Kinetics of Silver Accumulation in Tissues of Laboratory Mice after Long-Term Oral Administration of Silver Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
3. Scheme of the Experiment
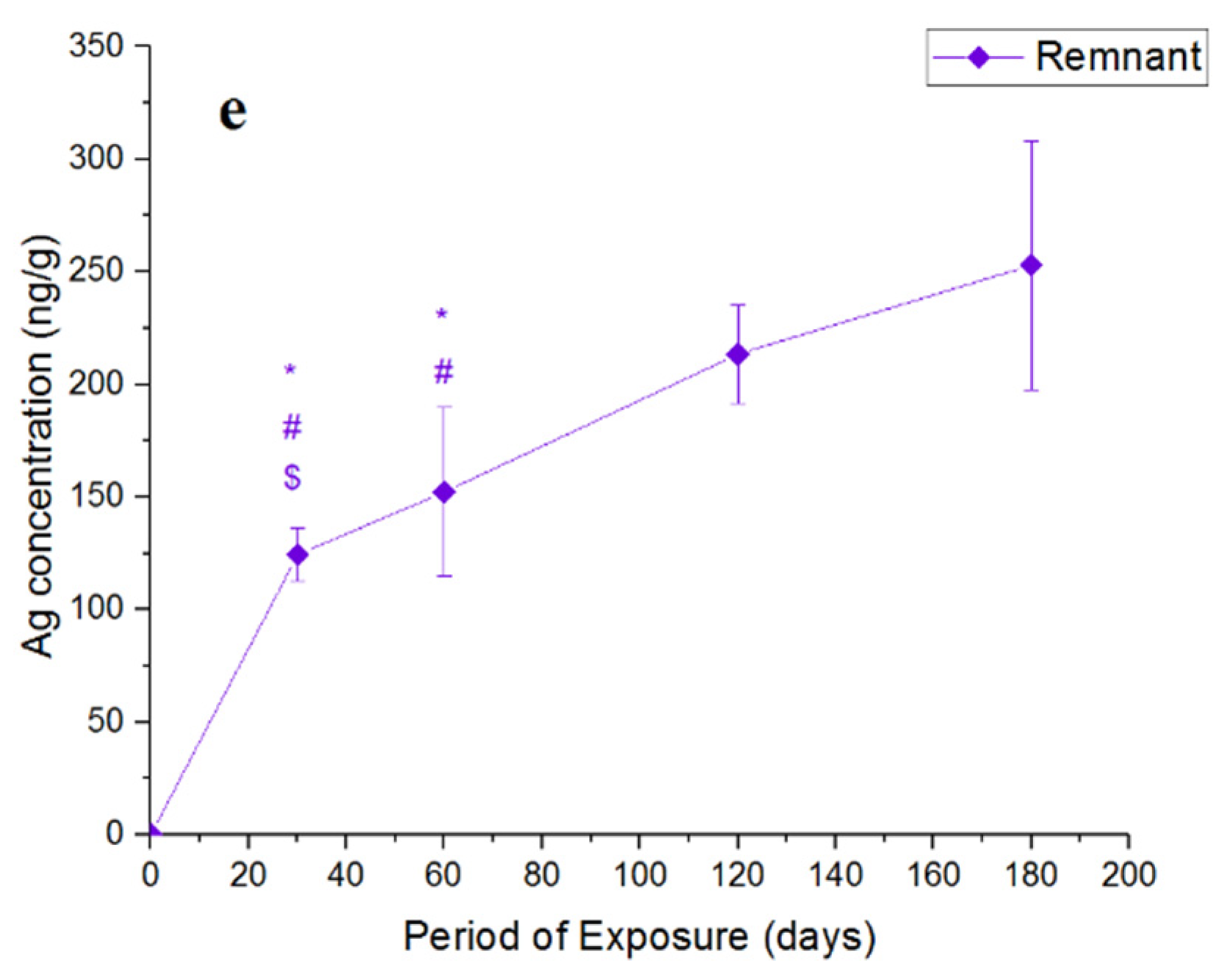
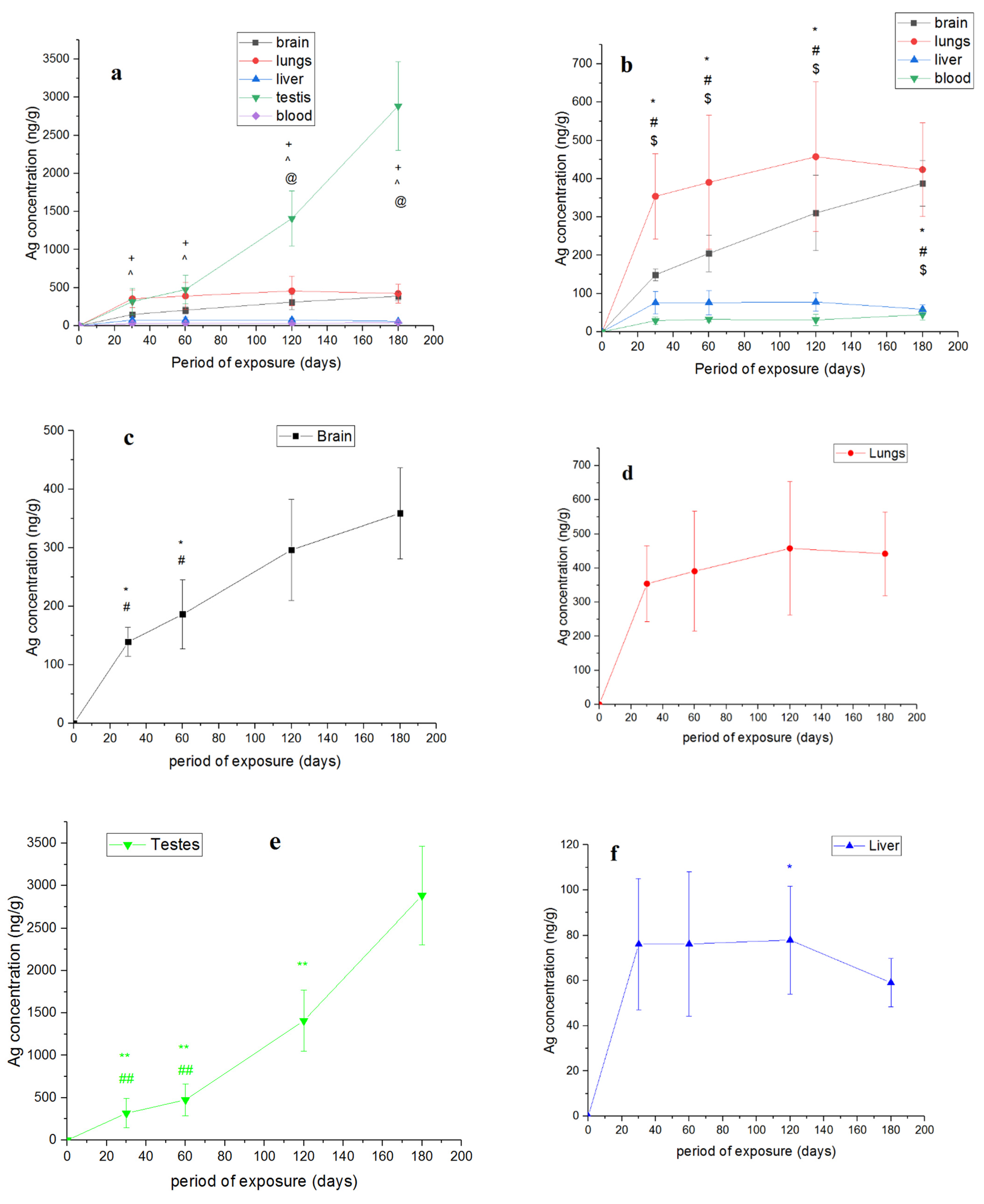
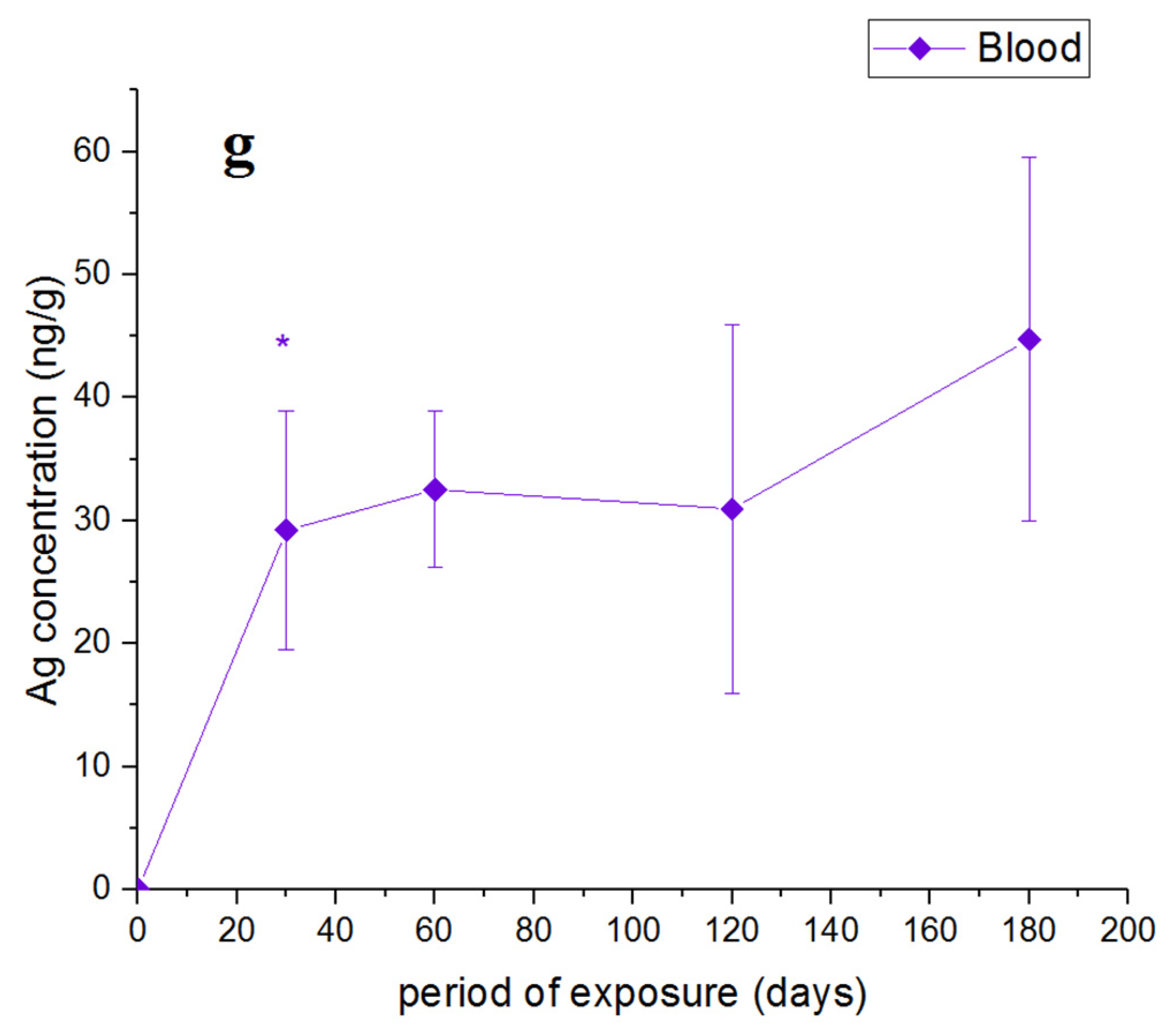
4. Results and Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Just, J.; Szniolis, A. Germicidal properties of silver in water. J. Am. Water Work. Assoc. 1936, 28, 492–506. [Google Scholar] [CrossRef]
- Mao, B.H.; Chen, Z.Y.; Wang, Y.J.; Yan, S.J. Silver nanoparticles have lethal and sublethal adverse effects on development and longevity by inducing ROS-mediated stress responses. Sci. Rep. 2018, 8, 2445. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.N.; Thomas, D.G.; Jolley, H.; Kodali, V.K.; Littke, M.H.; Munusamy, P.; Baer, D.R.; Gaffrey, M.J.; Thrall, B.D.; Teeguarden, J.G. All that is silver is not toxic: Silver ion and particle kinetics reveals the role of silver ion aging and dosimetry on the toxicity of silver nanoparticles. Part. Fibre Toxicilogy 2018, 15, 47. [Google Scholar] [CrossRef]
- Vazquez-Muñoz, R.; Borrego, B.; Juárez-Moreno, K.; García-García, M.; Mota Morales, J.D.; Bogdanchikova, N.; Huerta-Saquero, A. Toxicity of silver nanoparticles in biological systems: Does the complexity of biological systems matter? Toxicol. Lett. 2017, 276. [Google Scholar] [CrossRef]
- Drake, P.L.; Hazelwood, K.J. Exposure-Related Health Effects of Silver and Silver Compounds: A Review. Ann. Occup. Hyg. 2005, 49, 575–585. [Google Scholar]
- Behra, R.; Sigg, L.; Clift, M.J.D.; Herzog, F.; Minghetti, M.; Johnston, B.; Petri-Fink, A.; Rothen-Rutishauser, B. Bioavailability of silver nanoparticles and ions: From a chemical and biochemical perspective. J. R. Soc. Interface 2013, 10, 20130396. [Google Scholar] [CrossRef]
- Sun, X.; Shi, J.; Zou, X.; Wang, C.; Yang, Y.; Zhang, H. Silver nanoparticles interact with the cell membrane and increase endothelial permeability by promoting VE-cadherin internalization. J. Hazard. Mater. 2016, 317, 570–578. [Google Scholar] [CrossRef]
- Boruczkowski, M.; Zurawski, J. Current Knowledge of Silver and Gold Nanoparticles in Laboratory Research—Application, Toxicity, Cellular Uptake. Nanomaterials 2021, 11, 2454. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef] [Green Version]
- Loza, K.; Epple, M. Silver nanoparticles in complex media: An easy procedure to discriminate between metallic silver nanoparticles, reprecipitated silver chloride, and dissolved silver species. RSC Adv. 2018, 8, 24386–24391. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Ryu, D.Y. Silver nanoparticle-induced oxidative stress, genotoxicity and apoptosis in cultured cells and animal tissues. J. Appl. Toxicol. 2013, 33, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Jarak, I.; Carrola, J.; Barros, A.S.; Gil, A.M.; Pereira, M.L.; Corvo, M.L.; Duarte, I.F. From the Cover: Metabolism Modulation in Different Organs by Silver Nanoparticles: An NMR Metabolomics Study of a Mouse Model. Toxicol. Sci. 2017, 159, 2, 422–435. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.J.; Lee, S.J.; Yun, S.J.; Jang, J.-Y.; Kang, H.; Kim, K.; Choi, I.-H.; Park, S. Silver nanoparticles affect glucose metabolism in hepatoma cells through production of reactive oxygen species. Int. J. Nanomed. 2015, 11, 55–68. [Google Scholar] [CrossRef] [Green Version]
- Hadrup, N.; Sharma, A.K.; Loeschner, K. Toxicity of silver ions, metallic silver, and silver nanoparticle materials 2 after in vivo dermal and mucosal surface exposure: A review. Regul. Toxicol. Pharmacol. 2018, 98, 257–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.M.; Lee, S.H. Generalized argyria after habitual use of AgNO3. J. Dermatol. 1994, 21, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Costa, B.; Ferreira, M.V.; Miguéis, D.; Louros, J.M.S.; Durazzo, A.; Lucarini, M.; Eder, P.; Chaud, M.V.; Morsink, M.; et al. Nanotoxicology and Nanosafety: Safety-By-Design and Testing at a Glance. Int. J. Environ. Res. Public Health 2020, 17, 4657. [Google Scholar] [CrossRef] [PubMed]
- Antsiferova, A.; Buzulukov, Y.; Demin, V.; Kashkarov, P.; Kovalchuk, M.; Petritskaya, E. Extremely low level of Ag nanoparticle excretion from mice brain in in vivo experiments. In IOP Conference Series: Materials Science and Engineering; IOP Publishing Ltd.: Bristol, UK, 2015; Volume 98. [Google Scholar] [CrossRef]
- Available online: http://vector-vita.com/argovit.html (accessed on 12 November 2021).
- Sadauskas, E.; Jacobsen, N.R.; Danscher, G.; Stoltenberg, M.; Vogel, U.; Larsen, A.; Kreyling, W.; Wallin, H. Biodistribution of gold nanoparticles in mouse lung following intratracheal instillation. Chem. Cent. J. 2009, 3, 16. [Google Scholar] [CrossRef] [Green Version]
- Zinicovscaia, I.; Grozdov, D.; Yushin, N.; Ivlieva, A.; Petritskaya, E.; Rogatkin, D. Neutron activation analysis as a tool for tracing the accumulation of silver nanoparticles in tissues of female mice and their offspring. J. Radioanal. Nucl. Chem. 2019, 322, 1079–1083. [Google Scholar] [CrossRef]
- Antsiferova, A.A.; Buzulukov, Y.P.; Kashkarov, P.K.; Kovalchuk, M.V. Experimental and theoretical study of the transport of silver nanoparticles at their prolonged administration into a mammal organism. Crystallogr. Rep. 2016, 616, 1020–1026. [Google Scholar] [CrossRef]
- Antsiferova, A.A.; Buzulukov, Y.P.; Demin, V.A.; Demin, V.F.; Rogatkin, D.A.; Petritskaya, E.N.; Abaeva, L.F.; Kashkarov, P.K. Radiotracer methods and neutron activation analysis for the investigation of nanoparticle biokinetics in living organisms. Nanotechnol. Russ. 2015, 10, 100–108. [Google Scholar] [CrossRef]
- Beaudoin, G.; Lee, S.H.; Singh, D.; Yuan, Y.; Ng, Y.G.; Reichardt, L.F.; Arikkath, J. Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nat. Protoc. 2012, 7, 1741–1754. [Google Scholar] [CrossRef]
- Phillips, A.M.; Kim, T.; Vargas, E.; Petrou, S.; Reid, C.A. Spike-and-wave discharge mediated reduction in hippocampal HCN1 channel function associates with learning deficits in a genetic mouse model of epilepsy. Neurobiol. Dis. 2014, 64, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Lamana, J.; Laborda, F.; Bolea, E.; Abad-Álvaro, I.; Castillo, J.R.; Bianga, J.; He, M.; Bierla, K.; Mounicou, S.; Ouerdane, L.; et al. An insight into silver nanoparticles bioavailability in rats. Metallomics 2014, 6, 2242–2249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Y.; Zhang, S.; Huang, Y.; Zhang, T.; Liu, X.; Hu, Y.; Zhang, Z.; Tang, M. Acute toxic effects and gender-related biokinetics of silver nanoparticles following an intravenous injection in mice. J. Appl. Toxicol. 2012, 32, 890–899. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.S.; Song, K.S.; Ryu, H.R.; Sung, J.H.; Park, J.D.; Park, H.M.; Song, N.W.; Shin, B.S.; Marshak, D.; et al. Biopersistence of silver nanoparticles in tissues from Sprague–Dawley rats. Part Fibre Toxicol. 2013, 10, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Zande, M.; Vandebriel, R.J.; Van Doren, E.; Kramer, E.; Herrera Rivera, Z.; Serrano-Rojero, C.S.; Gremmer, E.R.; Mast, J.; Peters, R.J.B.; Hollman, P.C.H.; et al. Distribution, Elimination, and Toxicity of Silver Nanoparticles and Silver Ions in Rats after 28-Day Oral Exposure. ACS Nano 2012, 6, 7427–7442. [Google Scholar] [CrossRef]
- Lovaković, B.T.; Barbir, R.; Pem, B.; Goessler, W.; Ćurlin, M.; Micek, V.; Debeljak, Ž.; Božičević, L.; Ilić, K.; Pavičić, I.; et al. Sex-related response in mice after sub-acute intraperitoneal exposure to silver nanoparticles. NanoImpact 2021, 23, 100340. [Google Scholar] [CrossRef]
- Antsiferova, A.A.; Kopaeva, M.Y.; Kochkin, V.N.; Kashkarov, P.K.; Kovalchuk, M.V. Disturbance in Mammalian Cognition Caused by Accumulation of Silver in Brain. Toxics 2021, 9, 30. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Pavlov, S.S.; Frontasyeva, M.V.; Ivlieva, A.L.; Petritskaya, E.N.; Rogatkin, D.A.; Demin, V.A. Accumulation of silver nanoparticles in mice tissues studied by neutron activation analysis. J. Radioanal. Nucl. Chem. 2018, 318, 985–989. [Google Scholar] [CrossRef]
- Recordati, C.; De Maglie, M.; Cella, C.; Argentiere, S.; Paltrinieri, S.; Bianchessi, S.; Losa, M.; Fiordaliso, F.; Corbelli, A.; Milite, G.; et al. Repeated oral administration of low doses of silver in mice: Tissue distribution and effects on central nervous system. Part Fibre Toxicol. 2021, 18, 23. [Google Scholar] [CrossRef]
- Elsharkawy, E.E.; El-Nasser, M.A.; Kamaly, H.F. Silver nanoparticles testicular toxicity in rat. Environ. Toxicol. Pharmacol. 2019, 70, 103194. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; Abdelrahman, S.A.; Shalaby, S.M. Evaluating the effect of silver nanoparticles on testes of adult albino rats (histological, immunohistochemical and biochemical study). J. Mol. Hist. 2017, 48, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Fathi, N.; Hoseinipanah, S.M.; Alizadeh, Z.; Assari, M.J.; Moghimbeigi, A.; Mortazavi, M.; Hosseini, M.H.; Bahmanzadeh, M. The effect of silver nanoparticles on the reproductive system of adult male rats: A morphological, histological and DNA integrity study. Adv. Clin. Exp. Med. 2019, 28, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Melnik, E.A.; Buzulukov, Y.P.; Demin, V.F.; Demin, V.A.; Gmoshinski, I.V.; Tyshko, N.V.; Tutelyan, V.A. Transfer of Silver Nanoparticles through the Placenta and Breast Milk during in vivo Experiments on Rats. ActaNaturae 2013, 5, 107–115. [Google Scholar] [CrossRef]
- Węsierska, M.; Dziendzikowska, K.; Gromadzka-Ostrowska, J.; Dudek, J.; Polkowska-Motrenko, H.; Audinot, J.N.; Gutleb, A.C.; Lankoff, A.; Kruszewski, M. Silver ions are responsible for memory impairment induced by oeal administration of solver nanoparticles. Toxicol. Lett. 2018, 290, 133–144. [Google Scholar] [CrossRef]
- Antsiferova, A.; Kopaeva, M.; Kashkarov, P. Effects of Prolonged Silver Nanoparticle Exposure on the Contextual Cognition and Behavior of Mammals. Materials 2018, 11, 558. [Google Scholar] [CrossRef] [Green Version]
- Greish, K.; Alqahtani, A.A.; Alotaibi, A.F.; Abdulla, A.M.; Bukelly, A.T.; Alsobyani, F.M.; Alharbi, G.H.; Alkiyumi, I.S.; Aldawish, M.M.; Alshahrani, T.F.; et al. The Effect of Silver Nanoparticles on Learning, Memory and Social Interaction in BALB/C Mice. Int. J. Environ. Res. Public Health 2019, 16, 148. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.T.; Minai-Tehrani, A.; Hwang, S.K.; Kim, J.E.; Shin, J.Y.; Yu, K.N.; Chang, S.H.; Kim, D.S.; Kwon, Y.T.; Choi, I.J.; et al. Acute pulmonary toxicity and body distribution of inhaled metallic silver nanoparticles. Toxicol. Res. 2012, 28, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Seiffert, J.; Hussain, F.; Wiegman, C.; Li, F.; Bey, L.; Baker, W.; Porter, A.; Ryan, M.P.; Chang, Y.; Gow, A.; et al. Pulmonary Toxicity of Instilled Silver Nanoparticles: Influence of Size, Coating and Rat Strain. PLoS ONE 2015, 10, e0119726. [Google Scholar] [CrossRef] [Green Version]
- Wiemann, M.; Vennemann, A.; Blaske, F.; Sperling, M.; Karst, U. Silver Nanoparticles in the Lung: Toxic Effects and Focal Accumulation of Silver in Remote Organs. Nanomaterials 2017, 7, 441. [Google Scholar] [CrossRef] [Green Version]
- Braakhuis, H.M.; Gosens, I.; Krystek, P.; Boere, J.A.F.; Cassee, F.R.; Fokkens, P.H.B.; Post, J.A.; van Loveren, H.; Park, M.V.D.Z. Particle size dependent deposition and pulmonary inflammation after short-term inhalation of silver nanoparticles. Part Fibre Toxicol. 2014, 11, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demin, V.A.; Gmoshinsky, I.V.; Demin, V.F.; Anciferova, A.A.; Buzulukova, Y.P.; Khotimchenko, S.A.; Tutelyan, V.A. Modeling Interorgan Distribution and Bioaccumulationof Engineered Nanoparticles(Using the Example of Silver Nanoparticles). Nanotechnol. Russ. 2015, 10, 288–296. [Google Scholar] [CrossRef]
- Demin, V.A.; Antsiferova, A.A.; Buzulukov, Y.P.; Gmoshinsky, I.V.; Demin, V.F.; Kashkarov, P.K. Mathematical Simulation of the Biokinetics of Selenium Nanoparticles and Salt Forms in Living Organisms. Nanotechnol. Russ. 2017, 12, 305–314. [Google Scholar] [CrossRef]
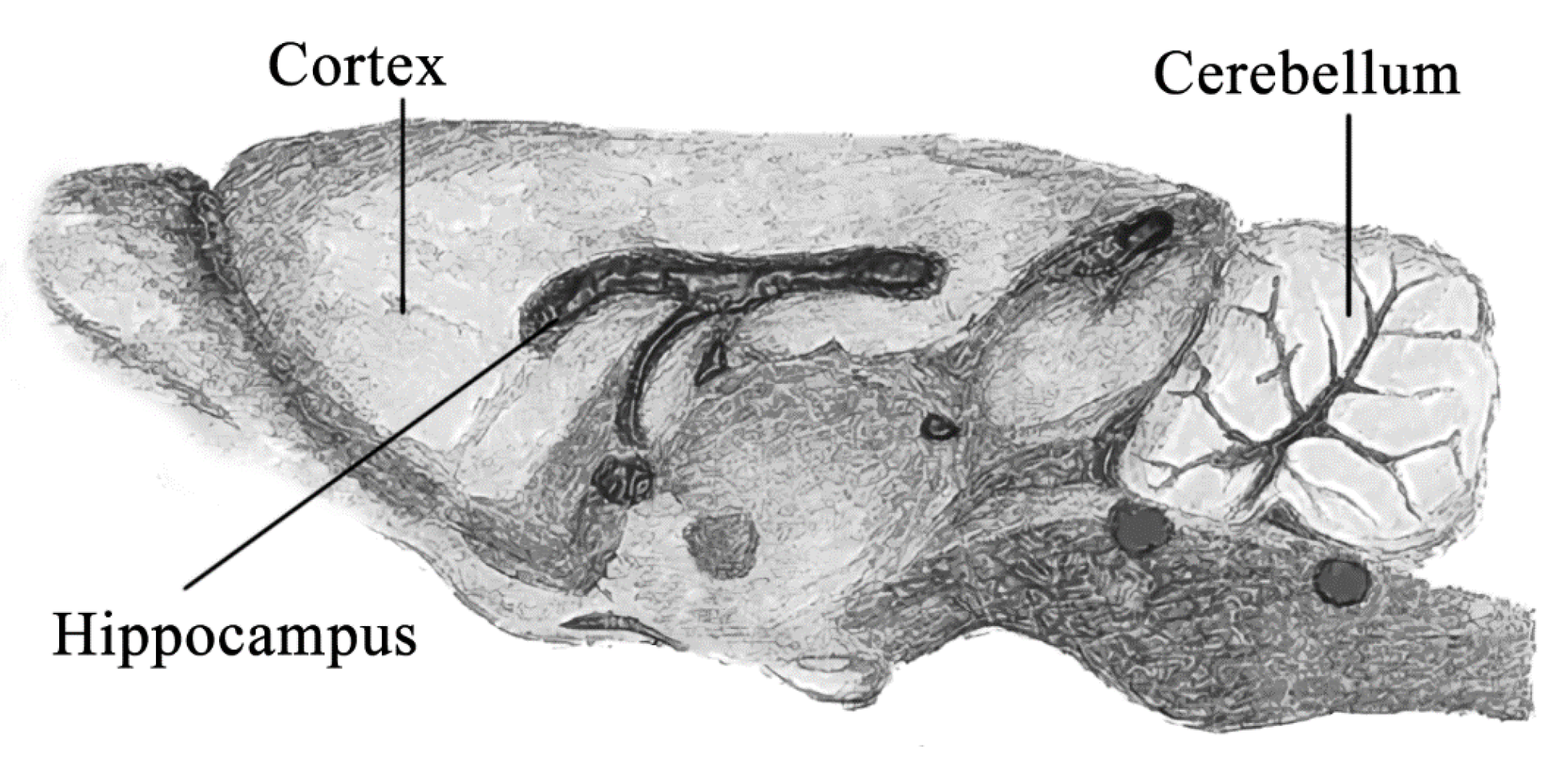
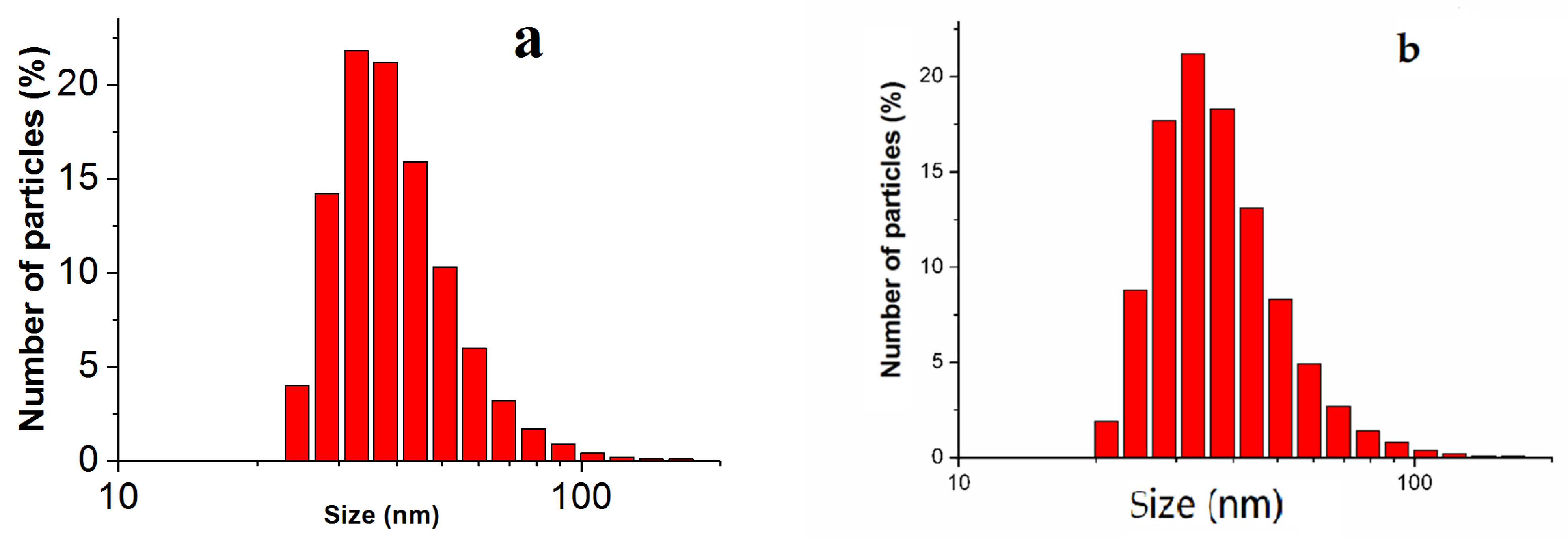
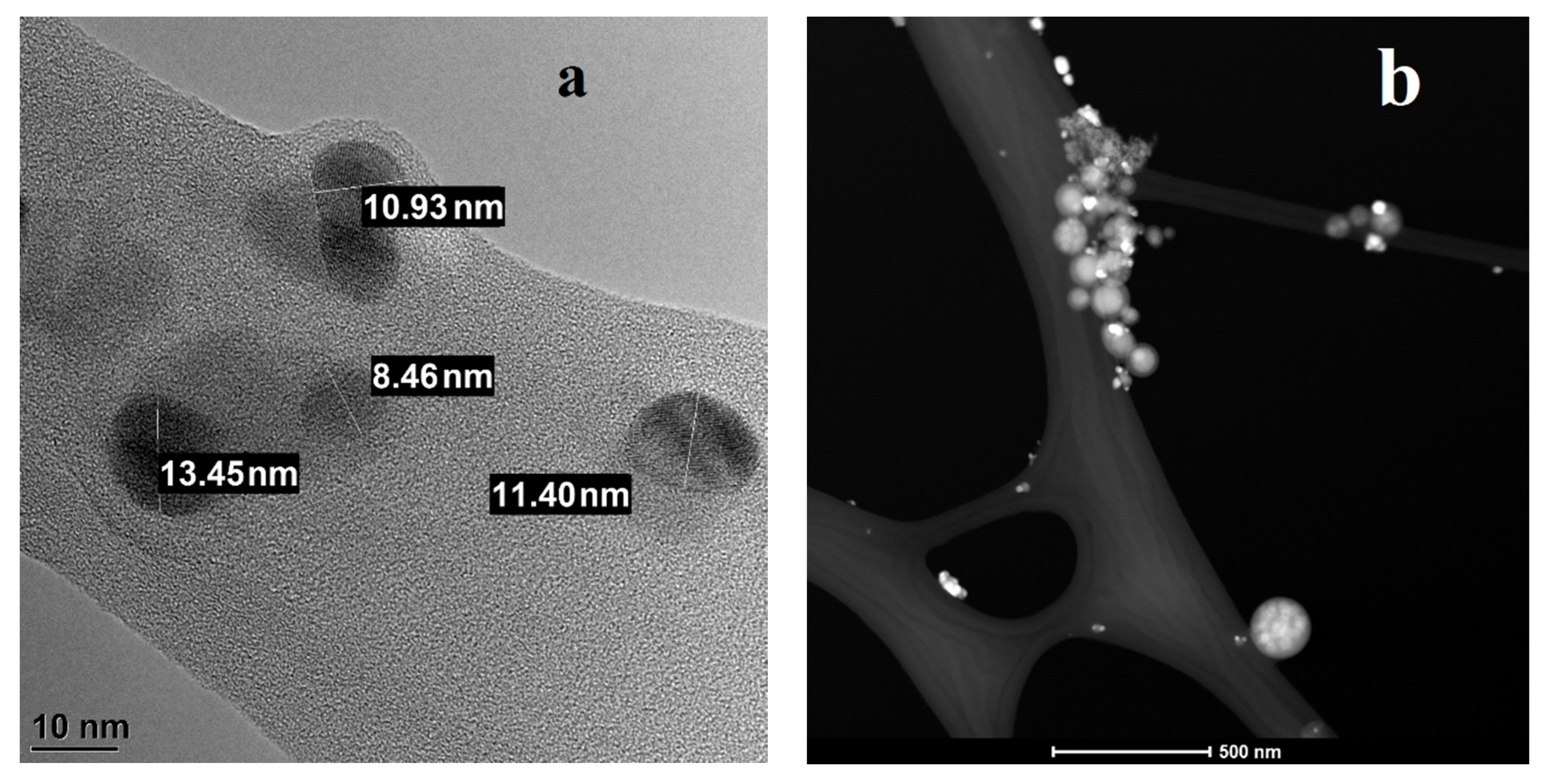
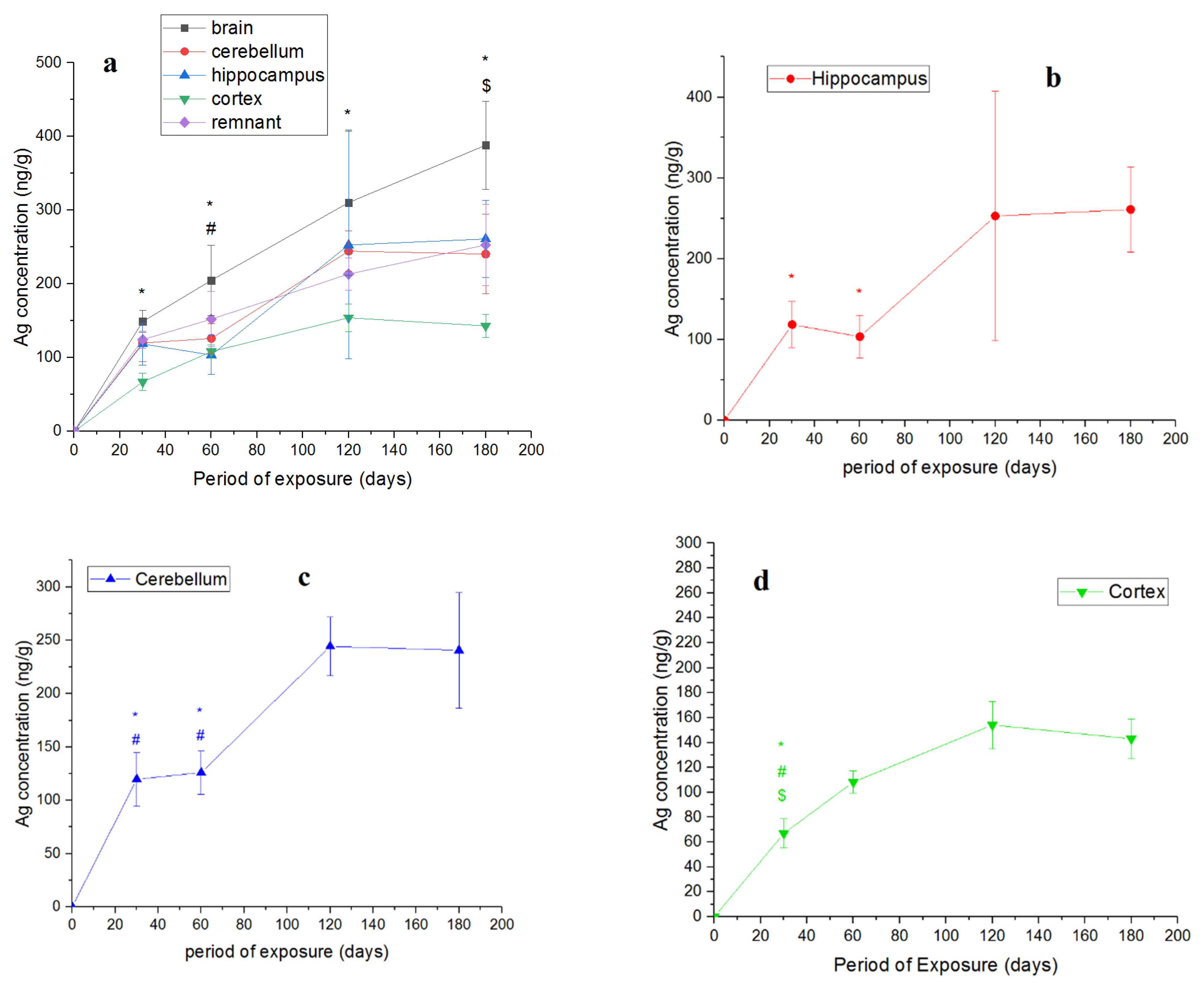
| Period of Exposure, Days | Silver Mass, ng (Mean ± SD) | |||
|---|---|---|---|---|
| Hippocampus | Cerebellum | Cortex | Remnant | |
| 30 | 6.4 ± 1.5 | 16.3 ± 3.4 | 10.4 ± 3.1 | 28 ± 2.5 |
| 60 | 5.8 ± 1.5 | 15 ± 2.4 | 16.9 ± 1.5 | 30.3 ± 7.4 |
| 120 | 13.4 ± 8 | 27.6 ± 3 | 20.5 ± 6.0 | 42.9 ± 4.2 |
| 180 | 12.4 ± 2.3 | 8.2 ± 2.1 | 31.9 ± 11.3 | 65.9 ± 14.3 |
| Period of Exposure, Days | Silver Mass, ng (Mean ± SD) | ||||
|---|---|---|---|---|---|
| Brain | Lungs | Testis | Liver | Blood | |
| 30 | 63.1 ± 5.3 | 63.7 ± 19.8 | 61.8 ± 33.2 | 105 ± 38.3 | 18.5 ± 5.5 |
| 60 | 84.7 ± 19.1 | 78.1 ± 34.2 | 87.8 ± 31.5 | 102.1 ± 40.9 | 20.1 ± 3.7 |
| 120 | 137.3 ± 41.2 | 96.1 ± 38.7 | 267.8 ± 66.7 | 113 ± 33.4 | 19.1 ± 9 |
| 180 | 166 ± 24 | 89 ± 19.3 | 519.1 ± 95.4 | 90.7 ± 15.1 | 32.2 ± 8.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antsiferova, A.A.; Kopaeva, M.Y.; Kochkin, V.N.; Kashkarov, P.K. Kinetics of Silver Accumulation in Tissues of Laboratory Mice after Long-Term Oral Administration of Silver Nanoparticles. Nanomaterials 2021, 11, 3204. https://doi.org/10.3390/nano11123204
Antsiferova AA, Kopaeva MY, Kochkin VN, Kashkarov PK. Kinetics of Silver Accumulation in Tissues of Laboratory Mice after Long-Term Oral Administration of Silver Nanoparticles. Nanomaterials. 2021; 11(12):3204. https://doi.org/10.3390/nano11123204
Chicago/Turabian StyleAntsiferova, Anna A., Marina Yu. Kopaeva, Vyacheslav N. Kochkin, and Pavel K. Kashkarov. 2021. "Kinetics of Silver Accumulation in Tissues of Laboratory Mice after Long-Term Oral Administration of Silver Nanoparticles" Nanomaterials 11, no. 12: 3204. https://doi.org/10.3390/nano11123204
APA StyleAntsiferova, A. A., Kopaeva, M. Y., Kochkin, V. N., & Kashkarov, P. K. (2021). Kinetics of Silver Accumulation in Tissues of Laboratory Mice after Long-Term Oral Administration of Silver Nanoparticles. Nanomaterials, 11(12), 3204. https://doi.org/10.3390/nano11123204








