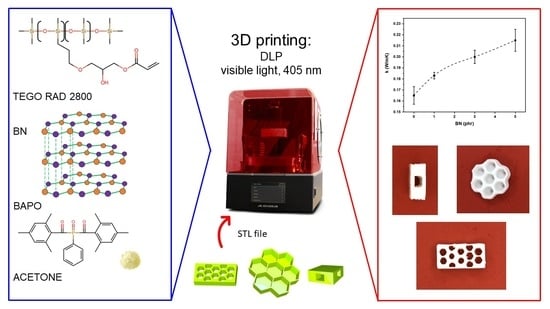
3D Printing of PDMS-Like Polymer Nanocomposites with Enhanced Thermal Conductivity: Boron Nitride Based Photocuring System
Abstract
1. Introduction
2. Materials and Methods
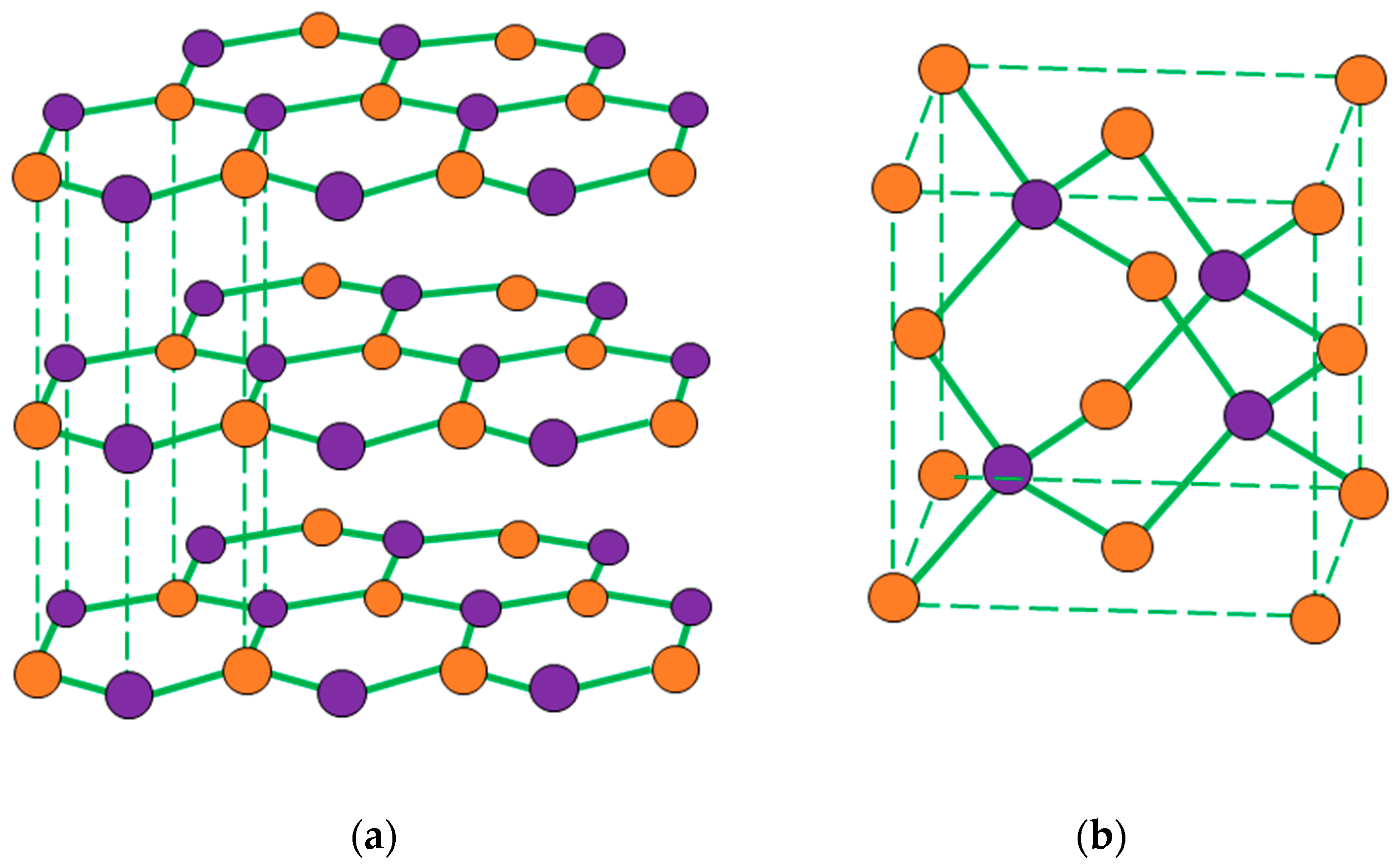
2.1. Materials
2.2. Formulation Preparation and 3D-Printing
2.3. Charcaterization
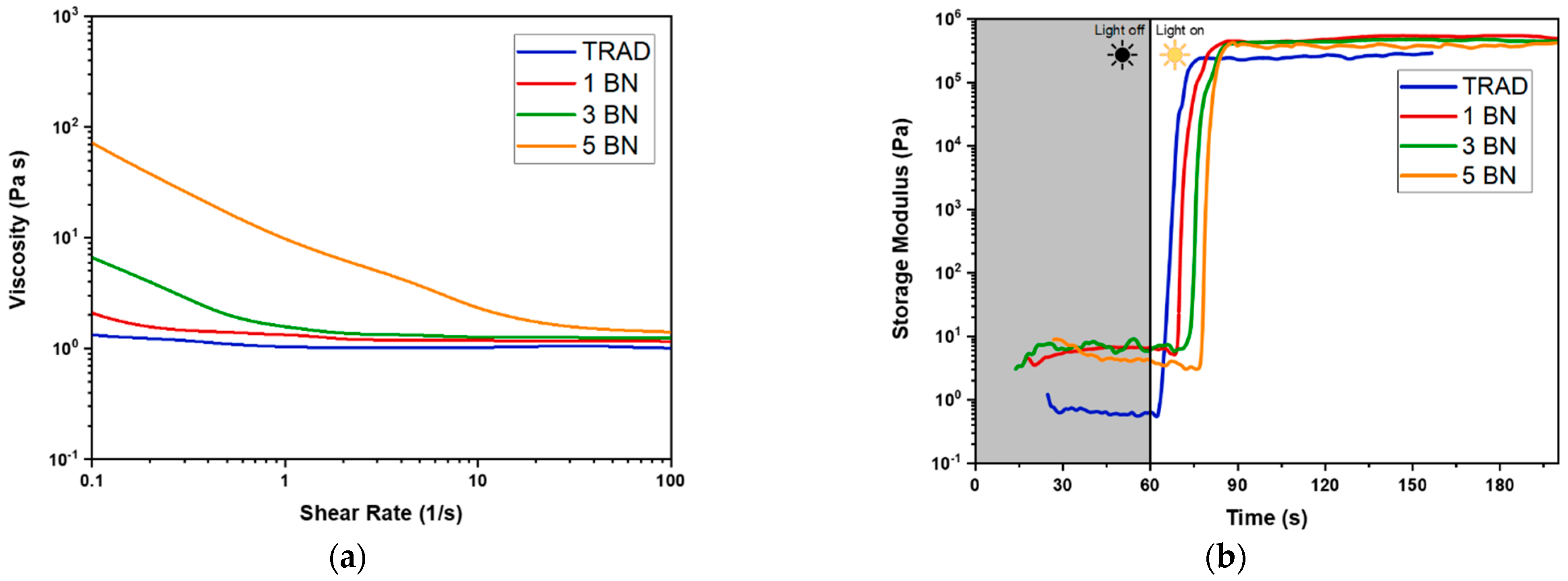
2.3.1. Photorheological and Viscosity Test
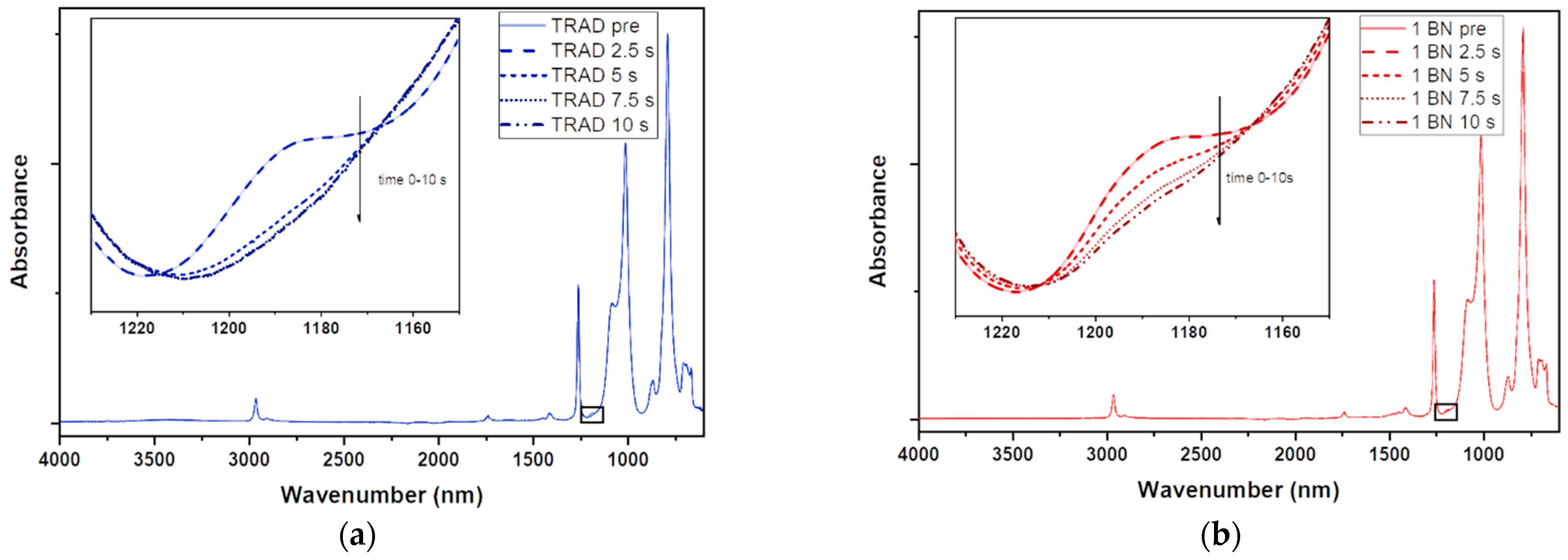
2.3.2. ATR-FTIR Spectroscopy
2.3.3. Extraction of Unreacted Resin
2.3.4. Scanning Electron Microscopy
2.3.5. Dynamic Mechanical Thermal Analysis (DMTA)
2.3.6. Tensile Analysis
2.3.7. Thermal Analysis
3. Results and Discussion
3.1. Characterization of the Photocurable Formulations
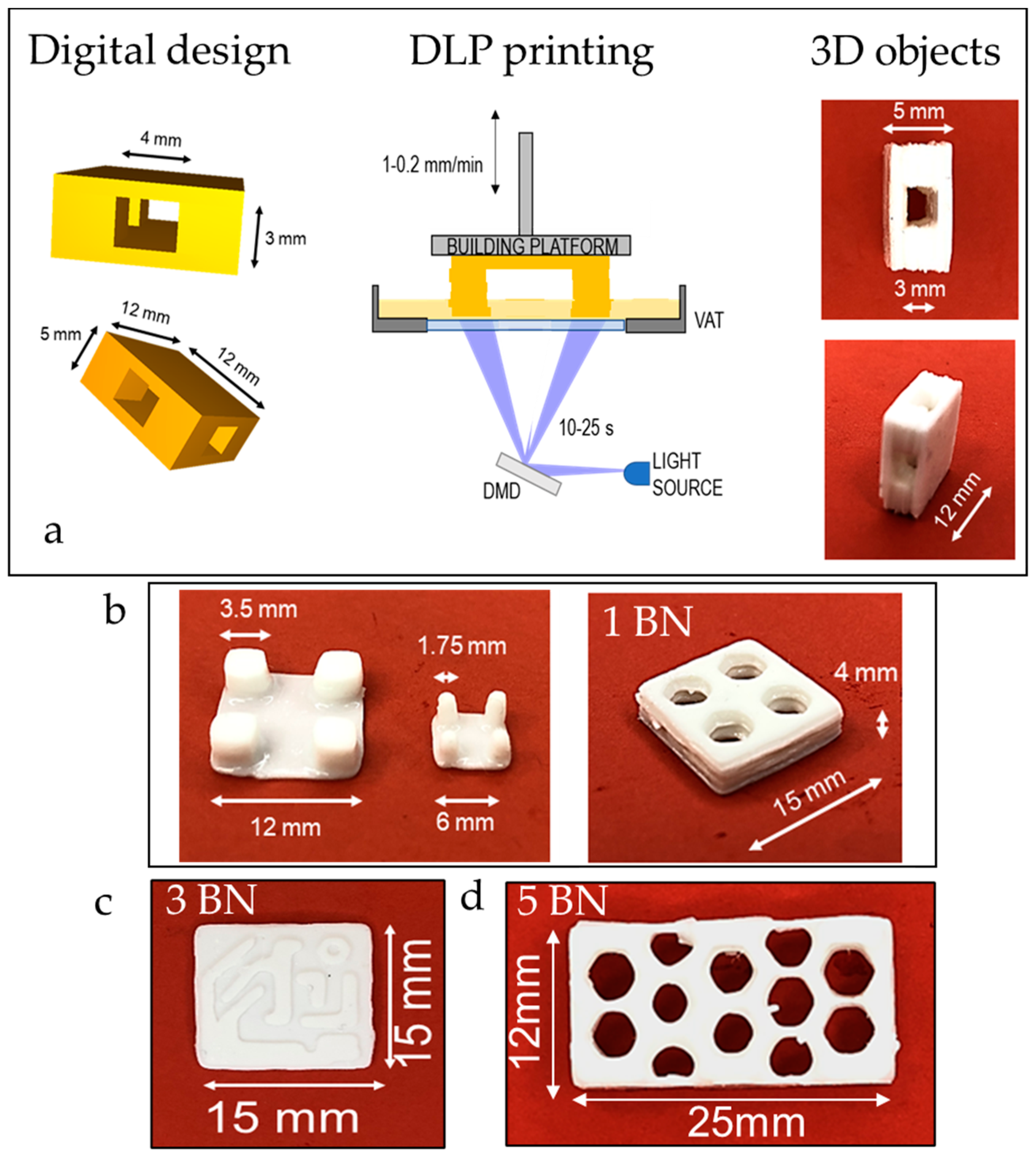
3.2. DLP 3D Printing
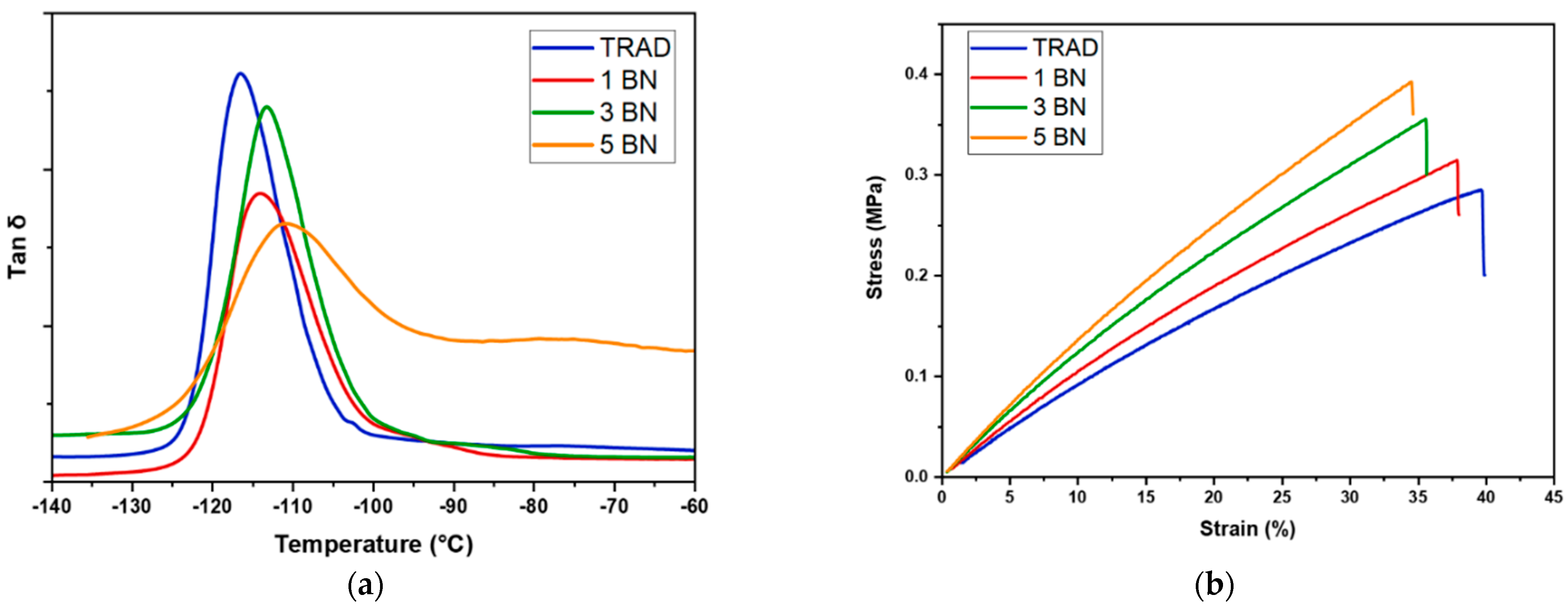
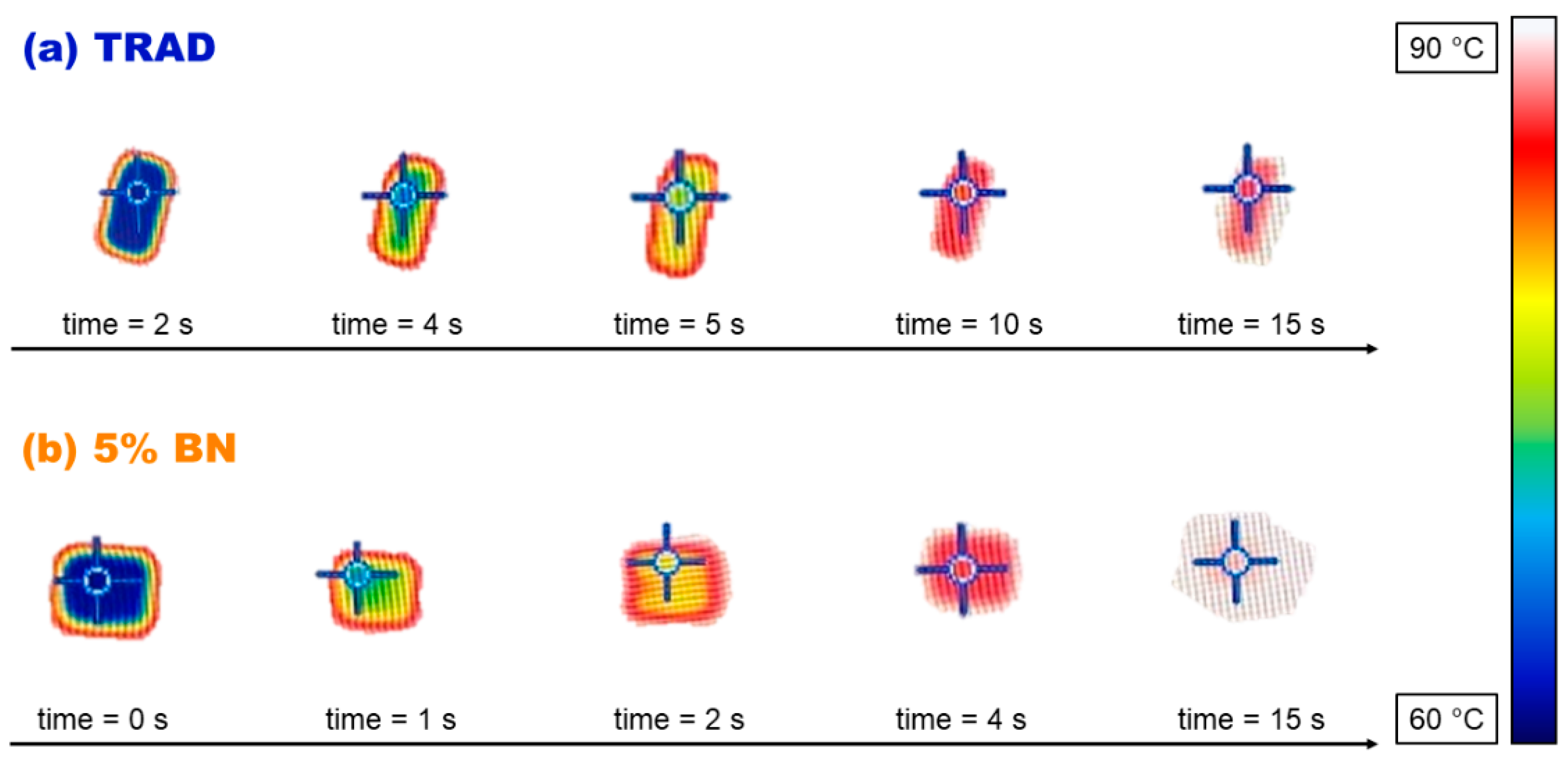
3.3. Characterization of the Printed Materials
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Ginzburg, V.V.; Yang, J.; Yang, Y.; Liu, W.; Huang, Y.; Du, L.; Chen, B. Thermal conductivity of polymer-based composites: Fundamentals and applications. Prog. Polym. Sci. 2016, 59, 41–85. [Google Scholar] [CrossRef]
- Byon, C.; Jeon, S.; Byon, C. Thermal conductivity of transparent and flexible polymers containing fillers: A literature review. Int. J. Heat Mass Transf. 2016, 98, 219–226. [Google Scholar] [CrossRef]
- Guo, Y.; Ruan, K.; Shi, X.; Yang, X.; Gu, J. Factors affecting thermal conductivities of the polymers and polymer composites: A review. Compos. Sci. Technol. 2020, 193, 108134. [Google Scholar] [CrossRef]
- Sim, L.C.; Ramanan, S.R.; Ismail, H.; Seetharamu, K.N.; Goh, T.J. Thermal characterization of Al2O3 and ZnO reinforced silicone rubber as thermal pads for heat dissipation purposes. Thermochim. Acta 2005, 430, 155–165. [Google Scholar] [CrossRef]
- Viswanath, R.; Wakharaka, V.; Eatwe, A.; Lebonheur, V. Thermal perfomance challenges from silicon to system. Intel. Tech. 2000, 3, 1–16. [Google Scholar]
- Wolf, M.P.; Salieb-Beugelaar, G.B.; Hunziker, P. PDMS with designer functionalities—Properties, modifications strategies, and applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Che, J.; Wu, K.; Lin, Y.; Wang, K.; Fu, Q. Largely improved thermal conductivity of HDPE/expanded graphite/carbon nanotubes ternary composites via filler network-network synergy. Compos. Part A Appl. Sci. Manuf. 2017, 99, 32–40. [Google Scholar] [CrossRef]
- Yang, K.; Gu, M. Enhanced thermal conductivity of epoxy nanocomposites filled with hybrid filler system of triethylenetetramine-functionalized multi-walled carbon nanotube/silane-modified nano-sized silicon carbide. Compos. Part A Appl. Sci. Manuf. 2010, 41, 215–221. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, P.; Tanaka, T. A review of dielectric polymer composites with high thermal conductivity. IEEE Electr. Insul. Mag. 2011, 27, 8–16. [Google Scholar] [CrossRef]
- Yi, P.; Awang, R.A.; Rowe, W.S.T.; Kalantar-Zadeh, K.; Khoshmanesh, K. PDMS nanocomposites for heat transfer enhancement in microfluidic platforms. Lab Chip 2014, 14, 3419–3426. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Qian, X.; Yang, R. Thermal conductivity of polymers and polymer nanocomposites. Mater. Sci. Eng. R Rep. 2018, 132, 1–22. [Google Scholar] [CrossRef]
- Liu, C.H.; Huang, H.; Wu, Y.; Fan, S.S. Thermal conductivity improvement of silicone elastomer with carbon nanotube loading. Appl. Phys. Lett. 2004, 84, 4248–4250. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef]
- Zhou, W.; Qi, S.-H.; Zhao, H.-Z.; Liu, N.-L. Thermally conductive silicone rubber reinforced with boron nitride particle. Polym. Compos. 2007, 28, 23–28. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, C.; An, Q.; Ou, H. Thermal Properties of Heat Conductive Silicone Rubber Filled with Hybrid Fillers. J. Compos. Mater. 2008, 42, 173–187. [Google Scholar] [CrossRef]
- Kemaloglu, S.; Özkoç, G.; Aytac, A. Thermally conductive boron nitride/SEBS/EVA ternary composite: “Processing and characterization”. Polym. Compos. 2010, 31, 1398–1408. [Google Scholar] [CrossRef]
- Sim, L.C.; Lee, C.K.; Ramanan, S.R.; Ismail, H.; Seetharamu, K.N. Cure characteristics, mechanical and thermal properties of Al2O3 and ZnO reinforced silicone rubber. Polymer-Plast. Technol. Eng. 2006, 45, 301–307. [Google Scholar] [CrossRef]
- Liu, M.; Chiang, S.-W.; Chu, X.; Li, J.; Gan, L.; He, Y.; Li, B.; Kang, F.; Du, H. Polymer composites with enhanced thermal conductivity via oriented boron nitride and alumina hybrid fillers assisted by 3-D printing. Ceram. Int. 2020, 46, 20810–20818. [Google Scholar] [CrossRef]
- Gu, J.; Meng, X.; Tang, Y.; Li, Y.; Zhuang, Q.; Kong, J. Hexagonal boron nitride/polymethyl-vinyl siloxane rubber dielectric thermally conductive composites with ideal thermal stabilities. Compos. Part A Appl. Sci. Manuf. 2017, 92, 27–32. [Google Scholar] [CrossRef]
- Kemaloglu, S.; Özkoç, G.; Aytac, A. Properties of thermally conductive micro and nano size boron nitride reinforced silicon rubber composites. Thermochim. Acta 2010, 499, 40–47. [Google Scholar] [CrossRef]
- Raman, C.; Meneghetti, P. Boron nitride finds new applications in thermoplastic compounds. Plast. Addit. Compd. 2008, 10, 26–31. [Google Scholar] [CrossRef]
- Guerra, V.; Wan, C.; McNally, T. Thermal conductivity of 2D nano-structured boron nitride (BN) and its composites with polymers. Prog. Mater. Sci. 2019, 100, 170–186. [Google Scholar] [CrossRef]
- Yuan, F.-Y.; Zhang, H.-B.; Li, X.; Li, X.-Z.; Yu, Z.-Z. Synergistic effect of boron nitride flakes and tetrapod-shaped ZnO whiskers on the thermal conductivity of electrically insulating phenol formaldehyde composites. Compos. Part A Appl. Sci. Manuf. 2013, 53, 137–144. [Google Scholar] [CrossRef]
- Hou, J.; Li, G.; Yang, N.; Qin, L.; Grami, M.E.; Zhang, Q.; Wang, N.; Qu, X. Preparation and characterization of surface modified boron nitride epoxy composites with enhanced thermal conductivity. RSC Adv. 2014, 4, 44282–44290. [Google Scholar] [CrossRef]
- Gigot, A.N.; Morra, A.; Castellino, M.; Pirri, C.F.; Mittal, V.; Dietliker, K.; Sangermano, M. Photolatent base catalyzed Michael-addition and concomitant in situ graphene oxide reduction to obtain electrically and thermally conductive UV-cured composite. Polymer 2017, 108, 251–256. [Google Scholar] [CrossRef]
- Sadej, M.; Gojzewski, H.; Gajewski, P.; Vancso, G.J.; Andrzejewska, E. Photocurable acrylate-based composites with enhanced thermal conductivity containing boron and silicon nitrides. Express Polym. Lett. 2018, 12, 790–807. [Google Scholar] [CrossRef]
- Sangermano, M.; Razza, N.; Graham, G.; Barandiaran, I.; Kortaberria, G. Electrically insulating polymeric nanocomposites with enhanced thermal conductivity by visible-light curing of epoxy-boron nitride nanotube formulations. Polym. Int. 2017, 66, 1935–1939. [Google Scholar] [CrossRef]
- Sangermano, M.; Calvara, L.; Chiavazzo, E.; Ventolà, L.; Asinari, P.; Mittal, V.; Rizzoli, R.; Ortolani, L.; Morandi, V. Enhancement of electrical and thermal conductivity of Su-8 photocrosslinked coatings containing graphene. Prog. Org. Coatings 2015, 86, 143–146. [Google Scholar] [CrossRef]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-curing 3D printing technique and its challenges. Bioact. Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Layani, M.; Wang, X.; Magdassi, S. Novel Materials for 3D Printing by Photopolymerization. Adv. Mater. 2018, 30, e1706344. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [PubMed]
- Thrasher, C.J.; Schwartz, J.J.; Boydston, A.J. Modular Elastomer Photoresins for Digital Light Processing Additive Manufacturing. ACS Appl. Mater. Interfaces 2017, 9, 39708–39716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, Q.; Wang, S.; Tao, J.; Gou, M. Digital Light Processing Based Three-dimensional Printing for Medical Applications. Int. J. Bioprint. 2019, 6, 242. [Google Scholar] [CrossRef]
- González, G.; Baruffaldi, D.; Martinengo, C.; Angelini, A.; Chiappone, A.; Roppolo, I.; Pirri, C.F.; Frascella, F. Materials Testing for the Development of Biocompatible Devices through Vat-Polymerization 3D Printing. Nanomaterials 2020, 10, 1788. [Google Scholar] [CrossRef]
- Sears, N.A.; Seshadri, D.R.; Dhavalikar, P.S.; Cosgriff-Hernandez, E. A Review of Three-Dimensional Printing in Tissue Engineering. Tissue Eng. Part B Rev. 2016, 22, 298–310. [Google Scholar] [CrossRef]
- Kadry, H.; Wadnap, S.; Xu, C.; Ahsan, F. Digital light processing (DLP) 3D-printing technology and photoreactive polymers in fabrication of modified-release tablets. Eur. J. Pharm. Sci. 2019, 135, 60–67. [Google Scholar] [CrossRef]
- Gul, J.Z.; Sajid, M.; Rehman, M.M.; Siddiqui, G.U.; Shah, I.; Kim, K.-H.; Lee, J.-W.; Choi, K.H. 3D printing for soft robotics—A review. Sci. Technol. Adv. Mater. 2018, 19, 243–262. [Google Scholar] [CrossRef]
- He, Y.; Wu, Y.; Fu, J.-Z.; Gao, Q.; Qiu, J.-J. Developments of 3D Printing Microfluidics and Applications in Chemistry and Biology: A Review. Electroanalysis 2016, 28, 1658–1678. [Google Scholar] [CrossRef]
- Rusling, J.F. Developing Microfluidic Sensing Devices Using 3D Printing. ACS Sens. 2018, 3, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, G.; Chiappone, A.; Roppolo, I.; Fantino, E.; Bertana, V.; Perrucci, F.; Scaltrito, L.; Pirri, F.; Sangermano, M. Development of 3D printable formulations containing CNT with enhanced electrical properties. Polymer 2017, 109, 246–253. [Google Scholar] [CrossRef]
- González, G.; Chiappone, A.; Dietliker, K.; Pirri, C.F.; Roppolo, I. Fabrication and Functionalization of 3D Printed Polydimethylsiloxane-Based Microfluidic Devices Obtained through Digital Light Processing. Adv. Mater. Technol. 2020, 5, 1–10. [Google Scholar] [CrossRef]
- Gillono, M.; Chiappone, A.; Mendola, L.; Gomez, M.G.; Scaltrito, L.; Pirri, C.F.; Roppolo, I. Study on the Printability through Digital Light Processing Technique of Ionic Liquids for CO2 Capture. Polymers 2019, 11, 1932. [Google Scholar] [CrossRef]
- Mu, Q.; Wang, L.; Dunn, C.K.; Kuang, X.; Duan, F.; Zhang, Z.; Qi, H.J.; Wang, T. Digital light processing 3D printing of conductive complex structures. Addit. Manuf. 2017, 18, 74–83. [Google Scholar] [CrossRef]
- Roppolo, I.; Frascella, F.; Gastaldi, M.; Castellino, M.; Ciubini, B.; Barolo, C.; Scaltrito, L.; Nicosia, C.; Zanetti, M.; Chiappone, A. Thiol–yne chemistry for 3D printing: Exploiting an off-stoichiometric route for selective functionalization of 3D objects. Polym. Chem. 2019, 10, 5950–5958. [Google Scholar] [CrossRef]
- Stassi, S.; Fantino, E.; Calmo, R.; Chiappone, A.; Gillono, M.; Scaiola, D.; Pirri, C.F.; Ricciardi, C.; Chiadò, A.; Roppolo, I. Polymeric 3D Printed Functional Microcantilevers for Biosensing Applications. ACS Appl. Mater. Interfaces 2017, 9, 19193–19201. [Google Scholar] [CrossRef]
- Hu, G.; Cao, Z.; Hopkins, M.; Lyons, J.G.; Brennan-Fournet, M.; Devine, D.M. Nanofillers can be used to enhance the thermal conductivity of commercially available SLA resins. Procedia Manuf. 2019, 38, 1236–1243. [Google Scholar] [CrossRef]
- Mubarak, S.; Dhamodharan, D.; Kale, M.B.; Divakaran, N.; Senthil, T.; Wu, L.; Wang, J. A Novel Approach to Enhance Mechanical and Thermal Properties of SLA 3D Printed Structure by Incorporation of Metal–Metal Oxide Nanoparticles. Nanomaterials 2020, 10, 217. [Google Scholar] [CrossRef]
- Mubarak, S.; Dhamodharan, D.; Divakaran, N.; Kale, M.B.; Senthil, T.; Wu, L.; Wang, J. Enhanced Mechanical and Thermal Properties of Stereolithography 3D Printed Structures by the Effects of Incorporated Controllably Annealed Anatase TiO2 Nanoparticles. Nanomaterials 2020, 10, 79. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. Standard Test Method for Tensile Properties of Thin Plastic Sheeting. 2002. Available online: https://www.astm.org/Standards/D882.htm (accessed on 15 November 2020). [CrossRef]




| Group | Filler | Thermal Conductivity (W/mK) |
|---|---|---|
| Carbon-based fillers | Carbon nanotubes (CNT) | 2000–6000 |
| Graphene | 3000 | |
| Diamond | 2000 | |
| Carbon black (CB) | 6–174 | |
| Metallic fillers | Copper (Cu) | 483 |
| Silver (Ag) | 450 | |
| Gold (Au) | 345 | |
| Aluminum (Al) | 204 | |
| Ceramic filler | Boron nitride (BN) | 250–300 |
| Beryllium oxide | 260 | |
| Aluminum nitride (AlN) | 200 | |
| Aluminum oxide (Al2O3) | 30 |
| Formulation Name | Tegorad Matrix | Filler BN | Photoinitiator BAPO (phr *) | Solvent Acetone (phr *) | ||
|---|---|---|---|---|---|---|
| (phr *) | (wt % **) | (phr *) | (wt % **) | |||
| TRAD | 100 | (100) | 0 | (0.00) | 0.6 | 2.5 |
| 1 BN | 100 | (99.01) | 1 | (0.99) | 0.6 | 2.5 |
| 3 BN | 100 | (97.09) | 3 | (2.91) | 0.6 | 2.5 |
| 5 BN | 100 | (95.24) | 5 | (4.76) | 0.6 | 2.5 |
| Formulation Name | Light Intensity (mW/cm2) | Layer Thickness (μm) | Exposure Time (s) | Exposure Time Burn-in Layers 1 (s) | Platform Movement (mm/s) |
|---|---|---|---|---|---|
| 1 BN | 40 | 75 | 10 | 15 | 1 |
| 3 BN | 40 | 75 | 20 | 25 | 0.5 |
| 5 BN | 40 | 50 | 25 | 30 | 0.2 |
| CAD | 1 BN | 3 BN | 5 BN |
|---|---|---|---|
 |  |  |  |
| Nominal dimension: Base width: 20 mm Cell wall thickness: 1 mm | Measured values: Base width: 20.274 mm Cell wall thickness: 1.25 ± 0.10 mm | Measured values: Base width: 20.210 mm Cell wall thickness: 1.12 ± 0.08 mm | Measured values: Base width: 20.150 mm Cell wall thickness: 1.07 ± 0.09 mm |
| Formulation Name | % gel 1 | Tg 2 (°C) | E 3 (MPa) | UTS 3 (MPa) | Elongation at Break 3 (%) |
|---|---|---|---|---|---|
| TRAD | 96 ± 1 | −115 ± 2 | 0.85 ± 0.06 | 0.28 ± 0.07 | 40 ± 5 |
| 1 BN | 93 ± 2 | −116 ± 2 | 0.96 ± 0.09 | 0.34 ± 0.02 | 38 ± 5 |
| 3 BN | 94 ± 1 | −114 ± 1 | 0.98 ± 0.06 | 0.36 ± 0.06 | 36 ± 6 |
| 5 BN | 95 ± 2 | −111 ± 1 | 1.24 ± 0.05 | 0.39 ± 0.05 | 34 ± 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pezzana, L.; Riccucci, G.; Spriano, S.; Battegazzore, D.; Sangermano, M.; Chiappone, A. 3D Printing of PDMS-Like Polymer Nanocomposites with Enhanced Thermal Conductivity: Boron Nitride Based Photocuring System. Nanomaterials 2021, 11, 373. https://doi.org/10.3390/nano11020373
Pezzana L, Riccucci G, Spriano S, Battegazzore D, Sangermano M, Chiappone A. 3D Printing of PDMS-Like Polymer Nanocomposites with Enhanced Thermal Conductivity: Boron Nitride Based Photocuring System. Nanomaterials. 2021; 11(2):373. https://doi.org/10.3390/nano11020373
Chicago/Turabian StylePezzana, Lorenzo, Giacomo Riccucci, Silvia Spriano, Daniele Battegazzore, Marco Sangermano, and Annalisa Chiappone. 2021. "3D Printing of PDMS-Like Polymer Nanocomposites with Enhanced Thermal Conductivity: Boron Nitride Based Photocuring System" Nanomaterials 11, no. 2: 373. https://doi.org/10.3390/nano11020373
APA StylePezzana, L., Riccucci, G., Spriano, S., Battegazzore, D., Sangermano, M., & Chiappone, A. (2021). 3D Printing of PDMS-Like Polymer Nanocomposites with Enhanced Thermal Conductivity: Boron Nitride Based Photocuring System. Nanomaterials, 11(2), 373. https://doi.org/10.3390/nano11020373