Porous Clay Heterostructure with Alginate Encapsulation for Toluene Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PCH
2.3. Encapsulation of PCH Using Alginate
2.4. Characterization of Alginate PCH Bead
2.5. Toluene Adsorption–Desorption Experiments
3. Results
3.1. Characterization of Bentonite, PCH, and Alg-PCH
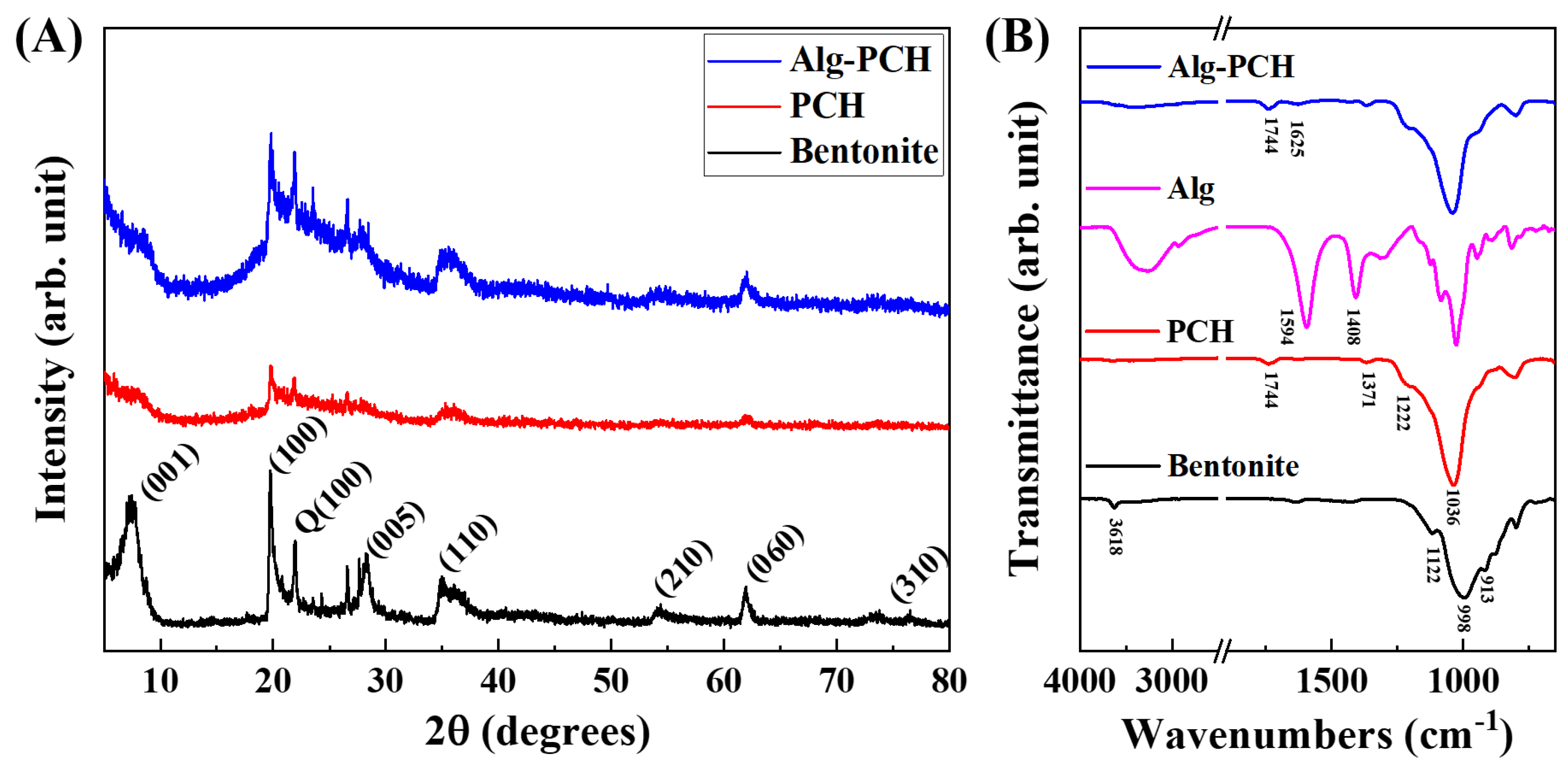
3.1.1. Physicochemical Property Analysis
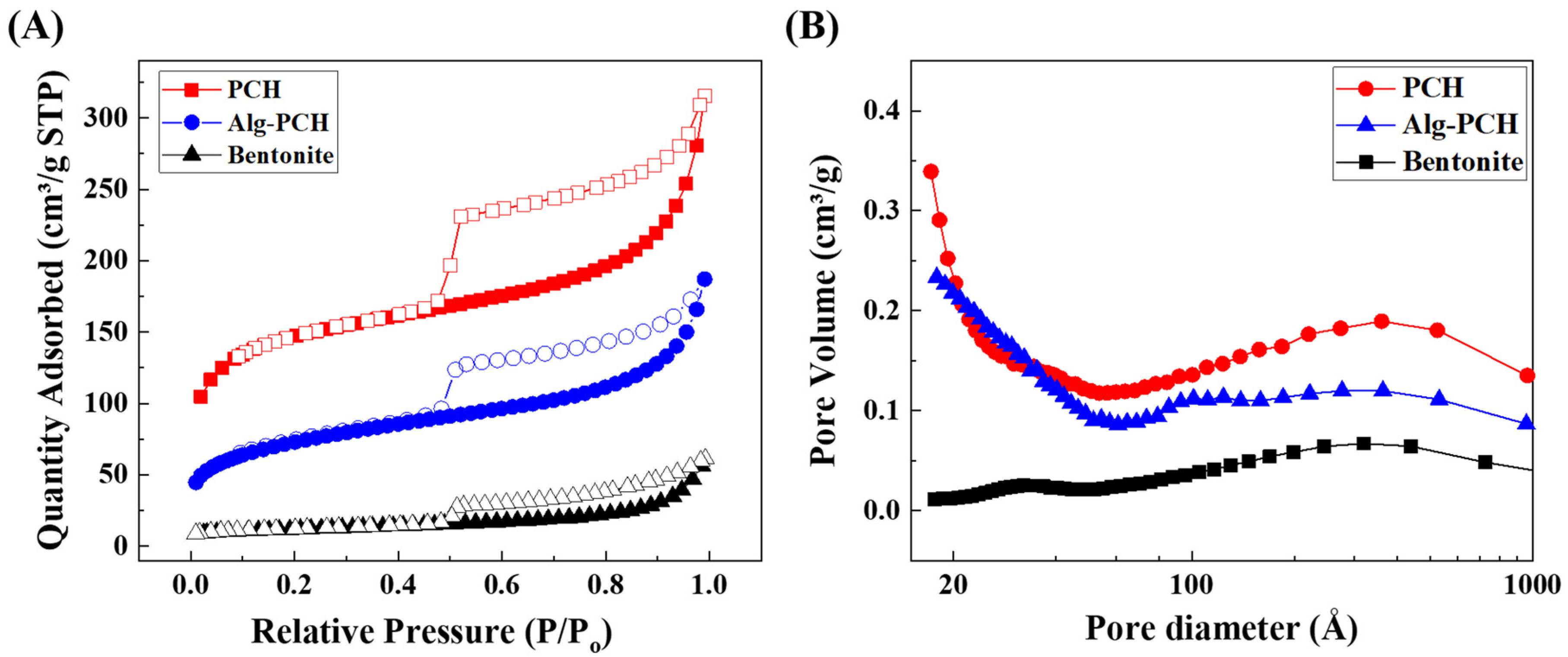
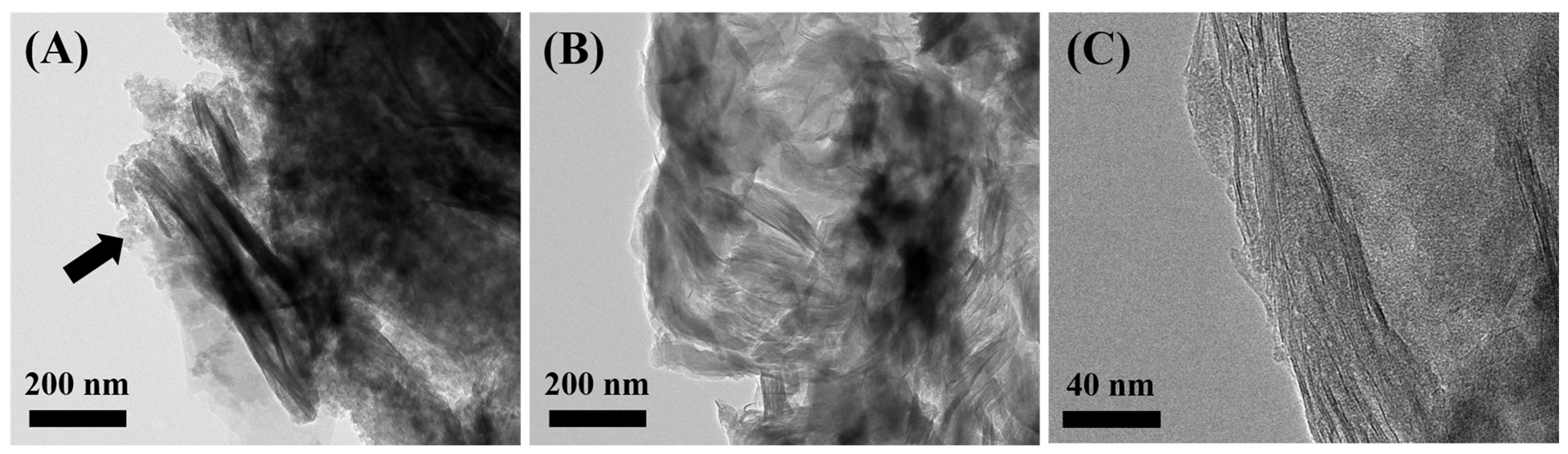
3.1.2. Pore Structure and Morphological Analysis
3.1.3. Thermal Analysis
3.2. Optimization of Alg-PCH Preparation Condition for Toluene Adsorption
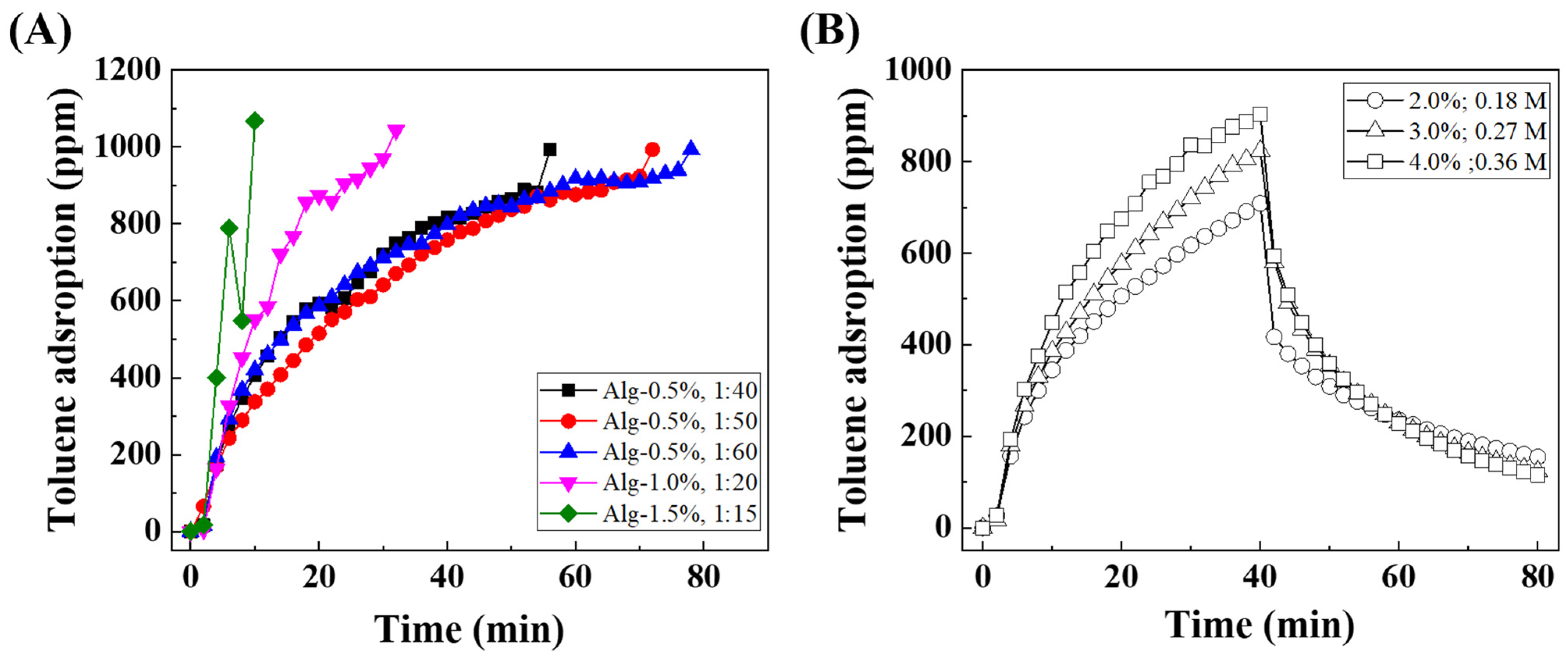
3.2.1. Alginate Concentration and Alg:PCH Ratio
3.2.2. CaCl2 Concentration
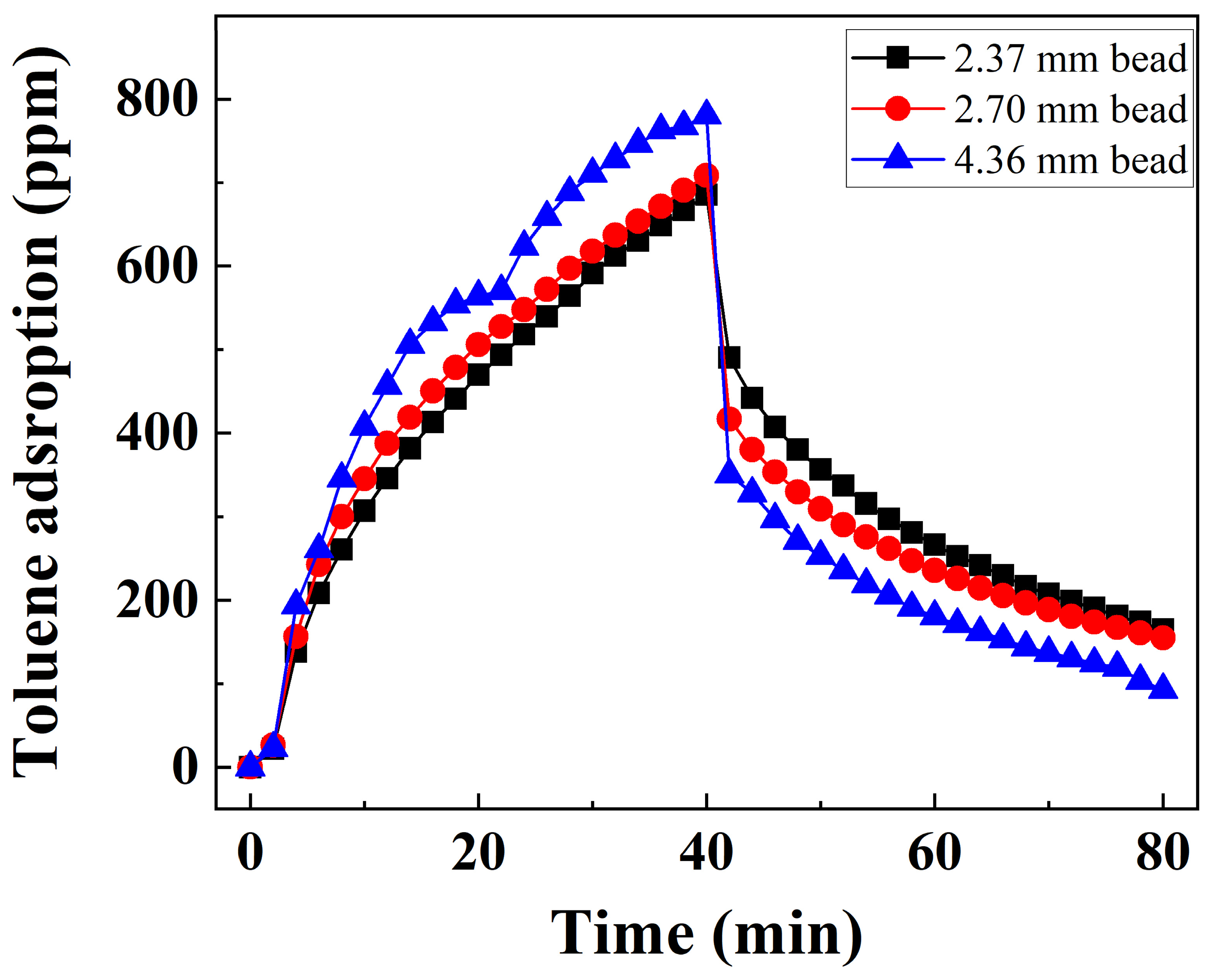
3.2.3. Bead Size
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Nie, L.; Li, J.; Wang, Y.; Wang, G.; Wang, J.; Hao, Z. Characterization and assessment of volatile organic compounds (VOCs) emissions from typical industries. Chin. Sci. Bull. 2013, 58, 724–730. [Google Scholar] [CrossRef]
- Fiore, A.M.; Naik, V.; Spracklen, D.V.; Steiner, A.; Unger, N.; Prather, M.; Bergmann, D.; Cameron-Smith, P.; Cionni, I.; Collins, W.J.; et al. Global air quality and climate. Chem. Soc. Rev. 2012, 41, 6663–6683. [Google Scholar] [CrossRef] [PubMed]
- Derwent, R.G. Sources, distributions, and fates of VOCs in the atmosphere. Issues Environ. Sci. Technol. 1995, 4, 1–16. [Google Scholar] [CrossRef]
- Bari, M.A.; Kindzierski, W.B. Ambient volatile organic compounds (VOCs) in Calgary, Alberta: Sources and screening health risk assessment. Sci. Total Environ. 2018, 631, 627–640. [Google Scholar] [CrossRef] [PubMed]
- An, T.; Huang, Y.; Li, G.; He, Z.; Chen, J.; Zhang, C. Pollution profiles and health risk assessment of VOCs emitted during e-waste dismantling processes associated with different dismantling methods. Environ. Int. 2014, 73, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Batterman, S.; Godwin, C. VOCs in industrial, urban and suburban neighborhoods, Part 1: Indoor and outdoor concentrations, variation, and risk drivers. Atmos. Environ. 2008, 42, 2083–2100. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Air Pollutant Emissions Trends Data. Available online: https://www.epa.gov/air-emissions-inventories/air-pollutant-emissions-trends-data (accessed on 5 January 2021).
- Li, M.; Liu, H.; Geng, G.; Hong, C.; Liu, F.; Song, Y.; Tong, D.; Zheng, B.; Cui, H.; Man, H.; et al. Anthropogenic emission inventories in China: A review. Nat. Sci. Rev. 2017, 4, 834–866. [Google Scholar] [CrossRef]
- Im, J.; Kim, H.; Kim, M.; Lee, J.; Lee, S.; Lee, C. A Study on the Variation of Hazardous Pollutant Emissions in Korea from 2006 to 2015. J. Environ. Health Sci. 2018, 44, 15–23. [Google Scholar] [CrossRef]
- Parrish, D.D. Critical evaluation of US on-road vehicle emission inventories. Atmos. Environ. 2006, 40, 2288–2300. [Google Scholar] [CrossRef]
- Unwin, J.; Cocker, J.; Scobbie, E.; Chambers, H. An Assessment of Occupational Exposure to Polycyclic Aromatic Hydrocarbons in the UK. Ann. Occup. Hyg. 2006, 50, 395–403. [Google Scholar] [CrossRef]
- Singh, P.; Chauhan, S.R. Carbonyl and aromatic hydrocarbon emissions from diesel engine exhaust using different feedstock: A review. Renew. Sustain. Energy Rev. 2016, 63, 269–291. [Google Scholar] [CrossRef]
- Samet, J. Environmental Controls and Lung Disease: Report of the ATS Workshop on Environmental Controls and Lung Disease, Santa Fe, New Mexico, March 24–26, 1988. Am. Rev. Respir. 1990, 142, 915–939. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Shao, M.; Lu, S.; Wang, B. Source profiles of volatile organic compounds associated with solvent use in Beijing, China. Atmos. Environ. 2010, 44, 1919–1926. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Yang, Z.; Wang, P.; Yan, Y.; Ran, J. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: A review. Sep. Purif. Technol. 2020, 235, 116213. [Google Scholar] [CrossRef]
- Chuang, C.L.; Chiang, P.C.; Chang, E.E. Modeling VOCs adsorption onto activated carbon. Chemos. Oxford 2003, 53, 17–27. [Google Scholar] [CrossRef]
- Detchanamurthy, S.; Gostomski, P.A. Biofiltration for treating VOCs: An overview. Rev. Environ. Sci. Bio. Technol. 2012, 11, 231–241. [Google Scholar] [CrossRef]
- Parthasarathy, G.; El-Halwagi, M. Optimum mass integration strategies for condensation and allocation of multicomponent VOCs. Chem. Eng. Sci. 2000, 55, 881–895. [Google Scholar] [CrossRef]
- Tichenor, B.A.; Palazzolo, M.A. Destruction of volatile organic compounds via catalytic incineration. Environ. Prog. 1987, 6, 172–176. [Google Scholar] [CrossRef]
- Ruhl, M.J. Recover VOCs via adsorption on activated carbon. Chem. Eng. Prog. 1993, 89, 37–41. [Google Scholar]
- Gupta, V.K.; Verma, N. Removal of volatile organic compounds by cryogenic condensation followed by adsorption. Chem. Eng. Sci. 2002, 57, 2679–2696. [Google Scholar] [CrossRef]
- Khan, F.I.; Ghoshal, A.K. Removal of volatile organic compounds from polluted air. J. Loss Prev. Process Ind. 2000, 13, 527–545. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mat. 2017, 338, 102–123. [Google Scholar] [CrossRef] [PubMed]
- Djilani, C.; Zaghdoudi, R.; Modarressi, A.; Rogalski, M.; Djazi, F.; Lallam, A. Elimination of organic micropollutants by adsorption on activated carbon prepared from agricultural waste. Chem. Eng. J. 2012, 189, 203–212. [Google Scholar] [CrossRef]
- Nasrullah, A.; Bhat, A.H.; Naeem, A.; Isa, M.H.; Danish, M. High surface area mesoporous activated carbon-alginate beads for efficient removal of methylene blue. Int. J. Biol. Macromol. 2018, 107, 1792–1799. [Google Scholar] [CrossRef] [PubMed]
- Cazetta, A.L.; Junior, O.P.; Vargas, A.M.M.; Da Silva, A.P.; Zou, X.; Asefa, T.; Almeida, V.C. Thermal regeneration study of high surface area activated carbon obtained from coconut shell: Characterization and application of response surface methodology. J. Anal. App. Pyrolysis 2013, 101, 53–60. [Google Scholar] [CrossRef]
- Jarraya, I.; Fourmentin, S.; Benzina, M.; Bouaziz, S. VOC adsorption on raw and modified clay materials. Chem. Geol. 2010, 275, 1–8. [Google Scholar] [CrossRef]
- Yang, X.; Yi, H.; Tang, X.; Zhao, S.; Yang, Z.; Ma, Y.; Feng, T.; Cui, X. Behaviors and kinetics of toluene adsorption-desorption on activated carbons with varying pore structure. J. Environ. Sci. 2018, 67, 104–114. [Google Scholar] [CrossRef]
- Takahashi, N.; Ushiki, I.; Hamabe, Y.; Ota, M.; Sato, Y.; Inomata, H. Measurement and prediction of desorption behavior of five volatile organic compounds (acetone, n-hexane, methanol, toluene, and n-decane) from activated carbon for supercritical carbon dioxide regeneration. J. Supercrit. Fluids 2016, 107, 226–233. [Google Scholar] [CrossRef]
- Zaitan, H.; Bianchi, D.; Achak, O.; Chafik, T. A comparative study of the adsorption and desorption of o-xylene onto bentonite clay and alumina. J. Hazard. Mat. 2008, 153, 852–859. [Google Scholar] [CrossRef]
- Qu, F.; Zhu, L.; Yang, K. Adsorption behaviors of volatile organic compounds. J. Hazard. Mat. 2009, 170, 7. [Google Scholar] [CrossRef]
- Pires, J.; Bestilleiro, M.; Pinto, M.; Gil, A. Selective adsorption of carbon dioxide, methane and ethane by porous clays heterostructures. Sep. Purif. Technol. 2008, 61, 161–167. [Google Scholar] [CrossRef]
- Bellir, K.; Lehocine, M.B.; Meniai, A.-H. Zinc removal from aqueous solutions by adsorption onto bentonite. Desalin. Water Treat. 2013, 51, 5035–5048. [Google Scholar] [CrossRef]
- Okada, T.; Seki, Y.; Ogawa, M. Designed nanostructures of clay for controlled adsorption of organic compounds. J. Nanosci. Nanotechnol. 2014, 14, 2121–2134. [Google Scholar] [CrossRef]
- Celis, R.; Hermosin, M.C.; Cornejo, J. Heavy metal adsorption by functionalized clays. Environ. Sci. Technol. 2000, 34, 4593–4599. [Google Scholar] [CrossRef]
- Gier, S.; Johns, W.D. Heavy metal-adsorption on micas and clay minerals studied by X-ray photoelectron spectroscopy. App. Clay Sci. 2000, 16, 289–299. [Google Scholar] [CrossRef]
- Chen, H.; Koopal, L.K.; Xiong, J.; Avena, M.; Tan, W. Mechanisms of soil humic acid adsorption onto montmorillonite and kaolinite. J. Colloid Interface Sci. 2017, 504, 457–467. [Google Scholar] [CrossRef]
- Arellano-Cárdenas, S.; Gallardo-Velázquez, T.; Osorio-Revilla, G.; López-Cortéz, M.D.S.; Gómez-Perea, B. Adsorption of Phenol and Dichlorophenols from Aqueous Solutions by Porous Clay Heterostructure (PCH). J. Mex. Chem. Soc. 2005, 49, 287–291. [Google Scholar]
- Cecilia, J.A.; García-Sancho, C.; Franco, F. Montmorillonite based porous clay heterostructures: Influence of Zr in the structure and acidic properties. Microporous Mesoporous Mat. 2013, 176, 95–102. [Google Scholar] [CrossRef]
- Rezaei, F.; Webley, P. Optimum structured adsorbents for gas separation processes. Chem. Eng. Sci. 2009, 64, 5182–5191. [Google Scholar] [CrossRef]
- Shim, W.G.; Lee, J.W.; Moon, H. Adsorption equilibrium and column dynamics of VOCs on MCM-48 depending on pelletizing pressure. Microporous Mesoporous Mat. 2006, 88, 112–125. [Google Scholar] [CrossRef]
- Küsgens, P.; Zgaverdea, A.; Fritz, H.G.; Siegle, S.; Kaskel, S. Metal-organic frameworks in monolithic structures. J. Am. Ceram. Soc. 2010, 93, 2476–2479. [Google Scholar] [CrossRef]
- Zaitseva, N.; Zaitsev, V.; Walcarius, A. Chromium(VI) removal via reduction–sorption on bi-functional silica adsorbents. J. Hazard. Mat. 2013, 250, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Suchithra, P.S.; Vazhayal, L.; Peer Mohamed, A.; Ananthakumar, S. Mesoporous organic–inorganic hybrid aerogels through ultrasonic assisted sol–Gel intercalation of silica–PEG in bentonite for effective removal of dyes, volatile organic pollutants and petroleum products from aqueous solution. Chem. Eng. J. 2012, 200, 589–600. [Google Scholar] [CrossRef]
- Samiey, B.; Cheng, C.-H.; Wu, J. Organic–Inorganic hybrid polymers as adsorbents for removal of heavy metal ions from solutions: A review. Materials 2014, 7, 673–726. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Huang, X.; Tang, Z.; Huang, Q.; Niu, F.; Wang, X. Polymer-based nanocomposites for heavy metal ions removal from aqueous solution: A review. Polymer Chem. 2018, 9, 3562–3582. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog Polymer Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Kwon, O.-H.; Kim, J.-O.; Cho, D.-W.; Kumar, R.; Baek, S.H.; Kurade, M.B.; Jeon, B.-H. Adsorption of As(III), As(V) and Cu(II) on zirconium oxide immobilized alginate beads in aqueous phase. Chemosphere 2016, 160, 126–133. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef]
- Yang, P.; Song, M.; Kim, D.; Jung, S.P.; Hwang, Y. Synthesis conditions of porous clay heterostructure (PCH) optimized for volatile organic compounds (VOC) adsorption. Korean J. Chem. Eng. 2019, 36, 1806–1813. [Google Scholar] [CrossRef]
- Park, J.-A.; Kang, J.-K.; Kim, J.-H.; Kim, S.-B.; Yu, S.; Kim, T.-H. Bacteriophage removal in various clay minerals and clay-amended soils. Environ. Eng. Res. 2015, 20, 133–140. [Google Scholar] [CrossRef]
- Lee, B.-B.; Ravindra, P.; Chan, E.-S. Size and Shape of Calcium Alginate Beads Produced by Extrusion Dripping. Chem. Eng. Technol. 2013, 36, 1627–1642. [Google Scholar] [CrossRef]
- Alabarse, F.G.; Conceição, R.V.; Balzaretti, N.M.; Schenato, F.; Xavier, A.M. In-situ FTIR analyses of bentonite under high-pressure. App. Clay Sci. 2011, 51, 202–208. [Google Scholar] [CrossRef]
- Mandal, S.; Patil, V.S.; Mayadevi, S. Alginate and hydrotalcite-like anionic clay composite systems: Synthesis, characterization and application studies. Microporous Mesoporous Mat. 2012, 158, 241–246. [Google Scholar] [CrossRef]
- He, Y.; Wu, Z.; Tu, L.; Han, Y.; Zhang, G.; Li, C. Encapsulation and characterization of slow-release microbial fertilizer from the composites of bentonite and alginate. App. Clay Sci. 2015, 109, 68–75. [Google Scholar] [CrossRef]
- Basir, N.M.; Lintang, H.O.; Endud, S. Phosphotungstic acid supported on acid-leached porous kaolin for friedel-crafts acylation of anisole. J. Teknol. 2015, 76. [Google Scholar] [CrossRef]
- Gârea, S.A.; Mihai, A.I.; Vasile, E.; Nistor, C.; Sârbu, A.; Mitran, R. Synthesis of new porous clay heterostructures: The influence of co-surfactant type. Mat. Chem. Phys. 2016, 179, 17–26. [Google Scholar] [CrossRef]
- Kusuktham, B.; Prasertgul, J.; Srinun, P. Morphology and Property of Calcium Silicate Encapsulated with Alginate Beads. Silicon 2014, 6, 191–197. [Google Scholar] [CrossRef]
- Ambroz, F.; Macdonald, T.J.; Martis, V.; Parkin, I.P. Evaluation of the BET Theory for the Characterization of Meso and Microporous MOFs. Small Methods 2018, 2, 1800173. [Google Scholar] [CrossRef]
- Kaufhold, S.; Dohrmann, R.; Klinkenberg, M.; Siegesmund, S.; Ufer, K. N2-BET specific surface area of bentonites. J. Colloid Interface Sci. 2010, 349, 275–282. [Google Scholar] [CrossRef]
- Huff, W.D.; Whiteman, J.A.; Curtis, C.D. Investigation of a K-Bentonite by X-Ray Powder Diffraction and Analytical Transmission Electron Microscopy. Clays Clay Miner. 1988, 36, 83–93. [Google Scholar] [CrossRef]
- Pereira, R.; Tojeira, A.; Vaz, D.C.; Mendes, A.; Bártolo, P. Preparation and characterization of films based on alginate and aloe vera. Int. J. Polymer Anal. Charact. 2011, 16, 449–464. [Google Scholar] [CrossRef]
- Lee, D.W. Formulation and Characterization of the Metal-Organic Compound UiO-66. Master’s Thesis, University of Oslo, Oslo, Norway, 1 August 2017. [Google Scholar]
- Zhang, X.; Yang, Y.; Lv, X.; Wang, Y.; Liu, N.; Chen, D.; Cui, L. Adsorption/desorption kinetics and breakthrough of gaseous toluene for modified microporous-mesoporous UiO-66 metal organic framework. J. Hazard. Mat. 2019, 366, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhou, K.; Li, H.; Xu, X.; Liu, B.; Li, H.; Zeng, Z.; Ma, W.; Li, L. Pressure swing adsorption properties of activated carbon for methanol, acetone and toluene. Chem. Eng. J. 2020, 127384. [Google Scholar] [CrossRef]
- Wang, C.-M.; Chang, K.-S.; Chung, T.-W.; Wu, H. Adsorption Equilibria of Aromatic Compounds on Activated Carbon, Silica Gel, and 13X Zeolite. J. Chem. Eng. Data 2004, 49, 527–531. [Google Scholar] [CrossRef]
- Liu, C.; Cai, W.; Liu, L. Hydrothermal carbonization synthesis of Al-pillared montmorillonite@carbon composites as high performing toluene adsorbents. App. Clay Sci. 2018, 162, 113–120. [Google Scholar] [CrossRef]
- Vellingiri, K.; Kumar, P.; Deep, A.; Kim, K.-H. Metal-organic frameworks for the adsorption of gaseous toluene under ambient temperature and pressure. Chem. Eng. J. 2017, 307, 1116–1126. [Google Scholar] [CrossRef]
- Stuckert, N.R.; Yang, R.T. CO2 Capture from the Atmosphere and Simultaneous Concentration Using Zeolites and Amine-Grafted SBA-15. Environ. Sci. Technol. 2011, 45, 10257–10264. [Google Scholar] [CrossRef]
- Lu, X.; He, J.; Xie, J.; Zhou, Y.; Liu, S.; Zhu, Q.; Lu, H. Preparation of hydrophobic hierarchical pore carbon–Silica composite and its adsorption performance toward volatile organic compounds. J. Environ. Sci. 2020, 87, 39–48. [Google Scholar] [CrossRef]

| Sample | BET Surface Area (m2/g) | BJH Pore Volume (cm3/g) | BJH Pore Size (nm) |
|---|---|---|---|
| Bentonite | 43 | 0.087 | 13.46 |
| PCH | 538 | 0.336 | 7.32 |
| Alg-PCH | 256 | 0.242 | 6.44 |
| Alginate Concentration (%) | Alginate:PCH (w/w) | Adsorption Capacity (mg/g) | Formation |
|---|---|---|---|
| 0.5% | 1:10 | N.D. | × |
| 1:20 | N.D. | △ | |
| 1:40 | 59.13 | ○ | |
| 1:50 | 71.86 | ○ | |
| 1:60 | 64.73 | ○ | |
| 1.0% | 1:20 | 31.43 | ○ |
| 1:30 | N.D. | × | |
| 1.5% | 1:15 | 15.32 | ○ |
| 1:20 | N.D. | × |
| CaCl2 Concentration (w/v) | Adsorption Capacity (mg/g) | Desorption Capacity (mg/g) | Desorption Efficacy (%) |
|---|---|---|---|
| 2% | 61.63 | 29.66 | 48.5 |
| 3% | 53.34 | 27.64 | 51.9 |
| 4% | 44.55 | 31.87 | 71.5 |
| Sample | Fixed Bed Adsorption | BET Surface Area (m2/g) | Ref. | |
|---|---|---|---|---|
| Qad (mg/g) | Pr (P/P0) | |||
| Granular activated carbon | 87.9 | 0.045 | - | [66] |
| Granular silica gel | 37.5 | 0.045 | - | [66] |
| Granular 13X zeolite | 7.9 | 0.091 | - | [66] |
| Al-Mt@C powder | 39.9 | 0.029 | 163 | [67] |
| MOF-5 powder | 32.9 | 0.0026 | 424 | [68] |
| MIL-101(Fe) powder | 98.3 | 0.0026 | 377 | [68] |
| PCH powder | 199.7 | 0.029 | 538 | This study |
| Alg-PCH bead | 64.7 | 0.029 | 256 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, Y.; Kim, T.-H.; Kim, D.; Hwang, Y. Porous Clay Heterostructure with Alginate Encapsulation for Toluene Removal. Nanomaterials 2021, 11, 388. https://doi.org/10.3390/nano11020388
Son Y, Kim T-H, Kim D, Hwang Y. Porous Clay Heterostructure with Alginate Encapsulation for Toluene Removal. Nanomaterials. 2021; 11(2):388. https://doi.org/10.3390/nano11020388
Chicago/Turabian StyleSon, Yeongkyun, Tae-Hyun Kim, Daekeun Kim, and Yuhoon Hwang. 2021. "Porous Clay Heterostructure with Alginate Encapsulation for Toluene Removal" Nanomaterials 11, no. 2: 388. https://doi.org/10.3390/nano11020388
APA StyleSon, Y., Kim, T.-H., Kim, D., & Hwang, Y. (2021). Porous Clay Heterostructure with Alginate Encapsulation for Toluene Removal. Nanomaterials, 11(2), 388. https://doi.org/10.3390/nano11020388










