Accumulation and Effect of Silver Nanoparticles Functionalized with Spirulina platensis on Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanoparticles
2.2. Cyanobacterial Strain
2.3. Production of AgNPs Functionalized with Spirulina platensis Biomass (AgNPs—Spirulina)
2.4. Extraction of the Protein Fraction from Spirulina Biomass Enriched with AgNPs
2.5. Animals and Experimental Design
2.6. Methods for Nanoparticles Analysis and Silver Content Determination
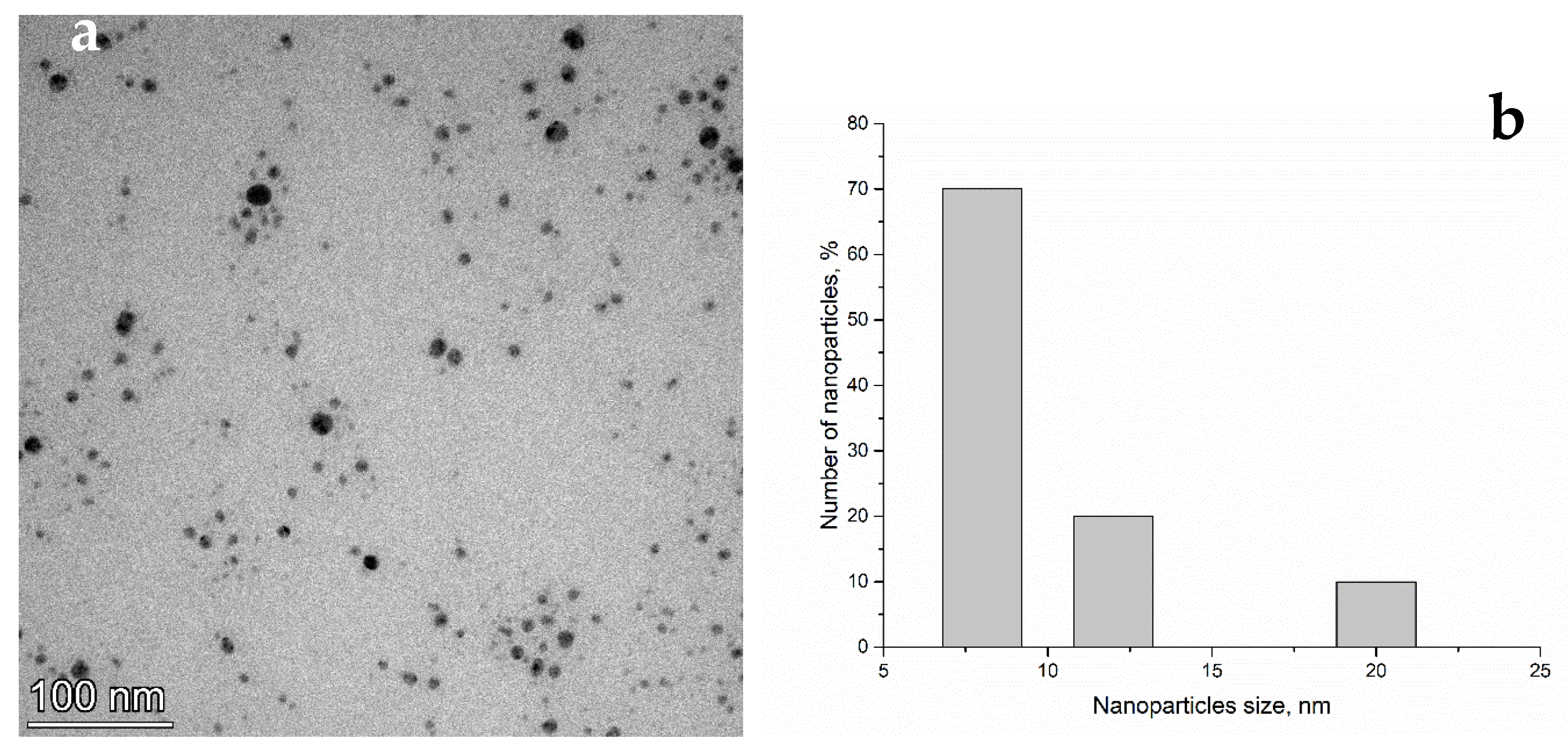
Transmission Electron Microscopy (TEM)
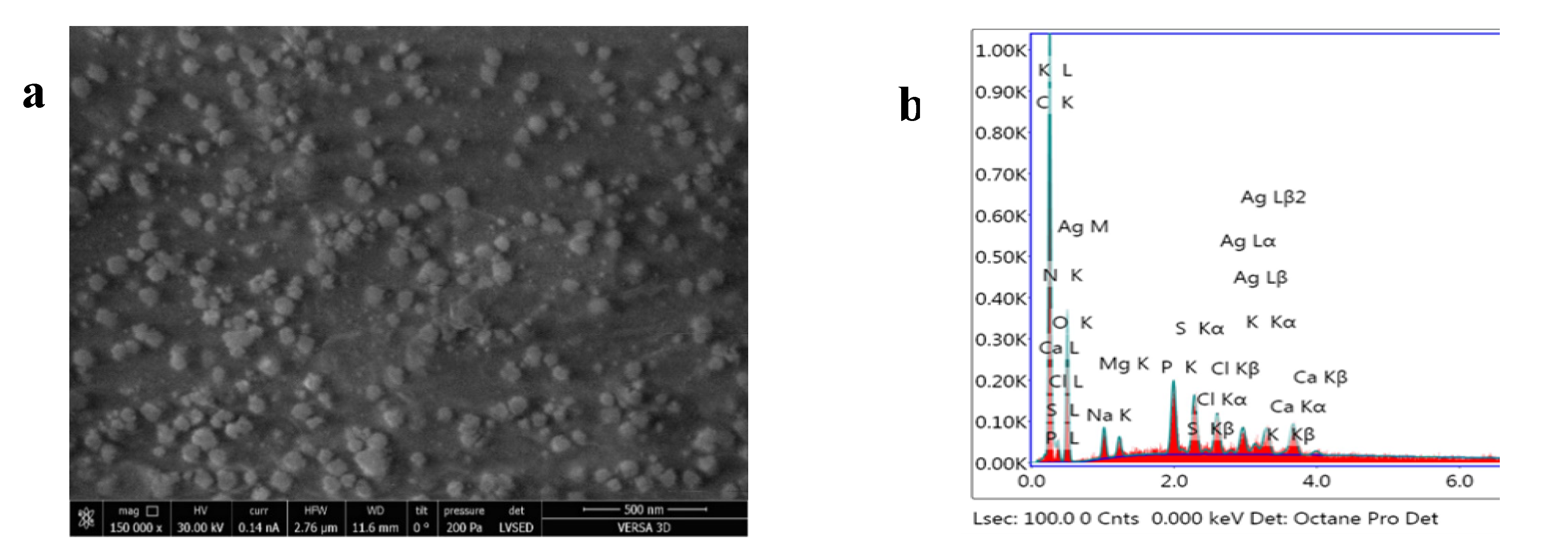
Scanning Electron Microscopy (SEM)
Energy-Dispersive Analysis of X-rays (EDAX)
Neutron Activation Analysis
2.7. Blood Hematology and Biochemistry
2.8. Statistical Analysis
3. Results
3.1. Nanoparticle Characterization
3.2. Characterization of AgNPs-Spirulina and Determination of the Concentration of Nanoparticles Administered to Animals
3.3. Biological Parameters of Animals and Organs after AgNPs and AgNPsSp Administration
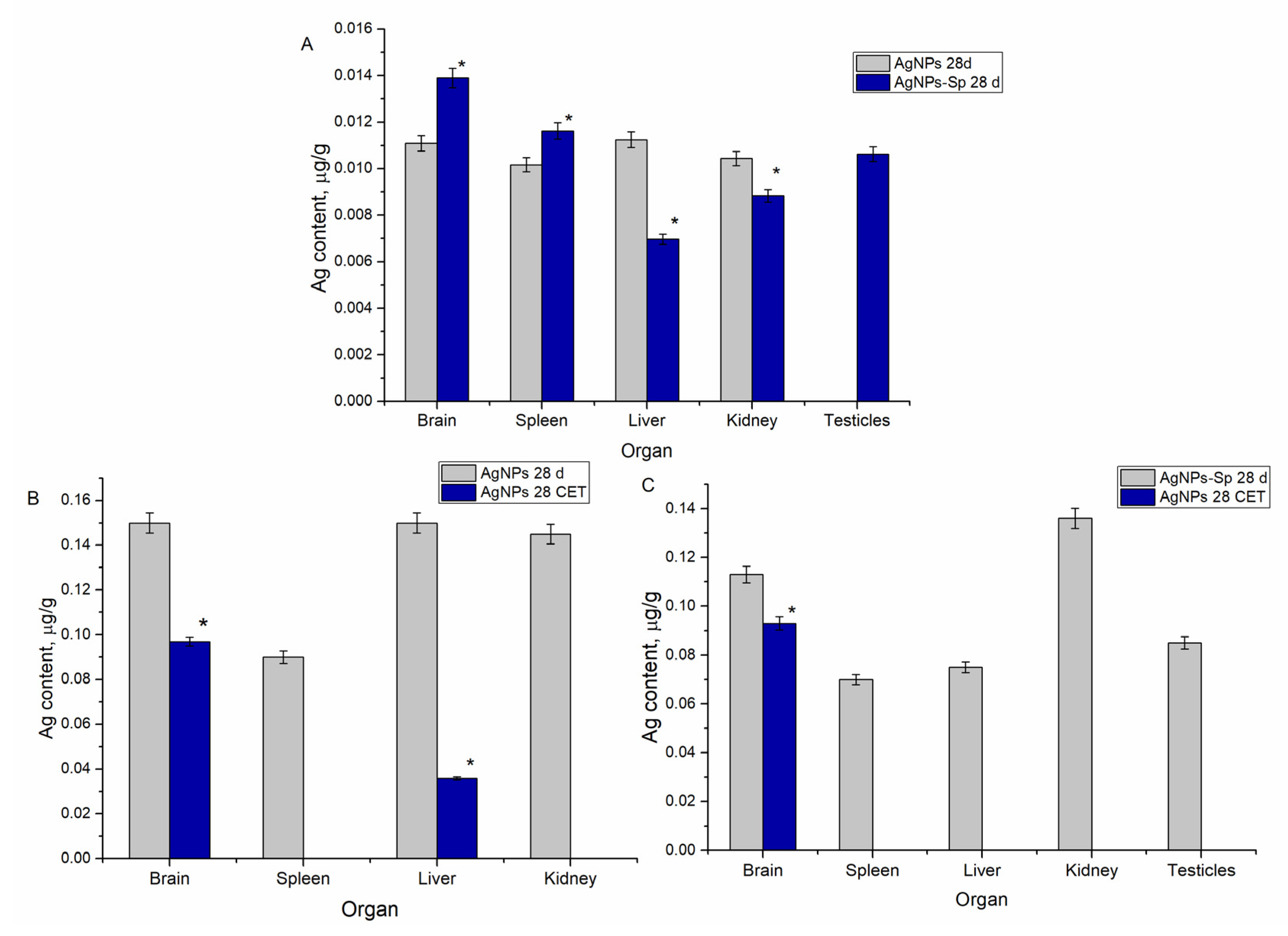
3.4. Content of Silver in Animal Organs Following Administration of AgNPs and after a Period of Clearance
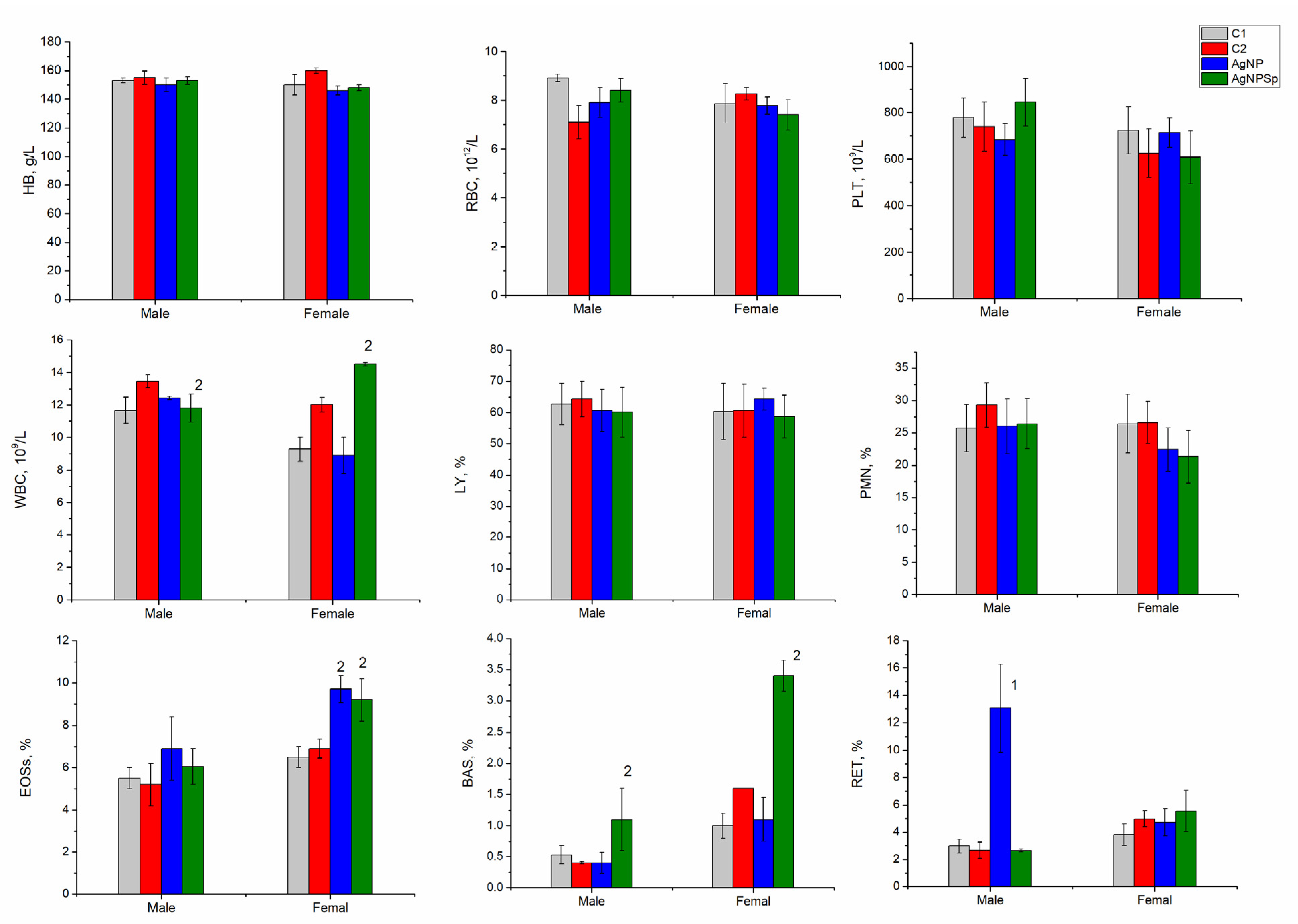
3.5. Hematological Test Results for Laboratory Animals Administrated with AgNPs
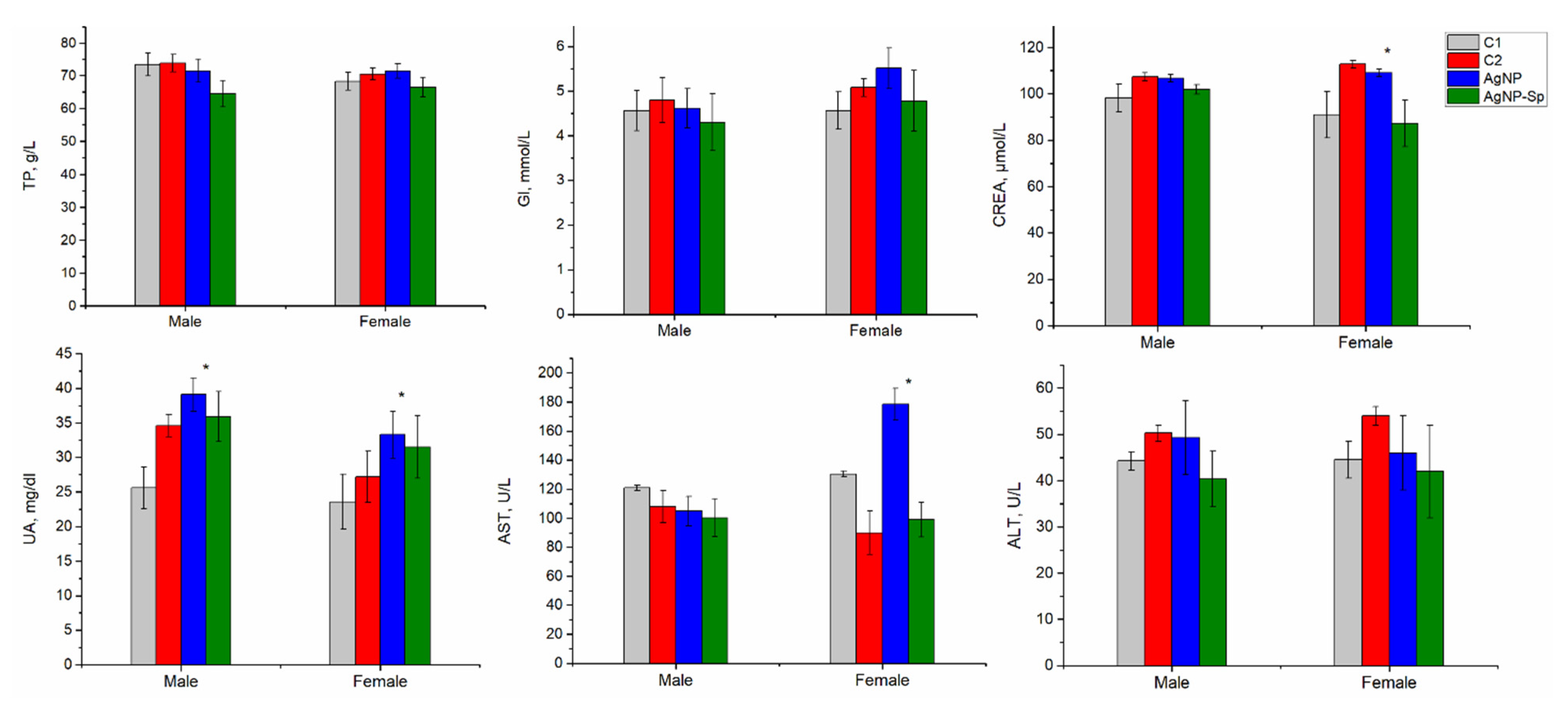
3.6. Biochemical Test Results for Laboratory Animals Administrated with AgNPs
3.7. Hematological and Biochemical Indicators in Laboratory Animals at the End of the Clearance Period
4. Discussion
5. Conclusions
- Spirulina biomass can be used for the biofunctionalization of AgNPs stabilized with polyethylene glycol, as approximately 80% of them were trapped to the proteinic fraction of biomass.
- Both types of nanoparticles (biofunctionalized using spirulina culture and AgNPs stabilized by polyethylene glycol) were accumulated in the brain, spleen, liver, and kidneys. Biofunctionalized particles showed a higher affinity for the brain and spleen, whereas the unmodified ones showed higher affinity for the liver and kidneys.
- There was no accumulation of nanoparticles in the ovaries, while in the testicles biofunctionalized nanoparticles were accumulated only. This selectivity can serve as a basis for the development of preparations based on targeted AgNPs.
- During the clearance period, silver in the form of AgNPs-Spirulina was excreted from all organs, except the brain (where 82.3% of accumulated content remained), whereas unmodified AgNPs were excreted completely from the spleen and kidneys, but 24% of the silver accumulated in the liver and 65% in the brain remained. Thus, both types of studied AgNPs easily crossed the blood–brain barrier in the direction of the brain, while the reverse flow was very low.
- Based on the level of silver accumulation and elimination from the kidneys and liver, the involvement of these two organs in AgNPs elimination and the preferential renal pathway in the case of AgNPs-Spirulina can be assumed.
- The hematological and biochemical parameters of blood in rats after administration of silver nanoparticles remained within the normal parameters for the species; however, statistically significant differences were observed for some of them.
- The change in the level of eosinophils, basophils, and reticulocytes indicated moderate immune reactions.
- The changes in the renal and hepatic parameters pointed to the probable toxicity of the studied nanoparticles relative to these organs.
- AgNPs should be used with caution by taking into account their persistence in the brain and evidence for some delayed or prolonged effects over time.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 147–166. [Google Scholar] [CrossRef]
- Calderón-Jiménez, B.; Johnson, M.E.; Montoro Bustos, A.R.; Murphy, K.E.; Winchester, M.R.; Baudrit, J.R.V. Silver nanoparticles: Technological advances, societal impacts, and metrological challenges. Front. Chem. 2017, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Keat, C.L.; Aziz, A.; Eid, A.M.; Elmarzugi, N.A. Biosynthesis of nanoparticles and silver nanoparticles. Bioresour. Bioprocess. 2015, 2, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver nanoparticles and their antibacterial applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Liao, S.; Zhang, Y.; Pan, X.; Zhu, F.; Jiang, C.; Liu, Q.; Cheng, Z.; Dai, G.; Wu, G.; Wang, L.; et al. Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa. Int. J. Nanomed. 2019, 14, 1469. [Google Scholar] [CrossRef] [Green Version]
- Almofti, M.R.; Ichikawa, T.; Yamashita, K.; Terada, H.; Shinohara, Y. Silver ion induces a cyclosporine A-insensitive permeability transition in rat liver mitochondria and release of apoptogenic cytochrome c. J. Biochem. 2003, 134, 43–49. [Google Scholar] [CrossRef]
- Antony, J.J.; Sivalingam, P.; Chen, B. Toxicological effects of silver nanoparticles. Environ. Toxicol. Pharmacol. 2015, 40, 729–732. [Google Scholar] [CrossRef]
- Haberl, N.; Hirn, S.; Wenk, A.; Diendorf, J.; Epple, M.; Johnston, B.D.; Krombach, F.; Kreyling, W.G.; Schleh, C. Cytotoxic and proinflammatory effects of PVP-coated silver nanoparticles after intratracheal instillation in rats. Beilstein J. Nanotechnol. 2013, 4, 933–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.F.; Wang, J.; Patterson, T.A.; Saini, U.T.; Robinson, B.L.; Newport, G.D.; Murdock, R.C.; Schlager, J.J.; Hussain, S.M.; Ali, S.F. Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles. Toxicol. Lett. 2009, 187, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Monopoli, M.P.; Bombelli, F.B.; Dawson, K.A. Nanoparticle coronas take shape. Nat. Nanotechnol. 2011, 6, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef] [Green Version]
- Ravindran, A.; Chandran, P.; Khan, S.S. Biofunctionalized silver nanoparticles: Advances and prospects. Colloids Surf. B Biointerfaces 2013, 105, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Dinparvar, S.; Bagirova, M.; Allahverdiyev, A.M.; Abamor, E.S.; Safarov, T.; Aydogdu, M.; Aktas, D. A nanotechnology-based new approach in the treatment of breast cancer: Biosynthesized silver nanoparticles using Cuminum cyminum L. seed extract. J. Photochem. Photobiol. B Biol. 2020, 208, 111902. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Raman, J.; Abd Malek, S.N.; John, P.A.; Vikineswary, S. Green synthesis of silver nanoparticles using Ganoderma neo-japonicum Imazeki: A potential cytotoxic agent against breast cancer cells. Int. J. Nanomed. 2013, 8, 4399–4413. [Google Scholar] [CrossRef] [Green Version]
- Opris, R.; Toma, V.; Olteanu, D.; Baldea, I.; Baciu, A.M.; Lucaci, F.I.; Berghian-Sevastre, A.; Tatomir, C.; Moldovan, B.; Clichici, S.; et al. Effects of silver nanoparticles functionalized with Cornus mas L. extract on architecture and apoptosis in rat testicle. Nanomedicine 2019, 14, 275–299. [Google Scholar] [CrossRef]
- Cepoi, L.; Rudi, L.; Chiriac, T.; Valuta, A.; Zinicovscaia, I.; Duca, G.H.; Kirkesali, E.; Frontasyeva, M.; Culicov, O.; Pavlov, S.; et al. Biochemical changes in cyanobacteria during the synthesis of silver nanoparticles. Can. J. Microbiol. 2014, 61, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Furmaniak, M.A.; Misztak, A.E.; Franczuk, M.D.; Wilmotte, A.; Waleron, M.; Waleron, K.F. Edible cyanobacterial genus Arthrospira: Actual state of the art in cultivation methods, genetics, and application in medicine. Front. Microbiol. 2017, 8, 2541. [Google Scholar] [CrossRef] [PubMed]
- Cepoi, L.; Zinicovscaia, I.; Rudi, L.; Chiriac, T.; Rotari, I.; Turchenko, V.; Djur, S. Effects of PEG-coated silver and gold nanoparticles on spirulina platensis biomass during its growth in a closed system. Coatings 2020, 10, 717. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Pavlov, S.S.; Frontasyeva, M.V.; Ivlieva, A.L.; Petritskaya, E.N.; Rogatkin, D.A.; Demin, V.A. Accumulation of silver nanoparticles in mice tissues studied by neutron activation analysis. J. Radioanal. Nucl. Chem. 2018, 318, 985–989. [Google Scholar] [CrossRef]
- Frontasyeva, M.V. Epithermal Neutron activation analysis at the ibr-2 reactor of the frank laboratory of neutron physics at the joint institute for nuclear research (Dubna). Phys. At. Nucl. 2008, 71, 1684–1693. [Google Scholar] [CrossRef]
- Delwatta, S.L.; Gunatilake, M.; Baumans, V.; Seneviratne, M.D.; Dissanayaka, M.L.B.; Batagoda, S.S.; Udagedara, A.H.; Walpola, P.B. Reference values for selected hematological, biochemical and physiological parameters of Sprague-Dawley rats at the Animal House, Faculty of Medicine, University of Colombo, Sri Lanka. Anim. Model. Exp. Med. 2018, 1, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Wolford, S.T.; Schroer, R.A.; Gohs, F.X.; Gallo, P.P.; Brodeck, M.; Falk, H.B.; Ruhren, R. Reference range data base for serum chemistry and hematology values in laboratory animals. J. Toxicol. Environ. Health 1986, 18, 161–188. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Tripathy, S.; Adhikary, J.; Chattopadhyay, S.; Mandal, D.; Dash, S.K.; Das, S.; Dey, A.; Dey, S.K.; Das, D.; et al. Surface modification minimizes the toxicity of silver nanoparticles: An in vitro and in vivo study. JBIC J. Biol. Inorg. Chem. 2017, 22, 893–918. [Google Scholar] [CrossRef] [PubMed]
- Garza-Ocañas, L.; Ferrer, D.A.; Burt, J.; Diaz-Torres, L.A.; Ramírez Cabrera, M.; Rodríguez, V.T.; Rangel, R.L.; Romanovicz, D.; Jose-Yacaman, M. Biodistribution and long-term fate of silver nanoparticles functionalized with bovine serum albumin in rats. Metallomics 2010, 2, 204–210. [Google Scholar] [CrossRef]
- Gurunathan, S.; Jeong, J.-K.; Han, J.W.; Zhang, X.-F.; Park, J.H.; Kim, J.-H. Multidimensional effects of biologically synthesized silver nanoparticles in Helicobacter pylori, Helicobacter felis, and human lung (L132) and lung carcinoma A549 cells. Nanoscale Res. Lett. 2015, 10, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Garcia, T.; Lafuente, D.; Blanco, J.; Sánchez, D.J.; Sirvent, J.J.; Domingo, J.L.; Gómez, M. Oral subchronic exposure to silver nanoparticles in rats. Food Chem. Toxicol. 2016, 92, 177–187. [Google Scholar] [CrossRef]
- Loeschner, K.; Hadrup, N.; Qvortrup, K.; Larsen, A.; Gao, X.; Vogel, U.; Mortensen, A.; Lam, H.R.; Larsen, E.H. Distribution of silver in rats following 28 days of repeated oral exposure to silver nanoparticles or silver acetate. Part. Fibre Toxicol. 2011, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Wen, H.; Dan, M.; Yang, Y.; Lyu, J.; Shao, A.; Cheng, X.; Chen, L.; Xu, L. Acute toxicity and genotoxicity of silver nanoparticle in rats. PLoS ONE 2017, 12, e0185554. [Google Scholar] [CrossRef] [Green Version]
- Qin, G.; Tang, S.; Li, S.; Lu, H.; Wang, Y.; Zhao, P.; Li, B.; Zhang, J.; Peng, L. Toxicological evaluation of silver nanoparticles and silver nitrate in rats following 28 days of repeated oral exposure. Environ. Toxicol. 2017, 32, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Recordati, C.; De Maglie, M.; Cella, C.; Argentiere, S.; Paltrinieri, S.; Bianchessi, S.; Losa, M.; Fiordaliso, F.; Corbelli, A.; Milite, G.; et al. Repeated oral administration of low doses of silver in mice: Tissue distribution and effects on central nervous system. Part. Fibre Toxicol. 2021, 18, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, J.S.; Cho, H.S.; Rha, D.S.; Kim, J.M.; Park, J.D.; Choi, B.S.; Lim, R.; Chang, H.K.; Chung, Y.H.; et al. Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal. Toxicol. 2008, 20, 575–583. [Google Scholar] [CrossRef]
- Hassanen, E.I.; Khalaf, A.A.; Tohamy, A.F.; Mohammed, E.R.; Farroh, K.Y. Toxicopathological and immunological studies on different concentrations of chitosan-coated silver nanoparticles in rats. Int. J. Nanomed. 2019, 14, 4723–4739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, W.H.; Van Der Ven, L.T.M.; Sleijffers, A.; Park, M.V.D.Z.; Jansen, E.H.J.M.; Van Loveren, H.; Vandebriel, R.J. Systemic and immunotoxicity of silver nanoparticles in an intravenous 28 days repeated dose toxicity study in rats. Biomaterials 2013, 34, 8333–8343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasgupta, N.; Ranjan, S.; Ramalingam, C.; Gandhi, M. Silver nanoparticles engineered by thermal co-reduction approach induces liver damage in Wistar rats: Acute and sub-chronic toxicity analysis. 3 Biotech 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Torous, D.K.; Avlasevich, S.L.; Khattab, M.G.; Baig, A.; Saubermann, L.J.; Chen, Y.; Bemis, J.C.; Lovell, D.P.; Walker, V.E.; MacGregor, J.T.; et al. Human blood PIG-A mutation and micronucleated reticulocyte flow cytometric assays: Method optimization and evaluation of intra- and inter-subject variation. Environ. Mol. Mutagen. 2020, 61, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Kuang, H.; Zhang, W.; Aguilar, Z.P.; Wei, H.; Xu, H. Comparisons of the biodistribution and toxicological examinations after repeated intravenous administration of silver and gold nanoparticles in mice. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
| AgNPs 28d | AgNPs CET | AgNPs-Sp 28d | AgNPs-Sp CET | |
|---|---|---|---|---|
| HB, g/L | 149 ± 4.67 | 148 ± 0.71 | 152 ± 0.67 | 148.5 ± 7.78 |
| RBC, 1012/L | 7.77 ± 0.11 | 8.07 ± 0.77 | 8.4 ± 0.61 | 7.99 ± 0.68 |
| WBC, 109/L | 10.82 ± 1.28 | 15.69 ± 3.98 | 11.72 ± 1.92 | 10.16 ± 1.7 |
| PLT, 109/L | 647.3 ± 68.2 | 886.5 ± 7.78 | 812.6 ± 106.2 | 730.0 ± 46.67 |
| PMN, % | 25.07 ± 2.49 | 48.25 ± 4.74 | 24.0 ± 2.81 | 33.7 ± 8.34 |
| LY % | 61.83 ± 3.49 | 36.35 ± 0.71 | 59.63 ± 1.51 | 49.7 ± 4.53 |
| EOS, % | 8.3 ± 1.98 | 4.1 ± 0.05 * | 7.60 ± 2.23 | 12.45 ± 5.45 |
| BAS, % | 0.75 ± 0.13 | 0.25 ± 0.07 * | 2.25 ± 1.63 | 0.35 ± 0.071 * |
| RET, % | 8.9 ± 5.3 | 2.95 ± 0.73 | 4.1 ± 2.05 | 2.93 ± 0.69 |
| TP, g/L | 75.17 ± 10.02 | 71.15 ± 11.19 | 65.03 ± 4.52 | 73.8 ± 9.48 |
| GL, mmol/L | 4.83 ± 0.61 | 6.87 ± 0.5 * | 4.42 ± 0.54 | 6.06 ± 0.83 * |
| CREA, µmol/L | 107.3 ± 12.42 | 105.5 ± 5.09 | 98.25 ± 7.3 | 98.25 ± 5.16 |
| UA, mg/dL | 37.67 ± 6.67 | 35.48 ± 1.85 | 34.82 ± 8.53 | 37.67 ± 2.47 |
| ALT, U/L | 47.65 ± 2.35 | 50.95 ± 10.21 | 41.15 ± 1.20 | 220.6 ± 42.3 * |
| AST, U/L | 141.66 ± 52.08 | 66.6 ± 8.72 | 99.6 ± 0.99 | 115.35 ± 29.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudi, L.; Zinicovscaia, I.; Cepoi, L.; Chiriac, T.; Peshkova, A.; Cepoi, A.; Grozdov, D. Accumulation and Effect of Silver Nanoparticles Functionalized with Spirulina platensis on Rats. Nanomaterials 2021, 11, 2992. https://doi.org/10.3390/nano11112992
Rudi L, Zinicovscaia I, Cepoi L, Chiriac T, Peshkova A, Cepoi A, Grozdov D. Accumulation and Effect of Silver Nanoparticles Functionalized with Spirulina platensis on Rats. Nanomaterials. 2021; 11(11):2992. https://doi.org/10.3390/nano11112992
Chicago/Turabian StyleRudi, Ludmila, Inga Zinicovscaia, Liliana Cepoi, Tatiana Chiriac, Alexandra Peshkova, Anastasia Cepoi, and Dmitrii Grozdov. 2021. "Accumulation and Effect of Silver Nanoparticles Functionalized with Spirulina platensis on Rats" Nanomaterials 11, no. 11: 2992. https://doi.org/10.3390/nano11112992









