Menon, D.; Nair, S.V.; Rao, C.N.R.; Koyakutty, M. Hemocompatibility and macrophage response of pristine and functionalized graphene. Small 2012, 8, 1251–1263. [Google Scholar] [CrossRef]
- Sopotnik, M.; Leonardi, A.; Križaj, I.; Dušak, P.; Makovec, D.; Mesarič, T.; Ulrih, N.P.; Junkar, I.; Sepčić, K.; Drobne, D. Comparative study of serum protein binding to three different carbon-based nanomaterials. Carbon N. Y. 2015, 95, 560–572. [Google Scholar] [CrossRef]
- Leso, V.; Fontana, L.; Iavicoli, I. Nanomaterial exposure and sterile inflammatory reactions. Toxicol. Appl. Pharmacol. 2018, 355, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Aggarwal, P.; Hall, J.B.; McNeil, S.E. Preclinical Studies To Understand Nanoparticle Interaction with the Immune System and Its Potential Effects on Nanoparticle Biodistribution. Mol. Pharm. 2008, 5, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Keshavan, S.; Calligari, P.; Stella, L.; Fusco, L.; Delogu, L.G.; Fadeel, B. Nano-bio interactions: A neutrophil-centric view. Cell Death Dis. 2019, 10. [Google Scholar] [CrossRef]
- Lin, H.; Ji, D.K.; Lucherelli, M.A.; Reina, G.; Ippolito, S.; Samorì, P.; Bianco, A. Comparative Effects of Graphene and Molybdenum Disulfide on Human Macrophage Toxicity. Small 2020, 16. [Google Scholar] [CrossRef] [PubMed]
- Furze, R.C.; Rankin, S.M. The role of the bone marrow in neutrophil clearance under homeostatic conditions in the mouse. FASEB J. 2008, 22, 3111–3119. [Google Scholar] [CrossRef]
- Chang, T.K.; Lu, Y.C.; Yeh, S.T.; Lin, T.C.; Huang, C.H.; Huang, C.H. In vitro and in vivo biological responses to graphene and graphene oxide: A murine calvarial animal study. Int. J. Nanomed. 2020, 15, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, S.R.R.; Pinto, S.R.; de Menezes, F.D.; Martinez-Manez, R.; Ricci-Junior, E.; Alencar, L.M.R.; Helal-Neto, E.; da Silva de Barros, A.O.; Lisboa, P.C.; Santos-Oliveira, R. Senescence and the Impact on Biodistribution of Different Nanosystems: The Discrepancy on Tissue Deposition of Graphene Quantum Dots, Polycaprolactone Nanoparticle and Magnetic Mesoporous Silica Nanoparticles in Young and Elder Animals. Pharm. Res. 2020, 37. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Deng, Y.; Liao, X.; Zhang, J.; Cheng, B.; Cao, Z.; Lu, H. Graphene oxide nanoparticles induce hepatic dysfunction through the regulation of innate immune signaling in zebrafish (Danio rerio). Nanotoxicology 2020, 14, 667–682. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, Y.; Li, J.; Kong, H.; Yan, Q.; Zhang, J.; Li, W.; Ren, N.; Cui, Y.; Zhang, T.; et al. Blood exposure to graphene oxide may cause anaphylactic death in non-human primates. Nano Today 2020, 35. [Google Scholar] [CrossRef]
- El-Yamany, N.A.; Mohamed, F.F.; Salaheldin, T.A.; Tohamy, A.A.; Abd El-Mohsen, W.N.; Amin, A.S. Graphene oxide nanosheets induced genotoxicity and pulmonary injury in mice. Exp. Toxicol. Pathol. 2017, 69. [Google Scholar] [CrossRef]
- Sasidharan, A.; Swaroop, S.; Koduri, C.K.; Girish, C.M.; Chandran, P.; Panchakarla, L.S.; Somasundaram, V.H.; Gowd, G.S.; Nair, S.; Koyakutty, M. Comparative in vivo toxicity, organ biodistribution and immune response of pristine, carboxylated and PEGylated few-layer graphene sheets in Swiss albino mice: A three month study. Carbon N. Y. 2015, 95. [Google Scholar] [CrossRef]
- Xiaoli, F.; Qiyue, C.; Weihong, G.; Yaqing, Z.; Chen, H.; Junrong, W.; Longquan, S. Toxicology data of graphene-family nanomaterials: An update. Arch. Toxicol. 2020, 94. [Google Scholar] [CrossRef]
- Mohamed, H.R.H.; Welson, M.; Yaseen, A.E.; El-Ghor, A. Induction of chromosomal and DNA damage and histological alterations by graphene oxide nanoparticles in Swiss mice. Drug Chem. Toxicol. 2019, 1–11. [Google Scholar] [CrossRef]
- Mao, L.; Hu, M.; Pan, B.; Xie, Y.; Petersen, E.J. Biodistribution and toxicity of radio-labeled few layer graphene in mice after intratracheal instillation. Part. Fibre Toxicol. 2016, 13. [Google Scholar] [CrossRef]
- Krajnak, K.; Waugh, S.; Stefaniak, A.; Schwegler-Berry, D.; Roach, K.; Barger, M.; Roberts, J. Exposure to graphene nanoparticles induces changes in measures of vascular/renal function in a load and form-dependent manner in mice. J. Toxicol. Environ. Heal. Part A 2019, 82, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.F.; Newman, L.; Jasim, D.; Mukherjee, S.P.; Wang, J.; Vacchi, I.A.; Ménard-Moyon, C.; Bianco, A.; Fadeel, B.; Kostarelos, K.; et al. Size-Dependent Pulmonary Impact of Thin Graphene Oxide Sheets in Mice: Toward Safe-by-Design. Adv. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Z.; Liu, Y.; Chu, M.; Yang, C.; Li, W.; Shao, Y.; Yue, Y.; Xu, R. The short- and long-term effects of orally administered high-dose reduced graphene oxide nanosheets on mouse behaviors. Biomaterials 2015, 68. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Bonaccorso, F.; Fal’ko, V.; Novoselov, K.S.; Roche, S.; Bøggild, P.; Borini, S.; Koppens, F.H.L.; Palermo, V.; Pugno, N.; et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.; Jasim, D.A.; Prestat, E.; Lozano, N.; De Lazaro, I.; Nam, Y.; Assas, B.M.; Pennock, J.; Haigh, S.J.; Bussy, C.; et al. Splenic Capture and in Vivo Intracellular Biodegradation of Biological-Grade Graphene Oxide Sheets. ACS Nano 2020, 14, 10168–10186. [Google Scholar] [CrossRef] [PubMed]
- Bussy, C.; Ali-Boucetta, H.; Kostarelos, K. Safety considerations for graphene: Lessons learnt from carbon nanotubes. Acc. Chem. Res. 2013, 46, 692–701. [Google Scholar] [CrossRef]
- Pinto, A.M.; Gonçalves, C.; Sousa, D.M.; Ferreira, A.R.; Moreira, J.A.; Gonçalves, I.C.; Magalhães, F.D. Smaller particle size and higher oxidation improves biocompatibility of graphene-based materials. Carbon N. Y. 2016, 99. [Google Scholar] [CrossRef]
- Yan, L.; Zhao, F.; Li, S.; Hu, Z.; Zhao, Y. Low-toxic and safe nanomaterials by surface-chemical design, carbon nanotubes, fullerenes, metallofullerenes, and graphenes. Nanoscale 2011, 3. [Google Scholar] [CrossRef]
- Qu, Y.; He, F.; Yu, C.; Liang, X.; Liang, D.; Ma, L.; Zhang, Q.; Lv, J.; Wu, J. Advances on graphene-based nanomaterials for biomedical applications. Mater. Sci. Eng. C 2018, 90. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Mozafari, M. Biological response to carbon-family nanomaterials: Interactions at the nano-bio interface. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef]
- Wojtoniszak, M.; Chen, X.; Kalenczuk, R.J.; Wajda, A.; Łapczuk, J.; Kurzewski, M.; Drozdzik, M.; Chu, P.K.; Borowiak-Palen, E. Synthesis, dispersion, and cytocompatibility of graphene oxide and reduced graphene oxide. Colloids Surfaces B Biointerfaces 2012, 89, 79–85. [Google Scholar] [CrossRef]
- Di Luca, M.; Vittorio, O.; Cirillo, G.; Curcio, M.; Czuban, M.; Voli, F.; Farfalla, A.; Hampel, S.; Nicoletta, F.P.; Iemma, F. Electro-responsive graphene oxide hydrogels for skin bandages: The outcome of gelatin and trypsin immobilization. Int. J. Pharm. 2018, 546. [Google Scholar] [CrossRef]
- Cirillo, G.; Curcio, M.; Spizzirri, U.G.; Vittorio, O.; Tucci, P.; Picci, N.; Iemma, F.; Hampel, S.; Nicoletta, F.P. Carbon nanotubes hybrid hydrogels for electrically tunable release of Curcumin. Eur. Polym. J. 2017, 90. [Google Scholar] [CrossRef]
- Li, Y.; Feng, L.; Shi, X.; Wang, X.; Yang, Y.; Yang, K.; Liu, T.; Yang, G.; Liu, Z. Surface coating-dependent cytotoxicity and degradation of graphene derivatives: Towards the design of non-toxic, degradable nano-graphene. Small 2014, 10, 1544–1554. [Google Scholar] [CrossRef]
- Hu, W.; Peng, C.; Lv, M.; Li, X.; Zhang, Y.; Chen, N.; Fan, C.; Huang, Q. Protein Corona-Mediated Mitigation of Cytotoxicity of Graphene Oxide. ACS Nano 2011, 5, 3693–3700. [Google Scholar] [CrossRef]
- Chong, Y.; Ge, C.; Yang, Z.; Antonio Garate, J.; Gu, Z.K.; Weber, J.; Liu, J.; Zhou, R. Reduced Cytotoxicity of Graphene Nanosheets Mediated by Blood-Protein Coating. ACS Nano 2015, 9, 5713–5724. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.C.; Lee, I.; Kwon, S.J.; Wee, Y.; Kwon, K.Y.; Jeon, C.; An, H.J.; Jung, H.T.; Ha, S.; Dordick, J.S.; et al. Fabrication of enzyme-based coatings on intact multi-walled carbon nanotubes as highly effective electrodes in biofuel cells. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Bussy, C.; Kostarelos, K. Culture Media Critically Influence Graphene Oxide Effects on Plasma Membranes. Chem 2017, 2, 322–323. [Google Scholar] [CrossRef][Green Version]
- Bhattacharya, K.; Mukherjee, S.P.; Gallud, A.; Burkert, S.C.; Bistarelli, S.; Bellucci, S.; Bottini, M.; Star, A.; Fadeel, B. Biological interactions of carbon-based nanomaterials: From coronation to degradation. Nanomed. Nanotechnol. Biol. Med. 2016, 12. [Google Scholar] [CrossRef] [PubMed]
- Punetha, V.D.; Rana, S.; Yoo, H.J.; Chaurasia, A.; McLeskey, J.T.; Ramasamy, M.S.; Sahoo, N.G.; Cho, J.W. Functionalization of carbon nanomaterials for advanced polymer nanocomposites: A comparison study between CNT and graphene. Prog. Polym. Sci. 2017, 67, 1–47. [Google Scholar] [CrossRef]
- Kiew, S.F.; Kiew, L.V.; Lee, H.B.; Imae, T.; Chung, L.Y. Assessing biocompatibility of graphene oxide-based nanocarriers: A review. J. Control. Release 2016, 226, 217–228. [Google Scholar] [CrossRef]
- Cirillo, G.; Peitzsch, C.; Vittorio, O.; Curcio, M.; Farfalla, A.; Voli, F.; Dubrovska, A.; Iemma, F.; Kavallaris, M.; Hampel, S. When polymers meet carbon nanostructures: Expanding horizons in cancer therapy. Future Med. Chem. 2019, 11, 2205–2231. [Google Scholar] [CrossRef]
- Madni, A.; Noreen, S.; Maqbool, I.; Rehman, F.; Batool, A.; Kashif, P.M.; Rehman, M.; Tahir, N.; Khan, M.I. Graphene-based nanocomposites: Synthesis and their theranostic applications. J. Drug Target. 2018, 26, 858–883. [Google Scholar] [CrossRef]
- Yang, H.; Bremner, D.H.; Tao, L.; Li, H.; Hu, J.; Zhu, L. Carboxymethyl chitosan-mediated synthesis of hyaluronic acid-targeted graphene oxide for cancer drug delivery. Carbohydr. Polym. 2016, 135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, K.; Feng, L.; Liu, Z. In vitro and in vivo behaviors of dextran functionalized graphene. Carbon N. Y. 2011, 49. [Google Scholar] [CrossRef]
- Nezakati, T.; Seifalian, A.; Tan, A.M.; Seifalian, A. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef]
- Mahajan, S.; Patharkar, A.; Kuche, K.; Maheshwari, R.; Deb, P.K.; Kalia, K.; Tekade, R.K. Functionalized carbon nanotubes as emerging delivery system for the treatment of cancer. Int. J. Pharm. 2018, 548. [Google Scholar] [CrossRef]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Majidinia, M.; Sadeghpour, A.; Yousefi, B. The roles of signaling pathways in bone repair and regeneration. J. Cell. Physiol. 2018, 233, 2937–2948. [Google Scholar] [CrossRef]
- Lichte, P.; Pape, H.C.; Pufe, T.; Kobbe, P.; Fischer, H. Scaffolds for bone healing: Concepts, materials and evidence. Injury 2011, 42, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Depan, D.; Girase, B.; Shah, J.S.; Misra, R.D.K. Structure–process–property relationship of the polar graphene oxide-mediated cellular response and stimulated growth of osteoblasts on hybrid chitosan network structure nanocomposite scaffolds. Acta Biomater. 2011, 7, 3432–3445. [Google Scholar] [CrossRef]
- Arnold, A.M.; Holt, B.D.; Daneshmandi, L.; Laurencin, C.T.; Sydlik, S.A. Phosphate graphene as an intrinsically osteoinductive scaffold for stem cell-driven bone regeneration. Proc. Natl. Acad. Sci. USA 2019, 116, 4855–4860. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, H.; Pan, Q.; Gao, C.; Wang, Y.; Yang, S.; Zan, X.; Guan, Y. Graphene Oxide and Lysozyme Ultrathin Films with Strong Antibacterial and Enhanced Osteogenesis. Langmuir 2019, 35, 6752–6761. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Crook, J.M.; Wallace, G.G. 3D Printing of Cytocompatible Graphene/Alginate Scaffolds for Mimetic Tissue Constructs. Front. Bioeng. Biotechnol. 2020, 8, 824. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, A.; Liu, Y.; Jiao, D.; Zeng, D.; Wang, X.; Cao, L.; Jiang, X. Enhanced bone regeneration of the silk fibroin electrospun scaffolds through the modification of the graphene oxide functionalized by BMP-2 peptide. Int. J. Nanomed. 2019, 14, 733–751. [Google Scholar] [CrossRef]
- Chal, J.; Pourquié, O. Making muscle: Skeletal myogenesis in vivo and in vitro. Development 2017, 144, 2104–2122. [Google Scholar] [CrossRef] [PubMed]
- Ciciliot, S.; Schiaffino, S. Regeneration of mammalian skeletal muscle. Basic mechanisms and clinical implications. Curr. Pharm. Des. 2010, 16, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Corona, B.T.; Rivera, J.C.; Owens, J.G.; Wenke, J.C.; Rathbone, C.R. Volumetric muscle loss leads to permanent disability following extremity trauma. J. Rehabil. Res. Dev. 2015, 52, 785–792. [Google Scholar] [CrossRef]
- Gilbert, P.M.; Havenstrite, K.L.; Magnusson, K.E.G.; Sacco, A.; Leonardi, N.A.; Kraft, P.; Nguyen, N.K.; Thrun, S.; Lutolf, M.P.; Blau, H.M. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 2010, 329, 1078–1081. [Google Scholar] [CrossRef]
- Jun, I.; Jeong, S.; Shin, H. The stimulation of myoblast differentiation by electrically conductive sub-micron fibers. Biomaterials 2009, 30, 2038–2047. [Google Scholar] [CrossRef]
- Nikolić, N.; Skaret Bakke, S.; Tranheim Kase, E.; Rudberg, I.; Flo Halle, I.; Rustan, A.C.; Thoresen, G.H.; Aas, V. Electrical Pulse Stimulation of Cultured Human Skeletal Muscle Cells as an In Vitro Model of Exercise. PLoS ONE 2012, 7, e33203. [Google Scholar] [CrossRef]
- Jo, S.B.; Erdenebileg, U.; Dashnyam, K.; Jin, G.-Z.; Cha, J.-R.; El-Fiqi, A.; Knowles, J.C.; Patel, K.D.; Lee, H.-H.; Lee, J.-H.; et al. Nano-graphene oxide/polyurethane nanofibers: Mechanically flexible and myogenic stimulating matrix for skeletal tissue engineering. J. Tissue Eng. 2020, 11, 2041731419900424. [Google Scholar] [CrossRef]
- Park, J.; Choi, J.H.; Kim, S.; Jang, I.; Jeong, S.; Lee, J.Y. Micropatterned conductive hydrogels as multifunctional muscle-mimicking biomaterials: Graphene-incorporated hydrogels directly patterned with femtosecond laser ablation. Acta Biomater. 2019, 97, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Manoharan, V.; Cheung, L.; Lee, S.; Cha, B.-H.; Newman, P.; Farzad, R.; Mehrotra, S.; Zhang, K.; Khan, F.; et al. Nanoparticle-Based Hybrid Scaffolds for Deciphering the Role of Multimodal Cues in Cardiac Tissue Engineering. ACS Nano 2019, 13, 12525–12539. [Google Scholar] [CrossRef]
- Choe, G.; Kim, S.-W.; Park, J.; Park, J.; Kim, S.; Kim, Y.S.; Ahn, Y.; Jung, D.-W.; Williams, D.R.; Lee, J.Y. Anti-oxidant activity reinforced reduced graphene oxide/alginate microgels: Mesenchymal stem cell encapsulation and regeneration of infarcted hearts. Biomaterials 2019, 225, 119513. [Google Scholar] [CrossRef]
- McFarlane, S. Attraction vs. repulsion: The growth cone decides. Biochem. Cell Biol. 2000, 78, 563–568. [Google Scholar] [CrossRef]
- Zuchero, J.B.; Barres, B.A. Glia in mammalian development and disease. Development 2015, 142, 3805–3809. [Google Scholar] [CrossRef] [PubMed]
- Mietto, B.S.; Mostacada, K.; Martinez, A.M.B. Neurotrauma and inflammation: CNS and PNS responses. Mediat. Inflamm. 2015, 2015, 251204. [Google Scholar] [CrossRef]
- Yiu, G.; He, Z. Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 2006, 7, 617–627. [Google Scholar] [CrossRef]
- Rolls, A.; Shechter, R.; Schwartz, M. The bright side of the glial scar in CNS repair. Nat. Rev. Neurosci. 2009, 10, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.; Ming, G. Roles of channels and receptors in the growth cone during PNS axonal regeneration. Exp. Neurol. 2010, 223, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.M.; Knight, W.A. 4th Emergency Neurological Life Support: Traumatic Spine Injury. Neurocrit. Care 2017, 27, 170–180. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Nori, S.; Tetreault, L.; Wilson, J.; Kwon, B.; Harrop, J.; Choi, D.; Fehlings, M.G. Traumatic Spinal Cord Injury-Repair and Regeneration. Neurosurgery 2017, 80, S9–S22. [Google Scholar] [CrossRef]
- Qian, Y.; Zhao, X.; Han, Q.; Chen, W.; Li, H.; Yuan, W. An integrated multi-layer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration. Nat. Commun. 2018, 9, 323. [Google Scholar] [CrossRef]
- Shah, S.; Yin, P.T.; Uehara, T.M.; Chueng, S.-T.D.; Yang, L.; Lee, K.-B. Guiding stem cell differentiation into oligodendrocytes using graphene-nanofiber hybrid scaffolds. Adv. Mater. 2014, 26, 3673–3680. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Teshima, T.F.; Nakashima, H.; Ueno, Y. Graphene-based neuron encapsulation with controlled axonal outgrowth. Nanoscale 2019, 11, 13249–13259. [Google Scholar] [CrossRef]
- Javadi, M.; Gu, Q.; Naficy, S.; Farajikhah, S.; Crook, J.M.; Wallace, G.G.; Beirne, S.; Moulton, S.E. Conductive Tough Hydrogel for Bioapplications. Macromol. Biosci. 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.; Mo, X.; Wang, H. Reduced Graphene Oxide-Encapsulated Microfiber Patterns Enable Controllable Formation of Neuronal-Like Networks. Adv. Mater. 2020, 32, 2004555. [Google Scholar] [CrossRef]
- Bisht, G.S.; Canton, G.; Mirsepassi, A.; Kulinsky, L.; Oh, S.; Dunn-Rankin, D.; Madou, M.J. Controlled continuous patterning of polymeric nanofibers on three-dimensional substrates using low-voltage near-field electrospinning. Nano Lett. 2011, 11, 1831–1837. [Google Scholar] [CrossRef]
- Chamberlain, K.A.; Nanescu, S.E.; Psachoulia, K.; Huang, J.K. Oligodendrocyte regeneration: Its significance in myelin replacement and neuroprotection in multiple sclerosis. Neuropharmacology 2016, 110, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, M.; Mozafari, S.; Glatza, M.; Starost, L.; Velychko, S.; Hallmann, A.-L.; Cui, Q.-L.; Schambach, A.; Kim, K.-P.; Bachelin, C.; et al. Rapid and efficient generation of oligodendrocytes from human induced pluripotent stem cells using transcription factors. Proc. Natl. Acad. Sci. 2017, 114, E2243–E2252. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, K.; Das, G.; Khan, J.; Gupta, V.; Barman, S.; Adak, A.; Ghosh, S. Neuro-Regenerative Choline-Functionalized Injectable Graphene Oxide Hydrogel Repairs Focal Brain Injury. ACS Chem. Neurosci. 2019, 10, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.R.U.; Augustine, R.; Zahid, A.A.; Ahmed, R.; Tariq, M.; Hasan, A. Reduced Graphene Oxide Incorporated GelMA Hydrogel Promotes Angiogenesis For Wound Healing Applications. Int. J. Nanomed. 2019, 14, 9603–9617. [Google Scholar] [CrossRef]
- Li, J.; Zhou, C.; Luo, C.; Qian, B.; Liu, S.; Zeng, Y.; Hou, J.; Deng, B.; Sun, Y.; Yang, J.; et al. N-acetyl cysteine-loaded graphene oxide-collagen hybrid membrane for scarless wound healing. Theranostics 2019, 9, 5839–5853. [Google Scholar] [CrossRef]
- Kim, S.; Moon, J.-M.; Choi, J.S.; Cho, W.K.; Kang, S.M. Mussel-Inspired Approach to Constructing Robust Multilayered Alginate Films for Antibacterial Applications. Adv. Funct. Mater. 2016, 26, 4099–4105. [Google Scholar] [CrossRef]
- Yan, X.; Li, F.; Hu, K.-D.; Xue, J.; Pan, X.-F.; He, T.; Dong, L.; Wang, X.-Y.; Wu, Y.-D.; Song, Y.-H.; et al. Nacre-mimic Reinforced Ag@reduced Graphene Oxide-Sodium Alginate Composite Film for Wound Healing. Sci. Rep. 2017, 7, 13851. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellet, P.; Gasparotto, M.; Pressi, S.; Fortunato, A.; Scapin, G.; Mba, M.; Menna, E.; Filippini, F. Graphene-Based Scaffolds for Regenerative Medicine. Nanomaterials 2021, 11, 404. https://doi.org/10.3390/nano11020404
Bellet P, Gasparotto M, Pressi S, Fortunato A, Scapin G, Mba M, Menna E, Filippini F. Graphene-Based Scaffolds for Regenerative Medicine. Nanomaterials. 2021; 11(2):404. https://doi.org/10.3390/nano11020404
Chicago/Turabian StyleBellet, Pietro, Matteo Gasparotto, Samuel Pressi, Anna Fortunato, Giorgia Scapin, Miriam Mba, Enzo Menna, and Francesco Filippini. 2021. "Graphene-Based Scaffolds for Regenerative Medicine" Nanomaterials 11, no. 2: 404. https://doi.org/10.3390/nano11020404
APA StyleBellet, P., Gasparotto, M., Pressi, S., Fortunato, A., Scapin, G., Mba, M., Menna, E., & Filippini, F. (2021). Graphene-Based Scaffolds for Regenerative Medicine. Nanomaterials, 11(2), 404. https://doi.org/10.3390/nano11020404







