Abstract
Background: TiO2 nanoparticles (TiO2 NPs) are the nanomaterial most produced as an ultraviolet (UV) filter. However, TiO2 is a semiconductor and, in nanoparticle size, is a strong photocatalyst, raising concerns about photomutagenesis. Mesoporous silica nanoparticles (MSN) were synthetized incorporating TiO2 NPs (TiO2@MSN) to develop a cosmetic UV filter. The aim of this study was to assess the toxicity of TiO2@MSN, compared with bare MSN and commercial TiO2 NPs, based on several biomarkers. Materials and Methods: Human peripheral blood mononuclear cells (PBMC) were exposed to TiO2@MSN, bare MSN (network) or commercial TiO2 NPs for comparison. Exposed PBMC were characterized for cell viability/apoptosis, reactive oxygen species (ROS), nuclear morphology, and cytokines secretion. Results: All the nanoparticles induced apoptosis, but only TiO2 NPs (alone or assembled into MSN) led to ROS and micronuclei. However, TiO2@MSN showed lower ROS and cytotoxicity with respect to the P25. Exposure to TiO2@MSN induced Th2-skewed and pro-fibrotic responses. Conclusions: Geno-cytotoxicity data indicate that TiO2@MSN are safer than P25 and MSN. Cytokine responses induced by TiO2@MSN are imputable to both the TiO2 NPs and MSN, and, therefore, considered of low immunotoxicological relevance. This analytical assessment might provide hints for NPs modification and deep purification to reduce the risk of health effects in the settings of their large-scale manufacturing and everyday usage by consumers.
1. Introduction
Until a few decades ago, microsized (approximately 0.1–10.0 µm) titanium dioxide (TiO2, titania) was incorporated in sunscreens as an inorganic ultraviolet (UV) filter [1]. In such form, its cosmetic profile was low due to its thick and chalky appearance on the skin. More recently, TiO2 has been prepared in the form of nanoparticles (<100 nm in size, TiO2 NPs), becoming one of the most produced nanomaterials, finding applications in a wide variety of technological fields, such as in cosmetics [2]. Indeed, TiO2 NPs are characterized by suitable bandgap energy leading to effective UV absorption exploited as a UV filter for sunscreen formulations. TiO2 NPs incorporated into sunscreen formulations are invisible to the naked eye after application on the skin, while remaining effective in photoprotecting against UV radiation [3]. Nowadays, according to European Union (EU) regulations, the maximum admitted total concentration of TiO2 (sum of micro and/or nanoform) in cosmetic formulations is 25 wt%. However, it is prohibited in applications that may result in exposure of the end user’s lungs by inhalation [4]. In addition to transparency on the skin, TiO2 NPs offer other advantages that make them cosmetically appealing, such as the absence of skin irritation and sensitization, non-comedogenicity, chemical stability, low reactivity in terms of toxicity profiles, and allergic reactions as well as a low cost [3,5]. However, TiO2 is a semiconductor material and, particularly in nanoparticle form, is a strong photocatalyst, mediated in the presence of light by formation of super-oxide anion radicals (O−2), hydroxyl radicals (•OH) and H2O2 [6,7] raising concerns about photomutagenesis [8]. From the point of view of applications, the photocatalytic activity of TiO2 NPs leads to mutually opposite effects: (i) beneficial effects when used in industrial applications, such as in organic waste and waste water treatment processes [9], self-cleaning surfaces [10] and as a potential tumor cell destructive agent [11]; and (ii) potential harmful effects when used in cosmetics as sun UV blocker, due to the photodegradation of the organic matrices present in sunscreen formulations [12] as well as the generation of reactive oxygen species (ROS) that can impact the cellular and genetic integrity of a living cell, possibly driving it toward apoptosis [13]. Oxidative stress is also responsible for genotoxic effects, even though DNA damage without generating ROS and the generation of ROS without DNA damage were reported [14]. The phototoxicity of the nanosized TiO2 filter and its inclusion in sunscreens has also raised safety questions, although toxicity concerns can only occur when TiO2 NPs are able to penetrate the stratum corneum entering the dermis [15]. The question of whether TiO2 NPs penetrate deeper levels of skin to any significant degree is not entirely resolved [16]: most of the currently available data suggest a lack of absorption across both intact and damaged (tape-stripped) skin [8] and a penetration only in the outermost corneocytes of healthy and psoriatic skin [17]. In order to minimize the formation of ROS, and to prevent potential cell damage when irradiated with UV light, different strategies were recently proposed. The use of non-semiconductor coating materials (such as silica), applied to the surface of a semiconductor, was demonstrated to be quite effective to limit its photocatalytic activity and to reduce the citotoxicity [13,18,19,20,21,22,23,24]. A different approach consists in the growth of the active UV filter into the pores of mesoporous silica nanoparticles (MSN). The control of the in situ growth of nanocrystalline particles into the pores of MSN has the advantage of a wide tunability in terms of pores and MSN sizes. The flexibility of MSN was exploited, for example, to reduce the citotoxicity and simultaneously improve the stability of metal halide perovskites [25,26] or to stabilize metastable crystalline phases [27]. In this context, TiO2 NPs embedded MSN (TiO2@MSN) were synthesized as a case study [20]. TiO2@MSN nanocomposite may have different properties with respect to its single NPs constituents [19], leading to unpredictable outcomes when it interacts with biological tissues. Therefore, immunotoxicity studies on TiO2@MSN, bare MSN and a commercial nano-TiO2 are crucial to assess its potential as a commercial UV filter and highly desirable to ensure the maximum protection of the skin in the perspective of sustainable nanotechnology.
In vitro immunotoxicity was observed in RAW 264.7 murine leukemic monocyte macrophages exposed to TiO2 NPs by simultaneous induction of immunocyte apoptosis and multiple toll-like receptors (TLRs) signaling through oxidative stress-dependent SAPK/JNK (Stress-activated protein kinases (SAPK)/Jun amino-terminal kinases (JNK)) and p38 mitogen-associated protein kinase activation [28]. Animal studies showed that TiO2 NPs can translocate to the lymph nodes by lymphatic vessels and can activate dendritic cells [29].
In vivo studies in rodents showed that topical, inhalant or intratracheal applied TiO2 NPs alone worsened atopic dermatitis, induced lung injury (Interleukin-4 (IL-4) independent) and T helper-dependent inflammatory responses through ROS production and apoptosis. Moreover, chronically inhaled TiO2 NPs induced pulmonary inflammation and fibrosis, increased expression NF-κB along with a large number of inflammatory and fibrotic cytokines in the lung. MSN resulted in being both non-toxic and non-inflammagenic, inducing only very low immune responses in splenocytes as determined by surface expression of activation markers and release of pro-inflammatory cytokines such as Interleukin-6, -12 and -1β [30]. In addition, MSN were not noxious to chronically administered animals [31]. Nevertheless, MSN are captured by phagocytic monocyte cells and potentially cause toxicity to the immune system by ROS overproduction and proinflammatory response. Some studies showed primary or secondary dose- and size-related genotoxic effects (DNA alkylation, apoptosis, DNA strands break and chromosomal aberrations, micronuclei) of silica nanoparticles in phagocytic cells and in animals [32]. An in vitro study on human lung epithelial cells A549 exposed to TiO2@MSN nanocomposites showed that the different ratio among silica and titania played a crucial role in the induced cytotoxicity [19]. In addition, TiO2/mesoporous silica nanotubes resulted in being biocompatible with mouse fibroblast cells, although still highly photocatalytic [33]. The aim of the present study was to evaluate aspects of genotoxicity and immunotoxicity of TiO2@MSN, compared to bare MSN and commercial nano-TiO2 NPs. To this end, investigations on these three systems of NPs concerned (i) the in vitro effects on cell metabolism/cytotoxic profile using the prototypical fibroblast-like murine L929 cells; and (ii) the ex vivo cytogenotoxic and immunotoxic responses using primary human peripheral blood mononuclear cells (PBMC) as target cells. The results suggest the use of TiO2@MSN as a potential UV nanofilter for cosmetic application.
2. Materials and Methods
2.1. Nanoparticle Synthesis and Characterization
MSN (≈100–150 nm, ordered pores, hexagonal symmetry, pore size ≈5 nm) were synthetized as described by Ma et al. [34]. Based on preliminary results [20], a TiO2@MSN nanocomposite (10 wt% of TiO2) was prepared by the impregnation method and fully characterized from a physicochemical point of view. For comparison, a commercially available nano-TiO2 Evonik Aeroxide® P25 (Evonik, Essen, Germany) labelled as P25 was tested.
Verification of the SiO2/TiO2 ratio of the synthesized material was performed by instrumental neutron activation analysis (INAA) at the Triga Mark II reactor of Pavia University [35]. The SiO2/TiO2 ratio experimentally determined (10.8) was in good agreement with the theoretical one.
The absence of bacterial and mycoplasma contamination was checked by seeding 100 μL of 2000 μg nanoparticles/mL stock suspension of the nanoparticles onto non-selective agar plate and growth for 4 days at 37 °C, followed by polymerase chain reaction (PCR) testing with mycoplasma-specific primers/agarose gel electrophoresis/fluorescent dye UV-visualization. Potential interference of MSN and TiO2@MSN on absorbance of ultraviolet–visible (UV–vis) light and the ability to induce the formation of cell-independent product and scattering in these assays was checked by analyzing control samples containing particles without cells. NPs’ cytotoxicity was evaluated by two complementary colorimetric assays measuring either mitochondrial enzyme activity (MTS) and lactate dehydrogenase release in the culture medium (LDH), using conventional fibroblast-like murine L929 cells [21]. The first is based on the reduction of tetrazolium salt (MTS, yellow) to formazan (purple) by mitochondrial succinate dehydrogenase enzyme; the second is based on cytoplasmic lactate dehydrogenase activity and detection of the product in culture medium (Table 1).

Table 1.
Physicochemical characterization and evaluation of contaminants and cytotoxic activity of TiO2@MSN.
2.2. Human Primary Mono/Lymphocytes
Human peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation on Lymphoprep medium (Axis Shield) [36] of freshly withdrawn ACD blood from a healthy donor. They were seeded in a 96-well plate (200,000 cells/well) in 200 µL complete culture medium (RPMI, 10% FCS), supplemented or not with the polyclonal activator phytohaemoagglutinin (PHA, 5 mg/mL) in presence of 0, 1, 25, 50, 100 µg NPs/mL in culture medium. Such NPs concentrations were obtained by dilution of a 10 mg NPs/mL stock suspension pre-filtered trough 22 µm nylon mesh filter (Millipore, Darmstadt, Germany). PHA is a polyclonal activator promoting agglutination by close contacts between cell membranes, hence stimulating cell division and metabolism, used as positive control for stimulation of (otherwise resting) human PBMC and induction of functional T-lymphocytes in vitro.
Under these conditions, lympho/monocytes (either unstimulated or PHA-activated), were used as target cells in vitro to evaluate the cyto/immunotoxicity after being induced by exposure for 6, 24, 48, 72 h in humified 5% CO2, 37 °C by TiO2@MSN, MSN and nano-TiO2 (Figure 1).
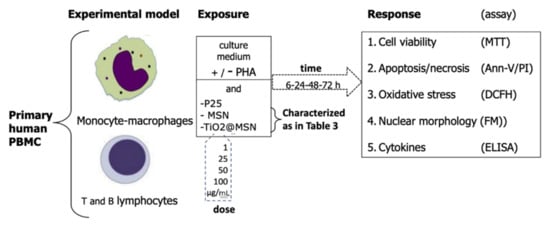
Figure 1.
Study outline.
2.3. Cell Viability/Cytotoxicity
Cell viability was evaluated by the MTT colorimetric technique [37]. Briefly, 20 μL of the yellow tetrazolium (MTT (3-(4,5-dimethylthiazol-2)-2,5 diphenyl tetrazolium bromide) (Sigma-Aldrich, Milano, Italy, 5 mg/mL in phosphate-buffered saline (PBS)), was added to each well. The plates were incubated for 3 h at 37 °C 5% CO2, for reduction of MTT by metabolically active cells. The medium was then carefully removed and solubilization of formazan crystals (insoluble purple product of MTT reduction) 200 μL DMSO (Dimethyl Sulfoxide) was added to each well. The plates were placed on a shaker for 15 min in order to achieve the complete solubilization of the crystals and then the optical density of each culture supernatant was determined. The quantity of formazan product was measured by the amount of 540 nm absorbance, which is directly proportional to the number of living cells in culture. Changes in cell viability measured by MTT were compared to that of PBMCs in complete RPMI culture medium used for preparing NPs suspensions and cells exposure.
2.4. Apoptosis
Apoptotic cells were detected and discriminated from viable cells by fluorochrome conjugated-Annexin V that can bind to phosphatildylserine residues exposed on the outer layer only on damaged cell membranes [38]. Necrotic cells were identified as cells positively stained with the DNA-specific fluorescent dye propidium iodide (PI, Sigma-Aldrich, Milano, Italy). Lymphocytes (0.5 × 106 cells) were washed with 1 mL of PBS, resuspended in 70 µL binding buffer (10 mM Hepes, 140 mM NaCl, 2.5 mM CaCl2, 0.1% BSA, pH 7.4), stained with 5 µL FITC-conjugated Annexin V (Valter Occhiena, Torino, Italy) and 0.5 µg/mL propidium iodide for 15 min, at room temperature, in the dark. As positive control, 1 mM H2O2 was added to unexposed cells and incubated for 3 h. Samples were analyzed immediately after staining by flow cytometry using the FASCanto cytofluorimeter (Becton Dickinson, Milano, Italy) and the acquired data (10,000 events within the viable cell gate, per sample) were analyzed with FacsDiva software v 6.1.3 (BD). Annexin V-positive/PI negative cells are considered apoptotic, whereas PI positive are considered as necrotic.
2.5. Oxidative Stress
Oxidative stress was evaluated by the level of intracellular ROS measured using the dye dichlorodihydrofluorescin diacetate (DCFH-DA) [39]. Briefly, 2 × 105 PBMC per well were seeded in a 96-well cell culture plate and treated with or without nanoparticles. After 24 h, cells were extensively washed with PBS and incubated with 10 µM DCFH-DA in PBS, for 30 min, at 37 °C. As positive control, 1 mM H2O2 was added to another duplicate of cultures. Fluorescence of the oxidized form of DCFH-DA (DCF) was measured using a FacsCanto cytofluorimeter (excitation wavelength: 485 nm; emission wavelength: 530 nm). For each sample, 10,000 events within the viable cells gate were acquired. Each condition was tested in quadruplicate.
2.6. Nuclear Staining
The nuclear morphology was examined using a confocal fluorescence microscope (IX71, Olympus, Düsseldorf, Germany) by staining cells with a fluorescent DNA-binding dye. After incubation in media containing different concentrations of NPs, the cultured PBMCs were treated with 100 mg/mL of the fluorescent DNA-biding dye 488 Green (Sigma-Aldrich, Merkgroup, Milano, Italy) and then observed.
2.7. Cytokines Secretion
PBMC (500,000 cells per well in 0.5 mL culture medium + PHA) were incubated for 48 h with nanoparticles (25 µg/mL, non cytotoxic concentration) in triplicate wells and, in parallel samples, no NPs were added to set the baseline levels. Cell culture supernatants were harvested and stored at −70 °C until analyzed for the quantifications of different cytokines (Interleukin-17 (IL-17), IL-23, Interferon- γ (IFN-γ), IL-10, IL-13, IL-1-β, IL-2, IL-6, Tumor Necrosis Factor- α (TNF-α) by enzyme-linked immunosorbent assay (ELISA) MAP Human Cytokine reagents and the Milliplex platform (Millipore, Darmstadt, Germany), according to the protocol provided by the manufacturer.
2.8. Statistical Analysis
All experiments were done in triplicate and the results were presented as mean ± standard error of the mean (SEM). To evaluate the differences between conditions of culture relatively to time and dose of exposure and treatment data were analyzed by two-way analysis of variance (ANOVA) followed by Bonferroni post-test using GraphPad Prism software version 4.00 (GraphPad Software, San Diego, CA, USA). Differences were considered significant at p < 0.05.
3. Results
3.1. In Vitro Exposure of Peripheral Blood Mononuclear Cells (PBMC)
3.1.1. Cell Viability
No cytotoxicity was observed after 6 and 24 h of incubation of TiO2@MSN, MSN and P25 for both PHA-stimulated and unstimulated PBMC (results not shown). After 48 h, different cytotoxicity profiles were found in PHA-activated or unstimulated PBMC. In PHA-stimulated PBMC, exposure to increasing concentrations of MSN were not associated with proportional changes of cell viability (−25% at 1 µg/mL, +33% at 100 µg/mL). Decrease of viable cells (−40%) was associated with the exposure to the highest doses (50 µg/mL and 100 µg/mL) of P25 and TiO2@MSN (Figure 2).
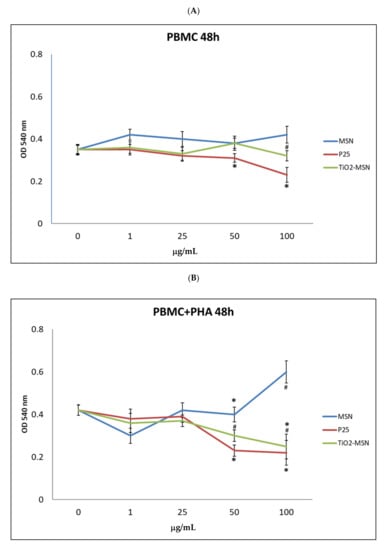
Figure 2.
MTT (3-(4,5-dimethylthiazol-2)-2,5 diphenyl tetrazolium bromide) viability assay. (A) without phytohaemoagglutinin (PHA) and (B) with PHA. Significance values: * or # = p < 0.05; error bars represent the standard error of the mean.
3.1.2. Apoptosis and Necrosis
PHA-unstimulated PBMCs ex vivo in culture incubated with TiO2@MSN, MSN and nano-TiO2 for up to 48 h maintained a high rate of viable cells (90–95%); the apoptosis rate for P25 is significantly higher at 50 and 100 µg/mL (approx. 14% and 17%, respectively) compared to TiO2@MSN (approx. 10%) (Figure 3). After 72 h, as expected for primary cells ex vivo, the percent of viable cells decreased to 80–85% (data not shown).
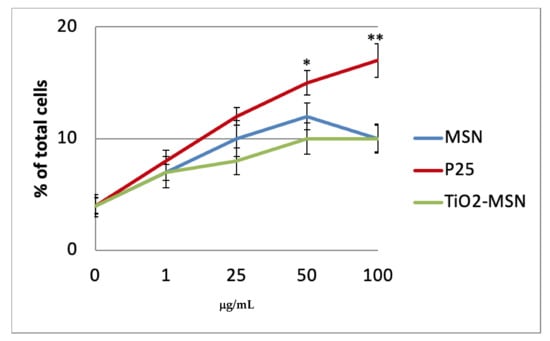
Figure 3.
Apoptosis detection of resting peripheral blood mononuclear cells (PBMC) by annexin V staining after 6 h culture without PHA. Significance values: * p < 0.05, ** p < 0.005; error bars represent the standard error of the mean.
Viability of PHA-activated PBMCs culture showed a more complex outline. The three types of NPs induced dose-dependent apoptosis within 6 h (independently from exposure to PHA).
After 24 h, a lower degree of apoptotic cells (3%) was detected among PHA-activated PBMCs. After 48 h, all three curves were almost congruent, but not dose-dependent, with the highest apoptosis shown by only P25 at 25 µg/mL (Figure 4).
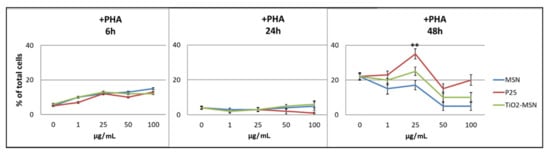
Figure 4.
Apoptosis detection of PHA-stimulated PBMC by Annexin V staining. Significance values: ** p < 0.005; error bars represent the standard error of the mean.
Necrosis was not observed after 6 h and appeared in low percentage after 24 h in any condition of exposure. After 48 h the highest rate of necrotic cells (approx. 18%) was associated with P25, while approx. 10% were detected for TiO2@MSN and MSN (Figure 5).
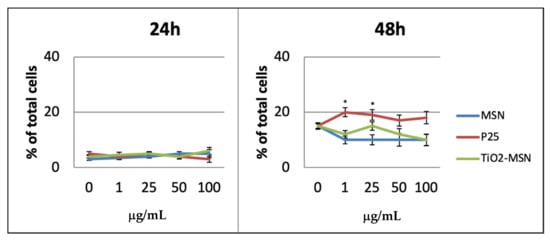
Figure 5.
Necrosis detection by propidium iodide staining. Significance values: * p < 0.05; error bars represent the standard error of the mean.
3.1.3. Oxidative Stress
ROS increased in in vitro PBMC exposed to P25 and TiO2@MSN in a time- and dose-dependent manner from 6 h (Figure 6) to 48 h.
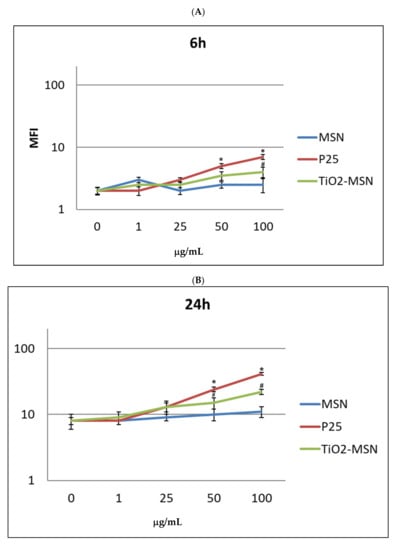
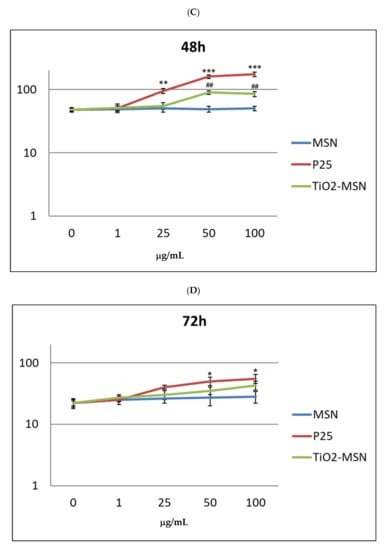
Figure 6.
Reactive oxygen species (ROS) detection by DCFDH probe at increasing time of exposure: (A) 6 h. (B) 24 h. (C) 48 h. (D) 72 h. Significance values: * or #: p < 0.05; ** or ##: p < 0.005; ***: p < 0.0005; error bars represent the standard error of the mean.
Remarkably, 1 to 50 μg/mL of P25 determined the sharpest increase of ROS, compared with equal doses of the other compound. MSN exposure was associated with increase of ROS level by time, but conversely, was independent of dose. In any condition, a proportional reduction of ROS levels was measured after 72 h. However, at this timepoint extensive cell damage appeared by optical microscope observation (40×) (not shown). As expected, PHA-stimulated PBMC showed a generally higher ROS level (not shown), compared to unstimulated PBMC. Notably, P25, in any time and culturing condition induced the highest level of ROS. The two highest doses of TiO2@MSN were associated with induction of a lower level of ROS at 24 h and 48 h.
3.1.4. Nuclear Morphology
Bright nuclei (interphase condensed chromatin) with organized nucleoli as well as enlarged nuclei (replicating chromatin), typical of viable cells, were visible in samples exposed to the lowest concentration (1 µg/mL) of either of the three types of NP. Beside those, weakly colored nuclei (i.e., less organized chromatin) characteristic of cells undergoing necrosis, were visible. Furthermore, bright micronuclei (MN, circles), i.e., small chromatin formations clearly distinguished from the nucleus, adjacent to it, with same coloration and size between 1/16 and 1/3 of the mean diameter of the nucleous [40], were apparent in the samples exposed to 25 µg/mL of P25 or TiO2@MSN, with a higher frequency for the former. Nuclear abnomalies (white arrows) were evident only in samples exposed to P25 (25 µg/mL). At the highest concentration (50 µg/mL), nuclear buds (NBUDs, red arrows) and chromatin bridges (yellow arrow) were also present. Dividing nuclei were observed in the sample exposed to 25 µg/mL P25 (m = metaphase) and 50 µg/mL MSN (a = anaphase) (Figure 7).
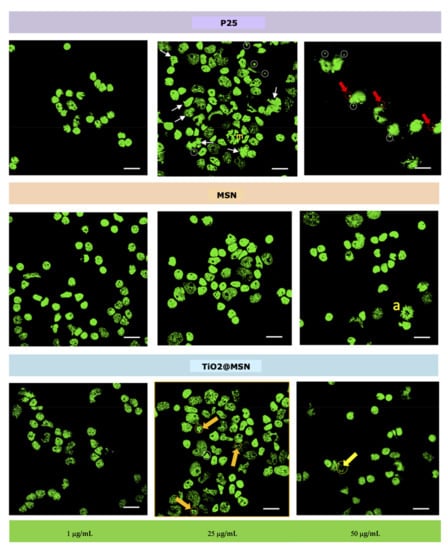
Figure 7.
Nuclear morphology. Bright micronuclei (MN, circles), nuclear anomalies (white arrows). Nuclear buds (NBUDs, red arrows) and chromatin bridges (yellow arrow). Dividing nuclei were observed (m = metaphase; a = anaphase).
3.1.5. Cytokines Profile
Unstimulated PBMC (no PHA) produced barely detectable (IL-2, IL-6, IL-1β, TNF-α, IL-4, IL-10, IL-23) or undetectable (IL-17 and IFN-γ) cytokine proteins in the culture medium upon exposure to TiO2@MSN, MSN or P25. The addition of T-cell polyclonal activator PHA to PBMC induced a strong increase in the secretion of IL-2, IL-4, IL-10, IL-17, IL-23, and IFN-γ and no quantitative changes were observed in IL-6, IL-1β and TNF-α expression, compared to the unstimulated control.
Addition of the three types of NP in the culture medium produced the following effects on the level of cytokines secreted by PHA-stimulated PBMCs (Table 2 and Figure 8):

Table 2.
Dose-dependent cytokine patterns associated with exposure of PBMC to TiO2@MSN, MSN and P25 and their target cells of the immune system.
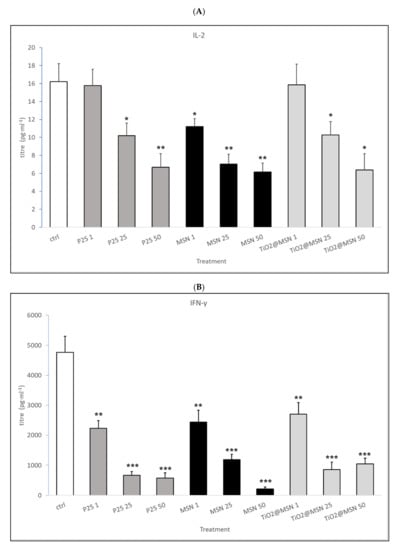
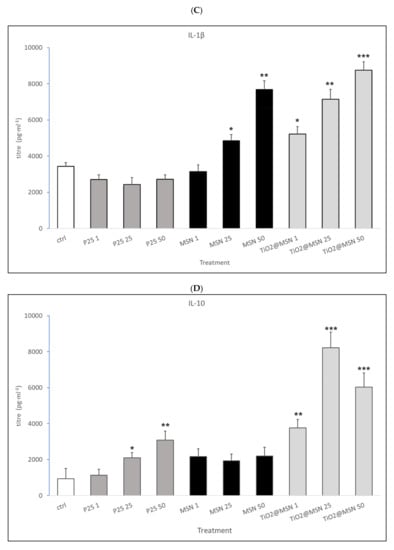
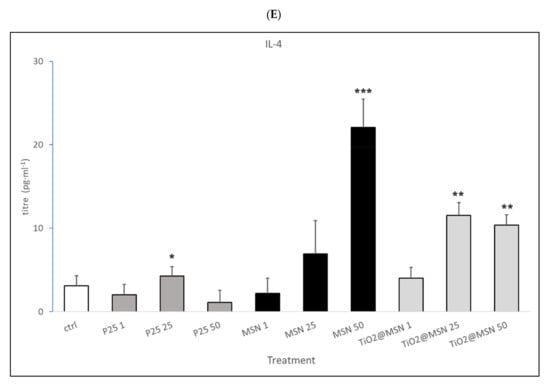
Figure 8.
Secreted cytokines pattern: titer of (A) IL-2; (B) INF-γ; (C) IL-1β; (D) IL-10; (E) IL-4. Significance values: * p < 0.05, ** p < 0.005, *** p < 0.0005; error bars represent the standard error of the mean.
- (i)
- MSN exposure was associated with strong increase of IL-1β and IL-4, a decrease of IL-2 and IFN-γ levels, in a dose-dependent manner. IL-17 and IL-23 were down-modulated while no significant changes in IL-6, TNF-α, IL-10 were detected (data not shown).
- (ii)
- P25 determined a decrease of IL-2 and IFN-γ, increase of IL-4 (at non cytotoxic concentrations, 1–50 µg/mL) and an obvious dose-dependent increase of IL-10, and TNF-α. IL-17 and IL-23 were downmodulated, whereas IL-6 and IL-1β were not affected (data not shown).
- (iii)
- TiO2@MSN induced dose-dependent reduction of IL-2 and IFN-γ, particularly high spikes of TNF-α and dose-dependent increase of IL-1β and IL-10. Moreover, it induced a dose- and time-dependent fluctuating levels of TNF-α and IL-4, an increase of IL-17 and IL-23 as well as no significant change of (limited) IL-6 (Figure 8).
4. Discussion
TiO2 NPs are an essential nanomaterial for numerous technological applications. In particular, they are appealing as protective UV filter in solar sunscreens, use of which would entail frequent and pervasive cutaneous (skin and hair) treatment with TiO2 NPs for over-exposed or inherently under-protected people, such as infants, the elderly and outdoor workers.
Regrettably, toxicological data gathered so far address potential pitfalls of these NPs related to its chemistry and size. In human skin cell-based experiments, often characterized by high doses of exposure, TiO2 causes oxidative stress and DNA damage [41] and, extracted from sunscreens, induces apoptosis of exposed UVA-irradiated cells, an effect that is dampened by pre-coating [42]. Furthermore, TiO2 NPs are phagocytized by monocytes/macrophages that consequently display oxidative stress and genotoxic effects [43]. In mouse brain microglia, at non-cytotoxic concentrations, TiO2 NPs form cytoplasmic aggregates and stimulate ROS [44]. In chronically exposed rodents, TiO2 induces pro-inflammatory cytokines and fibrosis of the lung [45], and TiO2 NPs appear to play a role in the onset and aggravation of allergies through a cyto-genotoxic mechanism [46]. Also, human lymphocytes, either from healthy individuals or with respiratory disease, are damaged by TiO2 NPs at the DNA level [47]. Human studies, regarding professionally exposed worker to TiO2 (nanoparticles), show oxidative damage of nucleic acids of Ti-containing exhaled breath condensate samples [48] and increased oxidative stress and overexpression of inflammatory and fibrogenic cytokines [49]. However, the aforementioned studies are limited in numbers, jagged/fragmentary and not specifically addressed to highlight TiO2 NPs in humans.
Hence, to improve the efficiency of TiO2 NPs as a UV filter and, concurrently, its biosafety, we synthetized a nanotitania composite by growing TiO2 NPs inside MSN. In fact, MSN are largely used in therapeutic and diagnostic applications for their high surface area, large pore size, good biocompatibility and biodegradability, and stability in aqueous dispersions [50]. TiO2@MSN was submitted to key geno-immunotoxic assessment by comparative testing against MSN and P25 in human PBMC, using a combination of damage/defense responses of immune cells against noxious agents (i.e., apoptosis, ROS, nuclear morphology evaluation and released cytokines detection).
In previous studies, we showed that primary human PBMC were useful for ex vivo evaluation of the genotoxic and immunotoxic potential of NPs [51,52,53,54,55]. Also, PBMC testing was proposed for monitoring the health protection of potentially exposed workers at higher risk of asthma and atopic dermatitis [56] and as a diagnostic biomarker [57]. Recently, the pre-clinical evaluation of cytokines released by PBMC in vitro has been recommended by the FDA (Food and Drug Administration) for large (protein) therapeutic molecules [58]. In addition, we have shown that PBMC produce ROS, undergo apoptosis and characteristic dysregulation of cell cycle and cytokine expression when exposed to NPs [52,53,54,59,60,61,62,63,64,65,66].
Apoptosis occurs as a defence mechanism at a low dose preventing genotoxic mutation from becoming mutagenesis [67]. Moreover, nuclear abnormalities was detected as signs of genetic damage caused by NPs [68]. Amongst these are micronuclei, acentric fragments or entire chromosomes unable to migrate to the poles during cell division, and nuclear buds, that instead remain linked to the nucleus through a “bridge” of nucleoplasmic material [40].
Abnormal cytokine patterns can reflect genetic dysregulation and cancer [69] even ending in death (cytokine storm, cytokine release syndrome [70]). The expression of cytokines is firstly regulated at the transcription level through transcription factors that respond to signalling pathways activated by pathogen-derived ligands or endogenous inflammatory mediators [69]. Notably, NPs can mimic pathogens and bind surface receptors able to trigger those pathways [60]. Conversely, mutation (or inhibition) of a single transcription factor (TF) binding site can upheaval cytokine expression and lead to immune disorders [69]. All the aforementioned were detected in our study for either of two TiO2-based nanoparticles tested, and for MSN. Although the exact mechanism(s) of TiO2 NPs genotoxicity remain to be determined, oxidative stress might initiate it as suggested by the parallel increase of ROS. Nano-TiO2-induced genotoxicity could rely also on the inhibition of DNA base excision repair systems [71], known to operate when metal(oxide)-NP-based DNA damage occur [72].
It cannot be excluded that soluble compounds from aggregates of TiO2 NPs might have contributed to the observed effect, but these appear to be sensibly reduced by the growth of TiO2 NPs inside MSN.
ROS were detected also in cells exposed to the two highest concentrations of TiO2@MSN and, since MSN alone do not induce as much ROS as the TiO2@MSN, the geno-cytotoxicity appears to be mediated by nano-TiO2.
Our findings strongly suggest that “typical” ROS-induced genotoxicity of TiO2 NPs is abrogated when it is incorporated inside MSN nanopores and that, in such a form, it is associated with higher concentrations corresponding to aggregates formation; also, the biological modifications associated with TiO2@MSN exposure are detectable when the activation of the target lymphocytes is turned on. If confirmed, the use of MSN as a scaffold might represent a way to lower the toxic potential of TiO2 NPs and others.
The higher toxicity observed for TiO2@MSN might be related to the formation of larger aggregates observed when dispersed in culture medium supplemented with serum, a phenomenon being underlined as the most relevant in the assessment of nanoparticles toxicity and potential human effects. On the other hand, residual toxicity of the novel nanomaterial could be due to titania impurities, and chemically instability and loss of morphology in culture medium.
All three samples analyzed downmodulate IL-2 and IFN-γ cytokines. Likely, this reflects suppression of T helper 1 (Th1) cells that are central players in specific acquired immune response against microbial pathogens and tumor cells. On the other hand, over-secreted IL-4 might critically stimulate Th2 cells, physiologically involved in the fight against parasites but, together with B-cells, also targets of this cytokine, leading to detrimental allergic and fibrogenic responses too. Beside those shared effects, MSN and TiO2 NPs appear to display polarized behaviors, although hindering each other. In fact, MSN-containing NPs are associated with the increased release of IL-1β, as elsewhere described [63], known to be produced by and activate monocytes/macrophages to capture and annihilate intruding particulate matters generating inflammation in situ. Instead, P25 and TiO2@MSN appear to stimulate IL-10 and, therefore, might favor immune tolerance, antigen presentation and fibrosis mediated by responding regulatory T cells, antigen processing cells and epithelial cells, respectively.
5. Conclusions
This study shows that the novel nanocomposite TiO2@MSN, with improved physicochemical properties of TiO2 NPs, also shows improved biocompatibility in terms of lower cyto-genotoxic effects. Moreover, the observed Th2-skewed and pro-fibrotic responses upon acute exposure to TiO2@MSN are both likely imputable to the non-encapsulated precursor of TiO2 NPs, when analyzed comparatively. Furthermore, the observed cytokine pattern induced by TiO2@MSN is not dissimilar and typical of that of any other particulate material [63] and, therefore, to be considered of low immunotoxicological relevance. Collectively, this study shows that TiO2@MSN represents an advanced TiO2-based nanocomposite material suitable for bio-applications.
The pre-production evaluations described here based on complex interplaying human cellular populations along with the assessment of the true “biologic” NPs size allows rapid monitoring of innovative NPs biocompatibility. This analytical assessment also might provide hints for NPs modification (i.e., by encapsulation, anti-oxidant molecules etc.), and deep purification to reduce the risk of health effects in the settings of their large-scale manufacturing and everyday usage by consumers.
Author Contributions
Conceptualization C.P., M.D.G., E.S., Q.N. and methodology C.P., G.Z., G.V., F.G., A.M.G.P., F.C.; Software C.P., G.V., F.C.; validation C.P. and formal analysis E.S., S.M.; Investigation C.P., S.M., G.Z., G.V.; resources C.P., A.B.; data curation G.Z., G.V.; writing—original draft preparation C.P., E.S., M.D.G.; writing—review and editing C.P., G.V., G.Z., A.B., E.S., Q.N., L.D.G.; visualization C.P., E.S., A.M.G.P.; supervision C.P., E.S., A.B.; project administration C.P.; founding acquisition, M.D.G. and C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Smijs, T.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Applications. 2011, 4, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Lux-Research. Nanomaterials State of the Market: Stealth Success, Broad Impact. 2 Report. 2018. Available online: rchinc.com/research/ (accessed on 20 March 2018).
- Trivedi, B.B.M.; Murase, J. Titanium Dioxide in Sunscreen. IntechOpen 2017, 26, 61–71. [Google Scholar] [CrossRef]
- European Union. COMMISSION REGULATION (EU) 2016/1143 of 13 July 2016 amending Annex VI to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products. Off. J. Eur. Union 2016, 189, 40–43. [Google Scholar]
- Sungur, Ş. Titanium Dioxide Nanoparticles. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Kharissova, O., Martínez, L., Kharisov, B., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Lewicka, Z.A.; Yu, W.W.; Oliva, B.L.; Contreras, E.Q.; Colvin, V.L. Photochemical behavior of nanoscale TiO2 and ZnO sunscreen ingredients. J. Photochem. Photobiol. A Chem. 2013, 263, 24–33. [Google Scholar] [CrossRef]
- Falcaro, P.; Zaccariello, G.; Stoyanova, V.; Benedetti, A.; Costacurta, S. Temperature matters: An infrared spectroscopic investigation on the photocatalytic efficiency of titania coatings. Sci. Adv. Mater. 2014, 6, 1330–1337. [Google Scholar] [CrossRef]
- Schneider, S.L.; Lim, H.W. A review of inorganic UV filters zinc oxide (ZnO) and titanium dioxide (TiO2). Photodermatol. Photoimmunol. Photomed. 2018, 35, 442–446. [Google Scholar] [CrossRef]
- Vela, N.; Pérez-Lucas, G.; Fenoll, J.; Navarro, S. Recent Overview on the Abatement of Pesticide Residues in Water by Photocatalytic Treatment Using TiO2. Appl. Titan. Dioxide 2017, 147–177. [Google Scholar] [CrossRef]
- Padmanabhan, N.T.; John, H. Titanium dioxide based self-cleaning smart surfaces: A short review. J. Environ. Chem. Eng. 2020, 8, 104211. [Google Scholar] [CrossRef]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.J.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef]
- De La Vega, A.C.S.; Molins-Delgado, D.; Barceló, D.; Diaz-Cruz, M.S. Nanosized titanium dioxide UV filter increases mixture toxicity when combined with parabens. Ecotoxicol. Environ. Saf. 2019, 184, 109565. [Google Scholar] [CrossRef]
- Chen, L.; Wang, S. Nanotechnology in Photoprotection. In Nanoscience in Dermatology; Academic Press: Cambridge, MA, USA, 2016; pp. 229–236. [Google Scholar] [CrossRef]
- Wani, M.R.; Shadab, G. Titanium dioxide nanoparticle genotoxicity: A review of recent in vivo and in vitro studies. Toxicol. Ind. Health 2020, 36, 514–530. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.D.; Stotland, M.; Ellis, J.I. The safety of nanosized particles in titanium dioxide- and zinc oxide-based sunscreens. J. Am. Acad. Dermatol. 2009, 61, 685–962. [Google Scholar] [CrossRef]
- Musial, J.; Krakowiak, R.; Mlynarczyk, D.T.; Goslinski, T.; Stanisz, B.J. Titanium Dioxide Nanoparticles in Food and Personal Care Products—What Do We Know about Their Safety? Nanomaterials 2020, 10, 1110. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.; Pirot, F.; Bertholle, V.; Roussel, L.; Falson, F.; Padois, K. Commonly Used UV Filter Toxicity on Biological Functions: Review of Last Decade Studies. Int. J. Cosmet. Sci. 2013, 35, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Ortelli, S.; Poland, C.A.; Baldi, G.; Costa, A.L. Silica matrix encapsulation as a strategy to control ROS production while preserving photoreactivity in nano-TiO2. Environ. Sci. Nano 2016, 3, 602–610. [Google Scholar] [CrossRef]
- Bengalli, R.; Ortelli, S.; Blosi, M.; Costa, A.L.; Mantecca, P.; Fiandra, L. In Vitro Toxicity of TiO2:SiO2 Nanocomposites with Different Photocatalytic Properties. Nanomaterials 2019, 9, 1041. [Google Scholar] [CrossRef] [PubMed]
- Zaccariello, G.; Moretti, E.; Storaro, L.; Riello, P.; Canton, P.; Gombac, V.; Montini, T.; Rodriguez-Castellon, E.; Benedetti, A. TiO2-mesoporous silica nanocomposites: Cooperative effect in the photocatalytic degradation of dyes and drugs. RSC Adv. 2014, 4, 37826–37837. [Google Scholar] [CrossRef]
- Zaccariello, G.; Back, M.; Zanello, M.; Canton, P.; Cattaruzza, E.; Riello, P.; Alimonti, A.; Benedetti, A. Formation and controlled growth of bismuth titanate phases into mesoporous silica nanoparticles: An efficient self-sealing nanosystem for UV filtering in cosmetic formulation. ACS Appl. Mater. Interfaces 2016, 9, 1913–1921. [Google Scholar] [CrossRef]
- Zaccariello, G.; Back, M.; Benedetti, A.; Canton, P.; Cattaruzza, E.; Onoda, H.; Glisenti, A.; Alimonti, A.; Bocca, B.; Riello, P. Bismuth titanate-based UV filters embedded mesoporous silica nanoparticles: Role of bismuth concentration in the self-sealing process. J. Colloid Interface Sci. 2019, 549, 1–8. [Google Scholar] [CrossRef]
- Back, M.; Casagrande, E.; Brondin, C.A.; Ambrosi, E.; Cristofori, D.; Ueda, J.; Tanabe, S.; Trave, E.; Riello, P. Lanthanide-Doped Bi2SiO5@SiO2 Core-Shell Upconverting Nanoparticles for Stable Ratiometric Optical Thermometry. ACS Appl. Nano Mater 2020, 3, 2594–2604. [Google Scholar] [CrossRef]
- Casagrande, E.; Back, M.; Cristofori, D.; Ueda, J.; Tanabe, S.; Palazzolo, S.; Rizzolio, F.; Canzonieri, V.; Trave, E.; Riello, P. Upconversion-Mediated Boltzmann Thermometry in Double-Layered Bi2SiO5:Yb3+, Tm3+@SiO2 Hollow Nanoparticles. J. Mater. Chem. C 2020, 8, 7828–7836. [Google Scholar] [CrossRef]
- Malgras, V.; Tominaka, S.; Ryan, J.W.; Henzie, J.; Takei, T.; Ohara, K.; Yamauchi, Y. Observation of quantum confinement in monodisperse methylammonium lead halide perovskite nanocrystals embedded in mesoporous silica. J. Am. Chem. Soc. 2016, 138, 13874–13881. [Google Scholar] [CrossRef] [PubMed]
- Dirin, D.N.; Protesescu, L.; Trummer, D.; Kochetygov, I.V.; Yakunin, S.; Krumeich, F.; Stadie, N.P.; Kovalenko, M.V. Harnessing defect-tolerance at the nanoscale: Highly luminescent lead halide perovskite nanocrystals in mesoporous silica matrixes. Nano Lett. 2016, 16, 5866–5874. [Google Scholar] [CrossRef] [PubMed]
- Back, M.; Trave, E.; Zaccariello, G.; Cristofori, D.; Canton, P.; Benedetti, A.; Riello, P. Bi2SiO5@g-SiO2 upconverting nanoparticles: A bismuth-driven core-shell self-assembly mechanism. Nanoscale 2019, 11, 675–687. [Google Scholar] [CrossRef]
- Dhupal, M.; Oh, J.-M.; Tripathy, D.R.; Kim, S.-K.; Koh, S.-B.; Park, K.-S. Immunotoxicity of titanium dioxide nanoparticles via simultaneous induction of apoptosis and multiple toll-like receptors signaling through ROS-dependent SAPK/JNK and p38 MAPK activation. Int. J. Nanomed. 2018, 13, 6735–6750. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, R.K.; Gaur, K.; Torres, J.F.C.; Loza-Rosas, S.A.; Torres, A.; Saxena, M.; Julin, M.; Tinoco, A.D. Fueling a Hot Debate on the Application of TiO2 Nanoparticles in Sunscreen. Materials 2019, 12, 2317. [Google Scholar] [CrossRef]
- Heidegger, S.; Gössl, D.; Schmidt, A.; Niedermayer, S.; Argyo, C.; Endres, S.; Bein, T.; Bourquin, C. Immune response to functionalized mesoporous silica nanoparticles for targeted drug delivery. Nanoscale 2016, 8, 938–948. [Google Scholar] [CrossRef]
- Murugadoss, S.; Lison, D.; Godderis, L.; Brule, S.V.D.; Mast, J.; Brassinne, F.; Sebaïhi, N.; Hoet, P. Toxicology of silica nanoparticles: An update. Arch. Toxicol. 2017, 91, 2967–3010. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Zhang, Y.; Zhang, G.; Kang, Y.; Chen, A.; Feng, X.; Shao, L. The Toxicity of Silica Nanoparticles to the Immune System. Nanomedicine 2018, 13, 1939–1962. [Google Scholar] [CrossRef]
- Cendrowski, K. Titania/mesoporous silica nanotubes with efficient photocatalytic properties. J. Chem. Technol. 2018, 20, 103–108. [Google Scholar] [CrossRef]
- Ma, S.; Wang, Y.; Zhu, Y. A simple room temperature synthesis of mesoporous silica nanoparticles for drug storage and pressure pulsed delivery. J. Porous Mater. 2010, 18, 233–239. [Google Scholar] [CrossRef]
- Cammi, A.; Ponciroli, R.; Di Tigliole, A.B.; Magrotti, G.; Prata, M.; Chiesa, D.; Previtali, E. A zero dimensional model for simulation of TRIGA Mark II dynamic response. Prog. Nucl. Energy 2013, 68, 43–54. [Google Scholar] [CrossRef]
- Böyum, A. Separation of Leukocytes from Blood and Bone Marrow. Introduction. Scand. J. Clin. Lab. Investig. 1968, 97, 7. [Google Scholar]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Koopman, G.; Reutelingsperger, C.; Kuijten, G.; Keehnen, R.; Pals, S.; Van Oers, M. Annexin V for Flow Cytometric Detection of Phosphatidylserine Expression on B Cells Undergoing Apoptosis. Blood 1994, 84, 1415–1420. [Google Scholar] [CrossRef]
- Lebel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the Probe 2′,7′-Dichlorofluorescin as an Indicator of Reactive Oxygen Species Formation and Oxidative Stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef]
- Fenech, M. Cytokinesis-Block Micronucleus Cytome Assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef]
- Tiano, L.; Armeni, T.; Venditti, E.; Barucca, G.; Mincarelli, L.; Damiani, E. Modified TiO2 Particles Differentially Affect Human Skin Fibroblasts Exposed to UVA Light. Free Radic. Biol. Med. 2010, 49, 408–415. [Google Scholar] [CrossRef]
- Rampaul, A.; Parkin, I.P.; Cramer, L.P. Damaging and Protective Properties of Inorganic Components of Sunscreens Applied to Cultured Human Skin Cells. J. Photochem. Photobiol. A Chem. 2007, 191, 138–148. [Google Scholar] [CrossRef]
- Nohynek, G.; Dufour, E.; Roberts, M.S. Nanotechnology, Cosmetics and the Skin: Is There a Health Risk? Ski. Pharmacol. Physiol. 2008, 21, 136–149. [Google Scholar] [CrossRef]
- Long, T.C.; Saleh, N.; Tilton, R.D.; Lowry, G.V.; Veronesi, B. Titanium Dioxide (P25) Produces Reactive Oxygen Species in Immortalized Brain Microglia (BV2): Implications for Nanoparticle Neurotoxicity. Environ. Sci. Technol. 2006, 40, 4346–4352. [Google Scholar] [CrossRef] [PubMed]
- Pelclova, D.; Zdimal, V.; Fenclova, Z.; Vlckova, S.; Turci, F.; Corazzari, I.; Kacer, P.; Schwarz, J.; Zikova, N.; Makes, O.; et al. Markers of Oxidative Damage of Nucleic Acids and Proteins among Workers Exposed to TiO2 (Nano) Particles. Occup. Environ. Med. 2016, 73, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Ji, L.; Zhou, Y.; Wang, L. Retracted: Pulmonary Fibrosis of Mice and Its Molecular Mechanism Following Chronic Inhaled Exposure to TiO2 Nanoparticles. Environ. Toxicol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Osman, I.F.; Najafzadeh, M.; Sharma, V.; Shukla, R.K.; Jacob, B.K.; Dhawan, A.; Anderson, D. TiO2 NPs Induce DNA Damage in Lymphocytes from Healthy Individuals and Patients with Respiratory Diseases—An Ex Vivo/In Vitro Study. J. Nanosci. Nanotechnol. 2018, 18, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Uga, H.; Mori, A.; Kurata, H. Increased Serum IL-17A and Th2 Cytokine Levels in Patients with Severe Uncontrolled Asthma. Eur. Cytokine Netw. 2017, 28, 8–18. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, Y.; Jia, G.; Xu, H.; Zhou, J.; Tang, S.; Xu, Z.; Kong, F.; Liu, G.; Zhang, Y.; et al. Cardiopulmonary Effects Induced by Occupational Exposure to Titanium Dioxide Nanoparticles. Nanotoxicology 2018, 12, 169–184. [Google Scholar] [CrossRef]
- Jafari, S.; Derakhshankhah, H.; Alaei, L.; Fattahi, A.; Varnamkhasti, B.S.; Saboury, A.A. Mesoporous Silica Nanoparticles for Therapeutic/Diagnostic Applications. Biomed. Pharmacother. 2019, 109, 1100–1111. [Google Scholar] [CrossRef]
- Petrarca, C.; Clemente, E.; Di Giampaolo, L.; Mariani-Costantini, R.; Leopold, K.; Schindl, R.; Lotti, L.V.; Mangifesta, R.; Sabbioni, E.; Niu, Q.; et al. Palladium Nanoparticles Induce Disturbances in Cell Cycle Entry and Progression of Peripheral Blood Mononuclear Cells: Paramount Role of Ions. J. Immunol. Res. 2014, 2014, 295092. [Google Scholar] [CrossRef]
- Petrarca, C.; Perrone, A.; Verna, N.; Verginelli, F.; Ponti, J.; Sabbioni, E.; Di Giampaolo, L.; Dadorante, V.; Schiavone, C.; Boscolo, P.; et al. Cobalt Nano-Particles Modulate Cytokine in Vitro Release by Human Mononuclear Cells Mimicking Autoimmune Disease. Int. J. Immunopathol. Pharmacol. 2006, 19 (Suppl. 4), 11–14. [Google Scholar]
- Boscolo, P.; Bellante, V.; Leopold, K.; Maier, M.; Di, L.G.; Antonucci, A.; Iavicoli, I.; Tobia, L.; Paoletti, A.; Montalti, M.; et al. Effects of Palladium Nanoparticles on the Cytokine Release from Peripheral Blood Mononuclear Cells of Non-Atopic Women. J. Biol. Regul. Homeost. Agents 2010, 24, 207–214. [Google Scholar] [CrossRef]
- Di Gioacchino, M.; Perrone, A.; Petrarca, C.; Verna, N.; Esposito, D.; Ponti, J.; Sabbioni, E.; Di Giampaolo, L.; Boscolo, P.; Costantini, R.M. In Vitro Cytokine Modulation by Cobalt Nano- and Microparticles and Solutions. G. Ital. Di Med. Del Lav. Ed. Ergon. 2006, 28, 316–318. [Google Scholar]
- Di Gioacchino, M.; Verna, N.; Di Giampaolo, L.; Di Claudio, F.; Turi, M.; Perrone, A.; Petrarca, C.; Mariani-Costantini, R.; Sabbioni, E.; Boscolo, P. Immunotoxicity and Sensitizing Capacity of Metal Compounds Depend on Speciation. Int. J. Immunopathol. Pharmacol. 2007, 20 (Suppl. 2), 15–22. [Google Scholar] [CrossRef] [PubMed]
- Olson, W.C.; Smolkin, M.E.; Farris, E.M.; Fink, R.J.; Czarkowski, A.; Fink, J.H.; Chianese-Bullock, K.A.; Slingluff, C.L. Shipping Blood to a Central Laboratory in Multicenter Clinical Trials: Effect of Ambient Temperature on Specimen Temperature, and Effects of Temperature on Mononuclear Cell Yield, Viability and Immunologic Function. J. Transl. Med. 2011, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Esfandyarpour, R.; Kashi, A.; Nemat-Gorgani, M.; Wilhelmy, J.; Davis, R.W. A Nanoelectronics-Blood-Based Diagnostic Biomarker for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Proc. Natl. Acad. Sci. USA 2019, 116, 10250–10257. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Mankus, C.I.; Vermilya, A.M.; Soheilian, F.; Clogston, J.D.; Dobrovolskaia, M.A. Feraheme® Suppresses Immune Function of Human T Lymphocytes through Mitochondrial Damage and MitoROS Production. Toxicol. Appl. Pharmacol. 2018, 350, 52–63. [Google Scholar] [CrossRef]
- Malorni, L.; Guida, V.; Sirignano, M.; Genovese, G.; Petrarca, C.; Pedata, P. Exposure to Sub-10nm Particles Emitted from a Biodiesel-Fueled Diesel Engine: In Vitro Toxicity and Inflammatory Potential. Toxicol. Lett. 2017, 270, 51–61. [Google Scholar] [CrossRef]
- Petrarca, C.; Clemente, E.; Amato, V.; Pedata, P.; Sabbioni, E.; Bernardini, G.; Iavicoli, I.; Cortese, S.; Niu, Q.; Otsuki, T.; et al. Engineered Metal Based Nanoparticles and Innate Immunity. Clin. Mol. Allergy 2015, 13, 13. [Google Scholar] [CrossRef]
- Sabbioni, E.; Fortaner, S.; Farina, M.; Del Torchio, R.; Petrarca, C.; Bernardini, G.; Mariani-Costantini, R.; Perconti, S.; Di Giampaolo, L.; Gornati, R.; et al. Interaction with Culture Medium Components, Cellular Uptake and Intracellular Distribution of Cobalt Nanoparticles, Microparticles and Ions in Balb/3T3 Mouse Fibroblasts. Nanotoxicology 2014, 8, 88–99. [Google Scholar] [CrossRef]
- Pedata, P.; Petrarca, C.; Garzillo, E.M.; Di Gioacchino, M. Immunotoxicological Impact of Occupational and Environmental Nanoparticles Exposure: The Influence of Physical, Chemical, and Combined Characteristics of the Particles. Int. J. Immunopathol. Pharmacol. 2016, 29, 343–353. [Google Scholar] [CrossRef]
- Di Gioacchino, M.; Petrarca, C.; Lazzarin, F.; Di Giampaolo, L.; Sabbioni, E.; Boscolo, P.; Mariani-Costantini, R.; Bernardini, G. Immunotoxicity of Nanoparticles. Int. J. Immunopathol. Pharmacol. 2011, 24 (Suppl. 1), 65S–71S. [Google Scholar]
- Di, L.G.; Di, M.G.; Mangifesta, R.; Gatta, A.; Tinari, N.; Grassadonia, A.; Niu, Q.; Paganelli, R.; Sabbioni, E.; Otsuki, T.; et al. Occupational Allergy: Is There a Role for Nanoparticles? J. Biol. Regul. Homeost. Agents 2019, 33, 661–668. Available online: http://www.ncbi.nlm.nih.gov/pubmed/31179676 (accessed on 3 July 2019).
- Perconti, S.; Aceto, G.M.; Verginelli, F.; Napolitano, F.; Petrarca, C.; Bernardini, G.; Raiconi, G.; Tagliaferri, R.; Sabbioni, E.; Di, M.G.; et al. Distinctive Gene Expression Profiles in Balb/3T3 Cells Exposed to Low Dose Cobalt Nanoparticles, Microparticles and Ions: Potential Nanotoxicological Relevance. J. Biol. Regul. Homeost. Agents 2013, 27, 443–454. [Google Scholar]
- Di Gioacchino, M.; Petrarca, C.; Gatta, A.; Scarano, G.; Farinelli, A.; Della Valle, L.; Lumaca, A.; Del Biondo, P.; Paganelli, R.; Di Giampaolo, L. Nanoparticle-Based Immunotherapy: State of the Art and Future Perspectives. Expert Rev. Clin. Immunol. 2020, 16, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicologic Pathology. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Netzer, K.; Jordakieva, G.; Kataeva, N.; Schotter, J.; Ertl, P.; Girard, A.M.; Budinsky, A.C.; Pilger, A.; Richter, L.; Godnic-Cvar, J. Next-Generation Magnetic Nanocomposites: Cytotoxic and Genotoxic Effects of Coated and Uncoated Ferric Cobalt Boron (FeCoB) Nanoparticles In Vitro. Basic Clin. Pharmacol. Toxicol. 2018, 122, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Pro, S.C.; Imedio, A.D.; Santoso, C.S.; Gan, K.A.; Sewell, J.A.; Martinez, M.; Sereda, R.; Mehta, S.; Bass, J.I.F. Global Landscape of Mouse and Human Cytokine Transcriptional Regulation. Nucleic Acids Res. 2018, 46, 9321–9337. [Google Scholar] [CrossRef]
- Canna, S.; Behrens, E.M. Making Sense of the Cytokine Storm: A Conceptual Framework for Understanding, Diagnosing, and Treating Hemophagocytic Syndromes. Pediatric Clin. N. Am. 2012, 59, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Biola-Clier, M.; Beal, D.; Caillat, S.; Libert, S.; Armand, L.; Herlin-Boime, N.; Sauvaigo, S.; Douki, T.; Carriere, M. Comparison of the DNA Damage Response in BEAS-2B and A549 Cells Exposed to Titanium Dioxide Nanoparticles. Mutagenesis 2016, 32, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Nelson, B.C.; Scanlan, L.D.; Coskun, E.; Jaruga, P.; Doak, S.H. Exposure to Engineered Nanomaterials: Impact on DNA Repair Pathways. Int. J. Mol. Sci. 2017, 18, 1515. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).