The Influence of Aryl Substituents on the Supramolecular Structures and Photoluminescence of Cyclic Trinuclear Pyrazolato Copper(I) Complexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Techniques
2.2. Instrumentation
2.3. Preparation of Complexes
2.3.1. [Cu(μ-L5pz)]3
2.3.2. [Cu(μ-L6pz)]3
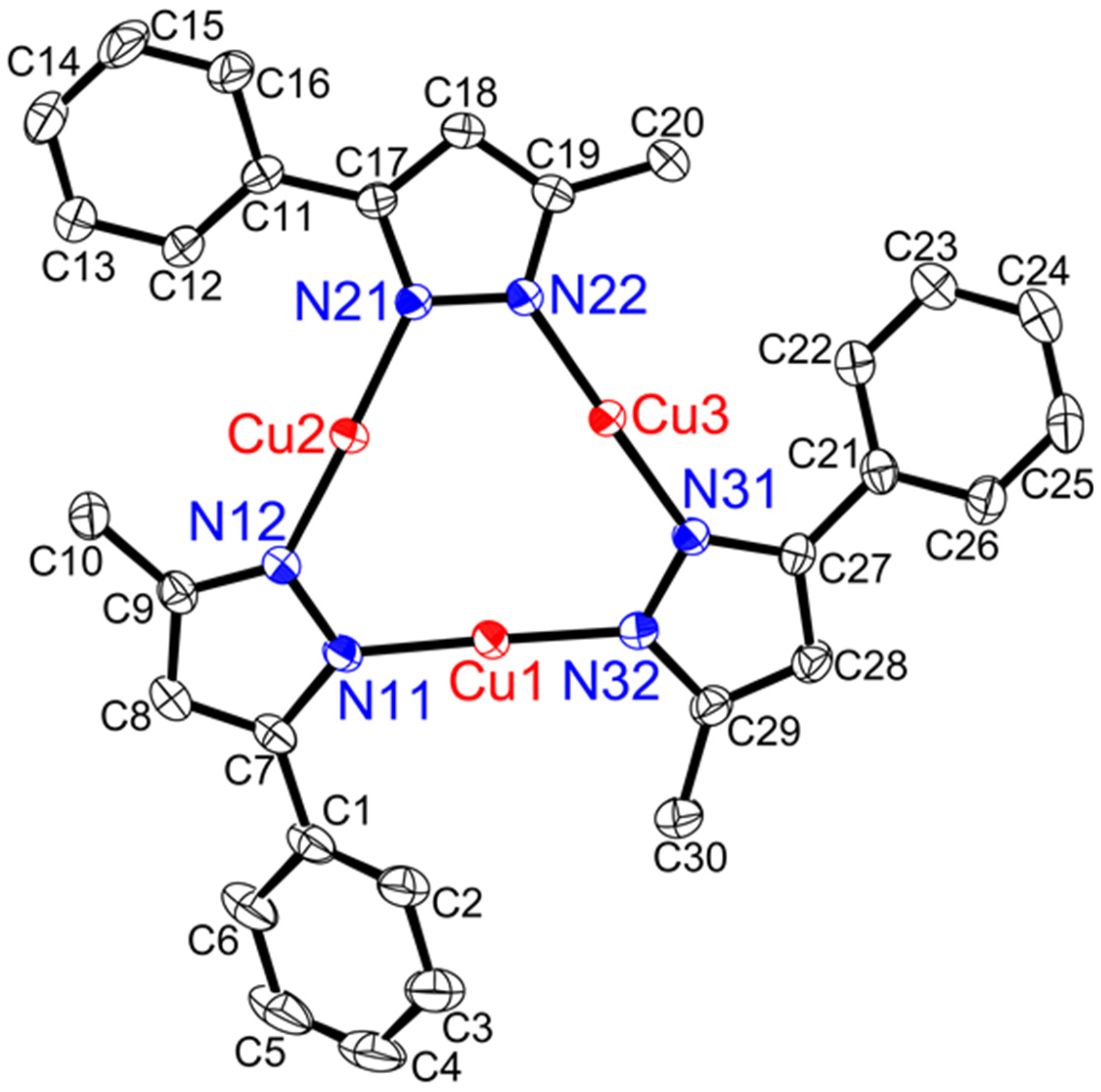
2.4. X-ray Crystal Structures
3. Results and Discussion
3.1. Synthesis
3.2. Structure
3.3. Solution-State Properties
3.4. Solid-State Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References and Notes
- Zheng, J.; Lu, Z.; Wu, K.; Ning, G.-H.; Li, D. Coinage-metal-based cyclic trinuclear complexes with metal−metal interactions: Theories to experiments and structures to functions. Chem. Rev. 2020, 120, 9675–9742. [Google Scholar] [CrossRef] [PubMed]
- Omary, M.A.; Determan, J.J.; Gamage, C.S.P.; Sinha, P.; Li, S.; Patterson, M.R.; Nestero, V.N.; Wilson, A.K.; Dias, H.V.R. Is a high photoluminescence quantum yield good enough for OLEDs? Can luminescence rigidochromism be manifest in the solid state? An Optoelectronic device Screening case study for diphosphine/pyrazolate copper(I) complexes. Comments Inorg. Chem. 2020, 40, 1–24. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, H.; Xie, M.; Li, D. The π-acidity/basicity of cyclic trinuclear units (CTUs): From a theoretical perspective to potential applications. Chem. Commun. 2019, 55, 7134–7146. [Google Scholar] [CrossRef] [PubMed]
- Galassi, R.; Rawashdeh-Omary, M.A.; Dias, H.V.R.; Omary, M.A. Homoleptic cyclic trinuclear d10 complexes: From self-association via metallophilic and excimeric bonding to the breakage thereof via oxidative addition, dative bonding, Quadrupolar, and heterometal bonding interactions. Comments Inorg. Chem. 2019, 39, 287–348. [Google Scholar] [CrossRef] [Green Version]
- Elguero, J.; Alkorta, I. A computational study of metallacycles formed by pyrazolate ligands and the coinage metals M = Cu(I), Ag(I) and Au(I): (pzM)n for n = 2, 3, 4, 5 and 6. Comparison with structures reported in the Cambridge Crystallographic Data Center (CCDC). Molecules 2020, 25, 5108. [Google Scholar] [CrossRef]
- Yu, S.-Y.; Lu, H.-L. From metal-metal bonding to supra-metal-metal bonding directed self-assembly: Supramolecular architectures of group 10 and 11 metals with ligands from mono- to poly-pyrazoles. Isr. J. Chem. 2019, 59, 166–183. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Zhang, Y.-B.; Lin, J.-B.; Chen, X.-M. Metal azolate frameworks: From crystal engineering to functional materials. Chem. Rev. 2012, 112, 1001–1033. [Google Scholar] [CrossRef]
- Fustero, S.; Sánchez-Roselló, M.; Barrio, P.; Simón-Fuentes, A. From 2000 to mid-2010: A fruitful decade for the synthesis of pyrazoles. Chem. Rev. 2011, 111, 6984–7034. [Google Scholar] [CrossRef] [PubMed]
- Halcrow, M.A. Pyrazoles and pyrazolides—Flexible synthons in self-assembly. Dalton Trans. 2009, 2059–2073. [Google Scholar] [CrossRef]
- Fustero, S.; Simón-Fuentes, A.; Sanz-Cervera, J.F. Recent advances in the synthesis of pyrazoles. A review. Org. Prep. Proced. Int. 2009, 41, 253–290. [Google Scholar] [CrossRef]
- Raptis, R.G.; Fackler, J.P., Jr. Structure of tris(μ-3,5-diphenylpyrazolato-N,N′)tricopper(I). structural comparisons with the silver(I) and gold(I) pyrazolate trimers. Inorg. Chem. 1988, 27, 4179–4182. [Google Scholar] [CrossRef]
- Murray, H.H.; Raptis, R.G.; Fackler, J.P., Jr. Syntheses and X-ray structures of group 11 pyrazole and pyrazolate complexes. X-ray crystal structures of bis(3,5-diphenylpyrazole)copper(II) dibromide, tris(μ-3,5-diphenylpyrazolato-N,N′)trisilver(I)-2-tetrahydrofuran, tris (μ-3,5-diphenylpyrazolato-N,N′)trigold(I), and hexakis(μ-3,5-diphenylpyrazolato-N,N′)hexagold(I). Inorg. Chem. 1988, 27, 26–33. [Google Scholar]
- Moga, T.G. Counting on copper. Nat. Chem. 2012, 4, 334. [Google Scholar] [CrossRef]
- Xu, Z.-L.; Li, H.-X.; Ren, Z.-G.; Du, W.-Y.; Xu, X.-C.; Lang, J.-P. Cu(OAc)2·H2O-catalyzed N-arylation of nitrogen-containing heterocycles. Tetrahedron 2011, 67, 5282–5888. [Google Scholar] [CrossRef]
- Morawitz, M.; Lerner, H.-W.; Bolte, M. cyclo-Tris(μ2-3-phenyl-1H-pyrazole)tricopper(I). Acta Cryst. 2006, E62, m1474–m1476. [Google Scholar] [CrossRef]
- Ehlert, M.K.; Rettig, S.J.; Storr, A.; Thompson, R.C.; Trotter, J. Synthesis and X-ray crystal structure of the 3,5-dimethylpyrazolato copper(I) trimer, [Cu(pz″)]3. Can. J. Chem. 1990, 68, 1444–1449. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Yin, Y.-G.; Wu, T.; Li, D.; Huang, X.-C. Design and solvothermal synthesis of luminescent copper(I)-pyrazolate coordination oligomer and polymer frameworks. Chem. Commun. 2006, 2845–2847. [Google Scholar] [CrossRef]
- Fujisawa, K.; Ishikawa, Y.; Miyashita, Y.; Okamoto, K. Crystal structure of pyrazolato-bridged copper(I) polynuclear complexes. Chem. Lett. 2004, 33, 66–67. [Google Scholar] [CrossRef]
- Fujisawa, K.; Ishikawa, Y.; Miyashita, Y.; Okamoto, K. Pyrazolate-bridged group 11 metal(I) complexes: Substituent effects on the supramolecular structures and physicochemical properties. Inorg. Chim. Acta 2010, 363, 2977–2989. [Google Scholar] [CrossRef]
- Dias, H.V.R.; Diyabalanage, H.V.K.; Eldabaja, M.G.; Elbjeirami, O.; Rawashdeh-Omary, M.A.; Omary, M.A. Brightly phosphorescent trinuclear copper(I) complexes of pyrazolates: Substituent effects on the supramolecular structure and photophysics. J. Am. Chem. Soc. 2005, 127, 7489–7501. [Google Scholar] [CrossRef]
- Ehlert, M.K.; Rettig, S.J.; Storr, A.; Thompson, R.C.; Trotter, J. Polynuclear pyrazolate complexes of copper. crystal and molecular structures of [Cu(tmpz)]3, [Cu(3-CO2dmpz)(tmpzH)]2Cu, and [Cu(4-Br-3-CO2mepz)(4-BrdmpzH)2], (where mepz = methylpyrazolate, dmpz = dimethylpyrazolate, and tmpz = trimethylpyrazolate) and magnetic susceptibility studies on the dinuclear complex. Can. J. Chem. 1992, 70, 2161–2173. [Google Scholar]
- Gong, F.; Wang, Q.; Chen, J.; Yang, Z.; Liu, M.; Li, S.; Yang, G.; Bai, L.; Liu, J.; Dong, Y. Exploring intertrimer Cu⋯Cu interactions and further phosphorescent properties of aryl trimer copper(I) pyrazolates via substituent changing and external pressure. Inorg. Chem. 2010, 49, 1658–1666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, H.; Xiang, J. Facile halogenation of pyrazolate-bridged copper(I) complexes: Synthesis, crystal structure, and photoluminescent properties. Z. Anorg. Allg. Chem. 2016, 642, 1173–1177. [Google Scholar] [CrossRef]
- Ardizzoia, G.A.; Cenini, S.; Monica, G.L.; Masciocchi, M.; Maspero, A.; Moret, M. Syntheses, structures, and reactivity of polynuclear pyrazolato copper(I) complexes, including an ab-Initio XRPD Study of [Cu(dmnpz)]3 (Hdmnpz = 3,5-dimethyl-4-nitropyrazole). Inorg. Chem. 1998, 37, 4284–4292. [Google Scholar] [CrossRef] [PubMed]
- Dias, H.V.R.; Polacha, S.A.; Wang, Z. Coinage metal complexes of 3,5-bis(trifluoromethyl)pyrazolate ligand synthesis and characterization of {[3,5-(CF3)2Pz]Cu}3 and {[3,5-(CF3)2Pz]Ag}3. J. Fluor. Chem. 2000, 103, 163–169. [Google Scholar] [CrossRef]
- Omary, M.A.; Rawashdeh-Omary, M.A.; Gonser, M.W.A.; Elbjeirami, O.; Grimes, T.; Cundari, T.R.; Diyabalanage, H.V.K.; Gamage, C.S.P.; Dias, H.V.R. Metal effect on the supramolecular structure, photophysics, and acid-base character of trinuclear pyrazolato coinage metal complexes. Inorg. Chem. 2005, 44, 8200–8210. [Google Scholar] [CrossRef] [Green Version]
- Bondi, A. van der Waals volume and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Pyykkö, P. Strong closed-shell interactions in inorganic chemistry. Chem. Rev. 1997, 97, 597–636. [Google Scholar] [CrossRef]
- Pyykkö, P.; Runeberg, N.; Mendizabal, F. Theory of the d10–d10 closed-shell attraction: 1. dimers near equilibrium. Chem. Eur. J. 1997, 3, 1451–1457. [Google Scholar] [CrossRef]
- Jerabek, P.; von der Esch, B.; Schmidbaur, H.; Schwerdtfeger, P. Influence of relativistic effects on bonding modes in M(II) dinuclear complexes (M = Au, Ag, and Cu). Inorg. Chem. 2017, 56, 14624–14631. [Google Scholar] [CrossRef]
- Ardizzoia, G.A.; Cenini, S.; Monica, G.L.; Masciocchi, N.; Moret, M. Synthesis, X-ray structure, and catalytic properties of the unprecedented tetranuclear copper(I) species [Cu(dppz)]4 (Hdppz = 3,4-diphenylpyrazole). Inorg. Chem. 1994, 33, 1458–1463. [Google Scholar] [CrossRef]
- Rheingold, A.L.; Ostrander, R.L.; Haggerty, B.S.; Trofimenko, S. Homoscorpionate (tris(pyrazolyl)borate) ligands containing tethered 3-phenyl groups. Inorg. Chem. 1994, 33, 3666–3676. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 7th ed.; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- Kitajima, N.; Fujisawa, K.; Fujimoto, C.; Moro-oka, Y.; Hashimoto, S.; Kitagawa, T.; Toriumi, T.; Tatsumi, K.; Nakamura, A. A new model for dioxygen binding in hemocyanin. Synthesis, characterization, and molecular structure of the μ-η2:η2 peroxo dinuclear copper(II) complexes, [Cu(HB(3,5-R2pz)3)]2(O2) (R = i-Pr and Ph). J. Am. Chem. Soc. 1992, 114, 1277–1291. [Google Scholar] [CrossRef]
- CrystalClear: Data Collection and Processing Software; Rigaku Corporation: Tokyo, Japan, 2001.
- CrysAlisPro: Data Collection and Processing Software; Rigaku Corporation: Tokyo, Japan, 2015.
- SIR2008: Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Siliqi, D.; Spagna, R. IL MILIONE: A suite of computer programs for crystal structure solution of proteins. J. Appl. Cryst. 2007, 40, 609–613. [Google Scholar] [CrossRef]
- SIR2004: Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Spagna, R. SIR2004: An improved tool for crystal structure determination and refinement. J. Appl. Cryst. 2005, 38, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Crystal Structure 4.3: Crystal Structure Analysis Package; Rigaku Corporation: Tokyo, Japan, 2003.
- SHELXL Version 2018/3: Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]





| Complex | d Intra (Cu⋯Cu) /Å | Cu⋯Cu⋯Cu /Deg | d Inter (Cu⋯Cu) /Å a | Npz−Cu−Npz b /Deg | Torsion Angle b Cu–Npz–Npz–Cu/Deg | Ref. |
|---|---|---|---|---|---|---|
| [Cu(μ-L5)]3 | 3.1567(11), 3.1727(12), 3.1563(12) | 59.82(3), 59.84(3), 60.34(3) | n.a. | 177.3(3), 175.2(3), 177.6(2) | −49.2(5), 11.0(6), 12.7(6) | this work |
| [Cu(μ-L5)]3 •2(CH2Cl2) | 3.1078(7), 3.1247(6), 3.2651(8) | 63.19(2), 58.16(2), 58.66(2) | n.a. | 173.5(2), 176.9(2), 176.4(2) | 11.8(3), 7.0(4), −40.9(3) | this work |
| [Cu(μ-L6)]3 •0.5(CH2Cl2) | 3.1867(5), 3.2452(4), 3.1848(5), 3.2063(5), 3.2344(4), 3.1946(4) | 59.35(1), 59.41(1), 61.24(1), 59.47(1), 59.83(1), 60.70(1) | 2.9099(4), 2.9622(4) | 177.1(1), 173.5(1), 175.4(1), 177.8(1), 174.6(1), 171.9(1) | 0.8(2), 4.4(2), 0.5(2), 10.8(2), −6.2(2), −3.3(2) | this work |
| [Cu(μ-L5)]3 •1/3(C6H14) | 3.280(1), 3.406(1), 3.332(1) | 62.02, 59.74, 58.24 | n.a. | 178.6(3), 169.6(3), 169.2(3) | 5.6, −39.6(6), 7.0(7) | [11] |
| [Cu(μ-3,4-Ph2pz)]3 | 3.147, 3.259, 3.213, 3.325, 3.138, 3.235 | 60.18, 58.19, 61.63, 59.99, 62.86, 57.15 | 3.483(2), 3.483(2), 2.921(1), 2.921(1) | 177.9(3), 177.6(3), 174.0(3), 171.6(3), 174.0(3), 175.4(3) | 0.0(8), −4.9(8), −7.3(7), 18.8(8), 12.8(7), 0.6(8) | [14] |
| [Cu(μ-3-Phpz)]3 | 3.2076, 3.2083, 3.2402 | 59.66, 60.67, 59.68 | 3.099(2), 3.559(2) | 177.8(3), 178.5(3), 173.6(3) | 0.7(8),−10.1(8), −0.7(8) | [15] |
| [Cu(μ-3,5-Me2pz)]3 | 3.207, 3.197, 3.257, 3.195, 3.204, 3.258 | 61.15, 59.57, 59.28, 61.22, 59.25, 59.53 | 2.944(2), 2.947(2) | 173.4(2), 175.3(2) 173.5(2), 174.7(2) 175.2(2), 173.6(2) | −0.7(6), 0.8(6), 0.4(6), −1.0(5), 0.2(6), −0.3(6) | [16] |
| [Cu(μ-3,5-Me2pz)]3 | 3.1950, 3.2582, 3.2061, | 59.57, 59.23, 61.20 | 2.9534(6), 2.9534(6) | 175.4(1), 173.9(1), 173.8(1) | 0.9(3), 0.3(3), 0.5(3) | [17] |
| [Cu(μ-3,5-iPr2pz)]3 | 3.1907(6), 3.1997(7), 3.2370(6) | 59.43(1), 59.70(1), 60.87(1) | 3.0250(7), 3.0250(7) | 169.6(1), 171.4(1), 176.9(1) | −4.9(3), 13.0(3), 1.7(3) | [18,19] |
| [Cu(μ-3,5-iPr2pz)]3 | 3.195, 3.211, 3.235 | 59,42, 59.92, 60.66 | 2.989, 2.989 | 169.84, 171.24, 176.88 | 1.4(1), 13.9(1), −6.1(1) | [20] |
| [Cu(μ-3,4,5-Me3pz)]3 | 3.155, 3.272, 3.207 | 59.84, 58.27, 61.89 | 3.069(1), 3.069(1) | 174.6(2), 173.9(2), 174.7(2) | 3.2(5), 3.0(6), 5.4(5) | [21] |
| [Cu(4-Ph-μ-3,5-Me2pz)]3 •1/2(CHCl3) | 3.284, 3.214, 3.322 | 61.49, 60.29, 58.23 | 3.671(1), 3.494(1) | 178.7(2), 176.4(2), 176.3(2) | −10.3(4), 6.1(4),−10.6(4) | [22] |
| [Cu(4-I-μ-3,5-Me2pz)]3 | 3.214, 3.180, 3.202 | 60.10, 60.47, 59.42 | 3.897(4), 3.631(4) | 179.4(6), 177.9(5), 175.5(5) | −9(1), 2(1), 5(1) | [23] |
| [Cu(4-NO2-μ-3,5-Me2pz)]3 | 3.185, 3.255, 3.185 | 59.2, 59.2, 61.7 | 3.329(7), 3.329(7) | 177.6, 177.4(7), 177.4(7) | 8, 8(2), 8 | [24] |
| [Cu(μ-3-CF3pz)]3 | 3.216, 3.246, 3.231, 3.214, 3.248, 3.264 | 59.99, 59.54, 60.46, 60.68, 59.14, 60.18 | 3.100(2), 3.345(1) | 177.9(3), 174.5(3), 176.8(3), 178.2(3), 177.9(3), 178.1(3) | 5.9(9), −7.4(9), 2.1(8), −0.6(8), −0.6(8), −7.2(8) | [20] |
| [Cu(μ-3-CF3-5-Mepz)]3 | 3.2052, 3.2009, 3.2451 | 60.87, 59.63, 59.50 | 3.7040(5), 3.9150(6) | 178.7(1), 178.1(1), 179.0(1) | 1.9(3), 1.4(3), −3.8(3) | [20] |
| [Cu(μ-3-CF3-5-Phpz)]3 | 3.2197, 3.1473, 3.2580 | 61.54, 60.32, 58.14 | 3.8482 | 175.8(1), 175.3(1), 174.2(1) | −8.2(2), 6.5(2), 24.9(2) | [20] |
| [Cu(μ-3,5-(CF3)2pz)]3 | 3.232, 3.242, 3.221 | 59.67, 60.01, 60.33 | 3.879, 3.893 | 179.2(2), 179.0(2), 178.7(2) | 2.6(6), 2.8(6), −4.7(6) | [25] |
| [Cu(μ-3,5-(CF3)2pz)]3 | 3.2309, 3.2184, 3.2474 | 60.47, 59.96, 59.57 | 3.813(1), 3.987(1) | 178.4(1), 178.6(1), 178.4(1) | −7.0(3), 4.2(3), 6.3(3) | [20,26] |
| Complex | [Cu(μ-L5pz)]3 | [Cu(μ-L5pz)]3 •2(CH2Cl2) | [Cu(μ-L6pz)]3 •0.5(CH2Cl2) |
|---|---|---|---|
| CCDC number | 2,117,510 | 2,117,511 | 2,117,512 |
| Empirical formula | C45H33Cu3N6 | C47H37Cl4Cu3N6 | C30.5H28Cl1Cu3N6 |
| Formula weight | 848.43 | 1018.30 | 704.69 |
| Crystal system | Monoclinic | Monoclinic | Triclinic |
| Space group | P21/c (#14) | P21/n (#14) | (#2) |
| a/Å | 12.7491(8) | 13.5299(4) | 14.65231(15) |
| b/Å | 15.9322(11) | 14.4842(4) | 15.23581(19) |
| c/Å | 18.4769(13) | 22.9784(7) | 15.50482(13) |
| α/° | 90 | 90 | 117.8200(10) |
| β/° | 100.173(6) | 105.293(3) | 103.3700(8) |
| γ/° | 90 | 90 | 98.7900(10) |
| V/Å3 | 3694.0(4) | 4343.6(2) | 2838.24(7) |
| Z | 4 | 4 | 4 |
| Dcalc/g cm−3 | 1.525 | 1.557 | 1.649 |
| μ(MoKα)/cm−1 | 17.539 | 17.433 | 23.542 |
| Temperature/°C | −95.0 | −70.0 | −95.0 |
| 2θ range/° | 6–55 | 6–55 | 6–55 |
| Reflections collected | 56,293 | 34,206 | 92,558 |
| Unique reflections | 8478 | 9949 | 13,014 |
| Rint | 0.1526 | 0.0428 | 0.0284 |
| Number of variables | 487 | 541 | 730 |
| Refls./Para ratio | 17.41 | 18.39 | 17.83 |
| Residuals: R1 (I > 2 σ (I)) | 0.0747 | 0.0588 | 0.301 |
| Residuals: R (All refl.) | 0.1763 | 0.0805 | 0.0322 |
| Residuals: wR2 (All refl.) | 0.1568 | 0.1789 | 0.858 |
| Goodness of fit ind. | 1.035 | 1.026 | 1.039 |
| Max/min peak,/e Å−3 | 0.62/−0.54 | 2.08/−1.67 | 2.32/−1.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujisawa, K.; Saotome, M.; Ishikawa, Y.; Young, D.J. The Influence of Aryl Substituents on the Supramolecular Structures and Photoluminescence of Cyclic Trinuclear Pyrazolato Copper(I) Complexes. Nanomaterials 2021, 11, 3101. https://doi.org/10.3390/nano11113101
Fujisawa K, Saotome M, Ishikawa Y, Young DJ. The Influence of Aryl Substituents on the Supramolecular Structures and Photoluminescence of Cyclic Trinuclear Pyrazolato Copper(I) Complexes. Nanomaterials. 2021; 11(11):3101. https://doi.org/10.3390/nano11113101
Chicago/Turabian StyleFujisawa, Kiyoshi, Mai Saotome, Yoko Ishikawa, and David James Young. 2021. "The Influence of Aryl Substituents on the Supramolecular Structures and Photoluminescence of Cyclic Trinuclear Pyrazolato Copper(I) Complexes" Nanomaterials 11, no. 11: 3101. https://doi.org/10.3390/nano11113101
APA StyleFujisawa, K., Saotome, M., Ishikawa, Y., & Young, D. J. (2021). The Influence of Aryl Substituents on the Supramolecular Structures and Photoluminescence of Cyclic Trinuclear Pyrazolato Copper(I) Complexes. Nanomaterials, 11(11), 3101. https://doi.org/10.3390/nano11113101







