Spatial Period of Laser-Induced Surface Nanoripples on PET Determines Escherichia coli Repellence
Abstract
:1. Introduction
2. Materials and Methods
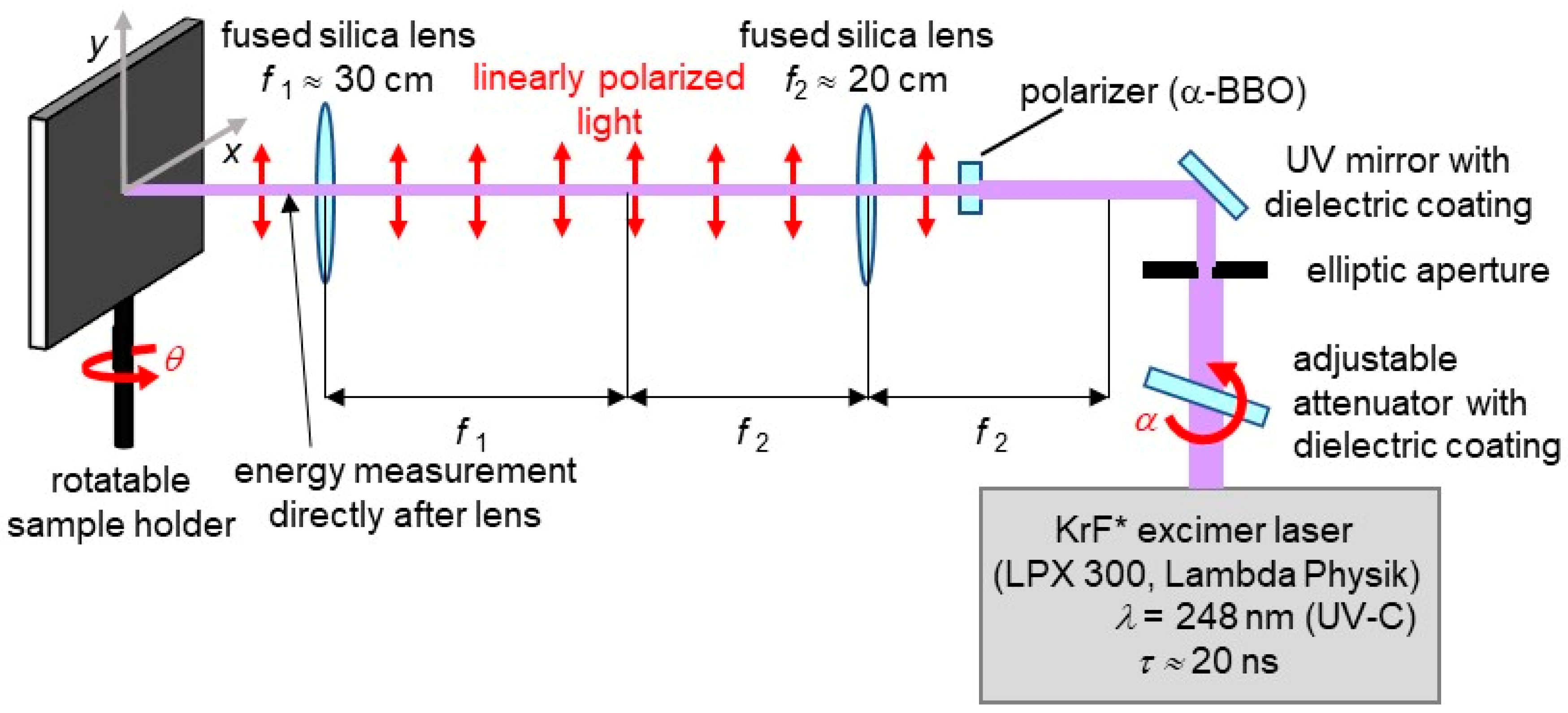
2.1. Laser Processing of Poly(Ethylene Terephthalate) (PET) Foils
2.2. Surface Microscopy
2.2.1. Scanning Electron Microscopy (SEM) of PET Foils
2.2.2. Calculation of Spatial Periods Λ from SEM Data
2.2.3. Atomic Force Microscopy (AFM) of PET Foils
2.3. X-ray Photoelectron Spectroscopy (XPS)
2.4. Attenuated Total Reflection Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.5. Bacterial Strains and Biofilm Cultivation
2.6. Optical Light Microscopy and Biofilm Quantification
2.7. Scanning Electron Microscopy (SEM) of Attached Biofilms
3. Results
3.1. Topographic Characterization of Laser Processed PET Foils
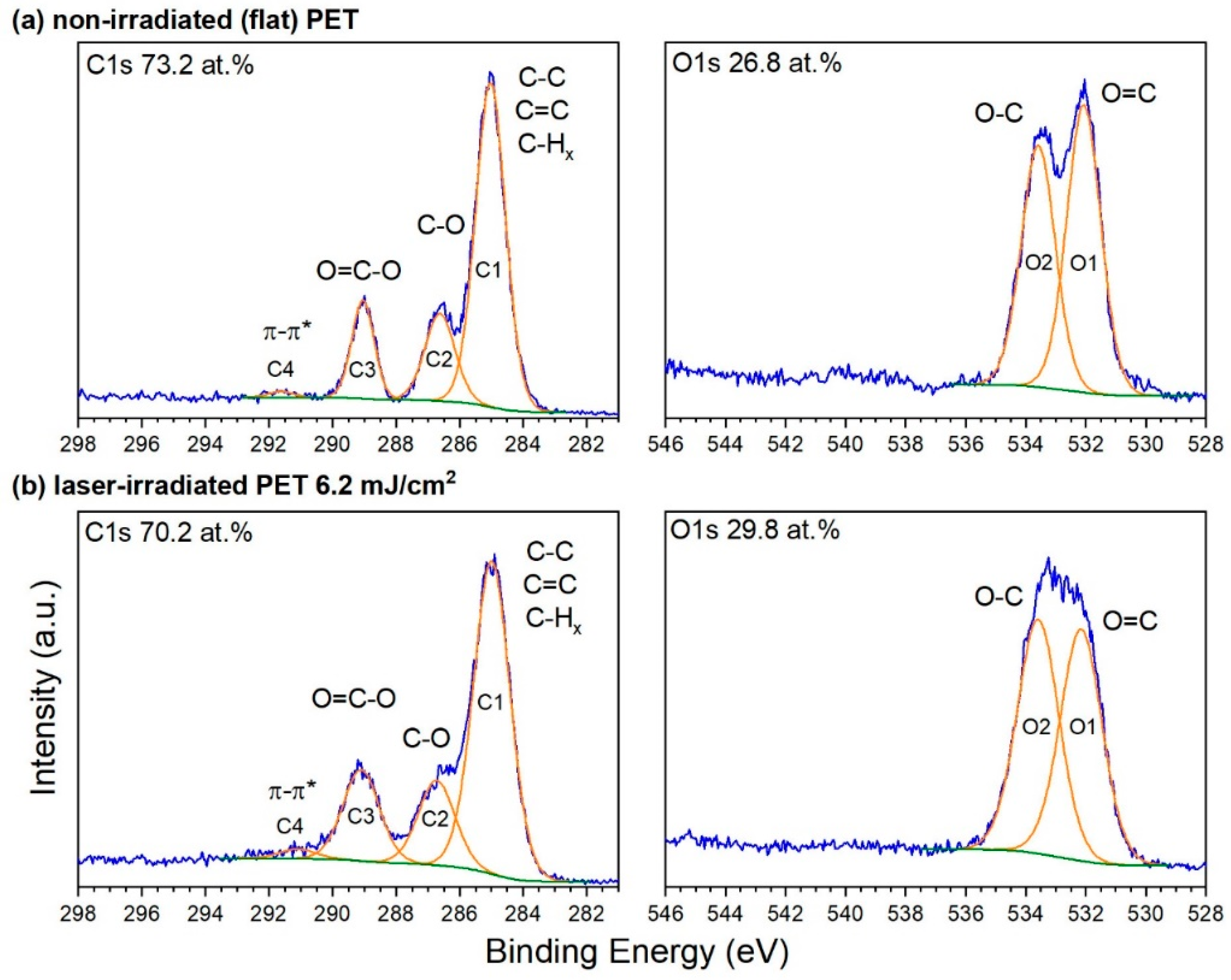
3.2. XPS Chemical Characterization of Laser Processed PET Foils
3.3. ATR-FTIR Structural Characterization of Laser Processed PET Foils
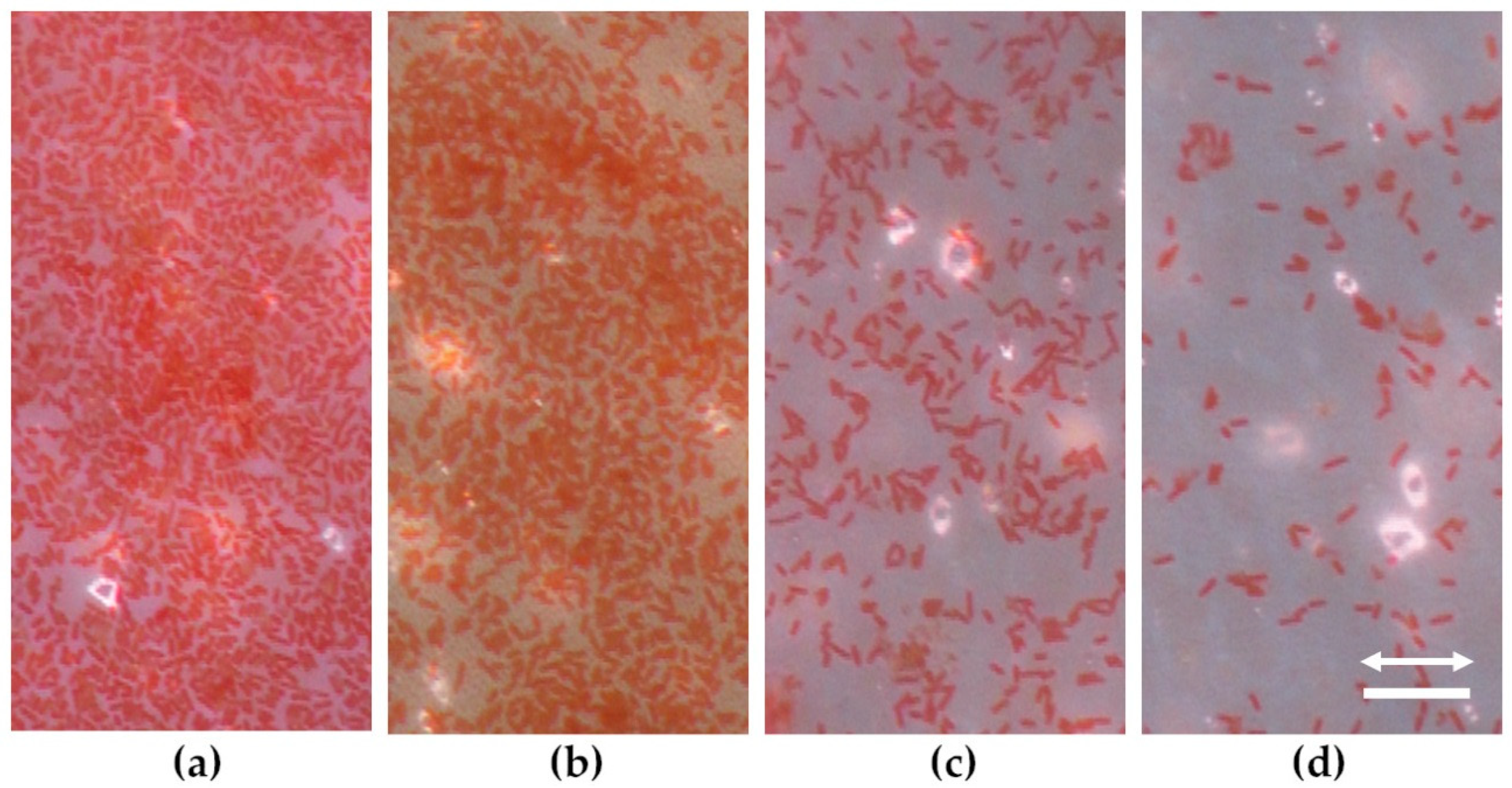
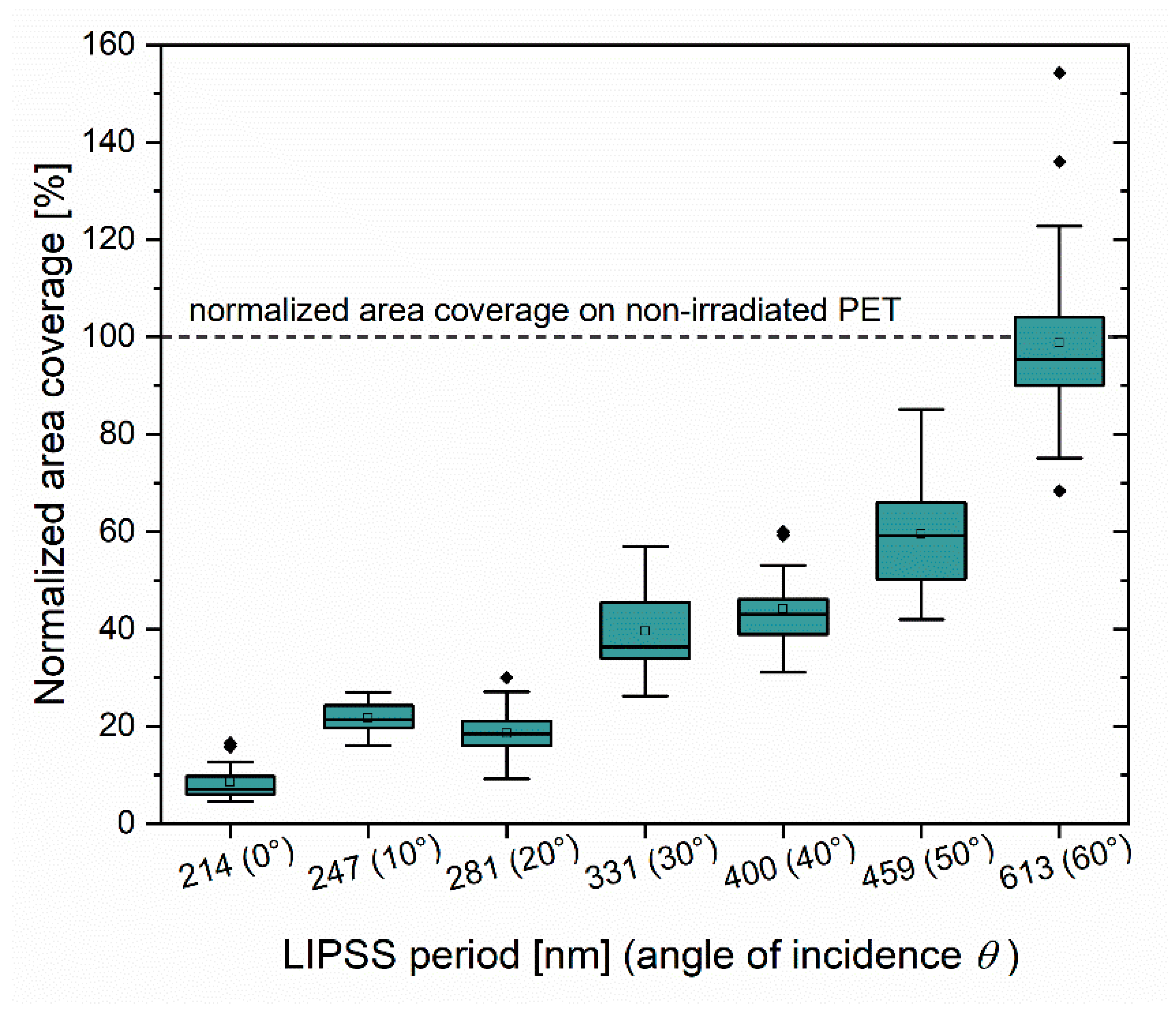
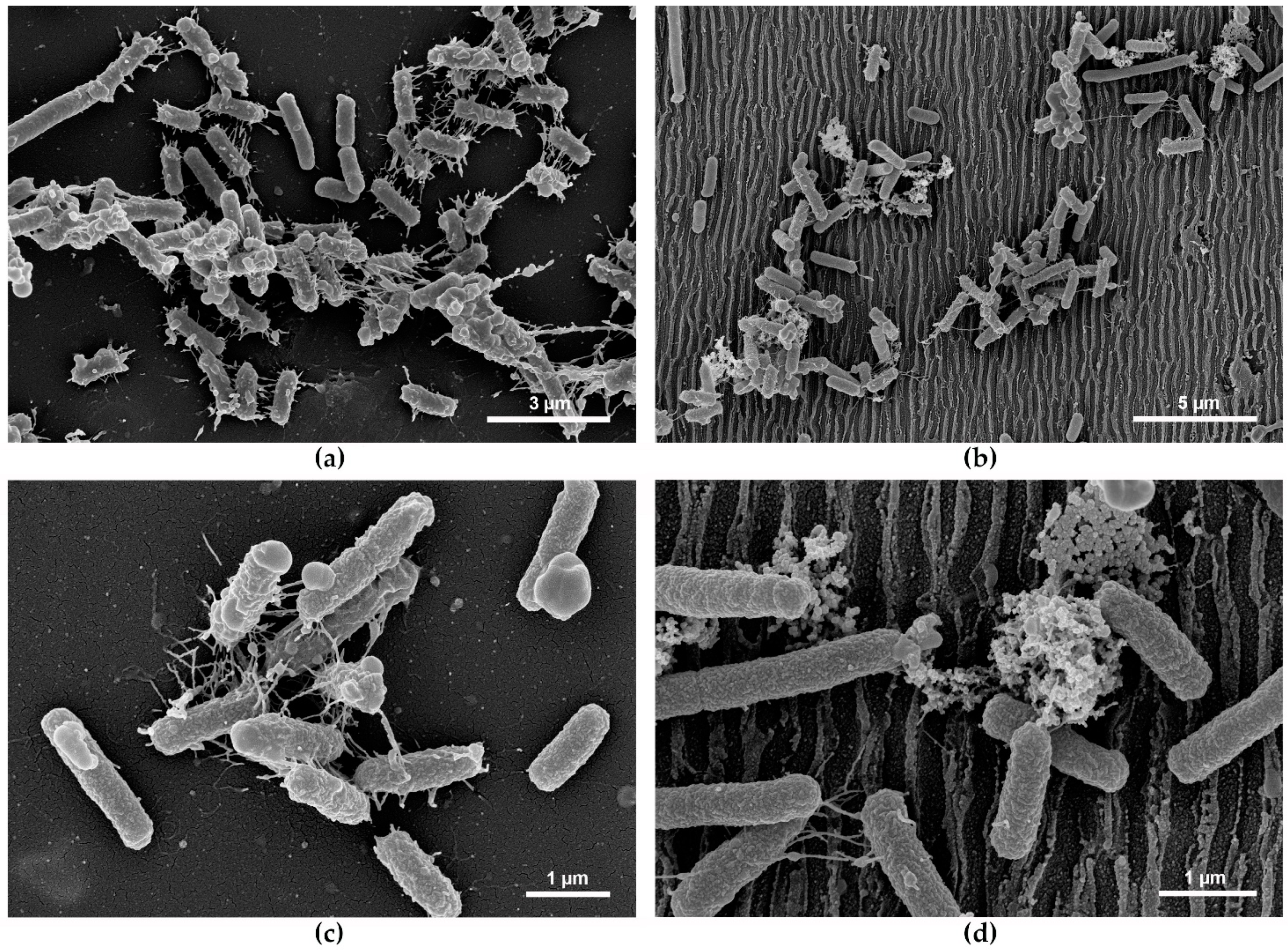
3.4. Bacterial Attachment on Laser Processed PET
3.5. Bacterial Response upon Attachment and Role of Pili
4. Discussion
4.1. Determination of Surface Physico-Chemical Parameters
4.2. Bacterial Attachment Depends on the Ripples Spatial Period
4.3. LIPSS Mainly Affect the Nanofiber-Mediated Adhesion
4.4. Extrapolymeric Substances Help to Augment Bacterial Adhesion on Otherwise Unfavorable Surfaces
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Tolker-Nielsen, T. Tolerance and Resistance of Pseudomonas aeruginosa Biofilms to Antimicrobial Agents-How P. aeruginosa Can Escape Antibiotics. Front. Microbiol. 2019, 10, 913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef] [Green Version]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Friedlander, R.S.; Vlamakis, H.; Kim, P.; Khan, M.; Kolter, R.; Aizenberg, J. Bacterial flagella explore microscale hummocks and hollows to increase adhesion. Proc. Natl. Acad. Sci. USA 2013, 110, 5624–5629. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.K.; de Anda, J.; Baker, A.E.; Bennett, R.R.; Luo, Y.; Lee, E.Y.; Keefe, J.A.; Helali, J.S.; Ma, J.; Zhao, K.; et al. Multigenerational memory and adaptive adhesion in early bacterial biofilm communities. Proc. Natl. Acad. Sci. USA 2018, 115, 4471–4476. [Google Scholar] [CrossRef] [Green Version]
- O’Toole, G.A.; Wong, G.C.L. Sensational biofilms: Surface sensing in bacteria. Curr. Opin. Microbiol. 2016, 30, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuson, H.H.; Weibel, D.B. Bacteria-surface interactions. Soft Matter 2013, 9, 4368–4380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Ag. 2010, 35, 322–332. [Google Scholar] [CrossRef] [Green Version]
- Crabbé, A.; Jensen, P.Ø.; Bjarnsholt, T.; Coenye, T. Antimicrobial Tolerance and Metabolic Adaptations in Microbial Biofilms. Trends Microbiol. 2019, 27, 850–863. [Google Scholar] [CrossRef]
- Benčina, M.; Mavrič, T.; Junkar, I.; Bajt, A.; Krajnović, A.; Lakota, K.; Žigon, P.; Sodin-Šemrl, S.; Kralj-Iglič, V.; Iglič, A. The Importance of Antibacterial Surfaces in Biomedical Applications; Iglič, A., Rappolt, M., García-Sáez, A.J., Eds.; Advances in Biomembranes and Lipid Self-Assembly; Elsevier: Amsterdam, The Netherlands, 2018; Volume 28. [Google Scholar]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.; Benoliel, C.; Drider, D.; Dhulster, P.; Chihib, N.E. Biofilm formation and persistence on abiotic surfaces in the context of food and medical environments. Arch. Microbiol. 2014, 196, 453–472. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Meier, M.; Schild, T. Mini-review: Microbial problems in paper production. Biofouling 2013, 29, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Wingender, J.; Flemming, H.C. Biofilms in drinking water and their role as reservoir for pathogens. Int. J. Hyg. Environ. Health 2011, 214, 417–423. [Google Scholar] [CrossRef]
- Bai, X.J.; Nakatsu, C.H.; Bhunia, A.K. Bacterial Biofilms and Their Implications in Pathogenesis and Food Safety. Foods 2021, 10, 2117. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial Biofilms in the Food Industry—A Comprehensive Review. Int. J. Environ. Res. Public Health 2021, 18, 2014. [Google Scholar] [CrossRef]
- Li, X.; Wu, B.; Chen, H.; Nan, K.H.; Jin, Y.Y.; Sun, L.; Wang, B.L. Recent developments in smart antibacterial surfaces to inhibit biofilm formation and bacterial infections. J. Mater. Chem. B 2018, 6, 4274–4292. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef]
- Wu, C.Z.; Schwibbert, K.; Achazi, K.; Landsberger, P.; Gorbushina, A.; Haag, R. Active Antibacterial and Antifouling Surface Coating via a Facile One-Step Enzymatic Cross-Linking. Biomacromolecules 2017, 18, 210–216. [Google Scholar] [CrossRef]
- Mi, G.J.; Shi, D.; Wang, M.; Webster, T.J. Reducing Bacterial Infections and Biofilm Formation Using Nanoparticles and Nanostructured Antibacterial Surfaces. Adv. Healthc. Mater. 2018, 7, 1800103. [Google Scholar] [CrossRef] [PubMed]
- Pallavicini, P.; Dacarro, G.; Taglietti, A. Self-Assembled Monolayers of Silver Nanoparticles: From Intrinsic to Switchable Inorganic Antibacterial Surfaces. Eur. J. Inorg. Chem. 2018, 45, 4846–4855. [Google Scholar] [CrossRef]
- Wang, Y.R.; Wei, T.; Qu, Y.C.; Zhou, Y.; Zheng, Y.J.; Huang, C.B.; Zhang, Y.X.; Yu, Q.; Chen, H. Smart, Photothermally Activated, Antibacterial Surfaces with Thermally Triggered Bacteria-Releasing Properties. ACS Appl. Mater. Interfaces 2020, 12, 21283–21291. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Tang, Z.; Yu, Q.; Chen, H. Smart Antibacterial Surfaces with Switchable Bacteria-Killing and Bacteria-Releasing Capabilities. ACS Appl. Mater. Interfaces 2017, 9, 37511–37523. [Google Scholar] [CrossRef]
- Linklater, D.P.; Baulin, V.A.; Juodkazis, S.; Crawford, R.J.; Stoodley, P.; Ivanova, E.P. Mechano-bactericidal actions of nanostructured surfaces. Nat. Rev. Microbiol. 2020, 19, 8–22. [Google Scholar] [CrossRef]
- Modaresifar, K.; Azizian, S.; Ganjian, M.; Fratila-Apachitei, L.E.; Zadpoor, A.A. Bactericidal effects of nanopatterns: A systematic review. Acta Biomater. 2019, 83, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Lazzini, G.; Romoli, L.; Lutey, A.H.A.; Fuso, F. Modelling the interaction between bacterial cells and laser-textured surfaces. Surf. Coat. Technol. 2019, 375, 8–14. [Google Scholar] [CrossRef]
- Stratakis, E.; Bonse, J.; Heitz, J.; Siegel, J.; Tsibidis, G.D.; Skoulas, E.; Papadopoulos, A.; Mimidis, A.; Joel, A.C.; Comanns, P.; et al. Laser engineering of biomimetic surfaces. Mater. Sci. Eng. R 2020, 141, 100562. [Google Scholar] [CrossRef]
- Cunha, A.; Elie, A.M.; Plawinski, L.; Serro, A.P.; Rego, A.M.B.; Almeida, A.; Urdaci, M.C.; Durrieu, M.C.; Vilar, R. Femtosecond laser surface texturing of titanium as a method to reduce the adhesion of Staphylococcus aureus and biofilm formation. Appl. Surf. Sci. 2016, 360, 485–493. [Google Scholar] [CrossRef]
- Epperlein, N.; Menzel, F.; Schwibbert, K.; Koter, R.; Bonse, J.; Sameith, J.; Krüger, J.; Toepel, J. Influence of femtosecond laser produced nanostructures on biofilm growth on steel. Appl. Surf. Sci. 2017, 418, 420–424. [Google Scholar] [CrossRef]
- Schwibbert, K.; Menzel, F.; Epperlein, N.; Bonse, J.; Krüger, J. Bacterial Adhesion on Femtosecond Laser-Modified Polyethylene. Materials 2019, 12, 3107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutey, A.H.A.; Gemini, L.; Romoli, L.; Lazzini, G.; Fuso, F.; Faucon, M.; Kling, R. Towards Laser-Textured Antibacterial Surfaces. Sci. Rep. 2018, 8, 10112. [Google Scholar] [CrossRef] [PubMed]
- Meinshausen, A.K.; Herbster, M.; Zwahr, C.; Soldera, M.; Müller, A.; Halle, T.; Lasagni, A.F.; Bertrand, J. Aspect ratio of nano/microstructures determines Staphylococcus aureus adhesion on PET and titanium surfaces. J. Appl. Microbiol. 2021, 131, 1498–1514. [Google Scholar] [CrossRef] [PubMed]
- Slepička, P.; Siegel, J.; Lyutakov, O.; Kasálková, N.S.; Kolská, Z.; Bačáková, L.; Švorčík, V. Polymer nanostructures for bioapplications induced by laser treatment. Biotechnol. Adv. 2018, 36, 839–855. [Google Scholar] [CrossRef]
- Pryjmaková, J.; Kaimlová, M.; Vokatá, B.; Hubáček, T.; Slepička, P.; Švorčík, V.; Siegel, J. Bimetallic Nanowires on Laser-Patterned PEN as Promising Biomaterials. Nanomaterials 2021, 11, 2285. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yao, S.L.; Zhang, H.J.; Cai, M.Y.; Liu, W.J.; Pan, R.; Chen, C.H.; Wang, X.M.; Wang, L.N.; Zhong, M.L. Biocompatible nano-ripples structured surfaces induced by femtosecond laser to rebel bacterial colonization and biofilm formation. Opt. Laser Technol. 2020, 124, 105973. [Google Scholar] [CrossRef]
- Jaggessar, A.; Shahali, H.; Mathew, A.; Yarlagadda, P.K.D.V. Bio-mimicking nano and micro-structured surface fabrication for antibacterial properties in medical implants. J. Nanobiotechnol. 2017, 15, 64. [Google Scholar] [CrossRef] [Green Version]
- Tripathy, A.; Sen, P.; Su, B.; Briscoe, W.H. Natural and bioinspired nanostructured bactericidal surfaces. Adv. Colloid Interfaces. 2017, 248, 85–104. [Google Scholar] [CrossRef]
- Fadeeva, E.; Truong, V.K.; Stiesch, M.; Chichkov, B.N.; Crawford, R.J.; Wang, J.; Ivanova, E.P. Bacterial Retention on Superhydrophobic Titanium Surfaces Fabricated by Femtosecond Laser Ablation. Langmuir 2011, 27, 3012–3019. [Google Scholar] [CrossRef]
- Reddy, S.T.; Chung, K.K.; McDaniel, C.J.; Darouiche, R.O.; Landman, J.; Brennan, A.B. Micropatterned Surfaces for Reducing the Risk of Catheter-Associated Urinary Tract Infection: An In Vitro Study on the Effect of Sharklet Micropatterned Surfaces to Inhibit Bacterial Colonization and Migration of Uropathogenic Escherichia coli. J. Endourol. 2011, 25, 1547–1552. [Google Scholar] [CrossRef] [Green Version]
- Vasudevan, R.; Kennedy, A.J.; Merritt, M.; Crocker, F.H.; Baney, R.H. Microscale patterned surfaces reduce bacterial fouling-microscopic and theoretical analysis. Colloid Surf. B 2014, 117, 225–232. [Google Scholar] [CrossRef]
- Green, D.W.; Lee, K.K.H.; Watson, J.A.; Kim, H.Y.; Yoon, K.S.; Kim, E.J.; Lee, J.M.; Watson, G.S.; Jung, H.S. High Quality Bioreplication of Intricate Nanostructures from a Fragile Gecko Skin Surface with Bactericidal Properties. Sci. Rep. 2017, 7, 41023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.F.; Lamb, R.N.; Baulin, V.A.; Watson, G.S.; et al. Bactericidal activity of black silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef]
- Diu, T.; Faruqui, N.; Sjöström, T.; Lamarre, B.; Jenkinson, H.F.; Su, B.; Ryadnov, M.G. Cicada-inspired cell-instructive nanopatterned arrays. Sci. Rep. 2014, 4, 7122. [Google Scholar] [CrossRef] [Green Version]
- Bandara, C.D.; Singh, S.; Afara, I.O.; Wolff, A.; Tesfamichael, T.; Ostrikov, K.; Oloyede, A. Bactericidal Effects of Natural Nanotopography of Dragonfly Wing on Escherichia coli. ACS Appl. Mater. Interfaces 2017, 9, 6746–6760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joel, A.C.; Meyer, M.; Heitz, J.; Heiss, A.; Park, D.; Adamova, H.; Baumgartner, W. Biomimetic Combs as Antiadhesive Tools to Manipulate Nanofibers. ACS Appl. Nano Mater. 2020, 3, 3395–3401. [Google Scholar] [CrossRef]
- Silverman, P.M. Towards a structural biology of bacterial conjugation. Mol. Microbiol. 1997, 23, 423–429. [Google Scholar] [CrossRef]
- Bäuerle, D. Laser Processing And Chemistry, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- van Driel, H.M.; Sipe, J.E.; Young, J.F. Laser-Induced Periodic Surface-Structure on Solids—A Universal Phenomenon. Phys. Rev. Lett. 1982, 49, 1955–1958. [Google Scholar] [CrossRef]
- Bonse, J.; Gräf, S. Maxwell Meets Marangoni-A Review of Theories on Laser-Induced Periodic Surface Structures. Laser Photonics Rev. 2020, 14, 2000215. [Google Scholar] [CrossRef]
- Pérez, S.; Rebollar, E.; Oujja, M.; Martín, M.; Castillejo, M. Laser-induced periodic surface structuring of biopolymers. Appl. Phys. A 2013, 110, 683–690. [Google Scholar] [CrossRef]
- Bäuerle, D.; Arenholz, E.; Svorcik, V.; Heitz, J.; Lukyanchuck, B.; Bityurin, N. Laser-Induced Surface Modification, Structure Formation, and Ablation of Organic Polymers. Proc. SPIE 1995, 2403, 312–320. [Google Scholar]
- Csete, M.; Bor, Z. Laser-induced periodic surface structure formation on polyethylene-terephthalate. Appl. Surf. Sci. 1998, 133, 5–16. [Google Scholar] [CrossRef]
- Lippert, T.; Dickinson, J.T. Chemical and spectroscopic aspects of polymer ablation: Special features and novel directions. Chem. Rev. 2003, 103, 453–485. [Google Scholar] [CrossRef] [PubMed]
- Rebollar, E.; Pérez, S.; Hernández, J.J.; Martín-Fabiani, I.; Rueda, D.R.; Ezquerra, T.A.; Castillejo, M. Assessment and Formation Mechanism of Laser-Induced Periodic Surface Structures on Polymer Spin-Coated Films in Real and Reciprocal Space. Langmuir 2011, 27, 5596–5606. [Google Scholar] [CrossRef] [Green Version]
- Bonse, J.; Höhm, S.; Kirner, S.V.; Rosenfeld, A.; Krüger, J. Laser-Induced Periodic Surface Structures-A Scientific Evergreen. IEEE J. Sel. Top. Quant. Electron. 2017, 23, 9000615. [Google Scholar] [CrossRef]
- Mirzadeh, H.; Moghadam, E.V.; Mivehchi, H. Laser-modified nanostructures of PET films and cell behavior. J. Biomed. Mater. Res. A 2011, 98, 63–71. [Google Scholar] [CrossRef]
- Petit, S.; Laurens, P.; Amouroux, J.; Arefi-Khonsari, F. Excimer laser treatment of PET before plasma metallization. Appl. Surf. Sci. 2000, 168, 300–303. [Google Scholar] [CrossRef]
- Watanabe, H.; Takata, T.; Tsuge, M. Polymer Surface Modification Due to Excimer Laser-Radiation—Chemical and Physical Changes in the Surface-Structure of Poly(Ethylene Terephthalate). Polym. Int. 1993, 31, 247–254. [Google Scholar] [CrossRef]
- Wong, W.; Chan, K.; Yeung, K.W.; Lau, K.S. Chemical surface modification of poly (ethylene terephthalate) by excimer irradiation of high and low intensities. Mater. Res. Innov. 2001, 4, 344–349. [Google Scholar] [CrossRef]
- Ghigo, J.M. Natural conjugative plasmids induce bacterial biofilm development. Nature 2001, 412, 442–445. [Google Scholar] [CrossRef]
- Trevors, J.T. Plasmid Curing in Bacteria. Fems Microbiol. Lett. 1986, 32, 149–157. [Google Scholar] [CrossRef]
- Pratt, L.A.; Kolter, R. Genetic analysis of Escherichia coli biofilm formation: Roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 1998, 30, 285–293. [Google Scholar] [CrossRef]
- Rédei, G.P. M9 Bacterial Minimal Medium. In Encyclopedia of Genetics, Genomics, Proteomics and Informatics; Springer: Dordrecht, The Netherlands, 2008; p. 1135. [Google Scholar]
- Serra, D.O.; Richter, A.M.; Klauck, G.; Mika, F.; Hengge, R. Microanatomy at cellular resolution and spatial order of physiological differentiation in a bacterial biofilm. MBio 2013, 4, e00103–e00113. [Google Scholar] [CrossRef] [Green Version]
- Barb, R.A.; Hrelescu, C.; Dong, L.; Heitz, J.; Siegel, J.; Slepicka, P.; Vosmanska, V.; Svorcik, V.; Magnus, B.; Marksteiner, R.; et al. Laser-induced periodic surface structures on polymers for formation of gold nanowires and activation of human cells. Appl. Phys. A 2014, 117, 295–300. [Google Scholar] [CrossRef]
- Briggs, D. Surface Analysis of Polymers by XPS and Static SIMS; Cambridge University Press: New York, NY, USA, 1998. [Google Scholar]
- Beamson, G.; Briggs, D. High Resolution XSP of Organic Polymers. In The Scienta ESCA 300 Database; John Wiley & Sons: Chichester, UK, 1992. [Google Scholar]
- Siegel, J.; Slepička, P.; Heitz, J.; Kolská, Z.; Sajdl, P.; Švorčík, V. Gold nano-wires and nano-layers at laser-induced nano-ripples on PET. Appl. Surf. Sci. 2010, 256, 2205–2209. [Google Scholar] [CrossRef]
- Roberts, C.; Edwards, S.; Vague, M.; León-Zayas, R.; Scheffer, H.; Chan, G.; Swartz, N.A.; Mellies, J.L. Environmental Consortium Containing Pseudomonas and Bacillus Species Synergistically Degrades Polyethylene Terephthalate Plastic. Msphere 2020, 5, e01151-20. [Google Scholar] [CrossRef] [PubMed]
- Falkenstein, P.; Gräsing, D.; Bielytskyi, P.; Zimmermann, W.; Matysik, J.; Wei, R.; Song, C. UV Pretreatment Impairs the Enzymatic Degradation of Polyethylene Terephthalate. Front. Microbiol. 2020, 11, 689. [Google Scholar] [CrossRef] [PubMed]
- Day, M.; Wiles, D.M. Photochemical Degradation of Poly(ethylene Terephthalate). II. Effect of Wavelength and Environment on Decomposition Process. J. Appl. Polym. Sci. 1972, 16, 191–202. [Google Scholar] [CrossRef]
- Blais, P.; Day, M.; Wiles, D.M. Photochemical Degradation of Poly(Ethylene Terephthalate).IV. Surface Changes. J. Appl. Polym. Sci. 1973, 17, 1895–1907. [Google Scholar] [CrossRef]
- Harr, B.; Schlötterer, C. Gene expression analysis indicates extensive genotype-specific crosstalk between the conjugative F-plasmid and the E. coli chromosome. BMC Microbiol. 2006, 6, 80. [Google Scholar] [CrossRef] [Green Version]
- May, T.; Okabe, S. Escherichia coli Harboring a Natural IncF Conjugative F Plasmid Develops Complex Mature Biofilms by Stimulating Synthesis of Colanic Acid and Curli. J. Bacteriol. 2008, 190, 7479–7490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Lazare, S.; Srinivasan, R. Surface-Properties of Poly(Ethylene Terephthalate) Films Modified by Far-Ultraviolet Radiation at 193-nm (Laser) and 185-nm (Low Intensity). J. Phys. Chem. 1986, 90, 2124–2131. [Google Scholar] [CrossRef]
- Neděla, O.; Slepička, P.; Kasálková, N.S.; Sajdl, P.; Kolská, Z.; Rimpelová, S.; Švorčík, V. Antibacterial properties of angle-dependent nanopatterns on polystyrene. React. Funct. Polym. 2019, 136, 173–180. [Google Scholar] [CrossRef]
- Wilkes, R.A.; Aristilde, L. Degradation and metabolism of synthetic plastics and associated products by Pseudomonas sp.: Capabilities and challenges. J. Appl. Microbiol. 2017, 123, 582–593. [Google Scholar] [CrossRef] [Green Version]
- Helbig, R.; Günther, D.; Friedrichs, J.; Rössler, F.; Lasagni, A.; Werner, C. The impact of structure dimensions on initial bacterial adhesion. Biomater. Sci. 2016, 4, 1074–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callow, M.E.; Jennings, A.R.; Brennan, A.B.; Seegert, C.E.; Gibson, A.; Wilson, L.; Feinberg, A.; Baney, R.; Callow, J.A. Microtopographic cues for settlement of zoospores of the green fouling alga Enteromorpha. Biofouling 2002, 18, 237–245. [Google Scholar] [CrossRef]
- Scardino, A.J.; Guenther, J.; de Nys, R. Attachment point theory revisited: The fouling response to a microtextured matrix. Biofouling 2008, 24, 45–53. [Google Scholar] [CrossRef]
- Peter, A.; Lutey, A.H.A.; Faas, S.; Romoli, L.; Onuseit, V.; Graf, T. Direct laser interference patterning of stainless steel by ultrashort pulses for antibacterial surfaces. Opt. Laser Technol. 2020, 123, 105954. [Google Scholar] [CrossRef]
- Beloin, C.; Roux, A.; Ghigo, J.M. Escherichia coli biofilms. Curr. Top. Microbiol. 2008, 322, 249–289. [Google Scholar] [CrossRef] [Green Version]
- Clarke, M.; Maddera, L.; Harris, R.L.; Silverman, P.M. F-pili dynamics by live-cell imaging. Proc. Natl. Acad. Sci. USA 2008, 105, 17978–17981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silverman, M.; Simon, M. Characterization of Escherichia coli Flagellar Mutants That Are Insensitive to Catabolite Repression. J. Bacteriol. 1974, 120, 1196–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prigent-Combaret, C.; Prensier, G.; Le Thi, T.T.; Vidal, O.; Lejeune, P.; Dorel, C. Developmental pathway for biofilm formation in curli-producing Escherichia coli strains: Role of flagella, curli and colanic acid. Environ. Microbiol. 2000, 2, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Mitik-Dineva, N.; Wang, J.; Mocanasu, R.C.; Stoddart, P.R.; Crawford, R.J.; Ivanova, E.P. Impact of nano-topography on bacterial attachment. Biotechnol. J. 2008, 3, 536–544. [Google Scholar] [CrossRef] [PubMed]


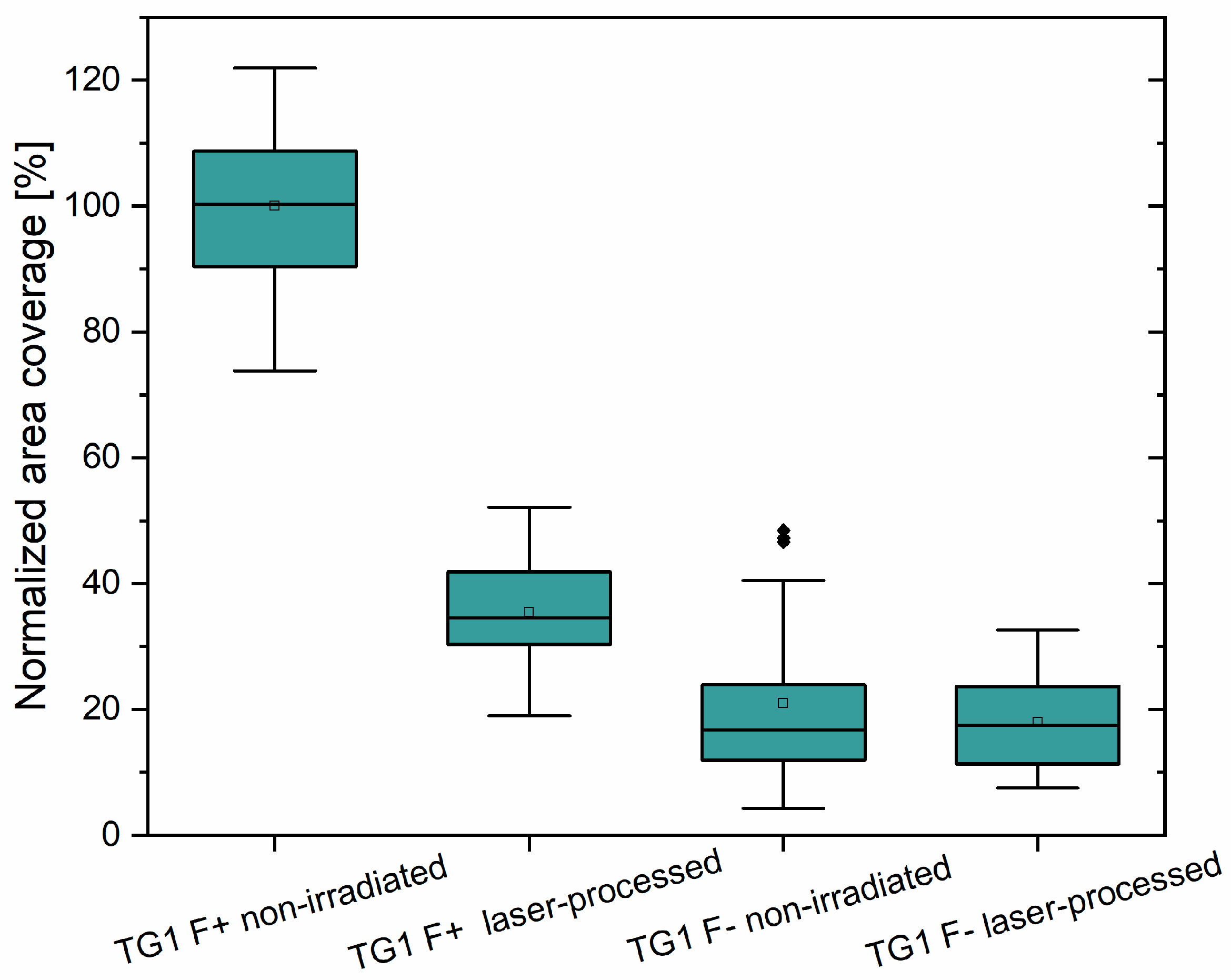
| Angle of Incidence θ [°] | 0° | 10° | 20° | 30° | 40° | 50° | 60° |
|---|---|---|---|---|---|---|---|
| Spatial period Λ [nm] | 214 | 247 | 281 | 331 | 400 | 459 | 613 |
| height h [nm] | 64 ± 8 | 42 ± 15 | 107 ± 11 | 84 ± 6 | 111 ± 18 | 72 ± 12 | 117 ± 52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richter, A.M.; Buchberger, G.; Stifter, D.; Duchoslav, J.; Hertwig, A.; Bonse, J.; Heitz, J.; Schwibbert, K. Spatial Period of Laser-Induced Surface Nanoripples on PET Determines Escherichia coli Repellence. Nanomaterials 2021, 11, 3000. https://doi.org/10.3390/nano11113000
Richter AM, Buchberger G, Stifter D, Duchoslav J, Hertwig A, Bonse J, Heitz J, Schwibbert K. Spatial Period of Laser-Induced Surface Nanoripples on PET Determines Escherichia coli Repellence. Nanomaterials. 2021; 11(11):3000. https://doi.org/10.3390/nano11113000
Chicago/Turabian StyleRichter, Anja M., Gerda Buchberger, David Stifter, Jiri Duchoslav, Andreas Hertwig, Jörn Bonse, Johannes Heitz, and Karin Schwibbert. 2021. "Spatial Period of Laser-Induced Surface Nanoripples on PET Determines Escherichia coli Repellence" Nanomaterials 11, no. 11: 3000. https://doi.org/10.3390/nano11113000
APA StyleRichter, A. M., Buchberger, G., Stifter, D., Duchoslav, J., Hertwig, A., Bonse, J., Heitz, J., & Schwibbert, K. (2021). Spatial Period of Laser-Induced Surface Nanoripples on PET Determines Escherichia coli Repellence. Nanomaterials, 11(11), 3000. https://doi.org/10.3390/nano11113000







