Development of Polythiourethane/ZnO-Based Anti-Fouling Materials and Evaluation of the Adhesion of Staphylococcus aureus and Candida glabrata Using Single-Cell Force Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Polymer Composites Preparation
2.2. AFM Surface Imaging
2.3. Confocal Laser Scanning Microscopy (CLSM) Imaging
2.4. Microorganism Strains and Culture Conditions
2.5. Single-Cell Force Spectroscopy Experiments
2.6. Tensile Testing
2.7. Scanning Electron Microscopy (SEM) Imaging and Energy Dispersive X-ray Spectroscopy (EDX)
2.8. Photometric Determination of Potential Zn-Ion (Zn2+) Release
2.9. Wetting Properties
2.10. Surface Charge Evaluation
3. Results and Discussion
3.1. PTU/ZnO Composites Have Anti-Adhesive Surface Properties
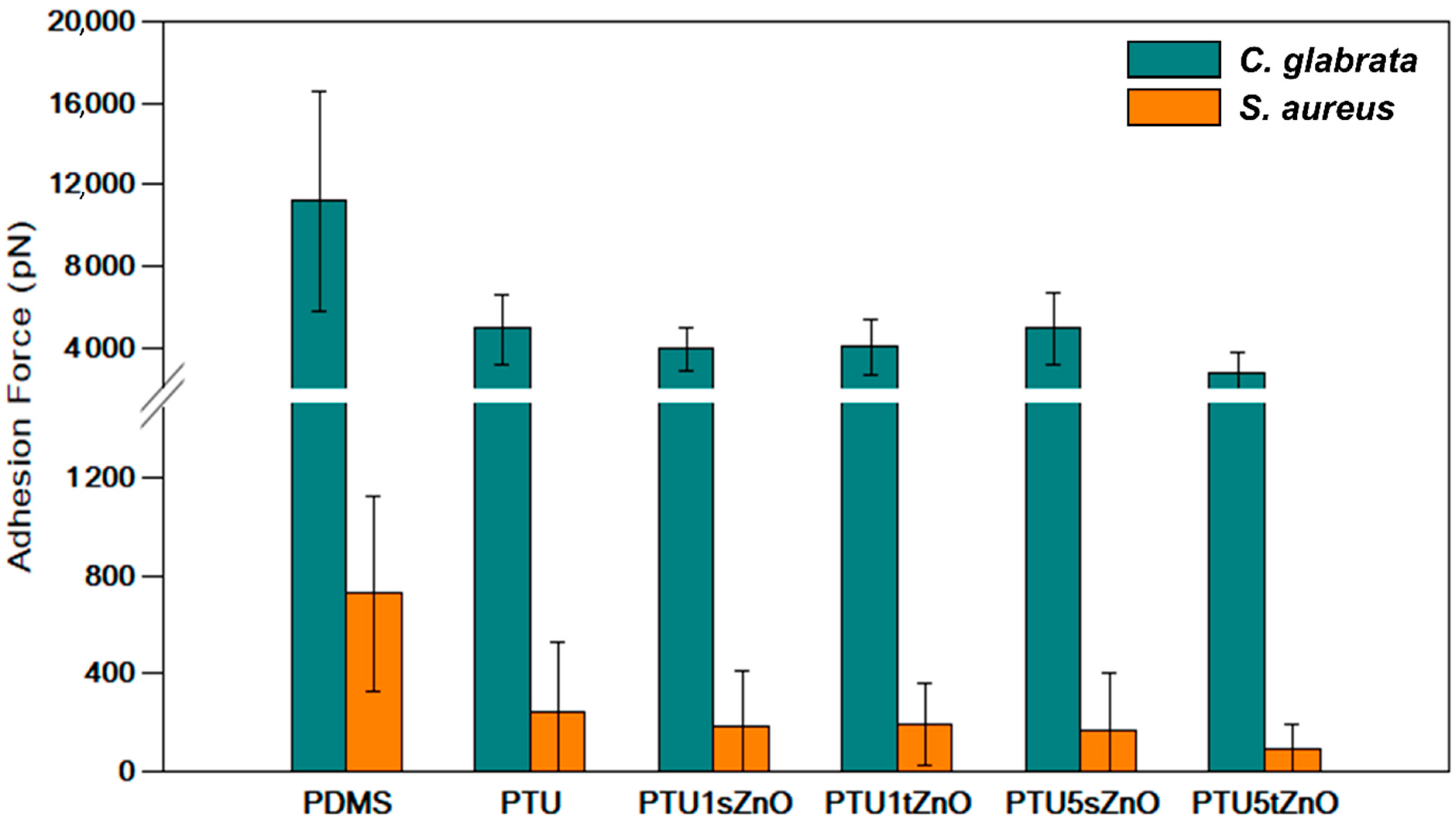
3.2. Adhesion of S. aureus to PTU/ZnO Composites Is Significantly Decreased Compared to PDMS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, H.A.; Ahmad, A.; Mehboob, R. Nosocomial Infections and Their Control Strategies. Asian Pac. J. Trop. Biomed. 2015, 5, 509–514. [Google Scholar] [CrossRef]
- Chen, M.; Yu, Q.; Sun, H. Novel Strategies for the Prevention and Treatment of Biofilm Related Infections. Int. J. Mol. Sci. 2013, 14, 18488–18501. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, M.; Teixeira, M.C. Candida Biofilms: Threats, Challenges, and Promising Strategies. Front. Med. 2018, 5, 28. [Google Scholar] [CrossRef]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-Associated Infections, Medical Devices and Biofilms: Risk, Tolerance and Control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Carniello, V.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Physico-Chemistry from Initial Bacterial Adhesion to Surface-Programmed Biofilm Growth. Adv. Colloid Interface Sci. 2018, 261, 1–14. [Google Scholar] [CrossRef]
- Terada, A.; Yuasa, A.; Kushimoto, T.; Tsuneda, S.; Katakai, A.; Tamada, M. Bacterial Adhesion to and Viability on Positively Charged Polymer Surfaces. Microbiology 2006, 152, 3575–3583. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for Combating Bacterial Biofilms: A Focus on Anti-Biofilm Agents and Their Mechanisms of Action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef]
- Kaplan, J.B. Biofilm Matrix-Degrading Enzymes. Methods Mol. Biol. 2014, 1147, 203–213. [Google Scholar] [CrossRef]
- Kalia, V.C. Quorum Sensing Inhibitors: An Overview. Biotechnol. Adv. 2013, 31, 224–245. [Google Scholar] [CrossRef]
- Ong, K.S.; Mawang, C.I.; Daniel-Jambun, D.; Lim, Y.Y.; Lee, S.M. Current Anti-Biofilm Strategies and Potential of Antioxidants in Biofilm Control. Expert Rev. Anti-Infect. Ther. 2018, 16, 855–864. [Google Scholar] [CrossRef]
- Formosa-Dague, C.; Speziale, P.; Foster, T.J.; Geoghegan, J.A.; Dufrêne, Y.F. Zinc-Dependent Mechanical Properties of Staphylococcus Aureus Biofilm-Forming Surface Protein SasG. Proc. Natl. Acad. Sci. USA 2016, 113, 410–415. [Google Scholar] [CrossRef]
- Feuillie, C.; Formosa-Dague, C.; Hays, L.M.C.; Vervaeck, O.; Derclaye, S.; Brennan, M.P.; Foster, T.J.; Geoghegan, J.A.; Dufrêne, Y.F. Molecular Interactions and Inhibition of the Staphylococcal Biofilm-Forming Protein SdrC. Proc. Natl. Acad. Sci. USA 2017, 114, 3738–3743. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.; De Pascale, G.; Ranieri, V.M.; Pelaia, P.; Tufano, R.; Piazza, O.; Zangrillo, A.; Ferrario, A.; De Gaetano, A.; Guaglianone, E.; et al. Comparison of Triple-Lumen Central Venous Catheters Impregnated with Silver Nanoparticles (AgTive®) vs Conventional Catheters in Intensive Care Unit Patients. J. Hosp. Infect. 2012, 82, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Legeay, G.; Poncin-Epaillard, F.; Arciola, C.R. New Surfaces with Hydrophilic/Hydrophobic Characteristics in Relation to (No)Bioadhesion. Int. J. Artif. Organs 2018. [Google Scholar] [CrossRef] [PubMed]
- Desrousseaux, C.; Sautou, V.; Descamps, S.; Traoré, O. Modification of the Surfaces of Medical Devices to Prevent Microbial Adhesion and Biofilm Formation. J. Hosp. Infect. 2013, 85, 87–93. [Google Scholar] [CrossRef]
- Geoghegan, M.; Andrews, J.S.; Biggs, C.A.; Eboigbodin, K.E.; Elliott, D.R.; Rolfe, S.; Scholes, J.; Ojeda, J.J.; Romero-González, M.E.; Edyvean, R.G.J.; et al. The Polymer Physics and Chemistry of Microbial Cell Attachment and Adhesion. Faraday Discuss. 2008, 139, 85–103. [Google Scholar] [CrossRef]
- Xu, L.-C.; Siedlecki, C.A. Staphylococcus Epidermidis Adhesion on Hydrophobic and Hydrophilic Textured Biomaterial Surfaces. Biomed. Mater. 2014, 9, 035003. [Google Scholar] [CrossRef]
- Hizal, F.; Rungraeng, N.; Lee, J.; Jun, S.; Busscher, H.J.; van der Mei, H.C.; Choi, C.-H. Nanoengineered Superhydrophobic Surfaces of Aluminum with Extremely Low Bacterial Adhesivity. ACS Appl. Mater. Interfaces 2017, 9, 12118–12129. [Google Scholar] [CrossRef]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling Coatings: Recent Developments in the Design of Surfaces That Prevent Fouling by Proteins, Bacteria, and Marine Organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef]
- Vendra, V.; Wu, L.; Krishnan, S. Polymer Thin Films for Biomedical Applications. In Nanostructured Thin Films and Surfaces; Wiley: Hoboken, NJ, USA, 2011; Volume 5, pp. 1–51. ISBN 978-3-527-32155-1. [Google Scholar]
- Herold, D.A.; Keil, K.; Bruns, D.E. Oxidation of Polyethylene Glycols by Alcohol Dehydrogenase. Biochem. Pharmacol. 1989, 38, 73–76. [Google Scholar] [CrossRef]
- Berne, C.; Ellison, C.K.; Ducret, A.; Brun, Y.V. Bacterial Adhesion at the Single-Cell Level. Nat. Rev. Microbiol. 2018, 16, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Ercan, B.; Taylor, E.; Alpaslan, E.; Webster, T.J. Diameter of Titanium Nanotubes Influences Anti-Bacterial Efficacy. Nanotechnology 2011, 22, 295102. [Google Scholar] [CrossRef] [PubMed]
- Puckett, S.D.; Taylor, E.; Raimondo, T.; Webster, T.J. The Relationship between the Nanostructure of Titanium Surfaces and Bacterial Attachment. Biomaterials 2010, 31, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Fadeeva, E.; Truong, V.K.; Stiesch, M.; Chichkov, B.N.; Crawford, R.J.; Wang, J.; Ivanova, E.P. Bacterial Retention on Superhydrophobic Titanium Surfaces Fabricated by Femtosecond Laser Ablation. Langmuir 2011, 27, 3012–3019. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wen, C.; Hodgson, P.; Li, Y. Biocompatibility of TiO2 Nanotubes with Different Topographies. J. Biomed. Mater. Res. Part A 2014, 102, 743–751. [Google Scholar] [CrossRef]
- Pavličević, J.; Špírková, M.; Bera, O.; Jovičić, M.; Pilić, B.; Baloš, S.; Budinski-Simendić, J. The Influence of ZnO Nanoparticles on Thermal and Mechanical Behavior of Polycarbonate-Based Polyurethane Composites. Compos. Part B Eng. 2014, 60, 673–679. [Google Scholar] [CrossRef]
- Hölken, I.; Hoppe, M.; Mishra, Y.K.; Gorb, S.N.; Adelung, R.; Baum, M.J. Complex Shaped ZnO Nano- and Microstructure Based Polymer Composites: Mechanically Stable and Environmentally Friendly Coatings for Potential Antifouling Applications. Phys. Chem. Chem. Phys. 2016, 18, 7114–7123. [Google Scholar] [CrossRef]
- Kultys, A.; Rogulska, M.; Pikus, S. The Synthesis and Characterization of New Thermoplastic Poly(Thiourethane-Urethane)s. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 1770–1782. [Google Scholar] [CrossRef]
- Rogulska, M.; Kultys, A.; Olszewska, E. New Thermoplastic Poly(Thiourethane-Urethane) Elastomers Based on Hexane-1,6-Diyl Diisocyanate (HDI). J. Therm. Anal. Calorim. 2013. [Google Scholar] [CrossRef]
- Hölken, I. Mechanically Stable and Environmentally Friendly Polymer/Particle Composites for the Application as Low-Fouling Coating in the Marine Sector. Ph.D. Thesis, Technischen Fakultät der Christian-Albrechts-Universität zu Kiel, Kiel, Germany, 2016. [Google Scholar]
- Hölken, I.; Hoppe, M.; Adelung, R.; Baum, M. Functional Ecofriendly Coatings for Marine Applications. Proceedings on the 3rd International Conference on Nanotechnologies and Biomedical Engineering, Chisinau, Republic of Moldova, 23–26 September 2015. [Google Scholar] [CrossRef]
- Qiu, H.; Hölken, I.; Gapeeva, A.; Filiz, V.; Adelung, R.; Baum, M. Development and Characterization of Mechanically Durable Silicone-Polythiourethane Composites Modified with Tetrapodal Shaped ZnO Particles for the Potential Application as Fouling-Release Coating in the Marine Sector. Materials 2018, 11, 2413. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Adelung, R. ZnO Tetrapod Materials for Functional Applications. Mater. Today 2018, 21, 631–651. [Google Scholar] [CrossRef]
- Antoine, T.E.; Hadigal, S.R.; Yakoub, A.M.; Mishra, Y.K.; Bhattacharya, P.; Haddad, C.; Valyi-Nagy, T.; Adelung, R.; Prabhakar, B.S.; Shukla, D. Intravaginal Zinc Oxide Tetrapod Nanoparticles as Novel Immunoprotective Agents against Genital Herpes. J. Immunol. 2016, 196, 4566–4575. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nanomicro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Papavlassopoulos, H.; Mishra, Y.K.; Kaps, S.; Paulowicz, I.; Abdelaziz, R.; Elbahri, M.; Maser, E.; Adelung, R.; Röhl, C. Toxicity of Functional Nano-Micro Zinc Oxide Tetrapods: Impact of Cell Culture Conditions, Cellular Age and Material Properties. PLoS ONE 2014, 9, e84983. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.N.; Fang, M.; Jiao, K.; Tang, L.H.; Xiao, Y.H.; Shen, L.J.; Chen, J.H. Tetrapod-like Zinc Oxide Whisker Enhancement of Resin Composite. J. Dent. Res. 2010, 89, 746–750. [Google Scholar] [CrossRef]
- Song, F.; Koo, H.; Ren, D. Effects of Material Properties on Bacterial Adhesion and Biofilm Formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic Force Microscope. Phys. Rev. Lett. 1986, 56, 930–934. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcal Biofilms. In Bacterial Biofilms; Romeo, T., Ed.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 207–228. ISBN 978-3-540-75418-3. [Google Scholar]
- Rodrigues, C.F.; Silva, S.; Henriques, M. Candida Glabrata: A Review of Its Features and Resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 673–688. [Google Scholar] [CrossRef]
- Beaussart, A.; El-Kirat-Chatel, S.; Sullan, R.M.A.; Alsteens, D.; Herman, P.; Derclaye, S.; Dufrêne, Y.F. Quantifying the Forces Guiding Microbial Cell Adhesion Using Single-Cell Force Spectroscopy. Nat. Protoc. 2014, 9, 1049–1055. [Google Scholar] [CrossRef]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.-J.; Park, W.-T.; Yoon, Y.-J. Polymeric Biomaterials for Medical Implants and Devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef]
- Mata, A.; Fleischman, A.J.; Roy, S. Characterization of Polydimethylsiloxane (PDMS) Properties for Biomedical Micro/Nanosystems. Biomed. Microdevices 2005, 7, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.; Wada, S.; Tanaka, S.; Takeda, M.; Ishikawa, T.; Tsubota, K.; Imai, Y.; Yamaguchi, T. In Vitro Blood Flow in a Rectangular PDMS Microchannel: Experimental Observations Using a Confocal Micro-PIV System. Biomed. Microdevices 2008, 10, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Mishra, Y.K.; Kaps, S.; Schuchardt, A.; Paulowicz, I.; Jin, X.; Gedamu, D.; Freitag, S.; Claus, M.; Wille, S.; Kovalev, A.; et al. Fabrication of Macroscopically Flexible and Highly Porous 3D Semiconductor Networks from Interpenetrating Nanostructures by a Simple Flame Transport Approach. Part. Part. Syst. Charact. 2013, 30, 775–783. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Modi, G.; Cretu, V.; Postica, V.; Lupan, O.; Reimer, T.; Paulowicz, I.; Hrkac, V.; Benecke, W.; Kienle, L.; et al. Direct Growth of Freestanding ZnO Tetrapod Networks for Multifunctional Applications in Photocatalysis, UV Photodetection, and Gas Sensing. ACS Appl. Mater. Interfaces 2015, 7, 14303–14316. [Google Scholar] [CrossRef]
- Hutter, J.L.; Bechhoefer, J. Calibration of Atomic-Force Microscope Tips. Rev. Sci. Instrum. 1993, 64, 1868–1873. [Google Scholar] [CrossRef]
- Delgado, A.V.; González-Caballero, F.; Hunter, R.J.; Koopal, L.K.; Lyklema, J. Measurement and Interpretation of Electrokinetic Phenomena (IUPAC Technical Report). Pure Appl. Chem. 2005, 77, 1753–1805. [Google Scholar] [CrossRef]
- Bhushan, B. Introduction to Tribology, 2nd ed.; Wiley: Chichester, West Sussex, UK, 2013; ISBN 978-1-119-94453-9. [Google Scholar]
- Bollen, C.M.; Papaioanno, W.; Van Eldere, J.; Schepers, E.; Quirynen, M.; van Steenberghe, D. The Influence of Abutment Surface Roughness on Plaque Accumulation and Peri-Implant Mucositis. Clin. Oral. Implant. Res. 1996, 7, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Popov, V.L. Contact Mechanics and Friction: Physical Principles and Applications; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-3-642-10803-7. [Google Scholar]
- Busscher, H.J.; Norde, W.; van der Mei, H.C. Specific Molecular Recognition and Nonspecific Contributions to Bacterial Interaction Forces. Appl. Environ. Microbiol. 2008, 74, 2559–2564. [Google Scholar] [CrossRef]
- Rosenberg, M.; Kjelleberg, S. Hydrophobic Interactions: Role in Bacterial Adhesion. In Advances in Microbial Ecology; Marshall, K.C., Ed.; Advances in Microbial Ecology; Springer: Boston, MA, USA, 1986; pp. 353–393. ISBN 978-1-4757-0611-6. [Google Scholar]
- Boks, N.P.; Busscher, H.J.; van der Mei, H.C.; Norde, W. Bond-Strengthening in Staphylococcal Adhesion to Hydrophilic and Hydrophobic Surfaces Using Atomic Force Microscopy. Langmuir 2008, 24, 12990–12994. [Google Scholar] [CrossRef]
- Oliveira, R.; Azeredo, J.; Teixeira, P.; Fonseca, A.P. The Role of Hydrophobicity in Bacterial Adhesion; Bioline: Toronto, ON, USA, 2001. [Google Scholar]
- Doyle, R.J. Contribution of the Hydrophobic Effect to Microbial Infection. Microbes Infect. 2000, 2, 391–400. [Google Scholar] [CrossRef]
- Rosenberg, M.; Gutnick, D.; Rosenberg, E. Adherence of Bacteria to Hydrocarbons: A Simple Method for Measuring Cell-Surface Hydrophobicity. FEMS Microbiol. Lett. 1980, 9, 29–33. [Google Scholar] [CrossRef]
- El-Kirat-Chatel, S.; Beaussart, A.; Derclaye, S.; Alsteens, D.; Kucharíková, S.; Van Dijck, P.; Dufrêne, Y.F. Force Nanoscopy of Hydrophobic Interactions in the Fungal Pathogen Candida Glabrata. ACS Nano 2015, 9, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Bos, R.; van der Mei, H.C.; Busscher, H.J. Physico-Chemistry of Initial Microbial Adhesive Interactions–Its Mechanisms and Methods for Study. FEMS Microbiol. Rev. 1999, 23, 179–230. [Google Scholar] [CrossRef]
- Olitzki, L. Electric Charge of Bacterial Antigens. J. Immunol. 1932, 22, 251–256. [Google Scholar]
- Francolini, I.; Piozzi, A.; Donelli, G. Efficacy Evaluation of Antimicrobial Drug-Releasing Polymer Matrices. In Microbial Biofilms: Methods and Protocols; Donelli, G., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2014; pp. 215–225. ISBN 978-1-4939-0467-9. [Google Scholar]
- Sousa, C.; Teixeira, P.; Oliveira, R. Influence of Surface Properties on the Adhesion of Staphylococcus Epidermidis to Acrylic and Silicone. Available online: https://www.hindawi.com/journals/ijbm/2009/718017/abs/ (accessed on 3 October 2019).
- Krasowska, A.; Sigler, K. How Microorganisms Use Hydrophobicity and What Does This Mean for Human Needs? Front. Cell. Infect. Microbiol. 2014, 4. [Google Scholar] [CrossRef]
- Maikranz, E.; Spengler, C.; Thewes, N.; Thewes, A.; Nolle, F.; Jung, P.; Bischoff, M.; Santen, L.; Jacobs, K. Different Binding Mechanisms of Staphylococcus Aureus to Hydrophobic and Hydrophilic Surfaces. Nanoscale 2020, 12, 19267–19275. [Google Scholar] [CrossRef]
- Chen, L.-C.; Kung, S.-K.; Chen, H.-H.; Lin, S.-B. Evaluation of Zeta Potential Difference as an Indicator for Antibacterial Strength of Low Molecular Weight Chitosan. Carbohydr. Polym. 2010, 82, 913–919. [Google Scholar] [CrossRef]
- Sonohara, R.; Muramatsu, N.; Ohshima, H.; Kondo, T. Difference in Surface Properties between Escherichia Coli and Staphylococcus Aureus as Revealed by Electrophoretic Mobility Measurements. Biophys. Chem. 1995, 55, 273–277. [Google Scholar] [CrossRef]
- Rijnaarts, H.H.M.; Norde, W.; Bouwer, E.J.; Lyklema, J.; Zehnder, A.J.B. Reversibility and Mechanism of Bacterial Adhesion. Coll. Surf. B. Biointerfaces 1995, 5–22. [Google Scholar] [CrossRef]
- Gross, M.; Cramton, S.E.; Götz, F.; Peschel, A. Key Role of Teichoic Acid Net Charge in Staphylococcus Aureus Colonization of Artificial Surfaces. Infect. Immun. 2001, 69, 3423–3426. [Google Scholar] [CrossRef]
- Siddiqui, S.; Chandrasekaran, A.; Lin, N.; Tufenkji, N.; Moraes, C. Microfluidic Shear Assay to Distinguish between Bacterial Adhesion and Attachment Strength on Stiffness-Tunable Silicone Substrates. Langmuir 2019, 35, 8840–8849. [Google Scholar] [CrossRef] [PubMed]
- Lichter, J.A.; Thompson, M.T.; Delgadillo, M.; Nishikawa, T.; Rubner, M.F.; Van Vliet, K.J. Substrata Mechanical Stiffness Can Regulate Adhesion of Viable Bacteria. Biomacromolecules 2008, 9, 1571–1578. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, A.; Isayeva, I.; Vorvolakos, K.; Das, S.; Li, Z.; Phillips, K.S. Interactions of Staphylococcus Aureus with Ultrasoft Hydrogel Biomaterials. Biomaterials 2016, 95, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Nikawa, H.; Sadamori, S.; Hamada, T.; Satou, N.; Okuda, K. Non-Specific Adherence of Candida Species to Surface-Modified Glass. J. Med. Vet. Mycol. 1989, 27, 269–271. [Google Scholar] [CrossRef] [PubMed]
| Ra Measured by CLSM (nm) | Ra Measured by AFM (nm) | |||
|---|---|---|---|---|
| PDMS | 110 ± 10 | 13.60 ± 4.60 | ||
| PTU | 110 ± 40 | 0.40 ± 0.08 | ||
| Type of particle | s-ZnO | t-ZnO | s-ZnO | t-ZnO |
| PTU/1 wt.% | 100 ± 10 | 150 ± 10 | 0.15 ± 0.34 | 0.41 ± 0.07 |
| PTU/ 5 wt.% | 140 ± 30 | 210 ± 50 | 0.78 ± 0.20 | 0.72 ± 0.18 |
| Material Variation | Elastic Modulus (MPa) |
|---|---|
| PDMS | 120 ± 9 |
| PTU | 624 ± 63 |
| PTU 1 wt.% s-ZnO | 641 ± 31 |
| PTU 5 wt.% s-ZnO | 630 ± 24 |
| PTU 1 wt.% t-ZnO | 611 ± 56 |
| PTU 5 wt.% t-ZnO | 689 ± 32 |
| PTU | PTU/1 wt.% t-ZnO | PTU/5 wt.% t-ZnO | PTU/1 wt.% s-ZnO | PTU/5 wt.% s-ZnO | PDMS | |
|---|---|---|---|---|---|---|
| Water Contact Angle (°) | 78.6 ± 2.4 | 77.9 ± 1.3 | 75.1 ± 1.7 | 76.0 ± 1.6 | 73.3 ± 2.3 | 114.9 ± 1.4 |
| Material Variation | ζ Zeta Potential (mV) |
|---|---|
| PDMS | −3.5 ± 0.6 |
| PTU | −23.0 ± 3.0 |
| PTU/5 wt.% t-ZnO | −23.5 ± 0.9 |
| PTU/5 wt.% s-ZnO | −22.0 ± 1.0 |
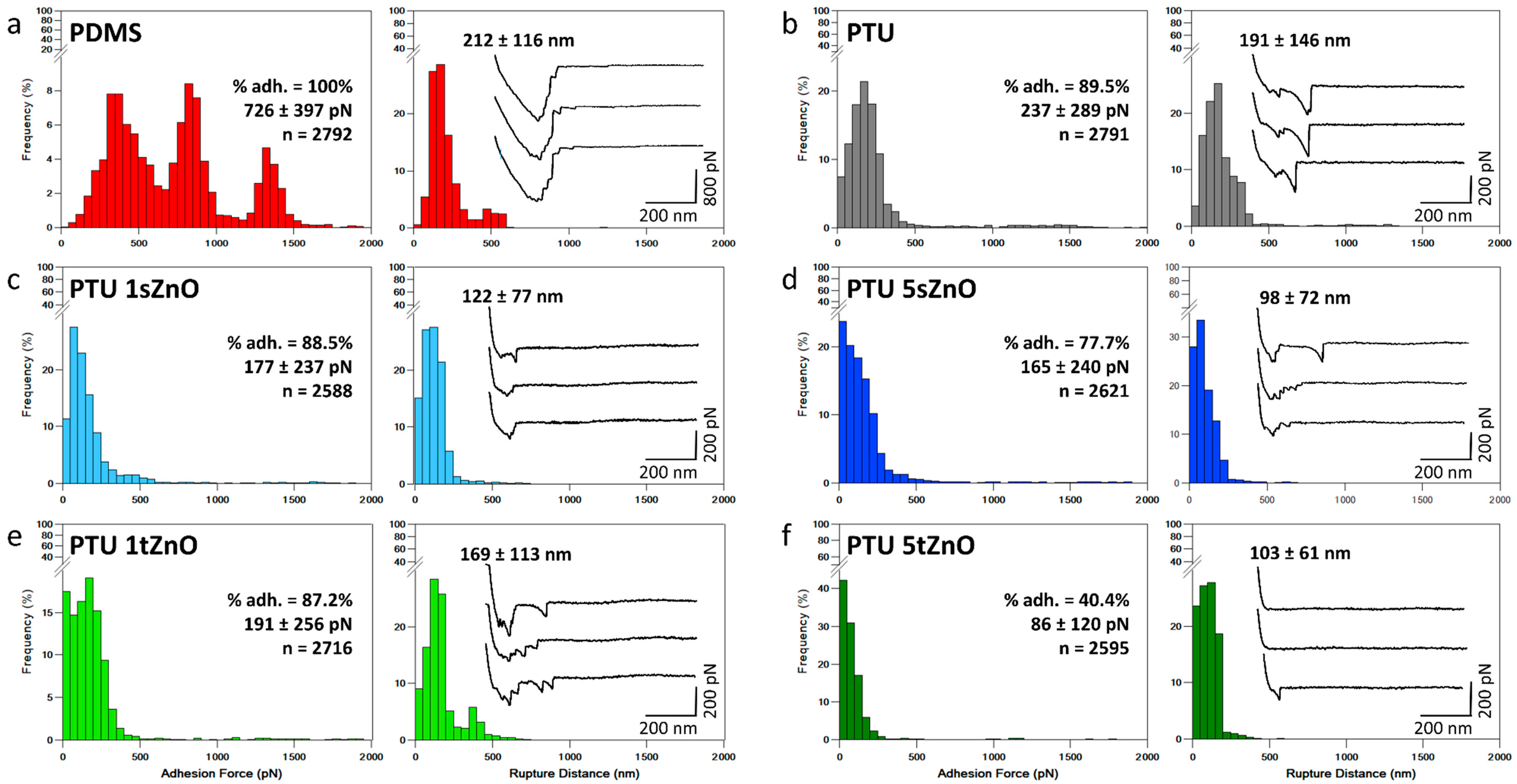
| Material Variation | Adhesion Force (pN) | Rupture Distance (nm) | % of Adhesion |
|---|---|---|---|
| PDMS | 726 ± 397 | 212 ± 116 | 100% |
| PTU | 237 ± 289 | 191 ± 146 | 89.5% |
| PTU/1 wt.% s-ZnO | 177 ± 237 | 122 ± 77 | 88.5% |
| PTU/1 wt.% t-ZnO | 191 ± 256 | 169 ± 113 | 87.2% |
| PTU/5 wt.% s-ZnO | 165 ± 240 | 98 ± 72 | 77.7% |
| PTU/5 wt.% t-ZnO | 86 ± 120 | 103 ± 61 | 40.4% |
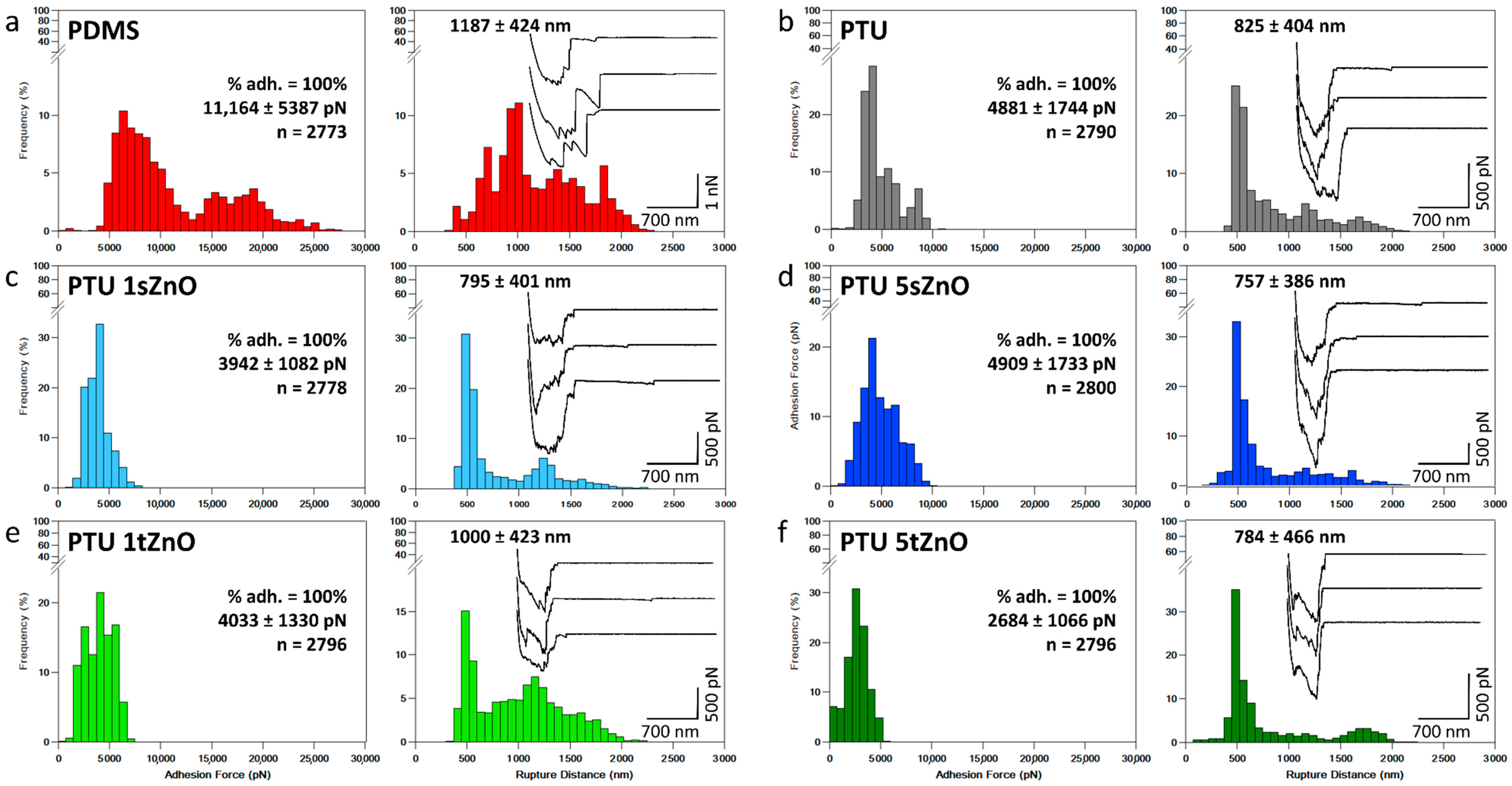
| Material Variation | Adhesion Force (pN) | Rupture Distance (nm) | % of Adhesion |
|---|---|---|---|
| PDMS | 11,164 ± 5387 | 1187 ± 424 | 100% |
| PTU | 4881 ± 1744 | 825 ± 404 | 100% |
| PTU/1 wt.% s-ZnO | 3942 ± 1082 | 795 ± 401 | 100% |
| PTU/1 wt.% t-ZnO | 4033 ± 1330 | 1000 ± 423 | 100% |
| PTU/5 wt.% s-ZnO | 4909 ± 1733 | 757 ± 386 | 100% |
| PTU/5 wt.% t-ZnO | 2684 ± 1066 | 784 ± 466 | 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klemm, S.; Baum, M.; Qiu, H.; Nan, Z.; Cavalheiro, M.; Teixeira, M.C.; Tendero, C.; Gapeeva, A.; Adelung, R.; Dague, E.; et al. Development of Polythiourethane/ZnO-Based Anti-Fouling Materials and Evaluation of the Adhesion of Staphylococcus aureus and Candida glabrata Using Single-Cell Force Spectroscopy. Nanomaterials 2021, 11, 271. https://doi.org/10.3390/nano11020271
Klemm S, Baum M, Qiu H, Nan Z, Cavalheiro M, Teixeira MC, Tendero C, Gapeeva A, Adelung R, Dague E, et al. Development of Polythiourethane/ZnO-Based Anti-Fouling Materials and Evaluation of the Adhesion of Staphylococcus aureus and Candida glabrata Using Single-Cell Force Spectroscopy. Nanomaterials. 2021; 11(2):271. https://doi.org/10.3390/nano11020271
Chicago/Turabian StyleKlemm, Sophie, Martina Baum, Haoyi Qiu, Zibin Nan, Mafalda Cavalheiro, Miguel Cacho Teixeira, Claire Tendero, Anna Gapeeva, Rainer Adelung, Etienne Dague, and et al. 2021. "Development of Polythiourethane/ZnO-Based Anti-Fouling Materials and Evaluation of the Adhesion of Staphylococcus aureus and Candida glabrata Using Single-Cell Force Spectroscopy" Nanomaterials 11, no. 2: 271. https://doi.org/10.3390/nano11020271
APA StyleKlemm, S., Baum, M., Qiu, H., Nan, Z., Cavalheiro, M., Teixeira, M. C., Tendero, C., Gapeeva, A., Adelung, R., Dague, E., Castelain, M., & Formosa-Dague, C. (2021). Development of Polythiourethane/ZnO-Based Anti-Fouling Materials and Evaluation of the Adhesion of Staphylococcus aureus and Candida glabrata Using Single-Cell Force Spectroscopy. Nanomaterials, 11(2), 271. https://doi.org/10.3390/nano11020271








