Ambient Climate Influences Anti-Adhesion between Biomimetic Structured Foil and Nanofibers
Abstract
1. Introduction
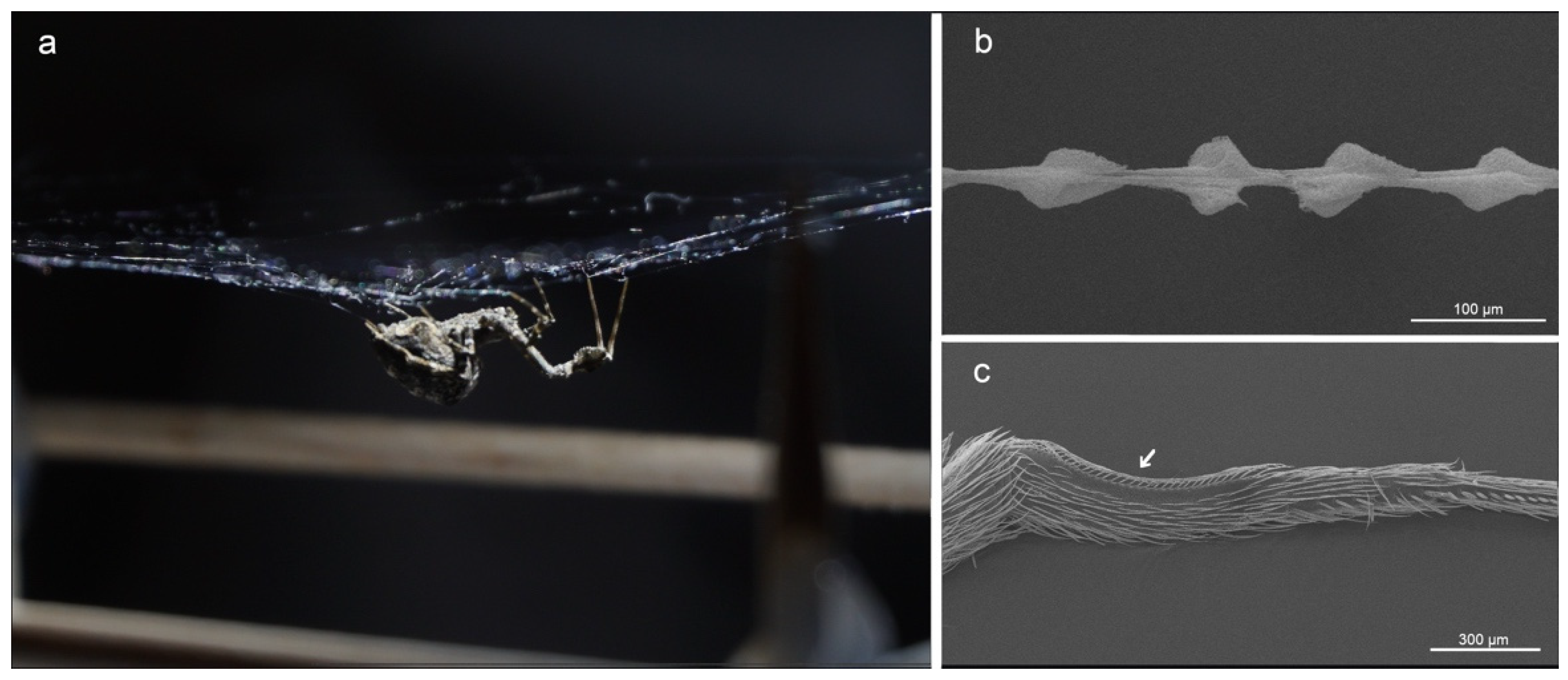
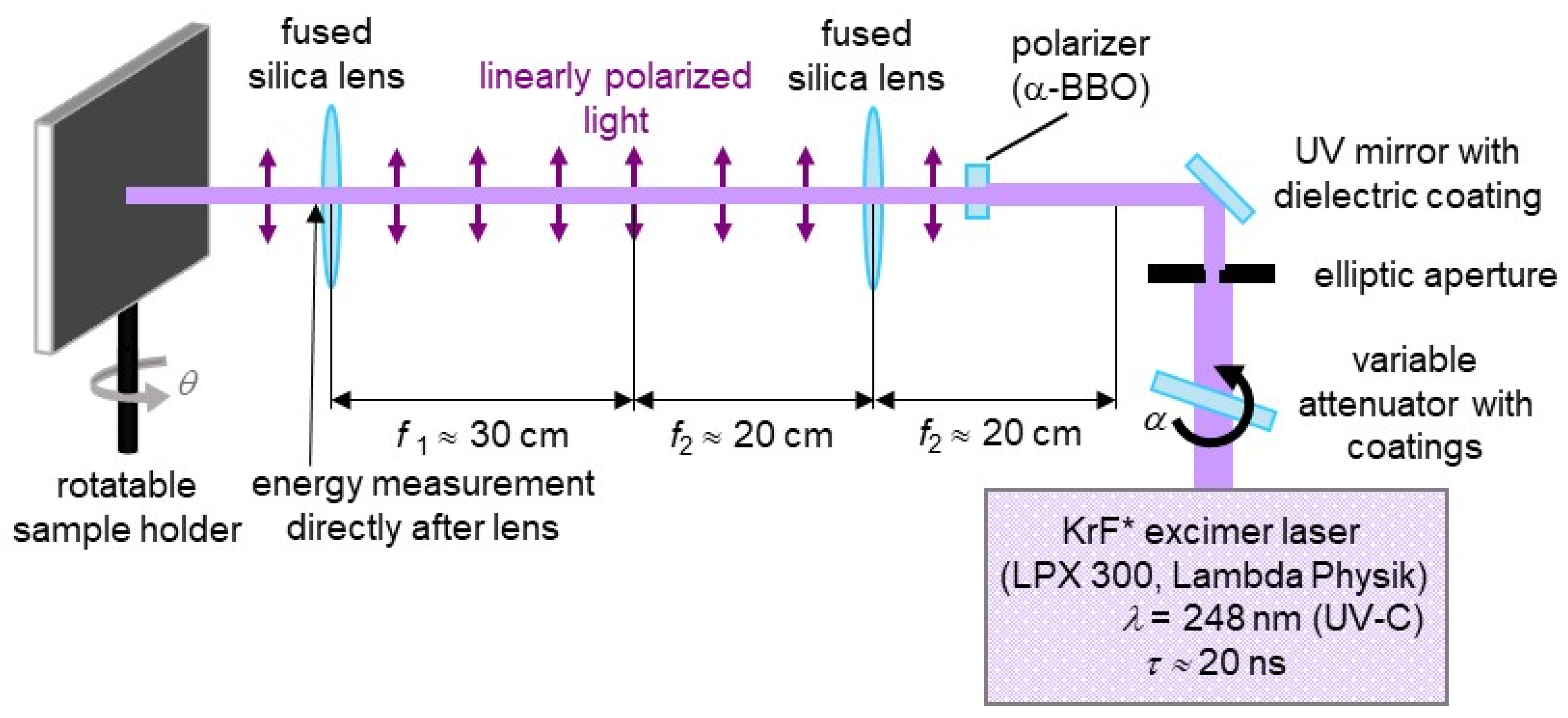
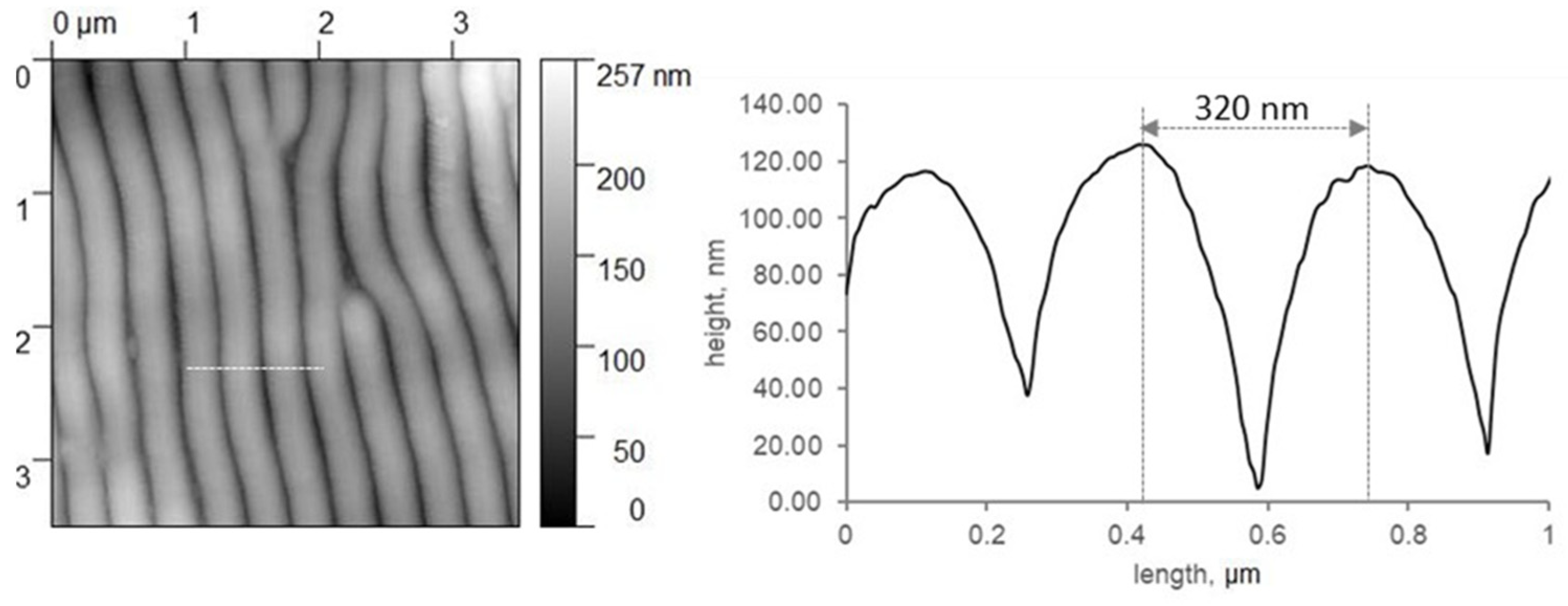
2. Materials and Methods
3. Results
3.1. Influence of Temperature and Humidity on the Mechanical Properties of the Cribellate Thread
3.2. Response to Changing Ambient Climate
4. Discussion
4.1. Influence of Humidity on Anti-Adhesion
4.2. Influence of Temperature on Anti-Adhesion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, C.; Wu, S.; Jian, M.; Xie, J.; Xu, L.; Yang, X.; Zheng, Q.; Zhang, Y. Silk nanofibers as high efficient and lightweight air filter. Nano Res. 2016, 9, 2590–2597. [Google Scholar] [CrossRef]
- Desai, K.; Kit, K.; Li, J.; Davidson, P.M.; Zivanovic, S.; Meyer, H. Nanofibrous chitosan non-wovens for filtration applications. Polymer 2009, 50, 3661–3669. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, L.; Guo, B.; Ma, P.X. Interwoven Aligned Conductive Nanofiber Yarn/Hydrogel Composite Scaffolds for Engineered 3D Cardiac Anisotropy. ACS Nano 2017, 11, 5646–5659. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Zarghami, N.; Yar, H.M.; Akbarzadeh, A. Nanofiber: Synthesis and biomedical applications. Artif. Cells Nanomed. Biotechnol. 2014, 44, 111–121. [Google Scholar] [CrossRef]
- Thavasi, V.; Singh, G.; Ramakrishna, S. Electrospun nanofibers in energy and environmental applications. Energy Environ. Sci. 2008, 1, 205–221. [Google Scholar] [CrossRef]
- Baji, A.; Zhou, L.; Mai, Y.W.; Yang, Z.; Yao, H. On the Adhesion performance of a single electrospun fiber. Appl Phys. A Mater. Sci. Process. 2015, 118, 51–56. [Google Scholar] [CrossRef]
- Shi, Q.; Wan, K.T.; Wong, S.C.; Chen, P.; Blackledge, T.A. Do electrospun polymer fibers stick? Langmuir 2010, 26, 14188–14193. [Google Scholar] [CrossRef]
- Hobza, P.; Zahradnik, R.; Heyrovsky, J. Intermolecular Interactions between Medium-Sized Systems. Nonempirical and Empirical Calculations of Interaction Energies: Successes and Failures. Chem. Rev. 1988, 88, 871–897. [Google Scholar] [CrossRef]
- Autumn, K.; Sitti, M.; Liang, Y.A.; Peattie, A.M.; Hansen, W.R.; Sponberg, S.; Kenny, T.W.; Fearing, R.; Israelachvili, J.N.; Full, R.J. Evidence for van der Waals adhesion in gecko setae. Proc. Natl. Acad. Sci. USA 2002, 99, 12252–12256. [Google Scholar] [CrossRef]
- Autumn, K.; Liang, Y.A.; Hsieh, S.T.; Zesch, W.; Chan, W.P.; Kenny, T.W.; Fearing, R.; Full, R.J. Adhesive force of a single gecko foot-hair. Nat. Cell Biol. 2000, 405, 681–685. [Google Scholar] [CrossRef]
- Peters, H.M. Fine Structure and Function of Capture Threads. In Ecophysiology of Spiders; Springer: Berlin/Heidelberg, Germany, 1987; pp. 187–202. [Google Scholar]
- Eberhard, W.; Pereira, F. Ultrastructure of Cribellate Silk of Nine Species in Eight Famiies and Possible Taxonomic Implications. J. Aarchnol. 1993, 21, 161–174. [Google Scholar]
- Friedrich, V.L.; Langer, R.M. Fine Structure of Cribellate Spider Silk. Am. Zool. 1969, 9, 91–96. [Google Scholar] [CrossRef][Green Version]
- Grannemann, C.C.F.; Meyer, M.; Reinhardt, M.; Ramírez, M.J.; Herberstein, M.E.; Joel, A.C. Small behavioral adaptations enable more effective prey capture by producing 3D-structured spider threads. Sci. Rep. 2019, 9, 17273. [Google Scholar] [CrossRef]
- Joel, A.C.; Kappel, P.; Adamova, H.; Baumgartner, W.; Scholz, I. Cribellate thread production in spiders: Complex processing of nano-fibres into a functional capture thread. Arthropod Struct. Dev. 2015, 44, 568–573. [Google Scholar] [CrossRef]
- Piorkowski, D.; Liao, C.P.; Joel, A.C.; Wu, C.L.; Doran, N.; Blamires, S.J.; Pugno, N.M.; Tso, I.-M. Adhesion of spider cribellate silk enhanced in high humidity by mechanical plasticization of the underlying fiber. J. Mech. Behav. Biomed. Mater. 2020, 114, 104200. [Google Scholar] [CrossRef]
- Piorkowski, D.; Blackledge, T.A.; Liao, C.-P.; Joel, A.-C.; Weissbach, M.; Wu, C.-L.; Tso, I.-M. Uncoiling springs promote mechanical functionality of spider cribellate silk. J. Exp. Biol. 2020, 223, jeb215269. [Google Scholar] [CrossRef]
- Peters, H.M. On the spinning apparatus and the structure of the capture threads of Deinopis subrufus (Araneae, Deinopidae). Zoomorphology 1992, 112, 27–37. [Google Scholar] [CrossRef]
- Bertkau, P. Über das Cribellum und Calamistrum. Arch. Für Nat. 1882, 1, 48. [Google Scholar]
- Michalik, P.; Piorkowski, D.; Blackledge, T.A.; Ramírez, M.J. Functional trade-offs in cribellate silk mediated by spinning behavior. Sci. Rep. 2019, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Joel, A.C.; Meyer, M.; Heitz, J.; Heiss, A.; Park, D.; Adamova, H.; Baumgartner, W. Biomimetic Combs as Antiadhesive Tools to Manipulate Nanofibers. ACS Appl. Nano Mater. 2020, 3, 3395–3401. [Google Scholar] [CrossRef]
- Bonse, J.; Kirner, S.V.; Krüger, J. Laser-Induced Periodic Surface Structures (LIPSS). In Handbook of Laser Micro- and Nano-Engineering; Springer Nature: Cham, Switzerland, 2020; pp. 1–59. [Google Scholar] [CrossRef]
- Joel, A.-C.; Scholz, I.; Orth, L.; Kappel, P.; Baumgartner, W. Morphological adaptation of the calamistrum to the cribellate spinning process in Deinopoidae (Uloboridae, Deinopidae). R. Soc. Open Sci. 2016, 3, 150617. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heiss, A.; Park, D.; Joel, A.C. The Calamistrum of the Feather-Legged Spider Uloborus plumipes Investigated by Focused Ion Beam and Scanning Electron Microscopy (FIB-SEM) Tomography. Microsc. Microanal. 2018, 24, 139–146. [Google Scholar] [CrossRef]
- Bäuerle, D. Laser Processing and Chemistry, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Bonse, J.; Hohm, S.; Kirner, S.V.; Rosenfeld, A.; Kruger, J. Laser-Induced Periodic Surface Structures-A Scientific Evergreen. IEEE J. Sel. Top. Quantum Electron. 2017, 23, 109–123. [Google Scholar] [CrossRef]
- Van Driel, H.M.; Sipe, J.E.; Young, J.F. Laser-induced periodic surface structure on solids: A universal phenomenon. Phys. Rev. Lett. 1982, 49, 1955–1958. [Google Scholar] [CrossRef]
- Bonse, J.; Gräf, S. Maxwell Meets Marangoni—A Review of Theories on Laser-Induced Periodic Surface Structures. Laser Photonics Rev. 2020, 14, 2000215. [Google Scholar] [CrossRef]
- Heitz, J.; Muck, M.; Vujovic, J.; Baumgartner, W.; Hassel, A.W.; Lone, S.A.; Steinhauser, B.; Hrelescu, C.; Klar, T.; Buchberger, G.; et al. Laser-induced periodic surface structures (LIPSS) for biomedical and sensing applications. In Proceedings of the 2020 22nd International Conference on Transparent Optical Networks (ICTON), Bari, Italy, 1–4 July 2020. [Google Scholar] [CrossRef]
- Rebollar, E.; Castillejo, M.; Ezquerra, T.A. Laser induced periodic surface structures on polymer films: From fundamentals to applications. Eur. Polym. J. 2015, 73, 162–174. [Google Scholar] [CrossRef]
- Barb, R.A.; Hrelescu, C.; Dong, L.; Heitz, J.; Siegel, J.; Slepicka, P.; Vosmanská, V.; Svorcik, V.; Magnus, B.; Marksteiner, R.; et al. Laser-induced periodic surface structures on polymers for formation of gold nanowires and activation of human cells. Appl. Phys. A Mater. Sci. Process. 2014, 117, 295–300. [Google Scholar] [CrossRef]
- Siegel, J.; Slepička, P.; Heitz, J.; Kolská, Z.; Sajdl, P.; Švorčík, V. Gold nano-wires and nano-layers at laser-induced nano-ripples on PET. Appl. Surf. Sci. 2010, 256, 2205–2209. [Google Scholar] [CrossRef]
- Pinon, A.V.; Wierez-Kien, M.; Craciun, A.D.; Beyer, N.; Gallani, J.L.; Rastei, M.V. Thermal effects on van der Waals adhesive forces. Phys. Rev. B 2016, 93, 35424. [Google Scholar] [CrossRef]
- Peng, Z.; Yang, Y.; Chen, S. Coupled effects of the temperature and the relative humidity on gecko adhesion. J. Phys. D Appl Phys. 2017, 50, 315402. [Google Scholar] [CrossRef]
- Niewiarowski, P.H.; Lopez, S.; Ge, L.; Hagan, E.; Dhinojwala, A. Sticky Gecko Feet: The Role of Temperature and Humidity. PLoS ONE. 2008, 3, e2192. [Google Scholar] [CrossRef]
- Persano, L.; Camposeo, A.; Tekmen, C.; Pisignano, D. Industrial Upscaling of Electrospinning and Applications of Polymer Nanofibers: A Review. Macromol. Mater. Eng. 2013, 298, 504–520. [Google Scholar] [CrossRef]
- MacDiarmid, A.G.; Jones, W.E.; Norris, I.D.; Gao, J.; Johnson, A.T.; Pinto, N.J.; Hone, J.; Han, B.; Ko, F.; Okuzaki, H.; et al. Electrostatically-generated nanofibers of electronic polymers. Synth. Met. 2001, 119, 27–30. [Google Scholar] [CrossRef]
- Elettro, H.; Neukirch, S.; Antkowiak, A.; Vollrath, F. Adhesion of dry and wet electrostatic capture silk of uloborid spider. Sci. Nat. 2015, 102, 41. [Google Scholar] [CrossRef]
- Zheng, Y.; Bai, H.; Huang, Z.; Tian, X.; Nie, F.Q.; Zhao, Y.; Zhai, J.; Jiang, L. Directional water collection on wetted spider silk. Nature 2010, 463, 640–643. [Google Scholar] [CrossRef]
- Joel, A.C.; Baumgartner, W. Nanofibre production in spiders without electric charge. J. Exp. Biol. 2017, 220, 2243–2249. [Google Scholar] [CrossRef]
- Richter, A.M.; Buchberger, G.; Stifter, D.; Duchoslav, J.; Hertwig, A.; Bonse, J.; Heitz, J.; Schwibbert, K. Spatial Period of Laser-Induced Surface Nanoripples on PET Determines Escherichia coli Repellence. Nanomaterials 2021, 11, 3000. [Google Scholar] [CrossRef]
- Wong, W.; Chan, K.; Yeung, K.W.; Lau, K.S. Chemical surface modification of poly (ethylene terephthalate) by excimer irradiation of high and low intensities. Mater. Res. Innov. 2016, 4, 344–349. [Google Scholar] [CrossRef]
- Watanabe, H.; Shimizu, H.; Takata, T. Surface Change of Aramid Fiber by Laser Ablation. Sen’i Gakkaishi 1993, 49, 616–620. [Google Scholar] [CrossRef][Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Plaza, G.R.; Guinea, G.V.; Pérez-Rigueiro, J.; Elices, M. Thermo-hygro-mechanical behavior of spider dragline silk: Glassy and rubbery states. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 994–999. [Google Scholar] [CrossRef]
- Hawthorn, A.C.; Opell, B.D. Evolution of adhesive mechanisms in cribellar spider prey capture thread: Evidence for van der Waals and hygroscopic forces. Biol. J. Linn. Soc. 2002, 77, 1–8. [Google Scholar] [CrossRef]
- Hawthorn, A.C.; Opell, B.D. van der Waals and hygroscopic forces of adhesion generated by spider capture threads. J. Exp. Biol. 2003, 206, 3905–3911. [Google Scholar] [CrossRef]
- Gosline, J.M.; Denny, M.W.; DeMont, M.E. Spider silk as rubber. Nat. Cell Biol. 1984, 309, 551–552. [Google Scholar] [CrossRef]
- Feiler, A.A.; Jenkins, P.; Rutland, M.W. Effect of relative humidity on adhesion and frictional properties of micro-and nano-scopic contacts. J. Adhes Sci. Technol. 2005, 19, 165–179. [Google Scholar] [CrossRef]
- McFarlane, J.; Tabor, D. Adhesion of solids and the effect of surface films. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1950, 202, 224–243. [Google Scholar] [CrossRef]
- Smith, T. The Hydrophilic Nature of a Clean Gold Surface. J. Colloid Interface Sci. 1980, 75, 51–55. [Google Scholar] [CrossRef]
- Liao, X.; Yin, G.; Huang, Z.; Yao, Y.; Gu, J.; Han, D. Supercontraction on cribellate spider spiral silk with wet-rebuilt micro-structure. Mater. Sci. Eng. C 2011, 31, 128–133. [Google Scholar] [CrossRef]
- Mailley, D.; Hébraud, A.; Schlatter, G. A Review on the Impact of Humidity during Electrospinning: From the Nanofiber Structure Engineering to the Applications. Macromol. Mater. Eng. 2021, 306, 2100115. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. Am. Chem. Soc. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Pelipenko, J.; Kristl, J.; Jankoví, B.; Baumgartner, S.; Kocbek, P. The impact of relative humidity during electrospinning on the morphology and mechanical properties of nanofibers. Int. J. Pharm. 2013, 456, 125–134. [Google Scholar] [CrossRef]
- World Spider Catalog; Version 22.5; Natural History Museum Bern: Bern, Switzerland, 2021; Available online: Http://Wsc.Nmbe.Ch (accessed on 6 November 2021). [CrossRef]

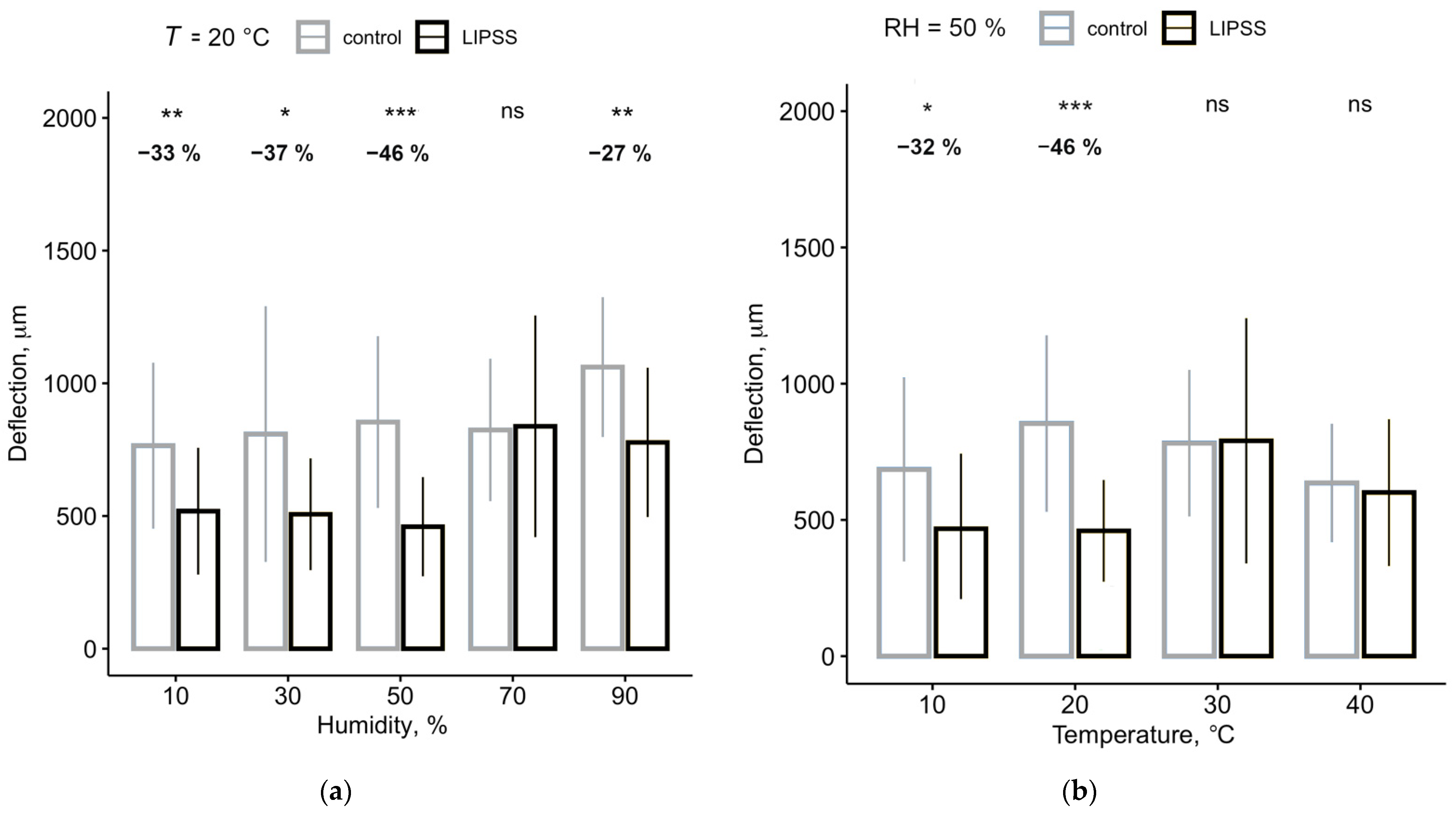

| Humidity | Deflection | p-Value | Diff. | |
|---|---|---|---|---|
| Control PET | LIPSS PET | |||
| 10% | 765 ± 312 µm | 518 ± 211 µm | 0.0036 | −32.63% |
| 30% | 809 ± 482 µm | 506 ± 239 µm | 0.0137 | −37.36% |
| 50% | 854 ± 323 µm | 459 ± 187 µm | 3.5 × 10−6 | −46.18% |
| 70% | 824 ± 268 µm | 838 ± 418 µm | ns. | +1.77% |
| 90% | 1061 ± 263 µm | 777 ± 282 µm | 0.0013 | −26.75% |
| Temperature | Deflection | p-Value | Diff. | |
|---|---|---|---|---|
| Control | LIPSS | |||
| 10 °C | 685 ± 337 µm | 468 ± 277 µm | 0.023 | −31.73% |
| 20 °C | 854 ± 323 µm | 460 ± 286 µm | 3.5 × 10−6 | −46.18% |
| 30 °C | 782 ± 269 µm | 791 ± 460 µm | ns. | +1.14% |
| 40 °C | 636 ± 217 µm | 600 ± 270 µm | ns. | −5.59% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer, M.; Buchberger, G.; Heitz, J.; Baiko, D.; Joel, A.-C. Ambient Climate Influences Anti-Adhesion between Biomimetic Structured Foil and Nanofibers. Nanomaterials 2021, 11, 3222. https://doi.org/10.3390/nano11123222
Meyer M, Buchberger G, Heitz J, Baiko D, Joel A-C. Ambient Climate Influences Anti-Adhesion between Biomimetic Structured Foil and Nanofibers. Nanomaterials. 2021; 11(12):3222. https://doi.org/10.3390/nano11123222
Chicago/Turabian StyleMeyer, Marco, Gerda Buchberger, Johannes Heitz, Dariya Baiko, and Anna-Christin Joel. 2021. "Ambient Climate Influences Anti-Adhesion between Biomimetic Structured Foil and Nanofibers" Nanomaterials 11, no. 12: 3222. https://doi.org/10.3390/nano11123222
APA StyleMeyer, M., Buchberger, G., Heitz, J., Baiko, D., & Joel, A.-C. (2021). Ambient Climate Influences Anti-Adhesion between Biomimetic Structured Foil and Nanofibers. Nanomaterials, 11(12), 3222. https://doi.org/10.3390/nano11123222






